Abstract
BACKGROUND:
Patients with heart failure (HF) with preserved ejection fraction (HFpEF) and obesity experience a high burden of symptoms and functional impairment, and a poor quality of life. In the STEP-HFpEF trial (Research Study to Investigate How Well Semaglutide Works in People Living With Heart Failure and Obesity), once-weekly semaglutide 2.4 mg improved symptoms, physical limitations, and exercise function, and reduced inflammation and body weight. This prespecified analysis investigated the effects of semaglutide on the primary and confirmatory secondary end points across the range of the Kansas City Cardiomyopathy Questionnaire (KCCQ) scores at baseline and on all key summary and individual KCCQ domains.
METHODS:
STEP-HFpEF randomly assigned 529 participants with symptomatic HF, an ejection fraction of ≥45%, and a body mass index of ≥30 kg/m2 to once-weekly semaglutide 2.4 mg or placebo for 52 weeks. Dual primary end points change in KCCQ-Clinical Summary Score (CSS) and body weight. Confirmatory secondary end points included change in 6-minute walk distance, a hierarchical composite end point (death, HF events, and change in KCCQ-CSS and 6-minute walk distance) and change in C-reactive protein. Patients were stratified by KCCQ-CSS tertiles at baseline. Semaglutide effects on the primary, confirmatory secondary, and select exploratory end points (N-terminal pro-brain natriuretic peptide) were examined across these subgroups. Semaglutide effects on additional KCCQ domains (Total Symptom Score [including symptom burden and frequency], Physical Limitations Score, Social Limitations Score, Quality of Life Score, and Overall Summary Score) were also evaluated.
RESULTS:
Baseline median KCCQ-CSS across tertiles was 37, 59, and 77 points, respectively. Semaglutide consistently improved primary end points across KCCQ tertiles 1 to 3 (estimated treatment differences [95% CI]: for KCCQ-CSS, 10.7 [5.4 to 16.1], 8.1 [2.7 to 13.4], and 4.6 [–0.6 to 9.9] points; for body weight, –11 [–13.2 to –8.8], –9.4 [–11.5 to –7.2], and –11.8 [–14.0 to –9.6], respectively; Pinteraction=0.28 and 0.29, respectively); the same was observed for confirmatory secondary and exploratory end points (Pinteraction>0.1 for all). Semaglutide-treated patients experienced improvements in all key KCCQ domains (estimated treatment differences, 6.7–9.6 points across domains; P≤0.001 for all). Greater proportion of semaglutide-treated versus placebo-treated patients experienced at least 5-, 10-, 15-, and 20-point improvements in all KCCQ domains (odds ratios, 1.6–2.9 across domains; P<0.05 for all).
CONCLUSIONS:
In patients with HFpEF and obesity, semaglutide produced large improvements in HF-related symptoms, physical limitations, exercise function, inflammation, body weight, and N-terminal pro-brain natriuretic peptide, regardless of baseline health status. The benefits of semaglutide extended to all key KCCQ domains.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT04788511.
Keywords: heart failure, diastolic; health status; obesity; quality of life; semaglutide; weight loss
Clinical Perspective.
What Is New?
In this prespecified analysis of the STEP-HFpEF trial (Research Study to Investigate How Well Semaglutide Works in People Living With Heart Failure and Obesity) that evaluated patients with heart failure with preserved ejection fraction and obesity, semaglutide consistently improved heart failure–related symptoms, physical limitations, and exercise function, and reduced body weight, C-reactive protein, and N-terminal pro-brain natriuretic peptide regardless of Kansas City Cardiomyopathy Questionnaire Clinical Summary Score at baseline.
Semaglutide-treated patients experienced large improvements in all key health status domains, which collectively reflect symptoms, physical limitations, social limitations, and quality of life.
In addition, a greater proportion of semaglutide-versus placebo-treated patients experienced small, moderate, large, and very large improvements across all of these domains.
What Are the Clinical Implications?
These findings allow for a more comprehensive review of semaglutide effects during clinician–patient discussions and shared decision-making.
Collectively, the results of the study provide additional support for semaglutide as a valuable treatment option in patients with heart failure with preserved ejection fraction and obesity.
Heart failure (HF) with preserved ejection fraction (HFpEF) currently represents the majority of all HF cases in the community, and its prevalence continues to increase, with few effective treatments available.1,2 Most people with HFpEF have obesity,3 and this group of patients is characterized by an especially high burden of HF-related symptoms and physical limitations, as well as adverse hemodynamics and a high risk for adverse HF events.3–10 Improvement in health status (symptoms, function, and quality of life) is a key goal of management in HF, and previous studies indicate that many patients with HF value improvement in these outcomes at least as much as survival.11–13 In the STEP-HFpEF trial (Research Study to Investigate How Well Semaglutide Works in People Living With Heart Failure and Obesity), treatment with once-weekly subcutaneous semaglutide 2.4 mg produced large improvements in symptoms, physical limitations, and exercise function, reduced inflammation, and resulted in greater weight loss compared with placebo in participants with HFpEF and obesity.14,15
However, it is not known whether these benefits of semaglutide in STEP-HFpEF vary depending on the degree of health status impairment at baseline (as had been previously observed with other HFpEF therapies).16 Furthermore, it is important to have a more granular understanding regarding the effects of semaglutide on all key aspects of health status, which include symptoms (burden and frequency), physical limitations, quality of life, and social limitations; and on the proportion of patients that experience deterioration, as well as small, moderate, large, and very large improvements across these domains.
Accordingly, this prespecified analysis of STEP-HFpEF had 2 chief objectives: (1) to investigate the efficacy of semaglutide versus placebo in patients with HFpEF and obesity on the dual primary, confirmatory secondary, and select exploratory trial outcomes across the different categories of health status impairment, as defined by the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS tertiles); and (2) to examine the effects of semaglutide across all of the key KCCQ domains, including the individual and summary scores that span symptoms, physical limitations, quality of life, and social limitations.
METHODS
Study Design
STEP-HFpEF (NCT04788511) was a randomized, international, double-blind, placebo-controlled trial that examined the efficacy and safety of semaglutide 2.4 mg once weekly compared with placebo in patients with HFpEF and obesity and without diabetes.14 The study design and the primary results have been published.14,15 Institutional review board ethics approval was obtained at each study site, and all participants provided informed consent.
Study Participants
Eligible participants were randomly assigned 1:1 to semaglutide 2.4 mg subcutaneously or matching placebo once weekly in addition to standard of care for 52 weeks.15 Patients were eligible if they had a documented history of HFpEF with a left ventricular ejection fraction ≥45%, New York Heart Association functional class II to IV, body mass index (BMI) ≥30 kg/m2, KCCQ-CSS <90 points, and at least 1 of the following criteria: (1) elevated left ventricular filling pressures (pulmonary artery wedge pressure or left ventricular end diastolic pressure ≥15 mm Hg at rest or ≥25 mm Hg with exercise documented during catheterization, or pulmonary artery diastolic pressure measured by an implantable monitor ≥15 mm Hg, assessed invasively); (2) elevated N-terminal pro-brain natriuretic peptide (NT-proBNP) levels (≥220 pg/mL for patients with BMI <35.0 and sinus rhythm, ≥660 pg/mL for patients with BMI <35.0 and persistent/permanent atrial fibrillation, ≥125 pg/mL for patients with BMI ≥35.0 and sinus rhythm, or ≥375 pg/mL for patients with BMI ≥35.0 and persistent/permanent atrial fibrillation, together with echocardiographic abnormalities [at least 1 of the following: {i} septal e′ <7 cm/s or lateral e′ <10 cm/s or average E/e′ ≥15; {ii} pulmonary artery systolic pressure >35 mm Hg; {iii} left atrial enlargement defined by local laboratory; {iv} left ventricular hypertrophy with septal thickness or posterior wall thickness ≥1.2 cm]); or (3) hospitalization for HF in the preceding 12 months plus requirement for ongoing diuretics and echocardiographic abnormalities (as defined above). Key exclusion criteria were prior or planned bariatric surgery, self-reported change in body weight >11 pounds (5 kg) within 90 days before randomization, or a systolic blood pressure of >160 mm Hg at screening. Patients were excluded from the trial if they had a glycated hemoglobin level ≥6.5% or a medical history of diabetes, because clinical characteristics and response to semaglutide may differ in patients with diabetes. A sister trial (STEP-HFpEF DM [Research Study to Look at How Well Semaglutide Works in People Living With Heart Failure, Obesity and Type 2 Diabetes]) is evaluating the effects of semaglutide in patients with HFpEF, obesity, and diabetes (NCT04916470). The STEP-HFpEF trial was sponsored by Novo Nordisk.
KCCQ Assessments
The KCCQ is a standardized 23-item, self-administered instrument that quantifies HF-related symptoms (including symptom burden and frequency scores [SBS and SFS, respectively], summarized by Total Symptom Score [TSS]), physical function (summarized by the Physical Limitations Score [PLS]), quality of life (summarized by the Quality of Life Score [QoLS]), and social function (summarized by the Social Limitations Score [SLS]).17 For each domain, the validity, reproducibility, responsiveness, and interpretability have been independently established for both HF with reduced ejection fraction and HFpEF populations.18 Scores are transformed to a range of 0 to 100, in which higher scores reflect better health status.19 KCCQ-CSS includes the symptom and physical function domains of the KCCQ, and the KCCQ Overall Summary Score (OSS) incorporates all of these domains. All KCCQ assessments were obtained at 20, 36, and 52 weeks after randomization.
Outcomes
The dual primary end points of STEP-HFpEF were change in KCCQ-CSS and percentage change in body weight from baseline to week 52.14,15 Confirmatory secondary end points included exercise function assessed by change in 6-minute walk distance (6MWD) from baseline to week 52, overall clinical benefit assessed using a hierarchical composite end point (all-cause death, HF events, several thresholds of change in KCCQ-CSS, and change in 6MWD ≥30 m from baseline to week 52), and change in C-reactive protein from baseline to week 52. Change in the levels of NT-proBNP between baseline and week 52 was one of the exploratory end points.
For this analysis, we also evaluated the change in all key summary (KCCQ-CSS, OSS, and TSS) and individual (SBS, SFS, PLS, SLS, and QoLS) KCCQ domains between baseline and 52 weeks.
In addition, to better understand the relationship between the degree of weight loss and extent of change in various KCCQ domains (excluding KCCQ-CSS, for which these results were previously reported),20 weight loss “dose-effect” analyses were performed according to the magnitude of body weight change during the trial. These analyses were confined to the semaglutide group, because the primary objective was to examine the effects of body weight change related to semaglutide treatment rather than spontaneous or other lifestyle-related weight changes (as in the placebo group).
Statistical Analysis
Baseline characteristics were evaluated according to the tertiles of KCCQ-CSS at baseline (≤48.4, 48.4 to ≤66.7, and >66.7 points) and tests for trend were performed across these subgroups; continuous variables used the Jonckheere–Terpstra trend test and binary variables used a Cochran–Armitage trend test. Efficacy end points for semaglutide compared with placebo, stratified by KCCQ-CSS tertiles at baseline, were assessed using the full analysis set and the treatment policy estimand (all randomized participants according to the intention-to-treat principle, regardless of treatment discontinuation). For change in KCCQ-CSS and 6MWD, missing observations at week 52 due to reasons other than cardiovascular death or previous HF events (if nonretrieved) were multiple imputed from retrieved participants in the same randomized treatment arm. For other end points, missing observations at week 52 were imputed irrespective of death or previous HF events using the same imputation method (see additional details on the imputation approach in the Supplemental Material). Subgroup analyses within the KCCQ-CSS tertiles for continuous end points were then performed using ANCOVA models, with an interaction term between treatment and KCCQ-CSS tertiles, adjusted for the baseline value of the relevant continuous outcome variable and BMI group (the stratification factor) using 1000 multiple imputations. For analyses of C-reactive protein and NT-proBNP, values were log-transformed. Estimates from the multiple imputations were derived using Rubin’s rule. Interaction P values were derived from an F-test of equality between the treatment differences across the 3 KCCQ-CSS tertiles. Furthermore, a linear contrast test was applied. Subgroup analyses of the hierarchical composite end point (win ratio) were performed based on direct comparisons of each participant randomly assigned to semaglutide versus each participant randomly assigned to placebo within each KCCQ tertile. For each of these participant pairs, a “treatment winner” on the basis of similar observation time was declared on the basis of the end point hierarchy (as previously reported).15 The win ratio (ie, the proportion of winners randomly assigned to semaglutide divided by the winners randomly assigned to placebo) was estimated independently within each KCCQ tertile using 1000 imputations. Test for equality of the KCCQ-CSS tertiles for the win ratio was performed using a Cochran’s Q-test. Analyses of all other KCCQ domains at week 52 used the same methodology and imputation methods as described above for KCCQ-CSS. In supportive analyses, the effects of semaglutide versus placebo on KCCQ domains were also evaluated using mixed models for repeated measurements with treatment adjusted for baseline of the end point variable and BMI group all nested within the trial visit using observed in-trial data. An unstructured covariance matrix was employed.
For the responder analyses, we examined the proportions of participants (on the basis of the observed data) who experienced a ≥5-point deterioration as well as ≥5-, 10-, 15-, and 20-point improvement across all key KCCQ domains (corresponding to at least small, at least moderate, large, and very large improvements) in semaglutide-treated and placebo-treated patients.19 For KCCQ-CSS, we also constructed the cumulative response curves that plot observed changes in KCCQ-CSS scores between baseline and week 52 against the cumulative proportions of participants in the semaglutide and placebo groups experiencing those changes. Logistic regression models were then used to calculate the odds ratios and corresponding 95% CIs for semaglutide effects on the likelihood of ≥5-point deterioration, as well as ≥5-, 10-, 15-, and 20-point improvement across all key KCCQ domains, with 1000 multiple imputations to account for missing data, adjusted for the baseline values for the relevant outcome variable and BMI group (the stratification factor). Estimates from the multiple imputations were derived using Rubin’s rule.
Change in body weight was analyzed as an ordinal variable using a linear regression model, including the following weight loss categories (from baseline to week 52): <5%, 5% to <10%, 10% to <15%, 15% to <20%, and ≥20%, and examining the association with changes in the KCCQ domains (OSS, TSS, PLS, QoLS, and SLS) at week 52 using observed in-trial data, adjusted for baseline body weight and the relevant KCCQ domain. A test for linearity was employed for these categorial weight change analyses.
P values <5% were considered significant and no adjustment for multiplicity was performed. All analyses were prespecified in the statistical analysis plan before the database lock.
Data Availability Statement
Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at https://www.novonordisk-trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the United States. Individual participant data will be shared in data sets in a deidentified/anonymized format.
RESULTS
Correlates of Health Status at Baseline
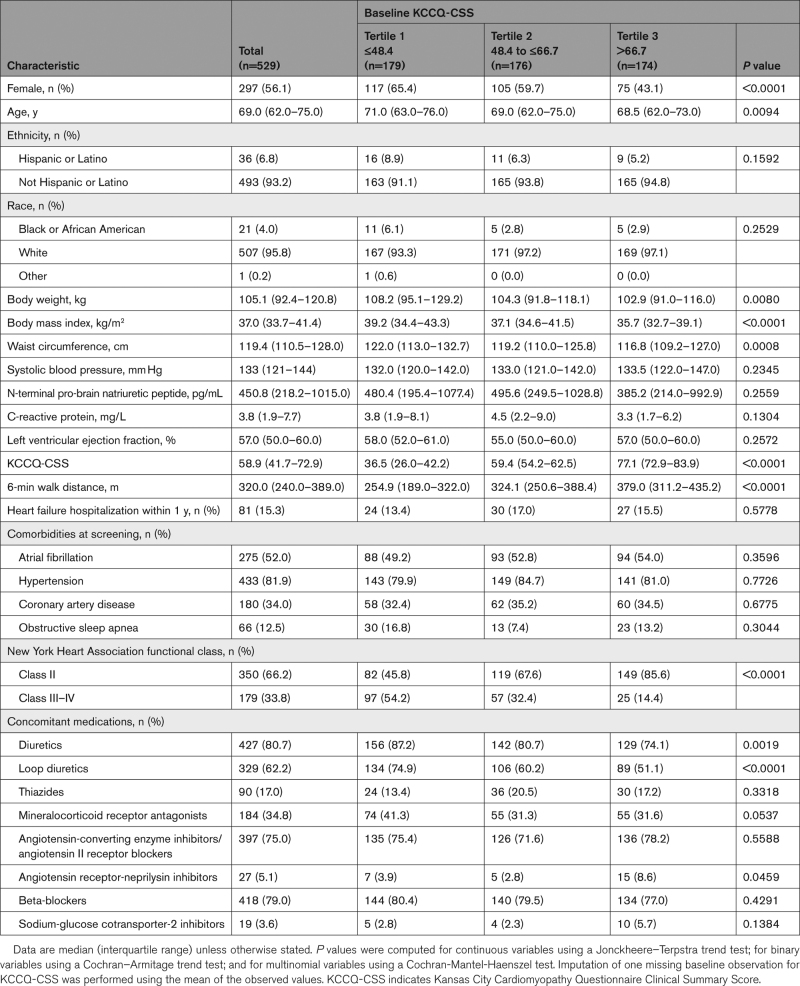
A total of 817 patients were screened, and 529 fulfilled eligibility criteria and were enrolled and randomly assigned between March 19, 2021, and March 09, 2022.15 Among the 529 STEP-HFpEF participants, 263 and 266 were randomly assigned to semaglutide and placebo, respectively; KCCQ data were available in 529 (100%) participants at baseline and 480 (91%) participants at week 52. The median KCCQ-CSS was 59 points; median KCCQ-CSS across tertiles was 37, 59, and 77 points, respectively. Compared with patients who had higher KCCQ-CSS at baseline, those with lower KCCQ-CSS were more likely to be women and older, with higher BMI, worse 6MWD and New York Heart Association functional class, and greater use of loop diuretics and mineralocorticoid receptor antagonists (Table). In contrast, there were no differences in NT-proBNP levels or the rates of atrial fibrillation or other comorbidities across participants in the 3 KCCQ-CSS tertiles.
Table.
Baseline Characteristics Stratified by KCCQ Tertiles
Treatment Effects by Baseline KCCQ-CSS
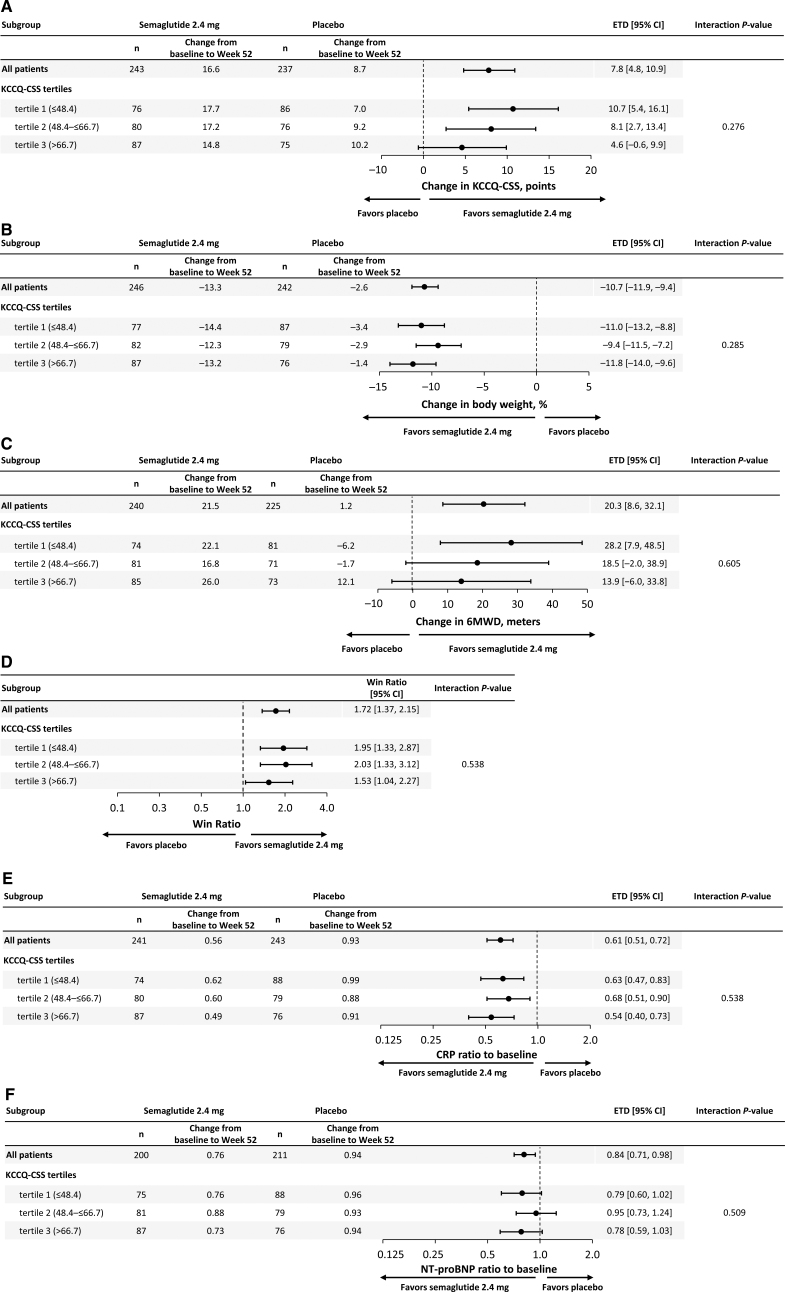
As compared with placebo, treatment with semaglutide improved KCCQ-CSS and reduced body weight across KCCQ-CSS tertiles (Figure 1A and 1B). Although the improvements in KCCQ-CSS were numerically greater in patients with the highest burden of symptoms and physical limitations, these differences were not statistically significant. Semaglutide also improved 6MWD, resulted in a greater number of wins versus placebo for the composite hierarchical end point, and reduced C-reactive protein and NT-proBNP across KCCQ-CSS tertiles, with no significant heterogeneity of treatment benefits (Figure 1C through 1F).
Figure 1.
Effects of semaglutide compared with placebo across KCCQ tertiles on heart failure symptoms and physical limitations (KCCQ-CSS; A); body weight (B); exercise function (6MWD; C); hierarchical composite end point (D); systemic inflammation (CRP; E); and NT-proBNP (F). Data are from the in-trial period for the full analysis set. Week 52 responses were analyzed using an ANCOVA model with randomized treatment, subgroup, and treatment by subgroup interaction as factors and baseline KCCQ-CSS as covariate. Missing observations for reasons other than cardiovascular death or previous heart failure events (if nonretrieved) were multiple (×1000) imputed from retrieved participants of the same randomized treatment arm. Missing observations due to cardiovascular death or previous heart failure events were imputed using a composite strategy with the least favorable value determined during the trial. P values for linear trend across the KCCQ tertiles were: 0.113 (KCCQ-CSS); 0.617 (body weight); 0.330 (6MWD); 0.478 (CRP), and 0.965 (NT-proBNP). 6MWD indicates 6-minute walk distance; CRP, C-reactive protein; CSS, Clinical Summary Score; ETD, estimated treatment difference; KCCQ, Kansas City Cardiomyopathy Questionnaire; and NT-proBNP, N-terminal pro-brain natriuretic peptide.
Semaglutide Effects Across KCCQ Domains
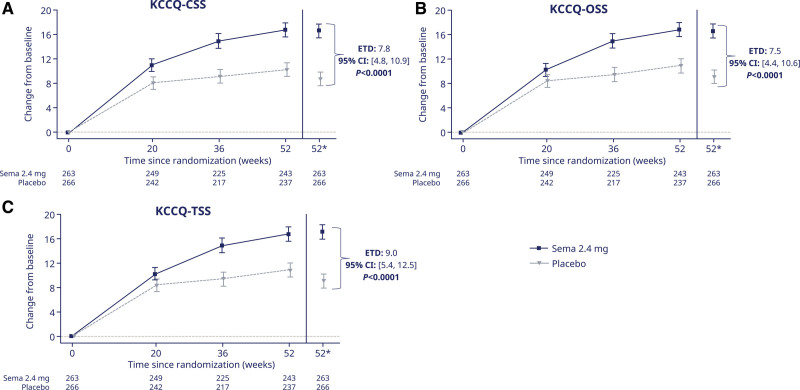
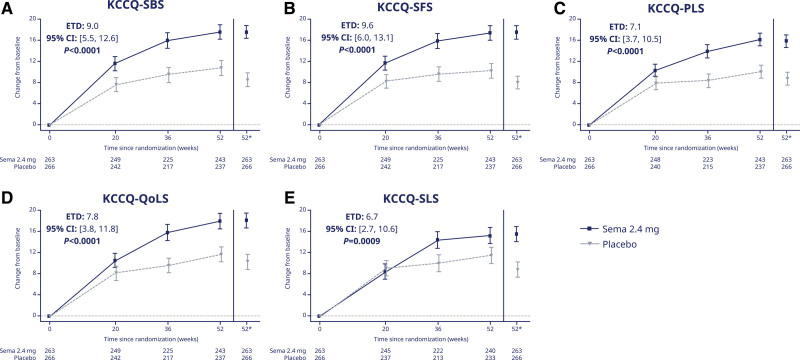
Treatment with semaglutide (versus placebo) improved all 3 KCCQ summary domains (estimated treatment differences and 95% CIs for KCCQ-CSS, OSS, and TSS: 7.8 [4.8 to 10.9], 7.5 [4.4 to 10.6], and 9.0 [5.4 to 12.5] points; Figure 2A through 2C, respectively; P<0.0001 for all). The same was the case for the individual domains of KCCQ-SBS, SFS, PLS, QoLS, and SLS (Figure 3A through 3E, respectively; P≤0.001 for all). The results were similar in supportive analyses that used mixed models for repeated measurements (Table S1).
Figure 2.
Change from baseline over time in KCCQ-CSS (A); KCCQ-OSS (B); and KCCQ-TSS (C). Observed data from the in-trial period. Error bars are ±SEM. *Estimated means are from the ANCOVA analysis. Numbers shown in the lower panel are subjects contributing to the mean. CSS indicates Clinical Summary Score; ETD, estimated treatment difference; KCCQ, Kansas City Cardiomyopathy Questionnaire; OSS, Overall Summary Score; Sema, semaglutide; and TSS, Total Symptom Score.
Figure 3.
Change from baseline over time in KCCQ-SBS (A); KCCQ-SFS (B); KCCQ-PLS (C); KCCQ-QoLS (D); and KCCQ-SLS (E). Observed data from the in-trial period. Error bars are ±SEM. *Estimated means are from the ANCOVA analysis. Numbers shown in the lower panel are subjects contributing to the mean. ETD indicates estimated treatment difference; KCCQ, Kansas City Cardiomyopathy Questionnaire; PLS, Physical Limitations Score; QoLS, Quality of Life Score; SBS, Symptom Burden Score; Sema, semaglutide; SFS, Symptom Frequency Score; and SLS, Social Limitations Score.
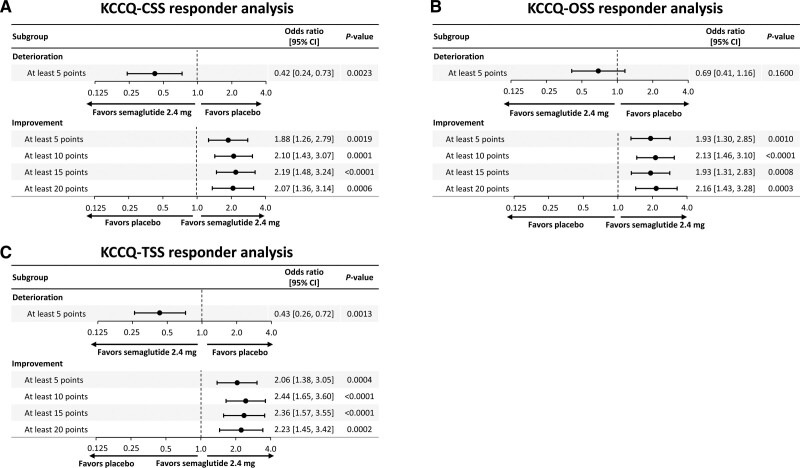
Fewer semaglutide-treated versus placebo-treated patients experienced ≥5-point deterioration across KCCQ domains (although these results did not reach statistical significance for OSS and QoLS). A greater proportion of semaglutide-treated versus placebo-treated patients experienced ≥5-, 10-, 15-, and 20-point improvements across all KCCQ domains (P<0.05 for all). The results of the responder analyses for KCCQ-CSS, OSS, and TSS are presented in Figure S1A through S1C, respectively, and for KCCQ-SBS, SFS, PLS, QoLS, and SLS in Figure S2A through S2E, respectively, for the observed proportions; the corresponding figures for the logistic regression models are Figure 4A through 4C and Figure S3A through S3E.
Figure 4.
Responder analysis for KCCQ-CSS (A); KCCQ-OSS (B); and KCCQ-TSS (C). Analysis of data from the in-trial period. responses were analyzed using a binary logistic regression model with randomized treatment and BMI group as factors and baseline KCCQ-CSS as covariate. Missing observations for reasons other than cardiovascular death or previous heart failure events (if nonretrieved) were multiple (×1000) imputed from retrieved participants of the same randomized treatment arm. Missing observations due to cardiovascular death or previous heart failure events were imputed using a composite strategy with the least favorable value determined during the trial. BMI indicates body mass index; CSS, Clinical Summary Score; KCCQ, Kansas City Cardiomyopathy Questionnaire; OSS, Overall Summary Score; and TSS, Total Symptom Score.
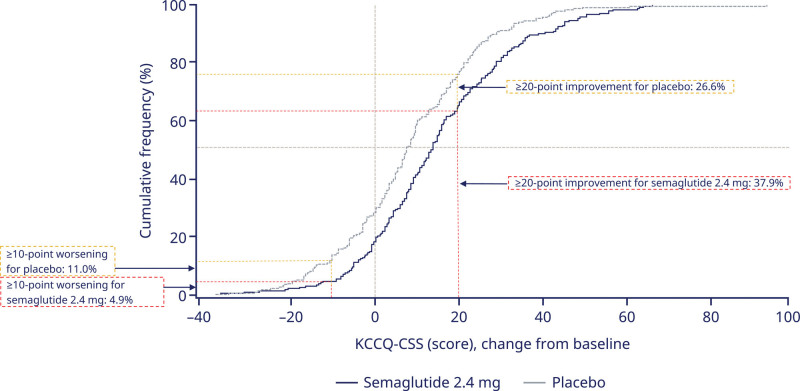
The cumulative response analysis showed continuous separation of KCCQ-CSS curves in favor of semaglutide versus placebo across the entire range of KCCQ change between baseline and week 52 (Figure 5). Of patients treated with semaglutide, 37.9% experienced an increase in KCCQ-CSS of ≥20 points, compared with 26.6% treated with placebo. Conversely, 4.9% of patients treated with semaglutide experienced a decrease in KCCQ-CSS of ≥10 points compared with 11% in the placebo group.
Figure 5.
KCCQ-CSS change from baseline to week 52 (cumulative response curves). Observed data from the in-trial period for the full analysis set. The graph shows cumulative frequency distributions of change from baseline in KCCQ-CSS. To interpret this graph, select a change in KCCQ-CSS on the x axis and find the corresponding proportion of semaglutide 2.4 mg and placebo participants who achieved that degree of improvement or worsening on the y axis. For example, note that the vertical line arising from 20-point improvement intersects with semaglutide and placebo curves at 62.1% and 73.4%, respectively. Therefore, 37.9% and 26.6% in the semaglutide and placebo groups achieved a ≥20-point improvement, respectively. KCCQ-CSS indicates Kansas City Cardiomyopathy Questionnaire Clinical Summary Score.
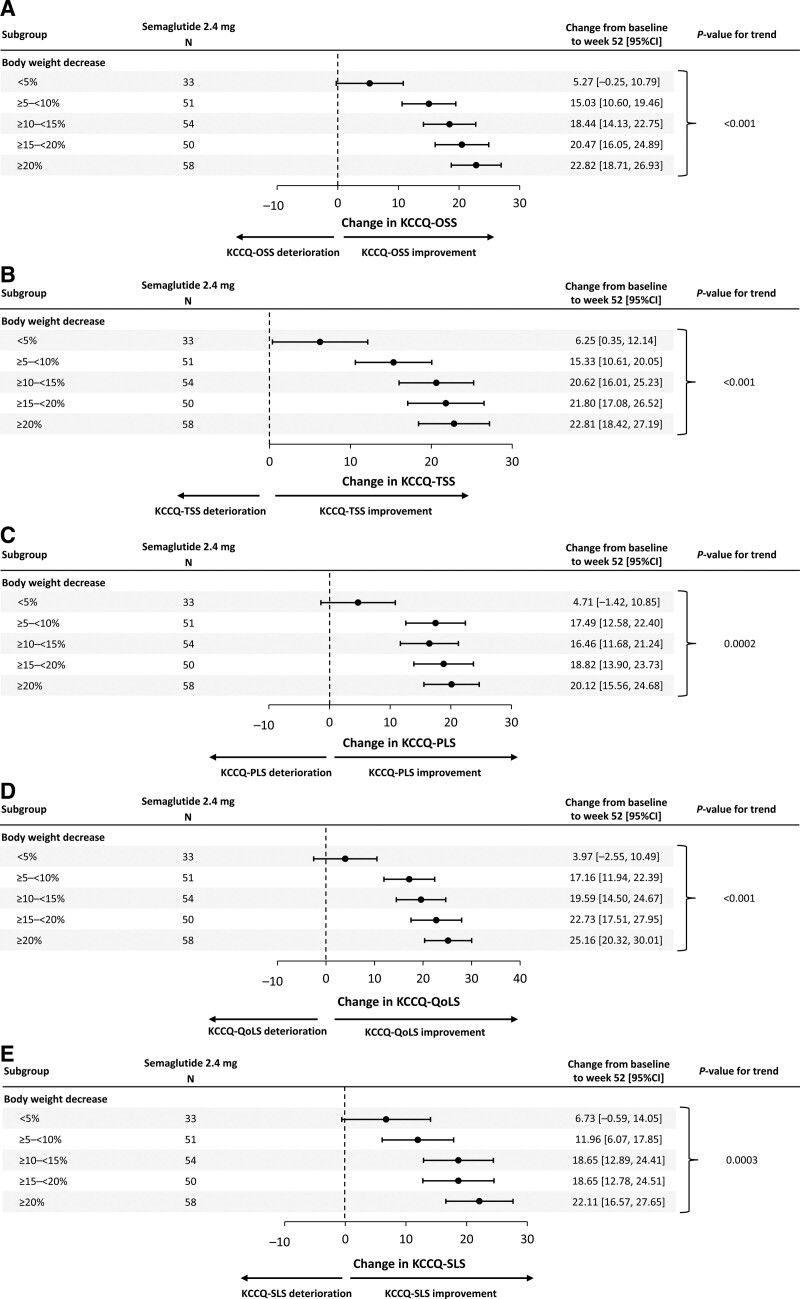
Relationship Between the Extent of Weight Loss and Change in KCCQ Domains in Semaglutide-Treated Patients
There was a significant, linear relationship between greater weight loss and larger improvements in KCCQ-OSS, TSS, PLS, QoLS, and SLS (P<0.001 for all; Figure 6A through 6E).
Figure 6.
Association of change from baseline to week 52 in KCCQ domains OSS (A); TSS (B); PLS (C); QoLS (D); SLS (E) and change in body weight. Analysis of data from the in-trial period. Participants with change from baseline in body weight at week 52 in the respective end point are included. The change from baseline to week 52 of each end point was analyzed using a linear regression model with treatment, subgroup, and treatment by subgroup interaction as factors and the baseline body weight and baseline value of the respective end point as covariates. P values are linear contrast test within treatment group. Results are shown for the semaglutide group only. KCCQ indicates Kansas City Cardiomyopathy Questionnaire; OSS, Overall Summary Score; PLS, Physical Limitations Score; QoLS, Quality of Life Score; SLS, Social Limitations Score; and TSS, Total Symptom Score.
DISCUSSION
In this prespecified analysis from the STEP-HFpEF trial, which evaluated patients with HFpEF and obesity, those with the poorest health status were more likely to be women, treated with diuretics, and living with greater obesity severity. Treatment with semaglutide (versus placebo) consistently improved HF-related symptoms, physical limitations, and exercise function, and reduced body weight, C-reactive protein, and NT-proBNP regardless of KCCQ-CSS at baseline. Furthermore, semaglutide produced large improvements in all key summary and individual domains of KCCQ, which together comprise symptoms (burden and frequency), physical limitations, quality of life, and social limitations. Finally, fewer semaglutide-treated patients experienced deterioration, and greater proportions of semaglutide-treated patients experienced at least small, moderate, large, and very large improvements across all key KCCQ domains compared with placebo.
These results have several important implications. First, our analyses of the dual primary and confirmatory secondary end points demonstrated no significant heterogeneity in the benefits of semaglutide according to the magnitude of symptomatic and functional impairment at baseline. Although some benefits of semaglutide (such as improvements in KCCQ-CSS) were numerically greater in patients with the highest burden of symptoms and physical limitations, these differences across subgroups were not statistically significant; and even those participants who had relatively mild KCCQ impairments experienced meaningful health status improvements.
Second, our findings substantially expand on the previously reported effects of semaglutide in patients with HFpEF and obesity,14 by evaluating a much broader range of KCCQ outcomes, as well as the detailed responder analyses. Specifically, when compared with other recent global clinical trial programs in HFpEF (such as those evaluating sodium-glucose cotransporter-2 inhibitors [SGLT2i]),16,21,22 we specifically undertook a more granular evaluation across all key summary and individual KCCQ domains, examining symptom burden and frequency, physical limitations, quality of life, and social limitations. This allows for a more comprehensive review of semaglutide effects during clinician–patient discussions and shared decision-making.
Third, and most importantly, the magnitude of improvements we observed with semaglutide versus placebo across all key KCCQ domains (when examining both mean effects and responder analyses) is substantial when considering other therapies that have been tested in HFpEF. Although cross-trial comparisons should be viewed with caution, the mean KCCQ effects (across examined domains) seen with sacubitril-valsartan, spironolactone, and SGLT2i, are generally in the range of 0.5 to 3.0 points; and the corresponding responder analyses for these treatments show similarly modest effects.21,23–26 The magnitude of both mean health status benefits seen with semaglutide in STEP-HFpEF (6.7- to 9.6-point increases across KCCQ domains) and the odds ratios for both deterioration (which range between 0.4 and 0.7) and very large improvements in KCCQ scores (which exceeded 2.0 for most KCCQ domains) in the responder analyses are considerably more pronounced. Of note (and in contrast with other tested HFpEF therapies), the beneficial effects of semaglutide on health status also appear to amplify over time, which is of clinical relevance. These findings collectively provide additional support for semaglutide as a valuable treatment option for patients with HFpEF and obesity.
The key hypothesis behind the STEP-HFpEF trial was that obesity is not simply a comorbidity but rather is a root cause for the development and progression of HFpEF in this patient group via a host of mechanisms6–8,27–29; therefore, targeting obesity can improve the key outcomes that define the HFpEF syndrome (ie, symptoms and functional limitations). Disentangling the extent to which the observed benefits of semaglutide are due to weight loss versus its other potential effects is challenging because these occur in parallel. In our previous report20 and in this study, we demonstrated that, in semaglutide-treated patients, a greater degree of weight loss was associated with larger improvements in various KCCQ domains, suggesting that weight loss is an important factor in the health status benefits of semaglutide. However, we also observed a significant reduction in NT-proBNP among semaglutide-treated versus placebo-treated participants (both overall, and across the tertiles of baseline KCCQ-CSS). Because NT-proBNP would be expected to increase with body weight reduction, as seen in previous trials of lifestyle-mediated weight loss,30 the reduction in NT-proBNP seen with semaglutide despite the substantial associated weight loss suggests disease-modifying, decongestive effects that extend beyond its effects on body weight.
The findings of this study should be considered in the context of several potential limitations. Although the proportions of women and men were balanced, most participants were White, and individuals with diabetes were excluded by design, which may affect the generalizability to non-White populations and people living with diabetes. A separate, ongoing trial is evaluating the effects of semaglutide in people with the HFpEF, obesity, and type 2 diabetes.15 The STEP-HFpEF trial was designed to evaluate effects of treatment on symptoms and physical limitations, exercise function, and inflammation, along with body weight, and was not powered to assess clinical end points such as HF hospitalizations. As with most clinical trials, STEP-HFpEF was designed to have the appropriate statistical power for the analyses of the key end points in the overall patient population, rather than within specific subgroups; subgroup analyses should be interpreted within the context of this limitation. The 52-week duration of treatment was relatively short, and whether the observed effects might have persisted (or become more amplified) with longer evaluation is not known. Use of SGLT2i was low in STEP-HFpEF, as patients with diabetes were excluded, and these agents were not yet approved for the treatment of HFpEF during the trial. Although semaglutide and SGLT2i have complementary and nonoverlapping mechanisms of action, the present study cannot determine whether background therapy with SGLT2i might have influenced the treatment benefits observed, which is an important question for future trials. Further insight into the effects of semaglutide in patients who receive background SGLT2i will be provided by the STEP-HFpEF DM trial, which includes a greater proportion (32%) of patients taking these agents.15 Nevertheless, given the proven benefits of SGLT2i in this patient population, it is reasonable to postulate that the future management of patients with HFpEF and obesity may include a combination therapy of SGLT2i and semaglutide.31
Conclusions
In patients with HFpEF and obesity, treatment with semaglutide produced large improvements in HF-related symptoms, physical limitations, and exercise function, and reduced body weight, inflammation, and natriuretic peptides regardless of baseline health status. Benefits of semaglutide extended to all key summary and individual KCCQ domains, with a significantly greater proportion of semaglutide-treated versus placebo-treated patients experiencing at least small, moderate, large, and very large improvements.
ARTICLE INFORMATION
Acknowledgments
This trial was sponsored by Novo Nordisk A/S and is registered at https://www.clinicaltrials.gov (NCT04788511). The sponsor took responsibility for activities related to trial conduct, data collection, and statistical analysis. The authors are indebted to the trial participants, the investigators, and the trial site staff who conducted the trial. Administrative support and development of figures and tables were provided by Casey McKeown RVN, FdSc, of Apollo, OPEN Health Communications, and funded by Novo Nordisk A/S, in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022). The academic members (Drs Kosiborod, Borlaug, Butler, Davies, Kitzman, Petrie, Shah, and Verma) of the steering committee, along with the sponsor, Novo Nordisk, conceived and designed the study. The first draft of the manuscript was prepared by Dr Kosiborod, who had full access to all study data. All authors interpreted the data, contributed to manuscript writing, approved the final version of the manuscript, vouched for data accuracy and fidelity to the protocol, and had final responsibility for the decision to submit for publication.
Sources of Funding
This trial was funded by Novo Nordisk A/S. Administrative support for manuscript development was funded by Novo Nordisk A/S. Dr Verma is supported by the Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada and holds the Canada Research Chair in Cardiovascular Surgery. Dr Borlaug is supported in part by the National Institutes of Health (NIH) grants R01 HL128526, R01 HL162828, and U01 HL160226, and by the US Department of Defense grant W81XWH2210245. Dr Davies is supported by the Leicester National Institute for Health Research (NIHR) Biomedical Research Centre, Leicester General Hospital, Leicester, UK. Dr Petrie is supported by the British Heart Foundation Centre of Research Excellence Award (RE/13/5/30177 and RE/18/6/34217+). Dr Shah was supported by NIH grants U54 HL160273, R01 HL107577, R01 HL127028, R01 HL140731, and R01 HL149423. Dr Kitzman was supported in part by the Kermit Glenn Phillips II chair in cardiovascular medicine and NIH grants U01AG076928, R01AG078153, R01AG045551, R01AG18915, P30AG021332, U24AG059624, and U01HL160272.
Disclosures
Dr Kosiborod served as a consultant or on an advisory board for 35Pharma, Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon Pharmaceuticals, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pfizer, Pharmacosmos, Sanofi, scPharmaceuticals, Structure Therapeutics, Vifor Pharma, and Youngene Therapeutics; has received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer; holds stocks in Artera Health and Saghmos Therapeutics; and has received honoraria from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk. He has also received other research support from AstraZeneca. Dr Verma has received research grants or consultancy fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Napp Pharmaceuticals, Novartis, Novo Nordisk, Pharmacosmos, Roche, and SQ Innovations; has served on committees for AbbVie, Akero, Alnylam, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, New Amsterdam, Novo Nordisk, Resverlogix, and Teikoku; and is Director of Global Clinical Trial Partners (GCTP). Dr Borlaug receives research grant funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, Rivus, and Tenax Therapeutics; has served as a consultant for Actelion, Amgen, Aria, Axon Therapies, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Imbria, Janssen, Merck, Novo Nordisk, NGM, NXT, and VADovations; and is a named inventor (US patent No. 10,307,179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure. Dr Butler is a consultant to 3live, Abbott, American Regent, Amgen, Applied Therapeutic, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardiac Dimension, Cardior, CVRx, Cytokinetics, Edwards, Element Science, Impulse Dynamics, Imbria, Innolife, Inventiva, Janssen, Lexicon, Lilly, LivaNova, Medtronics, Merck, Novartis, Novo Nordisk, Occlutech, Pfizer, Pharmacosmos, Pharmain, Roche, Sequana, SQ Innovation, and Vifor. Dr Davies has acted as a consultant, advisory board member, and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi; an advisory board member and speaker for AstraZeneca; an advisory board member for Medtronic, Pfizer, and ShouTi Pharma; and a speaker for Amgen, Novartis, and Sanofi. She has received grants in support of investigator and investigator-initiated trials from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, and Sanofi-Aventis. Dr Petrie has received research funding from AstraZeneca, Boehringer Ingelheim, Boston Scientific, Medtronic, Novartis, Novo Nordisk, Pharmacosmos, Roche, and SQ Innovations; and consultancy and committees payments for AbbVie, Akero, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardiorentis, Horizon Therapeutics, New Amsterdam, Novartis, Novo Nordisk, Pharmacosmos, Siemens, Takeda, Teikoku, and Vifor. Dr Shah reports receiving consulting fees from Abbott, Amgen, Aria CV, AstraZeneca, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Lilly, Merck, Metabolic Flux, MyoKardia, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sardocor, Shifamed, Tenax, Tenaya, and United Therapeutics. Drs Jensen, Rasmussen, and Marstrand are employees of and hold shares in Novo Nordisk. Dr Ito reports honoraria and consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Mochida, Novartis, and Novo Nordisk. Dr Schou reports speaker fees from AstraZeneca, Boehringer Ingelheim, Novartis, and Novo Nordisk. Dr Melenovský reports consulting fees from Bayer, MSD, and Novo Nordisk; research grants from Regeneron; and research support from the National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104), funded by the European Union – Next Generation EU. Dr Abhayaratna reports honoraria and consulting fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Novartis, and Novo Nordisk. Dr Kitzman reports receiving honoraria as a consultant for AstraZeneca, Bayer, Boehringer Ingelheim, Corvia Medical, Ketyo, Novartis, Novo Nordisk, Pfizer, and Rivus; received grant funding from AstraZeneca, Bayer, Novartis, Novo Nordisk, Pfizer, and Rivus; and has stock ownership in Gilead Sciences.
Supplemental Material
Additional information on the imputation methods to account for missing data, including references 32 and 33.
Table S1
Figure S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 6MWD
- 6-minute walk distance
- BMI
- body mass index
- CSS
- Clinical Summary Score
- HF
- heart failure
- HFpEF
- heart failure with preserved ejection fraction
- KCCQ
- Kansas City Cardiomyopathy Questionnaire
- NT-proBNP
- N-terminal pro-brain natriuretic peptide
- OSS
- Overall Summary Score
- PLS
- Physical Limitations Score
- QoLS
- Quality of Life Score
- SBS
- Symptom Burden Score
- SFS
- Symptom Frequency Score
- SGLT2i
- sodium-glucose cotransporter-2 inhibitor
- SLS
- Social Limitations Score
- TSS
- Total Symptom Score
Supplemental Material, the podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.123.067505.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
For Sources of Funding and Disclosures, see page 215.
Circulation is available at www.ahajournals.org/journal/circ
Presented as an abstract at the Scientific Sessions of the American Heart Association, Philadelphia, PA, November 11–13, 2023.
Contributor Information
Subodh Verma, Email: Subodh.Verma@unityhealth.to.
Barry A. Borlaug, Email: borlaug.barry@mayo.edu.
Javed Butler, Email: butlzih@gmail.com.
Melanie J. Davies, Email: melanie.davies@uhl-tr.nhs.uk.
Thomas Jon Jensen, Email: tjj@novonordisk.com.
Søren Rasmussen, Email: srrm@novonordisk.com.
Peter Erlang Marstrand, Email: PMRD@novonordisk.com.
Mark C. Petrie, Email: mark.petrie@glasgow.ac.uk.
Sanjiv J. Shah, Email: Sanjiv.shah@northwestern.edu.
Hiroshi Ito, Email: itomd1602@gmail.com.
Morten Schou, Email: morten.schou.04@regionh.dk.
Vojtěch Melenovský, Email: vome@ikem.cz.
Walter Abhayaratna, Email: Walter.P.Abhayaratna@act.gov.au.
Dalane W. Kitzman, Email: dkitzman@wakehealth.edu.
REFERENCES
- 1.Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. 2023;329:827–838. doi: 10.1001/jama.2023.2020 [DOI] [PubMed] [Google Scholar]
- 2.Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC Scientific Statement. J Am Coll Cardiol. 2023;81:1810–1834. doi: 10.1016/j.jacc.2023.01.049 [DOI] [PubMed] [Google Scholar]
- 3.Morgen CS, Haase CL, Oral TK, Schnecke V, Varbo A, Borlaug BA. Obesity, cardiorenal comorbidities, and risk of hospitalization in patients with heart failure with preserved ejection fraction. Mayo Clin Proc. 2023;98:1458–1468. doi: 10.1016/j.mayocp.2023.07.008 [DOI] [PubMed] [Google Scholar]
- 4.Dalos D, Mascherbauer J, Zotter-Tufaro C, Duca F, Kammerlander AA, Aschauer S, Bonderman D. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:189–199. doi: 10.1016/j.jacc.2016.04.052 [DOI] [PubMed] [Google Scholar]
- 5.Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol. 2016;68:200–203. doi: 10.1016/j.jacc.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 6.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/CIRCULATIONAHA.116.026807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy YNV, Lewis GD, Shah SJ, Obokata M, Abou-Ezzedine OF, Fudim M, Sun JL, Chakraborty H, McNulty S, LeWinter MM, et al. Characterization of the obese phenotype of heart failure with preserved ejection fraction: a RELAX trial ancillary study. Mayo Clin Proc. 2019;94:1199–1209. doi: 10.1016/j.mayocp.2018.11.037 [DOI] [PubMed] [Google Scholar]
- 8.Reddy YNV, Rikhi A, Obokata M, Shah SJ, Lewis GD, AbouEzzedine OF, Dunlay S, McNulty S, Chakraborty H, Stevenson LW, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22:1009–1018. doi: 10.1002/ejhf.1788 [DOI] [PubMed] [Google Scholar]
- 9.Adamson C, Kondo T, Jhund PS, de Boer RA, Cabrera Honorio JW, Claggett B, Desai AS, Alcocer Gamba MA, Al Habeeb W, Hernandez AF, et al. Dapagliflozin for heart failure according to body mass index: the DELIVER trial. Eur Heart J. 2022;43:4406–4417. doi: 10.1093/eurheartj/ehac481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borlaug BA, Jensen MD, Kitzman DW, Lam CSP, Obokata M, Rider OJ. Obesity and heart failure with preserved ejection fraction: new insights and pathophysiological targets. Cardiovasc Res. 2023;118:3434–3450. doi: 10.1093/cvr/cvac120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed SD, Fairchild AO, Johnson FR, Gonzalez JM, Mentz RJ, Krucoff MW, Vemulapalli S. Patients’ willingness to accept mitral valve procedure-associated risks varies across severity of heart failure symptoms. Circ Cardiovasc Interv. 2019;12:e008051. doi: 10.1161/CIRCINTERVENTIONS.119.008051 [DOI] [PubMed] [Google Scholar]
- 12.Forman DE, Arena R, Boxer R, Dolansky MA, Eng JJ, Fleg JL, Haykowsky M, Jahangir A, Kaminsky LA, Kitzman DW, et al. ; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e894–e918. doi: 10.1161/CIR.0000000000000483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4 [DOI] [PubMed] [Google Scholar]
- 14.Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, Hovingh GK, Kitzman DW, Lindegaard ML, Møller DV, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389:1069–1084. doi: 10.1056/nejmoa2306963 [DOI] [PubMed] [Google Scholar]
- 15.Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Christensen L, Davies M, Hovingh KG, Kitzman DW, Lindegaard ML, Møller DV, et al. Design and baseline characteristics of STEP-HFpEF program evaluating semaglutide in patients with obesity HFpEF phenotype. JACC Heart Fail. 2023;11:1000–1010. doi: 10.1016/j.jchf.2023.05.010 [DOI] [PubMed] [Google Scholar]
- 16.Kosiborod MN, Bhatt AS, Claggett BL, Vaduganathan M, Kulac IJ, Lam CSP, Hernandez AF, Martinez FA, Inzucchi SE, Shah SJ, et al. Effect of dapagliflozin on health status in patients with preserved or mildly reduced ejection fraction. J Am Coll Cardiol. 2023;81:460–473. doi: 10.1016/j.jacc.2022.11.006 [DOI] [PubMed] [Google Scholar]
- 17.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 18.Joseph SM, Novak E, Arnold SV, Jones PG, Khattak H, Platts AE, Dávila-Román VG, Mann DL, Spertus JA. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail. 2013;6:1139–1146. doi: 10.1161/circheartfailure.113.000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:2379–2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 20.Borlaug BA, Kitzman DW, Davies MJ, Rasmussen S, Barros E, Butler J, Einfeldt MN, Hovingh GK, Møller DV, Petrie MC, et al. Semaglutide in HFpEF across obesity class and by body weight reduction: a prespecified analysis of the STEP-HFpEF trial. Nat Med. 2023;29:2358–2365. doi: 10.1038/s41591-023-02526-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler J, Filippatos G, Jamal Siddiqi T, Brueckmann M, Böhm M, Chopra VK, Pedro Ferreira J, Januzzi JL, Kaul S, Piña IL, et al. Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the EMPEROR-Preserved trial. Circulation. 2022;145:184–193. doi: 10.1161/CIRCULATIONAHA.121.057812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–1960. doi: 10.1038/s41591-021-01536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wachter R, Shah SJ, Cowie MR, Szecsödy P, Shi V, Ibram G, Zhao Z, Gong J, Klebs S, Pieske B. Angiotensin receptor neprilysin inhibition versus individualized RAAS blockade: design and rationale of the PARALLAX trial. ESC Heart Fail. 2020;7:856–864. doi: 10.1002/ehf2.12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gronda E, Vanoli E, Iacoviello M. The PARAGON-HF trial: the sacubitril/valsartan in heart failure with preserved ejection fraction. Eur Heart J Suppl. 2020;22:L77–L81. doi: 10.1093/eurheartj/suaa140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. ; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 26.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. ; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 27.Sorimachi H, Burkhoff D, Verbrugge FH, Omote K, Obokata M, Reddy YNV, Takahashi N, Sunagawa K, Borlaug BA. Obesity, venous capacitance, and venous compliance in heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:1648–1658. doi: 10.1002/ejhf.2254 [DOI] [PubMed] [Google Scholar]
- 28.Reddy YNV, Obokata M, Testani JM, Felker GM, Tang WHW, Abou-Ezzeddine OF, Sun JL, Chakrabothy H, McNulty S, Shah SJ, et al. Adverse renal response to decongestion in the obese phenotype of heart failure with preserved ejection fraction. J Card Fail. 2020;26:101–107. doi: 10.1016/j.cardfail.2019.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation. 2017;135:825–838. doi: 10.1161/CIRCULATIONAHA.116.024822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertoni AG, Wagenknecht LE, Kitzman DW, Marcovina SM, Rushing JT, Espeland MA; Brain Natriuretic Peptide Subgroup of the Look AHEAD Research Group. Impact of the look AHEAD intervention on NT-pro brain natriuretic peptide in overweight and obese adults with diabetes. Obesity (Silver Spring). 2012;20:1511–1518. doi: 10.1038/oby.2011.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verma S, Borlaug BA, Butler J, Davies MJ, Kitzman DW, Petrie MC, Shah SJ, Dhingra NK, Kosiborod MN. A big STEP for treatment of heart failure with preserved ejection fraction. Cell Metab. 2023;35:1681–1687. doi: 10.1016/j.cmet.2023.08.003 [DOI] [PubMed] [Google Scholar]
- 32.McEvoy BW. Missing data in clinical trials for weight management. J Biopharm Stat. 2016;26:30–36. doi: 10.1080/10543406.2015.1094814 [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Tu W, Kim Y, Sinks S, He J, Cambon A, Crackel R, Hamilton K, Kettermann A, Clark J. Statistical methods for handling missing data to align with treatment policy strategy. Pharm Stat. 2023;22:650–670. doi: 10.1002/pst.2299 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared with bona fide researchers submitting a research proposal approved by the independent review board. Access request proposals can be found at https://www.novonordisk-trials.com. Data will be made available after research completion, and approval of the product and product use in the European Union and the United States. Individual participant data will be shared in data sets in a deidentified/anonymized format.