ABSTRACT
Research on membranous nephropathy truly exploded in the last 15 years. This happened because of the application of new techniques (laser capture microdissection, mass spectrometry, protein G immunoprecipitation, arrays) to the study of its pathogenesis. After the discovery of PLA2R as the major target antigen, many other antigens were identified and others are probably ongoing. Clinical and pathophysiology rebounds of new discoveries are relevant in terms of diagnosis and prognosis and it is time to make a first assessment of the innovative issues. In terms of classification, target antigens can be divided into: ‘membrane antigens’ and ‘second wave’ antigens. The first group consists of antigens constitutionally expressed on the podocyte membrane (as PLA2R) that may become a target of an autoimmune process because of perturbation of immune-tolerance. ‘Second wave’ antigens are antigens neo-expressed by the podocyte or by infiltrating cells after a stressing event: this allows the immune system to produce antibodies against them that intensify and maintain glomerular damage. With this abundance of target antigens it is not possible, at the moment, to test all antibodies at the bedside. In the absence of this possibility, the role of histological evaluation is still irreplaceable.
Keywords: glomerulonephritis, membranous nephropathy, oxidative systems, phospholipase-A2, superoxide dismutase
INTRODUCTION
Membranous nephropathy (MN) is the most common cause of nephrotic syndrome among Caucasian adults while it rarely occurs in the pediatric setting. It is an autoimmune condition characterized by the deposition of anti-podocyte antibodies on the subepithelial layer of the glomerular capillary wall that leads to the thickening of the glomerular basement membrane, complement activation and glomerular capillary injury with consequent proteinuria.
While around the 75% of patients with MN have the kidney as unique manifestation and have been classified as ‘idiopathic’ and then ‘primary’ after those specific antigens were identified, another 25% of cases need to be defined as ‘secondary’ because they are contemporarily associated with a causative disease, such as malignancies, infections, drug reactions, or systemic autoimmune diseases including systemic lupus erythematosus.
In past decades, the research on the pathogenesis of MN was characterized by a ‘wave’ trend, switching cycles of enthusiasm and disappointment. In the early 1960s, Heymann et al. presented an animal model able to closely reproduce the human disease, known as ‘Heymann nephritis’ (HN) [1], that boosted the research activity on MN worldwide. This model was useful to define a few fundamental aspects of pathogenesis: (i) the in situ formation of immune-complexes against the podocyte antigen megalin; (ii) the fundamental role of complement activation in glomerular damage; and (iii) the podocyte as the main and unique target of the autoimmune reaction. After that, different animal models of MN were proposed, each one having its own podocyte target antigen. However, circulating antibodies against podocyte antigens described as pathological in animal models (including megalin in HN), were not reported in human MN. For several decades, researchers worldwide failed to identify the podocyte antigens involved in the pathogenesis of human MN, therefore the disease maintained the definition of idiopathic [2].
In 2002, the lights were turned on again: Hanna Debiec et al. identified neutral endopeptidase (NEP) as the target antigen in an ante-natal form of MN [3]. During a first pregnancy, a mother genetically deficient in NEP, developed circulating anti-NEP antibodies that crossed the placenta and targeted NEP on the fetal kidney (possessing NEP) during her subsequent pregnancy, leading to nephrotic syndrome. However, this is an ultra-rare event, only a few other cases were subsequently described and a clinical screening on a large cohort of patients on circulating anti-NEP resulted as negative [4, 5]. The serendipity of this discovery was that NEP was the first identified human antigen in MN, but the pathogenesis was still far from being elucidated.
Identification of IgG4 autoantibodies against M-type phospholipase A2 receptor type 1 (PLA2R) and, later, against thrombospondin type 1 domain containing 7A (THSD7A), two podocyte-expressed proteins, represented a fundamental major step forward in defining the disease pathogenesis [6–8]. Recently, circulating antibodies targeting several other membrane antigens have been described in a limited number of patients representing further evolution on mechanisms of MN [8, 9]. MN associated with antibodies targeting specific glomerular antigens and without any other manifestation is now classified as primary.
However, several issues are still to be clarified. Among others, the natural history of the disease is extremely variable and it is only partially correlated with the presence and/or the levels of circulating autoantibodies. The hypothesis that further adaptive immune processes, linked to the development of antibodies blocking reparative processes, such as oxidative protection and macrophages, and how they may be involved in the pathogenesis of MN represent the main topic of the present review [10].
PLA2R1/anti-PLA2R1, the historical discovery
In the past 20 years, the development of new technologies played an essential role in the research on MN: in particular, laser capture micro-dissection allowed glomeruli to be obtained from renal biopsies and consented to elute only antibodies deriving from glomeruli. They were then tested against protein extracts of podocytes previously separated by two-dimensional electrophoresis (in non-denaturing and denaturing conditions) and analysed by mass spectrometry. With separation of non-denatured glomerular extracts, Beck et al. [6] identified PLA2R as the antigen responsible for 60–70% of cases of MN. Actually, PLA2R is identifiable also in glomeruli of healthy kidneys but in MN patients it probably delocalizes and co-stains with IgG in immune deposits [11, 12]. PLA2R is a type I transmembrane glycoprotein with a molecular mass of about 180 kDa that belongs to the mannose receptor family. Membrane PLA2R has an extracellular portion consisting of a NH2-terminal cysteine-rich domain, a fibronectin-like type II (FNII) domain, a tandem repeat of 8 C-type lectin-like domains (CTLD) and a short intracellular COOH-terminal region that acts as an internalization sequence [13]. Its biological function is not completely revealed, but experimental data suggest that PLA2R is involved in apoptosis, being a potential tumor suppressor gene [14]. The binding of circulating PLA2 IB (formerly identified as a digestive protein for its abundance in the pancreas) with PLA2R in glomeruli induces podocyte apoptosis via the ERK1/2-cPLA2α-AA-p53 signaling pathway. Another possible function of podocyte PLA2R is the interaction with collagen IV that promotes cellular adhesion to the GBM and anti-PLA2R may limit this binding [15, 16]. These findings suggest that the over-expression of podocyte PLA2R and/or its interaction with anti-PLA2R might contribute to kidney damage via podocyte apoptosis and/or to disarrangement of the glomerular filtration barrier.
As previously reported, circulating anti-PLA2R antibodies are found positive at diagnosis in about two thirds of patients with MN, with some differences according to the different study cohorts [17]. Therefore, the detection of anti-PLA2R antibodies, both in sera or in glomeruli, emerged as a fundamental tool to differentiate primary and secondary MN forms and for distinguishing MN from other glomerulonephritis. Many authors supported the opportunity to avoid kidney biopsy with the positivity of circulating anti-PLA2R antibodies, like in the most recent KDIGO guidelines [18]. This seems supported by many data since anti-PLA2R were almost negative in nephropathies different from MN; however, with the more and more widespread use of antibody testing, some authors reported sporadic non-specific positivities, such as in diabetic nephropathy, potentially due to technical problems with the ELISA test [19, 20]. The conclusion is that kidney biopsy is still fundamental to define MN and to characterize the histological score, in terms of extension of glomerual basement membrane deposits.
Since the first reports, many cohorts were tested to evaluate the potential prognostic power of the anti-PLA2R antibodies in respect of remission or recurrence, anticipating therefore the clinical manifestations of nephrotic syndrome. Another point of interest was whether both the presence and levels of anti-PLA2R antibodies could be used to decide on therapies. Overall, results from studies were not definitive [21]. In a recent report from the Netherlands [22], two cohorts of anti-PLA2R pos + MN patients were treated with the same standard protocols based on a six-month regimen with lower cyclophosphamide doses: circulating anti-PLA2R antibodies were evaluated every two months and the treatment was discontinued in a part of patients (the experimental cohort) if antibodies resulted negative. The rate of remission resulted similar in patients with protracted therapy and in those with anti-PLA2R antibody guided treatment suggesting the possibility to limit immunodepression on this basis. However, in the experimental cohort, a few patients showed a ‘clinical-serological dissociation’, both being anti-PLA2R negative but still nephrotic or in clinical remission but with circulating antibodies.
Beside the above-reported clinical limits of anti-PLA2R antibodies, several pathological mechanisms are still to be clarified. Among others, the reasons why only some subjects develop antibodies against PLA2R, that is an antigen commonly expressed in the kidney.
Attempts to reproduce PLA2R-induced MN in rats were only partially successful, as PLA2R1 is not expressed on podocytes membrane in rodents. In 2023, Tomas et al. [23] engineered a transgenic model where human PLA2R was expressed on mice podocytes. Animals spontaneously developed massive proteinuria and typical renal lesions of MN after four weeks on an immulogic basis, since de novo MN was completely abolished in Rag2-deficient mice that lack both mature B and T cells. Two issues in transgenic mice for human PLA2R remain to be explained: the first is why all mice develop MN that does not fit with the heterogeneity of human MN, the second is that circulating anti-human PLA2R do not recognize mouse-PLA2R, thus leading to consideration of a mechanism of xeno-immunization and not a proper model of human-PLA2R-membranous nephropathy.
Novel membrane antibodies in the clinical contests
After a few years from the first report on anti-PLA2R, in 2014, THSD7A was identified in a few cases of negative-PLA2R MN [7]. THSD7A is a 250-kDa multidomain trans-membrane protein expressed by podocytes in healthy conditions, and in analogy with PLA2R, the expression pattern of THSD7A changes in MN patients, becoming more granular and acquiring the co-localization with IgG in sub epithelial immune-deposits. Anti-THSD7A were found positive in about 3–5% of MN. Of interest, circulating anti-THSD7A resulted positive only in anti-PLA2R negative patients and, moreover, human anti-THSD7A can react with podocytes THSD7A in mice. In fact, only rare reports (overall, six cases) described the coexistence of THSD7A and PLA2R in immune deposits of the same patient [21], suggesting that they are independent in inducing MN lesions, probably based on different pathogenetic mechanisms. Differently to anti-PLA2R, anti-THSD7A antibodies are often associated with malignancies and neoplastic cells may express THSD7A. The ectopic expression of this protein may activate a ‘non-self’ immunization with consequent antibodies production and deposition on podocytse. The homology between mouse and human THSD7A is important because the pathogenetic involvement of anti-THSD7A was easily demonstrated, inducing MN in mice with the sole injections of human purified antibodies.
More recently, a bunch of different glomerular antigens have been described in MN. This happened because the technologies utilized in identifications of new antigens have been simplified in two steps, protein G immunoprecipitation of frozen kidney tissue and characterization by mass spectrometry [8]. Subsequent tissue co-localization with IgG acts as the validation test. A probably not fully comprehensive list of all MN antigens is: neural epidermal growth factor-like 1 (NELL1), exostosin 1/2 (EXT1/2), serine protease HTRA1, protocadherin 7A (PCDH7), semaphorin 3B (SEMA3B), neural cell adhesion molecule-1 (NCAM1), transforming growth factor beta receptor 3 (TGFBR3), protocadherin FAT1 (PCDH FAT1), contactin 1 (CNTN1), neural cell adhesion molecule-1 (NCAM1), netrin G1, vasorin (VASN), ficolin 3 (FCN3), macrophage stimulating 1 (MST1), natriuretic peptide receptor 3 (NPR3), early endosome antigen 1 (EEA1), cluster of differentiation 206 (CD206), seizure-related 6 homolog like 2 (SEZ6L2), proprotein convertase subtilisin/kexin type 6 (PCSK6) and neuron-derived neurotropic factor (NDNF) [8, 24–30] (Table 1).
Table 1:
Clinical and histological features of membrane antigens targets of autoimmunity in membranous nephropathy.
| Antigen | Prevalence in iMN (%) | Associations | Neo-expressed Ag | IgG subclass | Serum antibody in iMN |
|---|---|---|---|---|---|
| PLA2R | 65% | Rare with secondary MN | No | IgG4 | Yes |
| THSD7A | 1–3% | Cancer 10% | No | IgG4 | Yes |
| NELL1 | 1–2% | Cancer 30% | Yes | IgG1 | Yes |
| HTRA1 | 1% | unknown | No | IgG4 | Yes |
| PCDH7 | 1% | Cancer 21% Autoim. MN 14% |
Yes | IgG4/IgG1 | Yes |
| SEMA3B | <1% | Pediatric MN | No | IgG1 | Yes |
| EXT 1/2 | 2–3% | Lupus MN 17–38% |
Yes | IgG1 preval. | No |
| NCAM1 | 0.5–2% | Lupus MN 6% | No | IgG1 preval. | Yes |
| TGFBR3 | 0% | Lupus MN 5% | No | IgG1, IgG2, IgG3, IgG4 | No |
| FCN3 | 0.2%a | Lupus MN 2.8% | Yes | Unknown | Unknown |
| CD206 | 1 case | None | Yes | Unknown | Unknown |
| EEA1 | 1.2%a | Lupus MN 4.9% | Yes | Variable | Unknown |
| SEZ6L2 | 0.2%a | None | Yes | IgG4 > IgG1 | Unknown |
| NPR3 | 1 case | None | Yes | Unknown | Unknown |
| MST1 | 1.2%a | Lupus MN 2.1% | Yes | IgG1 > gG4 | Unknown |
| VASN | 1.5%a | Lupus MN 11% | Yes | IgG1 | Unknown |
| PCSK6 | 0.7%a | NSAID use | Yes | IgG4/IgG1 | Unknown |
| NDNF | 0.4%a | syphilis | Yes | IgG1, IgG2, IgG3 | Unknown |
means that prevalence was evaluated only in one case series.
Table 2:
Clinical and histological features of ‘second-wave’ antigens targets of autoimmunity in membranous nephropathy.
| Antigen | Prevalence in MN (%) | Associations | Neo-expressed podocyte antigen | IgG subclass | Serum antibody in MN |
|---|---|---|---|---|---|
| AR | 32% | Unknown | Yes | IgG4 | Yes |
| SOD2 | 28% | Unknown | Yes | IgG4 | Yes |
| AENO | 41% | Rheumatoid Arthritis IgG1/IgG3, 82% Lupus MN IgG2 | Yes | IgG4 | Yes |
| FMN-1 | 50% | FSGS, IgAN | No (antigen is present in tissue macrophages, not on podocytes) | Unknown | Yes |
The global prevalence of each of these antigens varies from a single case described to about 3% of MN cases. Some of them claim a brief description to discuss their peculiar aspects.
NELL1 is probably the most intriguing antigen. It is described in MN (prevalence 2%) but it has the higher known prevalence in cancer-associated MN (33%) and it has been described also in drug, autoimmune and infection-associated MN [31]. In all cases IgG1 is the prevalent isotype present in immune deposits. It is needed to highlight the fact that NELL-1 is not expressed by the podocyte in normal conditions [25]. All these characteristics (i.e. lack of specificity, IgG1 prevalence and podocyte neo-expression) raise the possibility that a unique disease pathogenesis lies under NELL-1-associated MN.
Another target protein that is not expressed by podocytes is PCDH7, a transmembrane protein highly present in the nervous system [27]. The estimated prevalence [25] of PCDH7 is 1% of MN cases and its peculiar characteristics are sub-nephrotic proteinuria and low complement deposition. Both of these two aspects may suggest that PCDH7 is a ‘late’ antigen that becomes identifiable after the causative antigen/antibody complex disappeared.
Among unique details that differentiate antigens, SEMA3B has been described only in pediatric MN and EXT1/2, NCAM1, TGFBR3 that have a high prevalence in membranous lupus nephritis (5–30%). It is, however, noteworthy that anti-EXT1/2 and TGFBR3 were never found in sera, neither in reducing nor not-reducing conditions, questioning whether they are real target antigens or only a histologic marker of the disease [2].
Activation of the complement cascade, a mechanism for proteinuria in MN
Complement cascade has a key role in MN and several components, such as C3 and C5b-9 or MAC, can be localized by immunofluorescence and by proteomics in biopsies of patients with MN [33]. The formation of MAC appears central to determine the acute glomerular damage and proteinuria that follow deposition of specific antibodies in MN and there is consensus on considering that complement participates to worsen renal function in either humans and animal models [32–35]. The direct correlation between complement and glomerular damage in MN has been clearly demonstrated in complement-deficient mice immunized with THSD7A which developed limited or no lesions compared with wild-type littermates [33].
However, complement involvement in MN appears as a paradox since IgG4, the typical isotypes of all MN autoantibodies, are poor activators of the complement system [36] and the complement pathway activated in MN is not completely elucidated. Classical pathway activation of C1q-by IgG binding in patients with both anti-PLA2R1 and anti-THSD7A MN was found, major for IgG1, IgG2, and IgG3 but not the IgG4 score. In the same studies, IgG4 were found to activate the lectin pathway via the mannose-binding lectin (MBL) and the associated serine proteases (MASPs) with a direct correlation with C4bC2b C3 convertase activation in a subset of cases. Therefore, in MN the complement may be activated through the classical pathway by IgG1-3 and through the MBL pathway by IgG4 on the other hand [33].
Other studies utilizing the combination of laser microdissection of glomeruli and proteomics of glomerular eluates confirmed the conclusions above. Sethi et al. [37] showed a relevant increase of either C3 or C4A complement components as a consequence of the MBL-pathway activation. In line with this, high circulating levels of MBL were also found in association with anti-PLA2R IgG4, but not in their absence [38]. In vitro, anti-PLA2R1 IgG4 autoantibodies were able to activate the MBL pathway and induce injury in podocytes [39]. Finally, anti-PLA2R IgG4 induced MN also in MBL-deficient mice [40], supporting the possibility of an alternance with the classical pathway. It has been recently shown that IgG4 can activate the classical complement cascade, but only at high antigen and antibody concentrations [41, 42], therefore confirming the concept on activation of the classical complement pathway in MN and proposing that also IgG4 at a high concentration may be involved in the mechanism.
Second-wave antibodies and their potential role in the adaptive contest
Anti-SOD2, anti-AR, anti-ENO
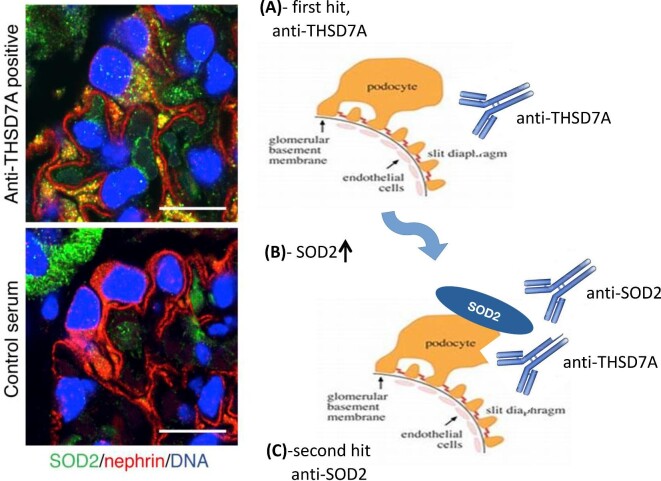
At the same time as the PLA2R discovery, using the same laser microdissection and separation of proteins in denaturing conditions, our research group identified three other target antigens that were neo-expressed by podocytes in MN and co-localized in the sub-epithelial deposits with IgG and C3, the major one of which was superoxyde dismutase 2 (SOD2) [9]. SOD2 is an antioxidant enzyme playing a major role in protecting cells from an oxidative injury that is generically up-regulated following inflammatory stimuli. An increased SOD2 expression was observed in glomeruli of mice injected with anti-THSD7A autoantibodies and in podocytes stimulated in vitro by IgG4 purified from MN patients [43], suggesting that an oxidative stress takes place following auto-antibody deposition in MN. SOD2 is critical to reduce intracellular lipid peroxidation and cell death. Because the antioxidative effect of SOD is strongly dependent on its expression levels [44], anti-SOD2 autoantibodies may alter the antioxidant protective effect by reducing SOD2, worsening the outcome. Aldose reductase (AR) and enolase (ENO) completed the panel of neo-expressed antigens in MN [9]. Circulating anti-ENO were first described in serum of MN patients in 1999 by Wakui et al. [45] and then confirmed by Kimura et al. [46] in 75% of primary and secondary MN, in association with anti-PLA2R in the former group. These authors could not detect ENO in sub-epithelial deposits.
Subsequent studies confirmed high circulating levels of IgG4 antibodies against all the neo-expressed podocyte antigens (SOD2, AR, and ENO) in a large cohort of MN patients [10, 47]. It was proved that antibodies coexist with different combinations in different patients, positive or negative for either circulating anti-PLA2R and anti-TSHD7A antibodies. Anti-SOD2 serum levels were associated with nephrotic proteinuria and anti-ENO with reduced renal function [10]. Overall, a multi-signature analysis better predicted a clinical outcome in MN patients [10]. This class of antibodies was defined as a ‘second wave’, to be distinguished from those classically involved in MN genesis (anti-PLA2R, anti-THSD7A, and potentially others) that target membrane proteins in glomeruli and represent the first hit of the disease (Fig. 1). A crucial characteristic of second-wave antibodies is that they are not specific for MN, but may be also involved in other glomerulonephrites, typically in lupus nephritis, as key adaptive phenomenon [48].
Figure 1:
Scheme reproducing the possible steps of MN and the interactive nature of first and second autoimmune hits.
Anti-FMNL1
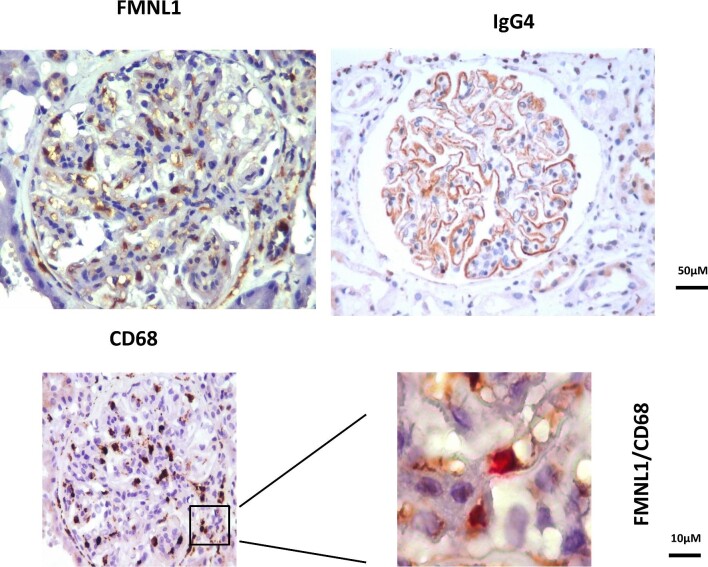
A further group of second-wave antibodies (the major of which are anti-formin-like 1 protein or FMNL1) has been recently discovered by utilizing ‘peptide arrays’, a new technology derived from pharmacology research and able to characterize binding epitopes of monoclonal antibodies [49, 50] and to define autoantibody signatures in several diseases [51]. Peptide arrays consist in 7 499 126 encompassing tiled peptides of 16 aminoacids each (covering the whole protein sequences coded by the human genome) linked to solid matrices to allow a direct interaction with human serum [52]. In this way, sera of MN were incubated with all the peptides of the customized array and the intensity of the relative fluorescence deriving from their interaction was aligned in sequence by informatic technologies to identify a single protein (34 peptides differing only by one amino acid can identify a protein). High anti-FMNL1 IgG4 were demonstrated in sera of MN patients and in glomeruli where they co-stained with CD68 that is the marker of macrophages (Fig. 2). FMNL1 is, in fact, a part of the formin family involved in actin-dependent processes, including migration, vesicle trafficking, and morphogenesis of macrophages [53, 54]. Circulating anti-FMNL1 IgG4 were associated with lack of remission of proteinuria in MN patients [52], strengthening the concept that autoantibodies directed to cells of the tissue repair have a definite role in determining the disease outcome.
Figure 2:
Stainings of a renal bioptic fragment from a patient with MN with anti-CDl8 and anti-FMNL1 antibodies immunoperoxidase staining.
The second-wave antibodies hypothesis in MN
Based on the data reported above, it is clear that the pathogenesis of MN is not so simple as previously speculated. The ‘one-size-fits-all’ hypothesis, with one antigen playing as the only actor on the stage, does not stand the test of experimental data. In fact, even if a single podocyte antigen probably stimulates the first-hit of the disease, it is highly probable that many other different antigens are involved in the pathological mechanisms and in the self-maintaining. Experimental data suggest that the first antigen is a protein normally expressed on the podocyte surface or on GBM that in healthy conditions is not immunogenic. This is either because it is recognized as a ‘self’ protein or because it is expressed in an area of ‘immunologic tolerance’, as the glomerular urinary area is considered. One perturbing event acting as a trigger may stimulate a self immunisation against this antigen, leading to podocytes damage and stimulation. Activated podocytes and stress conditions may favour the expression of many proteins on the cellular surface [55]. These neo antigens (ie. AR, SOD2, ENO, formin, and others) become targets of a new wave of immunization that leads to their co-localization with IgG and complement in immune deposits. Some of them were also found to be autoimmunity targets in other nephropathies such as lupus nephropathy [56]. This lack of specificity is not in contrast with their involvement in MN, but suggests a common pathway of podocyte deregulation and activation. As an example, the main difference found between MN and systemic lupus erythematosus is the IgG isotype: in these cases a few targets are the same but differ for the isotype of the antibody that is IgG4-predominant in MN and IgG2 in lupus nephritis. This may open the question about the reason for these differences that should be linked with extra-renal factors.
It is clear that all of these second-wave antigens are not involved in the first pathological steps of MN, but in the maintenance of the disease. Therefore, based on previous findings we can speculate a time-course between the primary antibodies that may have been cleared when the second wave is still present and worsened the glomerular damage. Based on that, it seems clear that focusing on only one antigen, as suggested by KDIGO guidelines, might not be enough to have a complete overview to provide a good treatment to the patient. This may be valid both in diagnosis and in the follow-up.
An important limitation of the ‘second wave’ theory is that it is largely based on data produced by a single group of research and have only limited confirmation outside [46]; a second issue is the ‘non-specificity’ of these antibodies. However, the presence of second-wave antibodies in other glomerulonephritis, such as in lupus nephritis, should not be a weakness if one accepts the idea that addictive mechanisms for progression of the renal damage may intervene during the evolution of the disease and may even be of the same importance as the first hit. Their association with the outcome in MN patients also strengthens the concept that addictive mechanisms of progression (such as the block of anti-oxidant mechanisms in the case of anti-SOD2 antibodies) have a key role and should become of interest for either pathologists and clinicians. The discovery of anti-FMNL1 antibodies also underscores the role of macrophages in glomerulonephritis that was recognized years ago either in experimental [57] and human [58] glomerulonephritis, with a differential effect depending on the M1 (pro-inflammatory) and M2a-M2c (protective) phenotypes [58]. It is probably time to extend our interest on mechanisms that are involved in protection (SOD2) and/or in regeneration of renal tissue that follows the inflammatory phase triggered by a first hit.
Clinical considerations
MN is a potentially evolving condition that may lead to end-stage kidney disease (ESKD) in 30% of cases generally over 10–15 years of disease. Therapeutical startegies targeting antibodies’ production with concomitant corticosteroids and cyclophosphamide and more recently with anti-CD20 monoclonal antibodies, appeared as equivalent in inducing partial or completed nephrotic syndrome remission [59]. However, kidney disease progression is not homogeneous and contextual with high levels of circulating anti-PLA2R and ‘second wave’ antibodies at diagnosis represent an independent risk factor of kidney failure [10].
As previously mentioned, 30% of patients with a diagnosis of MN have a spontaneous long-standing remission. They should be identified prior to administering any immunosuppressive therapies, with the aim to limit any possible adverse events. Levels and persistence of serum antibodies should correlate with the intensity of pro-inflammatory and oxidative effect in glomeruli, as animal models seem to indicate [43]. The concomitance of circulating anti-SOD antibodies plays a negative effect, limiting the antioxidant repairing effect of SOD [43]. Therefore, both anti-PLA2R and anti-SOD antibodies represent potential biomarkers to define the grade of renal injury and should be measured in MN patients at diagnosis. The results on the 1-year outcome of proteinuria and renal function in 285 patients of the Italian cohort of MN definitively strengthened this concept: the entire cohort was divided into four sub-groups depending on combination of anti-PLA2R and anti-SOD2 positivity (ie. PLA2R+/SOD2+, PLA2R-/SOD2-, PLA2R+/SOD2-, PLA2R-/SOD2+) and were followed for 1 year during which they received standard of care. As main results, 50% of PLA2R-/SOD2- had complete remission of proteinuria after 1 year compared to 25% of PLA2R+/SOD2- patients. Of relevance, single anti-SOD2 positivity (PLA2R-/SOD2+) or its combination with anti-PLA2R1 (PLA2R+/SOD2+) significantly increased the risk of reducing renal function under 60 ml/min at 12 months of follow-up [10]. Therefore, the definition of the multi-panel positivity referred to anti-PLA2R and anti-SOD2 antibody is key to predict the clinical outcome of MN patients. Patients negative for both antibodies have a good outcome, suggesting that positivity for other membrane antigens more recently discovered (not together with anti-PLA2R) does not modify the favourable clinical outcome. Despite an apparent interest, the clinical application of a multi-panel biomarker approach is limited by reasons already discussed in the preceding paragraph. It is possible that the application of a multi-panel approach could better predict the outcome and fills a space that is left unresolved by current research on causative antibodies (anti-PLA2R in this case). Personal unpublished observations on a predictive role of anti-FMNL1 in lupus nephritis go in this direction and may re-open a discussion on a wide mechanism for renal progression.
Also, therapies should be modeled considering that the antibody panel at diagnosis may influence the outcome. In clinical trials recruiting hundreds of patients, the random assignment to different therapeutic regimen should reduce or null the importance of this bias, but the presence or not of second-wave antibodies should be considered in cases of clinical studies with a limited number of enrolled MN patients.
Contributor Information
Corrado Murtas, Nephrology and Dialysis Unit, Ospedale Belcolle, ASL Viterbo, Viterbo, Italy.
Maurizio Bruschi, Department of Experimental Medicine (DIMES) University of Genoa, Italy; Nephrology, Dialysis and Transplantation Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy.
Sonia Spinelli, Nephrology, Dialysis and Transplantation Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy.
Xhuliana Kajana, Nephrology, Dialysis and Transplantation Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy.
Enrico E Verrina, Nephrology, Dialysis and Transplantation Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy.
Andrea Angeletti, Nephrology, Dialysis and Transplantation Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy.
Gianluca Caridi, Nephrology, Dialysis and Transplantation Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy.
Giovanni Candiano, Nephrology, Dialysis and Transplantation Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy.
Sandro Feriozzi, Nephrology and Dialysis Unit, Ospedale Belcolle, ASL Viterbo, Viterbo, Italy.
Marco Prunotto, Institute of Pharmaceutical Sciences of Western Switzerland, School of Pharmaceutical Sciences, University of Geneva, Geneva, Switzerland.
Gian Marco Ghiggeri, Nephrology, Dialysis and Transplantation Unit, IRCCS Istituto Giannina Gaslini, Genova, Italy.
FUNDING
A.A. is supported by ‘Ricerca Corrente 2023’ and ‘5XMille-5M-2019–23 680 431’. M.B. is supported by ‘Ricerca Corrente 2023’ and ‘5xMille, 5M-2019–23 680 420’.
AUTHORS’ CONTRIBUTIONS
All authors equally contributed to the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1. Heymann W, Hackel DB, Harwood S et al. Production of nephrotic syndrome in rats by freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med 1959;100:660–4. 10.3181/00379727-100-24736 [DOI] [PubMed] [Google Scholar]
- 2. Caza TN, Al-Rabadi LF, Beck LH Jr. How times have changed! A cornucopia of antigens for membranous nephropathy. Front Immunol 2021;12:800242. 10.3389/fimmu.2021.800242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Debiec H, Nauta J, Coulet F et al. Role of truncating mutations in MME gene in fetomaternal alloimmunisation and antenatal glomerulopathies. Lancet 2004;364:1252–9. 10.1016/S0140-6736(04)17142-0 [DOI] [PubMed] [Google Scholar]
- 4. Murtas C, Bruschi M, Candiano G et al. Coexistence of different circulating anti-podocyte antibodies in membranous nephropathy. Clin J Am Soc Nephrol 2012;7:1394–400. 10.2215/CJN.02170312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murtas C, Allegri L, Ghiggeri GM. Circulating antipodocyte antibodies in membranous nephropathy: new findings. Am J Kidney Dis 2013;62:12–5. 10.1053/j.ajkd.2013.01.030 [DOI] [PubMed] [Google Scholar]
- 6. Beck LH Jr., Bonegio RG, Lambeau G et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009;361:11–21. 10.1056/NEJMoa0810457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomas NM, Beck LH Jr., Meyer-Schwesinger C et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 2014;371:2277–87. 10.1056/NEJMoa1409354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caza TN, Storey AJ, Hassen SI et al. Discovery of seven novel putative antigens in membranous nephropathy and membranous lupus nephritis identified by mass spectrometry. Kidney Int 2023;103:593–606. 10.1016/j.kint.2023.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prunotto M, Carnevali ML, Candiano G et al. Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J Am Soc Nephrol 2010;21:507–19. 10.1681/ASN.2008121259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ghiggeri GM, Seitz-Polski B, Justino J et al. Multi-autoantibody signature and clinical outcome in membranous nephropathy. Clin J Am Soc Nephrol 2020;15:1762–76. 10.2215/CJN.02500220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Del Sordo R, Covarelli C, Brugnano R et al. PLA2R immunohistochemistry staining in membranous glomerulopathy: a challenging stain to interpret but a potentially useful diagnostic tool. Appl Immunohistochem Mol Morphol 2021;29:414–21. 10.1097/PAI.0000000000000892 [DOI] [PubMed] [Google Scholar]
- 12. Watanabe K, Watanabe K, Watanabe Y et al. Human soluble phospholipase A(2) receptor is an inhibitor of the integrin-mediated cell migratory response to collagen-I. Am J Physiol Cell Physiol 2018;315:C398–408. 10.1152/ajpcell.00239.2017 [DOI] [PubMed] [Google Scholar]
- 13. Ancian P, Lambeau G, Mattei MG et al. The human 180-kDa receptor for secretory phospholipases A2. Molecular cloning, identification of a secreted soluble form, expression, and chromosomal localization. J Biol Chem 1995;270:8963–70. 10.1074/jbc.270.15.8963 [DOI] [PubMed] [Google Scholar]
- 14. Augert A, Payre C, de Launoit Y et al. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep 2009;10:271–7. 10.1038/embor.2008.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skoberne A, Behnert A, Teng B et al. Serum with phospholipase A2 receptor autoantibodies interferes with podocyte adhesion to collagen. Eur J Clin Invest 2014;44:753–65. 10.1111/eci.12292 [DOI] [PubMed] [Google Scholar]
- 16. Hihara K, Iyoda M, Tachibana S et al. Anti-Phospholipase A2 receptor (PLA2R) antibody and glomerular PLA2R expression in japanese patients with membranous nephropathy. PLoS One 2016;11:e0158154. 10.1371/journal.pone.0158154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 2011;364:689–90. 10.1056/NEJMc1011678 [DOI] [PubMed] [Google Scholar]
- 18. Rovin BH, Adler SG, Barratt J et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int 2021;100:753–79. 10.1016/j.kint.2021.05.015 [DOI] [PubMed] [Google Scholar]
- 19. Caza TN, Larsen CP. False-positive anti-PLA2R ELISA testing in patients with diabetes mellitus. Kidney Int 2023;103:425. 10.1016/j.kint.2022.11.004 [DOI] [PubMed] [Google Scholar]
- 20. Dong D, Fan TT, Wang YY et al. Relationship between renal tissues phospholipase A2 receptor and its serum antibody and clinical condition and prognosis of idiopathic membranous nephropathy: a meta-analysis. BMC Nephrol 2019;20:444. 10.1186/s12882-019-1638-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaya B, Paydas S, Balal M et al. Renal expression of PLA2R, THSD7A, and igg4 in patients with membranous nephropathy and correlation with clinical findings. Int J Clin Pract 2021;75:e13855. 10.1111/ijcp.13855 [DOI] [PubMed] [Google Scholar]
- 22. Vink CH, Logt AV, van der Molen RG et al. Antibody-Guided therapy in phospholipase A2 receptor-associated membranous nephropathy. Kidney Int Rep 2023;8:432–41. 10.1016/j.ekir.2022.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tomas NM, Dehde S, Meyer-Schwesinger C et al. Podocyte expression of human phospholipase A2 receptor 1 causes immune-mediated membranous nephropathy in mice. Kidney Int 2023;103:297–303. 10.1016/j.kint.2022.09.008 [DOI] [PubMed] [Google Scholar]
- 24. Sethi S, Madden BJ, Debiec H et al. Exostosin 1/Exostosin 2-Associated membranous nephropathy. J Am Soc Nephrol 2019;30:1123–36. 10.1681/ASN.2018080852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sethi S, Debiec H, Madden B et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 2020;97:163–74. 10.1016/j.kint.2019.09.014 [DOI] [PubMed] [Google Scholar]
- 26. Sethi S, Debiec H, Madden B et al. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int 2020;98:1253–64. 10.1016/j.kint.2020.05.030 [DOI] [PubMed] [Google Scholar]
- 27. Sethi S, Madden B, Debiec H et al. Protocadherin 7-Associated membranous nephropathy. J Am Soc Nephrol 2021;32:1249–61. 10.1681/ASN.2020081165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sethi S. New ‘Antigens’ in membranous nephropathy. J Am Soc Nephrol 2021;32:268–78. 10.1681/ASN.2020071082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sethi S, Casal Moura M, Madden B et al. Proprotein convertase subtilisin/kexin type 6 (PCSK6) is a likely antigenic target in membranous nephropathy and nonsteroidal anti-inflammatory drug use. Kidney Int 2023;104:343–52. 10.1016/j.kint.2023.04.006 [DOI] [PubMed] [Google Scholar]
- 30. Sethi S, Madden B, Casal Moura M et al. Membranous nephropathy in syphilis is associated with neuron-derived neurotrophic factor. J Am Soc Nephrol 2023;34:374–84. 10.1681/ASN.0000000000000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sethi S. The many faces of NELL1 mN. Clin Kidney J 2023;16:442–6. 10.1093/ckj/sfac237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ayoub I, Shapiro JP, Song H et al. Establishing a case for anti-complement therapy in membranous nephropathy. Kidney Int Rep 2021;6:484–92. 10.1016/j.ekir.2020.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seifert L, Zahner G, Meyer-Schwesinger C et al. The classical pathway triggers pathogenic complement activation in membranous nephropathy. Nat Commun 2023;14:473. 10.1038/s41467-023-36068-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cybulsky AV, Rennke HG, Feintzeig ID et al. Complement-induced glomerular epithelial cell injury. Role of the membrane attack complex in rat membranous nephropathy. J Clin Invest 1986;77:1096–107. 10.1172/JCI112408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morita Y, Ikeguchi H, Nakamura J et al. Complement activation products in the urine from proteinuric patients. J Am Soc Nephrol 2000;11:700–7. 10.1681/ASN.V114700 [DOI] [PubMed] [Google Scholar]
- 36. van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by igg4 antibodies. Clin Exp Immunol 1986;64:415–22. [PMC free article] [PubMed] [Google Scholar]
- 37. Sethi S, Palma LMP, Theis JD et al. Proteomic analysis of complement proteins in glomerular diseases. Kidney Int Rep 2023;8:827–36. 10.1016/j.ekir.2023.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao Y, Cai M, Jiang Z et al. Association of serum mannose-binding lectin, anti-phospholipase A2 receptor antibody and renal outcomes in idiopathic membranous nephropathy and atypical membranous nephropathy: a single center retrospective cohort study. Ren Fail 2022;44:428–33. 10.1080/0886022X.2022.2048016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Haddad G, Lorenzen JM, Ma H et al. Altered glycosylation of igg4 promotes lectin complement pathway activation in anti-PLA2R1-associated membranous nephropathy. J Clin Invest 2021;131:e140453. 10.1172/JCI140453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bally S, Debiec H, Ponard D et al. Phospholipase A2 receptor-related membranous nephropathy and mannan-binding lectin deficiency. J Am Soc Nephrol 2016;27:3539–44. 10.1681/ASN.2015101155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oskam N, Damelang T, Streutker M et al. Factors affecting igg4-mediated complement activation. Front Immunol 2023;14:1087532. 10.3389/fimmu.2023.1087532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoxha E, Beck LH Jr., Wiech T et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-Specific antibodies in membranous nephropathy. J Am Soc Nephrol 2017;28:520–31. 10.1681/ASN.2016010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Buelli S, Perico L, Galbusera M et al. Mitochondrial-dependent autoimmunity in membranous nephropathy of igg4-related disease. EBioMedicine 2015;2:456–66. 10.1016/j.ebiom.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Motoori S, Majima HJ, Ebara M et al. Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res 2001;61:5382–8. [PubMed] [Google Scholar]
- 45. Wakui H, Imai H, Komatsuda A et al. Circulating antibodies against alpha-enolase in patients with primary membranous nephropathy (MN). Clin Exp Immunol 1999;118:445–50. 10.1046/j.1365-2249.1999.01080.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kimura Y, Miura N, Debiec H et al. Circulating antibodies to alpha-enolase and phospholipase A2 receptor and composition of glomerular deposits in japanese patients with primary or secondary membranous nephropathy. Clin Exp Nephrol 2017;21:117–26. 10.1007/s10157-016-1235-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bruschi M, Sinico RA, Moroni G et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo: alpha-enolase and annexin aI. J Am Soc Nephrol 2014;25:2483–98. 10.1681/ASN.2013090987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Angeletti A, Bruschi M, Moroni G et al. Second wave antibodies in autoimmune renal diseases: the case of lupus nephritis. J Am Soc Nephrol 2021; 32:3020–23. 10.1681/ASN.2021050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Forsstrom B, Axnas BB, Stengele KP et al. Proteome-wide epitope mapping of antibodies using ultra-dense peptide arrays. Mol Cell Proteomics 2014;13:1585–97. 10.1074/mcp.M113.033308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sahlstrom P, Hansson M, Steen J et al. Different hierarchies of anti-modified protein autoantibody reactivities in rheumatoid arthritis. Arthritis Rheumatol 2020;72:1643–57. 10.1002/art.41385 [DOI] [PubMed] [Google Scholar]
- 51. Yan SD, Zhu H, Zhu A et al. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat Med 2000;6:643–51. 10.1038/76216 [DOI] [PubMed] [Google Scholar]
- 52. Bruschi M, Cavalli A, Moll S et al. Discovery of anti-Formin-like 1 protein (FMNL1) antibodies in membranous nephropathy and other glomerular diseases. Sci Rep 2022;12:13659. 10.1038/s41598-022-17696-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gardberg M, Heuser VD, Iljin K et al. Characterization of leukocyte formin FMNL1 expression in human tissues. J Histochem Cytochem 2014;62:460–70. 10.1369/0022155414532293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Faix J, Grosse R. Staying in shape with formins. Dev Cell 2006;10:693–706. 10.1016/j.devcel.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 55. Tomas NM, Hoxha E, Reinicke AT et al. Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 2016;126:2519–32. 10.1172/JCI85265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bruschi M, Galetti M, Sinico RA et al. Glomerular autoimmune multicomponents of human lupus nephritis in vivo (2): planted antigens. J Am Soc Nephrol 2015;26:1905–24. 10.1681/ASN.2014050493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ikezumi Y, Hurst LA, Masaki T et al. Adoptive transfer studies demonstrate that macrophages can induce proteinuria and mesangial cell proliferation. Kidney Int 2003;63:83–95. 10.1046/j.1523-1755.2003.00717.x [DOI] [PubMed] [Google Scholar]
- 58. Hu W, Li G, Lin J et al. M2 macrophage subpopulations in glomeruli are associated with the deposition of IgG subclasses and complements in primary membranous nephropathy. Front Med (Lausanne) 2021;8:657232. 10.3389/fmed.2021.657232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Scolari F, Delbarba E, Santoro D et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. J Am Soc Nephrol 2021;32:972–82. 10.1681/ASN.2020071091 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.