Abstract
Objective:
Snakebites, a major health concern in developing countries, affect rural farming communities. Venom, primarily neurotoxin, injected during a snake bite disrupts the nervous system, causing symptoms like muscle weakness, paralysis, altered sensation, and coordination issues. This review focuses on evaluating neurological and neuro-ophthalmological manifestations associated with snakebites.
Methods:
A database search was conducted in EMBASE and PubMed for studies published from 2000 to 2023. The investigation centered on examining neurological and neuro-ophthalmological symptoms and signs, treatment approaches, treatment outcomes, and long-term complications of snake bites.
Results:
Neurological and neuro-ophthalmological symptoms were common in both neurotoxic and hemotoxic snake bites, especially in neurotoxic cases. Ptosis was a prevalent manifestation across various snake bites, along with respiratory paralysis, limb weakness, dysphasia, and visual disturbances in some instances. However, most patients improved without residual neurological symptoms after treatment.
Conclusions:
Understanding patterns of neurological manifestations contributes valuable insights for the comprehensive management of snakebite.
Keywords: neurological manifestations, neuro-ophthalmological manifestations, snake bite management, snake bites
Introduction
Highlights
Snake bites are major health concerns in developing countries, mainly rural farming communities.
Neurological and neuro-ophthalmological manifestations are prevalent regardless of toxin type.
Physicians should remain vigilant in monitoring and identifying neurological manifestations.
Neurological and neuro-ophthalmological manifestations improve following the administration of ASV treatment.
Snake bite is a major health concern, particularly in developing countries, affecting mainly rural farming communities1. It remains a neglected tropical disease that causes significant morbidity and mortality. Worldwide, an estimated 5.4 million snake bites occur annually, with half being envenoming, leading up to 138 000 preventable deaths and over 400 000 preventable amputations and other permanent disabilities1. In the United States (US), poison centres recorded over 7000–8000 snake envenomation in 2019, while in India, an average of 250 000 snake bites are recorded in a single year2,3. Snakes are found in deserts, forests, marshes, swamps, lakes, streams, and rivers of difficult terrains. The families of venomous snakes are Atractaspididae, Elapidae, Hydrophidae, and Viperidae 3. Snake venom can be classified into hematotoxic, neurotoxic, necrotoxic, cardiotoxic, and nephrotoxic, depending on the predominant effects of the venom4. The venom consists of various components, including protein, enzymes, neurotoxins, coagulants, anti-coagulants, and substances with cytotoxic effects5. Venoms are a complex mixture of enzymatic and toxic proteins, which trigger different clinical manifestations depending on the pathophysiological changes of certain species.
Hemotoxins affects the cardiovascular system causing bleeding or coagulation disorder whereas neurotoxins affect the nervous system. When neurotoxin enters the body by a snake bite, it causes neurological symptoms like muscle weakness, paralysis, altered sensation, and coordination issues4. It can also impact the visual system, resulting in neuro-ophthalmological manifestations such as blurred vision, double vision, drooping eyelids, and vision loss.
This systematic review aims to outline the nature and severity of neurological and neuro-ophthalmological symptoms and signs, as well as their treatment approaches, outcomes, and long-term complications associated with snake bites.
Methodology
This review article has been reported in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline6 and AMSTAR(Assessing the methodological quality of systematic reviews) guideline.
Study inclusion and exclusion criteria
The following requirements should have been met by the studies: (1) Included patients who had experienced a snake bite (2) Paediatric and adult patients (3) Information on one or more neurological manifestations.
The exclusion criteria were as follows: (1) Animal or in vitro studies (2) Insufficient data and language other than english (3) Duplicate publications (4) Reviews or meta-analyses.
Search methods and study selection
PubMed and EMBASE databases were searched for studies published from 2000 to 2023. Boolean logic was used for conducting a database search, and Boolean search operators “AND” and “OR” were used to link search terms: “snake bite”, “Neurological manifestation”.
The detailed PubMed search strategy was as follows: (((“neurologic manifestations”[MeSH Terms] OR (“neurologic”[All Fields] AND “manifestations”[All Fields]) OR “neurologic manifestations”[All Fields] OR (“neurological”[All Fields] AND “manifestation”[All Fields]) OR “neurological manifestation”[All Fields]) AND (“snake bites”[MeSH Terms] OR (“snake”[All Fields] AND “bites”[All Fields]) OR “snake bites”[All Fields] OR (“snake”[All Fields] AND “bite”[All Fields]) OR “snake bite”[All Fields]) AND (“loattrfull text”[Filter] AND 2000:2023 [Date - Publication])) NOT ((“case reports”[Publication Type] OR “case report”[All Fields]) AND “loattrfull text”[Filter])) AND (fft[Filter]). There was no restriction on language of publication.
We also searched the reference list of each included study to identify other potential material of interest. All shortlisted studies were then imported to the Mendeley, and duplicates were removed appropriately. Papers were initially reviewed by title, keywords, and abstract by two reviewers (K.P. and H.M.S.M.) independently and subsequently verified with a third reviewer (GN). Articles after the initial screen were subsequently reviewed in full by two reviewers (K.P. and H.M.S.M.). We resolved the final study selection differences between the two primary reviewers (K.P. and H.M.S.M.) by the discussion with a third reviewer (GN). An overall evaluation for potential overlap of the population was conducted based on authorship, hospital setting, and recruitment period. In cases of overlap, studies of higher quality or larger sample sizes were included. The work has been reported in line with AMSTAR (Assessing the methodological quality of systematic reviews) guidelines.
Data extraction
Two independent authors (K.P. and H.M.S.M.) rigorously reviewed selected studies for systematic review which met our inclusion criteria and extracted the precise information on different headings under one table depicting Author/Published year, study site, study design, sample size, participants (adults, paediatric), age (in years), sex, snake and venom type, neurological and neuro-ophthalmological manifestation, treatment, outcome and late complications were recorded in Microsoft Excel 2013 (Microsoft Corp).
Results
Search results and study selection
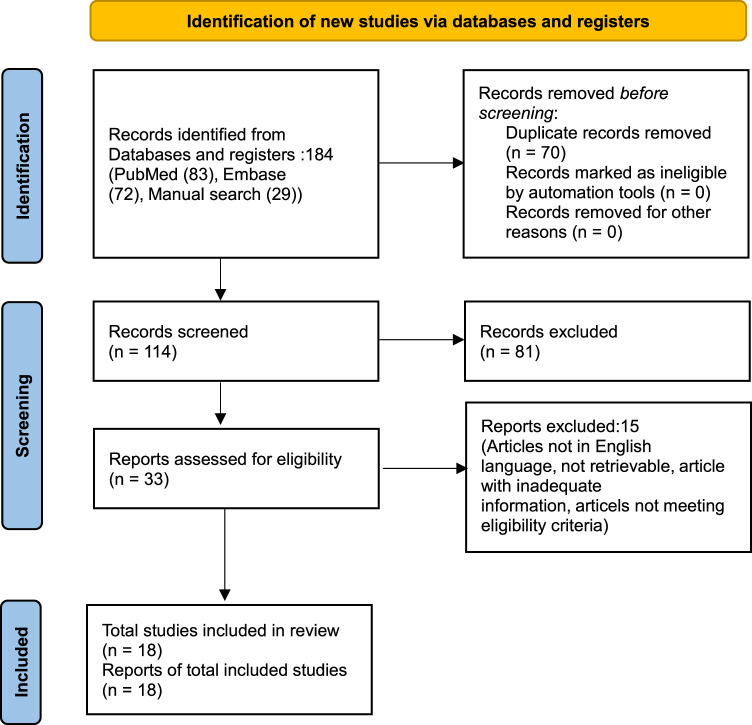
We found 184 studies through electronic database searches (83 from PubMed, 72 from EMBASE) and identified 29 additional studies through manual searches of reference lists and related systematic reviews. After removing duplicates, we screened 114 articles based on titles and abstracts. Subsequently, 33 full-text articles were retrieved and evaluated against predefined inclusion criteria. Ultimately, 18 articles met the criteria and were included in the review. The PRISMA diagram, illustrating the identification and selection process, is provided in Figure 1.
Figure 1.
The PRISMA diagram detailing the identification and selection process.
Study characterizations and patient’s demography
The review encompassed a total of 1,424 patients across 18 studies, comprising 15 observational studies and three case series. The mean age of participants ranged from 6 months to 80 years. Among these studies, four exclusively involved pediatric patients, two exclusively involved adult patients, and 12 included both pediatric and adult participants. The research was conducted in various countries, with five studies from India, four from Sri Lanka, three from the United States, two from Italy, and one each from Thailand, South Africa, Croatia, and Brazil. Table 1 provides a summary of the study characteristics.
Table 1.
Key methodological characteristics of studies included in this systematic review.
| Author and year | Country | Study design | Sample size | Snake type | Neurological manifestations | Treatment | outcomes | Late complications |
|---|---|---|---|---|---|---|---|---|
| Marano et al. (2021)6 | Italy | Observational | 24 Paediatric (1.5–16.2 years) |
Viper | Ptosis 2 (8.3%) Dysarthria 1 (4.2%) Nystagmus 1 (4.2%) |
Anti-venom in 12 (55%) of total cases | Improved | None |
| Tongpoo et al. (2018)7 | Thialand | Observational | 78 (1–76 years) | Krait | 69 (88.5%) developed neurological signs of motor weakness | Anti-venom:67 (85.90%) of total cases 75.6% of total cases required intubation and ventilator support |
Death:5 (7.2%) | None |
| Hernandez (2019)8 | USA | Observational | 72 children (6 months–15 years) |
Types of snake was not indentified | 5 (7%) developed progressive weakness | Anti-venom:1 (20%) of those who developed progressive weakness Fasciotomy 2 (40%) |
Improved | None |
| Samprathi et al. (2020)9 | India | Observational | 111 Children (5–10 years) |
Krait | 76 had neuroparalysis (68%) and 51 had EMNS of which 43 (84.3%) had bulbar palsy ,40 (78.4%) had ptosis. 35 had non EMNS of which 16 (45.7%) had bulbar palsy,24(68.5%) had ptosis. |
Anti-venom:111 (100%) | With EMNS 7(13.7%) death With non EMNS 3(8.57%) death |
None |
| Seneviratne et al. (2002)10 | Sri Lanka | Observational | 56 (13–65 years) | Viper (27) Krait (19) Cobra (3) Unidentified (7) |
Ptosis in 48 (85.7%) ophthalmoplegia 42 (75%) limb weakness 15 (26.8%) respiratory failure 10 (17.9%) palatal weakness 6(10.7%) neck muscle weakness 4 (7.1%), delayed sensory neuropathy 1 (1.8%). |
Anti-venom 10 cases required mechanical ventilation |
Improved 1 (1.79%) death due to Intracerebral haemorrhage |
None |
| Silva et al. (2016)11 | Sri Lanka | Observational | 33 (16–78 years) | Krait | Neurotoxicity None—8 (24.24%) Mild—8 (24.24%) Severe—17 (51.52%) Ptosis Partial – 12 (36.36%) Complete—13 (39.4%) Ophthalmoplegia Partial—4 (12.12%) Complete—14 (42.42%) Strabismus—11(33.33%) Facial Weakness—18(54.54%) Neck Flexon Weakness—15(45.45%) Difficulty swallowing—17 (51.51%) Low pitched voice—12 (36.36%) Tidal volume <250 ml—17 (51.51%) Reduced upper limb power—12 (36.36%) Reduced lower limb power (power <5)—5 (15.15%) Diminished or absent deep tendon reflexes 9 (27.27%) Autonomic features—6 (18.18%) |
Anti-venom—23 (69.70%) Intubation and mechanical ventilation—17 (51.51%) |
Improved Anaphylaxis—6 (18.18%) Intubated for anti-venom reaction—3 (9.10%) |
None |
| Walt (2019)12 | South Africa | Observational | 14 (1–65 years) | Berg Adder | Ptosis, mydriasis and visual disturbances and diminished motor power—13/13(100%) of patients who developed systemic sequelae following envenoming Dysphagia—9/10 (90%) of assessed patients Tendon reflexes diminished—7/9 (77.8%) of assessed patients‘ Respiratory Failure—10/13 (76.9%) of patients who developed systemic sequelae following envenoming |
Symptomatic | Improved | None |
| Karabuva et al. (2016)13 | Croatia | Observational | 160 children and adolescent (1--18 y) | Not mentioned | Ptosis—18 (11.2%) 7 (4.4%) ophthalmoplegia, 3 (2.0%) dysphagia, 1 (0.6%) dysphagia and dysphonia |
Anti-venom—100% Antibiotics—96% Corticosteriods – 84% Antihistamines—71% |
Improve 1 (0.625%)died Fasciotomy—12 (7.5%) |
None |
| Bisneto (2020)14 | Brazil | Case series | 7 (14–49 years) | Coral | dyspnoea/shallow breath (4 (51.14%)), palpebral ptosis (4 (57.14%)), blurred vision (3 (42.85%)), dysarthria (3 (42.85%)) and difficulty to walk (3 (42.85%)) |
Anti-venom—7 (100%) | Improved | None |
| Bawaskar et al. (2002)15 | India | Observational | 91 (10–62 years) | Kraits—20 (21.97%) Echis—9 (9.9%) Rest unknown 62 (68.13%) |
Paralysis—26 (28.6%) | Anti-venom | Deaths—10 (11%) | None |
| Lonati et al. (2014)16 | Italy | Case series | 24 (3–75 years) | Viper | Accommodation troubles and diplopia 24 (100%), ptosis 22 (91.7%), ophtalmoplegia 14(58.3%), dysphagia 5 (20.8%), drowsiness 4 (16.6%), cranial muscle weakness 3 (12.5%), dyspnoea 10 (4.2%) |
Anti-venom—19 (79.2%) | Improved | None |
| Anil et al. (2010)17 | India | Observational | 72 (15–65 years) | Krait | Altered sensorium- -4 (5.8%) Ptosis - 70 (97.1%) Weakness of neck flexors—50 (70.0%) Respiratory—10 (10.4%) |
Anti-venom NeostigminAtropine |
Improved Deaths—2 (2.78%) |
None |
| Roth (2016)18 | USA | Observational | 87 (2–79 years) | Copperheads | Pain, swelling, and disability, weakness, or residual venom effects of involved body parts in all patients. | Anti-venom—57 (65.51%) | Improved Anaphylaxis—2 (2.30%) Urticarial reaction—1 (1.15%) |
None |
| Bawaskar et al. (2014)19 | India | Cross-sectional | 141 Age range not mentioned All adults |
Krait | 37 (26%) victims had neuroparalysis (bulbar palsy). 16 (11.34%)victims had respiratory paralysis and needed ventilator support but seven of these victims died |
Anti-venom | 12.5% ( 7) died on the way to hospital 13.47% died during treatment |
None |
| Kularatne et al. (2014)20 | Sri Lanka | Observational | 55 (20–50 years) | Viper | Proven/Probable grp Ptosis: 30 (55%)/ 109 (71%) Ophthalmoplegia: 30 (55%)/ 111 (72%) Neck Muscle Weakness: 14 (25%)/50 (32%) Respiratory paralysis : 0/ 2 (1%) |
Anti-venom Proven Viper Bite : 64% Suspected Viper Bite : 75% |
Improved Reaction to Anti-venom: Proven/Probable grp Moderate : 31% / 6% Severe : 14% / 5% |
None |
| Sharma et al. (2005)21 | India | Observational | 142 (12–80 years) Paediatric and Adults |
Elapid(86 (60.56%)) and Viper (52 (36.61%)) Rest not mentioned in study. |
Ptosis : 75 (52.81%) Respiratory involvement: 65 (45.77%) Bulbar weakness: 59 (41.54%) Ophthalmoplegia: 42 (29.57%) LoC : 12 (8.45%) |
Equine polyvalent Anti-venom : 119 (83.8%) | Mortality in 5 patients (3.5%) 17 (11.97%) patients had ADR to anti-venom: Anaphylaxis: 2 (1.40%) Pyrexia; 6 (4.22%) Utricaria: 9 (6.33%) |
|
| Kularatne (2002)22 | Sri Lanka | Observational | 210 (10–30) years Paediatric and adults |
Krait | Ophthalmoplegia, Ptosis Muscle weakness in 101 (48.1%)patients Paralytic ileus in 42 (20%)patients |
Polyvalent Haffkine Anti-venom 101(48.1%) patients required mechanical ventilation |
Overall mortality of 7.6% ARDS in 6 (2.85%) patients Arrythmia in 20 (9.52%) patients |
None |
| Vohra et al. (2008)23 | USA | Case Series | 47 (5–70) years 7-paediatrics 40-Adults |
Rattle Snake | All patients presented with fasciculation in various parts (Face,eyelids,peri oral,oral,lingual,tongue,arms,legs,calf,shoulder,torso) 3(6.38%) Intubated for signs of respiratory distress |
Anti-venom: 46 (97.87%) Not Recorded: 1 (2.12%) |
Improved | None |
ADR, Adverse drug reaction; ARDS, Acute respiratory distress syndrome; EMNS, Early Morning Neuroparalytic Syndorme.
Snake / Venom types
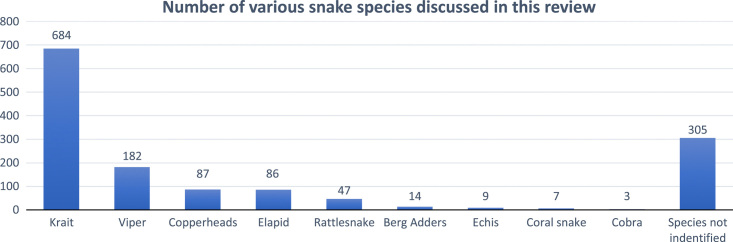
Out of 1424 cases, 1119 cases specified the snake species: 684 cases were caused by kraits (Bungarus caeruleus) (neurotoxin), 182 by vipers (Viperidae) (hematotoxin), 87 by copperheads (Agkistrodon contortrix) (hematotoxin), 86 by elapids (Elapidae) (neurotoxin), 47 by rattlesnakes (Crotalus cerastes) (neurotoxin and hematotoxin), 14 by berg adders (Bitis atropos) (neurotoxin), 9 by echis (Echis carinatus) (hematotoxin), 7 by coral snakes (Aspidelaps lubricus) (neurotoxin), and 3 by cobras (Naja naja) (neurotoxin). The remaining 305 cases did not identify the snake species. This data is illustrated in the Figure 2.
Figure 2.
showing number of various snake species discussed in this review.
Neurological and neuro-ophthalmological manifestations
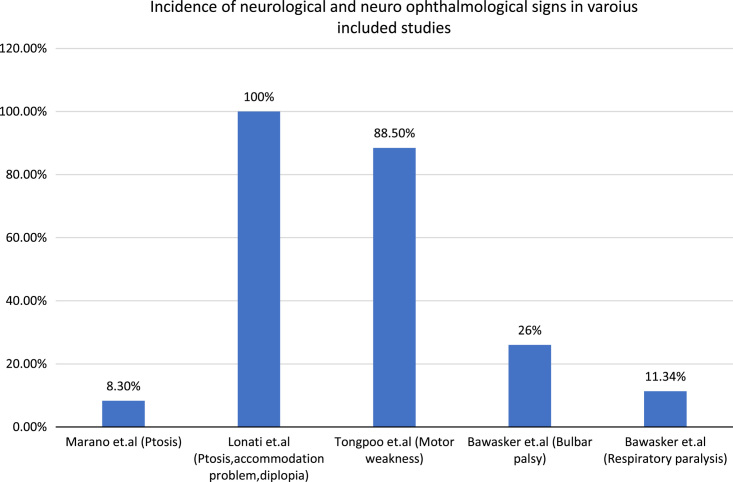
Marano et al.16 examined 24 cases of paediatric viper bites and found neurological signs in 8.3% of cases, with ptosis being the most common manifestation. Tongpoo et al.7 examined 78 cases of both paediatric and adult krait bites and found motor weakness to be present in 88.5% of cases. Lonati et al.11 studied 24 cases of both paediatric and adult viper bites and observed that all patients had accommodation problems and diplopia, with ptosis being the most common neurological manifestation. Similarly, Silva et al.17 studied 33 cases of krait bites and observed that ptosis was the most common manifestation. Anil et al.19 examined 72 cases of krait bites and found that most of the cases had ptosis. Bawaskar et al.12 studied 141 cases of krait bites and observed that 26% of cases had bulbar palsy and 11.34% had respiratory paralysis requiring mechanical ventilation. Additionally, this review included studies on Berg Adder bites, Copperheads bites, rattlesnake bites, and coral bites13,14,18,23. The detail can be found on Table 1. Incidence of various manifestations has been shown in Figure 3.
Figure 3.
showing incidence of various neurological and neuro-ophthalmological manifestations.
Treatment
Anti-snake venom (ASV) was administered in all the studies, and the patients were also provided with mechanical ventilation when necessary, except for a study conducted by Walt et al. 18, who managed 14 cases of Berg Adder bite symptomatically. In the study conducted by Karabuva et al.21, along with anti-snake venom, antibiotics, corticosteroids, and antihistamines were administered to the patients. However, Anil et al.19’s study on the effect of neostigmine in krait snake bites showed that the treatment was ineffective.
Outcome
We found that ASV effectively improved the neurological and neuro-ophthalmological manifestations. However, there have been reports of anaphylaxis caused by ASV in studies conducted by Sharma et al. 20, Kularatne et al. 10, Roth et al. 23, and Silva et al. 17. The studies have reported mortality rates ranging from 2.48 to 13.7%. Seneviratne et al.24 reported a case of mortality caused by intracranial haemorrhage. Nevertheless, the majority of patients improved without any residual neurological symptoms.
Discussion
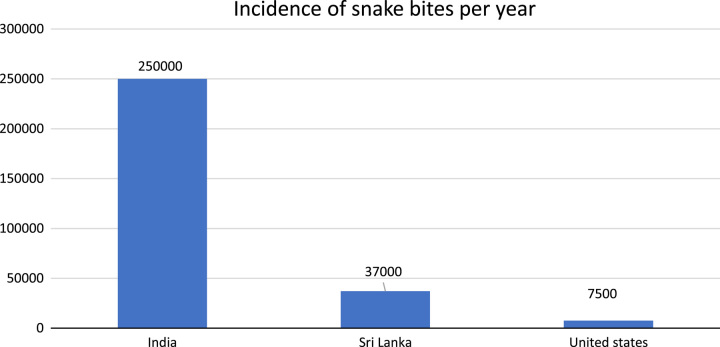
Our review showed that snake bites are prevalent in both children and adults. Most of the cases studied were of Krait bite. The majority of cases were reported from India, followed by Sri Lanka and the United States. In India alone, an average of 250 000 snake bites are reported each year3, while Sri Lankan hospitals admit around 37 000 snake bite cases annually25. This has been shown in Figure 4.
Figure 4.
showing the incidence rate of snake bite per year.
Neurotoxins present in snake venom bind to neuromuscular junctions both pre-synaptically and post-synaptically, resulting in muscle weakness. For instance, alpha-bungarotoxin found in krait venom binds to acetylcholine receptors, leading to a reduction in acetylcholine receptor sites in the postsynaptic membrane26. On the other hand, alpha-cobra toxin produces features of myasthenia gravis in experimental animals due to its similar action27. Neurotoxicity is caused by the pre-synaptic actions of phospholipase A2 enzyme and Beta-bungarotoxin22,28.
Hematotoxic venoms, on the other hand, can cause neurological symptoms like ptosis, ophthalmoplegia, and weakness due to three mechanisms: presynaptic inhibition of neurotransmission by phospholipase A2 in Russell’s viper venom, intracranial haemorrhage, or acute brain infarction29,30.
Most patients in this review report ptosis followed by neurological weakness of limbs and ophthalmoplegia, while nystagmus, delayed neuropathy, and dysarthria were reported in fewer patients. A moderate number of patients reported decrease in the visual acuity with snake bites. In neurotoxic snake bites, ptosis followed by neuroparalysis are the most common ocular signs and symptoms19,31, while hematotoxin snake bites can cause symptoms ranging from accommodation deficiency and ptosis to ophtalmoplegia, neck muscle weakness, and dysphagia10,20. According to Silva et al.17, patients who do not develop bulbar weakness or respiratory paralysis within 12 hours of the bite are unlikely to experience severe paralysis.
Several authors have reported muscle weakness after snake bites, which clinical and electrophysiological studies have attributed to defective neuromuscular transmission32,33. However, there have also been cases where muscular weakness was caused by rhabdomyolysis34. Snake bites can lead to potentially fatal respiratory muscle weakness, with krait bites being particularly notorious for rapidly causing respiratory failure24. However, in a series of ten patients with respiratory failure, all were successfully managed with mechanical ventilation, underscoring the importance of timely intervention24. Snake bites can also lead to Guillain-Barre syndrome and delayed neuropathy31. Neurotoxin snake bites commonly cause high blood pressure and tachycardia, which may be due to decreased parasympathetic activity35. Electrolyte imbalances, including hyponatremia, are also recognized clinical manifestations36.
ASV was administered to patients with systemic features, and our review revealed that it had a positive effect. Nevertheless, instances of anaphylaxis to ASV were also noted and were handled appropriately. The use of anti-venom has a high incidence of anaphylaxis37,38, as demonstrated in this review, which may limit its efficacy when widely used for snake bites and envenomation. The administration of anti-venom must be done in a high-care environment with constant monitoring and should be preceded by the administration of intramuscular adrenaline39.
The syndromic approach to managing snake bites, as popularized by Blaylock in 2005, divides management into three clinical syndromes that correspond to the three venom types40,41. The therapeutic triad, which includes elevation, intravenous fluids, and analgesia, is the mainstay of conservative management when the type of snake involved is unknown40. In severe cases of progressive painful swelling or bleeding, anti-venom administration and fasciotomy may be necessary42. Low dose anti-venom has been found to be as effective as high dose anti-venom for both neurotoxic and hematotoxic snake bites43. Early, empirical anti-venom is lifesaving, but good supportive care with mechanical ventilation is equally important and can lead to a good outcome44. The use of anti-coagulants in viper bites is controversial, with some reports suggesting it may increase the risk of haematoma and functional impairment9,45. The role of anticholinesterases in reversing neuroparalysis is also debated, with some studies showing ineffectiveness8,31.
Most cases in the reviewed studies recovered without neurological deficits. However, mortality rates were higher with neurotoxic snake bites compared to hemotoxic ones. Factors like younger age, ptosis, cardiac arrest, and lack of PICU beds were associated with higher mortality rates in children with EMNS, but prompt recognition, respiratory support, and PICU care can decrease mortality15.
To effectively prevent and treat snake bites, it is important to establish a proper documentation system such as a Snake Bite Registry that includes information on snake species, patient history, clinical signs, treatment, and final outcome. The North American Snakebite Registry (NASBR) is an example of such a registry46.
Conclusion
Ptosis is a prevalent neurological symptom in various snakebites, often accompanied by respiratory paralysis, limb weakness, dysphasia, and visual disturbances in certain cases. Notably, improvements are observed after ASV treatment, with almost no residual manifestations.
Ethical approval
Not Applicable for Systematic Review.
Consent
Informed consent was not required for this systematic review.
Source of funding
There is no any source of funding for this study.
Author contribution
K.P.: conceptualization, data curation, formal analysis, methodology, resources, validation, visualization, writing—original draft. A.R.: formal analysis, validation, methodology, writing—review and editing. H.M.S.M. and G.N.: conceptualization, resources and validation.
Conflicts of interest disclosure
None.
Research registration unique identifying number (UIN)
Name of the registry: Research registry Hyperlink https://www.researchregistry.com/browse-the-registry#home/registrationdetails/64c2a232f693a200273b1011/.
UIN: researchregistry9326.
Guarantor
Kamal Pandit.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Data availability
The authors declare no conflict of interest.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 22 November 2023
Contributor Information
Kamal Pandit, Email: kamalpandit33@gmail.com.
Aastha Rawal, Email: raastha57@gmail.com.
Himang Man Singh Maskey, Email: maskey.himang@gmail.com.
Gaurav Nepal, Email: drgauravnepal@gmail.com.
References
- 1.WHO . Snakebite Envenoming; 2021. https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming#:~:text=Key%20facts,are%20caused%20by%20snakebites%20annually
- 2.Warrell DA. Venomous bites, stings, and poisoning: an update. Infect Dis Clin 2019;33:17–38. [DOI] [PubMed] [Google Scholar]
- 3.Goswami PK, Samant M, Srivastava RS. Snake venom, anti-snake venom & potential of snake venom. Int J Pharm Pharm Sci 2014;6:4–7. [Google Scholar]
- 4.Kini RM. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon 2003;42:827–840. [DOI] [PubMed] [Google Scholar]
- 5.León G, Sánchez L, Hernández A, et al. Immune response towards snake venoms. Inflamm Allergy-Drug Targets (Formerly Curr Drug Targets-Inflammation Allergy)(Discontinued) 2011;10:381–398. [DOI] [PubMed] [Google Scholar]
- 6.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 7.Tongpoo A, Sriapha C, Pradoo A, et al. Krait envenomation in Thailand. Ther Clin Risk Manag. 2018:1711–1717. Published online. [DOI] [PMC free article] [PubMed]
- 8.Little M, Pereira P. Successful treatment of presumed death‐adder neurotoxicity using anticholinesterases. Emerg Med 2000;12:241–245. [Google Scholar]
- 9.Jollivet V, Hamel J-F, de Haro L, et al. European viper envenomation recorded by French poison control centers: A clinical assessment and management study. Toxicon 2015;108:97–103. [DOI] [PubMed] [Google Scholar]
- 10.Kularatne SAM, Silva A, Weerakoon K, et al. Revisiting Russell’s viper (Daboia russelii) bite in Sri Lanka: is abdominal pain an early feature of systemic envenoming? PLoS One 2014;9:e90198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lonati D, Giampreti A, Rossetto O, et al. Neurotoxicity of European viperids in Italy: Pavia poison control Centre case series 2001–2011. Clin Toxicol 2014;52:269–276. [DOI] [PubMed] [Google Scholar]
- 12.Bawaskar HS, Bawaskar PH, Bawaskar PH. Premonitory signs and symptoms of envenoming by common krait (Bungarus caeruleus). Trop Doct 2014;44:82–85. [DOI] [PubMed] [Google Scholar]
- 13.Bisneto PF, dos Santos Araújo B, da Silva Pereira H, et al. Envenomations by coral snakes in an Amazonian metropolis: Ecological, epidemiological and clinical aspects. Toxicon 2020;185:193–202. [DOI] [PubMed] [Google Scholar]
- 14.Vohra R, Cantrell FL, Williams SR. Fasciculations after rattlesnake envenomations: a retrospective statewide poison control system study. Clin Toxicol 2008;46:117–121. [DOI] [PubMed] [Google Scholar]
- 15.Samprathi M, Gupta V, Jayashree M, et al. Epidemiology and outcomes of early morning neuroparalytic syndrome following snake bite—a retrospective study. J Trop Pediatr 2020;66:435–440. [DOI] [PubMed] [Google Scholar]
- 16.Marano M, Pisani M, Zampini G, et al. Acute Exposure to European Viper Bite in Children: Advocating for a Pediatric Approach. Toxins (Basel) 2021;13:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva A, Maduwage K, Sedgwick M, et al. Neuromuscular effects of common krait (Bungarus caeruleus) envenoming in Sri Lanka. PLoS Negl Trop Dis 2016;10:e0004368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Walt AJ, Muller GJ. Berg adder (Bitis atropos) envenoming: an analysis of 14 cases. Clin Toxicol 2019;57:131–136. [DOI] [PubMed] [Google Scholar]
- 19.Anil A, Singh S, Bhalla A, et al. Role of neostigmine and polyvalent antivenom in Indian common krait (Bungarus caeruleus) bite. J Infect Public Health 2010;3:83–87. [DOI] [PubMed] [Google Scholar]
- 20.Sharma N, Chauhan S, Faruqi S, et al. Snake envenomation in a north Indian hospital. Emerg Med J 2005;22:118–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karabuva S, Vrkić I, Brizić I, et al. Venomous snakebites in children in southern Croatia. Toxicon 2016;112:8–15. [DOI] [PubMed] [Google Scholar]
- 22.Warrell DA. Injuries, envenoming, poisoning and allergic reactions caused by animals. In: Weatherall DJ, Ledingham JGG, Warrell DA, eds. Oxford Textbook of Clinical Medicine 1996:1142–1143. Published online.
- 23.Roth B, Sharma K, Onisko N, et al. Prospective evaluation of pain, swelling, and disability from copperhead envenomation. Clin Toxicol 2016;54:271–276. [DOI] [PubMed] [Google Scholar]
- 24.Seneviratne U, Dissanayake S. Neurological manifestations of snake bite in Sri Lanka. J Postgrad Med 2002;48:275. [PubMed] [Google Scholar]
- 25.Kasturiratne A, Pathmeswaran A, Fonseka MMD, et al. Estimates of disease burden due to land-snake bite in Sri Lankan hospitals. Southeast Asian J Trop Med Public Health 2005;36:733. [PubMed] [Google Scholar]
- 26.Drachman DB, Kao I, Pestronk A, et al. Myasthenia gravis as a receptor disorder. Ann N Y Acad Sci 1976;274:226–234. [DOI] [PubMed] [Google Scholar]
- 27.Satyamurti S, Drachman DB, Slone F. Blockade of acetylcholine receptors: a model of myasthenia gravis. Science (80- ) 1975;187:955–957. [DOI] [PubMed] [Google Scholar]
- 28.Warrell DA. Snake venoms in science and clinical medicine 1. Russell’s viper: biology, venom and treatment of bites. Trans R Soc Trop Med Hyg 1989;83:732–740. [DOI] [PubMed] [Google Scholar]
- 29.Warrell DA. Snake bite. Lancet 2010;375:77–88. [DOI] [PubMed] [Google Scholar]
- 30.Gawarammana I, Mendis S, Jeganathan K. Acute ischemic strokes due to bites by Daboia russelii in Sri Lanka–first authenticated case series. Toxicon 2009;54:421–428. [DOI] [PubMed] [Google Scholar]
- 31.Kularatne SAM. Common krait (Bungarus caeruleus) bite in Anuradhapura, Sri Lanka: a prospective clinical study, 1996–98. Postgrad Med J 2002;78:276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar S, Usgaonkar RS. Myasthenia gravis--like picture resulting from snake bite. J Indian Med Assoc. Published online 1968. [PubMed]
- 33.Sanmuganathan PS. Myasthenic syndrome of snake envenomation: a clinical and neurophysiological study. Postgrad Med J 1998;74:596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips RE, Theakston RDG, Warrell DA, et al. Paralysis, rhabdomyolysis and haemolysis caused by bites of Russell’s viper (Vipera russelli pulchella) in Sri Lanka: failure of Indian (Haffkine) antivenom. QJM An Int J Med 1988;68:691–715. [PubMed] [Google Scholar]
- 35.Laothong C, Sitprija V. Decreased parasympathetic activities in Malayan krait (Bungarus candidus) envenoming. Toxicon 2001;39:1353–1357. [DOI] [PubMed] [Google Scholar]
- 36.Höjer J, Tran Hung H, Warrell D. Life-threatening hyponatremia after krait bite envenoming–A new syndrome. Clin Toxicol 2010;48:956–957. [DOI] [PubMed] [Google Scholar]
- 37.Hon KLE, Chow CM, Cheung KL, et al. Snakebite in a child: could we avoid the anaphylaxis or the fasciotomies? Ann Med Singapore 2005;34:454. [PubMed] [Google Scholar]
- 38.Vongphoumy I, Chanthilat P, Vilayvong P, et al. Prospective, consecutive case series of 158 snakebite patients treated at Savannakhet provincial hospital, Lao People’s Democratic Republic with high incidence of anaphylactic shock to horse derived F (ab’) 2 antivenom. Toxicon 2016;117:13–21. [DOI] [PubMed] [Google Scholar]
- 39.Gutiérrez JM, Calvete JJ, Habib AG, et al. Snakebite envenoming. Nat Rev Dis Prim 2017;3:1–21. [DOI] [PubMed] [Google Scholar]
- 40.Blaylock RS. The identification and syndromic management of snakebite in South Africa. South African Fam Pract 2005;47:46–53. [Google Scholar]
- 41.Blaylock R. Epidemiology of snakebite in Eshowe, KwaZulu-Natal, South Africa. Toxicon 2004;43:159–166. [DOI] [PubMed] [Google Scholar]
- 42.Hsu C-P, Chuang J-F, Hsu Y-P, et al. Predictors of the development of post-snakebite compartment syndrome. Scand J Trauma Resusc Emerg Med 2015;23:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarin K, Dutta TK, Vinod K, V. Clinical profile & complications of neurotoxic snake bite & comparison of two regimens of polyvalent anti-snake venom in its treatment. Indian J Med Res 2017;145:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agrawal PN, Aggarwal AN, Gupta D, et al. Management of respiratory failure in severe neuroparalytic snake envenomation. Neurol India 2001;49:25–28. [PubMed] [Google Scholar]
- 45.Boels D, Hamel JF, Le Roux G, et al. Snake bites by European vipers in Mainland France in 2017–2018: Comparison of two antivenoms Viperfav® and Viperatab®. Clin Toxicol 2020;58:1050–1057. [DOI] [PubMed] [Google Scholar]
- 46.Ruha A-M, Kleinschmidt KC, Greene S, et al. The epidemiology, clinical course, and management of snakebites in the North American Snakebite Registry. J Med Toxicol 2017;13:309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare no conflict of interest.