Abstract
In attempts to enhance natural products as therapeutic agents, fluorination has emerged as a new tool for synthetic biologists and chemists. In recent articles published in Nature Chem. and Nature Chem. Bio., Grininger, Chang, and co-workers leveraged their expertise in engineering polyketide biosynthesis to incorporate fluorine into polyketide scaffolds.
Keywords: Polyketides, Secondary Metabolites, Fluorine, Chemical Biology, Synthetic Biology, Drug Discovery, Biosynthesis, Biocatalysis, Halogenation, Macrolides
Together, small molecules and biologics inspire drug leads and equip the pharmaceutical industry with broad arrays of tools to treat disease. Although research in recent decades has trended towards biologic development, small molecules remain crucial therapeutics. Of note, the chemical integration of fluorine continues to position small molecules as critical drug leads. One such drug, Florinef, was the first FDA-approved fluorinated drug in 1954. Since then, fluorinated drugs have increased to encompass an estimated 20% of pharmaceuticals in 2020.1 The most notable fluorinated pharmaceutical, Lipitor 1, was introduced in 1997, with estimated lifetime sales of >$150 billion, designating it the best-selling drug of all time.2 Contrasting hydrogen, fluorine substantially increases electronegativity while maintaining an atomic radius comparable to oxygen. Additionally, fluorine tends to reduce the pKa of neighboring functional groups via electron-withdrawing properties, leading to physiochemical changes in solubility, metabolism, and binding affinities. For example, the addition of 3-trifluoromethyl and 2,4,5-trifluorophenyl to a triazolopiperazine scaffold increased oral availability and potency, creating the Type II diabetes treatment, Sitagliptin.3 Synthetic approaches are typically used to produce fluorinated compounds. Unfortunately, these methods are often limited by poor enantioselectivity, scale-up feasibility, and multi-step routes resulting in low global yields. An alternative approach, the fusion of synthetic biology and chemical synthesis, enables the production of existing and novel fluorinated compounds.
Polyketides comprise many natural products with broad medicinal applications. The linear “assembly line”-type construction of polyketide scaffolds via Type I polyketide synthases (PKS) renders their derivatization an approachable means of pharmaceutical development and discovery. Solithromycin 2, a fluoroketolide developed to circumvent antibiotic resistance with key structural changes like C2 fluorination, more tightly binds to the 50S ribosomal subunit of bacterial pathogens, effectively treating infections like community-acquired bacterial pneumonia.4 However, construction requires 16 chemical steps from the fermentation product, Erythromycin A, where fluorination serves as the final step. While this method is typically robust, it is low-yielding with global yields of ~35% and produces toxic byproducts.5 Another example, flurithromycin 3, was created via C8 fluorination and demonstrated in vitro activity against 44 gram-negative pathogens, 85 gram-positive pathogens, and 125 anaerobes.6 While solithromycin and flurithromycin (Figure 1A) are produced semi-synthetically, alternative fluorination strategies remain desirable. Hypothetically, one method could involve tailoring enzymes, but enzymes that catalyze direct, regioselective fluorination of these scaffolds have yet to be discovered.
Figure 1. Demonstrated Methods of Polyketide Fluorine Incorporation.
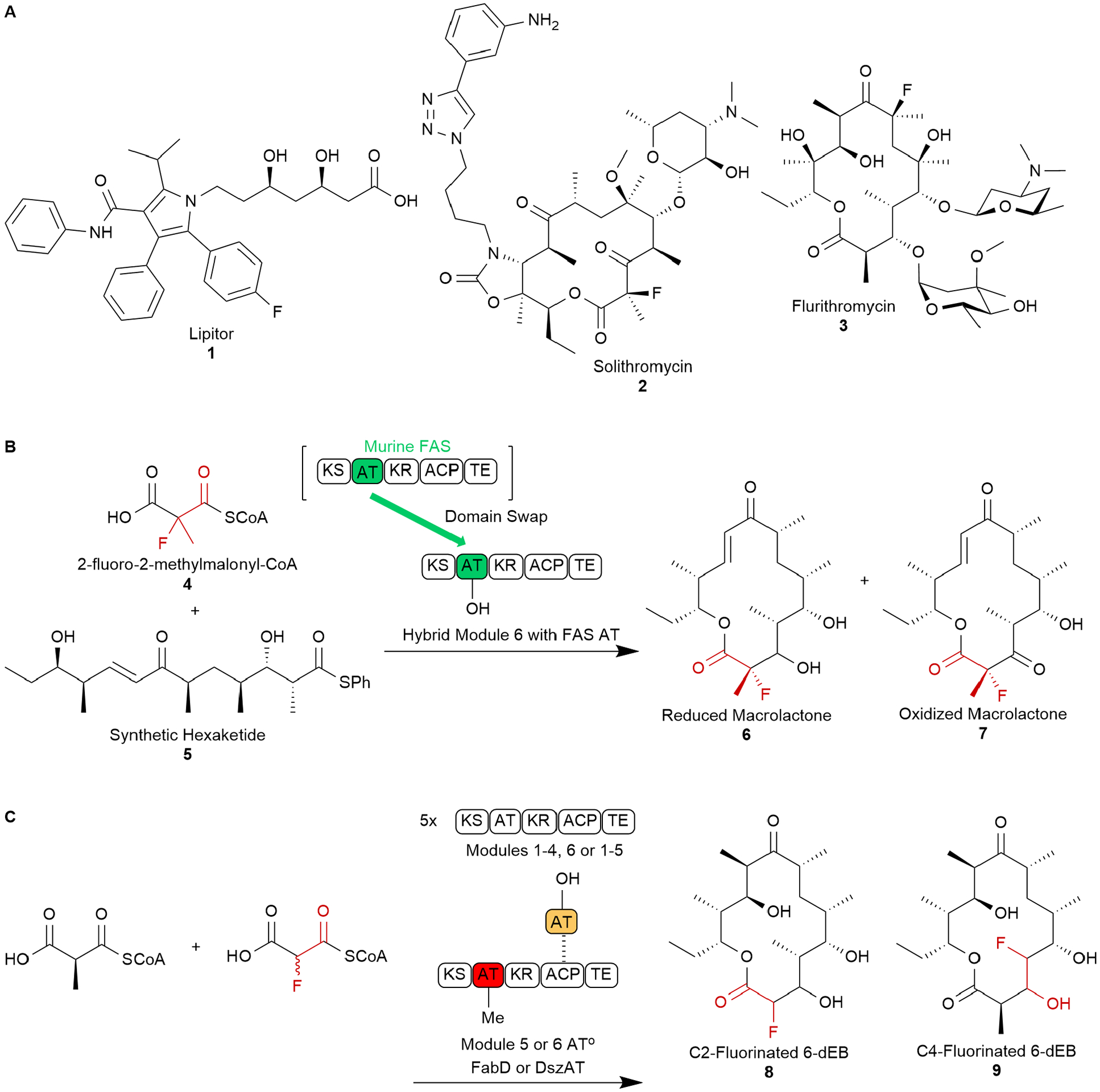
A) Designer macrolides solithromycin 1 and flurithromycin 2 with demonstrated antibacterial activity; B) Grininger et al leveraged an AT domain swap from a promiscuous FAS AT, with C2 stereochemistry assumed based on results with 12-membered macrolactones, while; C) Chang and co-workers employed a knock-out AT complemented with an engineered trans-AT, and both studies resulted in fluorinated macrolide products that could be utilized as antibiotic precursors.
Alternatively, recent work has demonstrated that alterations to acyltransferase (AT) domains in a PKS enable regioselective incorporation of non-natural extender units by altering or increasing substrate promiscuity.7,8,9 Since AT domains select for substituted malonyl-CoA extender units, a fluorinated extender unit (such as fluoromalonyl-CoA) used with a PKS module containing a promiscuous AT should allow for fluorine incorporation. The last decade of research has shown that AT domain swaps, random and rational mutagenesis, or supplementation with trans-ATs all prove effective means of non-natural extender unit incorporation. However, this work has not included fluorinated malonates until recently.
Installing fluorine into polyketide scaffolds remains a priority, but biological approaches represent considerable challenges due to the dearth of known fluorinases. Recently, two groups successfully demonstrated fluorine incorporation into polyketides. Recent studies from Chang et al. and Grininger et al. employed complementary approaches for fluorinated polyketide production.10,11 Both groups leveraged AT alterations to shift specificity toward fluorinated extender units. Previous work demonstrated that AT engineering can shift extender unit selectivity away from native substrates to non-canonical extender units.7,12 Grininger et al. leveraged a domain swap, where an AT with different extender substrate selectivity replaces the native AT. Identifying an AT with novel extender unit selectivity from a PKS biosynthetic gene cluster represents a challenge, considering a majority of ATs select for either malonyl or methylmalonyl-CoA.13 To overcome this obstacle, Grininger et al. identified a highly promiscuous acyltransferase from a metazoan fatty acid synthase (FAS) and investigated a series of mutations for impacts on substrate specificity and overall fold in protein structure.14 Once an ideal mutant was identified, the team characterized activity toward fluoromalonyl-CoA and measured its ability to transacylate the downstream acyl carrier protein (ACP). Further, a chimeric terminal module from the Erythromycin biosynthetic pathway was created by replacing the native AT for the promiscuous murine FAS AT. To demonstrate in vitro activity, hybrid modules were fed a chemically synthesized acceptor mimic (NDK) and fluoromalonyl-CoA to form triketide lactone (TKL) product. Following successful fluorinated TKL production, focus shifted to larger macrolides more reminiscent of natural product antibiotics. Using a synthetic pentaketide acceptor mimic, the chimeric module produced a 12-membered ring with a C2-fluorine and the preferred (2S,3S) stereochemistry. However, the electronic character afforded by fluorine created a bottleneck for microbial production, limiting fluorinated production to milligram scale. With a fluorine alpha to the ACP thioester, premature cleavage of the polyketide chain followed by hydrolysis and decarboxylation results in cyclic ketone shunt product formation. To solve the problem, racemic 2-fluoro-2-methylmalonyl-CoA 4 was synthesized to prevent enolate-induced cleavage. Gratifyingly, the hybrid PKS incorporated the novel extender unit and, crucially, shifted product formation toward the 12-membered macrolide and away from shunt products. Further cementing the utility of their hybrid PKS, a synthetic hexaketide substrate 5 tested with the novel fluorinated extender unit produced 14-membered macrolides 6 and 7, which represent potential intermediates for the production of solithromycin (Figure 1B). These products demonstrate the hybrid PKS’s capacity to incorporate fluorinated extender units and generate designer macrolides.11
In comparison, Chang et al. leveraged trans-AT expertise to affect fluorination of 6-deoxyerythronolide B (6-dEBs), the core scaffold of Erythromycin. Trans-ATs are independently expressed enzymes that transfer polyketide chains to target ACPs through protein-protein interactions. Taking the trans-AT DszAT from the disorazole biosynthetic pathway, its crystal structure was used to rationally guide protein engineering efforts.12 With a mutant library, TKL production experiments similar to Grininger’s study probed fluoromalonyl-CoA incorporation via DszAT and DEBS module 3 with an inactivated AT. One identified mutant, F190V, produced F-TKL with twenty-fold production over the nonfluorinated product, with F190V guiding further experiments. Larger fluorinated 6-dEB analogs were biosynthesized using mutant DszAT, while AT5 and AT6 within DEBS3 were sequentially inactivated, enabling selective fluorination at two locations (C2 8 and C4 9) within the 14-membered 6-dEB scaffold (Figure 1C). To construct an in vivo fluorination cassette, a malonate transporter and malonyl-CoA synthetase were needed. While investigating in vivo production of fluorinated 6-dEB, an engineered trans-AT, FabD, replaced DszAT since it is natively produced in E. coli. Using a system of four genes encoded across three plasmids used in previous AT research by Chang and others,12,15 an engineered E. coli strain successfully produced fluorinated 6-dEB analogs in vivo. However, yields were low, and the pathway requires additional engineering to relieve the burden of increased protein production and to balance protein expression between three plasmids.10 Additionally, further metabolic engineering is required to increase production beyond the milligram scale.
AT engineering has proven a valuable tool for polyketide diversification. By building an understanding of enzymatic activity and the extent to which they can be engineered while maintaining activity, work in the field has afforded countless opportunities for chemical biologists to probe PKSs. Fluorination of polyketides using engineered ATs has been effectively demonstrated, enabling future research to build upon engineering efforts and produce novel drug leads and intermediates. Leveraging entire domain swaps confers known activity from promiscuous domains and encodes control of fluorination sites, while trans-AT implementation potentially enables diversification of multiple sites in the polyketide backbone with fewer amino acid mutations. By leveraging two different methods of AT engineering, the Chang and Grininger labs have elucidated additional routes for the efficient production of new-to-nature polyketides.
Two new strategies for fluorine derivatization of polyketides were explored. Chang et al. introduced fluoromalonyl-CoA into a growing polyketide chain using an engineered trans-AT, while Grininger et al. utilized a promiscuous MAT domain swap to introduce both fluoromalonyl- and 2-fluoro-2-methylmalonyl-CoA. These approaches highlight the transfer of promiscuity afforded by direct cis-AT mutagenesis, which leverages native protein-protein interactions, in contrast to employing trans-AT enzymes, which take advantage of intermolecular protein interactions and diffusivity of smaller enzymes. In principle, both strategies could be employed in other parts of the assembly line or in other PKSs to enable further diversification of polyketides, but specific engineering for efficient incorporation would be required for optimized production. These strategies expand the toolbox with which biosynthetic chemists can generate novel semi-synthetic polyketides and provide a means of incorporating fluorine, an atom with an increasing presence in pharmaceuticals, typically used to improve activity and bioavailability.
While these studies serve as promising proofs of principle, future work is needed to increase product yields and couple with downstream tailoring enzymes. Further engineering, through directed evolution, increased integration of trans-ATs into orthogonal systems, and recovering activity lost through chimera construction all represent feasible ventures for future research. Switching specific motifs, rather than entire domains, may provide a way to change AT specificity with minimal disruption of crucial protein-protein interactions.7 Adaptations of these studies will likely provide additional, alternative derivatization methods. For instance, beyond fluorine, different AT mutations or domains can shift promiscuity towards amino or aromatic groups, which are currently underrepresented in polyketide scaffolds. These strategies can also be introduced to other biosynthetic pathways for fluorination or general derivatization of other pharmacotherapies, aiding drug discovery efforts.
ACKNOWLEDGMENTS
The authors acknowledge the support of the National Institutes of Health grants GM124112 (G.J.W) and 5T32GM141887 (S.D.W).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Inoue M Sumii Y Shibata N (2020). Contribution of Organofluorine Compounds to Pharmaceuticals. ACS Omega 5, 10633–10640. 10.1021/acsomega.0c00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brumley J (2017). The 15 All-Time Best Selling Prescription Drugs. Killinger; https://www.kiplinger.com/slideshow/investing/t027-s001-the-15-alltime-best-selling-prescription-drugs/index.html (accessed 8-22-22) [Google Scholar]
- 3.Hagmann W (2008). The Many Roles of Fluorine in Medicinal Chemistry. J. Med. Chem 51, 4359–4369. 10.1021/jm800219f [DOI] [PubMed] [Google Scholar]
- 4.Zhanel GG; Hartel E; Adam H; Zelenitsky S; Zhanel MA; Golden A; Schweizer F; Gorityala B; Lagacé-Wiens PR; Walkty AJ et al. (2016). Solithromycin: A Novel Fluoroketolide for the Treatment of Community-Acquired Bacterial Pneumonia, Drugs, 76 (18), 1737–1757. https://dio.org/10.1007/s40265-016-0667-z [DOI] [PubMed] [Google Scholar]
- 5.Fernandes P; Martens E; Bertrand D; Pereira D (2016). The Solithromycin Journey—It Is All in the Chemistry. Bioorganic & Medicinal Chemistry, 24 (24), 6420–6428. 10.1016/j.bmc.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Saverino D; Debbia EA; Pesce A; Lepore AM; Schito GC (1992). Antibacterial Profile of Flurithromycin, a New Macrolide. Journal of Antimicrobial Chemotherapy, 30 (3), 261–272. 10.1093/jac/30.3.261. [DOI] [PubMed] [Google Scholar]
- 7.Kalkreuter E; Bingham KS; Keeler AM; Lowell AN; Schmidt JJ; Sherman DH; Williams GJ(2021) Computationally-Guided Exchange of Substrate Selectivity Motifs in a Modular Polyketide Synthase Acyltransferase. Nature Communications 2021, 12 (1). 10.1038/s41467-021-22497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koryakina I; McArthur J; Randall S; Draelos MM; Musiol EM; Muddiman DC; Weber T; Williams GJ (2013). Poly Specific Trans-Acyltransferase Machinery Revealed via Engineered Acyl-COA Synthetases. ACS Chemical Biology, 8 (1), 200–208. 10.1021/cb3003489 [DOI] [PubMed] [Google Scholar]
- 9.Hemmerling F, Meoded R, Fraley A, Minas H, Dieterich C, Rust M, Ueoka R, Jensen J, Helfrich E, Bergande C et al. (2022). Modular Halogenation, α-Hydroxylation, and Acylation by a Remarkably Versatile Polyketide Synthase. 134. 10.1002/anie.202116614 [DOI] [PubMed] [Google Scholar]
- 10.Sirirungruang S, Ad O, Privalsky T, Ramesh S, Sax J, Dong H, Baidoo E, Amer B, Khosla C, Chang M (2022). Engineering siteselective incorporation of fluorine into polyketides. Nat. Chem. Bio 18, 886–893. 10.1038/s41589-022-01070-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rittner A, Mirko J, Schmidt J, Mayer L, Reiners S, Heid E, Herzberg D, Sherman D, Grininger M (2022). Chemoenzymatic Synthesis of fluorinated polyketides. Nat. Chem 1755–4349. 10.1038/s41557-022-00996-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thuronyi B, Chang M (2015), Synthetic biology approaches to fluorinated polyketides. (48) Acc. Chem. Res 584–592. 10.1021/ar500415c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn B, Khosla C (2013). Engineering the acyltransferase substrate specificity of assembly line polyketide synthases. (10). J. R. Soc. Interface 10.1098/rsif.2013.0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittner A, Paithankar KS, Huu KV & Grininger M (2018). Characterization of the polyspecific transferase of murine type I fatty acid synthase (FAS) and implications for polyketide synthase (PKS) engineering. (13). ACS Chem. Biol 723–732. 10.1021/acschembio.7b00718 [DOI] [PubMed] [Google Scholar]
- 15.Wong FT, Chen AY, Cane DE, Khosla C (2010). Protein-Protein Recognition between Acyltransferases and Acyl Carrier Proteins in Multimodular Polyketide Synthases. Biochemistry 49 (1), 95–102. 10.1021/bi901826g [DOI] [PMC free article] [PubMed] [Google Scholar]


