Abstract
Indazoles are a class of heterocyclic compounds with a bicyclic ring structure composed of a pyrazole ring and a benzene ring. Indazole-containing compounds with various functional groups have important pharmacological activities and can be used as structural motifs in designing novel drug molecules. Some of the indazole-containing molecules are approved by FDA and are already in the market. However, very few drugs with indazole rings have been developed against cardiovascular diseases. This review aims to summarize the structural and pharmacological functions of indazole derivatives which have shown efficacy against cardiovascular pathologies in experimental settings.
Keywords: Indazole derivatives, Cardiovascular diseases, Molecular mechanism, Cardiomyopathy, Drug discovery, N. sativa
1. INTRODUCTION
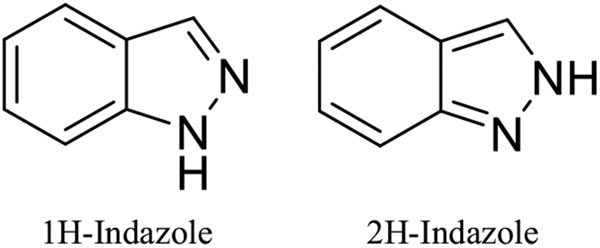
Indazoles, a class of heterocyclic compounds with a bicyclic ring structure composed of a pyrazole ring and a benzene ring, have a wide range of biological and pharmacological applications. Indazole was first defined by scientist Emil Fisher as a “pyrazole ring fused with the benzene ring.” Indazoles belong to the azoles family and contain carbon, hydrogen, and nitrogen atoms. There are generally two tautomeric variants of indazole, 1H-indazole, and 2H-indazole (Fig. 1). The main tautomer is 1H-indazole, which is thermodynamically more stable than 2H-indazole [1].
Fig. (1).
Two tautomeric variants of indazole: 1H-indazole and 2H-indazole.
Because of its unique chemical and biological features, indazole derivatives have been intensively researched. This nucleus, found in a number of synthetic compounds, has a wide range of pharmacological properties, e.g., metabolic (antihyperlipidemic, anti-obesity), cardiovascular (antiarrhythmic, antihypertensive), antimicrobial (antifungal, anti-bacterial, trichomonacidal), central nervous system (CNS) (antipsychotic, analgesic and antipyretic), anticancer as well as other activities (e.g. against rheumatoid arthritis) [2-15].
2. PHARMACOLOGY OF INDAZOLE DERIVATIVES
Indazole derivatives (Table 1) are important pharmacological agents and form the basic structure of several drug molecules. Indazole derivatives are building blocks of numerous therapeutic compounds as well, such as edaravone (MCI-186) for the treatment of amyotrophic lateral sclerosis (ALS) (NCT00424463, NCT00424463) [16]. Other examples of indazole derivatives are granisetron and benzydamine used as 5HT3 receptor antagonists (as an anti-emetic) and an anti-inflammatory agent, respectively. The indazole derivatives can be functionalized with high selectivity at different positions. Changing the planarity of the indazole ring, modifying the structure with side chain, and changing their length at different positions can afford a huge number of indazole derivatives, providing novel molecules with varieties of therapeutic properties. We will discuss different indazole derivatives obtained from natural products and synthetic sources.
Table 1.
Indazole derivatives explored in cardiovascular diseases.
| S. No | Indazole Derivative | Therapeutic Use | References |
|---|---|---|---|
| 1 | 7-nitroindazole | Vagal bradycardia/ hypertension | [59, 73] |
| 2 | DY-9760e 3-[2-[4-(3-Chloro-2-methylphenyl)-1-piperazinyl]ethyl]-5,6-dimethoxy-1-(4-imidazolylmethyl)-1H-indazole Dihydrochloride 3.5 Hydrate | Myocardial I/R injury Left ventricular hypertrophy | [31] |
| 3 | YC-1 | Hypoxia-induced cardiomyocytes | [74] |
| 4 | ARRY-797 / ARRY371797 -(2,4-difluorophenoxy)-N-[2-(dimethylamino)ethyl]-1-(2-methylpropyl)indazole-6-carboxamide | LMNA-related dilated cardiomyopathy | [38] |
| 5 | Indazole-Cl | Hypoxia -atherosclerosis | [44] |
| 6 | 7-nitroindazole, 7-Me-marsanidine | Hypertensive | [75, 76] |
| 7 | 7-chloro-1-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-1H-indazole (TCS-80) | Hypertension | [62] |
| 8 | (8R)-1-[(2S)-2-Aminopropyl]-8,9-dihydro-7H-pyrano [2,3-g]indazol-8-ol | Ocular hypotensive | [77] |
| 9 | Compound 38 | Hypertension and insulin resistance | [78] |
| 10 | PF-3882845 (3S,3aR)-2-(3-Chloro-4-cyanophenyl)-3-cyclopentyl-3,3a,4,5-tetrahydro-2H-benzo[g]indazole-7-carboxylic Acid | Hypertension and nephropathy | [79] |
| 11 | indazole, 5-aminoindazole and 6 -nitroindazole | Inflammation | [80] |
| 12 | 2-(1H-indazole-5-yl)amino-4-methoxy-6-piperazino triazine (DW1865) | Rho kinase inhibitor to attenuate stress fiber formation, cellular hypertrophy, and hypertension. | [81] |
| 13 | 6-Chloro-5-[4-(1-hydroxycyclobutyl)phenyl]-1H-indole-3-carboxylic Acid (PF-06409577) | Diabetic nephropathy | [82] |
| 14 | Compounds 10,11, 13 | Selective β3-AR agonistic activity against overactive bladder disorder. | [83] |
2.1. Natural Product Containing Indazole Ring
Nigella sativa (family: Ranunculaceae) is commonly known as black seed [17]. The extracts from N. sativa have been extensively explored for their therapeutic potential and shown to possess a wide spectrum of activities, viz. antihypertensive, bronchodilator, diuretic, renal protective, and antioxidant properties. Seeds from Nigella glandulifera contain pyrazole alkaloids or indazole ring-bearing alkaloids which include nigellicine, nigellidine, and nigeglanine (Fig. 2) [18-20]. The only natural source of indazole alkaloids is nigella species [21].
Fig. (2).
Indazole ring bearing alkaloids present in Nigella sativa.
2.2. Indazole Derivatives Derived from the Synthetic Route
2.2.1. DY-9760e
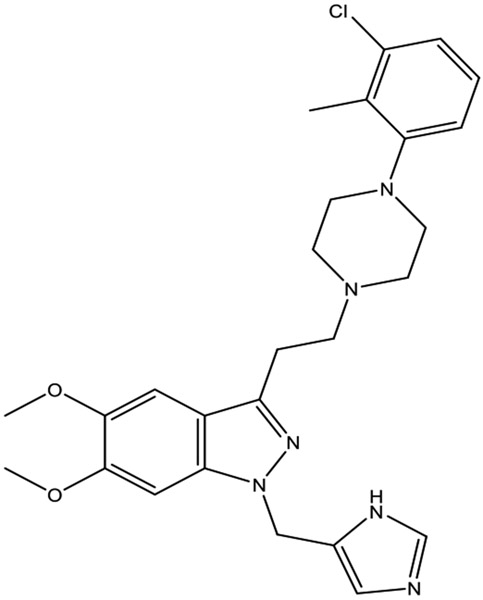
DY-9760e, 3-[2-[4-(3-chloro-2-methylphenyl)-1-piperazinyl] ethyl]-5,6-dimethoxy-1-(4-imidazolylmethyl)-1H-indazole dihydrochloride 3.5 hydrate, is a novel calmodulin antagonist (Fig. 3) [22]. DY-9760e has shown cardioprotection from ischemic/reperfusion injury.
Fig. (3).
Chemical structure of DY-9760e.
The activation of the CaMKII (Ca2+/calmodulin-dependent protein kinase II) is essential for myocardial contraction. Alteration of CaMKII activity in cardiomyocytes may result in myocardial disorder [23, 24]. For example, excessive activation of CaMKII can be characterized by increased activity of Ca2+ channel gating, leakage of Ca2+ from sarcoplasmic reticulum, and dysregulation of Ca2+ homeostasis, which may together cause arrhythmia and heart failure. Hashimoto et al. reported that increased levels of intracellular calcium due to ischemia activate the Ca2+ dependent calpains, also known as cysteine proteases, leading to degradation of structural proteins such as fodrin, thereby resulting in contractile dysfunction in the rat heart [22].
The molecule DY-9760e inhibits the degradation of the cytoskeletal protein fodrin and protects the heart from further damage. In general, fodrin binds to actin, calmodulin, and microtubules and is attached to the plasma membrane. Fodrin plays an important role in organelle organisation, molecular transportation, and secretion [25]. Fodrin is known to be proteolyzed by both calpains and caspase-3 [26]. Caspase-3 activation has a crucial role in the apoptosis process in the cardiomyocytes. Caspase-3 activity was dramatically decreased by DY-9760e therapy and thereby exerts cytoprotective effects by inhibiting fodrin degradation and tyrosine nitration in cardiac proteins (Fig. 4). DY-9760e has no effect on calpains, which are calmodulin (CaM)-independent enzymes [27]. CaM has been shown to stimulate calpain’s degradation of fodrin, but DY-9760e inhibits fodrin proteolysis by inhibiting CaM rather than calpains [28].
Fig. (4).
Schematic diagram showing the molecular mechanism of DY-9760e.
DY-9760e also protects dystrophin from degradation. Dystrophin is an essential protein for maintaining membrane structural integrity in cardiac and skeletal myocytes [29]. Calpain activation increases the breakdown of dystrophin and leads to myocardial injury [30]. This injury is restored by DY-9760e treatment [31]. DY-9760e has been shown to be neuroprotective and reduces infarct size in the permanent focal ischemia model of spontaneously hypertensive rats by decreasing proteolysis of fodrin [28].
DY-9836 is an active metabolite of DY-9760e. It has shown antioxidant properties by inhibiting the elevated levels of both superoxide and nitric oxide (NO) production in cultured cardiomyocytes [32]. The expression of hypertrophy-related genes such as atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) was elevated by endothelin-1-induced cardiomyocyte hypertrophy. These gene expressions were dramatically reduced by DY-9760e treatment [31, 33, 34].
Caveolin-3 is a caveolin component found in cardiac and skeletal muscle caveolae. Caveolin-3 is a scaffolding protein that interacts with and organises lipid and protein constituents in caveolae, including endothelial nitric-oxide synthase (eNOS). It is found in the sarcolemma and plays a role in the formation of caveolae membranes. Caveolin-3 binds to eNOS in caveolae and prevents it from generating NO. Feng Han et al. reported that significant transverse aortic constriction induced caveolin-3 breakdown in the left ventricle compared with control animals. This effect was significantly inhibited by DY-9760e treatment [31, 33, 34].
Phenylephrine-induced hypertrophy is associated with a caveolin-3 breakdown in cultured cardiomyocytes [34]. Cardiac-specific expression of caveolin-3 attenuates cardiac hypertrophy. DY-9760e inhibits the degradation of caveolin-3 and thereby shows its cardioprotection. Overall, DY-9760e, a novel calmodulin antagonist, effectively blocks activation of both calpain and caspase 3, and inhibits the degradation of fodrin, dystrophin, and caveolin-3 to provide its cardioprotective effect.
2.2.2. YC-1
YC-1, 3-(5'-hydroxymethyl-2'-furyl)-1-benzylindazole, (Fig. 5) is a molecule developed for the treatment of circulatory disorders. YC-1 inhibits platelet aggregation and vascular contraction. Yeo et al. reported that YC-1 administration in xenograft immunodeficient mice blocked angiogenesis and inhibited tumor growth [35].
Fig. (5).
Chemical structure of YC-1.
2.2.3. ARRY-797/ARRY371797
ARRY-797 (Fig. 6) is a highly potent (IC50 = 4.5 nM) and selective p38α (MAPK14) kinase inhibitor. The molecule was developed by Array BioPharma (a Pfizer subsidiary since mid-2019) for its therapeutic application against inflammation and pain. Currently, clinical trials are being conducted with this molecule to evaluate its therapeutic efficacy in humans. Array BioPharma has recently recruited patients to conduct a phase 2 clinical trial against Laminrelated dilated cardiomyopathy (NCT02351856) [36]. Lamin A-related-dilated cardiomyopathy is connected with arrhythmias, myocardial remodeling, and dilatation of the left ventricle (LV), which leads to poor cardiac function and heart failure. In another study, i.e., phase 3 clinical trial, the company is recruiting patients with symptomatic dilated cardiomyopathy due to Lamin A/C (LMNA) gene mutation (NCT03439514) [37].
Fig. (6).
Chemical structure of ARRY-371797.
Muchir et al. reported that ARRY-371797 treatment prevented left ventricular dilatation in LmnaH222P/H222P mice [38]. Left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD) were significantly reduced, while fractional shortening was significantly improved after the treatment. ARRY-371797 significantly reduced the expression of myosin light chain 1a and actin alpha 2, which are involved in sarcomere organization. Furthermore, the study found that phosphorylated p38α (MAPK14) levels were reduced in hearts after the treatment when compared to the placebo group [38].
2.2.4. Indazole-Cl
Indazole-Cl (In-Cl) (3-chloro-2-(4-hydroxyphenyl)-2H-indazol-5-ol), with a phenyl-2H-indazole core, is an estrogen receptor-β agonist (Fig. 7) [39]. Indazole-Cl was found to be effective among a series of nonsteroidal compounds synthesized by the Katzenellenbogen group.
Fig. (7).
Chemical structure of indazole -cl.
In-Cl is effective in preventing atherosclerosis through activation of estrogen receptor (ER)-β. Atherosclerosis is characterized by the deposition of fatty material on the inner walls of arteries. It is one of the most common causes of coronary artery disease and carotid artery disease, responsible for heart attack and stroke, respectively [40]. Vascular smooth muscle cells (VSMCs), the major components of blood vessels, are affected by hypoxia. The hypoxic condition affects VSMC inflammation, proliferation, and migration, which contribute to vascular stenosis and the atherosclerotic process. Estrogen receptor (ER)-β plays a key role in preventing the inflammatory response in VSMCs [41-44].
Through the hypoxia-inducible factor (HIF)-1-dependent pathway, hypoxia increases the expression of macrophage migration inhibitory factor MIF in VSMCs [45]. Macrophage migration inhibitory factor is a well-known proinflammatory factor that plays a crucial role in the progression of atherosclerosis. Treatment with In-Cl significantly reduced the hypoxia-induced reactive oxygen species (ROS) production and migration of VSMCs. Besides In-Cl’s effect as an ERβ agonist, the molecule also reduces ROS production induced under hypoxic condition. Increased cell migration and invasion by hypoxia were dramatically decreased following treatment with In-Cl. In-Cl also suppresses the inflammation in a model of endometriosis [46]. In-Cl significantly reduced the hypoxia-induced increase in COX-2 mRNA expression and NF-kB activity, which is mainly responsible for the generation of proinflammatory cytokines [47].
Platelet-derived growth factor (PDGF) is a cation glycoprotein mainly derived from platelets and also present in damaged endothelial cells, transitional fibroblasts, macrophages, smooth muscle cells, and mesangial cells [48]. The PDGF family consists of four gene products, PDGF-A, PDGF-B, PDGF-C, and PDGF-D, which selectively signal through two PDGF receptors (α,ß) to regulate diverse cellular functions. PDGF is a dimeric molecule consisting of disulfide-bonded A, B, C, and D polypeptide chains, and exists in homodimeric (PDGF-AA, BB, CC, and DD) as well as heterodimeric (PDGF-AB) isoforms [49-53]
Gallini et al. reported that PDGFA overexpression led to severe fibrosis, resulting in lethal cardiac failure. In contrast, PDGFB overexpression resulted in focal fibrosis and moderate cardiac hypertrophy [54]. When treated with estrogen receptor agonists, proliferation-related genes that are upregulated in hypoxia are down-regulated. In the hypoxic state, the PDGF B gene is elevated by 1.9-fold. However, after treatment with In-Cl, it is reduced by 0.78-fold. The indazole molecule, In-Cl, has shown axon myelination and neuroprotection in experimental autoimmune encephalomyelitis (EAE) in vivo studies [55]. Pang et al. reported that in patients with acute coronary syndrome, there are considerably higher levels of PDGF in their peripheral and coronary artery blood [48]. The serum PDGF concentration in individuals with the acute coronary syndrome was significantly increased, notably in the coronary artery, and it was linked to the severity of coronary artery disease (Fig. 8).
Fig. (8).
Schematic diagram showing the molecular effect of indazole-cl.
Heldin et al. reviewed that in mouse models, PDGF-AA or PDGF-BB in the heart has been demonstrated to cause cardiac fibrosis or elevated collagen deposition by interstitial cardiac fibroblasts. Pang et al. have shown that PDGF-BB contributes to the development of atherosclerosis through chemotaxis. Therefore, targeting PDGF with indazole derivatives could be the future strategy to reduce cardiovascular diseases.
2.2.5. 7-Nitroindazole
7-Nitroindazole is a heterocyclic indazole ring having a nitro group at position C7 (Fig. 9). The molecule inhibits neuronal nitric oxide synthase in a selective manner. 7-nitroindazole had an anti-hypertrophic effect on the heart and decreased wall thickness of thoracic aorta and carotid arteries. However, the long-term 7-nitroindazole treatment caused adverse effects like pressure-independent cardiac hypotrophy as well as arterial wall hypotrophy and reduced contractile efficiency due to decreased endothelial and smooth muscle cell mass [56, 57].
Fig. (9).
Chemical structure of 7-nitroindazole.
Administration of 7-nitroindazole markedly decreased the asymmetric dimethylarginine (ADMA) levels in the plasma of young spontaneously hypertensive rats (SHR). ADMA has been recognised to cause several adverse effects on cardiovascular disease. Chronic administration of 7-nitroindazole decreased homocysteine levels from 7.37 ± 0.467 μmol/l of control value to 4.890 ± 1.186 μmol/l. Plasma aspartate transaminase activities were reduced in the 7-nitroindazole-treated SHR group when compared to untreated controls. Administration of 7-nitroindazole improved the plasma redox status in comparison to both control and the NG-nitro-L-arginine methyl ester (NOS inhibitor) treated groups of animals. 7-nitroindazole treatment actively influenced cardiovascular biomarkers related to oxidative stress. 7-nitroindazole administration decreases ROS, homocysteine, and dimethylarginine levels. Similar to 7-nitroindazole, administration of another NOS inhibitor to young SHR led to a decrease in ADMA levels by 70%. It is known that higher production of homocysteine may induce oxidative stress, leading to elevated ADMA concentrations [58]. 7-nitroindazole treatment improves ROS, total thiol level (TTL), and the redox status i.e. ratio TTL/ROS [59]. 7-nitroindazole selective nNOS inhibition had no effect on total LV myocardial blood flow or its distribution in the subendocardial and subepicardial tissue layers [60].
Long-term 7-nitroindazole treatment resulted in pressure-independent cardiac hypotrophy as well as arterial wall hypotrophy and reduced contractile efficiency due to decreased endothelial and smooth muscle cell mass [56, 57]. Administration of 7-nitroindazole to rats has shown dose-dependent inhibition of penile erection. The high-dose group had a significantly decreased maximal rise in intracavernous pressure (mICP) when compared to the low-dose group [61].
2.2.6. TCS-80
Boblewski et al. developed a novel series of centrally acting agents and evaluated their effect on blood pressure and heart rate in rats. They synthesized two novel marsanidine analogues that decrease blood pressure and heart rate in rats. These two molecules are 7-chloro-1-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-1H-indazole (TCS-80) and 1-[(imidazolidin-2-yl)imino]-1H-indazole (marsanidine) (Figs. 10 and 11). Among all of these analogues, compound TCS-80 had the greatest affinity for the I1-imidazoline receptors [62].
Fig. (10).
Chemical structure of TCS-80.
Fig. (11).
Chemical structure of marsanidine.
2.2.7. Marsanidine Analogues
The adrenergic system controls neuronal, cardiovascular, endocrine, and metabolic functions [63]. α2-adrenoceptors (α2-ARs) are G-protein coupled receptors that mediate many of the central and peripheral actions. α2-ARs are present as three subtypes, α2A, α2B, and α2C ARs [64]. α2-ARs are involved in a variety of pathophysiological processes and regulate sympathetic nervous system activity, arterial blood pressure, body temperature, insulin secretion, and gastrointestinal motility [65].
Wasilewska et al. modified the selective α2-ARs agonists, i.e., 1-[(imidazolidin-2-yl)imino]-1H-indazole (marsanidine, Fig. 11A) and its methylene analogue 1-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-1H-indazole (Fig. 11B) by introducing a fluorine group at different positions on the indazole ring. It is reported that the compound having fluorine at C7 (Fig. 11C) showed the highest hypotensive and bradycardic activities in in vivo cardiac model [66].
Structure-activity relationship (SAR) analysis showed that substituting a chlorine or methyl group at the C7 atom of the indazole nucleus of marsanidine resulted in compounds with higher cardiovascular activity than the congeners [66-69].
3. FUTURE OF INDAZOL DERIVATIVES AS CARDIOPROTECTIVE AGENTS
Indazole derivatives are currently being explored as drug molecules in both preclinical and clinical studies (Tables 1 and 2). There is a huge scope to explore indazole derivatives for further development in cardiovascular as well as other diseases.
Table 2.
Indazole derivatives explored in diseases other than cardiovascular diseases.
As previously reported, ARRY-371797/ARRY-797 is a highly potent and selective p38ą (MAPK14) kinase inhibitor. According to previous studies, the p38 mitogenactivated protein kinase (MAPK) plays a key role in ischemic heart disease, right ventricular hypertrophy, and many other cardiac diseases. According to Kojonazarov et al., phosphorylated p38 MAPK is significantly enhanced in hypertrophied/failed right ventricles in both mice and humans. Inhibition of p38 MAPK may reduce collagen production and stress fibre formation in right ventricular fibroblasts. ARRY-371797/ARRY-797 also prevents the differentiation of right ventricular fibroblasts into myofibroblasts by inhibiting myocardin-related transcription factor A (MRTF-A), translocation of MRTF-A from the cytosol to the nucleus. p38 MAPK is considered as a potential drug target for the reduction of endothelial dysfunction, increased fibroblast activity, inflammation, and right ventricular failure [70]. Based on the previous studies, inhibition of p38 with indazole derivative ARRY-371797/ARRY-797 may show beneficial effects on cardiac diseases.
The beneficial effect of In-Cl could be attributed to its effect in reducing PDGF expression. Heldin et al. reviewed that both PDGF-AA and PDGF-BB in mouse hearts cause cardiac fibrosis with the increased collagen deposition by interstitial cardiac fibroblasts [71]. Park et al. reported that PDGF-BB treatment increased the cell proliferation in the vascular smooth muscle cell, and this was normalised with In-Cl treatment. Therefore, targeting PDGF with indazole derivatives could be a future strategy to reduce cardiac remodelling and heart failure. Further basic and clinical research should be undertaken to investigate the beneficial effect of indazole derivatives in cardiovascular diseases.
Though there are several studies that indicate the beneficial effects of indazole derivatives, there is only one toxicity study with one indazole derivative, i.e., 7-nitroindazole. The study reported that the administration of 7-nitroindazole to Wistar rats from the prenatal period to adulthood resulted in a reduction in the weight of the heart and kidneys, along with reduction of wall thickness/inner diameter ratio in the thoracic aorta and carotid artery [56]. The study indicates the requirement to conduct more toxicity studies to evaluate the adverse effect of other indazole derivatives.
CONCLUSION
Indazole derivatives are being used as drug molecules in various diseases including cancer, and cataracts (Table 3). Many indazole derivatives have shown potential beneficial effects against cardiovascular and metabolic diseases such as arrhythmia, ischemia-reperfusion injury, thrombosis, hypertension, hyperlipidemia, and obesity. One of the most promising indazole derivatives, DY-9760e, has shown cardioprotection against ischemic/reperfusion injury [22]. Another promising indazole derivative, YC-1, has been developed for its therapeutic use in circulatory disorders, platelet aggregation, and vascular contraction [35]. YC-1 has been identified as an activator of the physiological nitric oxide receptor, soluble guanylyl cyclase, a signalling molecule in the cardiovascular system [72]. Another indazole derivative, indazole-Cl, suppressed the inflammation in atherosclerosis [44]. 7-nitroindazole has an anti-hypertrophic effect on the heart and reduces wall thickness of the thoracic aorta and carotid arteries [56].
Table 3.
Approved indazole derivatives in the market.
| S. No | Indazole Derivatives | Chemical Structure | Mechanism of Action | Therapeutic Use |
|---|---|---|---|---|
| 1 | Bendazac |

|
Non-Steroidal Anti-Inflammatory | Inflammation |
| 2 | Benzyldamine |

|
NSAID | Cataracts |
| 3 | Bendazac Lysine Salt |

|
Anti-Denaturant | Eye drop to treat cataracts |
| 4 | Granisetron |

|
Serotonin 5-HT3 Receptor Antagonist | Nausea and vomiting |
| 5 | Axitinib |

|
VEGFR And PDGFR Inhibitor (TYROSINE KINASE INHIBITOR) | Cancer |
| 6 | Pazopanib |

|
VEGFR And C-Kit Inhibitor | Cancer |
| 7 | Niraparib |
|
inhibitor of poly (ADP-ribose) poly-merase (PARP) enzymes | Cancer |
Few indazole derivatives are in clinical trials and have shown promising results against cardiovascular diseases. ARRY-371797, a p38α (MAPK14)-selective kinase inhibitor, is now being tested in humans for LMNA-related dilated cardiomyopathy (NCT02351856, NCT03439514) [36, 37]. Indazole derivatives need to be explored further by researchers in academic institutes and pharma industries to develop novel drug molecules against newer targets in several cardiovascular diseases where adequate treatment is not available.
FUNDING
S.K. Banerjee is supported by the Department of Biotechnology [BT/PR22881/BRB/10/1654/2018]. S. Kumar is supported by the National Institute of Health [NIHR01HL152132]. MJ. Alam is supported by ICMR RA fellowship [3/1/2(19)/OBS/2019-NCD-II]
LIST OF ABBREVIATIONS
- ADMA
Asymmetric dimethylarginine
- CaM
Calmodulin
- eNOS
Endothelial nitric oxide synthase
- HIF-1
Hypoxia-inducible factor 1-alpha
- In-Cl
Indazole-Cl
- LMNA
Lamin A/C
- LV
Left ventricle
- MAPK
Mitogen-activated protein kinase
- PDGF
Platelet-derived growth factor
- ROS
Reactive oxygen species
- SHR
Spontaneously hypertensive rats
- VSMCs
Vascular smooth muscle cells
- α2-ARs
α2-Adrenoceptors
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- [1].Teixeira FC; Ramos H; Antunes IF; Curto MJM; Duarte MT; Bento I Synthesis and structural characterization of 1- and 2-substituted indazoles: ester and carboxylic acid derivatives. Molecules, 2006, 11(11), 867–889. 10.3390/11110867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Steffan RJ; Matelan E; Ashwell MA; Moore WJ; Solvibile WR; Trybulski E; Chadwick CC; Chippari S; Kenney T; Eckert A; Borges-Marcucci L; Keith JC; Xu Z; Mosyak L; Harnish DC Synthesis and activity of substituted 4-(indazol-3-yl)phenols as pathway-selective estrogen receptor ligands useful in the treatment of rheumatoid arthritis. J. Med. Chem, 2004, 47(26), 6435–6438. 10.1021/jm049194+ [DOI] [PubMed] [Google Scholar]
- [3].Giannouli V; Kostakis IK; Pouli N; Marakos P; Kousidou O.Ch.; Tzanakakis GN; Karamanos NK Design, synthesis, and evaluation of the antiproliferative activity of a series of novel fused xanthenone aminoderivatives in human breast cancer cells. J. Med. Chem, 2007, 50(7), 1716–1719. 10.1021/jm061410m [DOI] [PubMed] [Google Scholar]
- [4].Sikorski JA Oral cholesteryl ester transfer protein (CETP) inhibitors: a potential new approach for treating coronary artery disease. J. Med. Chem, 2006, 49(1), 1–22. 10.1021/jm058224l [DOI] [PubMed] [Google Scholar]
- [5].Leroy V; Lee GE; Lin J; Herman SH; Lee TB Facile preparation of 3-(1-piperazinyl)-1H-indazoles. Org. Process Res. Dev, 2001, 5(2), 179–183. 10.1021/op0002242 [DOI] [Google Scholar]
- [6].Schwan TJ; Honkomp LJ; Davis CS; Lougheed GS Synthesis and hypotensive activity of a series of 2-substituted 5,6-dimethoxyindazoles. J. Pharm. Sci, 1978, 67(7), 1022–1024. 10.1002/jps.2600670742 [DOI] [PubMed] [Google Scholar]
- [7].Kym PR; Iyengar R; Souers AJ; Lynch JK; Judd AS; Gao J; Freeman J; Mulhern M; Zhao G; Vasudevan A; Wodka D; Blackburn C; Brown J; Che JL; Cullis C; Lai SJ; LaMarche MJ; Marsilje T; Roses J; Sells T; Geddes B; Govek E; Patane M; Fry D; Dayton BD; Brodjian S; Falls D; Brune M; Bush E; Shapiro R; Knourek-Segel V; Fey T; McDowell C; Reinhart GA; Preusser LC; Marsh K; Hernandez L; Sham HL; Collins CA Discovery and characterization of aminopiperidinecoumarin melanin concentrating hormone receptor 1 antagonists. J. Med. Chem, 2005, 48(19), 5888–5891. 10.1021/jm050598r [DOI] [PubMed] [Google Scholar]
- [8].Duan J-X; Cai X; Meng F; Lan L; Hart C; Matteucci M Potent antitubulin tumor cell cytotoxins based on 3-aroyl indazoles. J. Med. Chem, 2007, 50(5), 1001–1006. 10.1021/jm061348t [DOI] [PubMed] [Google Scholar]
- [9].Wyrick SD; Voorstad PJ; Cocolas G; Hall IH Hypolipidemic activity of phthalimide derivatives. 7. Structure-activity studies of indazolone analogues. J. Med. Chem, 1984, 27(6), 768–772. 10.1021/jm00372a011 [DOI] [PubMed] [Google Scholar]
- [10].Arán VJ; Ochoa C; Boiani L; Buccino P; Cerecetto H; Gerpe A; González M; Montero D; Nogal JJ; Gómez-Barrio A; Azqueta A; López de Ceráin A; Piro OE; Castellano EE Synthesis and biological properties of new 5-nitroindazole derivatives. Bioorg. Med. Chem, 2005, 13(9), 3197–3207. 10.1016/j.bmc.2005.02.043 [DOI] [PubMed] [Google Scholar]
- [11].Badawey E-SA; El-Ashmawey IM Nonsteroidal antiinflammatory agents-part 1: antiinflammatory, analgesic and antipyretic activity of some new 1-(pyrimidin-2-yl)-3-pyrazolin-5-ones and 2-(pyrimidin-2-yl)-1, 2, 4, 5, 6, 7-hexahydro-3h-indazol-3-ones. Eur. J. Med. Chem, 1998, 33(5), 349–361. 10.1016/S0223-5234(98)80002-0 [DOI] [PubMed] [Google Scholar]
- [12].El-Hawash SA; Badawey SA; El-Ashmawey IM Nonsteroidal antiinflammatory agents-part 2 antiinflammatory, analgesic and antipyretic activity of some substituted 3-pyrazolin-5-ones and 1,2,4,5,6,7-3H-hexahydroindazol-3-ones. Eur. J. Med. Chem, 2006, 41(2), 155–165. 10.1016/j.ejmech.2005.09.006 [DOI] [PubMed] [Google Scholar]
- [13].Onifade AA; Jewell AP; Adedeji WA Nigella sativa concoction induced sustained seroreversion in HIV patient. Afr. J. Tradit. Complement. Altern. Med, 2013, 10(5), 332–335. 10.4314/ajtcam.v10i5.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bakathir HA; Abbas NA Detection of the antibacterial effect of Nigella sativa ground seeds with water. Afr. J. Tradit. Complement. Altern. Med, 2011, 8(2), 159–164. 10.4314/ajtcam.v8i2.63203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gaikwad DD; Chapolikar AD; Devkate CG; Warad KD; Tayade AP; Pawar RP; Domb AJ Synthesis of indazole motifs and their medicinal importance: An overview. Eur. J. Med. Chem, 2015, 90, 707–731. 10.1016/j.ejmech.2014.11.029 [DOI] [PubMed] [Google Scholar]
- [16].Mitsubishi Tanabe Pharma Corporation. An expanded controlled study of MCI-186 for treatment of amyotrophic lateral sclerosis in double-blind, parallel-group, placebo-controlled manner (phase 3) clinical trial registration NCT0042446, 2018.
- [17].Khare CP Encyclopedia of Indian Medicinal Plants: Rational Western Therapy, Ayurvedic and Other Traditional Usage, Botany; Springer, 2004. [Google Scholar]
- [18].Maiti S; Banerjee A; Nazmeen A; Kanwar M; Das S Activesite Molecular docking of Nigellidine with nucleocapsid- NSP2-MPro of COVID-19 and to human IL1R-IL6R and strong antioxidant role of Nigella-sativa in experimental rats. J. Drug Target, 2020, 1–23. 10.1080/1061186X.2020.1817040 [DOI] [PubMed] [Google Scholar]
- [19].Radl S. 12.10 - Bicyclic Systems with Two Bridgehead (Ring Junction) Nitrogen Atoms. In: Comprehensive Heterocyclic Chemistry III; Katritzky AR; Ramsden CA; Scriven EFV; Taylor RJK, Eds.; Elsevier: Oxford, 2008, pp. 365–479. 10.1016/B978-008044992-0.01110-X [DOI] [Google Scholar]
- [20].Salem EM; Yar T; Bamosa AO; Al-Quorain A; Yasawy MI; Alsulaiman RM; Randhawa MA Comparative study of Nigella Sativa and triple therapy in eradication of Helicobacter Pylori in patients with non-ulcer dyspepsia. Saudi J. Gastroenterol, 2010, 16(3), 207–214. 10.4103/1319-3767.65201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yuan T; Nahar P; Sharma M; Liu K; Slitt A; Aisa HA; Seeram NP Indazole-type alkaloids from Nigella sativa seeds exhibit antihyperglycemic effects via AMPK activation in vitro. J. Nat. Prod, 2014, 77(10), 2316–2320. 10.1021/np500398m [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hashimoto M; Takada Y; Takeuchi Y; Kasahara J; Hisa H; Shirasaki Y; Fukunaga K Cytoprotective effect of 3-[2-[4-(3-chloro-2-methylphenyl)-1-piperazinyl]ethyl]-5,6-dimethoxy-1-(4-imidazolylmethyl)-1H-indazole dihydrochloride 3.5 hydrate (DY-9760e) against ischemia/reperfusion-induced injury in rat heart involves inhibition of fodrin breakdown and protein tyrosine nitration. J. Pharmacol. Sci, 2005, 98(2), 142–150. 10.1254/jphs.FP0040551 [DOI] [PubMed] [Google Scholar]
- [23].Das DK; Engelman RM; Prasad MR; Rousou JA; Breyer RH; Jones R; Young H; Cordis GA Improvement of ischemia-reperfusion-induced myocardial dysfunction by modulating calcium-overload using a novel, specific calmodulin antagonist, CGS 9343B. Biochem. Pharmacol., 1989, 38(3), 465–471. 10.1016/0006-2952(89)90386-9 [DOI] [PubMed] [Google Scholar]
- [24].Schulman H; Anderson ME Ca/Calmodulin-dependent Protein Kinase II in Heart Failure. Drug Discov. Today Dis. Mech, 2010, 7(2), e117–e122. 10.1016/j.ddmec.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Derbala MH; Guo AS; Mohler PJ; Smith SA The role of βII spectrin in cardiac health and disease. Life Sci., 2018, 192, 278–285. 10.1016/j.lfs.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moore JD; Rothwell NJ; Gibson RM Involvement of caspases and calpains in cerebrocortical neuronal cell death is stimulus-dependent. Br. J. Pharmacol, 2002, 135(4), 1069–1077. 10.1038/sj.bjp.0704538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sugimura M; Sato T; Nakayama W; Morishima Y; Fukunaga K; Omitsu M; Miyamoto E; Shirasaki Y DY-9760e, a novel calmodulin antagonist with cytoprotective action. Eur. J. Pharmacol, 1997, 336(1), 99–106. 10.1016/S0014-2999(97)01251-X [DOI] [PubMed] [Google Scholar]
- [28].Takagi K; Sato T; Shirasaki Y; Narita K; Tamura A; Sano K Post-ischemic administration of DY-9760e, a novel calmodulin antagonist, reduced infarct volume in the permanent focal ischemia model of spontaneously hypertensive rat. Neurol. Res, 2001, 23(6), 662–668. 10.1179/016164101101198992 [DOI] [PubMed] [Google Scholar]
- [29].Houang EM; Sham YY; Bates FS; Metzger JM Muscle membrane integrity in Duchenne muscular dystrophy: Recent advances in copolymer-based muscle membrane stabilizers. Skelet. Muscle, 2018, 8(1), 31. 10.1186/s13395-018-0177-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Campos EC; O’Connell JL; Malvestio LM; Romano MMD; Ramos SG; Celes MRN; Prado CM; Simões MV; Rossi MA Calpain-mediated dystrophin disruption may be a potential structural culprit behind chronic doxorubicin-induced cardiomyopathy. Eur. J. Pharmacol, 2011, 670(2-3), 541–553. 10.1016/j.ejphar.2011.09.021 [DOI] [PubMed] [Google Scholar]
- [31].Han F; Lu Y-M; Hasegawa H; Kanai H; Hachimura E; Shirasaki Y; Fukunaga K Inhibition of dystrophin breakdown and endothelial nitric-oxide synthase uncoupling accounts for cytoprotection by 3-[2-[4-(3-chloro-2-methylphenyl)-1-piperazinyl] ethyl]-5,6-dimethoxy-1-(4-imidazolylmethyl)-1H-indazole dihydrochloride 3.5 hydrate (DY-9760e) in left ventricular hypertrophied Mice. J. Pharmacol. Exp. Ther, 2010, 332(2), 421–428. 10.1124/jpet.109.161646 [DOI] [PubMed] [Google Scholar]
- [32].Fukunaga K; Han F; Shioda N; Moriguchi S; Kasahara J; Shirasaki Y DY-9760e, a novel calmodulin inhibitor, exhibits cardioprotective effects in the ischemic heart. Cardiovasc. Drug Rev, 2006, 24(2), 88–100. 10.1111/j.1527-3466.2006.00088.x [DOI] [PubMed] [Google Scholar]
- [33].Lu Y-M; Shioda N; Han F; Kamata A; Shirasaki Y; Qin Z-H; Fukunaga K DY-9760e inhibits endothelin-1-induced cardiomyocyte hypertrophy through inhibition of CaMKII and ERK activities. Cardiovasc. Ther, 2009, 27(1), 17–27. 10.1111/j.1755-5922.2008.00068.x [DOI] [PubMed] [Google Scholar]
- [34].Lu Y-M; Han F; Shioda N; Moriguchi S; Shirasaki Y; Qin Z-H; Fukunaga K Phenylephrine-induced cardiomyocyte injury is triggered by superoxide generation through uncoupled endothelial nitric-oxide synthase and ameliorated by 3-[2-[4-(3-chloro-2-methylphenyl)-1-piperazinyl]ethyl]-5,6-dimethoxyindazole (DY-9836), a novel calmodulin antagonist. Mol. Pharmacol, 2009, 75(1), 101–112. 10.1124/mol.108.050716 [DOI] [PubMed] [Google Scholar]
- [35].Yeo E-J; Chun Y-S; Cho Y-S; Kim J; Lee J-C; Kim M-S; Park J-W YC-1: A potential anticancer drug targeting hypoxia-inducible factor 1. J. Natl. Cancer Inst, 2003, 95(7), 516–525. 10.1093/jnci/95.7.516 [DOI] [PubMed] [Google Scholar]
- [36].Pfizer. An open-label rollover study of arry-371797 in patients with symptomatic genetic dilated cardiomyopathy due to a lamin a/c gene mutation. Clinical trial registration: NCT02351856, 2021.
- [37].Pfizer. a phase 3, multinational, randomized, placebo-controlled study of arry-371797 (pf-07265803) in patients with symptomatic dilated cardiomyopathy due to a lamin a/c gene mutation. Clinical trial registration NCT03439514, 2021.
- [38].Muchir A; Wu W; Choi JC; Iwata S; Morrow J; Homma S; Worman HJ Abnormal p38α mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet, 2012, 21(19), 4325–4333. 10.1093/hmg/dds265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].De Angelis M; Stossi F; Carlson KA; Katzenellenbogen BS; Katzenellenbogen JA Indazole estrogens: Highly selective ligands for the estrogen receptor β. J. Med. Chem, 2005, 48(4), 1132–1144. 10.1021/jm049223g [DOI] [PubMed] [Google Scholar]
- [40].Lusis AJ Atherosclerosis. Nature, 2000, 407(6801), 233–241. 10.1038/35025203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chistiakov DA; Orekhov AN; Bobryshev YV Vascular smooth muscle cell in atherosclerosis. Acta Physiol. (Oxf.), 2015, 214(1), 33–50. 10.1111/apha.12466 [DOI] [PubMed] [Google Scholar]
- [42].Basatemur GL; Jørgensen HF; Clarke MCH; Bennett MR; Mallat Z Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol, 2019, 16(12), 727–144. 10.1038/s41569-019-0227-9 [DOI] [PubMed] [Google Scholar]
- [43].Harris HA; Albert LM; Leathurby Y; Malamas MS; Mewshaw RE; Miller CP; Kharode YP; Marzolf J; Komm BS; Winneker RC; Frail DE; Henderson RA; Zhu Y; Keith JC, Jr Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology, 2003, 744(10), 4241–4249. 10.1210/en.2003-0550 [DOI] [PubMed] [Google Scholar]
- [44].Park C; Park J; Shim MK; Rhyu M-R; Yoon B-K; Kim KS; Lee Y Indazole-Cl inhibits hypoxia-induced cyclooxygenase-2 expression in vascular smooth muscle cells. J. Mol. Endocrinol, 2019, 63(1), 27–38. 10.1530/JME-19-0018 [DOI] [PubMed] [Google Scholar]
- [45].Fu H; Luo F; Yang L; Wu W; Liu X Hypoxia stimulates the expression of macrophage migration inhibitory factor in human vascular smooth muscle cells via HIF-1α dependent pathway. BMC Cell Biol., 2010, 11(1), 66. 10.1186/1471-2121-11-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Madak-Erdogan Z; Kim SH; Gong P; Zhao YC; Zhang H; Chambliss KL; Carlson KE; Mayne CG; Shaul PW; Korach KS; Katzenellenbogen JA; Katzenellenbogen BS Design of pathway preferential estrogens that provide beneficial metabolic and vascular effects without stimulating reproductive tissues. Sci. Signal, 2016, 9(429), ra53. 10.1126/scisignal.aad8170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li Q; Verma IM NF-kappaB regulation in the immune system. Nat. Rev. Immunol, 2002, 2(10), 725–734. 10.1038/nri910 [DOI] [PubMed] [Google Scholar]
- [48].Pang S; Tao Z; Min X; Zhou C; Pan D; Cao Z; Wang X Correlation between the serum platelet-derived growth factor, angiopoietin-1, and severity of coronary heart disease. Cardiol. Res. Pract, 2020, 2020, 3602608. 10.1155/2020/3602608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Osornio-Vargas AR; Goodell AL; Hernández-Rodríguez NA; Brody AR; Coin PG; Badgett A; Bonner JC Platelet-derived growth factor (PDGF)-AA, -AB, and -BB induce differential chemotaxis of early-passage rat lung fibroblasts in vitro. Am. J. Respir. Cell Mol. Biol, 1995, 12(1), 33–40. 10.1165/ajrcmb.12.1.7811469 [DOI] [PubMed] [Google Scholar]
- [50].Kazlauskas A A new member of an old family. Nat. Cell Biol, 2000, 2(5), E78–E79. 10.1038/35010508 [DOI] [PubMed] [Google Scholar]
- [51].Li X; Pontén A; Aase K; Karlsson L; Abramsson A; Uutela M; Bäckström G; Hellström M; Boström H; Li H; Soriano P; Betsholtz C; Heldin CH; Alitalo K; Ostman A; Eriksson U PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat. Cell Biol, 2000, 2(5), 302–309. 10.1038/35010579 [DOI] [PubMed] [Google Scholar]
- [52].LaRochelle WJ; Jeffers M; McDonald WF; Chillakuru RA; Giese NA; Lokker NA; Sullivan C; Boldog FL; Yang M; Vernet C; Burgess CE; Fernandes E; Deegler LL; Rittman B; Shimkets J; Shimkets RA; Rothberg JM; Lichenstein HS PDGF-D, a new protease-activated growth factor. Nat. Cell Biol, 2001, 3(5), 517–521. 10.1038/35074593 [DOI] [PubMed] [Google Scholar]
- [53].Liu J; Wu LL; Li L; Zhang L; Song Z -E. Growth-promoting effect of platelet-derived growth factor on rat cardiac myocytes. Regul. Pept, 2005, 127(1-3), 11–18. 10.1016/j.regpep.2004.10.018 [DOI] [PubMed] [Google Scholar]
- [54].Gallini R; Lindblom P; Bondjers C; Betsholtz C; Andrae J PDGF-A and PDGF-B induces cardiac fibrosis in transgenic mice. Exp. Cell Res, 2016, 349(2), 282–290. 10.1016/j.yexcr.2016.10.022 [DOI] [PubMed] [Google Scholar]
- [55].Moore SM; Khalaj AJ; Kumar S; Winchester Z; Yoon J; Yoo T; Martinez-Torres L; Yasui N; Katzenellenbogen JA; Tiwari-Woodruff SK Multiple functional therapeutic effects of the estrogen receptor β agonist indazole-Cl in a mouse model of multiple sclerosis. Proc. Natl. Acad. Sci. USA, 2014, 111(50), 18061–18066. 10.1073/pnas.1411294111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kristek F; Cacanyiova S; Gerova M Hypotrophic effect of long-term neuronal NO-synthase inhibition on heart and conduit arteries of the Wistar rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc, 2009, 60(2), 21–27. [PubMed] [Google Scholar]
- [57].Kristek F; Malekova M; Ondrias K; Cacanyiova S Blood pressure-independent hypotrophy of the heart, kidneys and conduit arteries after 7-nitroindazole administration to Wistar rats from the prenatal period to adulthood. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc, 2013, 64(1), 35–39. [PubMed] [Google Scholar]
- [58].Edirimanne VER; Woo CWH; Siow YL; Pierce GN; Xie JY; O, K. Homocysteine stimulates NADPH oxidase-mediated superoxide production leading to endothelial dysfunction in rats. Can. J. Physiol. Pharmacol, 2007, 85(12), 1236–1247. 10.1139/Y07-112 [DOI] [PubMed] [Google Scholar]
- [59].Dovinová I; Hrabárová E; Jansen E; Kvandová M; Majzúnová M; Berenyiová A; Barančík M ADMA, homocysteine and redox status improvement affected by 7-nitroindazole in spontaneously hypertensive rats. Biomed. Pharmacother. Biomedecine Pharmacother, 2018, 106, 1478–1483. 10.1016/j.biopha.2018.07.096 [DOI] [PubMed] [Google Scholar]
- [60].Kingma JG Jr; Simard D; Rouleau JR Nitric oxide bioavailability affects cardiovascular regulation dependent on cardiac nerve status. Auton. Neurosci, 2015, 187, 70–75. 10.1016/j.autneu.2014.11.003 [DOI] [PubMed] [Google Scholar]
- [61].Spiess PE; Dion SB; Zvara P; Merlin SL; Chan PTK; Brock GB 7-Nitroindazole: a selective inhibitor of penile erection: An in vivo study in a rat animal model. Urology, 1996, 47(1), 93–96. 10.1016/S0090-4295(99)80389-6 [DOI] [PubMed] [Google Scholar]
- [62].Boblewski K; Lehmann A; Sączewski F; Sączewski J; Kornicka A; Marchwińska A; Rybczyńska A Circulatory effect of TCS-80, a new imidazoline compound, in rats. Pharmacol. Rep, 2016, 68(4), 715–719. 10.1016/j.pharep.2016.03.008 [DOI] [PubMed] [Google Scholar]
- [63].Hein L. Adrenoceptors and signal transduction in neurons. Cell Tissue Res, 2006, 326(2), 541–551. 10.1007/s00441-006-0285-2 [DOI] [PubMed] [Google Scholar]
- [64].Bylund DB; Eikenberg DC; Hieble JP; Langer SZ; Lefkowitz RJ; Minneman KP; Molinoff PB; Ruffolo RR Jr; Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev, 1994, 46(2), 121–136. [PubMed] [Google Scholar]
- [65].Ruffolo RR Jr; Nichols AJ; Stadel JM; Hieble JP Pharmacologic and therapeutic applications of alpha 2-adrenoceptor subtypes. Annu. Rev. Pharmacol. Toxicol, 1993, 33, 243–279. 10.1146/annurev.pa.33.040193.001331 [DOI] [PubMed] [Google Scholar]
- [66].Wasilewska A; Sączewski F; Hudson AL; Ferdousi M; Scheinin M; Laurila JM; Rybczyńska A; Boblewski K; Lehmann A Fluorinated analogues of marsanidine, a highly α2-AR/imidazoline I1 binding site-selective hypotensive agent. Synthesis and biological activities. Eur. J. Med. Chem, 2014, 87, 386–397. 10.1016/j.ejmech.2014.09.083 [DOI] [PubMed] [Google Scholar]
- [67].Saczewski F; Kornicka A; Rybczyńska A; Hudson AL; Miao SS; Gdaniec M; Boblewski K; Lehmann A 1-[(Imidazolidin-2-yl)imino]indazole. Highly alpha 2/I1 selective agonist: synthesis, X-ray structure, and biological activity. J. Med. Chem, 2008, 51(12), 3599–3608. 10.1021/jm800112s [DOI] [PubMed] [Google Scholar]
- [68].Sączewski F; Kornicka A; Hudson AL; Laird S; Scheinin M; Laurila JM; Rybczyńska A; Boblewski K; Lehmann A; Gdaniec M 3-[(Imidazolidin-2-yl)imino]indazole ligands with selectivity for the α(2)-adrenoceptor compared to the imidazoline I(1) receptor. Bioorg. Med. Chem, 2011, 19(1), 321–329. 10.1016/j.bmc.2010.11.020 [DOI] [PubMed] [Google Scholar]
- [69].Saczewski J; Hudson A; Scheinin M; Rybczynska A; Ma D; Saczewski F; Laird S; Laurila JM; Boblewski K; Lehmann A; Gu J; Watts H Synthesis and biological activities of 2-[(heteroaryl)methyl]imidazolines. Bioorg. Med. Chem, 2012, 20(1), 108–116. 10.1016/j.bmc.2011.11.025 [DOI] [PubMed] [Google Scholar]
- [70].Vanderpool RR; Tang H; Rischard F; Yuan JX-J Is p38 MAPK a dark force in right ventricular hypertrophy and failure in pulmonary arterial hypertension? Am. J. Respir. Cell Mol. Biol, 2017, 57(5), 506–508. 10.1165/rcmb.2017-0197ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Heldin C-H; Lennartsson J; Westermark B Involvement of platelet-derived growth factor ligands and receptors in tumorigenesis. J. Intern. Med, 2018, 283(1), 16–44. 10.1111/joim.12690 [DOI] [PubMed] [Google Scholar]
- [72].Shrivastava A; Chakraborty AK; Upmanyu N; Singh A Recent progress in chemistry and biology of indazole and its derivatives: A brief review. Austin J. Anal. Pharm. Chem, 2016, 3(4), 1076. [Google Scholar]
- [73].Farias M III; Jackson K; Johnson M; Caffrey JL Cardiac enkephalins attenuate vagal bradycardia: Interactions with NOS-1-cGMP systems in canine sinoatrial node. Am. J. Physiol. Heart Circ. Physiol, 2003, 285(5), H2001–H2012. 10.1152/ajpheart.00275.2003 [DOI] [PubMed] [Google Scholar]
- [74].Wang K; Ding R; Ha Y; Jia Y; Liao X; Wang S; Li R; Shen Z; Xiong H; Guo J; Jie W Hypoxia-stressed cardiomyocytes promote early cardiac differentiation of cardiac stem cells through HIF-1α/Jagged1/Notch1 signaling. Acta Pharm. Sin. B, 2018, 8(5), 795–804. 10.1016/j.apsb.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Boblewski K; Lehmann A; Marchwinska A; Kornicka A; Wasilewska A; Saczewski F; Rybczynska A Comparison of the effects of marsanidine derivatives on rat cardiovascular system. Acta Pol. Pharm, 2017, 74(2), 579–586. [PubMed] [Google Scholar]
- [76].Wróblewska M; Kasprzyk J; Sączewski F; Kornicka A; Boblewski K; Lehmann A; Rybczyńska A Marsanidine and 7-Me-marsanidine, the new hypotensive imidazolines augment sodium and urine excretion in rats. Pharmacol. Rep, 2013, 65(4), 1025–1032. 10.1016/S1734-1140(13)71085-5 [DOI] [PubMed] [Google Scholar]
- [77].May JA; Sharif NA; McLaughlin MA; Chen H-H; Severns BS; Kelly CR; Holt WF; Young R; Glennon RA; Hellberg MR; Dean TR Ocular hypotensive response in nonhuman primates of (8R)-1-[(2S)-2-aminopropyl]-8,9-dihydro-7H-pyrano [2,3-g]indazol-8-ol a selective 5-HT2 receptor agonist. J. Med. Chem, 2015, 58(22), 8818–8833. 10.1021/acs.jmedchem.5b00857 [DOI] [PubMed] [Google Scholar]
- [78].Lamotte Y; Faucher N; Sançon J; Pineau O; Sautet S; Fouchet M-H; Beneton V; Tousaint J-J; Saintillan Y; Ancellin N; Nicodeme E; Grillot D; Martres P Discovery of novel indazole derivatives as dual angiotensin II antagonists and partial PPARγ agonists. Bioorg. Med. Chem. Lett, 2014, 24(4), 1098–1103. 10.1016/j.bmcl.2014.01.004 [DOI] [PubMed] [Google Scholar]
- [79].Meyers MJ; Arhancet GB; Hockerman SL; Chen X; Long SA; Mahoney MW; Rico JR; Garland DJ; Blinn JR; Collins JT; Yang S; Huang HC; McGee KF; Wendling JM; Dietz JD; Payne MA; Homer BL; Heron MI; Reitz DB; Hu X Discovery of (3S,3aR)-2-(3-chloro-4-cyanophenyl)-3-cyclopentyl-3,3a,4,5-tetrahydro-2H-benzo[g]indazole-7-carboxylic acid (PF-3882845), an orally efficacious mineralocorticoid receptor (MR) antagonist for hypertension and nephropathy. J. Med. Chem, 2010, 53(16), 5979–6002. 10.1021/jm100505n [DOI] [PubMed] [Google Scholar]
- [80].Cheekavolu C; Muniappan M In vivo and in vitro anti-inflammatory activity of indazole and its derivatives. J. Clin. Diagn. Res, 2016, 10(9), FF01–FF06. 10.7860/JCDR/2016/19338.8465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Oh K-S; Oh BK; Park CH; Seo HW; Kang NS; Lee JH; Lee JS; Ho Lee B Cardiovascular effects of a novel selective Rho kinase inhibitor, 2-(1H-indazole-5-yl)amino-4-methoxy-6-piperazino triazine (DW1865). Eur. J. Pharmacol, 2013, 702(1-3), 218–226. 10.1016/j.ejphar.2013.01.027 [DOI] [PubMed] [Google Scholar]
- [82].Cameron KO; Kung DW; Kalgutkar AS; Kurumbail RG; Miller R; Salatto CT; Ward J; Withka JM; Bhattacharya SK; Boehm M; Borzilleri KA; Brown JA; Calabrese M; Caspers NL; Cokorinos E; Conn EL; Dowling MS; Edmonds DJ; Eng H; Fernando DP; Frisbie R; Hepworth D; Landro J; Mao Y; Rajamohan F; Reyes AR; Rose CR; Ryder T; Shavnya A; Smith AC; Tu M; Wolford AC; Xiao J Discovery and preclinical characterization of 6-chloro-5-[4-(1-hydroxycyclobutyl)phenyl]-1H-indole-3-carboxylic acid (PF-06409577), a direct activator of adenosine monophosphate-activated protein kinase (AMPK), for the potential treatment of diabetic nephropathy. J. Med. Chem, 2016, 59(17), 8068–8081. 10.1021/acs.jmedchem.6b00866 [DOI] [PubMed] [Google Scholar]
- [83].Wada Y; Shirahashi H; Iwanami T; Ogawa M; Nakano S; Morimoto A; Kasahara K; Tanaka E; Takada Y; Ohashi S; Mori M; Shuto S Discovery of novel indazole derivatives as highly potent and selective human β3-adrenergic receptor agonists with the possibility of having no cardiovascular side effects. J. Med. Chem, 2015, 58(15), 6048–6057. 10.1021/acs.jmedchem.5b00638 [DOI] [PubMed] [Google Scholar]
- [84].Rajesh KG; Sasaguri S; Suzuki R; Maeda H Antioxidant MCI-186 inhibits mitochondrial permeability transition pore and upregulates Bcl-2 expression. Am. J. Physiol. Heart Circ. Physiol, 2003, 285(5), H2171–H2178. 10.1152/ajpheart.00143.2003 [DOI] [PubMed] [Google Scholar]
- [85].Nath K; Guo L; Nancolas B; Nelson DS; Shestov AA; Lee S-C; Roman J; Zhou R; Leeper DB; Halestrap AP; Blair IA; Glickson JD Mechanism of antineoplastic activity of lonidamine. Biochim. Biophys. Acta, 2016, 1866(2), 151–162. 10.1016/j.bbcan.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tash JS; Chakrasali R; Jakkaraj SR; Hughes J; Smith SK; Hornbaker K; Heckert LL; Ozturk SB; Hadden MK; Kinzy TG; Blagg BS; Georg GI Gamendazole, an orally active indazole carboxylic acid male contraceptive agent, targets HSP90AB1 (HSP90BETA) and EEF1A1 (eEF1A), and stimulates Il1a transcription in rat Sertoli cells. Biol. Reprod, 2008, 78(6), 1139–1152. 10.1095/biolreprod.107.062679 [DOI] [PubMed] [Google Scholar]
- [87].Nya-Ngatchou J-J; Amory JK New approaches to male non-hormonal contraception. Contraception, 2013, 87(3), 296–299. 10.1016/j.contraception.2012.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Booth EA; Marchesi M; Knittel AK; Kilbourne EJ; Lucchesi BR The pathway-selective estrogen receptor ligand WAY-169916 reduces infarct size after myocardial ischemia and reperfusion by an estrogen receptor dependent mechanism. J. Cardiovasc. Pharmacol, 2007, 49(6), 401–407. 10.1097/FJC.0b013e3180544527 [DOI] [PubMed] [Google Scholar]
- [89].Grima J; Silvestrini B; Cheng CY Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol. Reprod, 2001, 64(5), 1500–1508. 10.1095/biolreprod64.5.1500 [DOI] [PubMed] [Google Scholar]
- [90].Wang L; Yan M; Li H; Wu S; Ge R; Wong CK; Silvestrini B; Sun F; Cheng CY The Non-Hormonal Male Contraceptive Adjudin Exerts Its Effects via MAPs and Signaling Proteins MTORC1/RpS6 and FAK-Y407. Endocrinology, 2021, 162(1), bqaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]