Abstract
Bacillus subtilis 168 is unable to grow on xylose and galactose as sole carbon sources, owing to the lack of specific transporters. We show that they are imported into the cell by the activity of AraE, an arabinose transporter whose synthesis is induced by l-arabinose.
The soil bacterium Bacillus subtilis is able to use a wide range of carbon sources, including even unusual sugar derivatives like the β-arylglucosides salicin and arbutin (5, 6). On the other hand d-xylose and d-galactose, two sugars frequently found in nature, are unable to serve as sole carbon sources (7, 13). This is very surprising, because B. subtilis synthesizes all proteins necessary to degrade both sugars (3, 4). This unusual feature is due to the fact that B. subtilis is unable to import these two sugars (4, 12).
We were interested in the toxic effects of galactose on galE-negative B. subtilis strains (4). In the course of this work, we coincidentally obtained spontaneous mutants of B. subtilis 168 which were able to grow on d-galactose as their sole carbon source. The characterization of these mutants revealed that a transporter for arabinose, AraE, functions as a transporter for at least three different sugars. B. subtilis is able to grow on both d-galactose and d-xylose when l-arabinose, the inducer of AraE synthesis, is added to the growth medium.
B. subtilis Gal+ is able to import d-galactose.
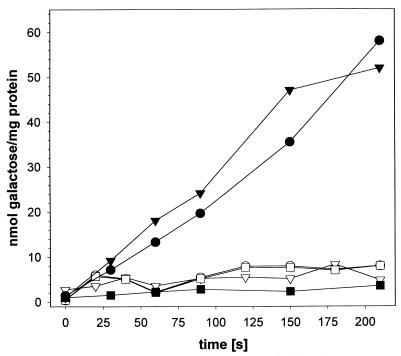
By chance, we isolated mutants capable of growth on d-galactose as the sole carbon source. One of these mutants (strain Gal+ [the strains and plasmids used in this study are listed in Table 1]) was further characterized. Transport of d-galactose into the cell was determined as described previously (12). The results of this experiment are depicted in Fig. 1. No d-galactose accumulation was detected for the B. subtilis wild-type strain. In contrast, significant d-galactose uptake was observed for the d-galactose-positive mutant. This transport activity was not dependent on the presence of d-galactose in the growth medium. Import of d-galactose was abolished when cells were grown in the presence of glucose. We further characterized the strain by using the API50CH kit with API 50 CHB medium (bioMérieux, Marcy l’Etoile, France) (data not shown). As expected, B. subtilis Gal+ was able to use d-galactose; surprisingly, it was also able to use d-xylose. Utilization of l-arabinose was slightly improved. No effect was detected for any of the other sugars tested. We then tested our strain for growth on d-xylose as its sole carbon source. In contrast to the wild type, the strain grew on minimal medium with d-xylose as a sole carbon source (data not shown). B. subtilis 168 encodes all genes necessary for xylose degradation (3). These genes are tightly regulated. High-level transcription is induced in the presence of xylose. However, utilization of xylose is not possible, because xylose is not transported into the cell. Spontaneous mutants capable of xylose importation are easily obtained (12). We concluded that a single mutation is sufficient to convert the B. subtilis wild-type strain into a derivative which is able to import both d-galactose and d-xylose.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant markers | Source or referencea |
|---|---|---|
| E. coli DH5α | hsdR17 endA1 recA1 gyrA96 thi relA1 supE44 φ80dlacZΔM15 Δ(lacZ-argF)U169 | BRL |
| B. subtilis strains | ||
| 168 | trpC2 | BGSC |
| Gal+ | trpC2; Gal+ Xyl+ mutant | This work |
| EP2 | trpC2 galE::aphA3 | 4 |
| Gal+ EP2 | trpC2 galE::aphA3 | This work |
| ΩaraR | trpC2 araR::pΩaraR | This work |
| Plasmids | ||
| pSGMU2 | bla cat | 2 |
| p14, p17, p35, p75 | spec | This work |
| pIC333 | bla Tn10spec′ | 1 |
| pΩaraR | bla cat ′ araR ′ | This work |
BRL, Bethesda Research Laboratories; BGSC, Bacillus Genetic Stock Center.
FIG. 1.
Galactose uptake of B. subtilis 168 (open symbols) and B. subtilis Gal+ (filled symbols). Cells were grown either in NB (circles), in NB with 0.2% galactose (triangles), or in NB with 0.2% glucose (squares). Uptake of 14C-labelled sugar was determined as described previously (12).
Inactivation of the araE gene renders Gal+ strains d-galactose negative.
B. subtilis strains without the functional galE gene are unable to grow in the presence of high concentrations of d-galactose (4). We therefore inactivated the galE gene of B. subtilis Gal+ by transforming B. subtilis Gal+ with chromosomal DNA from B. subtilis EP2 (4) and selecting for neomycin-resistant clones. This mutant is not capable of growth in the presence of even low concentrations of d-galactose (data not shown). We transformed this strain with plasmid pIC333 (1). This plasmid was constructed to allow easy and random transposon mutagenesis of B. subtilis. Mutagenesis was done as described previously (1). Integrants were obtained and grown on NB medium containing spectinomycin and 1 mM d-galactose. While a galE-negative wild-type strain was able to grow on such plates, the galE-negative Gal+ derivative of B. subtilis was not (data not shown). Several hundred integrants resistant to spectinomycin and d-galactose were obtained. We screened for candidates which were resistant to 1 mM d-galactose but which lysed in the presence of 10 mM d-galactose. Chromosomal DNAs from 10 clones were isolated and used to transform B. subtilis Gal+. Spectinomycin-resistant clones were isolated. Eight strains were unable to grow with d-galactose as the sole carbon source. Chromosomal DNA from four of these candidates was digested with HindIII, an enzyme which is unable to hydrolyze the transposon DNA itself, and religated. The religated DNA was transformed into Escherichia coli. One spectinomycin-resistant clone was isolated for each independent B. subtilis strain. The resulting plasmids were named p14, p17, p35, and p75. The plasmid DNA was sequenced with primers 333L (5′-CCCACTTATAAACAAAAGATCGG-3′) and 333R (5′-GGCCGATTCATTAATGCAGGGGG-3′). Both primers anneal to internal sequences of the mini-Tn10 transposon. The sequences were compared with the sequence of the SubtiList database (8). All transposons were integrated into the B. subtilis araE gene (p14, position 3483.424; p17, 3484.517; p35, 3484.349; p75, 3483.225 [nucleotide numbers are as given in reference 8]). The encoded protein is an arabinose transporter of B. subtilis. This indicates that AraE is directly or indirectly necessary for transport of d-galactose and d-xylose into B. subtilis Gal+. AraE is necessary for arabinose transport at low arabinose concentrations (11). At high arabinose concentrations, the AraNPQ transporter is sufficient to allow growth with arabinose as the sole carbon source. Our working hypothesis was that AraE is a transporter for several sugar molecules. l-arabinose, d-galactose, and d-xylose are of limited similarity. The simplest explanation for our results is that all three sugars are imported into the cell by the activity of AraE.
The d-galactose-positive phenotype is linked to araR.
If AraE is able to transport d-galactose and d-xylose into B. subtilis, a galactose- and xylose-positive phenotype should be obtained when araE, whose transcription is under the control of AraR (11), is expressed. AraE synthesis is repressed in the presence of glucose. This is in accordance with the fact that B. subtilis Gal+ grown in the presence of glucose is unable to transport d-galactose (Fig. 1). l-Arabinose-independent expression of araE is achieved by inactivating either araR or the AraR-binding sequence in front of araE (10). The regulatory gene araR is located next to araE (10). Due to the fact that there are no HindIII sites within araR, the entire gene is encoded on parts of the plasmids which were isolated by religating chromosomal DNA from the araE transposon insertions. We linearized plasmid p17 with HindIII, transformed it into B. subtilis 168, and selected for growth on d-xylose as the sole carbon source. Clones which were able to grow on d-xylose as the sole carbon source were obtained. This proves that the mutation which enables B. subtilis 168 to grow on d-xylose is closely linked to araE. To test whether an inactivation of araR is sufficient to obtain a galactose- and xylose-positive B. subtilis strain, we constructed plasmid pΩaraR by inserting the internal DraI/TaqI fragment of araR (positions 1173 to 1493 in reference 10) into AccI/SmaI-digested plasmid pSGMU2 (2). The resulting plasmid was transformed into B. subtilis 168. All tested chloramphenicol-resistant clones were able to grow on d-xylose as the sole carbon source. This proves that an inactivation of araR is sufficient to render B. subtilis 168 galactose and xylose positive.
B. subtilis 168 is able to utilize d-galactose and d-xylose in the presence of l-arabinose.
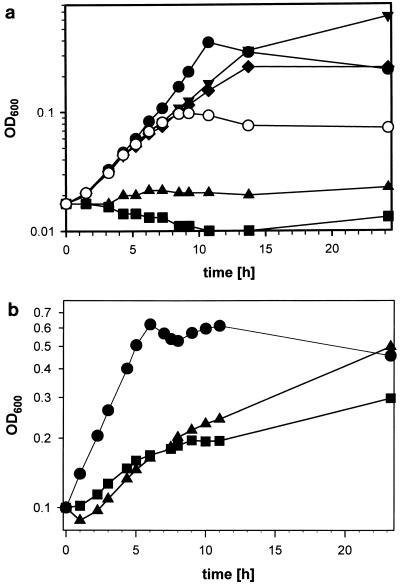
B. subtilis is able to use d-galactose and d-xylose as carbon sources when AraE is synthesized. d-Xylose, d-galactose, and l-arabinose are part of the cell wall of plants. We therefore hypothesized that, in nature, B. subtilis rarely encounters a situation in which d-galactose or d-xylose is the single carbon source. The likelihood is far greater that a mixture of several sugars is utilized as a carbon source. We therefore grew B. subtilis 168 either with 5 mM l-arabinose, d-galactose, or d-xylose as the sole carbon source or with 5 mM d-xylose or d-galactose in the presence of 1 mM l-arabinose (Fig. 2a). B. subtilis was able to grow at the expense of l-arabinose, but, as described previously (7, 13), we were unable to detect any growth with d-galactose or d-xylose as the sole carbon source. In the presence of inducing amounts of l-arabinose, B. subtilis 168 was able to utilize both d-galactose and d-xylose as carbon sources. Both sugars were also used as a carbon source when B. subtilis cells which were grown with l-arabinose as the sole carbon source were resuspended in media with either d-galactose or d-xylose as the sole carbon source (Fig. 2b). Therefore, it seems clear that B. subtilis is able to use both d-galactose and d-xylose as single carbon sources in nature. Possibly it streamlined its sugar uptake systems by creating a transporter with broad substrate specificity, the AraE protein. It seems that this protein is able to transport at least three structurally different sugar molecules, l-arabinose, d-galactose, and d-xylose. This is quite useful under natural conditions, because plant material is a polymeric mixture of different sugars. Natural rubber (Gummi arabicum) contains l-arabinose and d-galactose; hemicellulose, a major constituent of plant cell walls, contains mainly d-xylose and l-arabinose. Therefore, the presence of arabinose is quite often an indicator of the presence of xylose or galactose. The complex composition of natural C sources was not tested under simplified laboratory conditions. This artificial situation has given rise to the opinion that B. subtilis is unable to use two of the most important natural sugars.
FIG. 2.
Growth of B. subtilis 168 on different carbon sources. The optical density at 600 nm (OD600) is plotted against time. Circles, growth on 5 mM (closed symbols) or 1 mM (open symbols) arabinose; upward-pointing triangles, growth on 5 mM xylose; squares, growth on 5 mM galactose; downward-pointing triangles, growth on 5 mM xylose plus 1 mM arabinose; diamonds, growth on 5 mM galactose plus 1 mM arabinose. Cells were pregrown in MOPSO medium (9) with succinate (a) or arabinose (b) as the carbon source, washed, and resuspended in medium with the same carbon source.
Acknowledgments
This work was supported by the DFG (via Schwerpunkt “Molekulare Analyse von Regulationsnetzwerken in Bakterien”).
We thank K. Oliva for editing the manuscript.
REFERENCES
- 1.Dartois V, Djavakhishvili T, Hoch J A. Identification of a membrane protein involved in activation of the KinB pathway to sporulation in Bacillus subtilis. J Bacteriol. 1996;178:1178–1186. doi: 10.1128/jb.178.4.1178-1186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fort P, Errington J. Nucleotide sequence and complementation analysis of a polycistronic sporulation operon, spoVA, in Bacillus subtilis. J Gen Microbiol. 1985;131:1091–1105. doi: 10.1099/00221287-131-5-1091. [DOI] [PubMed] [Google Scholar]
- 3.Gärtner D, Geissendorfer M, Hillen W. Expression of the Bacillus subtilis xyl operon is repressed at the level of transcription and is induced by xylose. J Bacteriol. 1988;170:3102–3109. doi: 10.1128/jb.170.7.3102-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krispin O, Allmansberger R. The Bacillus subtilis galE gene is essential in the presence of glucose and galactose. J Bacteriol. 1998;180:2265–2270. doi: 10.1128/jb.180.8.2265-2270.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krüger S, Gertz S, Hecker M. Transcriptional analysis of bglPH expression in Bacillus subtilis: evidence for two distinct pathways mediating carbon catabolite repression. J Bacteriol. 1996;178:2637–2644. doi: 10.1128/jb.178.9.2637-2644.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krüger S, Hecker M. Regulation of the putative bglPH operon for aryl-β-glucoside utilization in Bacillus subtilis. J Bacteriol. 1995;177:5590–5597. doi: 10.1128/jb.177.19.5590-5597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindner C, Stülke J, Hecker M. Regulation of xylanolytic enzymes in Bacillus subtilis. Microbiology. 1994;140:753–757. doi: 10.1099/00221287-140-4-753. [DOI] [PubMed] [Google Scholar]
- 8.Moszer I, Glaser P, Danchin A. SubtiList: a relational database for the Bacillus subtilis genome. Microbiology. 1995;141:261–268. doi: 10.1099/13500872-141-2-261. [DOI] [PubMed] [Google Scholar]
- 9.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sa-Nogueira I, Mota L J. Negative regulation of l-arabinose metabolism in Bacillus subtilis: characterization of the araR (araC) gene. J Bacteriol. 1997;179:1598–1608. doi: 10.1128/jb.179.5.1598-1608.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sa-Nogueira I, Ramos S S. Cloning, functional analysis, and transcriptional regulation of the Bacillus subtilis araE gene involved in l-arabinose utilization. J Bacteriol. 1997;179:7705–7711. doi: 10.1128/jb.179.24.7705-7711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmiedel D, Hillen W. A Bacillus subtilis 168 mutant with increased xylose uptake can utilize xylose as sole carbon source. FEMS Microbiol Lett. 1996;135:175–178. [Google Scholar]
- 13.Steinmetz M. Carbohydrate catabolism: pathways, enzymes, genetic regulation, and evolution. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C: American Society for Microbiology; 1993. pp. 157–170. [Google Scholar]