Abstract
Background
Quantum computing and quantum machine learning (QML) are promising experimental technologies that can improve precision medicine applications by reducing the computational complexity of algorithms driven by big, unstructured, real-world data. The clinical problem of knee osteoarthritis is that, although some novel therapies are safe and effective, the response is variable, and defining the characteristics of an individual who will respond remains a challenge. In this study, we tested a quantum neural network (QNN) application to support precision data-driven clinical decisions to select personalized treatments for advanced knee osteoarthritis.
Methodology
After obtaining patients’ consent and Research Ethics Committee approval, we collected the clinicodemographic data before and after the treatment from 170 patients eligible for knee arthroplasty (Kellgren-Lawrence grade ≥3, Oxford Knee Score (OKS) ≤27, age ≥64 years, and idiopathic aetiology of arthritis) treated over a two-year period with a single injection of microfragmented fat. Gender classes were balanced (76 males and 94 females) to mitigate gender bias. A patient with an improvement ≥7 OKS was considered a responder. We trained our QNN classifier on a randomly selected training subset of 113 patients to classify responders from non-responders (73 responders and 40 non-responders) in pain and function at one year. Outliers were hidden from the training dataset but not from the validation set.
Results
We tested our QNN classifier on a randomly selected test subset of 57 patients (34 responders, 23 non-responders) including outliers. The no information rate was 0.59. Our application correctly classified 28 responders out of 34 and 6 non-responders out of 23 (sensitivity = 0.82, specificity = 0.26, F1 Statistic = 0.71). The positive and negative likelihood ratios were 1.11 and 0.68, respectively. The diagnostic odds ratio was 2.
Conclusions
Preliminary results on a small validation dataset showed that QML applied to data-driven clinical decisions for the personalized treatment of advanced knee osteoarthritis is a promising technology to reduce computational complexity and improve prognostic performance. Our results need further research validation with larger, real-world unstructured datasets, as well as clinical validation with an artificial intelligence clinical trial to test model efficacy, safety, clinical significance, and relevance at a public health level.
Keywords: quantum machine learning, biologic therapies, personalised precision medicine, joint preservation, osteoarthritis knee
Introduction
Applied research has shown that quantum computing applied to machine learning is a novel experimental digital medicine technology [1] which can reduce computational complexity [2,3] and improve data-driven clinical decisions [4].
Several biologic therapies, including the injection of microfragmented adipose tissue, have been shown to be safe and effective [5-7]. The outcomes of these treatments can be variable [8], with some patients showing dramatic improvements in their symptoms while others failing to respond [9].
Defining the characteristics of individuals who will respond remains a challenge. Applications and tools to support clinical decisions with predictive, data-driven, precision medicine can identify patients who will respond with reduction in pain and improvement in function to novel as well as established treatments based on pre-treatment clinicodemographic data [9]. This approach is key in developing personalized, evidence-based clinical pathways.
An earlier version of this article was previously posted to the medRxiv preprint server in 2021 [10].
Materials and methods
Ethics statement
This study was conducted in compliance with the rules of the Helsinki Declaration and International Ethical Regulations [11], including all subsequent amendments, under the approval of the Research Ethics Committee of the George Emil Palade University of Medicine, Pharmacy, Science and Technology of Targu Mures, Romania (approval number: 1464/2021).
Dataset
After obtaining patients’ consent and Research Ethics Committee approval, we collected clinicodemographic data before and after the treatment from 170 patients eligible for knee arthroplasty (Kellgren-Lawrence grade ≥3, Oxford Knee Score (OKS) ≤27, age ≥64 years, and idiopathic aetiology of arthritis) treated over a two-year period with a single injection of microfragmented fat (Table 1). Gender classes were balanced (76 males and 94 females) to mitigate gender bias. A patient with an improvement ≥7 OKS was considered a responder.
Table 1. Baseline data.
Age on procedure, preoperative Oxford Knee Score (OKS), and Kellgren-Lawrence (KL) grade of knee osteoarthritis. Source dataset 1464/2021.
| Mean ± SD | Range (minimum–maximum) | |
| Age | 74 ± 7 | 65–92 |
| Preoperative OKS | 21 ± 6 | 18–27 |
| KL grade | 4 ± 0.4 | 3–4 |
Oxford Knee Score
OKS comprises 12 questions that are scored 0-4, with 0 being severe compromise and 4 no compromise, covering pain and function of the knee. The best outcome is a score of 48 and the worst score possible is 0. This is a validated score or a measure of functional outcomes in patients undergoing knee arthroplasty. All 170 patients completed questionnaires before and at three months, six months, and one year following treatment [12].
Kellgren-Lawrence classification of the severity of osteoarthritis
The Kellgren-Lawrence system is a common method of classifying the severity of osteoarthritis using the following five grades [13]: (1) grade 0 (none): definite absence of X-ray changes of osteoarthritis; (2) grade 1 (doubtful): doubtful joint space narrowing and possible osteophytic lipping; (3) grade 2 (minimal): definite osteophytes and possible joint space narrowing; (4) grade 3 (moderate): moderate multiple osteophytes, definite narrowing of the joint space, and some sclerosis and possible deformity of bone ends; and (5) grade 4 (severe): large osteophytes, marked narrowing of the joint space, severe sclerosis, and definite deformity of bone ends. Osteoarthritis is deemed present at grade 2 although of minimal severity 1.
Model and circuit description
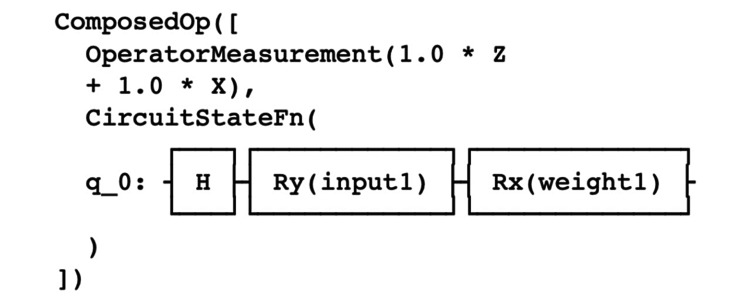
We applied the approach to quantum problem-solving developed by Zickert [14]. We utilized a Parameterized Quantum Circuit (PQC) based on Quantum Machine Learning Neural Networks Classifier (QNN) technology developed by IBM and Qiskit Machine Learning. More specifically, we utilized OpflowQNN which is a neural network-based application for the evaluation of quantum mechanical observables. The OpflowQNN circuit is described in Figure 1.
Figure 1. Circuit description. Source: IBM, Qiskit, and OpflowQNN.
Copyright notice: our code is an alteration of Qiskit Machine Learning which is a Copyright of IBM 2017, 2021. Qiskit is licensed under the Apache License, Version 2.0. All modifications of the code and derivative works are the responsibility of the authors.
q 0 = qubit initialization; Z, X = Pauli Z and X gates; H = Hadamard gate; Ry = single-qubit rotation gate through angle θ (radians) around the y-axis; Rx = single-qubit rotation gate through angle θ (radians) around the x-axis
Model training
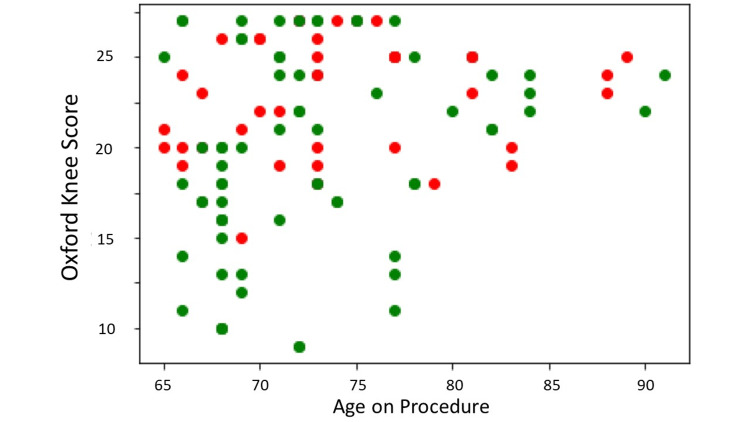
We trained our QNN classifier on a randomly selected training subset of 113 patients treated over a two-year period with a single injection of microfragmented fat classified as responders (R; OKS improvement ≥7) and non-responders (NR; OKS improvement <7) in pain and function at one year (73 R, 40 NR). Outliers were hidden from the training dataset but not from the validation set (Figure 2).
Figure 2. Training dataset.
X-axis = age on procedure; Y-axis = preoperative Oxford Knee Score; Green dots = responders with an improvement of ≥7 points in the Oxford Knee Score at year one; Red dots = non-responders with an improvement of <7 points in the Oxford Knee Score at year one. Source Dataset 1464/2021.
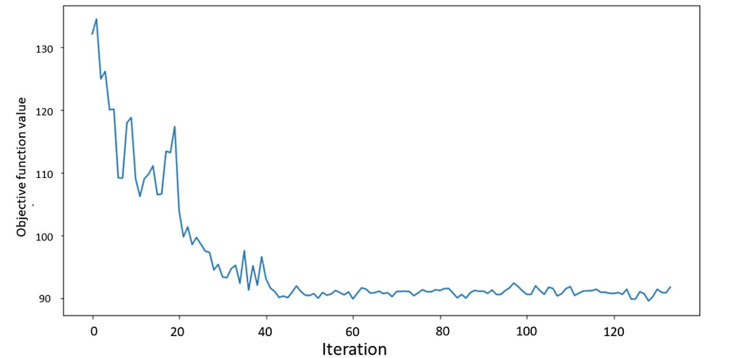
We initialized a 2-qubit Aer Simulator Backend with 1,024 shots. We utilized a Constrained Optimization BY Linear Approximation (COBYLA) optimizer to minimize the objective function value (Figure 3).
Figure 3. Objective function value against Iteration.
Constrained Optimization BY Linear Approximation11 (COBYLA) optimizer to minimize the objective function value. Source: IBM, Qiskit, and OpflowQNN.
Model validation
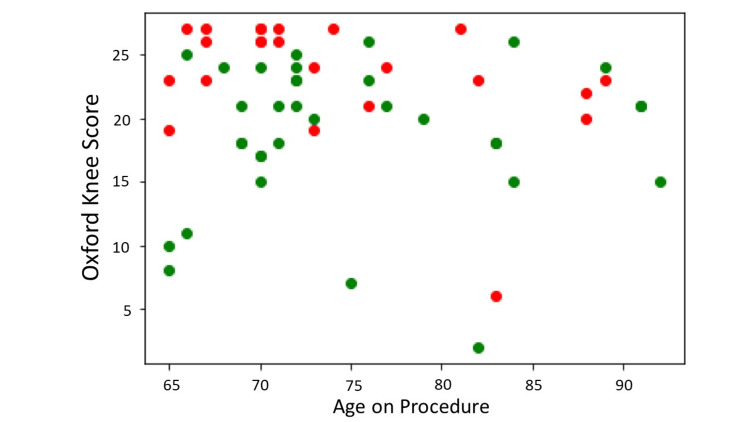
The application was tested on a balanced raw dataset including outliers of 57 patients (34 R, 23 NR) with Kellgren- Lawrence grades of 3 and 4, OKS ≤s27, age ≥64 years, and idiopathic aetiology of arthritis (Figure 4, Table 2).
Table 2. Test performance metrics. Source precisionKNEE QNN applied to the test set from dataset 1464/2021.
LR+ = positive likelihood ratio; LR- = negative likelihood ratio; DOR = diagnostic odds ratio
| Metric | Value |
| Sensitivity | 0.8235 |
| Specificity | 0.2609 |
| LR+ | 1.1142 |
| LR- | 0.6765 |
| DOR | 2.0000 |
| F1 | 0.7088 |
Figure 4. Test dataset.
X-axis = age on procedure; Y-axis = preoperative Oxford Knee Score; Green dots = responders with an improvement of ≥7 points in the Oxford Knee Score at year one; Red dots = non-responders with an improvement of <7 points in the Oxford Knee Score at year one. Source Dataset 1464/2021.
Results
Model results
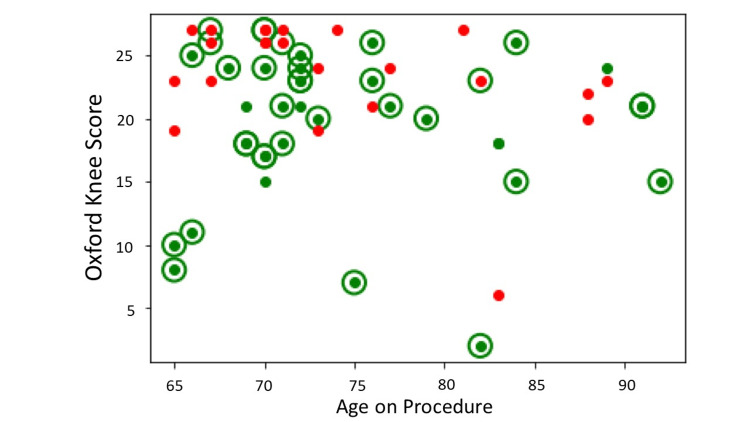
Our application correctly classified 28 responders out of 34 and 6 non-responders out of 23 (sensitivity = 0.82, specificity = 0.26, F1 statistic = 0.71). The positive (LR+) and negative (LR-) likelihood ratios were 1.11 and 0.68, respectively. The diagnostic odds ratio was 2 (Table 3, Figure 5).
Table 3. Confusion matrix.
Source precisionKNEE QNN applied to the test set from dataset 1464/2021.
R = responders; NR: non-responders
| Predicted | ||||
| R | NR | Observed | ||
| Observed | R | 28 | 6 | 34 |
| NR | 17 | 6 | 23 | |
| Predicted | 45 | 12 | 57 | |
Figure 5. Classification with precisionKNEE_QNN.
X-axis = age on procedure; Y-axis = preoperative Oxford Knee Score; Green dots = responders with an improvement of ≥7 points in the Oxford Knee Score at year one; Red dots = non-responders with an improvement of <7 points in the Oxford Knee Score at year one; Green circles = correct classification of either responders or non- responders; No circles = incorrect classification of either responders or non-responders. Source dataset 1464/2021.
Model interpretation
The use of the precisionKNEE QNN clinical decision-making tool requires external validation. This is a new technology best suited to large unstructured datasets. Some of the traditional methods of validation may not be appropriate for quantum machine learning tools such as ours [15].
The SPIRIT-AI [16], and CONSORT-AI [17] initiatives provide guidance to improve the transparency and completeness of reporting clinical trials evaluating interventions involving artificial intelligence. The utility of these initiatives is not clear for use in quantum machine learning.
Discussion
Since its original proposal by Richard Feinman [18], quantum computing has evolved from a concept to a practical technology [19] which is now being actively utilised with the potential to solve some of the more complex problems. These probably fall into the following three categories: (1) simulating nature - including chemistry, materials science, and physics; (2) processing data with a complex structure - including artificial intelligence/machine learning (AI/ML), factoring, and ranking; and (3) search and optimization - including pricing, risk analysis, and sampling [20].
A recent detailed review of quantum computer literature associated with the medical sciences [20] detailed three broad areas that most studies fit into, which include genomics and clinical research, diagnostics, and treatments and interventions.
Our study falls into the treatments and interventions category where we tested a QNN application to support precision data-driven clinical decisions to select personalized treatments for advanced knee osteoarthritis. QNNs were applied to clinicodemographic data from 170 individuals who were treated with microfragmented fat and followed up for over two years. In the assessment of the test’s diagnostic accuracy (Table 2), it was found to have a sensitivity of 82.35%, indicating a reasonably high ability to detect true positives. However, its specificity was notably low at 26.09%, suggesting a substantial rate of false positives. The LR+ of 1.1142 and LR- of 0.6765 further indicated that the test was only marginally more effective than chance in distinguishing between the presence and absence of the condition. The DOR was 2.0, reinforcing the test’s limited discriminative power. Despite these limitations, the F1 score of 0.7088 showed a moderate balance between precision and recall, reflecting an average overall accuracy in the test’s performance.
Healthcare practices today often rely on inferring insights from extensive datasets and applying these to individual cases. This approach is more refined than historical methods but still falls short of providing personalized treatment for each unique patient. The intricate nature of human biology means that medical interventions typically contribute just 10% to 20% to health outcomes. The remaining 80% to 90% is influenced by factors such as lifestyle choices, socioeconomic status, and environmental conditions [21].
There are many intersectional challenges at the nexus of quantum computing and healthcare, which will necessitate the refinement of quantum algorithms to adeptly manage complex datasets characterized by high dimensionality and variability [22], while simultaneously striving to minimize computational errors through enhanced physical devices and sophisticated software techniques. A pivotal goal is to expand quantum computational resources by increasing the available qubits, thereby enriching the capacity to encode complex features.
In tandem with these quantum-specific challenges, digital health strategies must prioritize transparent model explainability to demystify AI decision-making processes, ensuring that patient sovereignty over personal health data is uncompromised. There are indeed a multiplicity of technical and ethical challenges to the full deployment of quantum-enhanced health and medicine. This challenge has been taken out by the Cleveland Clinic and their collaboration with IBM to embed quantum computation into the workflow of providing personalised precision medicine [23].
Alongside modern technologies, it is important to recognise that there are broad and well-established principles that must be borne in mind when aiming to improve healthcare with the simultaneous pursuit of three aims, namely, improving the experience of care, improving the health of populations, and reducing per-capita costs of healthcare [24]. The more recent addition of the health and well-being of healthcare professionals [25] is a testament to how integral these individuals are in the delivery of healthcare as well as the innovation required to deliver the highly technological aspects of personalised precision medicine.
Limitations
Despite being one of the largest datasets of its kind, upon stratification we observed groups of patients with minimal observations and thus we cannot conclude external validity in the findings pertaining to these groups. Kellgren-Lawrence classification of arthritis was found to be an important feature during modelling. This measure is crude and correlates poorly with patient symptoms.
Microfragmented fat was the only treatment used in our model. With the inclusion of other treatment modalities, a true clinical decision-making support tool can be developed. Patients find these very useful and by providing a personalised prediction of the possible outcome, they will be able to play a more meaningful role in the decision-making process.
Conclusions
Preliminary results on a small validation dataset show that quantum machine learning applied to data-driven clinical decisions for the personalized treatment of advanced knee osteoarthritis is a promising technology to reduce computational complexity and improve prognostic performance.
Our results need further research validation with larger, real-world unstructured datasets, and clinical validation with an AI clinical trial to test model efficacy, safety, clinical significance, and relevance at a public health level.
Acknowledgments
We acknowledge Angela Cullen as the Data Manager and Analyst. Requests for the raw data will be considered on a case-by-case basis.
Some parts of the Quantum Circuits and Artificial Intelligence algorithms are copyrighted and the authors are likely to patent some of the contents.
Funding Statement
The authors (N.H., S.O., and D.M.) declare that they have received support for the present manuscript in terms of the provision of data and study materials. In the past 36 months, the authors have received grants from public research institutions and contracts from biomedical device companies pertaining to the fields of digital health, digital medicine, and osteoarthritis. The authors have received and are likely to receive in the future royalties, licenses, consulting fees, payment, or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events. Some parts of the Quantum Circuits and Artificial Intelligence algorithms are copyrighted and the authors are likely to patent some of the content. The authors hold stock and stock options directly and indirectly in publicly traded and private biotech, medtech, digital health, and healthcare companies. The authors declare no other competing interests.
Author Contributions
Concept and design: Nima Heidari, Mark Slevin, Leonard Azamfirei, Stefano Olgiati, Davide Meloni
Acquisition, analysis, or interpretation of data: Nima Heidari, Ali Noorani, Stefano Olgiati, Davide Meloni, Federico Pirovano
Drafting of the manuscript: Nima Heidari, Mark Slevin, Ali Noorani, Stefano Olgiati, Federico Pirovano
Critical review of the manuscript for important intellectual content: Nima Heidari, Leonard Azamfirei, Stefano Olgiati, Davide Meloni
Supervision: Nima Heidari, Mark Slevin, Leonard Azamfirei, Stefano Olgiati
Human Ethics
Consent was obtained or waived by all participants in this study. Research Ethics Committee, George Emil Palade University of Medicine, Pharmacy, Science and Technology issued approval 1464/2021
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Biology and medicine in the landscape of quantum advantages. Cordier BA, Sawaya NP, Guerreschi GG, McWeeney SK. J R Soc Interface. 2022;19:20220541. doi: 10.1098/rsif.2022.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quantum algorithm for quicker clinical prognostic analysis: an application and experimental study using CT scan images of COVID-19 patients. Sengupta K, Srivastava PR. BMC Med Inform Decis Mak. 2021;21:227. doi: 10.1186/s12911-021-01588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quantum circuits-an application in qiskit-python. Singh PN, Aarthi S. 2021 Third International Conference on Intelligent Communication Technologies and Virtual Mobile Networks. 2021:661–667. [Google Scholar]
- 4.The potential of quantum computing and machine learning to advance clinical research and change the practice of medicine. Solenov D, Brieler J, Scherrer JF. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6205278/ Mo Med. 2018;115:463–467. [PMC free article] [PubMed] [Google Scholar]
- 5.Patient-centered outcomes of microfragmented adipose tissue treatments of knee osteoarthritis: an observational, intention-to-treat study at twelve months. Heidari N, Noorani A, Slevin M, et al. Stem Cells Int. 2020;2020:8881405. doi: 10.1155/2020/8881405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gender-specific response in pain and function to biologic treatment of knee osteoarthritis: a gender-bias-mitigated, observational, intention-to-treat study at two years. Borg TM, Heidari N, Noorani A, et al. Stem Cells Int. 2021;2021:6648437. doi: 10.1155/2021/6648437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Microfragmented adipose tissue injection (MFAT) may be a solution to the rationing of total knee replacement: a prospective, gender-bias mitigated, reproducible analysis at two years. Heidari N, Borg TM, Olgiati S, et al. Stem Cells Int. 2021;2021:9921015. doi: 10.1155/2021/9921015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finnish Degenerative Meniscal Lesion Study (FIDELITY): a protocol for a randomised, placebo surgery controlled trial on the efficacy of arthroscopic partial meniscectomy for patients with degenerative meniscus injury with a novel 'RCT within-a-cohort' study design. Sihvonen R, Paavola M, Malmivaara A, Järvinen TL. BMJ Open. 2013;3:0. doi: 10.1136/bmjopen-2012-002510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.A gender-bias-mitigated, data-driven precision medicine system to assist in the selection of biological treatments of grade 3 and 4 knee osteoarthritis: development and preliminary validation of precisionKNEE. Heidari N, Parkin JM, Olgiati S, et al. medRxiv. 2021 doi: 10.7759/cureus.55832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A quantum-enhanced precision medicine application to support data-driven clinical decisions for the personalized treatment of advanced knee osteoarthritis: development and preliminary validation of precisionKNEE_QNN. Heidari N, Olgiati S, Meloni D, Pirovano F, Noorani A, Slevin M, Azamfirei L. https://www.medrxiv.org/content/10.1101/2021.12.13.21267704v1. medRxiv. 2021 doi: 10.7759/cureus.52093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2566407/pdf/11357217.pdf. Bull World Health Organ. 2001;79:373–374. [PMC free article] [PubMed] [Google Scholar]
- 12.Questionnaire on the perceptions of patients about total knee replacement. Dawson J, Fitzpatrick R, Murray D, Carr A. J Bone Joint Surg Br. 1998;80:63–69. doi: 10.1302/0301-620x.80b1.7859. [DOI] [PubMed] [Google Scholar]
- 13.Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Kohn MD, Sassoon AA, Fernando ND. Clin Orthop Relat Res. 2016;474:1886–1893. doi: 10.1007/s11999-016-4732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zickert F. PyQML. PyQML; 2021. Hands-on Quantum Machine Learning With Python. [Google Scholar]
- 15.Hidary JD. Quantum Computing: An Applied Approach.Springer. Cham: Springer; 2019. Quantum Computing: An Applied Approach. [Google Scholar]
- 16.Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI Extension. Rivera SC, Liu X, Chan AW, Denniston AK, Calvert MJ. BMJ. 2020;370:0. doi: 10.1136/bmj.m3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Liu X, Cruz Rivera S, Moher D, Calvert MJ, Denniston AK. Lancet Digit Health. 2020;2:0–48. doi: 10.1016/S2589-7500(20)30218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simulating physics with computers. Feynman RP. Int J Theor Phys. 1982;21:467–488. [Google Scholar]
- 19.Quantum computing: A taxonomy, systematic review and future directions. Gill SS, Kumar A, Singh H, Singh M, Kaur K, UsmanMand Buyya R. Software Pract Exp. 2022;52:66–114. [Google Scholar]
- 20.The state of quantum computing applications in health and medicine. Flöther FF. Res Directions Quantum Technol. 2023;1:0. [Google Scholar]
- 21.County health rankings: relationships between determinant factors and health outcomes. Hood CM, Gennuso KP, Swain GR, Catlin BB. https://www.ajpmonline.org/article/S0749-3797(15)00514-0/fulltext. Am J Prev Med. 2016;50:129–135. doi: 10.1016/j.amepre.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Quantum computing's potential for drug discovery: early stage industry dynamics. Zinner M, Dahlhausen F, Boehme P, Ehlers J, Bieske L, Fehring L. Drug Discov Today. 2021;26:1680–1688. doi: 10.1016/j.drudis.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Potential of quantum computing for drug discovery. Cao Y, Romero J, Aspuru-Guzik A. IBM J Res Dev. 2018;6:6. [Google Scholar]
- 24.The triple aim: care, health, and cost. Berwick DM, Nolan TW, Whittington J. Health Aff (Millwood) 2008;27:759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 25.From triple to quadruple aim: care of the patient requires care of the provider. Bodenheimer T, Sinsky C. Ann Fam Med. 2014;12:573–576. doi: 10.1370/afm.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]