ABSTRACT
Helicobacter pylori (H. pylori) causes a cascade from gastritis to gastric neoplasia. During the chronic stages, the oncogenic transcription factor forkhead box protein M1 (FOXM1) becomes increasingly expressed. Given certain strains of the gastric commensal Cutibacterium acnes (C. acnes) produce thiopeptides that directly inhibit FOXM1, we hypothesized that coinfection with thiopeptide-positive C. acnes would decrease Foxm1 expression and alter H. pylori-induced pathogenesis. C. acnes was isolated from 31% of gastric biopsies from Nicaraguan patients, and thiopeptide-positive strains were identified by whole genome sequencing. Germ-free INS-GAS mice were inoculated with thiopeptide-positive C. acnes, H. pylori SS1, H. pylori 1 week before C. acnes, C. acnes 2 weeks before H. pylori, or no bacteria. At 17 weeks post-infection, males dosed with C. acnes followed by H. pylori exhibited increased inflammation scores by histopathology; however, males dosed with H. pylori followed by C. acnes exhibited reduced gastric Foxm1, pro-inflammatory cytokine (Il-1β, Ifn-γ, Tnf-α, Il-17a, and iNOS), regulatory cytokine (Foxp3) gene expression, pro-inflammatory IgG2a antibodies, and gastric lymph node RORγT expression compared to H. pylori-monoinfected mice. Male mice coinfected with C. acnes followed by H. pylori exhibited reduced gastric inflammatory markers (IL-17, IL-10, GM-CSF, M-CSF, MCP-1, MIP-1α, MIP-2, RANTES, and VEGF) by cytokine array analysis and reduced H. pylori gastric colonization. These data show that thiopeptide-positive C. acnes exhibited anti-inflammatory and anti-FOXM1 properties in the context of H. pylori gastritis.
IMPORTANCE
H. pylori infects half of the world population and is the leading cause of gastric cancer. We previously demonstrated that gastric cancer risk is associated with gastric microbiota. Specifically, gastric urease-positive Staphylococcus epidermidis and Streptococcus salivarius had contrasting effects on H. pylori-associated gastric pathology and immune responses in germ-free INS-GAS mice. As gastritis progresses to gastric cancer, the oncogenic transcription factor Foxm1 becomes increasingly expressed. In this study, we evaluated the gastric commensal C. acnes, certain strains of which produce thiopeptides that directly inhibit FOXM1. Thiopeptide-positive C. acnes was isolated from Nicaraguan patient gastric biopsies and inoculated into germ-free INS-GAS mice with H. pylori. We, therefore, asked whether coinfection with C. acnes expressing thiopeptide and H. pylori would decrease gastric Foxm1 expression and pro-inflammatory cytokine mRNA and protein levels. Our study supports the growing literature that specific non-H. pylori gastric bacteria affect inflammatory and cancer biomarkers in H. pylori pathogenesis.
KEYWORDS: Helicobacter pylori, non-H. pylori gastric bacteria, Cutibacterium acnes, thiopeptide, gnotobiotic mouse model, mouse model of H. pylori-induced gastric cancer, Mus musculus
INTRODUCTION
Helicobacter pylori (H. pylori) is the leading cause of gastritis and gastric neoplasia in humans. H. pylori has an infection rate of 50% worldwide according to the CDC and up to 80%–90% in developing countries (1 – 3). Following colonization of the human stomach by H. pylori, the Correa cascade describes a progressive cascade from normal gastric tissue to superficial gastritis, intestinal metaplasia, and eventually gastric cancer (4). During this H. pylori-associated cascade in humans, H. pylori produces microRNAs that progressively increase oncogenic transcription factor forkhead box protein M1 (FOXM1) expression (5 – 7). FOXM1 then facilitates gastric cancer cell migration and invasion (8 – 10). Elevation in FOXM1 is a poor prognostic factor and mediates resistance to docetaxel, a chemotherapeutic (11, 12). FOXM1 has, therefore, been identified as a potential therapeutic target for gastric cancer. In C57BL/6J mice infected with H. pylori SS1, FOXM1 was elevated at a single time point of 8 months post-infection (5).
Previous studies in our laboratory have explored the role of non-H. pylori gastric bacteria in H. pylori pathogenesis and gastric cancer risk (13, 14). Staphylococcus epidermidis and Streptococcus salivarius isolated from patient gastric tissues had contrasting effects on H. pylori-induced pathogenesis in germ-free (GF) INS-GAS mice (15). At 5 months post-infection, mice coinfected with Streptococcus salivarius had higher histopathologic scores, and mice coinfected with Staphylococcus epidermidis had lower pro-inflammatory cytokine gene expression, compared to H. pylori monoinfection.
Cutibacterium acnes (C. acnes) is a commensal bacterium that colonizes multiple tissues, including skin, oral cavity, respiratory tract, and gastrointestinal tract (16 – 18). A novel thiopeptide from C. acnes was recently discovered. Thiopeptides are microbially produced peptides with antibacterial, immunomodulatory, and anticancer properties (19, 20). Prototype thiopeptides include thiostrepton from Streptomyces species, siomycin A from Streptomyces sioyaensis, and berninamycin from Streptomyces bernensis. The novel thiopeptide, named cutimycin, has a nearly identical structure to berninamycin A (18). Thiopeptides inhibit bacterial protein synthesis, with activity against Gram-positive and Gram-negative bacteria (19, 20). The anticancer action of thiopeptides is attributed to direct inhibition of FOXM1 and blockade of the proteasome (19, 20). Due to its instability in acidic environments, thiostrepton is not bioavailable when administered orally (21) but is FDA approved in a topical antibiotic formulation for veterinary use (Animax Ointment, Dechra Pharmaceuticals, Northwich, United Kingdom).
Thiopeptide-positive strains of C. acnes inhibited Staphylococcus aureus growth, supporting an antimicrobial role for C. acnes thiopeptide similar to previously evaluated thiopeptides (18). Thiopeptide-positive C. acnes also downregulated expression of the oncogenic transcription factor FOXM1 and inhibited cell proliferation in human primary prostate epithelial cells (22). Thus, thiopeptides and their bacterial producers, including C. acnes, have pharmacologic potential against infection and cancer, which we sought to explore in the context of H. pylori-induced gastric cancer. This study aims to characterize the effect of C. acnes, specifically a thiopeptide-positive strain, on H. pylori pathogenesis and continues our previous work elucidating the effect of non-H. pylori gastric bacteria on H. pylori pathogenesis. Given the potential ability for C. acnes thiopeptide to directly inhibit the oncogenic transcription factor FOXM1, which becomes progressively increased in human H. pylori infection, we hypothesized that coinfection of H. pylori and thiopeptide-positive C. acnes would alter inflammatory biomarkers and decrease FOXM1 expression in GF INS-GAS mice.
RESULTS
Gastric Foxm1 progressively increases in H. pylori-infected mice, paralleling H. pylori infection in humans
To demonstrate that Foxm1 is elevated in H. pylori-infected mice in parallel to what occurs in humans, Foxm1 gene expression was quantified in gastric tissue samples from our previous studies (14 – 16). Gastric Foxm1 was not elevated in INS-GAS male mice at 8 weeks or 5 months post-infection with H. pylori SS1 (Fig. 1A and B) but was elevated by 6 months post-infection (Fig. 1C). Gastric Foxm1 was also elevated at 6 months post-infection with H. pylori PMSS1, as well as in a different mouse strain and sex, C57BL/6 females, infected with H. pylori SS1 at 7–9 months post-infection (Fig. 1D and E).
Fig 1.
Retrospective Foxm1 expression. mRNA quantified by quantitative PCR using gastric tissue samples from previous studies. Gastric Foxm1 in INS-GAS male mice at (A) 8 weeks, (B) 5 months, and (C) 6 months post-infection with H. pylori SS1. (D) Gastric Foxm1 in INS-GAS male mice at 6 months post-infection with H. pylori PMSS1. (E) Gastric Foxm1 in C57BL/6 female mice at 7–9 months PI with H. pylori SS1. Gastric Foxm1 was progressively increased in male INS-GAS mice infected with H. pylori SS1 and was similarly increased with H. pylori PMSS1 infection and in females C57BL/6 mice. Hp, H. pylori. *P < 0.05, **P < 0.01.
Bacterial culture of gastric biopsy samples from Nicaraguan patients
Bacterial isolation was performed on 36 human gastric samples from Nicaragua. Bacteria cultured from these samples represented 22 genera, with the top five most frequently isolated genera being Streptococcus (n = 25), Rothia (n = 19), Actinomyces (n = 15), Cutibacterium (n = 11), and Veillonella (n = 7) (Table S1).
C. acnes was cultured from 11 gastric biopsies out of 36 (31%), which were from seven female patients and four male patients (Table 1). Most of these biopsies were positive for H. pylori by histopathology (n = 10/11) (Table 1), while only a subset was positive for H. pylori by culture (n = 3/11) (Table S2). Most samples had a histopathologic diagnosis of non-atrophic gastritis (NAG) (n = 7/11); the remaining showed gastric intestinal metaplasia (GIM) (n = 4/11) (Table 1).
TABLE 1.
C. acnes isolated from Nicaraguan gastric samples a
| C. acnes strain ID | Sex | H. pylori status | Histopathology | Thiopeptide PCR | WGS performed? |
|---|---|---|---|---|---|
| 18-1849-A2 | F | + | GIM | − | No |
| 18-1851-A4 | F | + | NAG | + | Yes |
| 18-1857-A1 | F | − | NAG | − | Yes |
| 18-1859-A1 | F | + | GIM | − | No |
| 18-1863-A1 | F | + | NAG | − | No |
| 18-1864-C6 | M | + | GIM | − | No |
| 18-1869-C3b | F | + | NAG | + | Yes |
| 18-1871-A4 | M | + | NAG | − | No |
| 18-1873-C1 | M | + | NAG | − | No |
| 18-1879-A3 | F | + | NAG | + | Yes |
| 18-1881-C2 | M | + | GIM | − | No |
Eleven strains of C. acnes were isolated from human antral biopsy samples. Shown for each gastric biopsy are the MIT accession number, clone ID, sex of the patient, H. pylori status, histopathologic diagnosis, results of thiopeptide PCR, and whether whole genome sequencing was performed. NAG, non-atrophic gastritis; GIM, gastric intestinal metaplasia.
Whole genome sequencing reveals thiopeptide biosynthetic gene cluster in C. acnes isolated from human stomachs
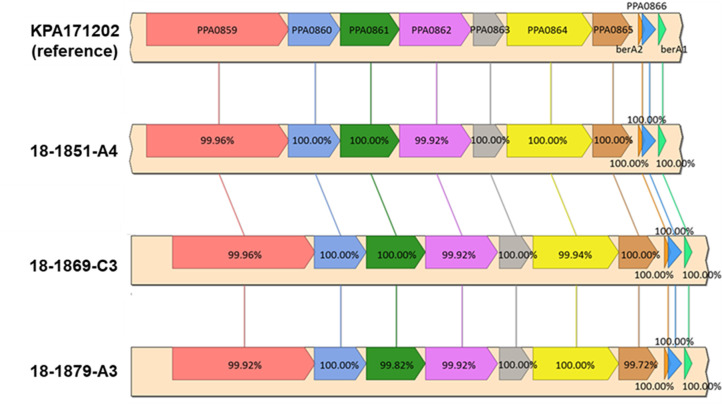
Whole genome sequencing of four C. acnes MIT isolates revealed that three were thiopeptide positive. All thiopeptide-positive strains were from gastric biopsies diagnosed with NAG. BLAST analysis and syntenic alignment confirmed that these C. acnes strains, named MIT 18-1851-A4, MIT 18-1869-C3, and MIT 18-1879-A3, had complete thiopeptide biosynthesis gene clusters with >99% identity compared to the reference strain KPA171202 (Fig. 2). None of the genes in the thiopeptide biosynthesis cluster were found in the genome of the fourth C. acnes strain, named MIT 18-1857-A1. Thiopeptide-encoding C. acnes MIT 18-1851-A4, MIT 18-1869-C3, and MIT 18-1879-A3 had larger genomes and more protein gene annotations compared to MIT 18-1857-A1 (thiopeptide negative) (Table 2).
Fig 2.
Sequencing of thiopeptide biosynthetic gene cluster. C. acnes MIT strains 18-1879-A3, 18-1869-C3, and 18-1851-A4 strains isolated from human gastric tissue have complete thiopeptide biosynthetic gene clusters by whole genome sequencing with greater than 99% identity compared to the reference C. acnes strain.
TABLE 2.
Whole genome sequencing of C. acnes strains a
| C. acnes strain ID | Contigs | Size | %GC content | Protein coding sequences (CDS) | RNA genes | GenBank |
|---|---|---|---|---|---|---|
| 18-1857-A1 | 10 | 2,483,440 | 60.1 | 2,447 | 48 | WOWG00000000 |
| 18-1851-A4 | 17 | 2,547,745 | 60.0 | 2,502 | 48 | WOWH00000000 |
| 18-1869-C3 | 14 | 2,547,366 | 60.0 | 2,492 | 48 | WOWI00000000 |
| 18-1879-A3 | 12 | 2,545,723 | 60.0 | 2,483 | 47 | WOWJ00000000 |
| C. acnes KPA171202 | 1 | 2,560,265 | 60.0 | 2,422 | 48 | AE017283 |
Genome summary statistics for four C. acnes MIT isolates and reference strain KPA171202.
Thiopeptide-positive C. acnes inhibited the growth of H. pylori in vitro
Given the known antimicrobial effects of thiopeptides, two of these thiopeptide-positive strains of C. acnes were tested for antibacterial activity against H. pylori. When cocultured with thiopeptide-positive C. acnes MIT 18-1879-A3 or MIT 18-1851-A4, H. pylori growth was reduced at a multiplicity of infection (MOI) of 1:5 for C. acnes MIT 18-1879-A3 and MOI of 1:5 or 1:1 for C. acnes MIT 18-1851-A4 (Fig. 3). Bacterial supernatant at concentrations of 5% or 10% from thiopeptide-positive C. acnes MIT 18-1879-A3 culture did not inhibit the growth of H. pylori (Fig. 3A).
Fig 3.
Thiopeptide-positive C. acnes inhibition assay. H. pylori PMSS1 (0.2 OD 600 ) was grown in monoculture or with thiopeptide-positive C. acnes MIT 18-1879-A3 (A) and MIT 18-1851-A4 (B) at MOI 1:5, 1:1, or 10:1. H. pylori PMSS1 was also incubated with 5% or 10% supernatant from C. acnes monoculture. CFU/mL were quantified following 72-hour incubation. Both thiopeptide-positive strains of C. acnes inhibited the growth of H. pylori in vitro. Hp, H. pylori PMSS1; Ca, C. acnes; sup, supernatant. *P < 0.05, ***P < 0.001.
Thiopeptides inhibit gastric cancer cell line growth and reduce Foxm1 expression
The direct effect of thiopeptides on cancer cell growth and Foxm1 expression was evaluated using thiostrepton, the thiopeptide produced by Streptomyces spp., as C. acnes thiopeptide is not commercially available. Thiostrepton inhibited MKN45 and AGS gastric cancer cell line proliferation in vitro in a dose-dependent manner (Fig. 4). AGS cells, both with and without concurrent H. pylori infection, exhibited decreased Foxm1 gene expression at 24 hours after exposure to thiostrepton 0.5 or 1 µM (Fig. 4).
Fig 4.
Effect of thiostrepton on gastric cancer cell growth and Foxm1 expression. Percent epithelial cell confluence of (A) MKN45 and (B) AGS gastric cancer cell lines after 72 hours following incubation with vehicle control or escalating concentrations of thiostrepton. Percent cell confluence was measured using the MTT assay. AGS gastric cancer cells incubated with thiostrepton at 0, 0.5, and 1 µM and incubated (C) with H. pylori strain SS1 or (D) uninfected at an MOI of 1:100 or vehicle control (DMSO). Following incubation at 37°C for 24 hours, fold changes of Foxm1 compared to the housekeeping gene Gapdh were determined by qPCR. Thiostrepton inhibited the proliferation of gastric cancer cell lines in a dose-dependent manner and decreased Foxm1 expression in H. pylori-infected and uninfected AGS cells. *P < 0.05, **P < 0.01.
Male mice coinfected with C. acnes prior to H. pylori exhibited decreased H. pylori gastric colonization
To evaluate the effect of thiopeptide-positive C. acnes on H. pylori-induced gastritis in vivo, GF INS-GAS mice were inoculated with thiopeptide-positive C. acnes MIT 18-1879-A3, H. pylori SS1, H. pylori 1 week before C. acnes (Hp + Ca), C. acnes 2 weeks before H. pylori (Ca + Hp), or no bacteria. Necropsies were performed at 17 weeks post-infection, and gastric colonization of H. pylori and C. acnes was quantified. C. acnes was isolated from the oral cavity, stomach, and feces of all tested C. acnes-colonized mice. C. acnes colonization did not differ between groups dosed with C. acnes alone or C. acnes with H. pylori (Fig. 5A). Male mice infected with C. acnes prior to H. pylori had decreased gastric H. pylori colonization compared to H. pylori alone (Fig. 5B and C). H. pylori colonization did not differ between male mice infected with H. pylori alone and coinfected with H. pylori followed by C. acnes or between H. pylori-monoinfected and coinfected females (Fig. 5B through D).
Fig 5.
C. acnes and H. pylori colonization in germ-free mice. (A) C. acnes CFU/mL in C. acnes or coinfected mice 17 weeks post-infection. H. pylori copy number per microgram of DNA in gastric tissue by qPCR from (B) male mice, (C) mice of both sexes, and (D) female mice infected with H. pylori alone or coinfected with C. acnes. Hp, H. pylori SS1 strain; Ca, C. acnes; Hp + Ca, mice infected with H. pylori followed by C. acnes; Ca + Hp, mice dosed with C. acnes prior to H. pylori. **P < 0.01, ***P < 0.001.
Coinfected male mice exhibited increased histopathologic inflammatory scores
At 17 weeks post-infection with H. pylori, gastric tissue from all groups was examined histologically. Histopathologic indices were increased in H. pylori-infected animals compared to uninfected and C. acnes controls (Fig. 6A through H). Gastric histologic activity indices (GHAI) did not differ between animals of both sexes in coinfection groups compared to H. pylori alone (Fig. 6A); however, male mice colonized with C. acnes followed by H. pylori had increased GHAI due to increased inflammation scores (Fig. 6E and F). Total histopathology, inflammation, and foveolar or glandular hyperplasia scores did not differ between coinfected and monoinfected females (Fig. S1A through C). Dysplasia and neoplasia scores were increased in females dosed with C. acnes prior to H. pylori compared to H. pylori alone (Fig. S1D).
Fig 6.
Gastric histopathology. Combined gastric histologic activity index (GHAI) as well as inflammation, foveolar and glandular hyperplasia, and dysplasia and neoplasia scores in both sexes (A–D) and males (E–H) 17 weeks post-infection. Gastric inflammation scores were increased in male mice colonized with C. acnes prior to H. pylori. Hp, H. pylori SS1 strain; Ca, C. acnes; Hp + Ca, mice infected with H. pylori followed by C. acnes; Ca + Hp, mice dosed with C. acnes prior to H. pylori. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Decreased inflammatory biomarkers and Foxm1 in the stomach of coinfected mice
The changes in gastric inflammatory biomarker and oncogene Foxm1 expression in response to H. pylori infection were measured by qPCR. Pro-inflammatory cytokine expression was increased in gastric tissue samples from H. pylori-infected animals compared to uninfected and C. acnes controls, especially for male mice (Fig. 7A through K; Fig. S2A through J). C. acnes-colonized male mice exhibited decreased Th1-associated cytokines (Ifn-γ and Tnf-α), Th17-associated cytokine (Il-17a), Treg-associated Foxp3, and Foxm1 compared to uninfected mice (Fig. 7F, G, H, I, and M). Foxm1 was increased in H. pylori-infected males compared to uninfected (Fig. 7L). Males infected with H. pylori and then C. acnes exhibited reduced pro-inflammatory gastric cytokines (Il-1β, Ifn-γ, Tnf-α, Il-17a, and iNOS), regulatory cytokines (Foxp3), and Foxm1 (Fig. 7D, E, F, G, H, I, K, L, and M) compared to monoinfection. Females infected with H. pylori and then C. acnes exhibited decreased Il-1β expression (Fig. S2B). Males infected with C. acnes followed by H. pylori had decreased Il-17a and Foxp3 compared to H. pylori alone (Fig. 7H and M).
Fig 7.
Gastric cytokine and Foxm1 gene expression. mRNA levels of Il-1β, Ifn-γ, Tnf-α, Il-17a, Foxm1, Foxp3, and iNOS in male and female mice (A–D and I–K) and male mice (E–H and L–N) 17 weeks post-infection. Gastric pro-inflammatory cytokine and Foxm1 expression was decreased in male mice infected with H. pylori followed by C. acnes compared to H. pylori monoinfection. Hp, H. pylori SS1 strain; Ca, C. acnes; Hp + Ca, mice infected with H. pylori followed by C. acnes; Ca + Hp, mice dosed with C. acnes prior to H. pylori. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Given the decrease in mRNA expression of gastric inflammatory markers observed in coinfected male mice, protein levels of gastric cytokines were also quantified in male mice by cytokine array. IFN-γ, eotaxin, G-CSF, IP-10, and MIG were increased in H. pylori-infected mice compared to uninfected and C. acnes-colonized controls (Fig. S3A through F). Male mice colonized with C. acnes followed by H. pylori showed decreased macrophage pro-inflammatory factors M-CSF, GM-CSF, MCP-1, MIP-1α, and MIP-2 (Fig. 8A through E), pro-inflammatory marker IL-17a (Fig. 8J), T-reg-associated IL-10 (Fig. 8K), and platelet-associated cytokines RANTES and VEGF (Fig. 8F and G) compared to H. pylori monoinfection. Gastric IL-6 was increased in male mice infected with H. pylori followed by C. acnes (Fig. 8L). Gastric levels of IL-1β, IL-2, IL-3, IL-4, IL-5, IL-7, IL-9, IL-12p40, IL-12p70, IL-13, IL-15, LIX, MIP-1β, and TNFα did not differ between groups of male mice (data not shown).
Fig 8.
Gastric inflammatory protein levels. (A) M-CSF, (B) GM-CSF, (C) MCP-1, (D) MIP-1α, (E) MIP-2, (F) RANTES, (G) VEGF-A, (H) LIF, (I) KC, (J) IL-17A, (K) IL-10, and (L) IL-6 in male germ-free INS-GAS mice 17 weeks post-infection. Pro-inflammatory gastric protein levels were decreased in male mice colonized with C. acnes prior to H. pylori. Hp, H. pylori SS1 strain; Ca, C. acnes; Hp + Ca, mice infected with H. pylori followed by C. acnes; Ca + Hp, mice dosed with C. acnes prior to H. pylori. *P < 0.05; **P < 0.01, ***P < 0.001, ****P < 0.0001.
Due to the decreased mRNA expression and protein levels of gastric Th1, Th17, and Treg-associated cytokines in the stomach between coinfected and H. pylori-monoinfected male mice, inflammatory cell subsets were quantified in gastric tissue by immunohistochemistry (IHC). F4/80+ macrophages, CD3+ T cells, FOXP3+ Treg cells, and CD45 B220+ B cells were increased in the gastric mucosa of animals infected with H. pylori compared to uninfected and C. acnes controls (Fig. 9; Fig. S6). Low numbers of MPO+ neutrophils were present in all groups (Fig. S6). There were no differences between groups by qualitative assessment of inflammatory cells. When comparing H. pylori monoinfection and coinfection groups, gastric immune cell numbers did not differ.
Fig 9.
Gastric IHC. IHC staining of (A) F4/80 for macrophages, (B) CD3 for T cells, (C) FOXP3 for Treg cells, and (D) CD45 B220 for B cells in the gastric tissue of germ-free INS-GAS mice that were uninfected, colonized by C. acnes, infected with H. pylori, infected with H. pylori prior to C. acnes, or dosed with C. acnes prior to H. pylori at 17 weeks post-infection. Quantification performed for A–D. Gastric inflammatory cells were increased in H. pylori-infected mice compared to uninfected and C. acnes controls. Hp, H. pylori SS1 strain; Ca, C. acnes; Hp + Ca, mice infected with H. pylori followed by C. acnes; Ca + Hp, mice dosed with C. acnes prior to H. pylori. *P < 0.05, **P < 0.01.
Coinfected males had exhibited less inflammatory serum antibody and draining lymph node responses to H. pylori infection
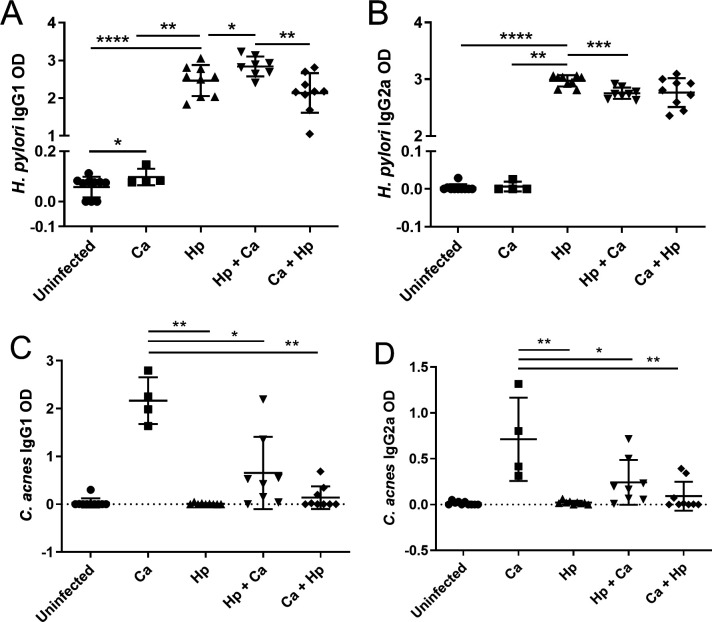
Based on the anti-inflammatory effect of coinfection on gastric cytokine responses in male mice, the serum antibody and draining lymph node T cell responses to H. pylori infection were also evaluated. In males infected with H. pylori prior to C. acnes, anti-inflammatory IgG1 H. pylori antibodies were increased, and pro-inflammatory IgG2a H. pylori antibodies were decreased compared to H. pylori alone (Fig. 10A and B). Coinfected females had decreased anti-inflammatory IgG1 H. pylori antibodies compared to H. pylori alone, but no difference in pro-inflammatory IgG2a antibodies between H. pylori-infected groups (Fig. S4A and B). Anti-C. acnes IgG1 and IgG2a antibodies were decreased in coinfected males compared to C. acnes alone (Fig. 10C and D). Coinfected females showed no differences in anti-C. acnes IgG1 and IgG2a antibodies compared to C. acnes alone (Fig. S4C and D).
Fig 10.
Serum antibody response to H. pylori and C. acnes. Anti-H. pylori and C. acnes serum anti-inflammatory IgG1 (A and C) and pro-inflammatory IgG2a (B and D) antibodies measured by ELISA in male germ-free INS-GAS mice that were uninfected, colonized by C. acnes, infected with H. pylori, infected with H. pylori prior to C. acnes, or dosed with C. acnes prior to H. pylori 17 weeks post-infection. Anti-inflammatory IgG1 antibodies were increased and pro-inflammatory IgG2a antibodies were decreased in male mice infected with H. pylori prior to C. acnes compared to H. pylori Monoinfection. Hp, H. pylori SS1 strain; Ca, C. acnes; Hp + Ca, mice infected with H. pylori followed by C. acnes; Ca + Hp, mice dosed with C. acnes prior to H. pylori. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
In the draining gastric lymph nodes (GLN), there were fewer RORγT+ Th17 cells for H. pylori and C. acnes-coinfected males compared to H. pylori alone (Fig. 11A). In H. pylori-infected animals, RORγT+ Th17 cells and RORγT+ FOXP3+ double-positive Treg cells were increased in GLNs compared to mesenteric lymph nodes (MLNs) (Fig. 11A and B). C. acnes- and/or H. pylori-colonized mice had an increased percentage of T-Bet+ Th1 cells and decreased percentage of FOXP3+ Treg cells compared to uninfected controls (Fig. S5). Percentage of T-Bet+ Th1 cells and FOXP3+ Treg cells in GLNs and MLNs did not differ between coinfected and monoinfected mice (Fig. S5).
Fig 11.
Flow cytometry of mesenteric and gastric lymph nodes. (A) Percent RORγT expression in CD4+ T cells in gastric and mesenteric lymph nodes. (B) Percent FOXP3 RORγT double-positive CD4+ T cells in gastric and mesenteric lymph nodes. Percent RORγT expression was decreased in gastric lymph nodes of mice coinfected with H. pylori followed by C. acnes compared to H. pylori monoinfection at 17 weeks post-infection. Percent RORγT and FOXP3 RORγT double-positive cells were increased in gastric lymph nodes from mesenteric for H. pylori-infected mice. Hp, H. pylori SS1 strain; Ca, C. acnes; Hp + Ca, mice infected with H. pylori followed by C. acnes; Ca + Hp, mice dosed with C. acnes prior to H. pylori. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
DISCUSSION
FOXM1 is a pro-oncogenic transcription factor overexpressed in many cancers, including H. pylori-induced gastric cancer (5 – 7). To ascertain whether gene expression of the oncogenic transcription factor Foxm1 is elevated in H. pylori-infected mice, we measured Foxm1 in mice previously studied by our laboratory (23 – 25). H. pylori infection of both sexes (INS-GAS males and C57BL/6 females) increased Foxm1 gene expression compared to uninfected controls. This was true for H. pylori PMSS1 and SS1, the latter of which is deficient in the bacterial type IV secretion system needed to deliver the oncogenic protein CagA to host epithelial cells, which suggests the mechanism of Foxm1 elevation does not depend on this phenomenon. H. pylori SS1 was chosen for the in vivo studies as PMSS1 results in varied colonization levels and duration and the loss of Cag expression during infection (26). Thus, mouse models accurately recapitulated the progressive increase in gastric FOXM1 seen in H. pylori-infected humans.
We then evaluated the prototype thiopeptide, thiostrepton, for anticancer properties, finding that thiostrepton decreased FOXM1 expression in AGS cells and inhibited the growth of MKN-45 and AGS gastric cancer epithelial cell lines. The decrease in FOXM1 expression may have been a direct effect of thiostrepton or caused by cellular growth inhibition. Cytotoxicity was less likely the cause of decreased gastric cancer cell growth because thiostrepton at the same concentrations decreased breast cancer cell growth without affecting normal breast epithelial cells (27). In addition, the FDA-approved Animax Ointment contains 2,500 units/mL of thiostrepton per the Safety Data Sheet, and thiostrepton has an activity of 900 units/mg per the USP reference standard with a molecular weight of 1,664.9 g/mol; thus, the thiostrepton concentration is 1.7 × 103 µM. This is approximately 1,000× the concentration used in our study and suggests that tumor suppression was not the result of thiostrepton toxicity.
In Nicaraguan gastric biopsies, 31% (11 of 36) were positive for C. acnes; by contrast, 5% (2 of 37) of gastric biopsies from our previously reported Colombian population were positive for C. acnes (15). Other bacterial isolates from Nicaraguan samples included Streptococcus spp., Actinomyces spp., and Prevotella spp., which are often associated with oral and respiratory microbiota. Whole genome sequencing found three of four C. acnes strains to be thiopeptide positive. PCR for the thiopeptide biosynthetic gene cluster aligned with whole genome sequencing results (Table 1). Interestingly, the three thiopeptide-positive biopsies had a diagnosis of non-atrophic gastritis, while five of nine thiopeptide-negative biopsies demonstrated the more severe histopathologic diagnosis of gastric intestinal metaplasia. This may suggest that thiopeptide-positive C. acnes is associated with less severe gastric pathology than thiopeptide-negative C. acnes, but further investigation is warranted.
The effect of thiopeptide-positive strains of C. acnes on the growth of H. pylori was tested in vitro using two positive strains isolated by our laboratory; both inhibited the growth of H. pylori in vitro in a dose-dependent manner, potentially due to direct effects or thiopeptide secretion. Thiopeptide-positive C. acnes 18-1879-A3 was inoculated into GF INS-GAS mice along with H. pylori SS1. Importantly, gastric Foxm1 expression was decreased in mice coinfected with H. pylori prior to C. acnes compared to H. pylori monoinfection. In GF INS-GAS mice infected with H. pylori, coinfection with C. acnes inhibited Foxm1 elevation, which is a key step in the progression of gastritis to gastric cancer. Future studies may compare Foxm1 expression and gastric cancer rates in H. pylori-colonized patients with and without concurrent thiopeptide-positive C. acnes colonization.
Consistent with previous studies, H. pylori infection of GF INS-GAS mice increased GHAI, which included inflammation, epithelial defects, foveolar and glandular hyperplasia, and dysplasia and neoplasia scores (15). An increase in the epithelial defect scores was seen in females dosed with C. acnes, but overall histopathology scores were not affected. Total histopathologic scores did not differ in male and female coinfected mice compared to H. pylori monoinfection. However, male mice colonized by C. acnes followed by H. pylori exhibited increased inflammation scores compared to H. pylori monoinfection, which translated to an increase in total histopathologic scores. This may be due to GF mice having more prominent immune responses to commensal bacteria than mice whose immune systems have previously been primed by the presence of microbiota. In fact, higher gastric histopathology scores were noted in H. pylori-infected GF INS-GAS mice colonized with Altered Schaedler’s Flora (Clostridium species ASF356, Bacteroides species ASF519, and Lactobacillus murinus ASF361) compared to H. pylori monocolonization (28). Decreased anti-C. acnes serum antibodies in coinfected mice compared to C. acnes alone also support there being immunomodulatory effects in coinfection. In addition, dosing mice with C. acnes prior to H. pylori decreased H. pylori colonization of the stomach in males. It is possible that increased inflammation in mice dosed with C. acnes prior to H. pylori led to selective clearing of H. pylori. However, macrophage, T and B lymphocyte, and neutrophil numbers did not differ between H. pylori monoinfection and coinfection by quantitative analysis of digital IHC images.
Gastric gene expression was quantified for Th1 (Il-1β, Ifn-γ, Tnf-α, and iNOS), Th17 (Il-17a and Il-22), and Treg (Foxp3 and Il-10) cytokines, which are increased in H. pylori infection (29 – 32). C. acnes alone had an anti-inflammatory effect in the stomach of GF INS-GAS mice with decreased mRNA expression of Ifn-γ, Tnf-α, Il-17a, and Foxp3 compared to uninfected mice. Consistent with previous studies, Il-1β, Ifn-γ, Tnf-α, Il-17a, and Foxp3 mRNA levels were increased in H. pylori-infected animals compared to uninfected controls (15). Coinfected male mice infected with H. pylori prior to C. acnes infection had decreased gastric mRNA expression of Il-1β, Ifn-γ, Tnf-α, Il-17a, iNOS, and Foxp3 compared to H. pylori monoinfection. Coinfected males dosed with C. acnes followed by H. pylori exhibited a decrease in both mRNA and protein expression of IL-17a, as well as mRNA expression of Foxp3, compared to H. pylori monoinfection. Female mice infected with H. pylori followed by C. acnes exhibited decreased gastric mRNA expression of Il-1β, but no other cytokines, compared to H. pylori-monoinfected female mice. As previously demonstrated in INS-GAS male mice and similar to human males, INS-GAS male mice showed more robust effects from H. pylori infection than females (33, 34).
Gastric inflammatory protein levels were quantified in male mice, including macrophage-associated proteins given histiocytic activation occurs in H. pylori infection (32, 35, 36). Gastric protein levels of GM-CSF and M-CSF, which regulate macrophage differentiation, MCP-1, a key monocyte chemoattractant, and MIP-1α and MIP-2, inflammatory proteins produced by macrophages, were decreased in male mice coinfected with C. acnes followed by H. pylori compared to H. pylori monoinfection. RANTES, a chemokine driving infiltration of inflammatory immune cells, and VEGF, which increases vascularity during inflammation, were also decreased in this coinfection group. Interestingly, gastric IL-6 was increased in mice infected with H. pylori followed by C. acnes compared to H. pylori monoinfection. While IL-6 is commonly regarded as a pro-inflammatory cytokine that is increased in H. pylori gastritis and gastric cancer (29, 37), the cytokine also has anti-inflammatory effects, including promoting alternative activation of macrophages to an anti-inflammatory phenotype (38). Overall, male coinfected mice had decreased gastric pro-inflammatory cytokine and protein expression. Interestingly, mice infected with H. pylori followed by C. acnes showed more effects on mRNA expression, while coinfection with C. acnes followed by H. pylori showed more effects on protein levels. The order in which bacteria are introduced appears to have an effect on H. pylori inflammatory biomarkers, potentially due to differences in the inflammatory state of the stomach when H. pylori is introduced.
The anti-inflammatory effect of C. acnes coinfection was also seen in the anti-H. pylori serum antibody response and Th17 cell differentiation in gastric lymph nodes. Coinfected male mice dosed with H. pylori prior to C. acnes infection had an increase in anti-inflammatory, Th2-associated IgG1 and a decrease in pro-inflammatory, Th1-associated IgG2a compared to H. pylori monoinfection. Mice in this coinfection group also had a decrease in RORγT expression in CD4+ T cells in gastric lymph nodes, suggesting that the anti-inflammatory effect of C. acnes extended to draining gastric lymph nodes.
Immune cell populations were also compared between gastric and mesenteric lymph nodes. The gastric lymph node, as the major draining lymph node of the stomach, showed increases in pro-inflammatory Th1 (T-BET+) and Th17 (RORγT+) cells, as well as decreases in Treg (FOXP3+) cells, with H. pylori infection. This is consistent with human gastric lymph nodes showing increased RORγT in CD4+ T cells in the early stages of gastric cancer (31). This study found elevated FOXP3 expression, while our study found decreased FOXP3 expression, which may reflect the inflammatory and regulatory balance of the immune system at the time of tissue collection. In addition, a recently described CD4+ T cell, which is double positive for Th17-associated transcription factor RORγT and Treg-associated transcription factor FoxP3, has been found in the gastrointestinal tract and exhibits enhanced regulatory function (39, 40). However, to the authors’ knowledge, this cell type has not been characterized in the context of H. pylori infection. We show that in H. pylori infection, RORγT FOXP3 double-positive CD4+ T cells were increased in the gastric lymph node compared to mesenteric lymph node in GF mice. These data support the evaluation of gastric lymph node in H. pylori infection, as there may be a more robust immune response than in mesenteric lymph nodes.
Further studies are needed to evaluate the effect of C. acnes on H. pylori in the timeframe of gastric cancer development, which occurs around 7 months post-infection in GF INS-GAS males (41). In Korean, Japanese, and Chinese populations, C. acnes was abundant in gastric cancer tissues; however, the thiopeptide status was not reported in these studies, and it is unclear whether C. acnes acts as a bystander or an active contributor in gastric cancer development (42 – 44).
In conclusion, this study demonstrated that thiopeptide-positive C. acnes has anti-inflammatory, antimicrobial, and anti-FOXM1 effects in vitro and in a GF mouse model of H. pylori gastritis. Comparison with a thiopeptide-negative strain of C. acnes will be needed to determine whether the overall anti-inflammatory effects of C. acnes may be due to thiopeptide inhibition, other bacterial secreted factors, or direct effects of the bacteria. Given we recently reported that Staphylococcus epidermidis and Streptococcus salivarius in GF INS-GAS mice have contrasting effects on H. pylori gastritis, this study adds to the body of literature supporting a role for non-H. pylori gastric bacteria in immunomodulating the progression of H. pylori-induced gastritis and gastric cancer.
MATERIALS AND METHODS
Gastric Foxm1 expression in H. pylori-infected mice
Gastric tissues were obtained from previous studies (23 – 25), including male INS-GAS mice 8 weeks (n = 5 uninfected, 5 infected), 5 months (n = 5 uninfected, 5 infected), and 6 months (n = 8 uninfected, 8 infected) post-infection with H. pylori SS1, male INS-GAS mice (n = 8 uninfected, 9 infected) 6 months post-infection with H. pylori PMSS1, and female C57BL/6 mice (n = 7 uninfected, 7 infected) 6 months post-infection with H. pylori PMSS1. RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA) and converted to cDNA using a high-capacity cDNA Archive kit following the manufacturer protocol (Thermo Fisher Scientific, Waltham, MA). cDNA levels for Foxm1 mRNA were measured by quantitative PCR using commercial primers and probes and compared to the housekeeping gene Gapdh.
Bacterial isolations and patient population
Thirty-six human antral biopsy specimens were collected from Nicaraguan patients. Participation was voluntary, and informed consent was obtained. The ethics committees of participating Nicaraguan hospitals and the Institutional Review Board of Vanderbilt University approved all study protocols. Subjects included 14 males and 22 females between 22 and 69 years old. Histopathologic diagnosis was non-atrophic gastritis in 20 biopsies, multifocal atrophic gastritis in two biopsies, and gastric intestinal metaplasia in 14 biopsies. Specimens were frozen at −80°C in thioglycolate, then thawed in an anaerobic atmosphere, and homogenized. For aerobic culture, homogenates were plated onto chocolate agar, blood agar, MacConkey agar, and Brucella broth medium containing 10% fetal calf serum. The plates were incubated at 37°C in 5% CO2 for 24–48 hours. For anaerobic culture, homogenates were plated onto pre-reduced Brucella blood agar plates and inoculated into thioglycolate broth. The cultures were incubated at 37°C in an anaerobic chamber (Coy Lab Products, Jackson County, Michigan) with mixed gas (10% CO2, 10% H2, 80% N2) for 48 hours. For microaerobic culture to detect the growth of H. pylori, homogenates were plated onto H. pylori-selective plates (45) and Brucella blood agar plates after passing through a 0.65-mm syringe filter. The plates were placed into a vented jar filled with mixed gas (10% CO2, 10% H2, 80% N2) and incubated at 37°C for up to 3 weeks. Isolated bacterial strains were identified by 16S rRNA sequencing.
Characterization of C. acnes strains by whole genome sequencing
DNA was isolated from C. acnes strains MIT 18-1857-A1, MIT 18-1851-A4, MIT 18-1869-C3, and MIT 18-1879-A3 using the Roche High Pure PCR product purification kit after prior incubation with 50 µL of lysozyme (50 mg/mL) and 10 µL of mutanolysin (2,500 units/mL) for 30 minutes at 37°C. Barcoded libraries were constructed using the QIAseq FX DNA library kit and sequenced with an Illumina MiSeq instrument (2 × 300 bp reads). Raw sequence reads were decontaminated of adapters and quality trimmed using Trim Galore (version: 0.6.1) and Cutadapt (version: 2.2) from the FastqUtils tool hosted by PATRIC (46). SPAdes (version: 3.10.0) was used for de novo contig assembly followed by genome annotation by Rapid Annotations using Subsystems Technology, both hosted by PATRIC (46). Average nucleotide identities (ANI) were calculated with JSpeciesWS (47). ANI values ≥95% were considered the same species. The syntenic alignment of the thiopeptide gene island between genomes was determined with SimpleSynteny (48) using C. acnes reference strain KPA171202.
In vitro assay for the effect of C. acnes on H. pylori growth
H. pylori PMSS1 was grown on 5% sheep blood agar plates under microaerobic conditions of 80% N2:10% H2:10% CO2 for 48 hours. Bacteria were collected into Brucella broth with 10% FBS. C. acnes MIT 18-1879-A3 and MIT 18-1851-A4 were cultured on 5% sheep blood agar plates for 24 hours under the same conditions as H. pylori. H. pylori (0.2 OD/mL) was cocultured with C. acnes at MOI 1:5, 1:1, or 10:1 OD600/mL. C. acnes MIT 18-1879-A3 was also grown in liquid culture of 10% fetal bovine serum in brain heart infusion (BHI) broth overnight. Bacterial supernatant was prepared by centrifuging the liquid culture at 12,000 rpm for 10 minutes, then passing through a 0.22-µm filter. Bacterial supernatant from C. acnes MIT 18-1879-A3 was added to H. pylori culture at concentrations of 5% or 10%. Mixtures were incubated in microaerobic conditions for 24 hours, after which 3 µL of serial dilutions was placed onto blood agar plates containing vancomycin (20 µg/mL), bacitracin (200 µg/mL), and nalidixic acid (20 µg/mL). H. pylori colonies were counted after 4 days of incubation.
The effect of thiostrepton on gastric cancer cell lines
MKN45 and AGS gastric cancer epithelial cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (ATCC, Manassas, VA) containing 10% fetal calf serum (Sigma-Aldrich, St. Louis, MO) and 1% antibiotic-antimycotic (100 units/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin B; Gibco/Thermo Fisher Scientific, Grand Island, NY) at 37°C with 5% CO2. MKN45 cells and AGS cells were exposed to vehicle control or 1.25, 2.5, 5, or 10 µM thiostrepton. The MTT assay for cell proliferation was performed as described previously (49).
The direct effect of thiostrepton on 12 human H. pylori strains was tested at a concentration of 1.0 µM of thiostrepton, and no inhibitory effect was observed (data not shown). Therefore, concentrations of thiostrepton of 1.0 µM or less were utilized to evaluate the effect of thiostrepton on uninfected and H. pylori-infected AGS gastric cancer cells. AGS gastric cancer cells were incubated with thiostrepton at 0, 0.5, or 1 µM and infected with H. pylori SS1 at MOI of 1:100 or vehicle control (DMSO). Mixtures were incubated at 37°C for 24 hours. Quantitative PCR was performed to measure Foxm1 mRNA levels as described above.
H. pylori and thiopeptide-positive C. acnes coinfection in GF INS-GAS mice
The animal protocol was approved by the Massachusetts Institute of Technology Committee on Animal Care. GF INS-GAS mice on an FVB/N background [Tg(Ins1-GAS)1Sbr] were maintained in a facility accredited by AAALAC International. GF mice were housed in sterile isolators on autoclaved hardwood bedding in solid-bottomed polycarbonate cages and fed autoclaved rodent diet (Prolab RMH 3000; PMI Nutrition International, St. Louis, MO). Sterile water was provided ad libitum. Every other week, GF control mice isolators were confirmed negative for microbial contaminants by culture, PCR using eubacterial primers, and Gram-stained fecal smears.
Sixty-nine mice (40 males, 29 females) were included in this study. Seven- to eight-week-old male and female INS-GAS mice were infected by oral gavage with 200 µL (~2 × 108 CFU) of H. pylori SS1 on alternate days for a total of 3 doses (n = 9 males, 4 females) (50). Coinfected mice were orally infected with thiopeptide-positive C. acnes MIT 18-1879-A3 200 µL (~2 × 108 CFU) either 2 weeks prior to (n = 9 males, 8 females) or 1 week after H. pylori infection (n = 8 males, 6 females). Control mice were either colonized by thiopeptide-positive C. acnes (MIT 18-1879-A3) (n = 4 males, 6 females) or remained uninfected (n = 10 males, 5 females). Mice were necropsied at 17 weeks post-infection with H. pylori.
Necropsy and histopathology
Immediately following CO2 euthanasia of the mice, blood was collected via cardiocentesis. Serum was separated and stored at −80°C. Gastric and mesenteric lymph nodes were collected for flow cytometry. The stomach and proximal duodenum were aseptically removed and incised along the greater curvature. Four linear gastric strips were sectioned from the lesser curvature and collected for culture, flash frozen for RNA analysis, stored at −80°C for DNA extraction, or preserved in 10% neutral buffered formalin for histopathologic evaluation (51). A comparative pathologist, who was blinded to sample identity, graded gastric lesions on an ascending scale from 0 to 4 for inflammation, epithelial defects, foveolar and glandular hyperplasia, and dysplasia and neoplasia. Scores were combined to generate a GHAI (15).
Quantification of gastric H. pylori
Colonization levels of gastric H. pylori SS1 were quantified using qPCR in the 7500 FAST real-time PCR system (Thermo Fisher Scientific, Waltham, MA) as previously described (34). Copy numbers of H. pylori were normalized to micrograms of mouse chromosomal DNA in the samples using the 18S rRNA gene-based primers and probe mixture (Thermo Fisher Scientific, Waltham, MA).
Quantitative C. acnes gastric colonization
Gastric tissue samples from nine C. acnes-colonized mice, four mice infected with H. pylori prior to C. acnes, and three mice dosed with C. acnes prior to H. pylori were weighed and homogenized in 1 mL of Brucella broth. Fifty microliters of 1:10 and 1:100 dilutions were grown in duplicate on 5% sheep blood agar plates under microaerophilic conditions. CFU counts were performed within 1 week when adequately sized colonies were present. Oral cavity swabs and feces from two animals per C. acnes-colonized group were cultured to confirm C. acnes colonization.
Gastric FOXM1 and cytokine mRNA expression profiles in gastric samples of GF INS-GAS mice
Il1β, Ifn-γ, Tnfα, Il17a, Il22, iNOS, Foxp3, and Foxm1 mRNAs were measured by quantitative PCR using commercial primers and probes for each cytokine as described previously (15).
Cytokine protein expression in gastric samples from GF INS-GAS mice by cytokine array analysis
Gastric tissue from male mice was homogenized in liquid nitrogen with a disposable pestle (Sigma-Aldrich, St. Louis, MO). One hundred fifty microliters of lysis buffer, 500 µL of RIPA buffer with protease inhibitor (Thermo Fisher Scientific, Waltham, MA), 5-µL protease inhibitor, and 5-µL 0.5 M EDTA were added. Samples were placed in a rotating mixer at 4°C for 1 hour. Supernatant was collected following centrifugation at 10,000 × g for 10 minutes at 4°C. Protein concentration was measured using a BCA kit (Thermo Fisher Scientific, Waltham, MA) and adjusted to 1 mg/mL. Thirty-two-plex gastric tissue cytokine array was performed to quantify eotaxin, G-CSF, GM-CSF, IFNγ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17A, IP-10, KC, LIF, LIX, MCP-1, M-CSF, MIG, MIP-1α, MIP-1β, MIP-2, RANTES, TNFα, and VEGF-A (Eve Technologies, Calgary, Alberta, Canada).
Serology
Serum Th1-associated IgG2a and Th2-associated IgG1 antibody response to sonicated antigens of H. pylori and C. acnes were quantified by enzyme-linked immunosorbent assay (ELISA). Antigen was prepared as previously described (10). In brief, H. pylori SS1 and C. acnes were grown under microaerobic conditions for 48 hours. Bacterial cells were pelleted, washed, resuspended, and sonicated and then filtered through a 0.2-µm syringe filter to remove cell debris.
For serum IgG measurement, 96-well Immulon II plates (Thermo Fischer Scientific, Waltham, MA) were coated with antigen at a concentration of 10 µg/mL and incubated overnight at 4°C. Wells were blocked with 2% bovine serum albumin (BSA) in PBS. Serum samples were diluted 1:100 with 1% BSA in PBS. Biotinylated monoclonal anti-mouse antibodies produced by clones A85-1 (specific for mouse IgG1) and 5.7 (specific for mouse IgG2; BD Biosciences, San Jose, CA) were diluted 1:2,000 and used as secondary antibodies. Incubation with ExtrAvidin-peroxidase (1:2,000; Sigma-Aldrich, St. Louis, MO) was followed by 2,29-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD) for color development at 405/562 nm.
Flow cytometry
GLN and MLN were manually dissociated into single cell suspensions. A total of 1–2 × 106 cells were incubated with Zombie violet (BioLegend, San Diego, CA) and Fc block (anti CD16/CD32, Clone 93, BioLegend, San Diego, CA). T cell surface markers, CD4 and CD3, were detected using CD4-PerCP (1:200; Clone RM4-5; BioLegend, San Diego, CA) and CD3-APC/Cy7 (1:100; Clone 17A2; BioLegend, San Diego, CA). Cells were fixed and permeabilized using Fix/Perm solution (BioLegend, San Diego, CA). Transcription factors were detected using the following antibodies: T-bet-FITC (1:100; Clone 4B10; BioLegend, San Diego, CA), RORγT-APC (1:100; Clone B2D; Thermo Fisher Scientific, Waltham, MA), or FOXP3-PE (1:100; Clone FJK-16s; Thermo Fisher Scientific, Waltham, MA). Samples were analyzed on a BD LSR II Flow Cytometer.
Gastric immunohistochemistry for immune cells
Paraffin-embedded gastric tissues from five control mice, four C. acnes-colonized mice, and eight mice from H. pylori and coinfected groups were stained using F4/80 antibody (1:100, Cell Signaling Technology, Danvers, MA) to detect macrophages, CD3 antibody (1:400, Agilent Dako, Santa Clara, CA) to detect T cells, FOXP3 antibody (1:100, Cell Signaling Technology, Danvers, MA) to detect Treg cells, CD45 B220 antibody (1:400, Thermo Scientific, Waltham, MA) to detect B cells, and MPO antibody (1:50, Thermo Scientific, Waltham, MA) to detect neutrophils by immunohistochemistry as previously described (5). Positive cells as a percentage of total cells within the gastric mucosa of whole digital slides were quantified using QuPath imaging software.
Statistical analyses
Statistical analysis and figure creation were performed using Prism (Version 9.0, GraphPad Software, La Jolla, CA). Results shown as mean ± standard deviation. GF gastric colonization, gastric histopathology scores, Foxm1 and cytokine mRNA expression, serology, flow cytometry % expression, and IHC results were analyzed using the Mann-Whitney U nonparametric test. Results were considered significant at P < 0.05 and were denoted by *P < 0.05, **P < 0.01, **P < 0.001, and ***P < 0001.
ACKNOWLEDGMENTS
We thank Andrea Vargas for maintenance and care of germ-free mice and Alejandra Aguilar for necropsy and tissue sample collections. We thank Ellen Buckley Jordan and Caroline Atkinson for laboratory support.
This research is supported by NIH grants P30-ES002109 (to J.G.F.), P01CA028842 (to J.G.F., B.M.P., and K.T.W.), R35 CA210088 (to T.C.W. and J.G.F.), and 1R01CA281732-01 (to R.M.P. and J.G.F.). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
James G. Fox, Email: jgfox@mit.edu.
Meera Unnikrishnan, University of Warwick, Coventry, United Kingdom .
DATA AVAILABILITY
Genomes for C. acnes strains MIT 18-1857-A1, 18-1851-A4, 18-1869-C3, and 18-1879-A3 were deposited under the following GenBank accessions, respectively: WOWG00000000, WOWH00000000, WOWI00000000, and WOWJ00000000.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/spectrum.03450-23.
Tables S1 and S2; Figures S1 to S6.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Duck WM, Sobel J, Pruckler JM, Song Q, Swerdlow D, Friedman C, Sulka A, Swaminathan B, Taylor T, Hoekstra M, Griffin P, Smoot D, Peek R, Metz DC, Bloom PB, Goldschmidt S, Parsonnet J, Triadafilopoulos G, Perez-Perez GI, Vakil N, Ernst P, Czinn S, Dunne D, Gold BD. 2004. Antimicrobial resistance incidence and risk factors among Helicobacter pylori–infected persons, United States. Emerg Infect Dis 10:1088–1094. doi: 10.3201/eid1006.030744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. 2017. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153:420–429. doi: 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 3. Fox JG, Wang TC. 2007. Inflammation, atrophy, and gastric cancer. J Clin Invest 117:60–69. doi: 10.1172/JCI30111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Correa P, Piazuelo MB. 2012. The gastric precancerous cascade. J Dig Dis 13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feng Y, Wang L, Zeng J, Shen L, Liang X, Yu H, Liu S, Liu Z, Sun Y, Li W, Chen C, Jia J. 2013. FoxM1 is overexpressed in Helicobacter pylori –induced gastric carcinogenesis and is negatively regulated by miR-370. Mol Cancer Res 11:834–844. doi: 10.1158/1541-7786.MCR-13-0007 [DOI] [PubMed] [Google Scholar]
- 6. Zeng J, Wang L, Li Q, Li W, Björkholm M, Jia J, Xu D. 2009. FoxM1 is up-regulated in gastric cancer and its inhibition leads to cellular senescence, partially dependent on p27kip 1 . J Pathol 218:419–427. doi: 10.1002/path.2530 [DOI] [PubMed] [Google Scholar]
- 7. Wang H, Huang C. 2015. Foxm1 and its oncogenic signaling in gastric cancer. Recent Pat Anticancer Drug Discov 10:270–279. doi: 10.2174/1574892810666150617112421 [DOI] [PubMed] [Google Scholar]
- 8. Huang L, Wang Z-Y, Pan D-D. 2019. Penicillin-binding protein 1A mutation-positive Helicobacter pylori promotes epithelial-mesenchymal transition in gastric cancer via the suppression of microRNA-134. Int J Oncol 54:916–928. doi: 10.3892/ijo.2018.4665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Zeng J, Pan J, Geng X, Li L, Wu J, Song P, Wang Y, Liu J, Wang L. 2016. MiR-320a inhibits gastric carcinoma by targeting activity in the FoxM1-P27KIP1 axis. Oncotarget 7:29275–29286. doi: 10.18632/oncotarget.8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang L, Cui M, Zhang L, Song L. 2017. FOXM1 facilitates gastric cancer cell migration and invasion by inducing cathepsin D. Oncotarget 8:68180–68190. doi: 10.18632/oncotarget.19254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li X, Ma K, Song S, Shen F, Kuang T, Zhu Y, Liu Z. 2018. Tight correlation between FoxM1 and FoxP3+ Tregs in gastric cancer and their clinical significance. Clin Exp Med 18:413–420. doi: 10.1007/s10238-018-0505-6 [DOI] [PubMed] [Google Scholar]
- 12. Li X, Qiu W, Liu B, Yao R, Liu S, Yao Y, Liang J. 2013. Forkhead box transcription factor 1 expression in gastric cancer: FOXM1 is a poor prognostic factor and mediates resistance to docetaxel. J Transl Med 11:1–13. doi: 10.1186/1479-5876-11-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang I, Woltemate S, Piazuelo MB, Bravo LE, Yepez MC, Romero-Gallo J, Delgado AG, Wilson KT, Peek RM, Correa P, Josenhans C, Fox JG, Suerbaum S. 2016. Different gastric microbiota compositions in two human populations with high and low gastric cancer risk in Colombia. Sci Rep 6:18594. doi: 10.1038/srep18594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mannion A, Sheh A, Shen Z, Dzink-Fox J, Piazuelo MB, Wilson KT, Peek R, Fox JG. 2023. Shotgun metagenomics of gastric biopsies reveals compositional and functional microbiome shifts in high- and low-gastric-cancer-risk populations from Colombia, South America. Gut Microbes 15:2186677. doi: 10.1080/19490976.2023.2186677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen Z, Dzink-Fox J, Feng Y, Muthupalani S, Mannion AJ, Sheh A, Whary MT, Holcombe HR, Piazuelo BM, Bravo LE, Josenhans C, Suerbaum S, Wilson KT, Peek RM, Wang TC, Fox JG, Young VB. 2022. Gastric non-Helicobacter pylori urease-positive Staphylococcus epidermidis and Streptococcus salivarius isolated from humans have contrasting effects on H. pylori-associated gastric pathology and host immune responses in a murine model of gastric cancer. mSphere 7:e0077221. doi: 10.1128/msphere.00772-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delgado S, Suárez A, Mayo B. 2011. Identification, typing and characterization of Propionibacterium strains from healthy mucosa of the human stomach. Int J Food Microbiol 149:65–72. doi: 10.1016/j.ijfoodmicro.2011.01.028 [DOI] [PubMed] [Google Scholar]
- 17. Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C. 2019. The microbiome of the upper respiratory tract in health and disease. BMC Biol 17:87. doi: 10.1186/s12915-019-0703-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Claesen J, Spagnolo JB, Ramos SF, Kurita KL, Byrd AL, Aksenov AA, Melnik AV, Wong WR, Wang S, Hernandez RD, Donia MS, Dorrestein PC, Kong HH, Segre JA, Linington RG, Fischbach MA, Lemon KP. 2020. A Cutibacterium acnes antibiotic modulates human skin microbiota composition in hair follicles. Sci Transl Med 12:1–14. doi: 10.1126/scitranslmed.aay5445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vinogradov AA, Suga H. 2020. Introduction to thiopeptides: biological activity, biosynthesis, and strategies for functional reprogramming. Cell Chem Biol 27:1032–1051. doi: 10.1016/j.chembiol.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 20. Bailly C. 2022. The bacterial thiopeptide thiostrepton. An update of its mode of action, pharmacological properties and applications. Eur J Pharmacol 914:174661. doi: 10.1016/j.ejphar.2021.174661 [DOI] [PubMed] [Google Scholar]
- 21. Deshayes C, Arafath MN, Apaire-Marchais V, Roger E. 2021. Drug delivery systems for the oral administration of antimicrobial peptides: promising tools to treat infectious diseases. Front Med Technol 3:778645. doi: 10.3389/fmedt.2021.778645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sayanjali B, Christensen GJM, Al-Zeer MA, Mollenkopf H-J, Meyer TF, Brüggemann H. 2016. Propionibacterium acnes inhibits FOXM1 and induces cell cycle alterations in human primary prostate cells. Int J Med Microbiol 306:517–528. doi: 10.1016/j.ijmm.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 23. Burns M, Muthupalani S, Ge Z, Wang TC, Bakthavatchalu V, Cunningham C, Ennis K, Georgieff M, Fox JG, Boneca IG. 2015. Helicobacter pylori infection induces anemia, depletes serum iron storage, and alters local iron-related and adult brain gene expression in male INS-GAS mice. PLoS ONE 10:e0142630. doi: 10.1371/journal.pone.0142630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burns M, Amaya A, Bodi C, Ge Z, Bakthavatchalu V, Ennis K, Wang TC, Georgieff M, Fox JG, Connor JR. 2017. Helicobacter pylori infection and low dietary iron alter behavior, induce iron deficiency anemia, and modulate hippocampal gene expression in female C57BL/6 mice. PLoS ONE 12:e0173108. doi: 10.1371/journal.pone.0173108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stair MI, Winn CB, Burns MA, Holcombe H, Artim SC, Ge Z, Shen Z, Wang TC, Muthupalani S, Franco-Mahecho O, Ennis K, Georgieff MK, Fox JG. 2023. Effects of chronic Helicobacter pylori strain PMSS1 infection on whole brain and gastric iron homeostasis in male INS-GAS mice. Microbes Infect 25:105045. doi: 10.1016/j.micinf.2022.105045 [DOI] [PubMed] [Google Scholar]
- 26. Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, Müller A. 2011. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology 140:199–209. doi: 10.1053/j.gastro.2010.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kwok JM-M, Myatt SS, Marson CM, Coombes RC, Constantinidou D, Lam EW-F. 2008. Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression. Mol Cancer Ther 7:2022–2032. doi: 10.1158/1535-7163.MCT-08-0188 [DOI] [PubMed] [Google Scholar]
- 28. Lertpiriyapong K, Whary MT, Muthupalani S, Lofgren JL, Gamazon ER, Feng Y, Ge Z, Wang TC, Fox JG. 2014. Gastric colonization with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut 63:54–63. doi: 10.1136/gutjnl-2013-305178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu B, Xiang L, Peppelenbosch MP, Fuhler GM. 2023. Overlapping cytokines in H. pylori infection and gastric cancer: a tandem meta-analysis. Front Immunol 14:1125658. doi: 10.3389/fimmu.2023.1125658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamamoto T, Kita M, Ohno T, Iwakura Y, Sekikawa K, Imanishi J. 2004. Role of tumor necrosis factor-alpha and interferon-gamma in Helicobacter pylori infection. Microbiol Immunol 48:647–654. doi: 10.1111/j.1348-0421.2004.tb03474.x [DOI] [PubMed] [Google Scholar]
- 31. Maruyama T, Kono K, Mizukami Y, Kawaguchi Y, Mimura K, Watanabe M, Izawa S, Fujii H. 2010. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci 101:1947–1954. doi: 10.1111/j.1349-7006.2010.01624.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dzierzanowska-Fangrat K, Michalkiewicz J, Cielecka-Kuszyk J, Nowak M, Celinska-Cedro D, Rozynek E, Dzierzanowska D, Crabtree JE. 2008. Enhanced gastric IL-18 mRNA expression in Helicobacter pylori-infected children is associated with macrophage infiltration, IL-8, and IL-1β mRNA expression. Eur J Gastroenterol Hepatol 20:314–319. doi: 10.1097/MEG.0b013e3282f340da [DOI] [PubMed] [Google Scholar]
- 33. Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, Varro A, Wang TC. 2003. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res 63:942–950. [PubMed] [Google Scholar]
- 34. Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, Dangler CA, Israel DA, Krishna U, Gaus K, Peek RM. 2003. Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology 124:1879–1890. doi: 10.1016/s0016-5085(03)00406-2 [DOI] [PubMed] [Google Scholar]
- 35. Kusugami K, Ando T, Imada A, Ina K, Ohsuga M, Shimizu T, Sakai T, Konagaya T, Kaneko H. 1999. Mucosal macrophage inflammatory protein‐1α activity in Helicobacter pylori infection. J Gastroenterol Hepatol 14:20–26. doi: 10.1046/j.1440-1746.1999.01810.x [DOI] [PubMed] [Google Scholar]
- 36. Whitney AE, Emory TS, Marty AM, O’Shea PA, Newman GW, Gold BD. 2000. Increased macrophage infiltration of gastric mucosa in Helicobacter pylori-infected children. Dig Dis Sci 45:1337–1342. doi: 10.1023/a:1005551903029 [DOI] [PubMed] [Google Scholar]
- 37. Piao J-Y, Lee HG, Kim S-J, Kim D-H, Han H-J, Ngo H-K-C, Park S-A, Woo J-H, Lee J-S, Na H-K, Cha Y-N, Surh Y-J. 2016. Helicobacter pylori activates IL-6-STAT3 signaling in human gastric cancer cells: potential roles for reactive oxygen species. Helicobacter 21:405–416. doi: 10.1111/hel.12298 [DOI] [PubMed] [Google Scholar]
- 38. Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Brönneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Brüning JC. 2014. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol 15:423–430. doi: 10.1038/ni.2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang BH, Hagemann S, Mamareli P, Lauer U, Hoffmann U, Beckstette M, Föhse L, Prinz I, Pezoldt J, Suerbaum S, Sparwasser T, Hamann A, Floess S, Huehn J, Lochner M. 2016. Foxp3+ T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol 9:444–457. doi: 10.1038/mi.2015.74 [DOI] [PubMed] [Google Scholar]
- 40. Bhaumik S, Mickael ME, Moran M, Spell M, Basu R. 2021. RORγt promotes Foxp3 expression by antagonizing the effector program in colonic regulatory T cells. J Immunol Res 207:2027–2038. doi: 10.4049/jimmunol.2100175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, Potter A, Varro A, Eibach D, Suerbaum S, Wang TC, Fox JG. 2011. Lack of commensal flora in H. pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 140:210–220. doi: 10.1053/j.gastro.2010.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Q, Wu W, Gong D, Shang R, Wang J, Yu H. 2021. Propionibacterium acnes overabundance in gastric cancer promote M2 polarization of macrophages via a TLR4/PI3K/Akt signaling. Gastric Cancer 24:1242–1253. doi: 10.1007/s10120-021-01202-8 [DOI] [PubMed] [Google Scholar]
- 43. Liu X, Shao L, Liu X, Ji F, Mei Y, Cheng Y, Liu F, Yan C, Li L, Ling Z. 2019. Alterations of gastric mucosal microbiota across different stomach microhabitats in a cohort of 276 patients with gastric cancer. EBioMedicine 40:336–348. doi: 10.1016/j.ebiom.2018.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gunathilake MN, Lee J, Choi IJ, Kim Y-I, Ahn Y, Park C, Kim J. 2019. Association between the relative abundance of gastric microbiota and the risk of gastric cancer: a case-control study. Sci Rep 9:13589. doi: 10.1038/s41598-019-50054-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. 1999. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res 59:4823–4828. [PubMed] [Google Scholar]
- 46. Olson RD, Assaf R, Brettin T, Conrad N, Cucinell C, Davis JJ, Dempsey DM, Dickerman A, Dietrich EM, Kenyon RW, et al. 2023. Introducing the bacterial and viral bioinformatics resource center (BV-BRC): a resource combining PATRIC, IRD and ViPR. Nucleic Acids Res 51:D678–D689. doi: 10.1093/nar/gkac1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Richter M, Rosselló-Móra R, Oliver Glöckner F, Peplies J. 2016. JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 32:929–931. doi: 10.1093/bioinformatics/btv681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Veltri D, Wight MM, Crouch JA. 2016. SimpleSynteny: a web-based tool for visualization of microsynteny across multiple species. Nucleic Acids Res 44:W41–W45. doi: 10.1093/nar/gkw330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mannion A, Shen Z, Feng Y, Artim SC, Ravindra K, Ge Z, Fox JG. 2019. Gamma‐glutamyltranspeptidase expression by Helicobacter saguini, an enterohepatic Helicobacter species isolated from cotton top tamarins with chronic colitis. Cell Microbiol 21:e12968. doi: 10.1111/cmi.12968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee C-W, Rao VP, Rogers AB, Ge Z, Erdman SE, Whary MT, Fox JG. 2007. Wild-type and interleukin-10-deficient regulatory T cells reduce effector T-cell-mediated gastroduodenitis in Rag2-/- mice, but only wild-type regulatory T cells suppress Helicobacter pylori gastritis. Infect Immun 75:2699–2707. doi: 10.1128/IAI.01788-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. 2005. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res 65:10709–10715. doi: 10.1158/0008-5472.CAN-05-1846 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2; Figures S1 to S6.
Data Availability Statement
Genomes for C. acnes strains MIT 18-1857-A1, 18-1851-A4, 18-1869-C3, and 18-1879-A3 were deposited under the following GenBank accessions, respectively: WOWG00000000, WOWH00000000, WOWI00000000, and WOWJ00000000.