Abstract
Müller glia are non-neuronal support cells that play a vital role in the homeostasis of the eye. Their radial-oriented processes span the width of the retina and respond to injury through a cellular response that can be detrimental or protective depending on the context. In some species, protective responses include the expression of stem cell-like genes which help to fuel new neuron formation and even restoration of vision. In many lower vertebrates including fish and amphibians, this response is well documented, however, in mammals it is severely limited. The remarkable plasticity of cellular reprogramming in lower vertebrates has inspired studies in mammals for repairing the retina and restoring sight, and recent studies suggest that mammals are also capable of regeneration, albeit to a lesser degree. Endogenous regeneration, whereby new retinal neurons are created from existing support cells, offers an exciting alternative approach to existing tissue transplant, gene therapy, and neural prosthetic approaches being explored in parallel. This review will highlight the role of Müller glia during retinal injury and repair. In the end, prospects for advancing retinal regeneration research will be considered.
Keywords: Müller glia, Retinal regeneration, Cellular reprogramming
1. Introduction
With nearly 285 million people suffering from impaired vision worldwide, retinal degenerations (RD) resulting from the loss of retinal photoreceptors (PR) or ganglion cells (RGCs) represent a major cause of permanent blindness (Pascolini & Mariotti, 2012). Although the cause of glaucoma, retinitis pigmentosa (RP), and age-related macular degeneration (AMD) can vary, close to 300 different genes and loci are implicated in RD, making this a difficult collection of disorders to address (Daiger et al., 1996). Current experimental approaches for restoring vision include gene therapy, cell or tissue transplantation and prosthetic stimulators, all of which are invasive and merely delay vision loss. Endogenous regeneration, which relies on the retina’s innate ability to repair itself, is an alternative with the potential to restore vision even after retinal neuron loss.
Following retinal injury, zebrafish and other lower vertebrates have the remarkable capability of restoring lost cells. They do so through cellular reprogramming of retinal pigment epithelial and Müller glial (MG) cells that dedifferentiate, transition into neural progenitors and finally differentiate into neurons. Although self-repair is common in fish and amphibians, this capability appears to be attenuated in mammals. A better understanding of self-repair mechanisms across species could pave the way for repair of the human retina.
2. Müller glia anatomy
2.1. Anatomy and function of Müller glia
Rod and cone PRs convert photons of light into electrical impulses that are transmitted through bipolar cells to the brain by way of RGC projections [Fig. 1]. Horizontal and amacrine interneurons modulate these signals through lateral inhibition, a feedback mechanism that disables the spreading of excitatory signals from adjacent PRs and bipolar cells and increases visual contrast. Radial MG spanning the retina regulate homeostatic properties and maintain retinal structural integrity. Although their cell bodies span the entire width of the retina, their nuclei reside in the inner nuclear layer (INL). MG have many activities, including neurotrophic factor release to support neurons, regulation of neuronal excitability by controlling the balance of extracellular ions and by blocking neural excitotoxicity (Sarthy & Ripps, 2001). This is accomplished via the recycling of glutamate and gamma-aminobutyric acid (GABA) neurotransmitters (Kawasaki et al., 2000). MG can also phagocytose dead cell debris through phosphatidylserine-Rac1 signaling (Nomura-Komoike et al., 2020). While the retinal pigment epithelium (RPE) regulates chromophore regeneration of rods and cones, MG also contribute to cone-specific visual pigment recycling (Wang et al., 2004).
Fig. 1.
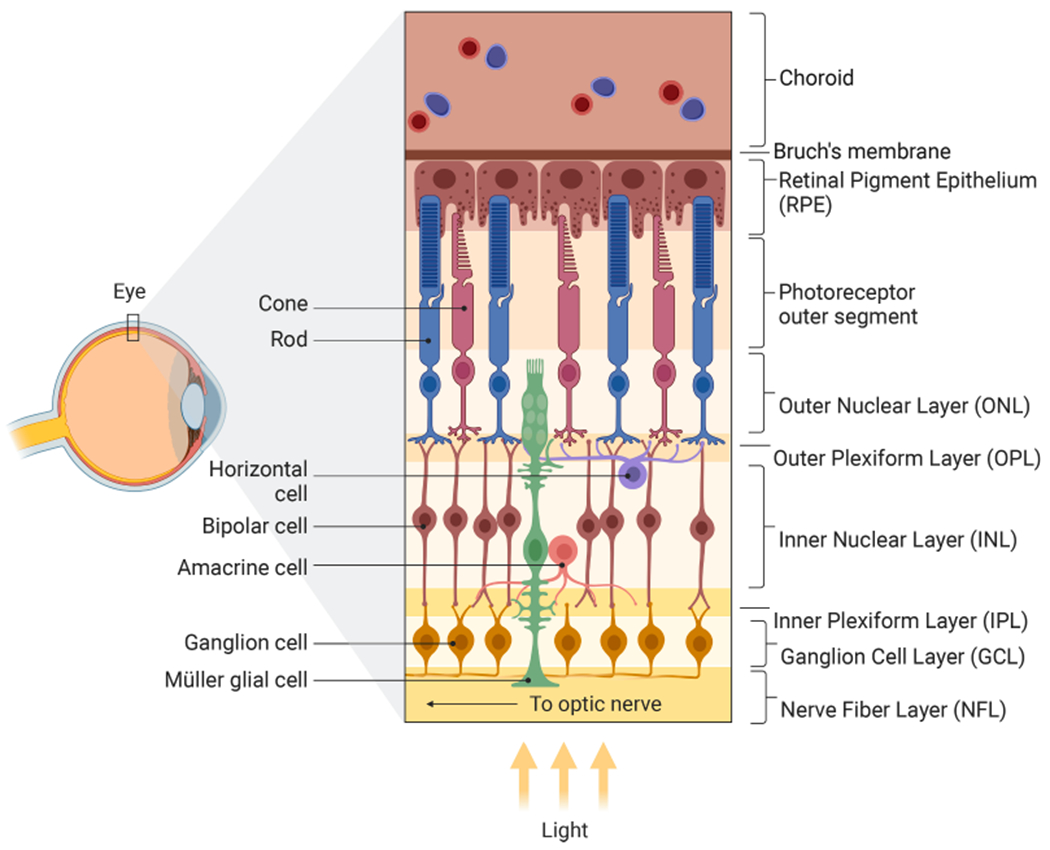
Structure of the Human Retina. The human retina has four nuclear layers: the outer, inner, ganglion cell, and retinal pigment epithelium layers (ONL, INL, GCL, RPE). Rod and cone photoreceptor (PR) cells, which are responsible for transducing light into electrical signals, are found in the outer retina between the retinal pigment epithelial (RPE) layer and the outer plexiform layer (OPL) where they connect with bipolar cells (BC) in the inner retina. Electrochemical signals then propagate through bipolar cells of the INL towards the inner plexiform layer (IPL) where they synapse with retinal ganglion cells (RGCs). The INL has horizontal and amacrine cell interneurons whose role is to modulate and refine vision through lateral inhibition and/or neuromodulation. RGC axons bundle emanating from the retinal ganglion cell layer (RGC) form the optic nerve which leaves the back of the eye, passes through the optic chiasm and connects with either the lateral geniculate nucleus (LGN) or to a lesser extent the superior colliculus (SC). From there nerve impulses connect to the visual cortex. In addition to neurons, two other types of cells found in the retina include Müller glia (MG) and RPE cells, both of which support the neurons of the retina. MG serve as the principle supporting glia of the eye and have nuclei in the INL, but cell bodies that span all layers of the retina. RPE cells absorb light while remaining in contact with PRs and serve a critical role in recycling pigmentation of PRs.
2.2. Response to retinal injury by Müller glia
MG are naturally resistant to lethal damage; however, retinal injury can lead to reactive gliosis, a process involving glial cell hypertrophy and retinal scarring. In the short term, neurotrophic factor release protects neurons from cell death but long term, gliosis can cause neuro-degeneration and cell debris accumulation. During proliferative gliosis in lower vertebrates, MG adopt a ‘stem-like’ state, proliferate and form multipotent progenitors which can differentiate into new neurons. This involves asymmetrical cell division and cell migration into the appropriate cell layers. Dedifferentiation into a proliferative state, as occurs in lower vertebrates, is a key feature for MG replacement.
3. Retinal regeneration models across species
Retinal cell fate determination of early and late-born cells is tightly controlled by a myriad of extrinsic and intrinsic cues that guide multipotent progenitors into various classes of neurons and glia (Cepko et al., 1996). These are specified in an overlapping fashion with RGCs being the first to be born followed by amacrine, horizontal, and cones. Rods, bipolar cells and MG take considerably longer to develop.
In mammals, MG typically do not generate new PR or RGC neurons. However, several model organisms including amphibians, birds and fish have long been recognized for their self-repair capability (Bernardos et al., 2007; Fausett & Goldman, 2006; Fischer & Reh, 2001; Yoshii et al., 2007). Insight from these species may hold important clues for controlling this process in humans.
3.1. Retinal regeneration in fish
Unlike mammals, teleost fish such as zebrafish and goldfish can rebuild damaged retinas and restore vision. While fish retinas share some characteristics with mammalian and avian retinas, notable differences exist. In fish, ciliary margin zone (CMZ) stem cells produce new neurons throughout life (Wan et al., 2016). MG also contribute to the regenerative potential of the eye by spontaneously generating rod progenitors at low frequency throughout life as well other retinal cells in response to injury (Thummel et al., 2008). Regeneration occurs after MG dedifferentiation and asymmetric cell division gives rise to new MG and multipotent daughter cells, the latter of which can reinstate lost neurons (Johns & Fernald, 1981).
3.2. Retinal regeneration in amphibians
In urodele amphibians (newts and salamanders) regeneration mediated by RPE transdifferentiation occurs through the activation of the extracellular signal-regulated kinase (ERK1/2) pathway and separately by regeneration through MG (Del Rio-Tsonis & Tsonis, 2003). Anuran amphibians (Xenopus tadpoles) with surgically excised retinas also demonstrate ERK1/2 mediated retina regeneration induced by exogenous FGF2 (Vergara & Del Rio-Tsonis, 2009). In addition, upon mechanical or chemical injury, frog MG readily proliferate to produce neural progenitors that later differentiate into photoreceptors (Langhe et al., 2017).
3.3. Retinal regeneration in birds
In birds, full retinal regeneration is possible only in early embryonic development. Later, the retina responds to injury by limited MG proliferation that is biased towards bipolar and amacrine cell generation. This asymmetric cell division is regulated by Notch signaling and new cells can be recognized by the marker genes achaete-scute homologue 1 (ASCL1), paired box 6 (PAX6) and visual system homeobox 2 (VSX2) (Ghai et al., 2010). This appears to be at least partially regulated by fibroblast growth factor 2 (FGF2), insulin and insulin-like growth factor 1 (IGF1) and downstream activation of ERK1/2 signaling pathways that stimulate MG proliferation and neural progenitor cell formation (Fischer et al., 2002).
3.4. Retinal regeneration in mammals
For many years, it was thought that regeneration in mammals does not occur. Retinal injury would lead to MG proliferation and expression of stem-like genes; however, this would not generate new retinal progenitors but rather MG undergoing reactive gliosis with subsequent retinal scarring, detachment, and further loss of vision. Despite the resistance to self-repair in the native retina, mouse MG are still partially capable of regenerating PRs and RGCs when transplanted into damaged retinas, provided they receive appropriate microenvironment cues, including growth factors and hormones such as EGF, FGF1, and insulin (Karl et al., 2008). Transcription factors can also regulate cell conversion, and in one study, de novo genesis of rods was observed in vivo when Adeno Associated Viruses (AAV) expressing beta (β)-catenin, followed by OTX2, CRX, and NRL were sequentially delivered to MG (Yao et al., 2018). Given the non-specific activity of viruses and a lack of genetic lineage-trace reporters for fate mapping, there are concerns regarding the accuracy of this AAV-based method for MG-derived regeneration (Blackshaw & Sanes, 2021; Le et al., 2022).
4. Mechanisms of cellular reprogramming
Evidence that MG respond to injury by dedifferentiating and reentering the cell cycle is supported by lineage tracing studies that used the thymidine analog BrdU to monitor cell division. More recently, genetic tracers have been developed using a Müller cell driven Cre recombination approach whereby a constitutively expressed fluorescent protein is blocked by a LoxP-stop-LoxP (LSL) sequence that is selectively excised in MG for permanent cellular tracing, thus allowing MG and their progeny to be tracked throughout reprogramming, proliferation and redifferentiation (Hoang et al., 2022). Upon retinal injury, MG transduce signals along interconnected signaling pathways such as Jak-Stat, Mapk-Erk, NF-κB, Notch, Pik3, Tgf-β, Wnt and Yap (Hippo) signaling to result in progenitor proliferation and exit from the cell cycle for neuronal differentiation across mammalian, fish, and avian species [Fig. 2].
Fig. 2.
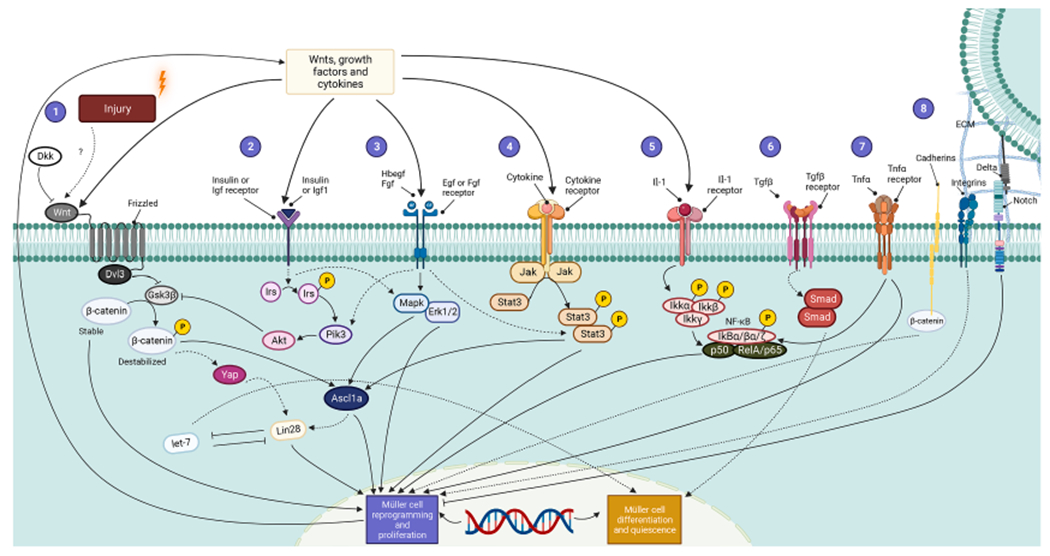
Signaling Pathways for Müller Cell Reprogramming and Proliferation. Regeneration is a highly regulated, multistep process that requires the expression of specific genetic programs at each stage and is influenced by the combined activity of secretory Wnt glycoproteins, inflammatory cytokines, and growth factors. (1) Wnts bound to Frizzled receptors activate Dishevelled Segment Polarity Protein 3 (Dvl3) to inhibit Gsk3β, which normally destabilizes β-catenin. A stabilized β-catenin is required for the proper linking of E-Cadherin to cytoskeletal structures to provide proper cell adhesion as well as gene expression leading to Müller cell reprogramming and retinal progenitor formation. β-catenin is a dual-function molecule that participates in cell adhesion to maintain structural integrity and polarity and as a transcriptional regulator that modulates gene expression; both contribute to MG dedifferentiation and progenitor formation. β-catenin can induce Yap/Taz (Hippo) signaling which can indirectly activate Lin28 to coax Müller glia de-differentiation. In addition, Wnt signaling is inhibited by its antagonist Dickkopf (Dkk). (2) Simultaneously, insulin and Igf1 signal through Insulin Receptor Substrate (IRS), Pik3, and Akt to inhibit Gsk3β. (3) Next, fibroblast growth factor (Fgf) and Hbegf bind to their receptors and control Egfr and mitogen-activated Mapk–Erk signaling that also end in reprogramming of Müller glia and cell division. (4) Meanwhile, cytokines acting via their respective receptors stimulate Jak proteins that phosphorylate Stat molecules to stimulate retinal regeneration by promoting gliogenesis. (5) Inhibition of NF-kB enhances Müller glia proliferation while its activation suppresses proliferating progenitor glia in a manner coordinated by microglia. (6) Furthermore, Tgf-β acts through the Smad pathway to coax Müller cells to exit quiescence, (7) interfering microRNA let-7, and RNA binding protein Lin28 are negatively regulated by each other and (8) Delta-Notch signaling complement modulated by the extracellular matrix (ECM); collectively these confer stem cell-like properties to Müller cells and stimulate retinal progenitor formation. Solid lines trace the pathways directly involved in Müller cell-mediated retinal regeneration, whereas dashed lines indicate the indirect ones.
4.1. Injury sensing or communication
Injury is a critical feature for regeneration across many species. Injury by light (UV or prolonged exposure to bright light), chemicals (N-methyl-d-aspartate (NMDA) or ouabain), mechanical trauma (needle poke), or toxic gene expression (e.g., nitroreductase) can stimulate the similar and sometimes convergent signaling pathways required to repair the retina (Fimbel et al., 2007; Montgomery et al., 2010; Vihtelic & Hyde, 2000). While acute exposure to bright light can destroy PRs, overstimulation of RGCs with NMDA or acute exposure to toxins can result in widespread collateral damage, and mechanical force usually annihilates all retinal cells in the affected area. During these many forms of injury, secretory factors including Wnts, adenosine diphosphate (ADP), ciliary neurotrophic factor (Cntf), heparin binding EGF-like growth factor (Hb-Egf), and tumor necrosis factor alpha (Tnfα) are produced at sites of injury where they communicate through paracrine and autocrine signaling to stimulate MG proliferation and reprogramming. While MG often remain in close contact with neighboring retinal cells in vivo, the disruption between injured cells and MG can activate nearby microglia which together infiltrate and phagocytose dead PRs. Importantly, microglia have been shown to suppress Ascl1-induced retinal regeneration in mice. To regenerate neurons across species, it will be necessary to better understand the many roles of microglia (Todd et al., 2020).
4.2. Activation of signal transduction pathways
Though the pathways and signaling molecules that regulate the cellular responses to injury and regeneration are still not completely understood, they involve a diverse cascade of signal transduction networks including Wnt-β-catenin, sonic hedgehog (SHH), epidermal growth factor (EGF), and ASCL1. The convergence of these networks at Ascl1 stimulates regeneration across a range of species through multiple pathways (Pollak et al., 2013). Pharmacological inhibition and gene disruption studies implicate glycogen synthase kinase 3β (Gsk3β)-β-catenin, Notch, Mapk–Erk and Jak–Stat signaling pathways as important for regulating regeneration [Fig. 2]. In NMDA-injured mouse retinas, Wnt3a initiates MG proliferation whereas subsequent treatment with growth factors (EGF, FGF1, or combined FGF1/insulin) leads to the formation of a small number of amacrine cells (Karl et al., 2008; Osakada et al., 2007). Overexpression of the Hippo pathway effector Yap also triggers MG cell cycle re-entry and proliferation (Hamon et al., 2019; Lourenco et al., 2021; Rueda et al., 2019). Furthermore, overexpression of downstream Ascl1 facilitates amacrine, bipolar and PR cell generation (Ueki et al., 2015; Jorstad et al., 2017). These effects can be augmented by inhibiting histone deacetylase (HDAC). Contrarily, a recent study showed that HDAC inhibition is not necessary since Atoh1 and Ascl1 overexpression in mouse MG can synergistically cooperate with each other to reprogram MG into light-responsive neurons in vivo in the absence of HDAC inhibition (Todd et al., 2020). Even in the absence of injury, β-catenin overexpression can contribute to de novo rod PR generation (Yao et al., 2018).
4.3. Cell cycle exit and differentiation
Cellular reprogramming stimulated by the Gsk3β–β-catenin, Notch, Mapk-Erk and Jak-Stat pathways regulates Ascl1 chromatin binding (Jorstad et al., 2020; Meyers et al., 2012; Nelson et al., 2012; Ramachandran et al., 2011), which in turn controls the expression of proneural Wnts, insulin-like growth factor-1 (Igf-1), fibroblast growth factor (Fgf), Heparin Binding EGF Like Growth Factor (Hbegf), Lin28, apolipoprotein B mRNA editing enzyme, catalytic polypeptide like 2b (Apobec2b), insulinoma-associated 1a (Insm1a), and phosphorylated Stat3. Ascl1 also regulates factors (e.g., let-7, Notch, Dkk, Insm1a, p57) that inhibit neural progenitor formation [Fig. 3]. Notably, the transcriptional repressor Insm1a plays a role in both MG reprogramming and cell cycle exit by driving p57 expression and Dkk inhibition. While activation of Gsk3β–β-catenin, Notch, Mapk–Erk and Jak–Stat encourages neural precursor formation, Gsk3β inhibition specifically promotes symmetrical division into two neurons which depletes the pool of dedifferentiated MG (Meyers et al., 2012). In most cases, proliferative MG undergoing reprogramming divide asymmetrically near the outer retina ventricular zone to generate a population of Müller cell-derived progenitors that undergo a finite number of cell divisions before differentiating into new retinal neurons. In addition to Ascl1a, other transcription factors including Pax6b, TGF-β induced factor 1 (Tgif1), and sine oculis homeobox homolog 3 (Six3) can contribute to MG expansion/conversion (Lenkowski et al., 2013; Thummel et al., 2010).
Fig. 3.
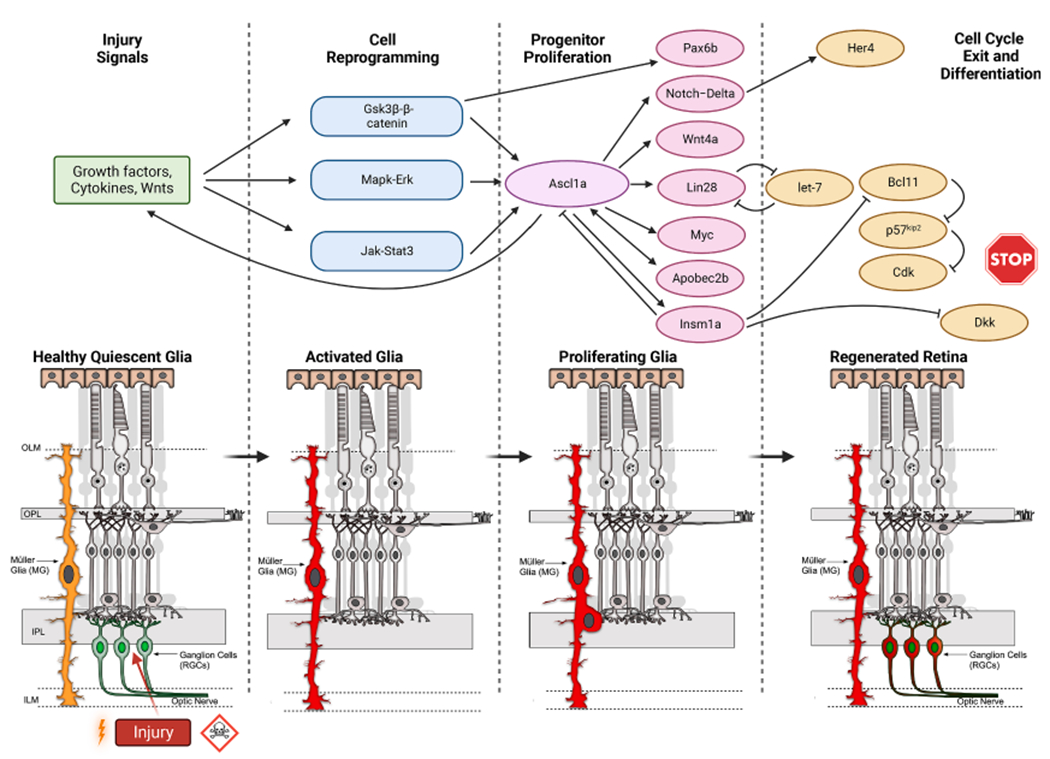
Transcriptional Signaling Cascades in Retinal Regeneration. Injury signals are transduced by growth factors, cytokines, and Wnts that impinge upon the Gsk3β–β-catenin, Mapk-Erk, and Jak-Stat signaling pathways which enable activation and reprogramming of Müller Glia. These pathways stimulate the injury-dependent expression of Ascl1a that participates in progenitor proliferation by regulating the Notch-Delta, Wnt4a, Lin28, Apobec2b, Insm1a genes. Ascl1a also controls factors that inhibit neural progenitor formation and help in cell cycle exit/differentiation such as microRNA let-7, Notch, Insm1a, Dkk, p57, etc.
5. Summary and conclusions
Most vertebrates can regenerate retinal neurons when MG sense injury or damage; this process involves progenitor cell formation, proliferation, cell cycle exit and neural differentiation. Studies of mechanical and pharmacological injury paradigms have led to the identification of growth and secretory factors that simulate cellular reprogramming and proliferation including Gsk3β–β-catenin, Notch, Mapk–Erk, and Jak–Stat signaling pathways. To better understand how regeneration can be controlled in mammals, further study of these pathways and their downstream effector genes across species is needed.
5.1. Future prospects
Retinal organoids derived from human pluripotent stem cells (hPSCs) mimic many aspects of human eye development and form all major classes of retinal cells in their proper laminar position (Nakano et al., 2012). Given their accessibility to experimental manipulation, 3D retinal organoids offer an exciting new platform for testing interventions that promote regeneration in human retinal degenerative diseases [Fig. 4]. Despite advancements in retinal regeneration, there are still knowledge gaps that have hindered progress in mammals. Recent work characterizing transcriptional profiles of injured/regenerating retinas across fish, birds, and mice may address some of this and has uncovered an important role for NF-κB in regulating MG proliferation (Hoang et al., 2020). NF-κB signaling can lead to SMAD3 and ID inhibition, resulting in the generation of Ascl1-positive neurons (Lee et al., 2020). During PR regeneration in chicken and mice, reactive microglia modulate NF-κB and regulate MG conversion (Palazzo et al., 2020; Palazzo et al., 2022). The RNA binding protein PTBP1 was also identified as a regulator of neural reprogramming with knockdown leading to de novo generation of RGCs following injury (Zhou et al., 2020). While this initially appeared to be mediated by MG conversion, subsequent studies using a Cre-LoxP-based lineage-traced reporter system failed to confirm a MG connection and suggested that new RGCs may not have originated from MG (Hoang et al., 2022; Xie & Chen, 2022; Xie et al., 2022). Reversing injury in the human retina, therefore, will require further knowledge of transcriptional differences between humans and other vertebrates and genetic fate-mapping lineage reporter experiments will be necessary to confirm MG-to-neuron conversion.
Fig. 4.
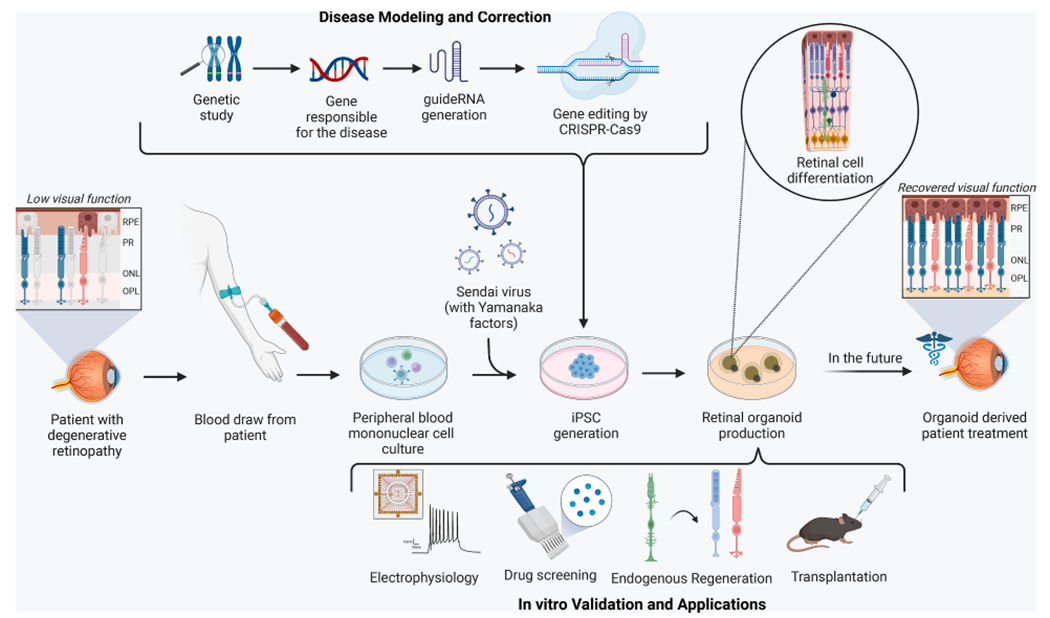
Generation and Application of Retinal Organoids. To generate retinal organoids, a blood draw is conducted in a patient with degenerative retinopathy, and peripheral blood mononuclear cells are extracted from the specimen. Sendai virus is used to reprogram the cells to form iPSCs which are later differentiated into 3D retinal organoids. CRISPR-Cas9 mediated gene editing can be utilized to model or correct disease states during the creation of organoids which can be validated by electrophysiology techniques or applied in drug screening, endogenous regeneration, and transplantation studies. These organoid-based approaches can potentially accelerate the development of treatments for retinal degeneration in the future.
Acknowledgement
All the figures in this review were generated using BioRender.
Funding
This work was supported by funding from the NIH (R01EY031318, P30EY022589), Altman Clinical and Translational Research Institute (ACTRI) grant #UL1TR001442, Foundation for Fighting Blindness, the Vision of Children Foundation, and the Richard C. Atkinson Laboratory for Regenerative Ophthalmology.
Footnotes
CRediT authorship contribution statement
Devansh Agarwal: Conceptualization, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. Hope Do: Conceptualization, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Kevin W. Mazo: Conceptualization, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. Manan Chopra: Conceptualization, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. Karl J. Wahlin: Conceptualization, Funding acquisition, Project administration, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA, 2007. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J. Neurosci 27 (26), 7028–7040. 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S, Sanes JR, 2021. Turning lead into gold: reprogramming retinal cells to cure blindness. J. Clin. Investig 131 (3) 10.1172/JCI146134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D, 1996. Cell fate determination in the vertebrate retina. PNAS 93 (2), 589–595. 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiger SP, Sullivan LS, Bowne SJ, Rossiter BJF, 1996. RetNet - Retinal Information Network. The University of Texas Health Science Center. [Google Scholar]
- Del Rio-Tsonis K, Tsonis PA, 2003. Eye regeneration at the molecular age. Dev. Dyn 226 (2), 211–224. 10.1002/dvdy.10224. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D, 2006. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J. Neurosci 26 (23), 6303–6313. 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR, 2007. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci 27 (7), 1712–1724. 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, McGuire CR, Dierks BD, Reh TA, 2002. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J. Neurosci 22 (21), 9387–9398. 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA, 2001. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci 4 (3), 247–252. 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ, 2010. Notch signaling influences neuroprotective and proliferative properties of mature Muller glia. J. Neurosci 30 (8), 3101–3112. 10.1523/JNEUROSCI.4919-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon A, Garcia-Garcia D, Ail D, Bitard J, Chesneau A, Dalkara D, Locker M, Roger JE, Perron M, 2019. Linking YAP to muller glia quiescence exit in the degenerative retina. Cell Rep. 27 (6), 1712–1725 e1716. 10.1016/j.celrep.2019.04.045. [DOI] [PubMed] [Google Scholar]
- Hoang T, Wang J, Boyd P, Wang F, Santiago C, Jiang L, Yoo S, Lahne M, Todd LJ, Jia M, Saez C, Keuthan C, Palazzo I, Squires N, Campbell WA, Rajaii F, Parayil T, Trinh V, Kim DW, Wang G, Campbell LJ, Ash J, Fischer AJ, Hyde DR, Qian J, Blackshaw S, 2020. Gene regulatory networks controlling vertebrate retinal regeneration. Science 370 (6519). 10.1126/science.abb8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Kim DW, Appel H, Pannullo NA, Leavey P, Ozawa M, Zheng S, Yu M, Peachey NS, Blackshaw S, 2022. Genetic loss of function of Ptbp1 does not induce glia-to-neuron conversion in retina. Cell Rep. 39 (11), 110849 10.1016/j.celrep.2022.110849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns PR, Fernald RD, 1981. Genesis of Rods in Teleost Fish Retina. Nature 293 (5828), 141–142. 10.1038/293141a0. [DOI] [PubMed] [Google Scholar]
- Jorstad NL, Wilken MS, Grimes WN, Wohl SG, VandenBosch LS, Yoshimatsu T, Wong RO, Rieke F, Reh TA, 2017. Stimulation of functional neuronal regeneration from Muller glia in adult mice. Nature 548 (7665), 103–107. 10.1038/nature23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorstad NL, Wilken MS, Todd L, Finkbeiner C, Nakamura P, Radulovich N, Hooper MJ, Chitsazan A, Wilkerson BA, Rieke F, Reh TA, 2020. STAT signaling modifies ascl1 chromatin binding and limits neural regeneration from muller glia in adult mouse retina. Cell Rep. 30 (7), 2195–2208 e2195. 10.1016/j.celrep.2020.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA, 2008. Stimulation of neural regeneration in the mouse retina. PNAS 105 (49), 19508–19513. 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki A, Otori Y, Barnstable CJ, 2000. Muller cell protection of rat retinal ganglion cells from glutamate and nitric oxide neurotoxicity. Invest. Ophthalmol. Vis. Sci 41 (11), 3444–3450. https://www.ncbi.nlm.nih.gov/pubmed/11006237. [PubMed] [Google Scholar]
- Langhe R, Chesneau A, Colozza G, Hidalgo M, Ail D, Locker M, Perron M, 2017. Muller glial cell reactivation in Xenopus models of retinal degeneration. Glia 65 (8), 1333–1349. 10.1002/glia.23165. [DOI] [PubMed] [Google Scholar]
- Le N, Appel H, Pannullo N, Hoang T, Blackshaw S, 2022. Ectopic insert-dependent neuronal expression of GFAP promoter-driven AAV constructs in adult mouse retina. Front. Cell Dev. Biol 10, 914386 10.3389/fcell.2022.914386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Wan J, Goldman D, 2020. Tgfb3 collaborates with PP2A and notch signaling pathways to inhibit retina regeneration. Elife 9. 10.7554/eLife.55137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkowski JR, Qin Z, Sifuentes CJ, Thummel R, Soto CM, Moens CB, Raymond PA, 2013. Retinal regeneration in adult zebrafish requires regulation of TGFbeta signaling. Glia 61 (10), 1687–1697. 10.1002/glia.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco R, Brandao AS, Borbinha J, Gorgulho R, Jacinto A, 2021. Yap regulates muller glia reprogramming in damaged zebrafish retinas. Front. Cell Dev. Biol 9, 667796 10.3389/fcell.2021.667796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JR, Hu L, Moses A, Kaboli K, Papandrea A, Raymond PA, 2012. beta-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev. 7, 30. 10.1186/1749-8104-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JE, Parsons MJ, Hyde DR, 2010. A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors. J. Comp. Neurol 518 (6), 800–814. 10.1002/cne.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y, 2012. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10 (6), 771–785. 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Gorsuch RA, Bailey TJ, Ackerman KM, Kassen SC, Hyde DR, 2012. Stat3 defines three populations of Muller glia and is required for initiating maximal muller glia proliferation in the regenerating zebrafish retina. J. Comp. Neurol. 520 (18), 4294–4311. 10.1002/cne.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura-Komoike K, Saitoh F, Fujieda H, 2020. Phosphatidylserine recognition and Rac1 activation are required for Muller glia proliferation, gliosis and phagocytosis after retinal injury [OriginalPaper]. Sci. Rep 10 (1), 1488. 10.1038/s41598-020-58424-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M, 2007. Wnt signaling promotes regeneration in the retina of adult mammals. J. Neurosci 27 (15), 4210–4219. 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo I, Deistler K, Hoang TV, Blackshaw S, Fischer AJ, 2020. NF-kappaB signaling regulates the formation of proliferating Muller glia-derived progenitor cells in the avian retina. Development 147 (10). 10.1242/dev.183418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo I, Todd LJ, Hoang TV, Reh TA, Blackshaw S, Fischer AJ, 2022. NFkB-signaling promotes glial reactivity and suppresses Muller glia-mediated neuron regeneration in the mammalian retina. Glia 70 (7), 1380–1401. 10.1002/glia.24181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP, 2012. Global estimates of visual impairment: 2010. Br. J. Ophthalmol 96 (5), 614–618. 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- Pollak J, Wilken MS, Ueki Y, Cox KE, Sullivan JM, Taylor RJ, Levine EM, Reh TA, 2013. ASCL1 reprograms mouse Muller glia into neurogenic retinal progenitors. Development 140 (12), 2619–2631. 10.1242/dev.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Zhao XF, Goldman D, 2011. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. PNAS 108 (38), 15858–15863. 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda EM, Hall BM, Hill MC, Swinton PG, Tong X, Martin JF, Poche RA, 2019. The hippo pathway blocks mammalian retinal muller glial cell reprogramming. Cell Rep 27 (6), 1637–1649 e1636. 10.1016/j.celrep.2019.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy V, Ripps H, 2001. The retinal Müller cell : structure and function. Kluwer Academic/Plenum Publishers, Publisher description; http://www.loc.gov/catdir/enhancements/fy0826/00058749-d.html. [Google Scholar]
- Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR, 2008. Characterization of Muller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp. Eye Res 87 (5), 433–444. 10.1016/j.exer.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel R, Enright JM, Kassen SC, Montgomery JE, Bailey TJ, Hyde DR, 2010. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp. Eye Res 90 (5), 572–582. 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Finkbeiner C, Wong CK, Hooper MJ, Reh TA, 2020. Microglia suppress ascl1-induced retinal regeneration in mice. Cell Rep 33 (11) 10.1016/j.celrep.2020.108507. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Wilken MS, Cox KE, Chipman L, Jorstad N, Sternhagen K, Simic M, Ullom K, Nakafuku M, Reh TA, 2015. Transgenic expression of the proneural transcription factor Ascl1 in Muller glia stimulates retinal regeneration in young mice. PNAS 112 (44), 13717–13722. 10.1073/pnas.1510595112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara MN, Del Rio-Tsonis K, 2009. Retinal regeneration in the Xenopus laevis tadpole: a new model system. Mol. Vis 15, 1000–1013 https://www.ncbi.nlm.nih.gov/pubmed/19461929. [PMC free article] [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR, 2000. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J. Neurobiol 44 (3), 289–307. 10.1002/1097-4695(20000905)44:3289::aid-neu13.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Wan Y, Almeida AD, Rulands S, Chalour N, Muresan L, Wu Y, Simons BD, He J, Harris WA, 2016. The ciliary marginal zone of the zebrafish retina: clonal and time-lapse analysis of a continuously growing tissue. Development 143 (7), 1099–1107. 10.1242/dev.133314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Iannaccone A, Jablonski MM, 2004. Contribution of muller cells toward the regulation of photoreceptor outer segment assembly. Neuron Glia Biol. 1 (3), 291–296. 10.1017/S1740925X05000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Chen B, 2022. Critical EXAMINATION OF MULLER GLIA-DERIVED IN VIVO NEUROGENESIS IN THE MOUSE REtina. Front. Cell Dev. Biol 10 10.3389/fcell.2022.830382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zhou J, Chen B, 2022. Critical examination of Ptbp1-mediated glia-to-neuron conversion in the mouse retina. Cell Rep. 39 (11) 10.1016/j.celrep.2022.110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K, Qiu S, Wang YV, Park SJH, Mohns EJ, Mehta B, Liu X, Chang B, Zenisek D, Crair MC, Demb JB, Chen B, 2018. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature 560 (7719), 484–488. 10.1038/s41586-018-0425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii C, Ueda Y, Okamoto M, Araki M, 2007. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev. Biol 303 (1), 45–56. 10.1016/j.ydbio.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Zhou H, Su J, Hu X, Zhou C, Li H, Chen Z, Xiao Q, Wang B, Wu W, Sun Y, Zhou Y, Tang C, Liu F, Wang L, Feng C, Liu M, Li S, Zhang Y, Xu H, Yang H, 2020. Glia-to-neuron conversion by CRISPR-CasRx alleviates symptoms of neurological disease in mice. Cell 181 (3), 590–603 e516. 10.1016/j.cell.2020.03.024. [DOI] [PubMed] [Google Scholar]
- Todd L, Hooper MJ, Haugan AK, Finkbeiner C, Jorstad N, Radulovich N, Wong CK, Donaldson PC, Jenkins W, Chen Q, Rieke F, & Reh TA (2021). Efficient stimulation of retinal regeneration from Muller glia in adult mice using combinations of proneural bHLH transcription factors. Cell Rep, 37(3), 109857. [DOI] [PMC free article] [PubMed] [Google Scholar]


