Abstract
To explore the risk factors and develop a nomogram to predict Double J stent encrustation incidence. The general demographic characteristics and underlying risk factors of 248 patients with upper urinary tract calculus who underwent endoscopic lithotripsy and Double J stenting at the Fifth Affiliated Hospital of Sun Yat-Sen University between January 1st, 2018 and January 1st, 2023 were retrospectively analyzed. Among them,173 patients were randomly selected to form the development cohort. A multivariate logistic regression model was employed to identify the independent risk factors associated with Double J stent encrustation, and a nomogram was developed for predicting its occurrence. Additionally, 75 patients were randomly selected to form the validation cohort to validate the nomogram. Multivariate logistic regression analysis revealed that several factors were significantly associated with Double J stent encrustation: indwelling time (odds ratio [OR]1.051; 95% confidence interval [CI] 1.030–1.073, P < .001), urine PH (OR 2.198; 95% CI 1.061–4.539, P = .033), fasting blood glucose (OR 1.590; 95% CI 1.300–1.943, P < .001), and total cholesterol (OR 2.676; 95% CI 1.551–4618, P < .001).Based on these findings, A nomogram was developed to predict the occurrence of Double J stent encrustation. The nomogram demonstrated good performance with an area under the curve of 0.870 and 0.862 in the development and validation cohorts, respectively. Furthermore, the calibration curve indicated a well-fitted model. We constructed and validated an accessible nomogram to assist urologists in evaluating the risk factors associated with Double J stent encrustation and predicting its likelihood.
Keywords: nomogram, obstruction, risk factors, stents, urolithiasis
1. Introduction
In the field of endourology today, Double J stenting has become one of the most commonly performed procedures for decompressing and relieving upper urinary tract obstruction. However, it is well-known that an indwelling Double J ureteric stent carries various complications, including stent encrustation, stone formation, hematuria, urinary tract infection, stent misplacement, and stent extraction failure.[1] Among these, the occurrences of stent encrustation and stone formation are particularly significant, as they can even develop within 1 to 2 weeks after insertion.[2] Once encrustation occurs, the stent becomes fragile and loses its flexibility, thereby increasing the risk of stent fracture and ureteral injuries during removal. Furthermore, stent encrustation can exacerbate lower urinary tract symptoms, secondary obstruction, infection, and impair renal function.[3] In severe cases, where encrustation spreads throughout the entire stent forming a Dumbbell-shaped structure, acute renal failure, sepsis and septic shock, and even death may occur. The management of Double J stent encrustation involves multimodular approaches such as extracorporeal shockwave lithotripsy with endoscopic intervention and even open surgeries.[4–6] These interventions impose significant physical and mental burdens on patients while adding to the strain on healthcare systems.
However, the risk factors and mechanism underlying Double J stent encrustation formation remain incompletely understood, and there is a lack of relevant guidelines for timely intervention. Therefore, it is of utmost importance to identify the risk factors and predict the probability of occurrence in order to effectively prevent complications related to Double J stent encrustation. While it is widely accepted that the indwelling time of double J stent placement is a primary risk factor,[7–9] there is inconsistency regarding other risk factors such as systemic or local urinary tract infections,[10–12] Physical and chemical parameters of the Double J stent, history of stone disease, and underlying medical history. These suggest that the formation of encrusted double J stents is influenced by multiple factors, and no single risk factor can accurately determine the likelihood of occurrence. Hence, the objective of our study is to identify the risk factors and develop a prediction model for preventing Double J stent encrustation-related complications. To achieve this, we retrospectively gathered data records from patients with upper urinary tract stones who underwent double J stenting at our institute. Subsequently, we analyzed the identified risk factors and established a nomogram for Double J stent encrustation.
2. Method
The study protocol was approved by the Ethics Committee of the Fifth Affiliated Hospital, Sun Yat-Sen University. Data were collected from patients with upper urinary tract stones who underwent endoscopic lithotripsy between January 1st, 2018 and January 1st, 2023.
2.1. Inclusion criteria
Patients who underwent endoscopic lithotripsy (ureteroscopic lithotripsy, retrograde intrarenal surgery, percutaneous lithotripsy, or a combination thereof).
The duration of Double J stent indwelling was between 2 weeks and 1 year.
The stent removal procedure was performed at our medical center.
2.2. Exclusion criteria
Patients with a history of renal transplantation, solitary kidney, urinary system malformation, spinal deformity, urinary or surrounding organ tumors, or severe lack of clinical information.
Patients who were pregnant.
Patients with apparent displacement of the Double J stent.
Patients under the age of 12 years old.
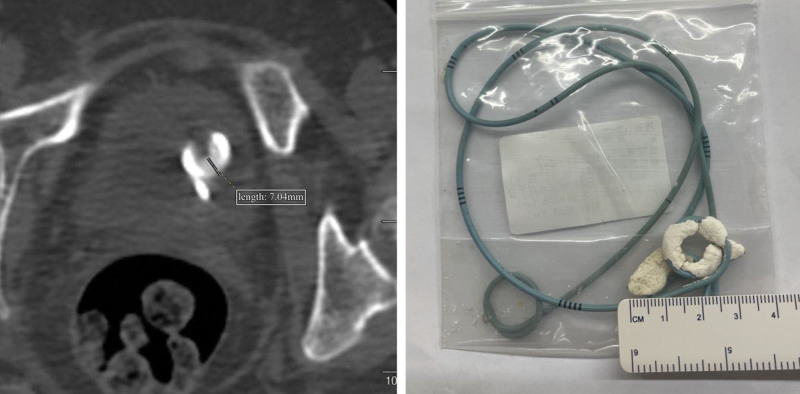
We categorized the included patients into 2 groups: the positive group and the negative group. The positive group consisted of patients with encrustation measurements ≥ 4 mm on computed tomography (CT) imaging or after stent removal, while the negative group included patients with encrustation diameters < 4 mm. These criteria were assessed by 2 urologists with over 10 years of clinical experience who reached a consensus for each subject. Figure 1 illustrates the 2 criteria for inclusion in the positive group.
Figure 1.
Illustration of two criteria for inclusion in the positive group.
We analyzed the medical records of the selected patients and collected the following factors as observational indicators: age, sex, history of urinary stone surgery, type of surgery, duration of surgery, brand and diameter of the stent, indwelling time of Double J stent, and the results of blood samples taken 1 week before lithotripsy. The blood sample parameters included red blood cell count, white blood cell count, platelet count, hemoglobin level, urine pH, urine white blood cell count, urine red blood cell count, urea, creatinine, uric acid, serum potassium, serum sodium, serum calcium, fasting blood glucose, alanine aminotransferase, albumin, triglyceride, and total cholesterol.
Statistical analysis was performed using IBM SPSS 25.0 software. Continuous variables with a normal distribution were presented as mean ± standard deviation and compared using independent-sample t-tests. For continuous variables with a non-normal distribution, median (interquartile range) was used, and group comparisons were conducted using the Mann–Whitney U test. Categorical data were expressed as number (%) and analyzed using the χ2 test or Fisher exact probability test. Missing data were handled using mean interpolation. To identify independent factors of Double J stent encrustation, univariate analysis was conducted in the development cohort to determine the significance of each variable. All variables showing a significant association with Double J stent encrustation were included as candidates for multivariate logistic regression analysis. All potential predictors were included in the analysis. The entry criterion for stepwise multivariate analysis was set at P < .05, and variables were retained in the logistic regression model if their P values were < .05. The results were reported as odds ratio with corresponding 95% confidence intervals (CIs).
R4.1.3 software was employed to develop a predictive model and construct a nomogram for predicting Double J stent encrustation. The predictive ability of the model was evaluated through discrimination and calibration. Discrimination was assessed by calculating the area under the receiver operating characteristic curve (AUC). Calibration was evaluated using the Hosmer–Lemeshow goodness-of-fit test, which measures the difference between the predicted probability and the observed probability. A calibration plot was generated based on these data. A P value > .05 indicates that there was no statistically significant difference between the predicted and actual probabilities, indicating a good fit of the model.
3. Result
After exclusions and eliminations, a total of 248 patients were enrolled in this study. The main reasons for exclusion were the absence of relevant surgical records or CT imaging before stent removal. Using the simple randomization method, the sample size was divided into 2 groups in a ratio of 7 to 3 by a second-year Master of Urology candidate. Consequently, the development cohort consisted of 173 patients (51 with double J stent encrustation), while the remaining 75 patients formed the validation cohort. The sample size meets the rule of “events per variable”.[13]
Table 1 presents the general demographic characteristics and observational factors of both the development and validation cohorts. Although there were statistical differences observed in indwelling time, serum sodium, and tube diameter, we strictly adhered to the principle of randomization. Overall, the majority of the observational factors did not significantly differ between the 2 groups. Therefore, we believe that the distribution of observational factors is comparable between the 2 groups.
Table 1.
General demographic characteristics and observation factors of the development and validation cohorts.
| Factor | Development Cohort(n = 173) |
Validation Cohort(n = 75) |
P value |
|---|---|---|---|
| Age[M(Q)] | 52 (17) | 50 (19) | .989 |
| Gender, n (%) | .355 | ||
| Male | 107 (61.8%) | 51 (68.0%) | |
| Female | 66 (38.2%) | 24 (32.0%) | |
| Stone surgery history, n (%) | .584 | ||
| Yes | 102 (59%) | 28 (37.3) | |
| No | 71 (41%) | 47 (62.7%) | |
| Surgery type, n (%) | .206 | ||
| URSL | 57 (32.9%) | 31 (41.3%) | |
| RIRS | 76 (43.9%) | 24 (32.0%) | |
| PNL | 40 (23.1%) | 20 (26.7%) | |
| Surgery duration [min, M(Q)] | 60 (61.5) | 55 (41) | .081 |
| Stent brand, n (%) | .481 | ||
| Brand 1 | 51 (29.5%) | 17 (22.7%) | |
| Brand 2 | 60 (34.7%) | 24 (32.0%) | |
| Brand 3 | 36 (20.8%) | 18 (24.0%) | |
| Brand 4 | 26 (15.0%) | 16 (21.3%) | |
| Lumen diameter, n (%) | .011 | ||
| F5 | 117 (67.6%) | 38 (50.7%) | |
| F6 | 56 (32.4%) | 37 (49.3%) | |
| Indwelling time [day, M(Q)] | 34 (17.5) | 26 (9) | <.001 |
| Red blood cell count [×1012/L, M(Q)] | 4.59 (0.9) | 4.70 (0.68) | .200 |
| White blood cell count [×109/L, M(Q)] | 6.85 (3.1) | 7 (3.32) | .921 |
| Platelet count [×109/L, M(Q)] | 239 (79.5) | 234 (87) | .687 |
| Urine PH [M(Q)] | 6 (0.5) | 6 (1) | .164 |
| Urine red blood cell count [/μl, M(Q)] | 349.8 (106.41) | 19.8 (118.72) | .101 |
| Urine white blood cell count [/μl, M(Q)] | 33.6 (93) | 37.62 (95.02) | .537 |
| Urea [mmol/L, M(Q)] | 5.3 (2.1) | 5.7 (2.63) | .080 |
| Creatine [μmol/L, M(Q)] | 81 (39.5) | 86 (34) | .351 |
| Uric acid [μmol/L, M(Q)] | 374 (135.5) | 377 (147) | .580 |
| Serum potassium [mmol/L, M(Q)] | 3.87 (0.5) | 3.8 (0.45) | .652 |
| Serum sodium [mmol/L, M(Q)] | 140 (3.6) | 138.9 (3.3) | .007 |
| Serum calcium [mmol/L, M(Q)] | 2.27 (0.1) | 2.27 (0.08) | .329 |
| Fasting blood glucose [mmol/L, M(Q)] | 5.36 (1.7) | 5.4 (1.64) | .687 |
| Alanine aminotransferase [U/L, M(Q)] | 19 (12) | 16.5 (12.1) | .829 |
| Albumin [g/L, M(Q)] | 41.7 (4.1) | 42.7 (3.71) | .290 |
| Total bilirubin [μmol/L, M(Q)] | 10.7 (5.2) | 9.7 (4.1) | .262 |
| Triglyceride [mmol/L, M(Q)] | 1.63 (0.9) | 1.48 (1.37) | .826 |
| Total cholesterol [mmol/L, M(Q)] | 4.46 (0.9) | 4.58 (1.1) | .158 |
RIRS = retrograde intrarenal surgery, URSL = ureteroscopic lithotripsy.
As shown in Table 2, Using univariate analysis, we analyzed the data of the 173 patients in the development cohort to explore the risk factors associated with Double J stent encrustation. The results showed that stent brand, indwelling time, urine PH, fasting blood glucose, and total cholesterol were related to Double J stent encrustation (P < .05). Therefore, the above 5 factors were included in the stepwise multivariate analysis.
Table 2.
Univariate analysis of factors related to encrustation (training cohort).
| Factor | Encrustation (n = 51) |
None-encrustation (n = 122) |
P value |
|---|---|---|---|
| Age[M(Q)] | 52 (15) | 50.5 (18.5) | .678 |
| Gender, n (%) | .120 | ||
| Male | 24 (47.1%) | 80 (65.6%) | |
| Female | 27 (52.9%) | 42 (34.4%) | |
| Stone surgery history, n (%) | .717 | ||
| Yes | 29 (56.9%) | 73 (59.8%) | |
| No | 22 (43.1%) | 49 (40.2%) | |
| Surgery type, n (%) | .505 | ||
| URSL | 10 (19.6%) | 30 (24.6%) | |
| RIRS | 21 (41.2%) | 55 (45.1%) | |
| PNL | 20 (39.2%) | 37 (30.3%) | |
| Surgery duration [min, M(Q)] | 65 (54) | 60 (65.75) | .515 |
| Stent brand, n (%) | .021 | ||
| Brand 1 | 23 (45.1%) | 28 (23%) | |
| Brand 2 | 13 (25.5%) | 47 (38.5%) | |
| Brand 3 | 11 (21.6%) | 25 (20.5%) | |
| Brand 4 | 4 (7.8%) | 22 (18.5%) | |
| Lumen diameter, n (%) | .595 | ||
| F5 | 33 (64.7%) | 84 (68.9%) | |
| F6 | 18 (35.3%) | 38 (31.1%) | |
| Indwelling time [day, M(Q)] | 39 (64) | 32.5 (14) | <.001 |
| Red blood cell count [×1012/L, M(Q)] | 4.46 (0.93) | 4.6 (0.82) | .745 |
| White blood cell count [×109/L, M(Q)] | 6.98 (3.0) | 6.76 (3.15) | .522 |
| Platelet count [×109/L, M(Q)] | 251 (64) | 233 (81.5) | .225 |
| Urine PH [M(Q)] | 6 (0.5) | 6 (0) | .004 |
| Urine red blood cell count [/μl, M(Q)] | 43.7 (231.28) | 51.92 (378.14) | .726 |
| Urine white blood cell count [/μl, M(Q)] | 29.01 (117.4) | 34.43 (83.9) | .903 |
| Urea [mmol/L, M(Q)] | 5.2 (2.01) | 5.3 (2.33) | .893 |
| Creatine [μmol/L, M(Q)] | 78 (39) | 82.5 (42.5) | .243 |
| Uric acid [μmol/L, M(Q)] | 375.9 (158) | 371.5 (129.25) | .650 |
| Serum potassium [mmol/L, M(Q)] | 3.9 (0.49) | 3.87 (0.52) | .226 |
| Serum sodium [mmol/L, M(Q)] | 140.1 (4) | 140 (3.13) | .597 |
| Serum calcium [mmol/L, M(Q)] | 2.26 (0.1) | 2.28 (0.11) | .614 |
| Fasting blood glucose [mmol/L, M(Q)] | 5.64 (3.43) | 5.2 (1.2) | <.001 |
| Alanine aminotransferase [U/L, M(Q)] | 19.27 (9) | 18.85 (14.08) | .383 |
| Albumin [g/L, M(Q)] | 42.3 (5) | 41.22 (3.93) | .102 |
| Total bilirubin [μmol/L, M(Q)] | 10.8 (5.4) | 10.6 (5.13) | .665 |
| Triglyceride [mmol/L, M(Q)] | 1.64 (1.26) | 1.62 (0.74) | .103 |
| Total cholesterol [mmol/L, M(Q)] | 4.78 (1.38) | 4.43 (0.83) | .001 |
RIRS = retrograde intrarenal surgery, URSL = ureteroscopic lithotripsy.
Multivariate logistic regression analysis with results reported as odds ratio (95% CI), indwelling time, urine PH, fasting blood glucose, and total cholesterol were significantly related to Double J stent encrustation. The results are shown in Table 3.
Table 3.
Multivariate analysis of factors related to encrustation.
| Factor | B | Wald χ² | P value | OR (95%CI) |
|---|---|---|---|---|
| Indwelling time | 0.050 | 23.426 | <.001 | 1.051(1.030–1.073) |
| Urine PH | 0.788 | 4.531 | .033 | 2.198 (1.064–4.539) |
| Fasting blood glucose | 0.463 | 20.424 | <.001 | 1.590 (1.300–1.943) |
| Total cholesterol | 0.984 | 12.503 | <.001 | 2.676 (1.551–4.618) |
CI = confidence interval, OR = odds ratio.
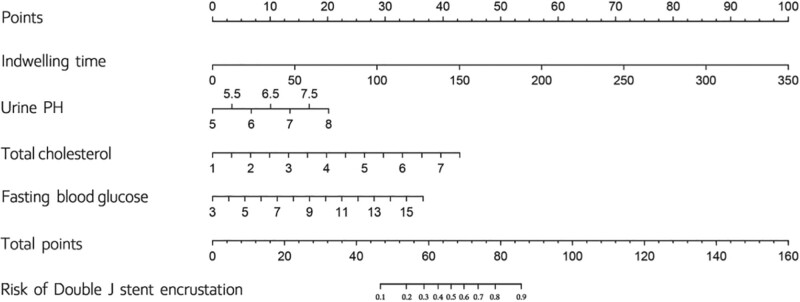
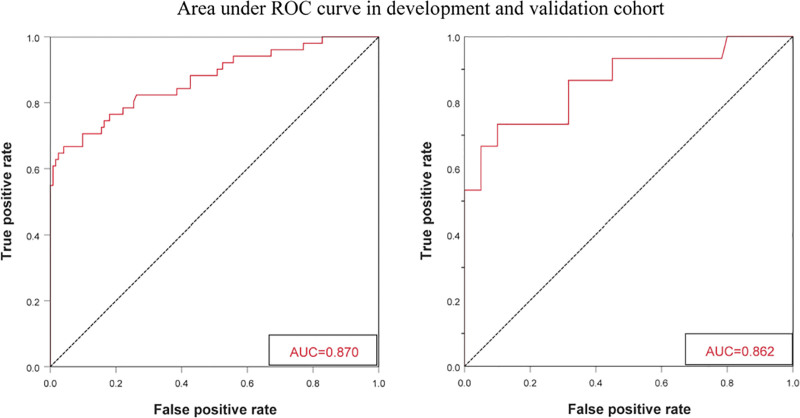
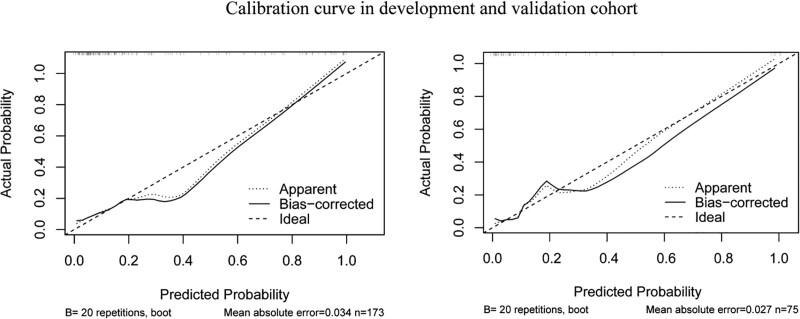
Figure 2 shows the nomogram formed to predict the risk of Ureteral stent encrustation based on these 4 parameters. The first step in using this nomogram is to find the corresponding position on the risk axis according to the clinical value of each risk factor, then draw a vertical line connecting the lower score axis to find the corresponding risk score, finally adding the 4 risk scores to obtain the total risk score for Double J encrustation. The nomogram showed good accuracy in estimating the risk of encrustation with an AUC of 0.87 (95% CI 0.805–0.934). in addition, Hosmer–Lemeshow goodness-of-fit test (X²=10.247, P = .248) and calibration plot demonstrated a high agreement between the predicted and actual results of Double J encrustation. In the validation cohort, the nomogram displayed an AUC of 0.862, and the calibration curve also showed a good performance. The receiver operator curve and calibration graph of the development and validation cohort are shown in Figure 3.and Figure 4.
Figure 2.
Nomogram for Double J stent encrustation.
Figure 3.
ROC curve and AUC for development and validation cohort. AUC = area under curve, ROC = receiver operator curve.
Figure 4.
Calibration curve for development and validation cohort.
4. Discussion
As one of the most common and harmful complications of Double J stenting, encrustation or stone, once formed, may bring serious consequences. In order to decrease stent complications, current research focuses on developing innovative products such as biodegradable, antibiofilm coatings and novel structure ureteral stents. While reports of fruitful efficacy for these products against encrustation exist, long-term and multi-center substantial research is still necessary to further verify their exact efficacy and safety,[3] which makes it challenging to popularize and apply in clinics in the short term. To resolve the current dilemma, the primary focus of our study is to elucidate the risk factors for stent encrustation and develop a model to predict its probability to guide clinicians to give effective and timely interventions. To our knowledge, this is the first model for predicting Double J stent encrustation.
The base of this study is how to accurately recognize the existence of encrustation. The previously established KUB scoring system is a system that uses renal CT or plain X-ray to predicate the severity of stent encrustation, it defines the encrustation as a maximum diameter of 5mm seen on CT or plain x-ray, and the effectiveness was proved by other hospital.[14,15]
Compared with the KUB scoring system, we have made 3 modifications: First, the “KUB” scoring system uses a plain abdominal x-ray as one of its tools to measure the largest diameter of Double J encrustation. However, the fact that plain abdominal film is challenging to make an accurate diagnosis due to its low resolution and poor anti-interference ability,[16] which tends to miss all uric acid stones. Thus, this study only uses 1 imaging tool (abdominal CT) as the evaluation standard. Secondly, even though the diagnostic performance of abdominal CT for Double J encrustation stones is excellent, there are still a small number of missed diagnoses. Hence, as a supplement, we added another diagnostic criterion: the measurement of the maximum diameter of Double J encrustation after extraction. Finally, lots of studies have used the “4mm” as the threshold for postoperative residual stone associated with postop complications such as infection and obstruction.[17] Therefore, we believe that the “maximum diameter of encrustation more than 4 mm” is more reasonable than the “maximum diameter of more than 5 mm” used in the KUB scoring system.
The risk factors of Double J encrustation are not fully understood. However, researchers generally believe that the Double J tube indwelling time is the most important independent risk factor.[7–9] In addition, infection, some physical and chemical parameters of the stent, certain underlying diseases of the patient, history of urinary calculi, metabolic disorders may also contribute to the formation of Double J encrustation.[10–12] In this study, we found that Double J indwelling time, urine PH, fasting glucose level, and total plasma cholesterol level had significant predictive value for Double J encrustation.
According to the results of regression analysis in our study, the longer the indwelling time of the double J stent, the higher the risk score for encrustation, and for every 1 additional day of indwelling time, the risk of encrustation formation increases by approximately 0.051 times. Additionally, based on the nomogram, among the 4 predictive factors, the indwelling time has the highest risk score and is the primary risk factor, and when the indwelling time exceeds 300 days, the probability of encrustation for the double J stent is at least 90%. Our conclusion is supported by many research. In 1991, El-Faqih SR et al[7] first compared the encrustation in 141 Double J stents and found that the probability of encrustation was 9.2%, 47.5%, and 76.3% when the Double J stent was left in situ for more than 6 weeks, between 6 and 12 weeks, and more than 12 weeks, respectively. In another similar study, Kawahara et al[8] set the same indwelling time interval as the above study and found the probability of encrustation to be 26.8%,56.9%, and 75.9%, respectively. Theoretically, appropriately shortening the indwelling time can reduce the risk of encrustation. However, it should be emphasized that as the duration of Double J stent placement shortens, its efficacy of urinary drainage and relief of ureteral edema will also be weakened, which may increase the risk of upper urinary tract obstruction tract infection and renal function impairment. The significance of shortening stent indwelling time differs from patient to patient,[18] and there is a lack of relevant studies to verify the optimal indwelling time. Thus, more relevant studies are needed.
The normal range of urine PH is 5.5 to 7.0, and its variation affects the solubility of crystalline components in urine. Low urine pH promotes the formation of uric acid stones and cystine stones, while high urine pH promotes the formation of calcium and magnesium stones, the latter being the main components of stent encrustation and playing a vital role in its progression.[19,20] However, in clinical practice, physicians often habitually alkalize urine to prevent encrustation formation, which is contrary to the view of our study too. In our study, regression analysis results suggest that elevated urine pH is an independent risk factor for the formation of Double J stent encrustation, and there is a positive correlation between the 2. For every 1 unit increase in urine pH, the risk of encrustation formation increases approximately 1.198 times. A recent RCT study also suggested that stent encrustation is less likely to develop when the urine pH is stable between a relevant low level of 5.5 and 6.2.[21] Based on similar view, Bard Company has introduced the pHreeCoat coated ureteral stent, which aims to prevent calcium salt deposition by maintaining urine pH at a lower level.[3]
Similar to urolithiasis, changes in certain metabolic levels in the body can also affect the formation of Double J stent encrustation. Akay et al found that even with routine prophylactic antimicrobial therapy, the probability of bacterial colony formation on Double J stents in diabetic patients was as high as 61%, and the probability of bacteriuria was more than 10 times higher than that in nondiabetic patients.[22] This conclusion was also confirmed by Kehinde et al[23] study. Our study suggests a positive correlation between blood glucose levels and the formation of Double J stent encrustation. For every 1 mmol/L increase in fasting blood glucose level, the probability of encrustation formation on Double J stents increases by approximately 0.590 times. This may be because high level of blood glucose stimulates the formation of colonies on double J stents and eventually result the encrustation core formation. In addition to glucose metabolism disorders, our study also found that serum cholesterol metabolism disorder is related to the formation of Double J stent encrustation. For every 1 mmol/L increase in plasma cholesterol, the probability encrustation formation on Double J stents increases by approximately 1.676 times, which is supported by a recent RCT study hold by Yoshida et al[2] The underlying principle may be that high levels of cholesterol ultimately promote the excretion of stone-forming components such as sodium, potassium, magnesium, calcium, and oxalate in urine, thereby promoting the deposition of encrustation of Double J stents immersed in urine. In summary, when providing medical education to patients about the Double J stent, physicians also need to emphasize the importance of controlling underlying metabolic disease, which urologists often overlook.
Nomogram has gained popularity among clinicians for their ability to present predictive models. As an example, if a patient undergoing upper urinary tract stone surgery with a Double J Double J Double J stenting was given a preoperative examination within a week before the surgery, in which his total plasma cholesterol level was 5 mmol/L, his fasting glucose value was 10 mmol/L, his urine pH was 5, and his expected to have stent indwelling time of 150 days. According to the nomogram, his total risk score is 87, with a risk of encrustation of 90%. Therefore, interventions, as described above, are essential. In conclusion, our nomogram can help urologists to develop a better management plan for patients at risk of Double J encrustation.
In the end, there are some shortcomings in this study. First, this is a retrospective single-center study. Second, the strict inclusion criteria for accurate enrollment made the sample size of this study small. However, our findings are supported by the corresponding literature to ensure the credibility of this study. Of course, to ensure the study’s quality, this study needs further sample size expansion and multi-center verification before being clinically applicable.
5. Conclusion
In this retrospective study, we established a mathematical prediction model that included 4 risk factors for Double J stent encrustation and developed a nomogram that can be used to calculate its occurrence. This nomogram can help urologists predict Double J stent encrustation and prevent the relevant complications. Although the nomogram has predictive value, further comprehensive analysis and dynamic monitoring are needed for patients planning to undergo Double J stenting.
Acknowledgments
We would like to thank the study participants, data collectors for their unreserved help. Finally, we are grateful to those who directly or indirectly supported us.
Author contributions
Conceptualization: Zicheng Liu, Yingbo Dai.
Data curation: Zicheng Liu, Junliang Qiu.
Formal analysis: Yingbo Dai.
Investigation: Zicheng Liu, Minbo Yan, Yingbo Dai.
Methodology: Zicheng Liu, Minbo Yan, Junliang Qiu.
Resources: Minbo Yan.
Software: Zicheng Liu, Yaser Naji, Haojie Wang, Yuteng Lin.
Supervision: Zicheng Liu, Minbo Yan.
Validation: Zicheng Liu, Minbo Yan, Yaser Naji.
Visualization: Zicheng Liu, Minbo Yan.
Writing – original draft: Zicheng Liu.
Writing – review & editing: Zicheng Liu, Yaser Naji.
Abbreviations:
- AUC
- area under curve
- CI
- confidence interval
- CT
- computed tomography
- OR
- odds ratio
- URSL
- ureteroscopic lithotripsy
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
ZL and MY contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Liu Z, Yan M, Naji Y, Qiu J, Wang H, Lin Y, Dai Y. Can Double J stent encrustation be predicted by risk analysis and nomogram?: A retrospective case–control study. Medicine 2024;103:2(e35303).
Contributor Information
Zicheng Liu, Email: liuzc327@163.com.
Minbo Yan, Email: yanminbo1986@163.com.
Yaser Naji, Email: dryanaji@gmail.com.
Junliang Qiu, Email: qjlstruggling@163.com.
Haojie Wang, Email: wonghaoj@163.com.
Yuteng Lin, Email: 312895406@qq.com.
References
- [1].Saltzman B. Ureteral stents: indications, variation and complications. J Urol. 1989;141:1278–1278. [PubMed] [Google Scholar]
- [2].Yoshida T, Takemoto K, Sakata Y, et al. A randomized clinical trial evaluating the short-term results of ureteral stent encrustation in urolithiasis patients undergoing ureteroscopy: micro-computed tomography evaluation. Sci Rep. 2021;11:10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tomer N, Garden E, Small A, et al. Ureteral stent encrustation: epidemiology, pathophysiology, management and current technology. J Urol. 2021;205:68–77. [DOI] [PubMed] [Google Scholar]
- [4].Singh I, Gupta NP, Hemal AK, et al. Severely encrusted polyurethane ureteral stents: management and analysis of potential risk factors. Urology. 2001;58:526–31. [DOI] [PubMed] [Google Scholar]
- [5].Vanderbrink BA, Rastinehad AR, Ost MC, et al. Encrusted urinary stents: evaluation and endourologic management. J Endourol. 2008;22:905–12. [DOI] [PubMed] [Google Scholar]
- [6].Xu C, Tang H, Gao X, et al. Management of forgotten ureteral stents with holmium laser. Lasers Med Sci. 2009;24:140–3. [DOI] [PubMed] [Google Scholar]
- [7].El-Faqih SR, Shamsuddin AB, Chakrabarti A, et al. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times J Urol. 1991;146:1487–91. [DOI] [PubMed] [Google Scholar]
- [8].Kawahara T, Ito H, Terao H, et al. Ureteral stent encrustation, incrustation, and coloring: morbidity related to indwelling times. J Endourol. 2012;26:178–82. [DOI] [PubMed] [Google Scholar]
- [9].Huang J, Wu W, Zhang S, et al. Characteristics of double-J stent encrustations and factors associated with their development. Urol J. 2021;19:22–7. [DOI] [PubMed] [Google Scholar]
- [10].Rebl H, Renner J, Kram W, et al. Prevention of encrustation on ureteral stents: which surface parameters provide guidance for the development of novel stent materials?. Polymers (Basel). 2020;12:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sighinolfi MC, Sighinolfi GP, Galli E, et al. Chemical and mineralogical analysis of ureteral stent encrustation and associated risk factors. Urology. 2015;86:703–6. [DOI] [PubMed] [Google Scholar]
- [12].Akay AF, Aflay U, Gedik A, et al. Risk factors for lower and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol. 2007;39:95–8. [DOI] [PubMed] [Google Scholar]
- [13].Peduzzi P, Concato J, Kemper E, et al. A simμlation study of the nμmber of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9. [DOI] [PubMed] [Google Scholar]
- [14].Arenas JL, Shen JK, Keheila M, et al. Kidney, Ureter, and Bladder (KUB): a novel grading system for encrusted ureteral stents. Urology. 2016;97:51–5. [DOI] [PubMed] [Google Scholar]
- [15].Kartal IG, Baylan B, Gok A, et al. The association of encrustation and ureteral stent indwelling time in urolithiasis and KUB grading system. Urol J. 2018;15:323–8. [DOI] [PubMed] [Google Scholar]
- [16].Weedin JW, Coburn M, Link RE. The impact of proximal stone burden on the management of encrusted and retained ureteral stents. J Urol. 2011;185:542–7. [DOI] [PubMed] [Google Scholar]
- [17].Chew BH, Brotherhood HL, Sur RL, et al. Natural history, complications and re-intervention rates of asymptomatic residual stone fragments after ureteroscopy: a report from the EDGE research consortiμm. J Urol. 2016;195(4 Part 1):982–6. [DOI] [PubMed] [Google Scholar]
- [18].Imam MS, Al Farooq MA, Sarwar MKA, et al. A comparison between short-and long-term DJ stent in Anderson–Hynes pyeloplasty for pelvi-ureteric junction obstruction. Pediatr Surg Int. 2020;36:1363–70. [DOI] [PubMed] [Google Scholar]
- [19].Bariol S, Farebrother T, Ruthven S, et al. Comparison of urinary stone and stent encrustation: biochemical analysis. J Endourol. 2003;17:741–3. [DOI] [PubMed] [Google Scholar]
- [20].Rouprêt M, Daudon M, Hupertan V, et al. Can ureteral stent encrustation analysis predict urinary stone composition?. Urology. 2005;66:246–51. [DOI] [PubMed] [Google Scholar]
- [21].Torrecilla C, Fernández-Concha J, Cansino JR, et al. Reduction of ureteral stent encrustation by modulating the urine pH and inhibiting the crystal film with a new oral composition: a multicenter, placebo controlled, double blind, randomized clinical trial. BMC Urol. 2020;20:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Akay AF, Aflay U, Gedik A, et al. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol. 2007;39:95–8. [DOI] [PubMed] [Google Scholar]
- [23].Kehinde EO, Rotimi VO, Al-Awadi KA, et al. Factors predisposing to urinary tract infection after J ureteral stent insertion. J Urol. 2002;167:1334–7. [PubMed] [Google Scholar]