ABSTRACT
Eravacycline is a synthetic fluorocycline approved by the U.S. Food and Drug Administration in 2018. This study aimed to describe clinical and microbiological outcomes in addition to associated adverse effects of eravacycline used in U.S. hospitals. Real-world, observational study involving patients receiving ≥72 h of eravacycline at 19 medical centers located in all 5 regions of the United States between October 2018 and August 2022. The primary outcome was clinical success, defined as survival and absence of microbiological recurrence at 30 days from the end of eravacycline therapy and clinical improvement within 96 h of eravacycline initiation. In total, 416 patients met study criteria and were evaluated. Index culture specimens were most often isolated from the respiratory tract (24.8%, n = 103/416), wound(s) (20.9%, n = 87/416), or blood (19.5%, n = 81/416). As definitive therapy, eravacycline was most often used to treat infections caused by Enterobacterales spp. (42.3%, n = 176/416; 24.4%, n = 43/176 carbapenem-resistant), Enterococci spp. (24.0%, n = 100/416; 49.0%, 49/100 vancomycin-resistant), and Acinetobacter spp. (23.3%, n = 97/416; 47.4%, n = 46/97 carbapenem-resistant). Clinical success occurred in 75.7% of patients (n = 315/416). Thirty-nine (9.4%, n = 39/416) patients experienced a treatment emergent adverse event (TEAE) potentially related to eravacycline with the majority (51.3%, n = 20/39) being gastrointestinal intolerance. Only 27 isolates (6.5%, n = 27/416) underwent eravacycline susceptibility testing. Eravacycline is being used to treat a broad range of Gram-negative and Gram-positive bacteria in the United States including those demonstrating multidrug-resistance with consistently low reported drug-related TEAE; however, antimicrobial susceptibility testing and subsequent in vitro susceptibility data of clinical isolates was sparingly performed.
IMPORTANCE
The rise of multidrug-resistant (MDR) pathogens, especially MDR Gram-negatives, poses a significant challenge to clinicians and public health. These resilient bacteria have rendered many traditional antibiotics ineffective, underscoring the urgency for innovative therapeutic solutions. Eravacycline, a broad-spectrum fluorocycline tetracycline antibiotic approved by the FDA in 2018, emerges as a promising candidate, exhibiting potential against a diverse array of MDR bacteria, including Gram-negative, Gram-positive, anaerobic strains, and Mycobacterium. However, comprehensive data on its real-world application remain scarce. This retrospective cohort study, one of the largest of its kind, delves into the utilization of eravacycline across various infectious conditions in the USA during its initial 4 years post-FDA approval. Through assessing clinical, microbiological, and tolerability outcomes, the research offers pivotal insights into eravacycline’s efficacy in addressing the pressing global challenge of MDR bacterial infections.
KEYWORDS: eravacycline, multidrug-resistant, antimicrobial stewardship
INTRODUCTION
Faced with the escalating challenge of antimicrobial resistance, which threatens an estimated 10 million lives annually by 2050 due to the spread of multidrug-resistant (MDR) pathogens, novel solutions are imperative (1 – 3). In this context, the U.S. Food and Drug Administration (FDA) approved eravacycline in August 2018 for treating complicated intraabdominal infections (cIAI) (4). As the first fully synthetic fluorocycline, eravacycline maintains stability against the efflux pumps and ribosomal protection proteins that typically confer resistance to other members of the tetracycline antibiotic class (5). Eravacycline has shown potent in vitro activity against a wide range of Gram-negative bacteria, including carbapenem-resistant isolates. This includes bacteria such as Enterobacterales that produce an extended-spectrum beta-lactamase or carbapenemase, Acinetobacter species, and other MDR Gram-negative pathogens. Several studies have reported low minimum inhibitory concentration (MIC90) values for eravacycline against these bacteria (6 – 10). Additionally, eravacycline has demonstrated activity in vitro against Gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE), nontuberculosis mycobacteria, and anaerobic bacteria such as Clostridioides difficile (11 – 13).
The efficacy of eravacycline in treating cIAIs was established through two phase III multicenter clinical randomized controlled trials (RCTs) (IGNITE I/IV), demonstrating noninferiority to ertapenem and meropenem, respectively (14, 15). Furthermore, real-world experience with eravacycline, although limited, has shown comparable clinical success and tolerability to the clinical trials (16). Retrospective studies utilizing real-world data provide valuable insights into real-world outcomes and usage, complementing RCTs by exploring long-term outcomes, rare adverse events, and complex relationships in diverse populations. The objective of this study is to describe the clinical use of eravacycline in United States hospitals in terms of clinical and microbiological response and drug-related adverse events in its first 4 years following FDA approval.
MATERIALS AND METHODS
Retrospective cohort study using inpatient data from October 2018 to August 2022 of adult patients admitted to a participating medical center and receipt of ≥72 consecutive hours of eravacycline therapy for any pathogen within the spectrum of eravacycline activity isolated from any infectious source. Participating centers encompassed a diverse range of medical institutions including academic/university-affiliated centers and community hospitals situated in both urban and suburban locales. Exclusion criteria included patients who were pregnant or nursing, prisoners, and those that received subsequent eravacycline courses not separated by at least 90 days from the end of the index eravacycline treatment course.
The primary outcome was clinical success, defined as survival with absence of microbiological recurrence at 30 days from the end of eravacycline therapy and clinical improvement within 96 hours of eravacycline initiation. Microbiological recurrence was defined as a positive culture for the same organism and infectious source within 30 days from the end of eravacycline therapy. Clinical improvement was defined as the resolution of infectious signs and symptoms including infection-related abnormal white blood cell count/temperature or as documented by the physician in clinical notes. Key secondary clinical, microbiological, and tolerability endpoints including hospital readmission, infection-related readmission, and possible eravacycline-related adverse effects using the common terminology criteria for adverse events were also evaluated (17). The relationship of possible treatment emergent adverse events (TEAEs) related to eravacycline was determined based on adverse event onset in relation to the initiation and possible discontinuation of eravacycline using medical record documentation. Concomitant therapy was defined as any therapy used in conjunction with eravacycline for ≥48 continuous hours for the primary organism that eravacycline therapy was used for.
To obtain information on patient demographics and baseline characteristics, we accessed the electronic health record (EHR) and recorded the data in Research Electronic Data Capture (REDCap) (18). The Charlson Comorbidity Index was used to estimate comorbidity burden, while measures of organ function and illness severity were assessed based on the highest Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) score within 48 h of index culture collection. Index culture was defined as the culture collected closest to eravacycline initiation. Immunosuppressive factors were defined as neutropenia (absolute neutrophil count <500), splenectomy (functional or surgical), or high dose corticosteroids (>prednisone 20 mg/day or equivalent). All cultures, bacterial identifications, and antibiotic susceptibilities were conducted according to local procedures at each center. Clinical Laboratory Standards Institute (CLSI) breakpoints were used to interpret MIC results, where applicable (19).
Descriptive statistics were employed to evaluate baseline characteristics. Frequencies and percentages were used to report discrete data, while continuous data were described using median and interquartile range (IQR) or mean and standard deviation (SD), depending on the normality of the distribution. IBM SPSS Statistics version 29 (IBM Corp., Armonk, NY) was used to carry out the analyses.
RESULTS
Demographics
A total of 416 patients receiving ≥72 h of eravacycline were included from 19 medical centers located in all five geographic regions of the United States. Baseline demographic data are displayed in Table 1. The mean (SD) age was 58.7 (15) years, and most patients were male (56.7%, n = 236/416), Caucasian (56.7%, n = 236/416), and admitted from home (57.2%, n = 238/416). The median (IQR) Charlson Comorbidity Index was 4.5 (2, 7) with diabetes (36.3%, n = 151/416), heart failure (18.3%, n = 76/416), and peripheral vascular disease (18%, n = 75/416) being the most common comorbid conditions. At least one immunosuppressive factor was identified in 16.6% (n = 69/416) of patients and 87.2% (n = 363/416) of patients had at least one MDR risk factor with the most common being ≥48 h hospitalization (55.3%, n = 230/416) and ≥24 h antibiotics (53.4%, n = 222/416) within 90 days prior to index positive culture collection for which eravacycline was used as definitive therapy.
TABLE 1.
| Parameter | Value |
|---|---|
| Age (mean) (years) ± SD | 58.7 ± 15 |
| Male | 236 (56.7) |
| Race | |
| African American | 122 (29.3) |
| Asian | 9 (2.2) |
| Caucasian | 236 (56.7) |
| Hispanic | 41 (9.9) |
| Other | 5 (1.2) |
| BMI (kg/m2) | 26.4 (22.6, 32.4) |
| Obese (BMI ≥30 kg/m2) | 133 (32) |
| Admitted from | |
| Home | 238 (57.2) |
| NH/LTC | 80 (19.2) |
| Transfer from outside hospital | 61 (14.7) |
| Other | 37 (8.9) |
| Severity scores | |
| APACHE II score | 16 (11, 25) |
| SOFA score | 4.5 (2.5, 7) |
| Charlson Comorbidity Index | 4.5 (2, 7) |
| Comorbid conditions | |
| Heart failure | 76 (18.3) |
| COPD | 65 (15.6) |
| Diabetes | 151 (36.3) |
| Chronic kidney disease | 71 (17.1) |
| HD dependent | 30 (7.2) |
| Clostridiodes difficile b | 27 (6.5) |
| Immunosuppression factors | |
| Neutropenia | 15 (3.6) |
| AIDS | 1 (0.2) |
| Splenectomy | 6 (1.4) |
| Solid organ transplant | 10 (2.4) |
| Bone marrow transplant | 3 (0.7) |
| Cytotoxic chemotherapy | 22 (5.3) |
| High dose corticosteroids | 12 (2.9) |
| MDR risk factors | |
| ≥24 h antibiotics within 90 days | 222 (53.4) |
| ≥48 h hospitalization within 90 days | 230 (55.3) |
| NH/LTC resident | 80 (19.2) |
| Home infusion | 18 (4.3) |
| Chronic dialysis | 24 (5.8) |
| Home wound care | 38 (9.1) |
| Surgery ≤30 days before index culture | 75 (18) |
| Colonization with resistant organism(s) | 46 (11.1) |
| Prior infection with resistant organism | 104 (25) |
Data presented as number (%) or median (IQR), as appropriate.
History of Clostridiodes difficile infection.
Immunosupression factors: neutropenia (absolute neutrophil count <500); splenectomy (functional or surgical); high dose corticosteroids (≥ prednisone 20 mg/day or equivalent).
SD, standard deviation; BMI, body mass index; LTAC, long-term acute care; NH/LTC, nursing home/long-term care facility; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, sequential organ failure assessment; COPD, chronic obstructive pulmonary disease; HD, hemodialysis; HIV, human immunodeficiency virus; IVDU, intravenous drug user; AIDS, acute immunodeficiency syndrome.
Clinical course and treatment characteristics
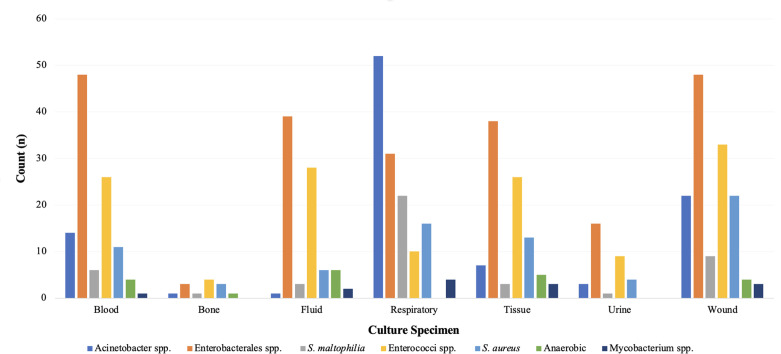
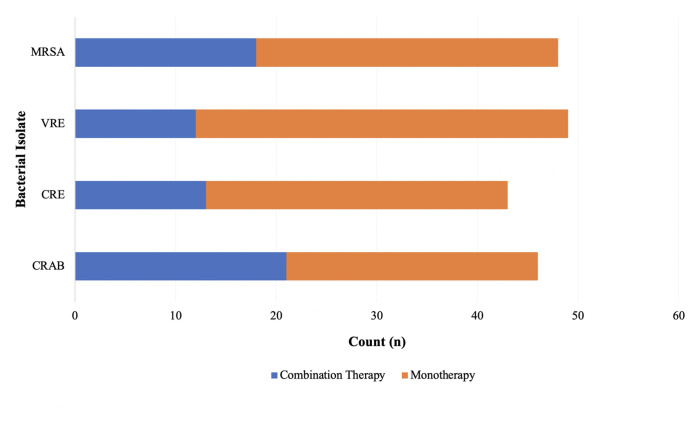
Clinical course and treatment characteristics are displayed in Table 2. In total, 42.5% (n = 177/416) of patients were admitted to the intensive care unit (ICU) at least once during the hospital admission with 26.9% (n = 112/416) being in the ICU at the time of index culture collection. Infections treated with eravacycline were classified as hospital-acquired in 49.5% (n = 206/416) of cases with a median (IQR) of 3 (1, 14) days from hospital admission to index culture collection. Index culture specimens were most often isolated from the respiratory tract (24.8%, n = 103/416), wound(s) (20.9%, n = 87/416), or blood (19.5%, n = 81/416). Figure 1 displays microbiological characteristics of index culture specimens. Infectious diseases and/or surgical consultations were initiated in 91.3% (n = 380/416) and 51.4% (n = 214/416) of patients, respectively. More than one-half of patients underwent a surgical procedure for source control (52.6%, n = 219/416) with incision and drainage (16.3%, n = 68/416) and debridement (12.3%, n = 51/416) being the most common procedures. Receipt of antimicrobial therapy with in vitro activity prior to the initiation of eravacycline occurred in 47.1% (n = 196/416) of patients and most often consisted of a carbapenem (29.6%, n = 58/196) or aminoglycoside (11.2%, n = 22/196) for isolated Gram-negative bacteria and vancomycin (16.8%, n = 70/196) or linezolid (12.8%, n = 25/196) for Gram-positive bacteria. The median (IQR) time from index culture collection to the first administration of eravacycline was 4 (2, 8) days. Consolidation of the antibiotic regimen was the most common reason for selecting eravacycline as definitive therapy (39.7%, n = 165/416). Most patients received eravacycline according to the package insert-recommended dosing of 1 mg/kg (96.9%, n = 403/416) administered every 12 h (95%, n = 395/416). The median duration of eravacycline therapy was 6.9 (4.1, 11.9) days. Approximately one-half of patients (50.7%, n = 211/416) received ≥48 h of concomitant antibiotic therapy with eravacycline, which was most often either meropenem (17.5%, n = 37/211) or amikacin (8.5%, n = 18/211). Figure 2 displays the use of combination therapy versus monotherapy for the treatment of select resistant bacterial isolates.
TABLE 2.
| Parameter | Value |
|---|---|
| ICU admissions | |
| At least one ICU admission during hospitalization | 177 (42.5) |
| Patients with >1 ICU admission during hospitalization | 43 (10.3) |
| In ICU at index culture collection | 112 (26.9) |
| ICU length of stay (days) | 14 (7, 32) |
| Mechanical ventilation for ≥48 h prior to index positive culture | 68 (16.3) |
| Duration of mechanical ventilation (days) | 22.5 (12, 36) |
| Culture information | |
| Hospital-acquired infection b | 206 (49.5) |
| Culture specimen | |
| Respiratory culture specimen | 103 (24.8) |
| Aspirate | 20 (4.8) |
| Bronchoalveolar lavage | 15 (3.6) |
| Sputum | 68 (16.3) |
| Wound | 87 (20.9) |
| Blood | 81 (19.5) |
| Fluid | 57 (13.7) |
| Tissue | 53 (12.7) |
| Urine | 17 (4.1) |
| Fecal | 13 (3.1) |
| Bone | 8 (1.9) |
| Catheter tip | 2 (0.5) |
| Concomitant bacteremia | 8 (1.9) |
| ID consult | 380 (91.3) |
| Surgery consult | 214 (51.4) |
| Surgical intervention c | 219 (52.6) |
| Active therapy before ERV d | 196 (47.1) |
| Aminoglycoside | 22 (11.2) |
| Carbapenem | 58 (29.6) |
| Cefepime | 44 (10.6) |
| Ceftazidime-avibactam | 16 (3.8) |
| Ceftolozane-tazobactam | 2 (0.5) |
| Meropenem-vaborbactam | 3 (0.7) |
| Polymyxins | 2 (1) |
| Quinolone | 11 (5.6) |
| Tigecycline | 8 (1.9) |
| Trimethoprim/sulfamethoxazole | 10 (2.4) |
| Daptomycin | 23 (5.5) |
| Linezolid/tedizolid | 25 (12.8) |
| Vancomycin | 70 (16.8) |
| Other | 72 (36.7) |
| ERV treatment specifics | |
| Rationale for ERV use | |
| Consolidation of regimen | 165 (39.7) |
| Lack of oral access | 16 (3.8) |
| Double CRE coverage | 31 (7.5) |
| ERV regimens | |
| Dose | |
| 1 mg/kg | 403 (96.9) |
| 1.5 mg/kg | 13 (3.1) |
| Frequency | |
| Every 12 h | 395 (95) |
| Every 12 h on day 1, then every 24 h | 14 (3.3) |
| Every 24 h | 7 (1.7) |
| ERV duration of therapy | 6.9 (4.1, 11.9) |
| Concomitant therapy e | 211 (50.7) |
| Amikacin | 18 (4.3) |
| Aztreonam | 2 (0.5) |
| Ciprofloxacin | 5 (1.2) |
| Colistin | 5 (1.2) |
| Ertapenem | 2 (0.5) |
| Gentamicin | 1 (0.2) |
| Imipenem | 5 (1.2) |
| Levofloxacin | 7 (1.7) |
| Meropenem | 37 (8.9) |
| Polymyxin B | 6 (1.4) |
| TMP/SMX | 9 (2.2) |
| Tobramycin | 9 (2.2) |
| Other | 111 (26.7) |
| Discharge disposition | |
| Home | 154 (37) |
| LTAC | 108 (26) |
| Rehab center | 35 (8.4) |
| Outside hospital | 1 (0.2) |
| Hospice | 28 (6.7) |
| Morgue | 82 (19.7) |
| Hospital length of stay (days) | 21 (11, 41) |
Data presented as number (%) or median (IQR), as appropriate.
Hospital-acquired infection: Index positive culture collected ≥48 h from hospital admission (includes time accrued at previous institution if the patient transferred from an outside hospital).
Surgical intervention: Incision and drainage (n = 68), debridement (n = 51), amputation (n = 10), valvular replacement (n = 2), invasive device removal (n = 6), other (n = 82).
Total may exceed n of 416 due to receipt of multiple antibiotics.
Active therapy: Demonstrated in vitro susceptibility.
Concomitant therapy: Antibiotic administered for ≥48 continuous hours while the patient received eravacycline.
ICU, intensive care units; ID, infectious diseases; ERV, eravacycline; CRE, carbapenem-resistant Enterobacterales; LTAC, long-term acute care.
Fig 1.
Microbiological isolates and culture specimen source.
Fig 2.
Use of combination therapy versus monotherapy for resistant bacterial isolates. Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci; CRE, carbapenem-resistant Enterobacterales; CRAB, carbapenem-resistant Acinetobacter baumannii.
Microbiological characteristics
All isolated organisms and those for which eravacycline was used as definitive therapy are displayed in Table 3. Eravacycline was most often ordered as definitive therapy to treat infections caused by Acinetobacter spp. [23.3%, n = 97/416; 47.4% of which were carbapenem-resistant Acinetobacter (n = 46/97)] or Enterococci spp. [24%, n = 100/416; 49% (n = 49/100) of which were vancomycin-resistant Enterococci]. Eravacycline was also often used as definitive therapy for carbapenem-resistant Enterobacterales (CRE) (10.3%, n = 43/416) and Stenotrophomonas maltophilia (9.9%, n = 41/416).
TABLE 3.
Definitive eravacycline therapy
| Parameter | Value |
|---|---|
| Gram-negative | |
| Achromobacter spp. | 4 (1) |
| Acinetobacter spp. | 97 (23.3) |
| Acinetobacter baumannii | 92 (22.1) |
| Carbapenem-resistant Acinetobacter spp. | 46 (11.1) |
| Enterobacterales | 176 (42.3) |
| Citrobacter freundii | 6 (1.4) |
| Enterobacter cloacae | 33 (7.9) |
| Escherichia coli | 50 (12) |
| Klebsiella aerogenes | 5 (1.2) |
| Klebsiella oxytoca | 12 (2.9) |
| Klebsiella pneumoniae | 54 (13) |
| Morganella morganii | 4 (1) |
| Proteus mirabilis | 5 (1.2) |
| Proteus vulgaris | 1 (0.2) |
| Providencia stuartii | 3 (0.7) |
| Serratia marcescens | 3 (0.7) |
| Carbapenem-resistant Enterobacterales | 43 (10.3) |
| Pseudomonas aeruginosa | 0 (0) |
| Stenotrophomonas maltophilia | 41 (9.9) |
| Gram-positive | |
| Enterococci | 100 (24) |
| Enterococcus faecalis | 45 (10.8) |
| Enterococcus faecium | 55 (13.2) |
| Vancomycin-resistant enterococci | 49 (11.8) |
| Staphylococcus aureus | 51 (12.3) |
| MRSA | 48 (11.5) |
| Coagulase negative staphylococci | 14 (3.4) |
| Streptococcus spp. | 18 (4.3) |
| S. anginosus | 9 (2.2) |
| Anaerobes | 16 (3.8) |
| Bacteroides fragilis | 6 (1.4) |
| Bacteroides ovatus | 1 (0.2) |
| Bacteroides thetaiotaomicron | 2 (0.5) |
| Clostridiodes difficile | 7 (16.8) |
| Fungal | 2 (0.5) |
| Mycobacterium spp. | |
| Mycobacterium abscessus | 14 (3.4) |
| Polymicrobial | 157 (37.7) |
Total may exceed n of 416 due to polymicrobial infections.
spp., species; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus.
Data are presented as number (%), as appropriate.
Isolate baseline MICs are displayed in Table 4. Notably, only 27 (6.5%) of isolates underwent susceptibility testing for eravacycline and of those, 88.9% (n = 24/27) were MIC testing and 11.1% (n = 3/27) were disk diffusion. Eravacycline susceptibility testing was most often conducted for A. baumannii (55.6%, n = 15/27) and carbapenem-resistant K. pneumoniae (22.2%, n = 6/27) isolates. In contrast, 49.8% (n = 207/416) of all isolates underwent susceptibility testing for minocycline (10%, n = 42/416) and/or tigecycline (46%, n = 191/416). The overall median (IQR) eravacycline MIC was 0.5 µg/mL (0.25, 1), MIC range was ≤0.125 to 2 µg/mL, and MIC90 was 1 µg/mL. For minocycline, the median (IQR) MIC was 2 µg/mL (4, 12), MIC range was 1 to 16 µg/mL, and MIC90 was 8 µg/mL. For tigecycline, the median (IQR) MIC was 2 µg/mL (1, 2), MIC range was 1 to 4 µg/mL, and MIC90 was 2 µg/mL.
TABLE 4.
Eravacycline MIC distribution by organism c
| Organism | MIC (μg/mL) a | Disk diffusion (mm) b | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤0.125 | 0.25 | 0.5 | 1 | 2 | 12 | 14 | 18 | |
| A. baumannii | 3 | 0 | 5 | 3 | 2 | 1 | 1 | 0 |
| E. cloacae | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| E. coli | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| K. pneumoniae | 2 | 4 | 0 | 0 | 0 | 0 | 0 | 1 |
| S. maltophilia | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Total | 5 | 4 | 5 | 6 | 4 | 1 | 1 | 1 |
MIC tests: Liofilchem MTS, Thermo Scientific Sensititre, and bioMerieux ETEST.
Disk diffusion test: HardyDisk.
MIC, minimum inhibitory concentration.
Clinical outcomes and tolerability
Clinical outcomes and tolerability data are displayed in Table 5. In total, 75.7% (n = 315/416) of patients demonstrated clinical success. Of those, survival and absence of microbiological recurrence within 30 days of eravacycline completion occurred in 94.7% (n = 394/416) and 94.5% (n = 393/416) of patients, respectively, while 84.1% (n = 350/416) improved clinically within 96 h of eravacycline initiation. Most patients that did not survive to 30 days following eravacycline completion (5.3%, n = 22/416) had a positive sputum (40.9%, n = 9/22) or blood (31.8%, n = 7/22) culture and/or the culture was positive for an Enterococci spp. (31.8%, n = 7/22), K. pneumoniae (18.2%, n = 4/22), or S. maltophilia (18.2%, n = 4/22).
TABLE 5.
Clinical outcomes and tolerability (n = 416)
| Parameter a | Value |
|---|---|
| Clinical success b | 315 (75.7) |
| 30-day survival | 394 (94.7) |
| Clinical improvement c | 350 (84.1) |
| Absence of microbiological recurrence d | 393 (94.5) |
| Microbiological recurrence | 23 (5.5) |
| Symptomatic | 19 (4.6) |
| Treated with antibiotic(s) | 19 (4.6) |
| Treatment-emergent resistance | 0 (0) |
| Hospital readmission | |
| 30-day | 77 (18.5) |
| 60-day e | 81 (23.9) |
| Positive culture on readmission f | 10 (6.3) |
| ERV on readmission f | 14 (8.9) |
| Adverse effects | 39 (9.4) |
| Nephrotoxicity | 4 (1) |
| Gastrointestinal intolerance g | 20 (4.8) |
| Electrolyte disturbance | 1 (0.2) |
| Encephalopathy | 3 (0.7) |
| Hepatotoxicity | 7 (1.7) |
| Dermatologic reaction | 1 (0.2) |
| Infusion site phlebitis | 2 (0.5) |
| Catheter site pain | 1 (0.2) |
| ERV discontinuation secondary to an adverse effect | 9/39 (23.1) |
| Gastrointestinal intolerance | 6/9 (66.7) |
| Hepatotoxicity | 3/9 (33.3) |
Data are presented as number (%).
Clinical success: Patient survival and absence of microbiological recurrence at 30 days from the end of eravacycline therapy and clinical improvement within 96 h of eravacycline initiation.
Clinical improvement: The resolution of infectious signs and symptoms including infection-related abnormal white blood cell count/temperature or as documented by the physician in clinical notes.
Microbiological recurrence: An isolate of the same bacteria (at species level) from the same culture source taken after a negative culture.
Patients with a 30-day readmission were muted from 60-day readmission total.
Percent based on a denominator of 158, representing the number of patients readmitted within 30 and/or 60 days.
Gastrointestinal intolerance defined as nausea, vomiting, and/or diarrhea.
Eravacycline-related TEAEs occurred in 9.4% (n = 39/416) of patients with the majority (51.3%, n = 20/39) being gastrointestinal in nature, while the remaining TEAEs occurred in <2% of the cohort. In total, 23.1% of patients (n = 9/39) had eravacycline discontinued secondary to the TEAE (gastrointestinal intolerance n = 6, hepatotoxicity n = 3). For hospital readmission, 18.5% (n = 77/416) and 23.9% (n = 81/339) were readmitted within 30 and 60 days of discharge, respectively, with 6.3% (n = 10/158) experiencing microbiological recurrence at 30 days and 8.9% (n = 14/158) receiving eravacycline upon readmission. Of the patients experiencing microbiological recurrence (5.5%, n = 23/416), positive cultures were isolated from the respiratory tract (56.5%, n = 13/23), blood (17.4%, n = 4/23), wound (17.4%, n = 4/23), and urine (8.7%, n = 2/23) and grew A. baumannii (56.5%, n = 13/23), S. maltophilia (17.4%, n = 4/23), E. faecium (13%, n = 3/23), or S. aureus (13%, n = 3/23).
DISCUSSION
This study provides valuable insight into the real-world use of eravacycline for the treatment of various infections in U.S. hospitals in the 4 years following its FDA approval. The data herein suggest that eravacycline is predominantly used as consolidation therapy for monomicrobial infections from a variety of sources. The broad activity and low MIC90 values of eravacycline against an array of Gram-negative and Gram-positive bacteria, including those demonstrating multidrug resistance, make eravacycline a therapeutic option in such challenging clinical scenarios.
These data also identified that eravacycline was commonly used as definitive therapy for infections caused by carbapenem-resistant A. baumannii and Enterobacterales spp., which make up greater than one-fifth of the study cohort. The use of eravacycline for carbapenem-resistant Acinetobacter spp. infections in the study cohort is particularly noteworthy since eravacycline has not yet earned an indication specifically for Acinetobacter spp., nor has it been assigned a CLSI or U.S. Food & Drug Administration breakpoint despite demonstrating in vitro activity against MDR A. baumannii isolates (20). Similarly, eravacycline does not have an approved indication for the treatment of respiratory or acute bacterial skin and skin structure infections, the two most common sites of positive culture attainment in this cohort (21). Additionally, compared to patients in the eravacycline IGNITE I/IV clinical trials, our study population required a significantly higher level of care. Almost half were admitted to the ICU, those on ventilators received eravacycline therapy for an average of over 3 weeks, and eravacycline was often used to treat pathogens that have historically been challenging to manage. Therefore, these findings enrich limited data suggesting that eravacycline could be a potential treatment for challenging cases, such as infections caused by CRE, carbapenem-resistant Acinetobacter spp., and Stenotrophomonas maltophilia even though recent therapeutic guidance does not currently recommend eravacycline for these conditions (22, 23). Additional data is warranted to establish the effectiveness of eravacycline in these specific patient and clinical scenarios.
The incidence of eravacycline-related adverse events and subsequent eravacycline discontinuation are like that reported in the IGNITE I/IV clinical trials with the most common being gastrointestinal disorders. While gastrointestinal disorders are more common with the tetracycline class of antibiotics, available RCT and observational data demonstrate that the incidence of drug-related gastrointestinal disorders is approximately two to five-fold lower than that reported for the other tetracyclines including omadacycline, minocycline, and tigecycline (24 – 28).
While this study is the largest report of eravacycline use in U.S. hospitals to date, it has important limitations including its retrospective, observational design, and a lack of control group to validate the role of eravacycline in reported clinical effectiveness and tolerability. Furthermore, this study highlights the limited antimicrobial susceptibility testing of eravacycline occurring in U.S. hospital-affiliated microbiology laboratories. Limited testing may be due to a lack of eravacycline breakpoints for most organisms including Acinetobacter and Stenotrophomonas maltophilia and/or limited available antimicrobial susceptibility tests for eravacycline. There are only two FDA-cleared commercial automated antimicrobial susceptibility tests (AST) with eravacycline on-panel (e.g., VITEK 2 AST Gram-Negative Panel Assay and MicroScan Neg Urine Combo 90) (29, 30), and other available AST are limited to HardyDisk, Liofilchem MTS, Thermo Scientific Sensititre, and bioMerieux ETEST (31 – 34). Unfortunately, there remains scant in vitro susceptibility data comparing isolate eravacycline MIC data to that of other novel and standard of care antibiotics. In the current study, approximately half of participating centers used the isolate tigecycline MIC to guide eravacycline use for Acinetobacter spp. and carbapenem-resistant K. pneumoniae infections, which may be problematic since tigecycline breakpoints are not established for eravacycline. Surveillance data of eravacycline in vitro activity against Gram-negative bacilli aligns with limited MIC data presented herein; however, more data are needed to elucidate the appropriateness of this practice at an organism and infectious source level.
In conclusion, eravacycline is being used in real-world clinical settings to treat a broad range of Gram-negative and Gram-positive aerobic and anaerobic bacteria in the United States, including those demonstrating multidrug-resistance, with consistently low reported drug-related TEAEs. This study adds to the growing body of evidence that supports the clinical success and tolerability of eravacycline in the treatment of complicated infections. However, the limited availability of antimicrobial susceptibility data highlights the need for continued monitoring and surveillance of antibiotic resistance patterns. Further studies are warranted to evaluate the long-term safety and efficacy of eravacycline in different patient populations and clinical settings.
ACKNOWLEDGMENTS
We thank Dr. Susan Davis (Wayne State University, Henry Ford Hospital, Detroit, MI) for her assistance with this research.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published. All authors have provided writing and editing assistance.
This study was funded by an investigator-initiated grant from Tetraphase Pharmaceuticals, Inc. M.J.R. is partially supported by NIAID R21 AI163726 and R01AI130056.
Contributor Information
Michael J. Rybak, Email: m.rybak@wayne.edu.
Ahmed Babiker, Emory University School of Medicine, Atlanta, Georgia, USA .
REFERENCES
- 1. Spagnolo F, Trujillo M, Dennehy JJ. 2021. Why do antibiotics exist? mBio 12:e0196621. doi: 10.1128/mBio.01966-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tacconelli E. 2017. World health organization. global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics 2017. Available from: https://www.who.int/medicines/publications/WHO-PPLShort_Summary_25Feb-ET_NM_WHO.pdf. :7
- 3. CDC . 2021. Antibiotic-resistant germs: new threats. Available from: https://www.cdc.gov/drugresistance/biggest-threats.html
- 4. XERAVA (eravacycline) for injection. 2020.
- 5. Grossman TH, Starosta AL, Fyfe C, O’Brien W, Rothstein DM, Mikolajka A, Wilson DN, Sutcliffe JA. 2012. Target- and resistance-based Mechanistic studies with TP-434, a novel fluorocycline antibiotic. Antimicrob Agents Chemother 56:2559–2564. doi: 10.1128/AAC.06187-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdallah M, Olafisoye O, Cortes C, Urban C, Landman D, Quale J. 2015. Activity of eravacycline against enterobacteriaceae and Acinetobacter Baumannii, including multidrug-resistant isolates, from New York City. Antimicrob Agents Chemother 59:1802–1805. doi: 10.1128/AAC.04809-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seifert H, Stefanik D, Sutcliffe JA, Higgins PG. 2018. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int J Antimicrob Agents 51:62–64. doi: 10.1016/j.ijantimicag.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 8. Clark JA, Kulengowski B, Burgess DS. 2020. In vitro activity of eravacycline compared with tigecycline against carbapenem-resistant enterobacteriaceae. Int J Antimicrob Agents 56:106178. doi: 10.1016/j.ijantimicag.2020.106178 [DOI] [PubMed] [Google Scholar]
- 9. Morrissey I, Olesky M, Hawser S, Lob SH, Karlowsky JA, Corey GR, Bassetti M, Fyfe C. 2020. In vitro activity of eravacycline against gram-negative Bacilli isolated in clinical laboratories worldwide from 2013 to 2017. Antimicrob Agents Chemother 64:e01699-19. doi: 10.1128/AAC.01699-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii - ClinicalKey. Available from: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0924857917302716?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0924857917302716%3Fshowall%3Dtrue&referrer=. Retrieved 6 Apr 2023 [DOI] [PubMed]
- 11. Zhanel GG, Baxter MR, Adam HJ, Sutcliffe J, Karlowsky JA. 2018. In vitro activity of eravacycline against 2213 gram-negative and 2424 gram-positive bacterial pathogens isolated in Canadian hospital laboratories: CANWARD surveillance study 2014-2015. Diagn Microbiol Infect Dis 91:55–62. doi: 10.1016/j.diagmicrobio.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 12. Zhuchenko G, Schmidt-Malan S, Patel R. 2020. Planktonic and biofilm activity of eravacycline against Staphylococci isolated from periprosthetic joint infections. Antimicrob Agents Chemother 64:e01304-20. doi: 10.1128/AAC.01304-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bassères E, Begum K, Lancaster C, Gonzales-Luna AJ, Carlson TJ, Miranda J, Rashid T, Alam MJ, Eyre DW, Wilcox MH, Garey KW. 2020. In vitro activity of eravacycline against common ribotypes of Clostridioides difficile. J Antimicrob Chemother 75:2879–2884. doi: 10.1093/jac/dkaa289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, Tsai L, Sutcliffe JA, Horn P. 2017. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating gram-negative infections treated with eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg 152:224–232. doi: 10.1001/jamasurg.2016.4237 [DOI] [PubMed] [Google Scholar]
- 15. Solomkin JS, Gardovskis J, Lawrence K, Montravers P, Sway A, Evans D, Tsai L. 2019. IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated Intraabdominal infections. Clin Infect Dis 69:921–929. doi: 10.1093/cid/ciy1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hobbs ALV, Gelfand MS, Cleveland KO, Saddler K, Sierra-Hoffman MA. 2022. A retrospective, multicentre evaluation of eravacycline utilisation in community and academic hospitals. J Glob Antimicrob Resist 29:430–433. doi: 10.1016/j.jgar.2021.10.020 [DOI] [PubMed] [Google Scholar]
- 17. Common terminology criteria for adverse events (CTCAE). 2017. [PubMed]
- 18. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDcap)--a metadata-driven methodology and workflow process for providing translational research Informatics support. J Biomed Inform 42:377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. EM100 connect - CLSI M100 ED32:2022. Available from: http://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSI%20M100%20ED32:2022&xormat=SPDF&src=BB. Retrieved 21 Jun 2022
- 20. Holger DJ, Kunz Coyne AJ, Zhao JJ, Sandhu A, Salimnia H, Rybak MJ. 2022. Novel combination therapy for extensively drug-resistant Acinetobacter baumannii necrotizing pneumonia complicated by empyema: a case report. Open Forum Infect Dis 9:fac092. doi: 10.1093/ofid/ofac092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. XERAVATM (eravacycline) for injection. Available from: https://www.xerava.com. Retrieved 25 May 2023
- 22. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2021. Infectious diseases society of America guidance on the treatment of extended-spectrum β-Lactamase producing enterobacterales (ESBL-E), carbapenem-resistant enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis 72:1109–1116. doi: 10.1093/cid/ciab295 [DOI] [PubMed] [Google Scholar]
- 23. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2022. Infectious diseases society of America guidance on the treatment of AmpC Β-lactamase-producing enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis 74:2089–2114. doi: 10.1093/cid/ciab1013 [DOI] [PubMed] [Google Scholar]
- 24. O’Riordan W, Cardenas C, Shin E, Sirbu A, Garrity-Ryan L, Das AF, Eckburg PB, Manley A, Steenbergen JN, Tzanis E, McGovern PC, Loh E, OASIS-2 Investigators . 2019. Once-daily oral omadacycline versus twice-daily oral linezolid for acute bacterial skin and skin structure infections (OASIS-2): a phase 3, double-blind, multicentre, randomised, controlled, non-inferiority trial. Lancet Infect Dis 19:1080–1090. doi: 10.1016/S1473-3099(19)30275-0 [DOI] [PubMed] [Google Scholar]
- 25. Smith CJ, Sayles H, Mikuls TR, Michaud K. 2011. Minocycline and doxycycline therapy in community patients with rheumatoid arthritis: prescribing patterns, patient-level determinants of use, and patient-reported side effects. Arthritis Res Ther 13:R168. doi: 10.1186/ar3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lauf L, Ozsvár Z, Mitha I, Regöly-Mérei J, Embil JM, Cooper A, Sabol MB, Castaing N, Dartois N, Yan J, Dukart G, Maroko R. 2014. Phase 3 study comparing tigecycline and ertapenem in patients with diabetic foot infections with and without osteomyelitis. Diagn Microbiol Infect Dis 78:469–480. doi: 10.1016/j.diagmicrobio.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 27. Matthews P, Alpert M, Rahav G. 2012. A randomized trial of tigecycline versus ampicillin- sulbactam or amoxicillin-clavulanate for the treatment of complicated skin and skin structure infections. Available from: https://scholarworks.bwise.kr/gachon/handle/2020.sw.gachon/17484. Retrieved 6 Apr 2023. [DOI] [PMC free article] [PubMed]
- 28. O’Riordan W, Mehra P, Manos P, Kingsley J, Lawrence L, Cammarata S. 2015. A randomized phase 2 study comparing two doses of delafloxacin with tigecycline in adults with complicated skin and skin-structure infections. Int J Infect Dis 30:67–73. doi: 10.1016/j.ijid.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 29. K191766.Pdf. Available from: https://www.accessdata.fda.gov/cdrh_docs/reviews/K191766.pdf. Retrieved 24 May 2023
- 30. beckman-coulter-microscan-panel-brochure.pdf. Available from: https://media.beckmancoulter.com/-/media/diagnostics/products/microbiology/conventional-panels/docs/beckman-coulter-microscan-panel-brochure.pdf?rev=cc6fd4c886724e7c9e276cba0c11e670&hash=1328F5CE0BBD48F7B88713C33934BDC2&_ga=2.266490062.1571211292.1684944614-67209015.1684944611. Retrieved 24 May 2023
- 31. Eravacycline, 20Μg hdx,hardydisk,1X50Disks F. Available from: https://hardydiagnostics.com/z9401. Retrieved 24 May 2023
- 32. Liofilchem MTS eravacycline [ERV] 0.002-32 G/mL - microbiology susceptibility testing, MIC test strips. Available from: https://www.fishersci.com/shop/products/mts-eravacycline-erv-0-002-32-g-ml-3/p-7146392. Retrieved 24 May 2023
- 33. Sensititre-gram-negative-MDRO-plates-Mdrgn2F-Mdrgnx2F-overview-EN-Lt2542A.Pdf. Available from: https://assets.fishersci.com/TFS-Assets/MBD/brochures/Sensititre-Gram-Negative-MDRO-Plates-MDRGN2F-MDRGNX2F-Overview-EN-LT2542A.pdf. Retrieved 24 May 2023
- 34. ETEST® ERAVACYCLINE. Available from: https://www.biomerieux-usa.com/product/etest-eravacycline. Retrieved 24 May 2023