Abstract
Introduction:
COVID-19 vaccination side effects are rare but important medical situations. Spine-affecting side effects are amongst the rarest, but exceedingly important. Haemorrhagic spinal manifestations of COVID-19 and its vaccines are less reported with little knowledge about them.
Case presentation:
An 80-year-old male who received his first shot of the COVID-19 vaccine had developed COVID-19 pneumonia, weakness, and sensory problems in his legs followed by sphincter incontinence within 5 days period. MRI showed a spontaneous epidural spinal epidural haematoma (SSEDH) in T10–L1. He underwent laminectomy and haematoma evacuation. One month follow-up showed no clinical improvement.
Discussion:
To our knowledge, this was the first post-vaccination SSEDH and second in haemorrhagic spinal complications following COVID-19 vaccination. Considering the neuropathogenesis pathway of COVID-19 and its vaccines, there are common mechanisms of action that could potentially justify post-vaccination SSEDH such as seen in COVID-19 infection, itself. Early Neurosurgical intervention and better preoperative neurological status could be a beneficial modifier for favourable clinical outcomes.
Conclusion:
SSEDH and COVID-19 vaccine coincidence is a rare clinical event, still no solid association could be scientifically explained. Further studies are required for a reliable pathophysiologic association. Early diagnosis, interdisciplinary medical approach, and faster intervention are the cornerstone of the treatment paradigm.
Keywords: Adverse Drug reactions, COVID-19 vaccines, COVID-19, laminectomy, SARS‑CoV‑2, spontaneous Spinal epidural haematoma, spontaneous spinal haemorrhage, vaccine side effects
Introduction
Highlights
Post-vaccination spinal haemorrhage is a rare medical condition with insidious clinical course. In this case we have presented a patient with spontaneous spinal epidural haematoma following COVID-19 vaccination.
Post-vaccination weakness is the hallmark of spinal events requiring thorough neurological investigation. In this case the patient developed paraparesis and incontinency due to spontaneous spinal epidural haematoma.
Operation consisted of laminectomy and haematoma evacuation with mild neurological improvement.
Long term clinical follow-up showed poor neurological recovery, emphasizing the importance of early diagnosis in similar cases.
SARS-CoV-2 or COVID-19 pandemic is a serious global concern that affects worldwide health systems. SARS-CoV-2 has both pulmonary and extra-pulmonary manifestations. Skin, gastrointestinal tract, bone marrow, and nervous system could be invaded by SARS-CoV-2 as well. Guillain–Barre’s syndrome, peripheral neuropathies, encephalopathies, ischaemic and haemorrhagic strokes are among SARS-CoV-2 nervous system manifestations1,2. Myelopathy, neuropathy, Guillain–Barre’s syndrome, demyelinating syndromes, haemorrhagic lesions, and myelitis are amongst the most common clinical manifestations of the spine invasion by virus3,4. Haemorrhagic spinal lesions including spontaneous spine epidural haematoma (SSEDH), intramedullary haematoma, and other lesions in the COVID-19 setting are rare clinical conditions with few reports describing the associations4–6.
COVID-19 vaccine emergence saved the world from the pandemic but there are reports on possible side effects. Injection site reactions, fever, and pain are the most commonly reported COVID-19 vaccine reactions but more serious side effects are also reported. Regarding nervous system presentations, cerebral venous thrombosis, intracerebral haemorrhage, and demyelinating syndromes such as transverse myelitis and neuromyelitis optica have been reported in association with COVID-19 vaccines7. To our best knowledge, there is only 1 report on haemorrhagic spinal side effects of the COVID-19 vaccine and this case is the second report on such a rare clinical entity8. In this manuscript, we report spontaneous spinal epidural haematoma(SSEDH) concurrency with COVID-19 vaccine injection in an octogenarian male who was treated surgically. The work has been reported in line with the Surgical Case Report (SCARE) Guidelines criteria9.
Case
Clinical presentation
An 80-year-old male known case of chronic kidney disease (CKD stage 4) was vaccinated with the Sputnik V COVID-19 vaccine on 20 April 2021. After 3 days of his first shot, cough, high-grade fever, and malaise were developed followed by shortness of breath and excessive sweating. He received oral Acetaminophen 500 mg every 8 h. Two days later, he experienced reduced sensation from his waist to his toes, followed by reduced muscle strength of his feet and urinary-faecal incontinence, which progressed over the next 36 hours. He was brought to the emergency department, his Glasgow coma scale (GCS) was 15/15, and the vital signs were as blood pressure (BP)=133/85, heart rate (HR)=98/min, O2 saturation=87%, T=39 C, respiratory rate=23/min. Coarse crackles in both lungs and respiratory distress were evident. Neurologic examination showed acute flaccid paraplegia (ASIA score: A), mute deep tendon reflexes, T10 sensory level, anaesthesia of both lower limbs, and faecal-urinary incontinence with lax anal tone. The rest of his clinical examination was unremarkable. His drug history was negative for anticoagulants or anti-platelets. Nasopharynx swabs were positive for SARS-CoV-2, confirmed by a lung high-resolution computed tomography scan. He was diagnosed with acute cauda equina syndrome concurrently with SARS-CoV-2 pneumonia. Lab results showed creatinine level =3 mg/dl and K=6 mEq/l. He was not receiving any anticoagulants or anti-platelets and the coagulation profile (PT, PTT, INR, platelet count, and BT) was in normal range regarding his medical condition. The rest of his lab results were in expected ranges considering CKD history and COVID-19 pneumonia. Considering the CKD status of the patient, he underwent an emergent low K, heparinized haemodialysis session, and was scheduled for surgery. The pre-ictus haemodialysis details and the underlying reason for CKD had no specific points.
Neurosurgical evaluation
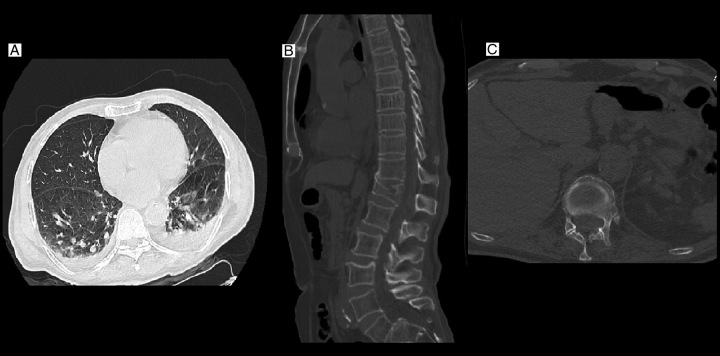
Full spine multidetector computed tomography was taken. An old T12 compression fracture with mild thoracolumbar junction kyphosis was detected which was revealed to be a neglected injury over the previous years. There was a suspicious isodense mass-like lesion posterior to the T10–T12 lamina, which had displaced the cord ventrally (Fig. 1).
Figure 1.
Lung high-resolution computed tomography of the patient shows bilateral severe COVID-19 pneumonia with transudative bilateral pleural effusions (A). spine multidetector computed tomography (B, C) shows an old T12 fracture with kyphotic deformity. There is an isodense lesion behind T10–L1 in favour of an epidural lesion.
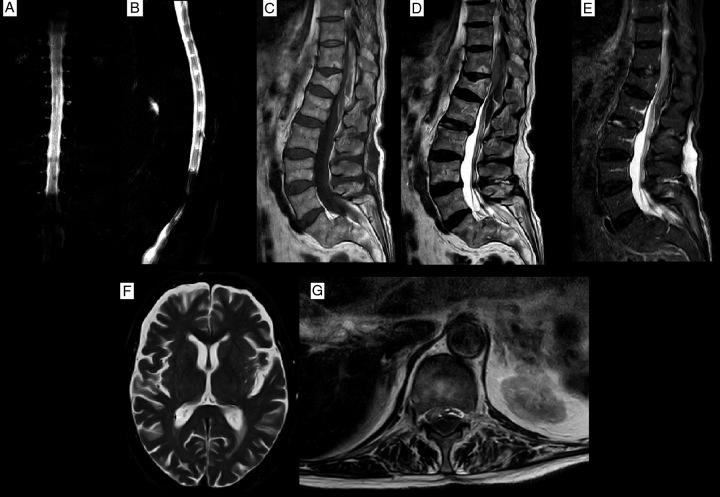
Brain and whole spine MRI were obtained. A bi-convex-shaped epidural lesion was detected in the thoracolumbar region which was iso-to-hyper intense in T1 sequences and had iso-to-hypo intensity signal changes in T2 images posterior to T10–L1 vertebral bodies. Epidural lesions had displaced the cord ventrolaterally and caused cerebrospinal fluid block in myelograms. T12 fracture was detected to be an old lesion in short-tau inversion recovery sequences. The brain and rest of the spine MRI had no specific findings (Fig. 2). Gadolinium-enhanced MRI sequences were not obtained due to CKD and the risk of nephrogenic systemic fibrosis (NSF). The most probable diagnosis was spontaneous spinal epidural haematoma according to the clinical and imaging findings while other scenarios such as epidural abscess or tumoral lesions were kept in mind as differential diagnoses. Multiple supplementary tests were obtained to investigate and weigh these differential diagnoses, however, they resulted in some degrees of obscurities. Due to clinical uncertainties and the need for urgent neurosurgical intervention for tissue confirmation, the surgery was prioritized to finalize the diagnosis and save the patient’s neurological reserve. Informed medical consent was obtained from the patient and his family members, then prepared for the operation.
Figure 2.
Preoperative MRI. (A, B) Show cerebrospinal fluid block, T1 image (C) shows mixed hypo-hyperintense epidural mass, and the T2 sequence shows mixed hypo-iso intensity signal changes. Short-tau inversion recovery view MRI (D) please notice hematoma had dislodged the conus, anteriorly (E) confirmed old T12 fracture (hypointense T12 body). T2-axial in T12 level (G) showed ventrolateral displacement of conus medullaris and cord with severe mass effect. Brain MRI had no remarkable findings (F).
Operation
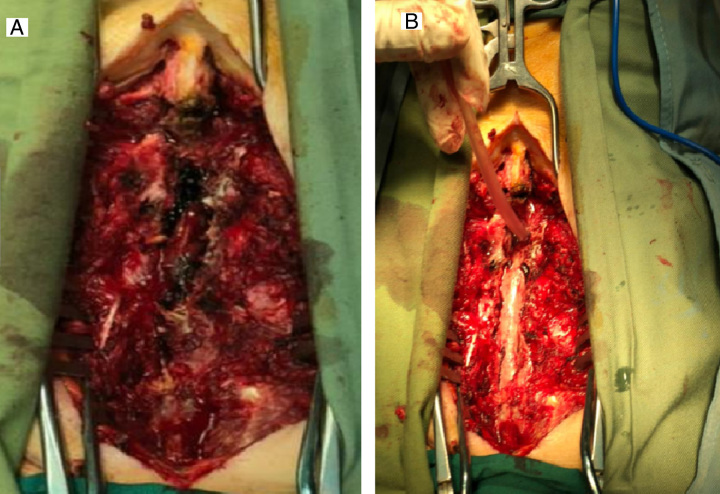
The patient was scheduled for emergency spine surgery. Under general anaesthesia and supine position, after prep and drape in a sterile fashion, skin incised over T10–L1 spinous processes in the midline plane, paravertebral muscles were stripped off, and bilateral laminectomy from T10 to L1 was performed. There was a massive dense epidural haematoma beneath the T10–L1 laminae, which was irrigated and evacuated (Fig. 3). The dura was intact but the thecal sac was shrunken into the spinal canal. Due to severe COVID-19 pneumonia and intra-operative hemodynamic instability, posterior instrumentation and kyphosis correction were postponed to another operation after further hemodynamic stability. A submuscular drain was inserted, and paravertebral muscles and fascia were approximated and repaired. Skin and subcutaneous tissues were repaired in anatomic layers.
Figure 3.
Intraoperative photographs showed a dense clot in the epidural space (A). The post-evacuation picture showed an intact dural sac with minor haematoma debris, removed via saline irrigation (B).
Postoperative period and follow-up
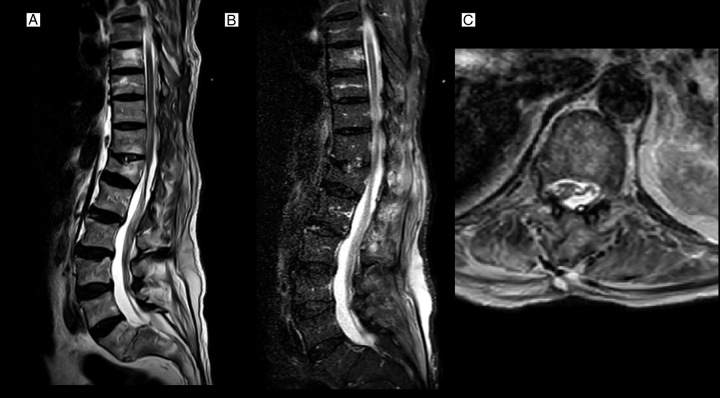
The patient was sent to the ICU for postoperative recovery. Early MRI (day 1 post-operation) showed resolution of epidural haematoma and decompression of the spinal cord (Fig. 4). On post-operation day 2, he showed no neurological improvement in motor (ASIA score: A) or sphincter function. One month follow-up showed no further clinical improvement.
Figure 4.
Immediate post-operation T2, short-tau inversion recovery view, and T2-axial MRI sequences (A–C graphs, respectively) showed decompression of cord and haematoma evacuation. Conus medullaris remodelling is considerable (C).
The pathologic evaluation of specimens showed no specific vascular, tumour, or inflammatory process but accumulated blood clots with no organized architecture.
Discussion
The central and peripheral nervous systems (CNS and PNS, respectively) are both affected by the COVID-19 virus. Headaches, encephalitis, myelitis, Guillain–Barre syndrome (GBS), neuropathies, intracranial haemorrhage(ICH), ischaemic strokes, seizure, neuropsychological presentations, cranial nerve dysfunction, ataxia are amongst the most common manifestations of COVID-19 in the nervous system10–12. Neuropathy, spinal cord myelopathy, transverse myelopathy, necrotizing myelopathy, haemorrhagic spinal lesions such as SSEDH/intramedullary haematoma and Guillen–Barre syndrome are amongst the most reported spinal manifestations of COVID-19 virus3–5,13–15.
Different pieces of literature have suggested that the virus can be transmitted to the nervous system via the olfactory nerve or even other cranial nerves in a retrograde spread pathway, using dynein and kinesin cell skeleton motor proteins16,17. Hematogenous spread is another mechanism by which the virus can reach the nervous system. Keyhanian and colleagues summarized multiple pathophysiological mechanisms to clarify hematogenous spread. Originating from a distant source of the virus such as the pulmonary system, SARS-CoV-2 virus or particles seed into the bloodstream, infecting all the body tissues that have proper surface antigen-receptor interactions, leading to virus implantation into tissues. SARS-CoV-2 infected leucocytes and blood-brain-barrier’s (BBB) endothelial interface are also hypothesized to be associated with CNS invasion of the COVID-19 virus16. In terms of the molecular interface between SARS-CoV-2 and the nervous system, angiotensin-converting enzyme 2 receptor(ACE-2R) is thought to be one of the key coupling sites for virus neurotropism18,19. Neuroinflammation, glial-neural cell damage, and vasculitis are also other suggested pathogenesis of SARS-CoV-2 nervous system presentations16. Cytokine release, cell-mediated-immune response, and humoral immunity play important roles in CNS Neuroinflammation resulting in local to extensive neural tissue damage16,19. On the other hand, endothelial injury (vascular damage) is one of the most determinant SARS-CoV-2 pathogenesis in the nervous system. The endothelium is rich in ACE-2 receptors and SARS-CoV-2 can adhere and enter the endothelial bed in the nervous system causing enthothelitis, BBB disruption, haemorrhagic or ischaemic events, collectively called vasculitis-like syndromes20–22.
Regarding the aforementioned mechanisms, multiple studies have tried to clear possible pathophysiological associations between the COVID-19 vaccine and neurological side effects23. Lu et al.24 reviewed the COVID-19 vaccine’s neurological side effects and suggested immune system-mediated responses can be responsible for such adverse reactions. Fan et al.25 conducted a systematic review with meta-analysis on the efficacy and safety of COVID-19 vaccines and found mRNA-based vaccines in comparison to vector/inactivated virus-based vaccines, had better protective properties against COVID-19 infection but were associated with higher adverse reactions. Post-vaccination myelitis and other demyelinating diseases are well-reported after COVID-19 vaccination. Viral neurotropism, cytokine-storming, viral re-activation, vasculitis-like syndromes, immune-complex mediated neuro-vascular damage such as SARS-CoV-2 spike domain S1 antibodies and endothelial dysfunction are the main hypothesized mechanisms of actions in the development of post COVID-19 vaccination nervous system manifestations myelitis7,26–28. Roman et al.29 compared the possible neuropathogenesis of transverse myelitis after COVID-19 infection and vaccination syndromes and concluded that the majority of these mechanisms are common. Still, there are multiple scientific gaps in complete understanding of possible mechanisms of actions requiring a thorough scientific investigations.
The Sputnik V COVID-19 vaccine or Gam-COVID-Vac is an adenovirus-based vector-type vaccine developed against COVID-19. Like other anti-COVID-19 vaccines, injection site reactions, flu-like symptoms, COVID-19 infection after vaccine injection, Bell’s palsy, Guillain–Barre syndrome, myocarditis, and other post-vaccination signs and symptoms are reported30–32.
In an interesting systematic review, Ghaderi and colleagues have summarized the nervous system complications of COVID-19 vaccines. Myelitis, spinal cord ischaemia, and myelopathy are amongst the most detected spinal side effects of the COVID-19 vaccines33.
Babamahmoodi and colleagues published a paper on 3236 adverse side effects out of 13435 shots of the Sputnik V vaccine on healthcare providers in Iran. They reported 26 cases of confirmed post-vaccination COVID-19 infection. Focusing on neurological adverse reactions of adenovirus-based vaccines, headache, myelitis, Bell’s palsy, seizure, ICH, sinus thrombosis, GBS, stroke, and other acute-sub-acute neurological presentations are reported but haemorrhagic spinal lesions are still extremely rare7,8. Compared to other vaccine types, there were reports concerning the safety and efficacy of this vaccine but due to the emergency of the COVID-19 pandemic, it was used in many countries30. Our patient developed severe COVID-19 infection 3 days after his first Sputnik V vaccine and was hospitalized for both COVID-19 and acute flaccid paralysis due to SSEDH.
SSEDH has a detrimental effect on the neurologic status of the patients. The exact aetiology is not well established, but trauma, tumours, vascular anomalies, and bleeding disorders are associated risk factors but the exact correlation between each specific risk factor and the incidence of SSEH is not yet strongly correlated34. Figueroa and DeVine reviewed the literature and reported prior anticoagulant use, vascular malformations, hemangioma, existing coagulopathy, pregnancy, haemophilia, and leukaemia are the predisposing factors that can be correlated with SSEH. They also reported considering attributable risk factors, in 40–60% of cases there are no identifiable risk factors, thus identified as idiopathic35. SSEDH and end-stage renal disease (ESRD) coincidence is a rare medical condition that has been reported previously. The exact pathogenesis or robust correlation between these two different entities is still controversial. Severe uraemia, uncontrolled hypertension, blood pressure fluctuation during haemodialysis, heparinization during haemodialysis and coagulopathy are suggested mechanisms to describe this situation. Deger et al.36 reported a 70-year-old patient with severe uraemia and stage 5 CKD(ESRD, Cr=7.5 mg/dl) who developed SSEDH in the cervicothoracic region and was treated surgically. In a similar report, Huang et al.37 reported a 71-year-old patient with ESRD (Cr=8.7) who developed cervical SSEDH and was treated surgically. Both of these cases had severe uraemia and ESRD which may be associated with an acquired platelet dysfunction and/or coagulopathy and lead to SSEH, however, the data on this correlation is not conclusive. Our case was a known case of CKD stage 4(Cr=3 mg/dl) with eGFR (estimated glomerular filtration rate)=20 mg/mmol which is not considered as ESRD and had no uraemia. He was not undergoing intensive haemodialysis sessions (only twice a week) rather than a high volume thrice a week for ESRD cases. His coagulation profile was in normal values. Sill the authors cannot deny nor approve the association between CKD and SSEDH in this case but it seems that in the absence of uraemia, being a non-ESRD and controlled medical condition, the development of SSEDH may be less attributable to the CKD status of our patient. The author hypothesized that another contributing factor (COVID-19 vaccine) could be the initiator.
Colliding SSEDH and COVID-19, the literature lacks robust reported data on such a rare medical situation. Shawn Wen-Yang Lim et al. reported the first case of SSEH and COVID-19 pneumonia that was treated conservatively with an acceptable outcome with steroid therapy6. Zubeyde et al.5 reported a thoracolumbar epidural haematoma following COVID-19 pneumonia. They reported the patient recovered after laminectomy and haematoma evacuation. In November 2023, a comprehensive systematic review was published and summarized all the existing data on spontaneous spinal haemorrhage following COVID-19 infection. The authors concluded that early neurosurgical intervention can be accounted as a salvage treatment for those with more severe deficits (ASIA≤C) while those with more stable neurological status can be expectedly managed. According to their review, sensory deficits had the best treatment response to neurosurgical care while sphincter dysfunction had the lowest recovery results38.
In 2021, the first case of spinal haemorrhage following the COVID-19 vaccine was detected. The authors reported a cervical intramedullary haematoma in a 67-year-old female 2 days after the Sinopharm vaccine injection. The patient was treated conservatively due to multiple medical complications. Her 6-month follow-up report showed acceptable neurological recovery8. There are no scientific reports in this regard but web-based pages resulted in few reported cases39. All 3 of them were over 60s females who had received 2 shots of COVID-19 vaccines and presented with pain and hypoesthesia. Due to the non-scientific format of these reports, we cannot rely on these reports but it would be beneficial for recording and spreading global awareness.
This study features a rare COVID-19 vaccine complication and thoroughly delineates possible pathophysiologic associations between live-attenuated virus-derived vaccines and spinal cord side effects scientifically.
In this report, the patient was a known case of CKD stage 4 who had received his first Sputnik V vaccine earlier. There was no history of recent trauma, anti-platelets/anticoagulants, or uncontrolled blood pressure. He was brought to the hospital days after his first neurologic symptoms and had an ASIA score of A on his admission. He underwent emergent laminectomy and evacuation of SSEDH at the T10–L1 level with no neurological recovery in follow-up.
Neural decompression via laminectomy and haematoma evacuation is the treatment of choice in SSEDH. Ventral or laterally located lesions, especially in thoracic and thoracolumbar zones, usually require further surgical exposure that may result in spinal column instability. Thus, spinal column fixation with pedicular screw-rod construct should be considered after decompressive surgery. In a review of previous publications, the preoperative neurological status of the patients is a significant determinant of postoperative neurological outcomes. Better preoperative neurological status (Frankel grade/ASIA score D, E) was correlated with better postoperative clinical outcomes40,41. Shin and colleagues investigated 14 cases of SSEDH (11 men, 3 women). They found a significant difference between poor and good preoperative Japanese Orthopedic Association (JOA) scores in the postoperative neurological outcome of the patients(P<0.05)42. In a recent systematic review on spontaneous spinal haematoma following COVID-19, the authors concluded that better preoperative neurological status and early and extensive neurosurgical decompression were associated with better clinical outcomes38.
Early decompression (<36 h) was associated with better neurosurgical results in previous reports. The time interval from the first symptom to surgical decompression is another predictor of postoperative functional outcome. Surgical decompression should be performed in the first 36 hours of the earliest symptoms. Laminectomy within the first 12 h of symptoms onset has better postoperative neurological outcomes than surgeries performed over 24–36 h after primary presentations42,43.
Non-operative treatments of SSEDH are also available but require specific case selection38. Groen compared functional outcomes in SSEDH patients treated conservatively (n=64) or operatively (n=474). The patients with mild clinical presentations (pain or negligible motor/sensory deficits) who preserve good neurological function and show no neurological decline could be followed conservatively. These patients should be observed closely in a neurosurgical setting. Any presentation denoting clinical deterioration requires immediate neurosurgical intervention.
Single/ multi-level status had no predictive value about the treatment modality (surgical versus conservative)44.
The authors would like to emphasize although the incidence of SSEDH and SARS-CoV-2 vaccination had a close temporal correlation it does not necessarily prove nor deny Sputnik V and SSEDH possible associations. There are emerging reports of the association between SARS-CoV-2 vaccines and thrombophilia/vasculitis in some vaccine products, but the data is not conclusive yet. The authors would like to reemphasize that the association between CKD stage 4 and SSEH in this case cannot be delineated yet. A higher number of reports and exact identification of molecular pathogenesis are required to picture possible correlations which are beyond the scope of this report and require a larger body of evidence. Finally, we would like to encourage all clinicians to consider any new neurological manifestation in SARS-CoV-2 infection as a possible neurological event and to approach the lesion(s) using the most precise modalities available.
Conclusion
SSEDH and COVID-19 vaccine coincidence is a rare clinical event, still, no solid association could be scientifically explained. Post-vaccination neurological events could be the harbinger of an ominous clinical situation. Any localizing neurological presentation should be thoroughly investigated by physical examination, MRI, and appropriate paraclinical modalities. Further studies are required for a reliable pathophysiologic association.
Ethics approval
All procedures performed were under the institutional and/or national research committee’s ethical standards and the 1964 Helsinki declaration and later amendments or comparable ethical standards. Isfahan University neurosurgery department board members supervised and approved this report on behalf of the Ethical Committee of Isfahan University of medical sciences.
Consent to participate
Informed consent was obtained from all individual participants included in the Study.
Consent for publication
An informed written consent was obtained from the patient. All authors have agreed on submission and or publication in the submitted journal.
Source of funding
Not applicable.
Author contribution
B.A. contributed to the conception of the work, data search, patient treatment, manuscript preparation, manuscript revision, final approval of the manuscript and agreed to be accountable for all aspects of the work. M.S. contributed to patient treatment, manuscript revision, final approval of the manuscript and agreed to be accountable for all aspects of the work. M.R. contributed to patient treatment, manuscript revision, final approval of the manuscript and agreed to be accountable for all aspects of the work. S.F. contributed to patient treatment, manuscript revision, final approval of the manuscript and agreed to be accountable for all aspects of the work. A.S. contributed to the conception of the work, data search, patient treatment, manuscript preparation, manuscript revision, final approval of the manuscript and agreed to be accountable for all aspects of the work. He is corresponding author of the manuscript. M.F. contributed to manuscript revision, final approval of the manuscript and agreed to be accountable for all aspects of the work. R.N. contributed to the conception of the work, data search, data gathering, patient treatment, manuscript preparation, manuscript revision, final approval of the manuscript and agreed to be accountable for all aspects of the work. M.S. contributed to patient treatment, funding processes, final approval of the manuscript and agreed to be accountable for all aspects of the work. S.B.M. contributed to patient treatment, manuscript revision, final approval of the manuscript and agreed to be accountable for all aspects of the work. A.S. contributed to manuscript revision, final approval of the manuscript and agreed to be accountable for all aspects of the work. S.V. contributed to manuscript revision, final approval of the manuscript and agreed to be accountable for all aspects of the work.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Not applicable.
Guarantor
Arman Sourani and Majid Rezvani.
Availability of data and material
Data and original images in the current study are available from the corresponding author on reasonable request. Authors can confirm that all relevant data are included in the article and/or its supplementary information files.
Provenance and peer review
Not invited.
Acknowledgements
This work is dedicated to our deceased professor Dr. Mahmoud Nourian who taught us everything until his final battle with COVID-19. The authors thank the patient and his family for their trust.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 8 December 2023
Contributor Information
Majid Rezvani, Email: M_rezvani@med.mui.ac.ir.
Masih Sabouri, Email: sabouri@med.mui.ac.ir.
Bahram Aminmansour, Email: aminmansour@med.mui.ac.ir.
Soheil Falahpour, Email: soheilfallahpour@gmail.com.
Arman Sourani, Email: Armansourani@gmail.com.
Mohammad Sharafi, Email: Sharafi.mohammad@gmail.com.
Sadegh Baradaran Mahdavi, Email: sadegh.b.mahdavi@gmail.com.
Mina Foroughi, Email: mina.f1994@gmail.com.
Roham Nik Khah, Email: Rohamnk101@gmail.com.
Armin Sourani, Email: arminsourani@gmail.com.
Shaahin Veisi, Email: ShaahinVeisi@outlook.com.
References
- 1.Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand 2020;142:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol 2020;19:767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mondal R, Deb S, Shome G, et al. COVID-19 and emerging spinal cord complications: a systematic review. Mult Scler Relat Disord 2021;51:102917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabouri M, Rezvani M, Aminmansour B, et al. Spontaneous intramedullary hematoma in a patient with COVID-19 infection: a case report. Clin Case Rep 2022;10:e05387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zübeyde Ö, Adem K, Samet D. A case of spontaneous thoracolumbar epidural hematoma in Covid-19 pneumonia. Int J Surg Case Rep 2022;90:106719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim SW, Wong E. Spontaneous epidural hematoma of the cervical spine in an elderly woman with recent COVID-19 infection: a case report. Am J Case Rep 2020;21:e926784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosseini R, Askari N. A review of neurological side effects of COVID-19 vaccination. Eur J Med Res 2023;28:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sourani A, Rezvani M, Foroughi M, et al. Spontaneous intramedullary hematoma following COVID-19 vaccination: a case report. Clinical Case Reports 2022;10:e6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agha RA, Franchi T, Sohrabi C, et al. The SCARE 2020 Guideline: updating Consensus Surgical CAse REport (SCARE) Guidelines. Int J Surg 2020;84:226–230. [DOI] [PubMed] [Google Scholar]
- 10.Shams Vahdati S, Ala A, Rahmanpour D, et al. Neurological manifestations of COVID-19 infection: an umbrella review. Egypt J Neurol Psychiatry Neurosurg 2021;57:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand 2020;142:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leasure AC, Khan YM, Iyer R, et al. Intracerebral hemorrhage in patients with COVID-19. Stroke 2021;52:e321–e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khedr EM, Karim AA, Soliman RK. Case report: acute spinal cord myelopathy in patients with COVID-19. Front Neurol 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow CCN, Magnussen J, Ip J, et al. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep 2020;13:e236720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang HY, Shah LM, McNally JS, et al. COVID-19-associated myelitis involving the dorsal and lateral white matter tracts: a case series and review of the literature. Am J Neuroradiol 2021;42:1912–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keyhanian K, Umeton RP, Mohit B, et al. SARS-CoV-2 and nervous system: from pathogenesis to clinical manifestation. J Neuroimmunol 2020;350:577436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 2020;11:995–998. [DOI] [PubMed] [Google Scholar]
- 18.Yachou Y, El Idrissi A, Belapasov V, et al. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci 2020;41:2657–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakhmola S, Indari O, Chatterjee S, et al. SARS-CoV-2, an underestimated pathogen of the nervous system. SN Compr Clin Med 2020;2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 2020;116:1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua AMU, Jamora RDG, Jose ACE, et al. Cerebral vasculitis in a COVID-19 confirmed postpartum patient: a case report. Case Rep Neurol 2021;13:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanafi R, Roger PA, Perin B, et al. COVID-19 neurologic complication with CNS vasculitis-like pattern. Am J Neuroradiol 2020;41:1384–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafiq A, Salameh MA, Laswi I, et al. Neurological immune-related adverse events after COVID-19 vaccination: a systematic review. J Clin Pharmacol 2022;62:291–303. [DOI] [PubMed] [Google Scholar]
- 24.Lu L, Xiong W, Mu J, et al. The potential neurological effect of the COVID-19 vaccines: a review. Acta Neurol Scand 2021;144:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan YJ, Chan KH, Hung IF. Safety and efficacy of COVID-19 vaccines: a systematic review and meta-analysis of different vaccines at phase 3. Vaccines (Basel) 2021;9:989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ismail II, Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J Neuroimmunol 2022;362:577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsiao Y-T, Tsai M-J, Chen Y-H, et al. Acute transverse myelitis after COVID-19 vaccination. Medicina (Kaunas) 2021;57:1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ismail II, Salama S. Association of CNS demyelination and COVID-19 infection: an updated systematic review. J Neurol 2022;269:541–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Román GC, Gracia F, Torres A, et al. Acute transverse myelitis (ATM):clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 Vaccine (AZD1222). Front Immunol 2021;12:653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cazzola M, Rogliani P, Mazzeo F, et al. Controversy surrounding the Sputnik V vaccine. Respir Med 2021;187:106569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akrami M, Hosamirudsari H, Faraji N, et al. Sputnik V vaccine-related complications and its impression on inflammatory biomarkers in healthcare providers. Indian J Med Microbiol 2023;43:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babamahmoodi F, Saeedi M, Alizadeh-Navaei R, et al. Side effects and immunogenicity following administration of the Sputnik V COVID-19 vaccine in health care workers in Iran. Sci Rep 2021;11:21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghaderi S, Mohammadi S, Heidari M, et al. Post-COVID-19 vaccination CNS magnetic resonance imaging findings: a systematic review. Can J Infect Dis Med Microbiol 2023;2023:1570830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raasck K, Habis AA, Aoude A, et al. Spontaneous spinal epidural hematoma management: a case series and literature review. Spinal Cord Ser Cases 2017;3:16043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figueroa J, DeVine JG. Spontaneous spinal epidural hematoma: literature review. J Spine Surg 2017;3:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deger SM, Emmez H, Bahadirli K, et al. A spontaneous spinal epidural hematoma in a hemodialysis patient: a rare entity. Intern Med 2009;48:2115–2118. [DOI] [PubMed] [Google Scholar]
- 37.Huang C-T, Lin C-H, Wang H-P, et al. Spontaneous cervical epidural hematoma in a patient with end-stage renal disease. Hemodial Int 2022;26:E5–E7. [DOI] [PubMed] [Google Scholar]
- 38.Sourani A, Vahdat N, Son C, et al. SARS-CoV-2 infection and spontaneous spinal hemorrhage: a systematic review. Neurosurg Rev 2023;46:300. [DOI] [PubMed] [Google Scholar]
- 39.https://www.ehealthme.com/vs/pfizer-biontech-covid-vaccine/spinal-epidural-haematoma/ COVID-19 vaccines spinal hematoma [Internet]. 2023.
- 40.Patel R, Kumar A, Nishizawa K, et al. Hemiparesis in spontaneous spinal epidural haematoma: a potential stroke imitator. BMJ Case Rep 2018;2018:bcr-2017-222686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimiwada T, Takahashi T, Shimizu H, et al. Clinical feature and surgical treatment of spontaneous spinal epidural hematoma. No Shinkei Geka 2004;32:333–338. [PubMed] [Google Scholar]
- 42.Shin JJ, Kuh SU, Cho YE. Surgical management of spontaneous spinal epidural hematoma. Eur Spine J 2006;15:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawton MT, Porter RW, Heiserman JE, et al. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg 1995;83:1–7. [DOI] [PubMed] [Google Scholar]
- 44.Groen RJ. Non-operative treatment of spontaneous spinal epidural hematomas: a review of the literature and a comparison with operative cases. Acta Neurochir (Wien) 2004;146:103–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and original images in the current study are available from the corresponding author on reasonable request. Authors can confirm that all relevant data are included in the article and/or its supplementary information files.