Abstract
Introduction:
Sickle cell disease (SCD) is a rare genetic disease with limited therapeutic options. Gene-based therapies are being investigated in clinical trials to evaluate their curative potential. The expected life-long benefits of one-time administration of genetically corrected stem cells present uncharted challenges in estimating value of these treatments. Our objective is to conduct a landscape analysis of clinical trials and prompt a discussion estimating the value of gene therapy as a therapeutic option for SCD.
Areas covered:
We searched Clinicaltrials.gov to identify and characterize clinical trials in gene therapies for SCD. We report available results and discuss current concerns and elements of value necessary to consider as these products come to market.
Expert opinion:
Gene therapies could represent a major advance in SCD treatment. Although clinical trials are ongoing, reports of serious adverse events have led to pause of these trials, emphasizing the need to prove long-term tolerability. Measured using the methods of health economic evaluation, we anticipate high up-front costs may be offset by potential life-long benefits of these treatments. During development and after treatment approval, attention should be focused on ensuring adequate availability and equitable access to emerging therapies in underserved areas and low-middle-income countries (LMIC).
Keywords: sickle cell disease, hematopoietic stem cell, gene therapy, gene addition, gene editing, value
1. Introduction
Gene-based therapies involve the introduction of exogenous genes into autologous or allogeneic cell types, altering the DNA of a cell, or modifying the expression of genes for the purpose of treating the underlying cause of a disease rather than simply treating its downstream symptoms[1]. Gene-based approaches to therapy are currently being evaluated for the purpose of correcting inherited genetic disorders such as cystic fibrosis, familial hypercholesterolemia, and hemoglobinopathies, including sickle cell disease (SCD)[1].
SCD is among the most prevalent and clinically significant of the hemoglobinopathies and occurs when a sickle hemoglobin (HbS) polymerizes on deoxygenation, reducing the deformability of red blood cells. This causes painful vasoocclusive episodes (VOEs), irreversible organ damage, poor quality of life, and reduced life expectancy. Although allogeneic hematopoietic stem-cell transplantation (HSCT) is a currently available curative option offered to certain children with SCD with a matched sibling donor, fewer than 18% of patients have access to a matched donor[2]. In addition, although HLA-mismatched donor transplants are possible, outcomes are not yet optimal and continue to be improved. Due to their potentially curative nature, without the need for a matched donor or risks of graft versus host disease, gene therapies may provide more accessible, long-term treatments for SCD. As gene therapies emerge, estimates of their value compared to available treatments will be useful in guiding treatment strategies that provide the greatest benefit to patients[3]. Within the National Heart, Lung and Blood Institute, National Institutes of Health, the Cure Sickle Cell Initiative (CureSCi), ‘is a collaborative, patient-focused research effort dedicated to accelerating development of treatments aimed at providing genetic-based cures in SCD’[4]. Investigators in the ‘blinded’ Consortium of the CureSCi are charged with creating a decision-modeling framework to compare estimates of value for these gene-based therapies to currently available therapies. The model will capture the disease and treatment trajectory of individuals with SCD over the lifetime horizon. In preparation for the modeling work, this landscape analysis is intended to systematically identify and characterize current gene therapy trials and their associated long-term follow-up studies in SCD, as represented in ClinicalTrials.gov. We first provide a general overview of gene-based therapies, followed by an overview of SCD treatments. We then accomplish two objectives. We first report the results of our landscape analysis and then discuss results of the gene-therapy trials in the context of SCD. We next discuss the potential value of using gene therapy in reducing lifetime healthcare use, improving productivity, and improving quality of life for SCD patients and families. We conclude with a discussion of the importance of ensuring adequate availability of and equitable access to these emerging therapies.
2. Overview of gene-based therapies
Current approaches to gene-based therapies consist of two molecular gene modification approaches: gene addition and gene editing. Both approaches are being intensively studied for their use in treating inherited genetic disorders such as SCD. Hematopoietic stem cells (HSC) are ideal targets for gene-based therapies due to their high potential for longevity and their ability of self-renewal[5]. HSCs are collected after mobilization into blood or via direct harvest from bone marrow and undergo ex vivo gene manipulation and effective gene modification[6]. Once the safety and quality of the modified product has been assessed, a conditioning regimen is used to weaken or eliminate the patients sickle cell-producing marrow and is then followed by infusion of the modified HSCs [7]. By infusing autologous cells, graft versus host disease (GVHD), that is often seen in allogeneic HSCT, has not been a factor to date in gene therapy trials.
Gene addition refers to the addition of an exogenous gene or genes into autologous cells to compensate for an abnormal gene causing a certain disease. In gene addition, a vector containing the transferred gene of interest, also known as a transgene, is used to deliver and integrate one or more genes into the genome of a patient’s stem cells to correct deficiencies by expressing itself for, ideally, the entirety of the patient’s life[5]. Unlike gene addition, gene editing may be conducted without the addition of transgenes into the human genome, allowing for direct, site-specific altering of the endogenous genomic sequence of a cell, decreasing the risks posed by randomly inserting genes. Several nucleases have been developed for genetic editing, and may be introduced into cells as a recombinant protein and are able to induce DNA breaks in a targeted region to enable an array of desired gene modifications such as gene disruption and correction[8]. However this is not without risk, as off target edits may impact normal gene function and lead to mutations.
To achieve stable engraftment of modified HSC after infusion, administration of a conditioning regimen, likely a chemotherapy- and/or radiotherapy-based preparation, is required for myeloablation[9]. Current conditioning regimens, such as the commonly used, busulfan, often contribute to the overall side effect profile of gene therapy as they cause severe pancytopenia such as severe neutropenia and thrombocytopenia, and later infertility. Current research effort is focused on strategies to assure engraftment of the therapeutic product while limiting toxicity.
Due to the sizable benefits of potentially curative therapies, including gene therapy, the Food and Drug Administration (FDA) has designed several pathways to accelerate marketing authorization and product launch, especially for rare diseases. Quinn et al. conducted a study to estimate future cell and gene therapy launches, penetration rate, and predicted size of eligible and treated patient populations for the launched products. Of the various indications studied, hematology was one of the leading indications with five expected launches by 2030[10].
3. Overview of sickle cell disease and treatments
SCD is one of the most commonly occurring, severe monogenic disorders worldwide. SCD affects multiple-organ systems and is associated with episodes of acute illness and progressive organ damage. SCD is the overarching term used to describe diseases of a characteristic clinical syndrome that can be specified by genotype. SCD affects approximately 100,000 individuals in the United States (US) with one SCD case occurring among 365 Black or African American births and one out of 16,300 Hispanic American births[11]. Global estimates of birth prevalence of homozygous SCD is 0.11% with a birth prevalence in Africa of 1,125 per 100 000 compared with 43.12 per 100,000 in Europe[12]. Nearly 75% of all SCD births occur in sub-Saharan Africa[13].
SCD is caused by a mutation in the β-globin gene, in which, HbS can polymerize following deoxygenation, resulting in the sickling and lysis of erythrocytes. The aggregation of mutated HbS-laden red cells leads to a series of alterations resulting in vaso-occlusion which promotes ischemia-reperfusion injury [14]. Along with the characteristic clinical syndromes such as severe pain episodes, acute chest syndrome, and stroke; natural disease progression involves other organ systems and complicated combinations of social determinants of health and the result of chronic illness with limited to no available therapies, which may contribute to mental health disorders[15].
The projected life expectancy and quality-adjusted life expectancy for patients with SCD in the US is 54 years and 33 years, respectively[16]. Using population-based surveillance data from California and Georgia from 2004 to 2008, the average age at death was 42.4 years (SD 16.9)[17]. The estimated mortality rate was 0.64 per 100 years of child observation with the highest rate of 7.3 per 100 seen in Africa[12]. A study investigating mortality rates in children and adults with SCD revealed that between 1975 and 2005, the mortality rate for adults 19 years and older increased by 1% per year, while in the pediatric population, mortality decreased by 3% per year[18]. The increased mortality rate in adults may reflect the possibility that SCD patients who would have died earlier in childhood, due to severity of disease, are now surviving to adulthood and thus shifting the curve as they are more likely to die sooner than those with less severe disease.
Current mainstay treatments for SCD are hydroxyurea, blood transfusions, and hematopoietic stem cell transplantation. Use of these therapies is limited by various barriers such as poor acceptability, low number of SCD specialists, increased numbers of health-care visits, and lack of matched donors. Moreover, availability of blood transfusions is limited to countries in which medical care is accessible on a long-term basis. In recent years, newer therapies have been approved in the US, such as crizanlizumab, voxelotor, and L-glutamine. Although approved for their positive impact on reduction of acute sickle cell events and hemoglobin response, these newer treatments have not yet been investigated for their efficacy in improving quality of life or prevent organ damage and death[19]. In addition, not all these newer agents are approved outside of the US.
4. Gene therapy in the context of sickle cell disease
Due to the limited availability of matched sibling donors and the high risk of transplantation-related risks associated with allogeneic HSCT, ex-vivo gene therapy is being extensively studied and tested in numerous clinical trials as a potential cure for SCD. Autologous HSCT would eliminate the need for donors, in theory, the risk of transplant rejection and GVHD, a potentially fatal condition, because the genetically modified cells are of patient origin[20]. Gene modification approaches could allow production of functionally improved RBCs in SCD. However, for gene therapy in SCD to be successful, three main objectives must be achieved to address technical complexity: (1) safe and efficient gene transfer or correction of long-term repopulating HSCs (2) high-level, appropriately regulated, stable gene expression and (3) reduction in risk of transplant rejection[21]. In addition, important factors such as long-term safety profiles, patient acceptance, unknown risks, and costs will affect the success of gene therapy in SCD and therefore must be considered.
Gene addition therapies studied in SCD involve anti-sickling β-globin or γ-globin gene transfer into lentiviral vectors. Lentiviral vectors provide stable gene addition, allow the ability to transduce nondividing HSCs, contain a larger capacity for DNA, and have a safe integration profile [21]. The defining mutation of the sickle allele occurs in the β-globin gene and therefore, developing a modified β-globin with antisickling activity may be advantageous. Use of the γ-globin gene is based on the observation that HbF (α2γ2) is a more potent anti-sickling hemoglobin when compared to the adult hemoglobin (α2β2), and therefore may require lower gene expression[21].
An additional gene therapy strategy used in SCD is gene editing. Targeted genome engineering using nucleases such as, zinc finger nucleases (ZFNs) and clustered regulatory-interspaced short palindromic repeat-associated nuclease/ CRISPR associated protein 9 (CRISPR/Cas9), are being studied in SCD to allow the introduction of site-specific DNA breaks into the human genome, followed by repair mechanisms, that ultimately correct the sickle mutation[21]. Instead of correction of the sickle mutation, a current target for gene editing in SCD is increased HbF endogenous production. A key protein in the regulation of HbF is BCL11A, which is required for γ-globin gene repression in adult erythropoiesis and therefore, HbF regulation in primary human cells. [21-23] Loss of erythroid-specific BCL11A in mouse models of SCD showed reversal of the hematologic and pathologic signs of the disease, thereby validating it as a therapeutic target[24]. In determining a therapeutic level of HbF, both the level per cell and percent of cells with this level are key (i.e. ability to achieve HbF pancelullarity). While these thresholds have not been definitively established, scientists have appreciated that SCD patients with increased levels of HbF >30% in a more pancellular pattern have an attenuated clinical course[25].
5. Landscape analysis current clinical trials for gene therapy in SCD
5.1. Methods
On 13 November 2020, we searched the Clinicaltrials.gov databases using a protocol that pre-specified treatment of SCD or thalassemia with gene therapy that were being conducted globally. We identified studies using at least one of the following search terms: ‘Sickle Cell Disease’ AND ‘Gene’; ‘Sickle Cell Disease’ AND ‘Gene therapy’; ‘Sickle Cell Thalassemia’ AND ‘gene’; ‘Sickle Cell Thalassemia’ AND ‘Gene therapy’; ‘Sickle Cell Disease’ AND ‘Autologous’; ‘Sickle Cell Disease’ AND ‘CRISPR’; ‘Sickle Cell Anemia’ AND ‘Gene’; ‘Sickle Cell Anemia’ AND ‘Gene therapy’; or ‘Sickle Cell Disease’ AND ‘BCL.’ Search terms were developed in consultation with a physician scientist and hematologist whose clinical practice consists of SCD patients (MAB).
One reviewer (DQ) extracted the main characteristics of each study. After duplicates were removed, the reviewer independently screened the titles, conditions, and intervention. We included multinational clinical trials that examined the safety and efficacy of gene addition therapy and gene-editing therapy for SCD. Studies were included if the clinical trials included a study intervention that was gene therapy treatment in SCD patients or if the long-term extension studies corresponded with a clinical trial for gene therapy in SCD patients. Studies were not included if identified as terminated. After the first reviewer extracted information populated from the searches and screened the trials for eligibility, an evidence synthesis methodologist (BD) and the clinician/scientist (MAB) confirmed the decisions. The content of this manuscript reflects the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement. Clinical trial results were continuously updated as reports were released.
5.2. Results
5.2.1. Study selection
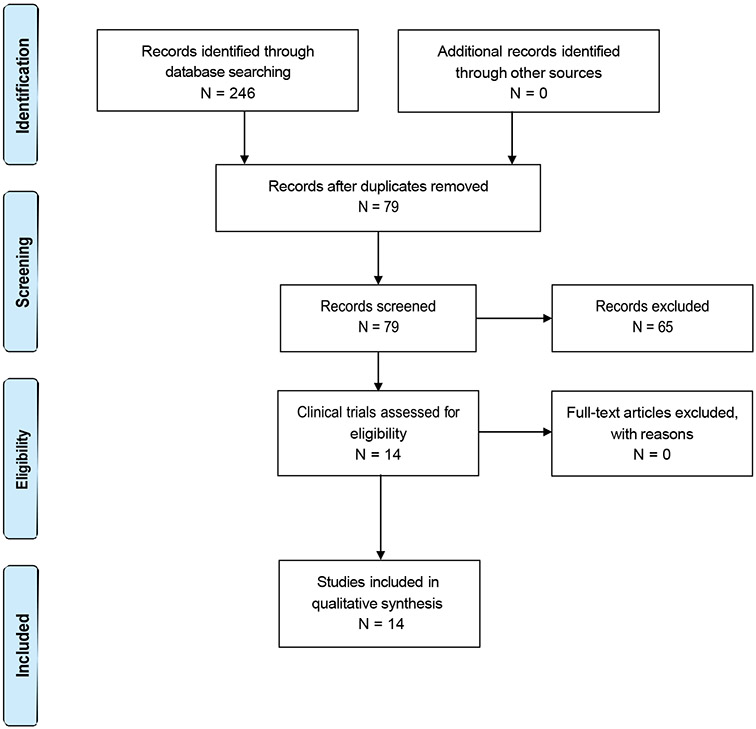
The PRISMA flow diagram of study selection and exclusion is presented in Figure 1. Our search identified 246 clinical trials or observational studies. After removing duplicates, we screened the titles, sickle cell conditions, and interventions of 79 studies. Sixty-five studies were excluded; 14 studies met all our inclusion criteria.
Figure 1.
PRISMA flow diagram.
5.2.2. Overview of included trials and observational studies
The study characteristics of the 14 included trials are found in Table 1. Of the 14 trials, 11 are interventional clinical trials and the remaining three studies are extension studies. Ten unique gene-based interventions are being evaluated (Table 2). Seven interventional trials evaluate gene addition therapy; of those, five use a β-globin-containing mobile gene element known as a gene cassette, and two use a γ-globin gene cassette. The remaining four interventional trials target BCL11A for gene editing or gene silencing. Table 3 provides detail on recruitment status, inclusion criteria, estimated study completion dates, and outcomes. For the clinical trials, severe disease is required for inclusion in all eleven. Inclusion criteria for the three long-term follow-up studies are simply that enrollees have been previously treated with a gene-based therapy product in the context of an interventional trial. Outcomes being evaluated are specific to each study, and range widely from overall survival and occurrence of adverse events, to successful neutrophil or platelet engraftment, from neutropenia, to malignancy, to number of required transfusions. Adverse events being evaluated include, but are not limited to, new malignancies, allergic reactions, infection, and organ toxicity.
Table 1.
Study characteristics.
| Phases | |||
|---|---|---|---|
| Phase 1 | 3 | ||
| Phase 1/2 | 6 | ||
| Phase 3 | 1 | ||
| Recruitment/enrollment status | |||
| Recruiting | 7 | Enroll by invitation | 3 |
| Completed | 1 | ||
| Active, not recruiting | 2 | ||
| Suspended | 1 | ||
| Enrollment | 3 to 50 patients | Enrollment | 85 to 94 patients |
| Farthest out completion date | 2035 | Farthest out completion date | 2039 |
| Population | |||
| Age | 3 to no upper limit | Age | No limit |
| Genders | All; one did not state | Genders | All |
| Location | |||
| Multinational | 2 | Multinational | 3 |
| US-based | 8 | ||
| France-based | 1 | ||
| Inclusion Criteria | |||
| Severe SCD | 10 | Treated with drug product | 3 |
| Hydroxyurea failure | 7 | ||
| Hydroxyurea willingness to DC | 2 | ||
| Hydroxyurea not mentioned | 2 |
Table 2.
Types of gene therapy in current clinical trials.
| Description (Trials) | Number of Trials | |
|---|---|---|
| Open-label trials | 11 | |
| Sickle Cell Disease (SCD) | 9 | |
| Sickle Cell Anemia | 3 | |
| Intervention | NCT Numbers | |
| Gene addition, modified β:β-AT87Q | 3 | 02,140,55402,151,52604,293,185 |
| Gene addition, modified β:β-AS3 | 1 | 03,964,792 |
| Gene addition, modified β:β-AS3-FB | 1 | 02,247,843 |
| Gene addition, modified γ:ARU-1801 | 1 | 02,186,418 |
| Gene addition, modified γ: with siRNA for selection:γ-G16D-RNA734 | 1 | 04,091,737 |
| Gene silencing, BCH-BB694 short hairpin RNA targeting:BCL11a | 1 | 03,282,656 |
| Zn finger editing of BCL11a:BIVV003 | 1 | 03,653,247 |
| CRISPR editing of BCL11a:CTX001 | 1 | 03,745,287 |
| Gene editing of BCL11a:OTQ923 and/or HIX763 | 1 | 04,443,907 |
| Description (Studies) | Number of Trials | |
| Long-term follow-up studies | 3 | |
| Disease | ||
| Sickle Cell Disease (SCD) | 3 | |
| Sickle Cell Anemia | 0 | |
| Intervention | ||
| γ-AT87Q | 2 | 02,633,94304,628,585 |
| CTX001 | 1 | 04,208,529 |
Table 3.
Clinical trials and study characteristics.
| NCT Number |
Title | Phase, Study Design, Sample Size. Intervention |
Recruitment/ Enrollment Status |
Disease/ Population/ Location | Primary Outcome | Key Inclusion Criteria |
|---|---|---|---|---|---|---|
| NCT 02140554 | A Study Evaluating the Safety and Efficacy of the bb1111 in Severe Sickle Cell Disease | Phase 1/2, open-label, n=50 Gene addition, modified β: β-AT87Q |
Active, not recruiting; Estimated completion date: 3/2023 |
SCD with either βS/βS or βS/β0 or (βS/β+ genotype Aged 12 to 50 years old All genders United States |
Proportion of subjects achieving complete resolution of severe vaso-occlusive events, between 6 months and 18 months after drug product infusion |
|
| NCT 02151526 | A Study Evaluating the Safety and Efficacy of LentiGlobin BB305 Drug Product in β-Thalassemia Major (Also Referred to as Transfusion-dependent β-Thalassemia [TDT]) and Sickle Cell Disease | Phase 1/2, open-label, n=7 Gene addition, modified β: β-AT87Q |
Completed; Completion date: 2/16/2019 | Severe SCD or transfusion dependent β-thalassemia major, regardless of the genotype with the diagnosis confirmed by Hb studies. (Transfusion dependence defined as requiring at least 100 mL/kg/year of packed red blood cells (pRBCs).) Aged 5 to 35 year old All genders France |
|
|
| NCT 02186418 | Gene Transfer for Patients with Sickle Cell Disease | Phase 1/2, open-label, n=10 Gene addition, modified γ: ARU-1801 | Recruiting; Estimated completion date: 6/2035 | Sickle cell disease (SCD) Aged 18 to 45 years old All genders United States, Canada, Jamaica |
|
|
| NCT 02247843 | Stem Cell Gene Therapy for Sickle Cell Disease | Phase 1/2, open-label, n=6 Gene addition, modified β: β-AS3-FB |
Recruiting; Estimated completion date: 8/2022 | SCD documented by genetic analysis (SIS, S/β-thalassemia-zero) Aged 18 years or older All genders University of California, Los Angeles (UCLA), United States |
|
|
| NCT 02633943 | Long term Follow-up of Subjects with Hemoglobinopathies Treated with Ex Vivo Gene Therapy | Follow-up, observational n=94 β-AT87Q |
Enrolling by invitation; Estimated completion date: 3/1/2031 |
β-thalassemias and SCD Up to the age of 50; all genders United States, Australia, France, Italy, Thailand |
|
|
| NCT 03282656 | Gene Transfer for Sickle Cell Disease | Phase 1, open-label, n=15 Gene silencing, BCH-BB694 lentiviral vector (short hairpin RNA targeting BCL11a) |
Active, not recruiting; Estimated completion date: 4/13/2024 |
SCD with genotype HbSS, HbS/0 thalassemia, HbSD, or HbSO Age 3 to 40 years old All genders Boston Children’s Hospital, United States |
Rescue of hematopoiesis after conditioning |
|
| NCT 03653247 | A Study to Assess the Safety, Tolerability, and Efficacy of BIVV003 for Autologous Hematopoietic Stem Cell Transplantation in Patients with Severe Sickle Cell Disease | Phase 1/2, open-label, n=8 Zn finger editing of BCL11a:BIVV003 |
Recruiting; Estimated completion date: 11/2023 |
SCD (HbSS or HbSβ0 genotype) Age 18 to 40 years old All genders UCSF Benioff Children’s Hospital; University of California Davis Comprehensive Cancer Center; Children’s Healthcare of Atlanta; Karmanos Cancer Institute, United States |
|
|
| NCT 03745287 | A Safety and Efficacy Study Evaluating CTX001 in Subjects with Severe Sickle Cell Disease | Phase 1/2, open-label, n=45 CRISPR editing of BCL11a:CTX001 |
Recruiting; Estimated completion date: 5/2022 |
Severe SCD (by genotype) Age 18 to 35 years old United States, Belgium, Canada, Germany, Italy |
|
|
| NCT 03964792 | Safety and Efficacy of Gene Therapy of the Sickle Cell Disease by Transplantation of an Autologous CD34+ Enriched Cell Fraction That Contains CD34+ Cells T ransduced ex Vivo with the GLOBE1 Lentiviral Vector Expressing the Œ≤AS3 Globin Gene in Patients with Sickle Cell Disease (DREPAGLOBE) | Phase 1, open-label, n=10 Gene addition, modified β: β-AS3 |
Recruiting Estimated completion date: 11/12/2024 | HbSS or S-β zero thalassemia Age 5 to 35 years old; All genders Department of Biotherapy Necker-Enfants Malades Hospital, France |
|
|
| NCT 04091737 | CSL200 Gene Therapy in Adults with Severe Sickle Cell Disease | Phase 1, open-label, n=3 Gene addition, modified gamma with siRNA for selection: Gamma globin G16D-RNA734 |
Active, not recruiting; Estimated completion date: 7/2021 |
SCD with homozygous HbSS or an HbSβ thalassemia variant (ie, HbSβ0 thalassemia or HbSβ+ thalassemia) Age 18 to 45 years old; All genders City of Hope Medical Center, United States |
|
|
| NCT 04208529 | A Long-term Follow-up Study in Subjects Who Received CTX001 | Follow-up, observational n=90 CTX001 |
Enrolling by invitation; Estimated completion date: 9/1/2039 |
Subjects must have received CTX001 infusion Age 18 years and older All genders United States, Germany, Italy |
|
|
| NCT 04293185 | A Study Evaluating Gene Therapy with BB305 Lentiviral Vector in Sickle Cell Disease | Phase 3, open-label, n=35 Gene addition, modified β: β-AT87Q |
Recruiting; Estimated completion date: 11/2023 |
SCD βS/βS, βS/β0 or βS/β+) Age 2 to 50 years old All genders University of Minnesota, Baylor College of Medicine; Texas Children’s Hospital, United States |
Proportion of subjects meeting Globin Response criteria |
|
| NCT 04443907 | Study of Safety and Efficacy of Genome-edited Hematopoietic Stem and Progenitor Cells in Sickle Cell Disease (SCD) | Phase 1/2, open-label, n=20 Gene editing of BCL11a:OTQ923 and/or HIX763 |
Recruiting; Estimated completion 11/20/2023 |
SCD (HbSS, HbSC, HbS/β0-thalassemia or others) Age 2 to 40 years old All genders Memphis, Tennessee, United States |
|
|
| NCT 04628585 | Long-term Follow-up of Subjects with Sickle Cell Disease Treated with Ex Vivo Gene Therapy | Follow-up, observational n=85 β-AT87Q |
Enrolling by invitation; Estimated completion date: 5/2037 |
SCD treated with ex vivo gene therapy product in bluebird bio-sponsored clinical studies Age 2 to 53 years old All genders Columbia University Medical Center, New York; Medical University of South Carolina, South Carolina; Hospital Necker, France |
Non-transfused total Hb over 15 years post-drug product infusion |
|
Key: ACS – acute chest syndrome; AE – adverse events; AESI – adverse event if special interest; dL – deciliter; ER – emergency room; FEV1 – forced expiratory volume in 1 second; FVC – forced volume capacity; GFR – glomerular filtration rate; gm – gram; Hb – hemoglobin; HbF – fetal hemoglobin; HU – hydroxyurea; HSC – hematopoietic stem cells; HSCT – hematopoietic stem cell transplantation; ICF – informed consent form; IU – intensive care unit; IV–intravenous; kg – kilogram; LVEF – left ventricular ejection fraction; m – meter; MRA – magnetic resistance angiography; MRI – magnetic resonance imaging; OS – overall survival; PI – private investigator; RBC – red blood cell; RCL – replication competent lentivirus; SCD – sickle cell disease; s – second; SAE serious adverse event; TRJ – tricuspid valve regurgitant jet; TRM – transplant related mortality; VCN – vector copy number; VOC – vaso-occlusive crises; VOE – vaso-occlusive episode; WBC – white blood cell
5.2.3. Results of included trials
The 14 studies explore various gene addition and editing therapies in the therapeutic area of SCD. Of the 14, only one study has been completed with results reported on Clinicaltrials.gov. In trial NCT02151526, conducted by Bluebird Bio, the anti-sickling gene therapy vector used is a lentiviral vector, LentiGlobin BB305 or bb1111, which encodes the human HBB variant βAT87Q. Three of the seven participants in the study carried the diagnosis of SCD. Neutrophil and platelet engraftment was achieved in all three participants with SCD. In terms of safety, all three participants experienced ‘any’ and ‘any serious’ adverse events; however, specific events are not yet disclosed. None of the three patients died during the trial. Results of the remainder of trials have not yet been reported on Clinicaltrials.gov as they are ongoing.
Among the 14 trials, five case reports are available, three summarizing non-oncologic follow-up and the latter two involving patients who were initially thought to have developed myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) related to the BB305 lentiviral vector (LVV) [2,26,27]. (Table 4) One case report from each of three trials (NCT02151526, NCT03745287, and NCT03282656) summarizes follow-up (treatment completion, survival, comorbid events, transfusions, and serious adverse events) in eight patients. One of the eight patients was treated with LentiGlobin BB305 (NCT02151526), one with CTX001 (NCT03745287), and six with BCH-BB694 lentiviral vector (NCT03282656). All eight patients survived the procedure and were able to complete treatment, once started. Patients were followed for an average of 16.5 months. When assessing clinical events of SCD since gene therapy infusion, such as vaso-occlusive crises, acute chest syndrome, or stroke, seven of the eight patients were symptom free. The eighth patient, treated with BCH-BB694 (NCT03282656), experienced several severe episodes of priapism, however, has not had priapism-related emergency department visits or hospitalizations since month eight. When assessing the need for transfusions, for the one patient each, treated with LentiGlobin BB305 and CTX001, transfusions were discontinued, with the last transfusion on day 88 and day 19, respectively. Of the six reported patients treated with BCH-BB694, five have not received a red-cell transfusion since engraftment. Serious adverse events were experienced in five of the eight patients (BB305, CTX001 and three receiving BCH-BB694). Serious adverse events include sepsis in the presence of neutropenia, cholelithiasis, abdominal pain, fever and influenza infection, recurrent priapism, and leg pain, all of which resolved with treatment or required readmission for less than 24 hours [2,26,27].
Table 4.
Summary of case report results and adverse events.
| Citation | Patient case reports included |
Mean follow-up time |
% pts survived |
% able to complete treatment, once started |
Among those who completed treatment, % who are symptom free from SCD |
% patients who became transfusion independent |
# of patients with severe AEs (list) |
|---|---|---|---|---|---|---|---|
| NCT02151526 | 1 | 15 months | 1 | 1 | 1 | 1 | 1
|
| NCT03745287 | 1 | 16.6 months | 1 | 1 | 1 | 1 | 1
|
| NCT03282656 | 6 | 18 months | 6 | 6 | 6 | 5 | 3
|
| NCT02140554 | 2 | 2 | 2 | 2
|
|||
| NCT04293185 | 1 | 1 | 1 | 1
|
Not reported in the three case reports above, are two more recently reported case reports that summarize incidents of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) in three patients. The first incident of MDS after receiving the BB305 lentiviral vector (NCT02140554) was reported in May 2020[28]. The patient was diagnosed with MDS 36 months post-infusion. Multiple independent cytogenetic and molecular assays were performed to investigate the cause of MDS. The results demonstrated the absence of vector integration and therefore, lentiviral vector-mediated oncogenesis was eliminated as the cause of the MDS; instead, the MDS was attributed to busulfan conditioning that occurred before transplantation of the modified gene[28].
As of February 2021, bluebird bio announced the temporary suspension of two of their trials (NCT02140554 and NCT04293185) following a report that a patient who was treated more than 5 years ago was diagnosed with AML, in addition to a second case of MDS in a separate patient, both in NCT02140554[29]. Bluebird bio later provided an update indicating that multiple independent analyses confirmed that vector insertion took place in the vesicle-associated membrane protein gene, a gene with ‘no known role in the development of AML or with any cellular process related to cancer’[30]. Based on these analyses, bluebird bio believes that the lentiviral vector BB305 is unlikely to be the cause of the AML and therefore has initiated a request to the FDA to resume their clinical trials. In April 2021, the treating investigator revised the case of suspected MDS to a diagnosis of transfusion-dependent anemia after reviewing results of additional tests[31]. Following the initial announcements regarding incidences of AML and MDS, the National Institutes of Health halted a similar, yet unrelated gene therapy trial (NCT03282656) being conducted at Boston’s Children’s Hospital, pending the investigation of the suspended bluebird bio trials. A recently proposed hypothesis suggests that some SCD patients may be predisposed to malignancy and therefore should be prescreened. However, evidence validating this hypothesis has not yet been collected[32].
After the U.S. FDA lifted clinical holds on the studies of LentiGlobin for SCD gene therapy in June 2021, bluebird bio announced they worked with study investigators and clinical trial sites to resume study activities[33]. According to ClinicalTrials.gov, the trial conducted at Boston’s Children’s Hospital, has resumed and is currently ‘active, not recruiting’ as of 9 February 2022[34].
6. Estimating the value of gene therapy in SCD
As the use of gene-based therapies in SCD is becoming increasingly investigated, estimations of their value compared to currently available therapies is warranted. Although limited outcomes data are available, a recent presentation conducted by Kanter et al. discusses the impact of gene therapy on health-related quality of life (HRQoL) based on patient reported outcomes (PRO) 12 months post-treatment of LentiGlobin BB305 in trial NCT02140554. Gene therapy improved HRQoL in all domains of the PRO Measurement Information System (PROMIS)-57 for patients whose baseline scores were ‘worse’ than the population norm at Month 6; these were sustained through Month 12. Clinically meaningful improvement was seen in all evaluable domains (pain intensity, pain interference, anxiety, depression, satisfaction with social roles, and physical function.)[35]. The potential long-term impacts of gene therapies in terms of better health, improved quality of life, as well as extended life expectancy, improved productivity, and a reduction in family and caregiver burden, provide sufficient incentive to pursue these novel therapies[36].
Although gene-based therapies for treatment of SCD are not yet approved or available globally, our analysis of clinical trials, via ClinicalTrials.gov, suggests the current clinical pipeline is active and some gene modification technologies may achieve market authorization in the coming years. However, the likely addition of curative therapies to the market presents financial challenges to existing funding mechanisms. To date, only one cost-efficacy analysis has been conducted in the context of gene therapy for a hemoglobin disorder. Coquerelle et al. performed a cost-efficacy analysis comparing β-thalassemia patients treated with gene therapy to those treated with an allogeneic HSCT between 2006 and 2016. This French-based study analyzed relevant cost components including bone-marrow collection, viral vector production, medical procedure costs, and follow-up costs. After a 2-year follow-up period, results suggest that patients who received gene therapy experienced fewer complications and hospital admissions than patients who underwent HSCT. Average costs were €608,086 for patients treated with gene therapy and €215,571 for HSCT. The vector comprised almost half of the total cost of gene therapy. The authors concluded that while gene therapy was more costly, it resulted in fewer complications than HSCT[37]. Although the costs for this analysis were gathered prior to the β-thalassemia gene therapy product being approved for marketing in September 2019, the results can advise us of what may be expected with the approval of gene therapies for SCD. More recent cost data from two different studies suggest that the cost of allogeneic HSCT may range from $78,702 to $618,367 and vary based on donor type and techniques used during the procedure. [38,39] Since no gene modification technologies are yet marketed for SCD, a useful approximation of cost can be made using the list price of the one currently marketed gene modification technology for β-thalassemia. Betibeglogene autotemcel costs approximately US$1.8 million per treatment[40]. Similarly, to betibeglogene autotemcel, it is likely that a gene modification approach approved for the treatment of SCD will have a high cost. According to a cost analysis conducted by the Institute for Clinical and Economic Review, the life-time treatment costs of crizanlizumab, voxelotor, and L-glutamine are $970,000, $1,100,000, and $299,000, respectively[19]. As there is not yet an estimate of the lifetime cost-effectiveness of betibeglogene autotemcel, let alone gene therapy for SCD, we are unable to compare the value of each treatment option over the lifetime.
Changes in future costs must be considered as the cost of gene therapy may also decrease overtime as manufacturing protocols are established and there is wider spread use and more competition. However, a discussion of cost only, is limiting. Long-term value includes comparative clinical effectiveness, estimated incremental cost-effectiveness, and contextual considerations such as access to treatment and resources in low-to-middle-income countries (LMIC), availability of other treatments, and ethical priorities. Benefits accrued over a lifetime must be considered as they may offset the expected high upfront costs. Benefits include measures beyond efficacy, such as reduced healthcare resource utilization over time, increased productivity, and reduction of caregiver burden. Although gene therapy has the potential to cure SCD and provide these benefits, irreversible consequences of the disease and unknown long-term risks of treatment may persist, such as infertility or secondary malignancy. These must also be considered and incorporated into the valuation of gene therapy in SCD. Although relevant, these elements may be difficult to quantify, especially as it pertains to gene therapy in SCD.
The standard economic measure used to quantify the benefits of therapies is the quality-adjusted life year (QALY), which measures both quantity and quality of life. QALY calculations can incorporate elements of efficacy, healthcare resource use, productivity, caregiver burden, and more[41]. Challenges to estimating QALYs in gene therapy in general, and SCD specifically, include the lack of long-term studies and large-scale experiences. Incremental QALYs, comparing two alternate treatment strategies, form the denominator of the incremental cost-effectiveness ratio, with incremental costs forming the numerator. This ratio is benchmarked against a willingness to pay (WTP) threshold. For example, World Health Organization (WHO) suggests a WTP threshold of 1 to 3 times gross domestic product (GDP) per capita for all countries. A 2016 study found that the thresholds estimated in LMIC are far less than that proposed by WHO. In Malawi, the country with the lowest income in the world, the estimated threshold was $3 to $116 and in Cambodia, a borderline low/low middle-income country, it was $4,485 to $8,018.38[40]. In countries with populations most impacted by SCD, such as those in Africa, there is limited available evidence to estimate WTP thresholds[42]. Therefore, similar estimates, alongside country-specific healthcare fund information, must be used to inform value-based decisions.
Salcedo et al. conducted a cost-effectiveness analysis of a hypothetical cell or gene therapy cure for SCD from the US healthcare sector perspective[43]. For the durable treatment arm, the intervention was assumed to be fully effective and therefore completely suppressed disease-related complications and costs, restored life expectancy, and HRQoL (measured in QALYs) to that of a comparable individual unaffected by the disease. Durable treatment resulted in an ICER of $140,977 per QALY gained, which is cost-effective at the WTP threshold of $150,000 per QALY gained. Their sensitivity analyses suggested that a median 20-year and 10-year duration scenario would result in an ICER of $410,607 per QALY/gained and $740,058 per QALY/ gained, which is not considered cost-effective[43]. The analysis is limited in that it did not incorporate the indirect costs of managing SCD or consider the potential long-term risks of gene therapy. However, the results provide some insight into whether there is a need to increase the WTP threshold for rare diseases.
The Institute for Clinical and Economic Review has discussed increasing the WTP threshold up to $500,000 per QALY gained due to the considerable potential health benefits and reduction of related burdens by therapies for rare diseases, such as SCD[44]. An even greater challenge presented is the lack of WTP threshold data in countries with populations most affected by SCD. Additional elements of value for gene therapy, beyond the traditional framework, need to be assessed and may support a higher value-based cost-effectiveness threshold. [45]
Although incremental cost-effectiveness ratios estimate value compared to the next best alternative, an additional necessary estimate is that of affordability, estimated using a budget impact[41]. A budget impact analysis performed from January 1 to 31 May 2020, using a $1.85 million list price for gene therapy in SCD as the base case, projected a mean 1-year budget impact of $29.96 million per state Medicaid program ($1.91 per member per month). This study suggests that gene therapy in SCD will likely present affordability and unique financial challenges, therefore, reinforcing the need to take steps to ensure patient access as these therapies will potentially offer substantial benefit to patients[46].
7. Conclusion
As experimentation of gene modification technologies in SCD increases, the sooner the expected high-cost treatments will achieve market authorization and therefore, the sooner the value of these potentially curative treatments for rare diseases must be assessed. A broader concept of value may provide rationale for a higher list price and, consequently a higher cost-effectiveness threshold, as these costs may be offset by the potential life-long benefits of gene therapy. The expected outcomes that increase the value of these treatments for SCD consist of increased patient eligibility for treatment, improved quality of life, extended life expectancy, improved productivity, and reduced family and caregiver burden. To estimate the true value, long-term efficacy and safety data and the social implications of treatment must be evaluated.
8. Expert opinion
Gene therapy is being studied in rare, chronic diseases, such as SCD. This landscape analysis of clinical trials for gene therapy in SCD has yielded information on the various gene modification approaches currently being investigated. The initial result of the single completed study is favorable and may indicate that gene therapy has potential to be a viable, curative treatment option for the SCD population. However, recent reports of AML and the reclassified MDS case report to transfusion-dependent anemia after treatment with gene therapy in SCD, leading to the suspension ongoing trials, gives pause to the interpretation of efficacy and safety of these emerging therapies[29]. Efforts to understand the risks and causes of adverse events are underway.
In addition to the recent reports of serious adverse events, current clinical trial design may also pose hesitation toward gene therapy in SCD. Current interventional trials of gene therapy in SCD have been single-arm and limited to a study population of 50 participants or less, which raises the issue of relative efficacy and generalizability, respectively, of the clinical evidence proposed. In addition, the lack of long-term studies, large-scale experiences, and the subjective nature of many assessment tools present a challenge to determining the value of gene therapy[47]. Although certain limitations, such as an open-label design, are sometimes unavoidable due to the nature of gene therapy technology, investigators will need to use additional approaches to find the necessary data to provide evidence of treatment effect. Drummond et al. suggests the use of a historical control cohort of patients as a supplement to assess comparative effectiveness and safety, however in gene therapy trials, it may be more beneficial to compare allogenic transplant treatment groups to conventional therapy[48]. An example would be the STRIDE 2 trial, comparing survival and sickle-related outcomes in patients with severe SCD after bone marrow transplantation and standard of care[49]. In addition, long-term effectiveness is often unknown despite the expected long-term health benefits of gene therapy. To mitigate this lack of clinical and safety data, registries of patients treated with gene therapy will be necessary to capture and monitor long-term outcomes, as is being done in a sub-study of the STRIDE 2 trial, creating a biorepository[50]. Additionally, registries of patients who do not undergo curative treatments may serve as contemporary controls for those who do undergo gene therapy to cure sickle cell disease.
The expected consequence of the one-time administration of gene therapy may represent a breakthrough in the treatment of SCD. However, due to the potentially curative nature of gene therapy and its implications on the entire SCD community, it is likely to be expensive. The challenging aspect of gene therapy is the expected high upfront costs, followed by health benefits that accrue over a patient’s full life span. The methodologies of pharmacoeconomic evaluations make it possible to consider the potential lifelong benefits. The potential lifelong benefits such as improved quality of life, extended life expectancy, reduced healthcare utilization, improved productivity, and reduction in family and caregiver burden create further value of gene therapy for the treatment of SCD, which provides the rationale for a higher list price, a higher WTP threshold, and increased experimentation in gene modification technologies. At this time, additional information is required to establish the differential cost-effectiveness ratio for gene therapies in SCD as well as to determine an appropriate efficiency threshold.
Additional unique elements of value for gene therapy must be considered and discussed in comparison to currently available chronic treatment options. Garrison et al. suggests that the value of full life may emerge from therapy that is able to cure a disease that may be otherwise fatal at a young age[45]. Other elements of value requiring a closer assessment for gene therapy are the real option value, value of hope, and value of knowing. The real option value suggests that therapies that increase life expectancy also provide patients with the option to benefit from future innovative therapies. If gene therapy were to result in an increased number of cures and reduced uncertainty about a response to an intervention, this would create the value of hope and the value of knowing for patients, respectively. The combination of these uncertainty-related elements of value supports adoption of a higher cost-effectiveness threshold as they may increase quality of life in these patients[45].
In the context of gene therapy for SCD, health care decision makers must also consider the availability of access to treatment and health fund resources in different socioeconomical areas. The current prospect of access to gene therapy in LMICs is unfavorable as, of the 14 clinical trials reported, none are open in Africa, although an estimated 75% of infants born with SCD live in sub-Saharan Africa. While gene therapy in SCD currently shows promise in the United States and other high-income countries, limitations to supportive care (e.g. transfusions and anti-microbials), infrastructure, technical complexity, and the high cost of these therapies significantly limits the ability to deploy these in LMICs. That said, multiple countries are prioritizing developing the support structures, and the development of automated systems for stem cell modification will speed up the use of gene therapy in Africa.
In sum, many trials are currently underway for gene therapy in SCD with some reporting results for patients who have completed the treatment procedure already available. Gene therapy in SCD may show promise, however, special attention needs to be given to potential adverse effects and result generalizability. As treatment may move toward approval, consideration of the likely high cost, potential life-long benefits, and, therefore, the potential need for revised WTP thresholds is required. Lastly, for gene therapy in SCD to be successful, concerns for access and affordability need to be addressed in underserved areas and LMICs where SCD is most prevalent. Ozuah writes, ‘The irony here is inescapable – some of the most underserved patients in the world are ideal candidates for the most advanced medical treatment yet conceived’[51].
Article highlights.
Gene therapy is an emerging area of science in which different approaches are taken to replace dysfunctional genes or add new ones. Gene therapies represent major advances in the treatment of genetic disorders, such as sickle cell disease (SCD), for which limited treatment options are available, as they hold curative promise; however, this is not without risk.
SCD is rare genetic disorder caused by an inherited structural defect in the beta globin gene. It is a lifetime disorder characterized by painful vaso-occlusive crises, anemia, and increased risk of stroke and other significant comorbidities, leading to increased utilization of health care resources.
Gene therapies are currently being studied for use in SCD. Both gene editing and gene addition are at early stages of development in clinical trials and may hold promise as cures and for additional life-long benefits such as increased quality of life and decreased disease burden.
Current mainstay treatments for SCD are hydroxyurea and blood transfusions. Other available treatment options include crizanlizumab, voxelotor, L-glutamine, and hematopoietic stem cell transplantation. Use of these therapies is limited by various barriers such as poor acceptability, increased number of health care visits, and lack of matched donors.
The one-time administration and expected upfront costs of gene therapy warrant accurate estimations of value including costs offset by lower lifetime health care resource use, improved productivity, and improved quality of life for patients and families.
A major challenge to estimating the value of gene therapies for SCD is determining the impact on quantity and quality of life without available long-term efficacy and safety data. In addition, challenges exist in establishing a willingness-to-pay (WTP) threshold for rare diseases in various regions, including LMICs.
Efforts to determine the value of these therapies and social influences affecting access to treatment are important to ensure adequate availability of and equitable access to these emerging therapies in regions most impacted by SCD.
Acknowledgments
The authors wish to acknowledge collaborators from the University of Washington and the Fred Hutchinson Cancer Research Center: Z. Baldwin, Z. Elsisi, K. Johnson, C. Henry, W. Wright. The authors also appreciate the valuable insights and suggestions provided by the members of the Clinical and Economic Analysis Initiative Expert Panel of the NHLBI Cure Sickle Cell Initiative.
Funding
This research was funded by the National Heart, Lung and Blood Institute, Cure Sickle Cell initiative (OTA Numbers: OT3HL152448, OT3HL151434)
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer on this manuscript declares acting as an advisory board member for Octapharma, Dova, Principia and Shionogi, a consultant for Novartis, Shionogi, Dova, Principia, Argenx, Rigel and Bayer, and has received research funding from Sysmex, Novartis, Rigel, Principia, Argenx, Dova, Octapharma and AstraZeneca (sickle cell disease study). Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Verma IM, Naldini L, Kafri T, et al. Gene therapy: promises, pro-blems and prospects. In: Boulyjenkov V, Berg K, Christen Y, editors. Genes and resistance to disease. Berlin Heidelberg: Springer; 2000. P. 147–157. doi: 10.1007/978-3-642-56947-0_13 [DOI] [Google Scholar]
- 2.Ribeil J, Hacein-Bey-Abina S, Payen E, et al. Gene therapy in a patient with sickle cell disease. . New England Journal of Medicine. 2017;376(9):848–855. [DOI] [PubMed] [Google Scholar]
- 3.Pearson SD. Effectiveness methods to determine value-based prices for potential cures: what are the options? Value Health. 2019;22(6):655–659. [DOI] [PubMed] [Google Scholar]
- 4.Cure sickle cell initiative ∣ advancing research in sickle cell disease ∣ cure sickle cell. curesickle.org. https://curesickle.org/. Published. 2021 2021. Jun 24.
- 5.Gonçalves GAR, Paiva RMA. Gene therapy: advances, challenges and perspectives. Einstein (Sao Paulo). Einstein (Sao Paulo, Brazil). 2017;15(3):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunbar CE. 2021. A plethora of gene therapies for hemoglobinopathies. . Nat Med. 202–204; 27,  10.1038/s41591-021-01235-7. [DOI] [PubMed] [Google Scholar]
- 7.Morgan R, Gray D, Lomova A, et al. Hematopoietic stem cell gene therapy: progress and lessons learned. cell stem cell. Cell Stem Cell. 2017;21(5):574–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu K, Natanson H, Dunbar C. Gene editing of human hematopoietic stem and progenitor cells: promise and potential hurdles. Hum Gene Ther. Human Gene Therapy. 2016;27(10):729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardo M, Aiuti A. The role of conditioning in hematopoietic stem-cell gene therapy. Hum Gene Ther. Human Gene Therapy. 2016;27(10):741–748. [DOI] [PubMed] [Google Scholar]
- 10.Quinn C, Young C, Thomas J, et al. Estimating the clinical pipeline of cell and gene therapies and their potential economic impact on the US healthcare system. Value Health. 2019;22 (6):621–626. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Data & Statistics on Sickle Cell Disease. Centers for Disease Control and Prevention. Published August 31, 2016. Accessed 2020 Dec 18. https://www.cdc.gov/ncbddd/sicklecell/data.htm [Google Scholar]
- 12.Wastnedge E, Waters D, Patel S, et al. The global burden of sickle cell disease in children under five years of age: a systematic review and meta-analysis. J. Glob Health 2018;8 (2):021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piel FB, Hay SI, Gupta S, et al. Global burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality and interventions. . PLoS Med. 2013;10(7):e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundd P, Gladwin MT, Novelli EM. Pathophysiology of Sickle Cell Disease. Annu Rev Pathol. 2019;14(1):263–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Society of Hematology; 2016. Rare patients with sickle cell disease live nearly twice as long as average. ; https://www.hematology.org/newsroom/press-releases/2016/rare-patients-with-sickle-cell-disease-live-nearly-twice-as-long-as-average. (Accessed: May 15, 2021).
- 16.Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Network Open. 2019. Nov 1; 2(11): e1915374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulukonis ST, Eckman JR, Snyder AB, et al. Defining sickle cell disease mortality using a population-based surveillance system, 2004 through 2008. Public Health Rep Wash DC. 1974;131 (2):367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanzkron S, Carroll CP, Haywood C Jr., Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. 433021 2013;128 (2):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bradt P, Spackman E, Synnott PG, et al. Crizanlizumab Voxelotor, and L-Glutamine for sickle cell disease: effectiveness and value. Institute for Clinical and Economic Review 2021. Jun 23 https://icer-review.org/material/sickle-cell-disease-draft-evidence-report/ ••This paper discusses the cost-effectiveness and value of newer sickle cell disease treatments
- 20.Demirci S, Uchida N, Tisdale J. Gene therapy for sickle cell disease: an update. Cytotherapy. 2018;20(7):899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoban M, Orkin S, Bauer D. Genetic treatment of a molecular disorder: gene therapy approaches to sickle cell disease. Blood. 2016;127(7):839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basak A, Sankaran VG. Regulation of the fetal hemoglobin silencing factor BCL11A. Ann. N Y Acad Sci 2016;1368(1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uda M, Galanello R, Sanna S, et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci USA. 2008;105(5):1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Peng C, Sankaran VG, et al. Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science. 2011;334(6058):993–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinberg MH, Chui DH, Dover GJ, et al. Fetal hemoglobin in sickle cell anemia: a glass half full? Blood. Blood. 2014;123(4):481–485. [DOI] [PubMed] [Google Scholar]
- 26. Frangoul H, Altshuler D, Cappellini M, et al. CRISPR-Cas9 gene editing for sickle cell disease and β-Thalassemia. N Engl J Med. 2021;384(3):252–260. • This paper discusses available results from the clinical trials of gene therapy in sickle cell disease.
- 27.Esrick E, Lehmann L, Biffi A, et al. Post-Transcriptional genetic silencing of BCL11A to treat sickle cell disease. . New England Journal of Medicine. 2021; 384: 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh M, Bonner M, Pierciey F, et al. Myelodysplastic syndrome unrelated to lentiviral vector in a patient treated with gene therapy for sickle cell disease. Blood Adv. Blood Advances. 2020;4 (9):2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Businesswire. bluebird bio Announces Temporary Suspension on Phase 1/2 and Phase 3 Studies of LentiGlobin Gene Therapy for Sickle Cell Disease (bb1111). Businesswire.com. Published 2021. 2021. Jul 1. https://www.businesswire.com/news/home/20210216005442/en/bluebird-bio-Announces-Temporary-Suspension-on-Phase-12-and-Phase-3-Studies-of-LentiGlobin-Gene-Therapy-for-Sickle-Cell-Disease-bb1111.
- 30.bluebird bio. Bluebird bio provides updated findings from reported case of Acute Myeloid Leukemia (AML) in lentiglobin for Sickle Cell Disease (SCD) gene therapy program.; 2021. Accessed 2021 Jun 13. https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-provides-updated-findings-reported-case-acute.
- 31.bluebird bio. bluebird bio Provides Update on Severe Genetic Disease Programs and Business Operations; 2021. 2021 May 30. https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-provides-update-severe-genetic-disease-programs-and.
- 32.Jones R, DeBaun M. Leukemia after gene therapy for sickle cell disease: insertional mutagenesis, busulfan, both or neither. Blood. 2021;doi: 10.1182/blood.2021011488. [DOI] [PubMed] [Google Scholar]
- 33.bluebird bio. bluebird bio announces the lifting of FDA clinical hold for sickle cell disease and β-Thalassemia Studies.; 2021. Accessed 2021 Jun 13. https://investor.bluebirdbio.com/news-releases/news-release-details/bluebird-bio-provides-updated-findings-reported-case-acute
- 34.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). (2018. Feb 13).). Gene transfer for sickle cell disease. Identifier NCT03282656, http://clinicaltrials.gov/ct/show/NCT03282656?order=1 [Google Scholar]
- 35. Kanter J, Tisdale J, Mapara M, et al. 365 improvements in health-related quality of life for patients treated with lentiglobin for sickle cell disease (bb1111) gene therapy. Poster presented at: American Society of Hematology Annual Meeting and Exposition; December 6; Virtual Meeting. https://ash.confex.com/ash/2020/webprogram/Paper136193.html. •• This poster discusses the impact of gene therapy on health-related quality of life (HRQoL) based on patient reported outcomes (PRO) 12 months post-treatment of LentiGlobin BB305
- 36.Harrison C. First gene therapy for β-thalassemia approved. Nat Biotechnol. 2019;37(10):1102–1103. [DOI] [PubMed] [Google Scholar]
- 37.Coquerelle S, Ghardallou M, Rais S, et al. Innovative curative treatment of beta thalassemia: cost-efficacy analysis of gene therapy versus allogeneic hematopoietic stem-cell transplantation. Hum Gene Ther. 2019;30(6):753–761. [DOI] [PubMed] [Google Scholar]
- 38.Arnold S, Brazauskas R, He N, et al. Clinical risks and healthcare utilization of hematopoietic cell transplantation for sickle cell disease in the USA using merged databases. Haematologica. Haematologica. 2017;102(11):1823–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saraf S, Ghimire K, Patel P, et al. Improved health care utilization and costs in transplanted versus non-transplanted adults with sickle cell disease. PLoS One. PloS one. 2020;15(2): e0229710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouyang W, Dong G, Zhao W, et al. Restoration of β-globin expression with optimally designed lentiviral vector for β-thalassemia treatment in Chinese patients. Hum Gene Ther. 2020; 10.1089/hum.2020.204. [DOI] [PubMed] [Google Scholar]
- 41.Neumann PG, Sanders GD, Russell LB, et al. Cost-effectiveness in health and medicine. 2nd ed. New York NY: Oxford University Press; 2017. [Google Scholar]
- 42.Woods B, Revill P, Sculpher M, et al. Country-level cost-effectiveness thresholds. Initial Estimates and the Need for Further Research. Value in Health. 2016;19:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salcedo J, Bulovic J, Young C. Cost-effectiveness of a hypothetical cell or gene therapy cure for sickle cell disease. . Sci Rep. 2021;11. 10.1038/s41598-021-90405-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. 2020 Value Assessment Framework Proposed Changes. Institute for Clinical and Economic Review; 2019. 2021 May 30. https://icer.org/wp-content/uploads/2020/10/ICER_2020_VAF_Proposals_082119-1.pdf. •• This paper discusses adjusting willingness-to-pay threshold due to the considerable potential health benefits and reduction of related burdens by therapies for rare diseases
- 45. Garrison L, Jackson T, Paul D, et al. , Value-based pricing for emerging gene therapies: the economic case for a higher cost-effectiveness threshold. J Manag Care Spec Pharm. 2019;25 (7):793–799. •• This paper focuses on the characterizing challenges for traditional approaches to assessing the value of one-time gene replacement therapies and provides a health economic rationale for a higher value-based cost-effectiveness threshold.
- 46.DeMartino P, Haag MB, Hersh AR, et al. A budget impact analysis of gene therapy for sickle cell disease: the medicaid perspec-tive. . JAMA Pediatr. published online 2021. Mar 22 10.1001/jamapediatrics.2020.7140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Salzman R, Cook F, Hunt T, et al. Addressing the value of gene therapy and enhancing patient access to transformative treatments. Mol Ther. 2018;26(12):2717–2726. • This paper focusses the value of gene therapy and patient access to transformative treatments.
- 48.Drummond M, Neumann P, Sullivan S, et al. Analytic considerations in applying a general economic evaluation reference case to gene therapy. Value Health. 2019;22(6):661–668. [DOI] [PubMed] [Google Scholar]
- 49.Clinicaltrials.gov. Bone marrow transplantation vs standard of care in patients with severe sickle cell disease (BMT CTN 1503). Betheseda, MD: National Library of Medicine; 2016. https://clinicaltrials.gov/ct2/show/NCT02766465. (Accessed: May 25, 2021). NLM identifier: NCT02766465. [Google Scholar]
- 50.Clinicaltrials.gov. STRIDE Biorepository. Bethesda, MD: National Library of Medicine. 2017; https://clinicaltrials.gov/ct2/show/NCT02843347. (Accessed May25, 2020). NLM Identifier: NCT02843347. [Google Scholar]
- 51.Gene OP. Therapy for sickle cell disease—a debt to be paid. JAMA Pediatr. 2021;175(6):565. [DOI] [PubMed] [Google Scholar]