Abstract
Background and Aims:
It is unknown whether young adults who vape nicotine and have poor mental health have greater risk of smoking initiation than expected based on individual risks of vaping and mental health alone. This study aims to estimate the joint association of vaping and mental health symptoms with smoking initiation among young adults, and test for additive interaction between vaping and mental health in smoking initiation risk.
Design:
Using five waves of the Population Assessment of Tobacco and Health (wave 1, 2013–2014; wave 2, 2014–2015; wave 3, 2015–2016; wave 4, 2016–2018; wave 5, 2018–2019), we estimated risk differences (RD) for the association of time-varying and time-lagged vaping and internalizing (e.g., anxiety, depressive) and externalizing (e.g., inattention/hyperactivity) mental health symptoms with cigarette smoking initiation at follow-up, over four 1-year intervals. We calculated interaction contrasts (IC) to estimate the excess risk of smoking initiation attributable to the interaction of vaping and mental health symptoms.
Setting:
United States.
Participants:
A total of 6,908 cigarette-naïve individuals aged 18–24 years.
Measurements:
Exposures included current (past-30 day) vaping and internalizing and externalizing mental health symptoms (high versus moderate/low symptoms). The outcome was smoking initiation (ever cigarette use) after one year.
Findings:
The per-interval risk of smoking initiation was 7.6% (1,039 cases/13,712 person-intervals). Compared to noncurrent vaping and moderate/low mental health symptoms, adjusted RDs for current vaping and high mental health symptoms were 17.2% (95%CI: 7.2% to 27.3%) for internalizing and 18.7% (95%CI: 8.1% to 19.2%) for externalizing symptoms. The excess risk attributed to interaction of current vaping and high externalizing symptoms was IC=11.3% (95%CI: 1.3% to 21.2%; p=0.018), with inconclusive findings for internalizing symptoms (IC=7.7% (95%CI: −2.2% to 17.7%; p=0.097).
Conclusions:
Findings indicate possible, but inconclusive, superadditivity between vaping and mental health in risk of smoking initiation, with implications for young adult smoking interventions.
Keywords: E-cigarette use, Mental Health, Cigarette Smoking, Interaction
INTRODUCTION
As cigarette smoking has declined in the United States (US) reaching historically low prevalence, the typical age of smoking initiation is occurring later.1,2 In the 20th Century, US smoking initiation nearly always occurred during adolescence.3 However, according to the National Survey on Drug Use and Health, the percentage of young adult smokers who initiated cigarette smoking between 18–23 years old increased from 20.6% in 2002 to 42.6% in 2018.1 This shift represents a positive transition; later age of smoking initiation is associated with reduced risk of nicotine dependence and long-term health effects.3 However, young adults have historically been overlooked in tobacco control efforts because of perceptions that nearly all smoking initiation begins in adolescence. Given national trends demonstrating a shift towards smoking initiation during early adulthood, identifying risk factors for young adult smoking initiation is critical to informing tobacco control efforts.4
Vaping5 and poor mental health6 have been identified as key risk factors for smoking initiation in early adulthood. Between 2014–2018, prevalence of current e-cigarette use among US adults 19–28 years old increased from 9% to 17%.7 There is a robust body of literature demonstrating a link between vaping and subsequent smoking initiation among young adults.5 Young adults who vape may smoke cigarettes to satisfy nicotine cravings, or because of normalized substance use behaviors.8 There may also be non-causal explanations for an association of vaping with smoking, including an underlying common liability for substance use. 5,9 Additionally, mental health has long been considered a potential cause of smoking initiation,6 and both internalizing (e.g., depression and anxiety) and externalizing (e.g., inattention/hyperactivity, antisocial behaviors) mental health symptoms increased among young adults over the last decade. For example, prevalence of major depressive disorder increased by 63% between 2009–2017 among US adults 18–25 years old.10 In a sample of ~5 million California adults, prevalence of ADHD diagnosis doubled between 2007–2016, and risk was greatest among individuals 18–24 years old.11 Young adults with mental health symptoms may smoke cigarettes as a form of self-medication, using nicotine for emotional regulation for internalizing problems,12 or behavioral control and cognitive enhancement for externalizing problems.13
While e-cigarettes and mental health have independently been identified as potential risk factors for smoking initiation, few studies have examined whether young adults who both vape nicotine and have poor mental health have greater risk of smoking initiation than would be expected based on the individual risks of e-cigarettes and poor mental health alone (i.e., synergism or superadditivity).14 It is plausible that interaction between vaping and mental health symptomology in smoking initiation risk may exist. Among young adults who use nicotine, those with mental health problems are more likely to develop nicotine dependence than those without mental health problems, even at low levels of nicotine exposure.15 Therefore, co-presentation (i.e., dual exposure) of e-cigarette use and mental health could lead to both greater nicotine dependence vulnerability and enhanced liability to seek out other forms of nicotine, including cigarettes. Understanding interactive effects of mental health symptoms and e-cigarette use on cigarette smoking initiation could inform interventions to prevent young adult smoking. If vaping and mental health confer a superadditive risk of smoking, interventions may seek to target populations of young adults who both vape and are suffering from mental health issues.
This study uses national cohort data to examine the synergistic effects of vaping and mental health symptoms in the risk of smoking initiation among US young adults 18–24 years old who have never smoked at baseline between 2013–2019. The aims were to (1) estimate the joint additive association of time-varying and time-lagged e-cigarette use and internalizing and externalizing mental health symptoms with subsequent incident cigarette smoking after one-year, and (2) quantify the interaction contrast (i.e., risk due to interaction) between vaping and mental health in risk of smoking initiation.
METHODS
Study Design
This study uses all five available waves from the adult cohort of the Population Assessment of Tobacco and Health (PATH) Study. PATH is a large national cohort study in which participants complete home-based computer-assisted personal interviews approximately 12-months apart on tobacco use and other health measures.16 PATH uses a four-stage stratified area probability sample design with oversampling of tobacco users, young adults, and Black/African-American individuals.16 At Wave 1 (2013–2014), 32,320 participants ≥18-years old were recruited into the adult cohort. Each subsequent wave includes participants originally recruited as adults, and participants who were recruited into the youth cohort and turned 18 over follow-up (i.e., “aged into” the adult cohort).
Data for the current study include adult cohort data at wave 1 (2013–2014), wave 2 (2014–2015), wave 3 (2015–2016), wave 4 (2016–2018), and wave 5 (2018–2019). Data were restructured into a long-form dataset in which each observation comprised an interval of two consecutive waves occurring approximately 12-months apart (i.e., Wave 1–Wave 2, Wave 2–Wave 3, Wave 3–Wave 4, and Wave 4–Wave 5). This nested trial design increases statistical efficiency by examining joint associations of e-cigarette use and mental health with smoking initiation at the subsequent wave simultaneously across four intervals.17
Participants
Participants were eligible for inclusion if at the “exposure” wave of each interval they 1) were 18–24yo; 2) had never smoked a cigarette; and 3) completed the survey at both the exposure and follow-up wave of the interval. Participants could contribute up to four person-intervals of observations if they met the inclusion criteria at the exposure wave of each interval. At wave 4, PATH included a replenishment sample to address study attrition among the wave 1 cohort and formed the new wave 4 cohort (33,822 adult participants). We included participants from the replenishment sample at wave 4, and youth who “aged into” the adult survey over follow-up. Prior to exclusions, there were 44,107 individual participants and 122,652 observations. After excluding observations without participation at consecutive exposure-outcome waves (n=17,091 ), >24yo (n=74,290), and ever smokers (n=17,559), the analytic sample included 6,908 individual participants and 13,712 person-intervals of observations (eFigure 1).
Measures
Internalizing and Externalizing Mental Health Symptoms
At each exposure wave, participants reported the last time they experienced significant problems with internalizing and externalizing mental health symptoms using the validated Global Appraisal of Individual Needs Short Screener (GAIN-SS).18,19 Internalizing problems (4-items) included depressive symptoms, sleep trouble, anxiety, and becoming distressed over the past. Externalizing problems (7-items) included lying/conning, not paying attention, not listening to instructions, bullying, physical fights, restlessness, and interrupting others. Response options for experiencing each individual symptom included a) past month, b) past 2–12 months, c) over a year ago, or d) never. Participants were defined as exhibiting the mental health symptom for the respective survey items if they selected past month or past 2–12 months, and symptoms were summed for two separate scores for internalizing and externalizing behaviors. Participants were subsequently classified as experiencing low (0–1), moderate (2–3), or high (≥4) internalizing/externalizing symptoms using previously established clinical thresholds.19,20 In primary analyses, internalizing and externalizing symptoms were dichotomized as high versus moderate or low symptoms to improve precision as in prior research.21
E-cigarette use
At each exposure wave, participants who reported ever using an e-cigarette were asked whether at the time of the survey, they used e-cigarettes a) everyday, b) some days, c) not at all. Current e-cigarette use was defined as currently using e-cigarettes everyday or some days.
Initiation of Combustible Cigarette Smoking
All participants had never smoked a cigarette at the exposure wave. At the follow-up wave of each interval, participants reported whether they ever smoked a combustible cigarette, even once or twice. As in prior studies,5,22,23 initiation of combustible cigarette smoking was defined as a report of ever smoking a cigarette at the follow-up wave of each interval. The outcome captures both experimenters and more regular smokers, however ‘ever cigarette use’ is a marker with adequate sensitivity (67%) and high specificity (92%) for predicting future progression to daily smoking.24
Covariates
We identified potential confounders, including time-invariant measures of sex at birth (female, male) and race/ethnicity as a proxy for social and/or structural racism that may influence mental health and substance use (non-Hispanic Black, non-Hispanic White, non-Hispanic all other races, Hispanic).25 More granular categories of race and ethnicity are not available in PATH public use data. We included time-varying measures of sexual identity (heterosexual, lesbian/gay/bisexual/other non-heterosexual identity [LGB+]), enrollment in a degree program (yes/no), employed at least part time (yes/no), living with a user of any type of tobacco product (yes/no), past 30-day use of: tobacco products other than cigarettes and e-cigarettes (yes/no), alcohol (yes/no), marijuana (yes/no), and illicit drugs (yes/no).
Analysis
We calculated the unadjusted risk of cigarette smoking initiation as the number of initiations per person-intervals of observation, and estimated risk by strata of mental health and e-cigarette use. We followed recommendations for presenting analyses of additive interaction,26 including calculating risk of smoking initiation and risk differences (RD) with confidence intervals (CI) for each stratum of mental health symptoms (high, moderate/low) and e-cigarette use (current, noncurrent) with a single reference category taken as the stratum with the hypothesized lowest risk of smoking initiation (i.e., moderate/low mental health symptoms and noncurrent e-cigarette use; “doubly unexposed”). We additionally calculated RDs for the association of high vs. moderate/low mental health symptoms within each stratum of e-cigarette use, and RDs for the association of current vs. noncurrent e-cigarette use within each stratum of mental health symptoms. To estimate the excess risk of smoking initiation attributable to the interaction between vaping and high mental health symptoms, we calculated the interaction contrast (IC; also known as the risk due to interdependence) and 95% CI for the IC based on stratum specific risks and variances.14 An IC>0 indicates superadditivity, or that the smoking risk in the doubly exposed is greater than the sum of the individuals risks for e-cigarette use and mental health alone.14
To estimate stratum-specific risks and RDs, we fit generalized estimating equations with a Poisson distribution, an identity link, and a repeated statement with an independent correlation matrix for robust standard errors (i.e., modified Poisson regression with sandwich error estimation).27 We fit unadjusted models, and sequentially adjusted multivariable models. Models were first adjusted for sex, race/ethnicity, enrollment in a degree program, employment status, and living with a tobacco product user. Models were subsequently adjusted for additional covariates that may act as confounders or mediators, depending on assumptions of temporal ordering, including past 30-day use of tobacco products other than cigarettes and e-cigarettes, alcohol, marijuana, illicit drugs, internalizing mental health symptoms (for externalizing models only), and externalizing symptoms (for internalizing models only). Adjustment for interval (i.e., exposure-wave) did not change estimates; thus interval was excluded as a covariate for model parsimony.
We do not incorporate PATH longitudinal survey weights, which require participation in all waves and would substantially reduce sample size. Representativeness is not always necessary to produce internally valid estimated associations.28 However, estimates should be interpreted as the effect in the survey population (not the US population).
In sensitivity analyses, we repeated primary analyses after restricting to participants with no past 30-day use of other tobacco products (to reduce potential for residual confounding) and after disaggregating moderate and low mental health symptoms. Participants who reported ever smoking at follow-up were asked how many cigarettes they ever smoked in their lifetime. In another sensitivity analysis, we reclassified participants as never smokers if they reported only ever smoking one or two puffs of a cigarette (i.e., never a whole cigarette). In another sensitivity analysis, we replaced e-cigarette exposure with cannabis use to assess whether associations are capturing common liability across substances, or if vaping has a unique association with later smoking. We tested for interaction by interval by examining a three-way interaction term (interval x mental health x e-cigarette use).
Restricting to never smokers at baseline could induce selection bias if vaping and mental health affect smoking at baseline and at follow-up (i.e., due to collider stratification, see eFigure 2).29 To address potential selection bias from conditioning on ever smoking at baseline, we constructed stabilized inverse probability of selection weights (IP-weights) using predicted probabilities from logistic regression models estimated among the full sample prior to excluding ever smokers (n=31,271 observations). The denominator was estimated by including never smoking at each exposure wave as the dependent variable, and predictors of smoking as independent variables (e-cigarette use, internalizing/externalizing mental health, and all covariates). The numerator was the probability of never smoking. IP-weighting creates a pseudopopulation in which participants selected into the study (i.e., never smokers) are weighted to account for themselves and for those with similar characteristics who were not selected (i.e., ever smokers), so that smoking status at baseline appears random with respect to exposures and measured covariates.29 We examined the distribution of e-cigarette use, mental health symptoms, and covariates by smoking status before and after IP-weighting.
We simulated missing values for covariates, exposures, and outcomes using Markov Monte Carlo method of multiple imputation with 10 imputed datasets. Data were missing for <2% of observations for all variables (eTable 1).
RESULTS
Among 6,908 participants who never smoked cigarettes (13,712 observations), 3.2% (n=440) reported current e-cigarette use, 18.5% (n=2,539) reported high internalizing mental health symptoms, and 20.2% (n=2,764) reported high externalizing mental health symptoms at exposure waves (Table 1). Participants with high (versus low) mental health symptoms were more likely to identify as LGB+, live with a tobacco user, and report past 30-day alcohol and drug use. Participants reporting current (versus noncurrent) e-cigarette use were more likely to be male, LGB+, live with a tobacco user, and report past 30-day tobacco, alcohol and drug use. eTable 2 presents covariate distribution across exposure waves. Over follow-up, 1,039 participants (7.6% per interval) initiated cigarette smoking (225 between W1-W2, 206 between W2-W3, 230 between W3-W4, and 378 between W3-W4) (eTable 3).
Table 1.
Baseline Covariates by Past 12-month Internalizing and Externalizing Symptoms and Current E-cigarette Use among 6,908 Young Adults 18–24yo who Never Smoked Cigarettes at Baseline, 2013–2019
| Internalizing Symptoms | Externalizing Symptoms | Current E-cigarette Use | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Covariates | Total | Low | Moderate | High | Low | Moderate | High | Yes | No |
| N observations | 13,712 | 7,613 | 3,560 | 2,539 | 7,022 | 3,926 | 2,764 | 440 | 13,272 |
|
| |||||||||
| N individual participants | 6,908 | 3,814 | 1,845 | 1,249 | 3,398 | 2,065 | 1,445 | 227 | 6,681 |
|
| |||||||||
| Time-invariant covariates a | |||||||||
| Female sex | 53.7 | 48.1 | 57.8 | 64.5 | 52.9 | 54.0 | 54.8 | 42.7 | 54.0 |
| Race/ethnicity | |||||||||
| NH Black | 18.4 | 19.3 | 17.9 | 16.3 | 20.8 | 17.0 | 14.8 | 14.5 | 18.5 |
| NH White | 45.1 | 44.0 | 46.0 | 47.0 | 40.5 | 47.8 | 52.0 | 52.0 | 44.8 |
| NH Asian or another race | 9.8 | 9.2 | 10.2 | 11.0 | 8.8 | 10.8 | 10.7 | 9.8 | 10.6 |
| Hispanic | 26.8 | 27.5 | 26.0 | 25.8 | 30.0 | 24.5 | 22.4 | 26.9 | 22.9 |
|
| |||||||||
| Time-varying covariates b | |||||||||
| Lesbian, Gay, Bisexual+ | 9.1 | 5.6 | 9.9 | 18.4 | 6.0 | 10.0 | 15.6 | 11.4 | 9.0 |
| Enrolled in a degree program | 49.8 | 48.2 | 53.8 | 48.7 | 45.0 | 54.1 | 55.7 | 49.8 | 49.8 |
| Employed at least part time | 62.3 | 63.6 | 61.6 | 59.3 | 62.8 | 63.0 | 59.8 | 69.6 | 62.0 |
| Lives with a tobacco user | 25.5 | 22.9 | 26.4 | 31.9 | 23.6 | 27.1 | 27.9 | 41.1 | 25.0 |
| Past 30-day other tobacco use | 10.6 | 10.9 | 10.1 | 10.7 | 10.5 | 10.3 | 11.6 | 53.4 | 9.2 |
| Past 30-day alcohol use | 38.2 | 34.7 | 42.6 | 42.6 | 32.8 | 42.4 | 46.2 | 58.0 | 37.6 |
| Past 30-day marijuana use | 10.6 | 9.0 | 11.7 | 14.1 | 8.2 | 12.2 | 14.7 | 34.3 | 9.9 |
| Past 30-day illicit drug use | 3.2 | 1.9 | 3.7 | 6.3 | 1.7 | 4.0 | 5.9 | 8.9 | 3.0 |
Estimates presented as unweighted column percentage of participants.
Estimates presented as unweighted column percentage of observations.
Additive Effect of E-cigarette use and Internalizing Mental Health Symptoms
The greatest unadjusted per-interval risk of smoking initiation was observed for participants who reported both current e-cigarette use and high internalizing mental health symptoms (“doubly exposed”, 31.5%); the lowest risk was among participants who reported no current e-cigarette use and moderate/low internalizing symptoms (“doubly unexposed”, 6.7%) (eTable 3). After adjusting for all confounders, RDs were 17.2% (95%CI: 7.2% to 27.3%) for participants who used e-cigarettes and reported high internalizing symptoms (compared to the doubly unexposed), 7.9% (95%CI: 3.1% to 12.7%) for participants who used e-cigarettes and reported moderate/low internalizing symptoms, and 1.6% (95%CI: 0.4% to 2.8%) for participants who were not current e-cigarette users and reported high internalizing mental health symptoms (Table 2).
Table 2.
Adjusted Additive Association of E-cigarette use and Mental Health Symptoms with Cigarette Smoking Initiation among 6,908 Young Adults 18–24yo who Never Smoked Cigarettes, 2013–2019
| Current E-cigarette use |
No Current E-cigarette use |
||||||
|---|---|---|---|---|---|---|---|
| Risk,%b | RD (95% CI)a | RD (95% CI)b | Risk,%b | RD (95% CI)a | RD (95% CI)b | RD (95% CI) for e-cig vs. no e-cig within mental health stratac | |
| Internalizing Symptoms d | |||||||
| High | 33.2 | 23.6 (13.4 to 33.7) | 17.2 (7.2 to 27.3) | 17.6 | 2.2 (1.0 to 3.4) | 1.6 (0.4 to 2.8) | 15.6 (5.5 to 25.7) |
| Moderate or Low | 23.9 | 13.0 (7.9 to 18.0) | 7.9 (3.1 to 12.7) | 16.0 | Reference | Reference | 7.9 (3.1 to 12.7) |
| RD (95% CI) for high vs. moderate/low symptoms within e-cig strata | - | 10.6 (−0.6 to 21.9) | 9.3 (−1.8 to 20.4) | - | 2.2 (1.0 to 3.4) | 1.6 (0.4 to 2.8) | - |
|
| |||||||
| Externalizing Symptoms e | |||||||
| High | 35.3 | 26.0 (15.3 to 36.7) | 18.7 (8.1 to 29.2) | 16.9 | 1.6 (0.5 to 2.8) | 0.3 (−0.8 to 1.4) | 18.3 (7.8 to 28.9) |
| Moderate or Low | 23.7 | 12.2 (7.3 to 17.0) | 7.1 (2.4 to 11.8) | 16.6 | Reference | Reference | 7.1 (2.4 to 11.8) |
| RD (95% CI) for high vs. moderate/low symptoms within e-cig strata | - | 13.8 (2.1 to 25.6) | 11.6 (0.1 to 23.1) | - | 1.6 (0.5 to 2.8) | 0.3 (−0.8 to 1.4) | - |
CI=Confidence Interval; E-cig=E-cigarette; RD=Risk Difference
Adjusted for sex, race/ethnicity, sexual identity, enrollment in degree program, employment status, living with a tobacco user
Adjusted for sex, race/ethnicity, sexual identity, enrollment in degree program, employment status, living with a tobacco user, past 30-day use of: other tobacco; cannabis; alcohol; any illicit drug, and internalizing mental health (for externalizing models), and externalizing mental health (for internalizing models).
Estimate is adjusted for all variables in footnote b. Partially adjusted risk difference e-cig vs. no e-cig among high internalizing symptoms=0.214 (0.112 to 0.316); externalizing symptoms=0.244 (0.137 to 0.351)
Interaction contrast (IC) for internalizing mental health=7.7% (95%CI: −2.2 to 17.7; p-value=0.096). IC calculated as R11- R10-R01+R00. Rii=risk of smoking initiation in each stratum of e-cigarette use and mental health.
IC for externalizing mental health=11.3% (95%CI: 1.3 to 21.2; p-value=0.018).
There was inconclusive evidence of additive interaction between e-cigarette use and internalizing symptoms. The individual risks associated with e-cigarette use and high internalizing mental health symptoms did not sum to the risk observed in the doubly exposed (IC>0; Figure 1). However, the IC estimate (7.7% (95%CI: −2.2 to 17.7%, p=0.097) was not statistically significant and the confidence interval was wide and included 0.
Figure 1.
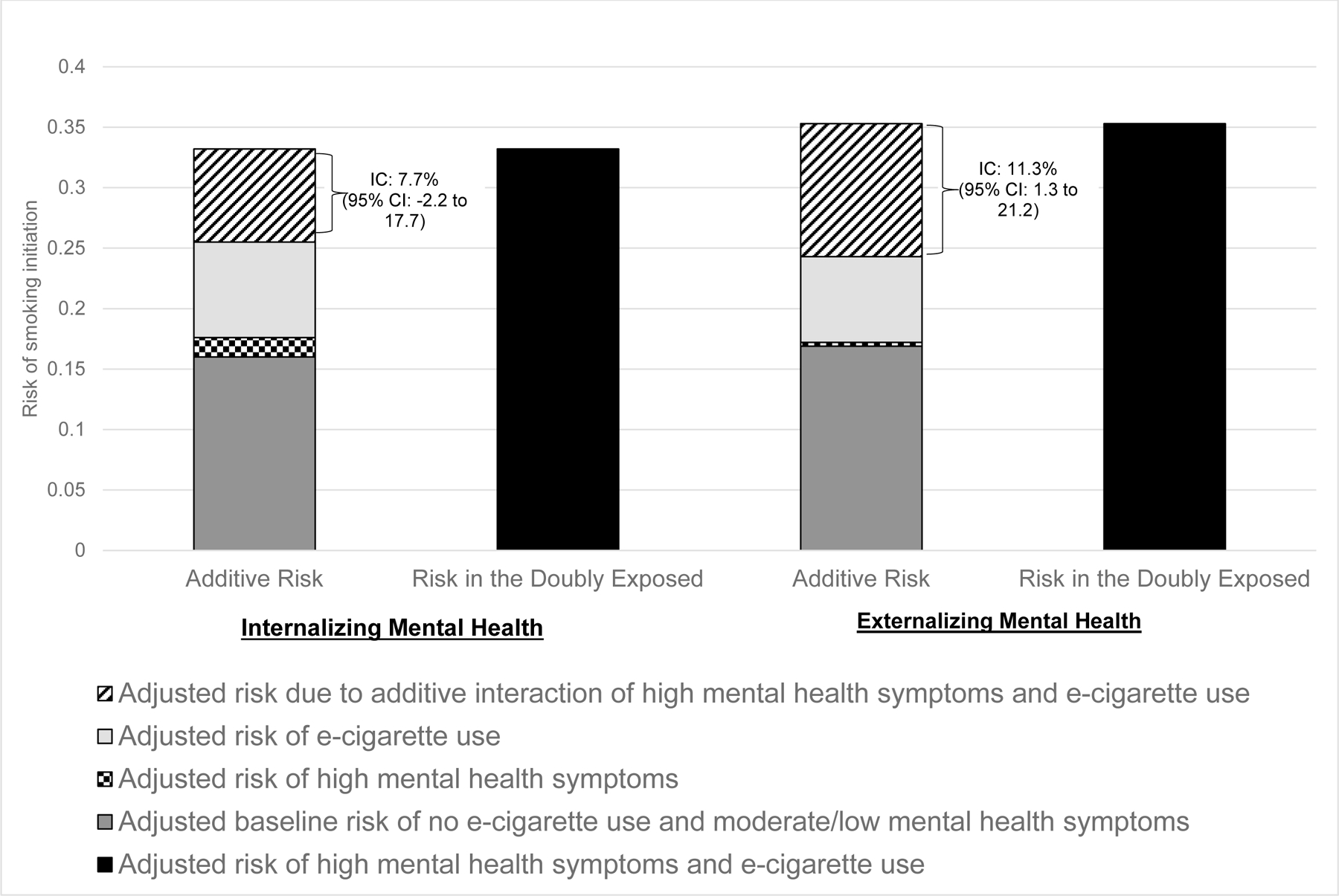
Additive Interaction of High Mental Health Symptoms and E-cigarette Use on Smoking Initiation among Young Adults in the United States, 2013–2019
IC=interaction contrast
Additive Effect of E-cigarette use and Externalizing Mental Health Symptoms
Results for externalizing mental health were similar to those for internalizing mental health. The unadjusted per-interval risk of smoking initiation was highest for participants who reported both current e-cigarette use and high externalizing symptoms (34.4%) and lowest among participants who reported no current e-cigarette use and moderate/low externalizing symptoms (6.7%) (eTable 3). Fully adjusted RDs were 18.7% (95%CI: 8.1% to 29.2%) for current e-cigarette users with high externalizing mental health symptoms (versus no current e-cigarette use and moderate/low externalizing symptoms), 7.1% (95%CI: 2.4% to 11.8%) for current e-cigarette users with moderate/low externalizing symptoms, and 0.3% (95%CI: −0.8% to 1.4%) for noncurrent e-cigarette users with high externalizing symptoms (Table 2). There was evidence of additive interaction between e-cigarette use and externalizing symptoms (Figure 1). The interaction between e-cigarette use and externalizing symptoms resulted in 11 excess cases of smoking initiation per 100 young adults (IC=11.3%, 95% CI: 1.3% to 21.2%, p=0.018). eTable 4a-b provides coefficients of all covariates included in multivariable models.
Secondary and Sensitivity Analyses
After excluding individuals who used other tobacco products at baseline (eTable 5) and reclassifying the outcome as ever smoking a whole cigarette (eTable 6), the RD patterns remained consistent with the original analysis; the greatest adjusted risk was observed among the doubly exposed. However, interaction contrast results were attenuated and became non-significant in these analyses.
In primary analyses, moderate and low mental health symptoms were collapsed to enhance precision. In analyses disaggregating moderate and low symptoms, results remained mostly unchanged. However, smoking initiation risk was slightly greater among current e-cigarette users with low versus moderate externalizing symptoms (eTable 7). When replacing e-cigarette use with cannabis use, risk differences and interaction contrasts were attenuated, suggesting that vaping may have a unique (and larger) association with smoking initiation beyond common substance liability (eTable 8). The interaction term p-values for interval x e-cigarette x mental health were p=0.1328 for internalizing and p=0.7370 for externalizing mental health, indicating no evidence that associations differed by exposure wave.
IP-weights (for selection bias) had a mean of 1.01 (range=0.47 to 34.5). E-cigarette use, mental health symptoms, and several covariates were strongly associated with baseline smoking status prior to IP-weighting. There were negligible differences in covariate distribution by smoking after weighting (eTable 9). In IP-weighted models, estimates in the doubly exposed group became stronger compared to the primary analysis, suggesting selection bias may have induced bias towards the null (Table 3). eTable 10 summarizes interaction contrast estimates for all models.
Table 3.
Adjusted and Inverse Probability of Selection Weighted Additive Effect of E-cigarette use and Mental Health Symptoms on Cigarette Smoking Initiation among 6,908 Young Adults 18–24yo who Never Smoked Cigarettes, 2013–2019
| Current E-cigarette Use |
Noncurrent E-cigarette Use |
|||
|---|---|---|---|---|
| Risk,%a | RD (95% CI)a | Risk,%a | RD (95% CI)a | |
| Internalizing Symptoms b | ||||
| High | 36.4 | 20.7 (15.5 to 25.9) | 16.9 | 1.3 (0.0 to 2.6) |
| Moderate or Low | 22.2 | 6.6 (4.0 to 9.2) | 15.6 | Reference |
|
| ||||
| Externalizing Symptoms c | ||||
| High | 42.2 | 26.5 (20.6 to 32.3) | 16.0 | 0.3 (−1.1 to 1.6) |
| Moderate or Low | 20.7 | 5.0 (2.5 to 7.4) | 15.7 | Reference |
CI=Confidence Interval; RD=Risk Difference
Adjusted for sex, race/ethnicity, sexual identity, enrollment in degree program, employment status, living with a tobacco user, past 30-day use of: other tobacco; cannabis; alcohol; any illicit drug, and internalizing mental health (for externalizing models), and externalizing mental health (for internalizing models). Additionally weighted by inverse probability of never smoking a cigarette at baseline.
Interaction contrast (IC) for internalizing mental health=12.9% (95%CI: −0.3 to 26.1; p-value=0.045). IC calculated as R11- R10-R01+R00. Rii=risk of smoking initiation in each stratum of e-cigarette use and mental health.
IC for externalizing mental health=21.2% (95%CI: 8.6 to 33.8; p-value=0.003).
DISCUSSION
In this prospective cohort study of young adults in the US who never smoked cigarettes at baseline, synergistic (i.e., superadditive) interaction between e-cigarette use and mental health in risk of cigarette smoking initiation was observed for externalizing mental health symptoms. In other words, the excess risk of smoking initiation among participants who used e-cigarettes at the time of the survey and reported high levels of adverse externalizing mental health symptoms in the past year was greater than could be explained by the individual risks associated with e-cigarette use, mental health symptoms, and the baseline risk of smoking initiation.
The excess risk of smoking initiation associated with e-cigarette use alone was greater than the risk associated with mental health alone. For example, e-cigarette use was associated with a >7% increase in the risk of cigarette smoking, while high internalizing and high externalizing mental health symptoms (separately) were associated with <2% increased risk of smoking initiation. Results are consistent with prior studies that find a strong association of e-cigarette use with smoking initiation among youth and young adults,5,30 even after accounting for numerous sources of potential bias, including time-dependent confounding, misclassification, and selection bias.22 Several prior studies used PATH data to examine associations of internalizing and externalizing mental health symptoms with tobacco product initiation among youth and/or young adults.20,21,31 In two studies among youth (<18y), both internalizing and externalizing mental health were strong predictors of smoking initiation.20,31 However, one prior study that combined youth and young adult populations (12–24y) similarly found a small effect for internalizing mental health and smoking, and little association between externalizing mental health and smoking.21
We observed that the smoking risk associated with e-cigarette use was greater among participants with high mental health symptoms than those with moderate or low mental health symptoms. This is inconsistent with three prior studies which show the relative risk of smoking initiation for e-cigarette use is greater among lower-risk and less smoking-susceptible youth.32–34 While one study included a measure of rebelliousness into their susceptibility construct (similar to externalizing symptoms),33 none of the prior studies included measures of internalizing mental health symptoms when assessing smoking susceptibility. Additionally, all three prior studies utilized relative measures of associations, and higher relative risks among lower-risk youth may be at least partially the result of a lower baseline risk of smoking in lower-risk youth.
Despite small independent effects of mental health symptomology on cigarette smoking initiation, externalizing symptoms appeared to interact with e-cigarette use to produce a greater risk of smoking initiation than can be explained by the individual risks alone. Interaction results for internalizing symptoms were not statistically significant. One potential mechanism underlying a causal association between e-cigarette use and smoking initiation is the development of nicotine dependence.8 Though the presence of additive interaction does not necessarily correspond to physical biologic interactions,35 nicotine dependence and mental health disorders do share underlying biologic pathways.36 For example, dopaminergic agents like the antidepressant buprioprion are used to treat both nicotine dependence and depression, and both cigarette smoking and depression are associated with decreased serotonin function.37 Additionally, psychiatric patients with nicotine dependence exhibit greater severity of mental health symptoms than patients without nicotine dependence,38 and in turn, smokers with mental health disorders (versus those without mental health disorders) often exhibit greater nicotine dependence symptoms.39,40 Thus it is plausible that the combined effect of vaping nicotine and mental disorders results in both greater nicotine dependence and severity of mental health symptoms, which work together to increase risk of smoking initiation. Investigating the mechanisms of our findings are beyond the scope of the current paper, but are an important future direction of this research. Additionally, it is possible there are non-causal explanations for the observed interaction effects, including the potential for residual confounding by unmeasured factors or reverse causation (e.g., vaping as a marker for future smoking).41 Interaction contrasts substantially attenuated in several sensitivity analyses, but became stronger when accounting for potential selection bias. IC confidence intervals were also wide. Additional studies are needed to confirm the presence and magnitude of interaction between vaping and mental health in risk of smoking initiation.
National trends showing a shift towards young adult smoking initiation highlight the importance of developing smoking interventions for young adults.1 Results of this study indicate that young adults who both vape and suffer from mental health problems may be an important target population for smoking interventions. When considering smoking interventions, program developers might consider incorporating both vaping and mental health components, and discussion of how the two factors may work together to increase risk. Focusing on vaping without consideration of mental health (and vice versa) may result in a loss of important information on how the two might interact to increase risk.42
This study has limitations. Estimation of interaction contrasts requires assumptions of three-way exchangeability, or no confounding. Residual confounding by unmeasured factors such as mental health treatment or symptom severity could partially or completely explain the observed interaction effects. Additionally, we were unable to adjust for age, as PATH public use files provide a categorical age variable that groups participants 18–24 years old together. There may also be misclassification of self-reported mental health symptoms, e-cigarette use, and cigarette smoking initiation. We did not incorporate measures of vaping or smoking frequency or intensity into this analysis, and PATH does not include validated measures for specific mental health disorders (e.g., generalized anxiety, major depression). Additionally, our sample was not adequately powered to examine interaction effects on transitions to established smoking (e.g., >100 lifetime cigarettes). Replicating this study using longer follow-up durations and transitions to regular smoking is an important direction for future research. There is also heterogeneity in e-cigarette product characteristics and nicotine concentration which we were not able to examine in this study. Exposure misclassification is likely to be non-differential with respect to the outcome given the prospective design, though outcome misclassification may be differential if participants who vape or report mental health symptoms have different probabilities of misreporting cigarette smoking. Additionally, while we addressed selection bias due to baseline smoking restrictions, selection bias could occur if loss to follow-up is associated with e-cigarette and mental health exposures and future smoking risk. However, our previous PATH study on e-cigarette use and smoking initiation found little evidence of selection bias due to loss-to-follow-up.25 Confidence intervals for interaction contrasts and some risk differences were imprecise, and some or all of the associations may be explained by random error. Furthermore, the magnitude of interaction contrasts attenuated in some sensitivity analyses. However, the greatest smoking risk was consistently observed among participants with high mental health symptoms and e-cigarette use in all sensitivity analyses. Finally, results may not generalize to other populations.
The shift from smoking initiation during adolescence towards young adulthood in the US warrants consideration of new smoking prevention programs that move away from traditional high school programs and target young adult populations. Young adults who suffer from mental health problems and also vape nicotine may be particularly at-risk for cigarette smoking initiation. Future research could examine underlying pathways through which e-cigarette use and mental health may interact to produce an excess risk of smoking initiation, and whether certain vaping device types or additives exacerbate risk further to inform regulations of products.
Supplementary Material
Source of funding:
Research was supported by the National Cancer Institute (NCI) and the FDA Center for Tobacco Products (CTP) under Award Number U54CA180905, NCI under award number R01CA229617, and the National Institute on Drug Addiction (NIDA), under award numbers K01DA042950 and K01DA058084. The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Declaration of competing interests: None declared
References
- 1.Barrington-Trimis JL, Braymiller JL, Unger JB, et al. Trends in the Age of Cigarette Smoking Initiation Among Young Adults in the US From 2002 to 2018. JAMA Netw Open 2020;3(10):e2019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harlow AF, McConnell R, Leventhal AM, Goodwin RD, Barrington-Trimis JL. Racial, Ethnic, and Education Differences in Age of Smoking Initiation Among Young Adults in the United States, 2002 to 2019. JAMA Netw Open 2023;6(3):e235742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2012. [Google Scholar]
- 4.Ganz O, Delnevo CD. Young Adults as a Tobacco Control Priority Population in the US. JAMA Netw Open 2020;3(10):e2019365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khouja JN, Suddell SF, Peters SE, Taylor AE, Munafò MR. Is e-cigarette use in non-smoking young adults associated with later smoking? A systematic review and meta-analysis. Tob Control 2020;30(1):8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paperwalla KN, Levin TT, Weiner J, Saravay SM. Smoking and depression. Med Clin 2004;88(6):1483–1494. [DOI] [PubMed] [Google Scholar]
- 7.Schulenberg J, Johnston L, O’Malley PM, Bachman JG, Miech R, Patrick M. Monitoring the Future National Survey Results on Drug Use, 1975–2018: Volume II, College Students and Adults Ages 19–60 The University of Michigan; 2018. [Google Scholar]
- 8.Schneider S, Diehl K. Vaping as a Catalyst for Smoking? An Initial Model on the Initiation of Electronic Cigarette Use and the Transition to Tobacco Smoking Among Adolescents. Nicotine Tob Res 2016;18(5):647–653. [DOI] [PubMed] [Google Scholar]
- 9.Khouja JN, Wootton RE, Taylor AE, Smith GD, Munafò MR. Association of genetic liability to smoking initiation with e-cigarette use in young adults: A cohort study. PLOS Med 2021;18(3):e1003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twenge JM, Cooper AB, Joiner TE, Duffy ME, Binau SG. Age, period, and cohort trends in mood disorder indicators and suicide-related outcomes in a nationally representative dataset, 2005–2017. J Abnorm Psychol 2019;128(3):185–199. [DOI] [PubMed] [Google Scholar]
- 11.Chung W, Jiang SF, Paksarian D, et al. Trends in the Prevalence and Incidence of Attention-Deficit/Hyperactivity Disorder Among Adults and Children of Different Racial and Ethnic Groups. JAMA Netw Open 2019;2(11):e1914344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leventhal AM, Zvolensky MJ. Anxiety, Depression, and Cigarette Smoking: A Transdiagnostic Vulnerability Framework to Understanding Emotion-Smoking Comorbidity. Psychol Bull 2015;141(1):176–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClernon FJ, Kollins SH. ADHD and smoking: from genes to brain to behavior. Ann N Y Acad Sci 2008;1141:131–147. doi: 10.1196/annals.1441.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenland S, Lash T, Rothman KJ. Concepts of Interaction. In: Modern Epidemiology 3rd ed. Lippincott Williams & Wilkins; 2008:71–83. [Google Scholar]
- 15.Dierker L, Donny E. The role of psychiatric disorders in the relationship between cigarette smoking and DSM-IV nicotine dependence among young adults. Nicotine Tob Res 2008;10(3):439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyland A, Ambrose BK, Conway KP, et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob Control 2017;26(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiol 2008;19(6):766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stucky BD, Edelen MO, Ramchand R. A psychometric assessment of the GAIN individual severity scale (GAIN-GISS) and short screeners (GAIN-SS) among adolescents in outpatient treatment programs. J Subst Abuse Treat 2014;46(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dennis ML, Chan YF, Funk RR. Development and validation of the GAIN Short Screener (GSS) for internalizing, externalizing and substance use disorders and crime/violence problems among adolescents and adults. Am J Addict 2006;15 Suppl 1(Suppl 1):80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riehm KE, Young AS, Feder KA, et al. Mental Health Problems and Initiation of E-cigarette and Combustible Cigarette Use. Pediatrics 2019;144(1):e20182935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green VR, Conway KP, Silveira ML, et al. Mental Health Problems and Onset of Tobacco Use Among 12- to 24-Year-Olds in the PATH Study. J Am Acad Child Adolesc Psychiatry 2018;57(12):944–954.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harlow AF, Stokes AC, Brooks DR, Benjamin EJ, Barrington-Trimis JL, Ross CS. e-Cigarette Use and Combustible Cigarette Smoking Initiation Among Youth: Accounting for Time-Varying Exposure and Time-Dependent Confounding. Epidemiol Camb Mass 2022;33(4):523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harlow AF, Lundberg D, Raifman JR, et al. Association of Coming Out as Lesbian, Gay, and Bisexual+ and Risk of Cigarette Smoking in a Nationally Representative Sample of Youth and Young Adults. JAMA Pediatr 2020;175(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sargent JD, Gabrielli J, Budney A, Soneji S, Wills T. Adolescent Smoking Experimentation as a Predictor of Daily Cigarette Smoking. Drug Alcohol Depend 2017;175:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones CP. Invited commentary: “race,” racism, and the practice of epidemiology. Am J Epidemiol 2001;154(4):299–304; discussion 305–306. [DOI] [PubMed] [Google Scholar]
- 26.Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol 2012;41(2):514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159(7):702–706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 28.Rothman KJ, Greenland S, Lash T. Validity in Epidemiologic Studies. In: Modern Epidemiology 3rd ed. Lippincott Williams & Wilkins; 2008:146–147. [Google Scholar]
- 29.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiol Camb Mass 2004;15(5):615–625. [DOI] [PubMed] [Google Scholar]
- 30.Soneji S, Barrington-Trimis JL, Wills TA, et al. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatr 2017;171(8):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buu A, Hu YH, Wong SW, Lin HC. Internalizing and Externalizing Problems as Risk Factors for Initiation and Progression of E-cigarette and Combustible Cigarette Use in the US Youth Population. Int J Ment Health Addict 2021;19(5):1759–1771. [Google Scholar]
- 32.Barrington-Trimis JL, Urman R, Berhane K, et al. E-Cigarettes and Future Cigarette Use. Pediatrics 2016;138(1):e20160379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wills TA, Sargent JD, Gibbons FX, Pagano I, Schweitzer R. E-cigarette use is differentially related to smoking onset among lower-risk adolescents. Tob Control 2016;26(5):534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aleyan S, Cole A, Qian W, Leatherdale ST. Risky business: a longitudinal study examining cigarette smoking initiation among susceptible and non-susceptible e-cigarette users in Canada. BMJ Open 2018;8(5):e021080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanderWeele TJ, Robins JM. The identification of synergism in the sufficient-component-cause framework. Epidemiol Camb Mass 2007;18(3):329–339. [DOI] [PubMed] [Google Scholar]
- 36.Nunes SOV, Vargas HO, Prado E, et al. The shared role of oxidative stress and inflammation in major depressive disorder and nicotine dependence. Neurosci Biobehav Rev 2013;37(8):1336–1345. [DOI] [PubMed] [Google Scholar]
- 37.Malone KM, Waternaux C, Haas GL, Cooper TB, Li S, Mann JJ. Cigarette smoking, suicidal behavior, and serotonin function in major psychiatric disorders. Am J Psychiatry 2003;160(4):773–779. [DOI] [PubMed] [Google Scholar]
- 38.Leventhal AM, Kahler CW, Ray LA, Zimmerman M. Refining the depression-nicotine dependence link: patterns of depressive symptoms in psychiatric outpatients with current, past, and no history of nicotine dependence. Addict Behav 2009;34(3):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lechner WV, Janssen T, Kahler CW, Audrain-McGovern J, Leventhal AM. Bi-directional associations of electronic and combustible cigarette use onset patterns with depressive symptoms in adolescents. Prev Med 2017;96:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kutlu MG, Gould TJ. Nicotine modulation of fear memories and anxiety: Implications for learning and anxiety disorders. Biochem Pharmacol 2015;97(4):498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun R, Mendez D, Warner KE. Is Adolescent E-Cigarette Use Associated With Subsequent Smoking? A New Look. Nicotine Tob Res 2022;24(5):710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.VanderWeele TJ. On the distinction between interaction and effect modification. Epidemiol 2009;20(6):863–871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


