Abstract
In animals, microRNAs are amongst the primary non-coding RNAs involved in regulating the gene expression of a cell. Most mRNAs in a cell are targeted by one or many miRNAs. Although several mechanisms can be attributed to the degradation of miRNA and mRNA within a cell, but the involvement of autophagy in the clearance of miRNA and its target mRNA is not known. We discover a leucine-responsive axis in blood cell progenitors that can mediate an autophagy-directed degradation of miRNA-bound mRNA in Drosophila melanogaster and Homo sapiens. This previously unknown miRNA clearance axis is activated upon amino acid deprivation that can traffic miRNA–mRNA-loaded Argonaute for autophagic degradation in a p62-dependent manner. Thus, our research not only reports a novel axis that can address the turnover of a catalytically active miRISC but also elucidates a slicer-independent mechanism through which autophagy can selectively initiate the clearance of target mRNA.
Graphical Abstract
Graphical Abstract.
Introduction
MicroRNAs (miRNAs) are short non-coding ribonucleic acids transcribed in an RNA pol II-dependent manner (1–3). Metazoan RNA silencing machinery utilizes miRNAs to regulate the expression of its genes (4–9). These molecules work in a cohort to impose a delicate regulatory balance over the whole network of genes, thus shaping the transcriptome and influencing multiple biological processes (10–13). Therefore, understanding the mechanisms that can regulate miRNA function is one of the most active fields in cell biology.
Before miRNA attains its functional state, a cell employs a variety of mechanisms to govern the turnover of miRNA during several pre-processing phases. Exonuclease and specific miRNA-modifying enzymes, such as methyltransferases, poly-A polymerases, and uridyltransferases, are among the well-known regulating mechanisms for miRNA turnover. (14–19) Even after extensive processing, miRNAs are only functional if loaded onto the Argonaute protein of the miRNA-induced silencing complex (miRISC) (20). Post-Argonaute loading, a miRNA attains the ability to bind to its cognate target and mediate repression.
Interestingly, despite numerous insights into the mechanisms underlying miRNA turnover, much remains to be learned about the clearance of mRNA following miRISC loading (miRNA–mRNA hybrids) (21). Moreover, despite the inherent significance of miRNA in gene regulation, the turnover of miRNA and miRNA–mRNA hybrid has never been substantiated through cell homeostatic mechanisms like ‘Autophagy’. Recent publications have demonstrated the presence of mRNAs in a cell's autophagosome; nonetheless, the physiological context and mechanism that can transport mRNA into autophagosome is a novel area of study (22,23). Studies so far demonstrate that the components involved in the formation of the miRNA-induced silencing complex can be regulated by autophagy (24–30). However, direct evidence of autophagy affecting the degradation of the miRNA–mRNA repressive complex has never been documented.
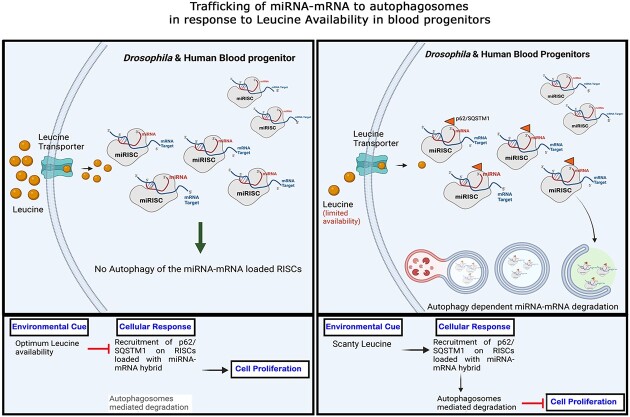
Our study unveils a functional association between an environmental signal of nutrient availability and active-miRNA machinery, quintessential for hematopoietic growth and proliferation. We show a proliferative and concentration-dependent role of leucine, a ‘branched-chain amino acid’ in Drosophila blood progenitors. This nutrient-responsive input can elicit autophagy, ‘a canonical proteostatic process’ to target the degradation of miRNA–mRNA-loaded RISC. Interestingly, we report that leucine-mediated autophagy also augments the interaction of miRNA-loaded Ago1 with the selective autophagy receptor p62. Additionally, this p62-targeted Ago1 interaction also encompasses mature miRNA engaged with its cognate target, thereby guiding its turnover through auto-lysosomal degradation. This process is quite distinct from the previously known TDMD (Target Directed miRNA Degradation) (31,32). While TDMD refers to the degradation of the miRNA bound to mRNA, our work sheds light on a process wherein the mRNA bound to miRNA is destined for autophagosome-dependent degradation. This phenomenon is not restricted only to Drosophila blood progenitors; upon leucine deprivation, human HSPCs (Hematopoietic Stem and Progenitor Cells) can also target miRNA-loaded RISC towards degradation via p62-dependent autophagy. This hitherto unknown conserved modality demonstrates how a cell homeostatic mechanism like autophagy through the nutrient-responsive feed can catalyze the degradation of active miRNAs along with its cognate targets.
Materials and methods
Larval culture and synchronization
For synchronized larvae culture, embryos were collected on a ‘Egg laying media’ made up of 2% agar, 5% sucrose prepared in mixed fruit juice containing 0.003% methyl paraben, and a bit of yeast paste applied onto the plate. Embryos from mated flies were collected onto these plates for 4 h, the collected embryos were then incubated for 20 h for hatching, both the collection and incubation of the embryos was done in 25°C. The hatched larvae were collected at 1 h interval and transferred onto fly food after reaching appropriate developmental time points these larvae are dissected and processed accordingly.
Preparation of standard fly food and semi-defined fly food
Standard fly food consists of 0.9% agar, 4.5% sucrose, 5.1% maize powder, 1.825% Type II Saccharomyces cerevisiae (Yeast), 0.75% Protein mix [37.60% EAA (4.52% isoleucine, 8.52% leucine, 4.61% valine, 7.48% lysine, 1.81% methionine, 2.47% phenylalanine, 5.48% threonine, 1.32% tryptophan, 1.40% histidine), 12.15% SEAA and 30.25% NEAA] with 0.003% (w/v) methyl paraben and 0.3% propionic acid. This composition makes up 1× of standard fly food.
Semi-defined fly food for protein supplementation experiments was modified from standard food with all the components remaining the same with exception of Type II yeast and protein mix. 1.6% of yeast and 0.5% of protein mix was added and 1.17 mM of individual essential amino acids (EAA) were added separately. For EAA exclusion screening, all EAAs were added except for the one which is being screened.
Preparation of cell culture media
Leucine deficient media
Premade media from Thermo Scientific Cat. No. 30030 (DMEM-LM) was purchased. To this media, a final working solution of 0.2 mM l-methionine was added to obtain leucine-deficient media. After preparation, a final working solution of 1 mM l-leucine was added to this culture media and used as a control for the leucine-deficient experiment.
Valine deficient media
We prepared valine deficient DMEM cell culture media by individually combining the specified components: Glycine (30.00 mg/l), l-arginine HCl (84.00 mg/l), l-cystine 2 HCl (48.00 mg/l), l-glutamine (584.00 mg/l), l-histidine HCl H2O (42.00 mg/l), l-isoleucine (105.00 mg/l), l-leucine (105.00 mg/l), l-lysine HCl (146.00 mg/l), l-methionine (30.00 mg/l), l-phenylalanine (66.00 mg/l), l-serine (42.00 mg/l), l-threonine (95.00 mg/l), l-tryptophan (16.00 mg/l), l-tyrosine 2 Na (89.47 mg/l). A final 4× MEM Vitamin Solution (Gibco-11120052, MEM Vitamin Solution (100×)) was added. A mix of inorganic salts consisting, CaCl2·2H2O (265.00 mg/l), Fe(NO3)3·9H2O (0.10 mg/l), KCl (400.00 mg/l), MgSO4 (97.67 mg/l), NaCl (6400.00 mg/l), NaHCO3 (3700.00 mg/l), and NaH2PO4 (109.00 mg/l), d-Glucose (4500.00 mg/l), and Phenol Red Sodium Salt (15.90 mg/l) was also added. These components were mixed, pH was equilibrated to 7.4, filtered and sterilized. After preparation, a final working solution of 1 mM l-valine was added to this culture media and used as a control for the deficient experiment.
Cell culture of human HSPC
Primary Bone Marrow CD34+ Hematopoietic Cells, Normal, Human were acquired from ATCC (PCS-800-012). Hereafter, these CD34+ cells were cultured in IMDM media with 20% FBS. Before any treatment, these cells were co-stained with antibodies directed against CD34, CD10 and Lineage Cocktail (CD3, CD14, CD16, CD19, CD20, CD56) (obtained from Biolegend). Only the CD34+ CD10-Lin cells were FACS sorted to obtain myeloid progenitor populations for downstream processing. Cell culture of myeloid-like AML cell lines (KG1) were cultured in IMDM media with 20% FBS before treatment in leucine containing (Control) and leucine-deficient media with variable culturing time as indicated in text and panels.
Tissue preparation and laser capture microdissection (LCM)
For the analysis of 48-h larval lymph glands (48 h LG), the specimens were meticulously dissected and placed onto a specialized dissection slide equipped with a Polyethylene Naphthalate (PEN) membrane coating to facilitate downstream manipulations. Subsequent to placing the lymph gland (LG) on the slide, a fixation step was undertaken by gently applying 10 μl of a 60% methanol solution. The fixation duration was carefully controlled within the range of 20–30 s to ensure optimal preservation of tissue architecture. Following the methanol fixation, a deliberate period was allotted for the methanol to evaporate, which contributed to the desiccation and flattening of the tissue atop the membrane slide. This preparatory step was crucial for subsequent laser capture microdissection (LCM) procedures.
The laser capture microdissection (LCM) was executed using the Zeiss Palm Microbeam system, meticulously dissecting the fixed and desiccated samples. The LCM procedure enabled the precise isolation of specific tissue regions. The dissected samples were directed into tubes containing TRIzol reagent (Invitrogen). These samples were then processed for subsequent RNA isolation in strict accordance with the manufacturer's protocol. Throughout the procedure, utmost care was taken to ensure the integrity of the tissue samples and minimize the introduction of potential contaminants. The combination of precise dissection, fixation and LCM facilitated the procurement of high-quality RNA samples for downstream analyses.
Subsequently, a 50 μl aliquot of chloroform was introduced into the homogenate, followed by gentle agitation to promote phase separation. The mixture was centrifuged at 15 000 × g for 15 min at 4°C. The resulting biphasic solution yielded an upper aqueous layer, which was carefully transferred into a fresh tube. 0.5 μl of Glycogen (Sigma) was added and gently mixed with the aqueous layer to enhance RNA recovery. For precipitation, an ice-cold isopropanol solution at 0.6 volumes was added to the mixture, which was subsequently stored at -20°C for an incubation period of 1 h. Post incubation, centrifugation was performed at 10 000 × g for 10 min at 4°C to collect the precipitated RNA. The supernatant was gently decanted, and the resultant pellet was washed twice with 200 μl of ice-cold 75% ethanol. A subsequent centrifugation step at 15 000 × g for 5 min at 4°C facilitated the removal of residual impurities. The resultant RNA pellet was air-dried and resuspended in DEPC-treated RNAse DNAse-free water (Sigma), ensuring RNA integrity.
RNA quality assessment and DNAse treatment
The purity and concentration of the extracted RNA were evaluated using a spectrophotometer, with specific attention to the A260/A280 ratio. DNAse treatment was carried out using the Qiagen RNAse-free DNAse set, following the manufacturer's guidelines to ensure the removal of any potential DNA contamination. The reaction was terminated through heat deactivation at 75°C for 15 min, supplemented with 1 mM EDTA.
cDNA synthesis from mRNA isolates
cDNA was synthesised using Verso cDNA synthesis kit, as per the manufacturer's protocol. 10–50 ng of DNAse treated RNA template was mixed with cDNA reaction mix (cDNA synthesis buffer, dNTP mix, anchored oligo dT primers, random hexamers, RT enhancer, Verso enzyme mix, nuclease-free water) for a final reaction volume of 20 μl. The reaction mix was proceeded at 42°C for 30 min, followed by an inactivation step (95°C, 2 min). Non-enzymatic control reactions were set alongside the main reaction.
cDNA synthesis from miRNA isolates
Multiplexed miRNA expression screening
MiRNA expression levels were analyzed in a multiplexing manner using QuantaBio's The qScript™ microRNA cDNA Synthesis Kit or Takarabio's Mir-X™ miRNA FirstStrand Synthesis qPCR kit following the manufacturer's protocol.
cDNA conversion for stem–loop-PCR for mature miRNA analysis
To comprehensively characterize mature microRNA (miRNA) expression profiles, the stem–loop reverse transcription polymerase chain reaction (RT-PCR) methodology was employed (33)—this technique was specifically designed for the precise quantification of mature miRNAs. Stem-loop primers were custom-designed for each target miRNA sequence following the protocol standardized by Kramer et al. (34), with the reverse transcription performed in a pulsed fashion. Firstly, samples were incubated at 16°C for 30 min. A pulsed RT of 60 cycles was then applied, with 30 s at 30°C, 30 s at 42°C, and 1 s at 50°C (35).
Real time quantitative PCR (RT-qPCR)
miRNA analysis
Each qPCR reaction was performed using Takara Bio TB Green Premix Ex Taq II as per the manufacturer's protocol. Template DNA was mixed with RT-qPCR master mix (TB-Green reaction mix, forward primer, reverse primer, nuclease-free water) for a final reaction volume of 20 μl. For high-throughput screening reverse primers were provided in the kit. For Stemloop, qRT-PCR reverse primer was designed according to Kramer et al. (34). Only primers with a Primer efficiency ranging from 90–110% were used. Replicates were taken for each cDNA condition and the reactions were performed in Bio-Rad CFX96 Touch Real-Time PCR Detection System. For each miRNA, the cycle threshold (Ct) values were obtained and normalized to an internal control [U6 snRNA for human cells, 2s rRNA for Drosophila] using the ΔCt method. The relative expression levels of miRNAs were calculated using the formula 2(–ΔΔCt), and the results were expressed as fold change compared to reference gene. Atleast three replicates were used, and all data were presented as mean ± standard deviation (SD).
mRNA analysis
The qPCR reactions were conducted using the Takara Bio TB Green Premix Ex Taq II, following the instructions provided by the manufacturer. A mixture was prepared by combining template DNA with RT-qPCR master mix, which consisted of TB-Green reaction mix, a forward primer, a reverse primer, and nuclease-free water. The resulting mixture had a final volume of 20 μl. We exclusively utilized primers with a Primer efficiency ranging from 90 to 110%. Multiple replicates were collected for each cDNA condition, and the reactions were conducted using the Bio-Rad CFX96 Touch Real-Time PCR Detection System. The Ct values for each miRNA were acquired and subsequently normalized to an internal reference, specifically Beta-Actin for Human cells and Rp49 transcript for Drosophila, utilizing the ΔCt method. The relative expression levels of microRNAs (miRNAs) were determined using the 2(–ΔΔCt) approach, and the outcomes were presented as fold change relative to a reference gene. The transcript levels were normalised with respect to rp49. Fold changes were determined using the ΔΔCt method and Pfaffl method. Details of the primers are mentioned in Supplementary Table S2. Multiple replicates were used, and all data were presented as mean ± standard deviation (SD).
After acquiring the data from both mRNA and miRNA analysis, statistical analysis was conducted using the Mann–Whitney U test to determine the significance of differences in the transcript expression levels.
End-point PCR and agarose gel electrophoresis
Template DNA was mixed with PCR reaction mix (standard Taq reaction buffer, dNTP mix, forward primers, reverse primers, Taq polymerase, nuclease-free water) for a final reaction volume of 20 μl. The reaction mix was amplified in a standard thermal cycler. To ensure the reliability of results, non-enzymatic control reactions were meticulously included alongside the primary reaction to account for potential non-specific amplification. After amplification, the resulting DNA products were evaluated via agarose-gel electrophoresis. The gel electrophoresis apparatus employed appropriate voltage and duration settings to facilitate the migration of DNA fragments through the gel matrix. Gel quantification was undertaken using ImageJ software, enabling accurate band intensities and molecular sizes assessment.
RNA sequencing and bioinformatics analysis
RNA extraction
RNA extraction was carried out using the ZR Quick RNA Mini-Prep Kit (Zymo Research) in strict accordance with the manufacturer's instructions. The quality and quantity of the isolated RNA samples were meticulously assessed using a NanoDrop spectrophotometer, followed by further validation using the Agilent TapeStation with High Sensitivity RNA ScreenTape.
Subsequently, the high-quality RNA samples were subjected to sequencing on the Illumina NovaSeq 6000 platform (from Eurofins Genomics India). The sequencing protocol encompassed the generation of 2 × 150 bp paired-end reads, ultimately yielding a data output of 3 GB. This comprehensive sequencing strategy ensured the acquisition of ample data for robust subsequent analyses.
Data preprocessing and alignment
After obtaining raw sequencing reads, an initial quality assessment was performed using FASTQC software. To ensure the reliability of downstream analyses, adapter sequences were removed from the reads using Trimmomatic. This step was essential to enhance the quality of the paired-end reads and improve the overall accuracy of subsequent alignment and assembly.
Read mapping and transcriptome assembly
The processed reads were then mapped to the human genome using the Tophat alignment tool, which facilitated accurate alignment and assessment of gene expression levels. Subsequently, transcriptome assembly was performed using Cufflinks, enabling comprehensive reconstruction of transcript structures and quantification of their expression.
Transcriptome integration and analysis
The Cuffmerge tool was employed to consolidate individual assemblies, enabling the integration of separate transcriptome assemblies into a unified dataset. Following this step, an extensive bioinformatic analysis was executed using the iDEP platform (36). This encompassed exploratory data analysis, visualization and interpretation of the transcriptomic profiles.
Immunostaining
Larval pull-outs containing the lymph glands were dissected in phosphate buffer saline (1× PBS, pH 7.2), and the pull-outs were transferred in a cavity block containing 1× PBS kept on ice. After dissection and pooling, the tissues were fixed in 4% paraformaldehyde <w/v> prepared in 1× PBS (pH 7.2) for 1 h at room temperature (RT), followed by two quick washes with 1× PBS. These tissues were then permeabilized with 0.3% PBT (0.3% Triton X-100 <v/v> in 1× PBS) for about 45 min (3 × 15 min washes) at RT. The pull-outs were then incubated in a Blocking solution made with 2% normal donkey serum, prepared in 0.3% PBT (37) for 1 h. Hereafter, the pull-outs were incubated in primary antibody diluted in Blocking solution for 12 h at 4°C (in the case of anti-Ago1 and anti-Ref(2)p, primary ab incubation was done at RT for 4 h) and washed with 0.3% PBT for 45 min (3 × 15 min washes) at RT. Next, the pull-outs were incubated in appropriate secondary antibody for 1.5–2 h at RT and subjected to three washes in 0.3% PBT for 15 min each, followed by incubation in Hoechst 33342 (1 μg/ml) solution (Invitrogen) for 1 h at RT. Excess Hoechst was washed off from the tissues by washing the samples with 1× PBS for 20 min (2 × 10 min washes). Lymph glands from these pull-outs were then mounted in Vectashield (Vector Laboratories), followed by image acquisition. The primary antibody used in this study are 1:100 mouse Anti-GFP (sigma), 1:500 rabbit Anti-GFP (Invitrogen-A11122), 1:2000 anti-Ref(2)p (Abcam), 1:2000 anti-Ago1 (Abcam), anti-mCherry (DSHB) and Secondary Abs (Jacksons ImmunoResearch).
EdU/OPP incorporation assay
Click-iT EdU and OPP plus (5- ethynyl-2′- deoxyuridine, a thymidine analog) kit from Invitrogen (C10639) was used to assay the proliferation rate of Lymph gland cells. Synchronized larvae at 36, 48 and 60 h AEH were used in this study. Larval pull-outs containing the lymph glands were immediately dissected and transferred to cavity blocks containing 1× PBS at RT. After that, the 1× PBS was dispensed, and tissues were resuspended in EdU solution (1:1000 in 1× PBS) for about 30 min at room temperature. After a quick 1× PBS wash, the pull-outs were fixed in 4% paraformaldehyde <w/v> prepared in 1× PBS (pH 7.2) for 1 h at RT. After fixation, pull-outs were permeabilized with 0.3% PBT for about 40 min (4 × 10 min washes), followed by blocking in 2% BSA (prepared in 0.3% PBT) for 1 h at RT. For detection of EdU incorporation, ‘EdU staining solution’ was prepared as per the manufacturer's instruction (for 50 μl staining solution, 44 μl of 1 × EdU Buffer, 1 μl of Copper protectant, 0.12 μl of Alexa Fluor Picolyl Azide, and 5 μl of 10 × EdU buffer additive). Staining of the pull-outs was done for 30 min at room temperature. After staining, the pull-outs were washed three times with 0.3% PBT, followed by a quick wash with PBS. The pull-outs were then incubated in Hoechst 33342 (1 μg/ml) for 1 h and mounted in Vectashield, followed by image acquisition.
EdU/OPP incorporation for cell culture
EdU/ OPP was added with the media at a final 1 mM concentration, and the cells were incubated for 4 h. Post-incubation, the cells were harvested, followed by a quick 1× PBS wash. The cells were transferred to Cyto-spin cartridges (Thermo scientific Cytospin 4) and centrifuged over a glass slide. The cells were then fixed in 4% paraformaldehyde <w/v> prepared in 1× PBS (pH 7.2) for 1 h at 4°C. After fixation, cells were permeabilized with 0.3% PBT for about 40 min (4 × 10 min washes), followed by blocking in 2% BSA (prepared in 0.3% PBT) for 1 h at RT. For detection of EdU incorporation, ‘EdU staining solution’ was prepared as per the manufacturer's instruction (for 50 μl staining solution, 44 μl of 1 × EdU Buffer, 1 μl of copper protectant, 0.12 μl of Alexa Fluor Picolyl Azide and 5 μl of 10× EdU buffer additive). Staining of the pull-outs was done for 30 min at room temperature. After staining, the pull-outs were washed three times with 0.3% PBT, followed by a quick wash with PBS. The pull-outs were then incubated in Hoechst 33342 (1 μg/ml) for 1h and mounted in Vectashield, followed by image acquisition.
LysoTracker staining in cell culture
LysoTracker Red from Invitrogen (L7528) was used to assay the amount of autolysosome generated in human cells. The cells plated were treated lysotracker red with a working concentration of 60 nM, followed by a 45-min incubation. After incubation, the cells were transferred to a glass bottom dish, and confocal images were acquired.
Cyto-ID staining in cell culture
CytoID staining was done as per the manufacturer's protocol (ENZ51031). The cells were harvested by centrifugation for 5 min at room temperature. The cells were then treated with 1 ul Cyto-ID green per mL of media and incubated for 1 h. The media was removed by centrifugation, and the cells were then washed twice with 1x Assay buffer provided with the kit. The cells were then resuspended in the detection reagent supplied with the kit and incubated for 30 min at 37°C. The cells were then plated on a coverslip bottom dish and imaged.
Co-immunoprecipitation and RNA immunoprecipitation
The samples collected in 1×PBS and centrifuged for 1 min at 2000 g. The supernatant was discarded, and Lysis Buffer (0.3% Igepal CA-630, 0.1% BSA, 1× Protease Inhibitor Cocktail (Roche), 5 units RNAseOUT (Invitrogen)) was added to the sample. The samples were incubated for 15 min with shaking followed by centrifugation at 10 000 g for 10 min; the supernatant obtained was transferred to a new tube and processed further for Co-IP. Ten percent of the volume was saved for Input analysis. Each sample was pre-cleared with unbound Protein A/G magnetic beads. After that, samples were distributed into equal volumes with the addition of 1 ug of appropriate antibody in one tube and no antibody in the other tube, acting as a ‘Beads only’ control. The samples were kept overnight at 4°C in a tube rotator. Magnetic beads (Protein A/G) were added to the solution and incubated for 1.5–2 h at room temperature. This treatment was followed by six 1× NRB buffer washes (50 mM HEPES–NaOH, 10 mM MgCl2, 1% Triton X-100, 0.01% SDS, 140 mM NaCl), followed by two 1× PBS wash.
RNA-IP experiments from human cells were performed using the Magna RIP kit (Sigma-17-700, Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit) as per the manufacturer's instruction. An additional crosslinking step is required for Drosophila tissues before performing cell lysis. Crosslinking was performed with 1% formaldehyde, followed by quenching of crosslinking with 2.66 M glycine. A pulsed sonication step was performed with an amplitude set at 10. Each pulse was performed for 4 s with 1-min incubation in ice and cycled four times. Following sonication, the solution was centrifuged and pre-cleared with unbound Protein A/G magnetic beads. Hereafter, the experiment was performed using the Magna RIP kit. The samples were then analyzed for the presence of the appropriate protein using western blotting and RNA isolation, followed by cDNA conversion and qPCR for RNA analysis.
Western blotting
The cell lysate was mixed with equal volumes of 2× Laemmli buffer (SDS, beta-mercaptoethanol, Tris–HCl (pH 6.8), Glycerol, Bromophenol blue) and heated at 95°C for 5 min. 10–20 ug of the samples were resolved in 10% SDS-PAGE (29:1 [acrylamide:bis-acrylamide]) gels with Tris-glycine as the running buffer (TrisBase, Glycine, SDS). The resolved proteins were electrophoretically transferred onto a PVDF membrane using a wet transfer setup. The blot containing the transferred proteins was then incubated for 1 h in the blocking solution of 5% skimmed milk in 0.05% TBS-T (0.05% Tween 20 in TBS [Tris Buffer Saline, NaCl]). The blot was washed with 0.05% TBS-T and incubated overnight in the primary antibody (made in blocking solution) at 40°C. The blot was washed 3 times for 10 min each with 0.05% TBS-T, followed by a 45 min incubation in 1:5000 dilution of HRP-conjugated secondary antibodies (anti-mouse IgG or anti-rabbit IgG) made in blocking solution. The blot was then washed in 0.3% TBS-T and developed in Image Quant LAS4000 post-acquisition; the band intensities were analysed using ImageJ software.
Microscopy and co-localization analysis
Confocal images were acquired in Zeiss LSM 780 and LSM 900, and the acquired images were processed in ImageJ and IMARIS. For co-localization analysis, image acquisition was done in super-resolution mode using the Airyscan 2 module in Zeiss LSM 900. A surface-surface co-localization analysis was done using IMARIS by volume rendering the super-resolution images, thereby creating a 3D volume of the acquired signal in one wavelength and followed by rendering the volume of signal present in different wavelengths. An object-based co-localization of these 3D-rendered images was done and statistically quantitated.
Statistical analysis
Statistical analysis was done using GraphPad Prism. The data points were tested for normality using Shapiro-Wilk's and Kolomogrov–Smirnov tests. Based on the normality of the data points, parametric and non-parametric tests were used. Parametric tests include One-way ANOVA with Tukey's for post hoc analysis for Multiple Individual comparisons. Two-way ANOVA was used with Tukey's test for post-hoc analysis for grouped comparisons. For two sample comparisons, the unpaired Student's t-test was used with Welch's correction. Multiple biological replicates were performed, however all data points are represented in the graph as mean ± standard deviation (SD), to account for total biological variability.
Results
miRNAs affect proliferation of Drosophila blood progenitors through Amino Acid Sensing
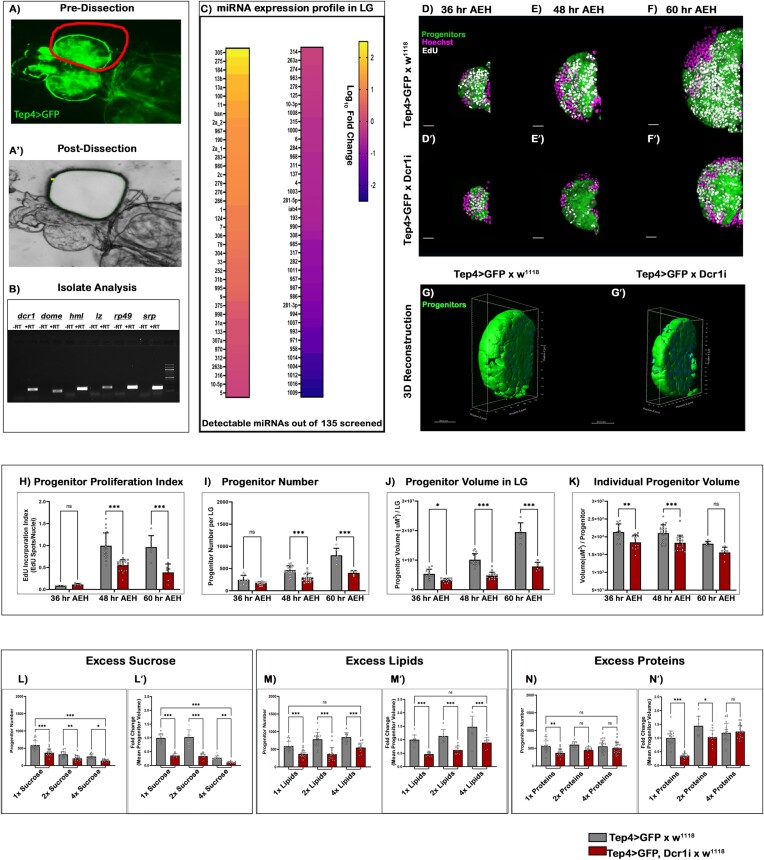
The lymph gland (LG) is the hematopoietic organ of Drosophila larvae and has emerged as an exemplary model for investigating hematopoiesis (38–41). However, LG’s miRNA expression profile and its regulatory involvement in hematopoietic development are still obscure. Due to its microscopic size and close-knit packing with its neighbouring tissues (dorsal vessel, pericardial cells, etc.), performing transcriptomic profiling of the lymph gland is difficult. Tissue dissociative techniques like FACS or single-Cell RNA-Seq have enabled us to perform high throughput transcriptomics in the LG (42,43). However, miRNA analysis in microscopic tissues poses an added difficulty, as miRNAs can exhibit instantaneous and dramatic changes in response to their environment (44). Therefore, rendering miRNA profiling inaccurate through tissue dissociation (45–47). To circumvent these limitations and to acquire an in vivo-like miRNA profile in LG, we standardized a fixed tissue laser capture micro-dissection (LCM) methodology to precisely dissect only the primary lobe of stage-selected lymph glands (Figure 1A–A′).
Figure 1.
Targeting miRNAs and amino acid sensing in Drosophila blood cell progenitors. (A–A') The primary lobe of the lymph gland was isolated from a 48 h AEH larvae by laser capture micro-dissection (LCM) technique. (B) Marker analysis was done after cDNA conversion of RNA isolates post-LCM dissection of the primary lobes, which is then visualized through Agarose gel electrophoresis. (C) Heatmap depicting the expression profile of the Drosophila miRNAs present in the primary lobe (48 h AEH) retrieved through LCM. (D–F') EdU incorporation analysis of lymph glands at (D, D') 36 h, (E, E') 48 h and (F, F') 60 h upon Dicer1 down regulation from the progenitor. (G–G') Volumetric comparison by 3D-reconstruction of progenitors in the wildtype (G) and (G') Dcr1 knockdown lymph gland. (H–K) Quantitative time-kinetic analysis of Wildtype and Dcr1KD progenitors depicting the (H) proliferation rate, (I) progenitor number, (J) progenitor volume and (K) individual progenitor cell volume observed at 36, 48 and 60 h AEH respectively. (L–N') Quantitative analysis of progenitor number and volume upon increasing concentration of Carbon sources. (L–L') in excess Carbohydrates, (M–M') in excess lipids, (N–N') in excess proteins. Scale, 20 μm in all images. Individual dots represent ‘n’ of the sample. Significance was evaluated using two-way ANOVA with Tukey's test was performed for grouped analyses. Error bar: standard deviation (SD). Data are mean ± SD. *P< 0.033, **P< 0.002 and ***P< 0.001. See also Supplementary Figure S1.
To identify relevant miRNA in the progenitor landscape, we concentrated on lymph glands of 48 ± 4 h AEH (after egg hatching). At this stage of development, most of the cells in LG are either core (DomeMesoGFP only) or intermediate progenitors (DomeMesoGFP, Hml-dsRed) (38) (Supplementary Figure S1A–A'''). qPCR based analyses of hemocyte-specific markers (like Domeless, Hemolectin and Serpent) (38,39,48,49) (Figure 1B) and non-hemocyte markers, like Z-band associated protein (ZASP: Pan-muscle) (50,51) and ELAV (embryonic lethal abnormal visual system: pan-neuronal) (52,53) were used to determine the relative purity of the recovered lobes (Supplementary Figure S1B). These samples were subsequently subjected to downstream qPCR screening of annotated Drosophila melanogaster mature miRNAs. Our analysis revealed that 61% (82 mature miRNAs) of the experimentally annotated Drosophila miRNAs were expressed at varying levels throughout the developing LG (Figure 1C, Supplementary Table S1).
To address the major functional consequence of these miRNAs within the hematopoietic progenitors, a UAS-Dicer1 shRNA construct was driven with a progenitor-specific Gal4 (TepIV-Gal4). This construct demonstrated a high Dicer1 Knockdown efficiency (Supplementary Figure S1C) that led to a significant decrease in miRNA levels (Supplementary Figure S1D). Analyzing the LG at 36 h, 48 h, and 60 h AEH revealed that miRNA disruption drastically compromised the progenitor pool, affecting its overall size compared to control (Figure 1D–F'). To address the decline in progenitor growth, an EdU incorporation assay was performed, and the total number of progenitors was counted. At 36 h AEH, the proliferation rate was comparable to the control (Figure 1D–D' and H). Thereafter, a significant decline in both the proliferation rate and the total number of progenitors was evident (Figure 1H–I). A 3D reconstruction of LG progenitors (Supplementary Movie 1) demonstrated a combined and individual volume of the cells (Figure 1G–G' and Supplementary Movie 2). Although there was no decline in cell number at 36 h AEH, a dramatic decrease in the cell volume was noticeable (Figure 1J-K).
The predominant phenotype upon functional disruption of miRNA activity is a significant decrease in cell size, followed by a decline in cell proliferation. The observed phenotype indicates cells reared in a condition of nutritional deprivation (54–58). Although the development of LG has been intricately linked to nutrient sensing pathways (59–61), a direct influence of individual nutrients has yet to be examined. To substantiate the role of nutrient availability as a determinant of miRNA-dependent progenitor growth, we first evaluated the responsiveness of LG to intermittent nutrient deprivation (4 and 12 h starvation pulse) (following the scheme shown in Supplementary Figure S1 E and H). Although 4 h of pulse starvation did not affect LG, upon 12 h of starvation, the mean progenitor number and volume dropped dramatically (Supplementary Figure S1F–G', I–J' and K–L). To investigate the involvement of nutrients as an effector, we progressively increased the concentration of three key carbon sources (carbohydrates, lipids and essential amino acids: EAA) in the fly food. Increasing carbohydrate and lipid concentrations altered the progenitor size and number in control. However, Dicer1 knockdown still led to a reduction in progenitor size and number (Figure 1L–M'). Interestingly, fly food with an excess of amino acids had no change in the size of the control LGs, but completely restored the number, and size of miRNA-disrupted progenitors (Figure 1N-N'). An independent RNAi against a different enzyme, ‘Argonaute-1’, the main effector of miRISC (Supplementary Figure S1M–N) (20,62), phenocopied Dicer1 KD from progenitors (Supplementary Figure S1O).
The major source of protein in fly food is yeast. An intermittent protein deprivation (4 and 12 h pulses) phenocopied the total starvation phenotype (Supplementary Figure S1P–R). Additionally, Dcr1 knockdown larvae, when reared in food with low levels of protein, showed no additive aggravation in the phenotype (Supplementary Figure S1). These results reveal that the inhibition in the growth of LG progenitor by miRNA interference and EAA deprivation are epistatic and functionally interdependent.
Leucine and miRNAs together control the growth of Drosophila blood progenitors
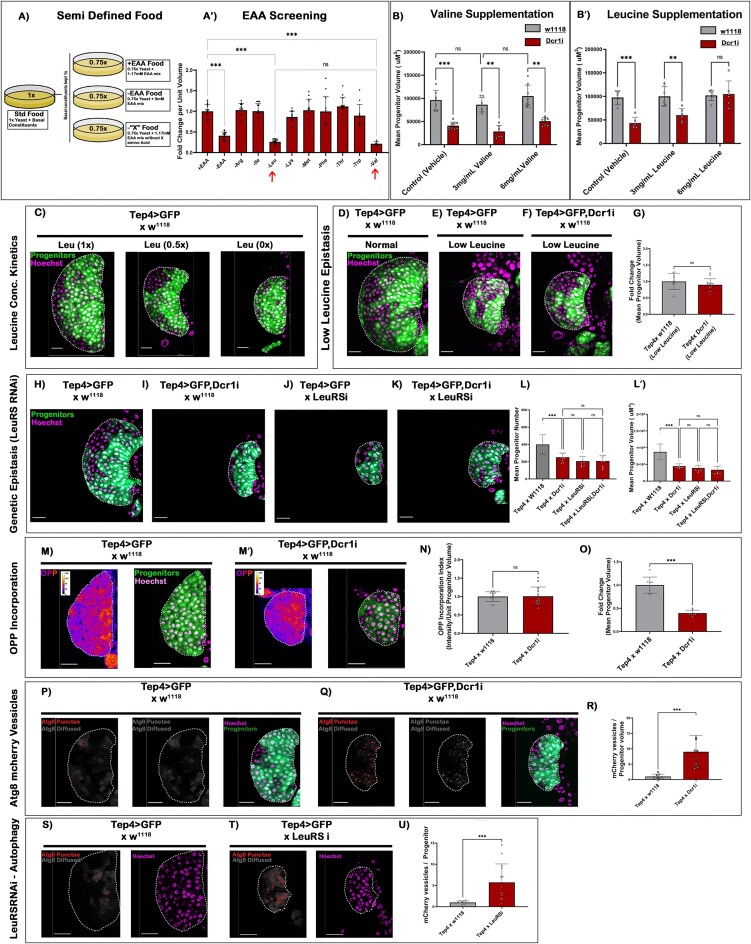
Larvae reared on a diet deficient in essential amino acids (–EAA) resulted in a significant reduction in both the size and number of the LG progenitors compared to those fed on control food (+EAA) (Figure 2A–A'). Interestingly, compared to other essential amino acids, valine and leucine-deficient food decreased the overall LG size similar to that observed in –EAA food (arrows in Figure 2A'), implicating that the availability of these two EAA is indispensable for the proper growth. We now exclusively supplemented the food source with an increasing concentration of valine and leucine in a Dcr1 KD condition. While no rescue was observed upon an incremental increase in valine concentration (Figure 2B), leucine demonstrated an effective concentration-dependent rescue of the Dcr1 KD phenotype (Figure 2B'). Additionally, LG cultured in a decremental concentration of leucine showed a high correlative decrease in its overall size (Figure 2C and Supplementary Figure S2A-A''). These outcomes motivated us to investigate the link between leucine and functional miRNAs in progenitor development. Interestingly, Dicer1 KD reared in leucine deficient food marked no phenotypic aggravation (Figure 2D–G). This observation set concludes a functional association of miRNAs to ‘leucine sensing’ leading up to the miRNA disruption phenotype seen in LG progenitors.
Figure 2.
Leucine and miRNAs together control the growth of Drosophila blood progenitor. (A) Schematic representation of semi-defined food with constituent variability only in Essential Amino Acids. (A') Effect of different Amino acids on the LG progenitor, arrows indicate the reduction of LG size in the absence of leucine and valine. (B–B') Quantitative estimation of the mean progenitor volume upon incremental increase in the concentration of (B) valine and (B') leucine. The grey bar indicates wild type lymph glands, while the red bar represents Dicer1 KD lymph glands. (C) Reduction in the size of wildtype LGs upon decreasing leucine concentration. (D–F) Effect of Dcr1 KD in LG progenitor upon rearing the larvae in low leucine food. (G) Quantitative measurement of total progenitor volume in Dcr1KD condition reared in low leucine food. (H–K) Epistatic analysis to check the phenotypic aggravation on LG progenitors upon double knockdown of Dcr1 and LeuRS (leucine sensor). (L–L') Quantitative representation of the progenitor number and volume upon of Dcr1 KD and LeuRS KD combinations. (M–M') OPP incorporation assay at 48 h AEH in Wildtype and Dcr1 KD progenitors. (N) Quantitative estimation of OPP incorporation per unit volume progenitors in a 48 h AEH lymph gland (O). The mean progenitor volume of the samples used to quantitate the OPP index (as in figure N). (P, Q) Expression of mCherry-Atg8 construct in a progenitor-dependent manner to quantitate the levels of autophagy vesicles upon Dcr1 KD. (R) Quantitative analysis of autophagic vesicles upon Dcr1 knockdown. (S, T) Progenitor-specific expression of mCherry-Atg8 construct in LeuRS downregulation background. (U) Quantitative analysis of autophagic vesicles upon LeuRS downregulation. Scale, 20 μm in all images. Individual dots represent ‘n’ of the sample. Two-way ANOVA was performed with Tukey's test for grouped analyses. One-way ANOVA was performed with Tukey's test for individual multiple comparisons. Error bar: standard deviation (SD). Data are mean ± SD. *P< 0.033, **P< 0.002 and ***P< 0.001. See also Supplementary Figure S2.
To rule out leucine's pleiotropic influence on LG, we performed Gal4-based genetic perturbations of the leucine-specific transporters and sensor within the LG progenitors. A qPCR-based analysis from LCM dissected primary lobes revealed that out of the three functionally annotated transporters of leucine (63,64), Minidisc (Mnd) expressed several magnitudes higher at 48 h AEH (Supplementary Figure S2B). We also found that LG expresses LeuRS (leucyl-tRNA synthetase), a validated leucine sensor (65,66) (Supplementary Figure S2C). Both Mnd and LeuRS showed no significant changes in the transcriptomic profile upon Dcr1 KD (Supplementary Figure S2C). However, shRNA constructs targeting Mnd and LeuRS (Supplementary Figure S2D, E) phenocopied the Dcr1 knockdown phenotype (Figure 2H–J, and Supplementary Figure S2F). In addition, a double knockdown condition of LeuRS with Dcr1 demonstrated a non-additive phenotype (Figure 2K and L–L' and Supplementary Figure S2G). These results genetically confirm an epistatic interaction between leucine deficiency and miRNA functionality, specifically within LG progenitors.
Leucine is majorly known to affect the metabolic adaptation of a cell by regulating two proteo-static processes, ‘Autophagy’ and ‘Translation’ (67–70) (Supplementary Figure S2H). Interestingly, the rate of protein synthesis within progenitors in a miRNA-depleted state at 48 h AEH was not affected (determined by OPP: O-propargyl-puromycin incorporation assay) (Figure 2M–O). Therefore, the flux of protein synthesis within the progenitors is not the primary cause of the defects observed upon miRNA depletion.
Since the translational rate was unaffected during the pheno-critical timepoint (48 h AEH), it was indicative of the unaltered activity of mTOR. Furthermore, we assayed the progenitor-specific involvement of mTOR through UAS-TOR-TED (an mTOR-Dominant Negative Construct). We found a significant reduction in proliferation phenocopying leucine deprivation conditions (Supplementary Figure S2I–I'). However, co-expression of TOR-TED and Dcr1i resulted in an additive aggravation of the phenotype (Supplementary Figure S2I''–J). These results confirm that the phenotype manifested at that time point of development by miRNA disruption is independent of mTOR.
We next assayed for autophagy, the other fundamental protein homeostatic process (71–74). The effect on autophagy was measured by the changes in the levels of ‘autophagic vesicles’ employing ‘UAS-mcherry-atg8’, a validated marker of autophagy (37,74–76). Interestingly, Dicer1 knockdown or progenitor-specific downregulation of LeuRS demonstrated a robust increase in the number of atg8-positive vesicles at 48 h AEH in the blood progenitors (Figure 2P–R and S–U). These results identify leucine-dependent autophagy and miRNA disruption as a functional offset to leucine-deprived growth arrest in blood progenitors.
Leucine sensing impinges on p62-dependent degradation of Argonaute
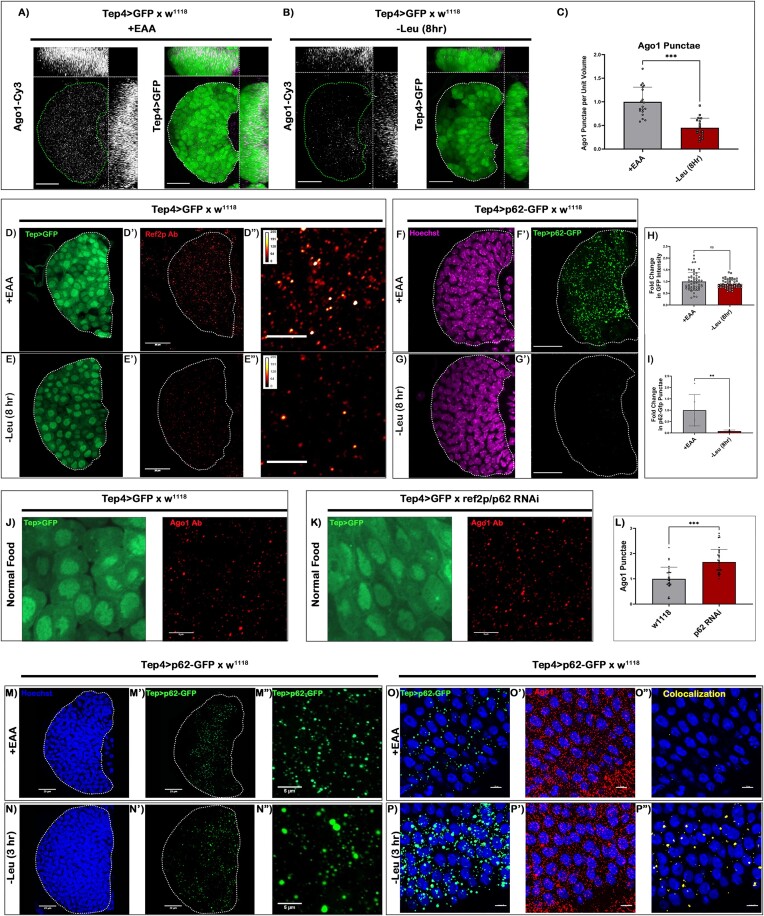
Our next objective was to elucidate the mechanistic basis of leucine's association with miRNA function in the hematopoietic progenitors. To address this, we assayed the effect of leucine deprivation on Ago1: the main slicer protein for miRISC (62). Ago1 expression was punctate and visibly enriched in Tep4 +ve progenitors (Figure 3A). Upon an 8 h pulse of leucine deprivation, a considerable drop in the levels of Ago1 was evident (Figure 3B and C).
Figure 3.
Leucine sensing targets p62-dependent degradation of Argonaute. (A, B) Levels of Ago-1 expression was analyzed using an antibody against Drosophila Ago-1 in (A) control (+EAA food) and (B) 8 h leucine deprived condition (–Leu food). (C) Quantitative measurement of the levels of Ago-1 upon (8 h) leucine deprivation per unit volume of LG progenitors. (D–E'') Ref(2)p expression was analyzed using an antibody against Drosophila Ref(2)p in (D–D'') control and (E–E'') 8 h leucine deprived condition. (F–G') Progenitor-specific expression (Tep4-Gal4) of p62-GFP construct was analyzed in (F–F') control and (G–G') 8 h leucine deprived condition. (H) GFP intensity measurement upon 8 h pulse of leucine deprivation in Tep4Gal4 > UAS-GFP background. (I) Same as in (H) but in the background of Tep4Gal4 > UAS-p62-GFP. (J, K) Levels of Ago-1 expression upon Ref(2)p knockdown in a progenitor-specific manner. (L) Quantitative analysis of Ago-1 punctae in (J, K). (M–N'') Qualitative analysis of the Ref(2)p punctae upon 3 h pulse of leucine deprivation, (N–N'') leucine deprived p62-GFP punctae form large aggregates w.r.t. undeprived control (M–M''). (O–P'') Object-based surface-surface co-localization of Ago-1 and p62 upon 3 h pulse of leucine deprivation. Green punctae represents p62-GFP, red punctae represents Ago-1, and yellow represents the co-localized fraction of Ago-1 within p62-GFP punctae (O–O'') represents undeprived control (+EAA food), and (P–P'') represents 3 h leucine deprived condition. Scale, 20 μm in all images. Individual dots represent ‘n’ of the sample. Unpaired Student's t-test with Welch correction. Error bar: standard deviation (SD). Data are mean ± SD. *P< 0.033, **P< 0.002 and ***P< 0.001. See also Supplementary Figure S3.
We also isolated the LG’s primary lobe at 48 h AEH with a similar pulse of leucine deprivation and analyzed the transcriptional profile of ago1, p62/ref(2)P known to interact with Ago1-dependent miRISC and leucine transporter (mnd). No change was observed in the transcription of ago1, p62 and mnd upon leucine deprivation (Supplementary Figure S3A). Therefore, we infer that leucine deprivation in LG progenitors causes a drop in Ago1 protein and an increase in autophagy, implicating an association between autophagy and functional miRISCs. This hypothesis gets strengthened with the observation that the protein levels of p62: a selective autophagy receptor and validated autophagy marker, also exhibit a drastic decline upon leucine deprivation (Figure 3D–E'' and Supplementary Figure S3B).
Ref(2)p/p62 is previously reported to interact with Argonaute-1, a crucial effector in the miRNA silencing complex (28,37,76); however, its cell biological and hematopoietic relevance in miRNA processing is unknown. To demonstrate that the reduction in Ref(2)P/p62 level is a consequence of a surge in Ref(2)p/p62 degradation, we first analyzed the GFP intensity of a progenitor-specific Gal4 (Tep4-Gal4 > UAS-GFP). Leucine deprivation has a minimal change in GFP intensity, signifying that Tep4-Gal4 activity is independent of leucine availability (Figure 3D–D'' and E–E'', J, K and H). Whereas, upon an 8 h pulse of leucine deprivation, the expression of a GFP-tagged Ref(2)p/p62 construct (Tep4Gal4 > UAS-p62-GFP) exhibited a drastic decrease in p62-GFP puncta in the progenitors (Figure 3F–G' and I). These findings suggest that a heightened degradation of Ref(2)p/p62 ensues upon leucine deficiency in the LG progenitors. A similar set of results was not obtained when the overall levels of Ago1 was analyzed systemically within similarly staged larvae (Da-Gal4 > UAS-p62-GFP), making LG progenitors one of the cell types to register such an effect upon leucine alteration (Supplementary Figure S3C, D).
The next step was to determine whether Ref(2)p/p62 impinges on Ago-1 as a cargo for degradation. Ref(2)p/p62 was downregulated from the progenitors using a high efficiency RNAi construct (Supplementary Figure S3E, F). Upon Ref(2)p/p62 knockdown, a significant increase in the overall level of Ago-1 within the progenitors (Figure 3J–L) was evidenced. Based on this analysis, we can infer that Ago1 can potentially be targeted by Ref (2)P/p62 for selective autophagy.
The Ref(2)p/p62 sequestered cargo is known to be trafficked to the autophagosome for degradation (37). To confirm whether, upon 3 h leucine deprivation, Ago1 is trafficked into autophagosomes, we visualized the colocalization of Ago1 and mCherry-atg8-expression. In addition to a surge in the mCherry-atg8-positive vesicles, an increased localization of Ago 1 in the autophagosomes was evident within the blood progenitors (Supplementary Figure S3J and K).
Additionally, quantitative volumetric density profiling post microscopic acquisition revealed that p62-GFP forms large aggregates within 3 h of leucine deprivation, followed by a reduction in punctae volume at extended hours (Figure 3 M–M'', N–N'', and Supplementary Figure S3H–I'). Co-Immunoprecipitation studies using Tep4-Gal4 > UAS-p62-GFP with anti-GFP as bait and immunoblotting with Ago-1 demonstrated an interaction between p62 with Ago-1 in the LG blood progenitors (Supplementary Figure S3G). Additionally, at this time point Ago1 exhibits a 2.1-fold increase in co-localization rate with p62 (Figure 3O–P'', and Supplementary Figure S3L). However, at a later time point (8–12 h post leucine deprivation), a significant reduction in overall Ref2p/p62 (Supplementary Figure S3I') and Ago1 levels (Figure 3A–C) was evident, but the co-localization index with p62 was still heightened (Supplementary Figure S3L).
These results demonstrate a propensity of p62 to target Ago-1 and direct its trafficking to autophagosomes upon sensing leucine deprivation.
Leucine-Autophagy axis can target the miRNA–mRNA complex in Drosophila blood progenitors
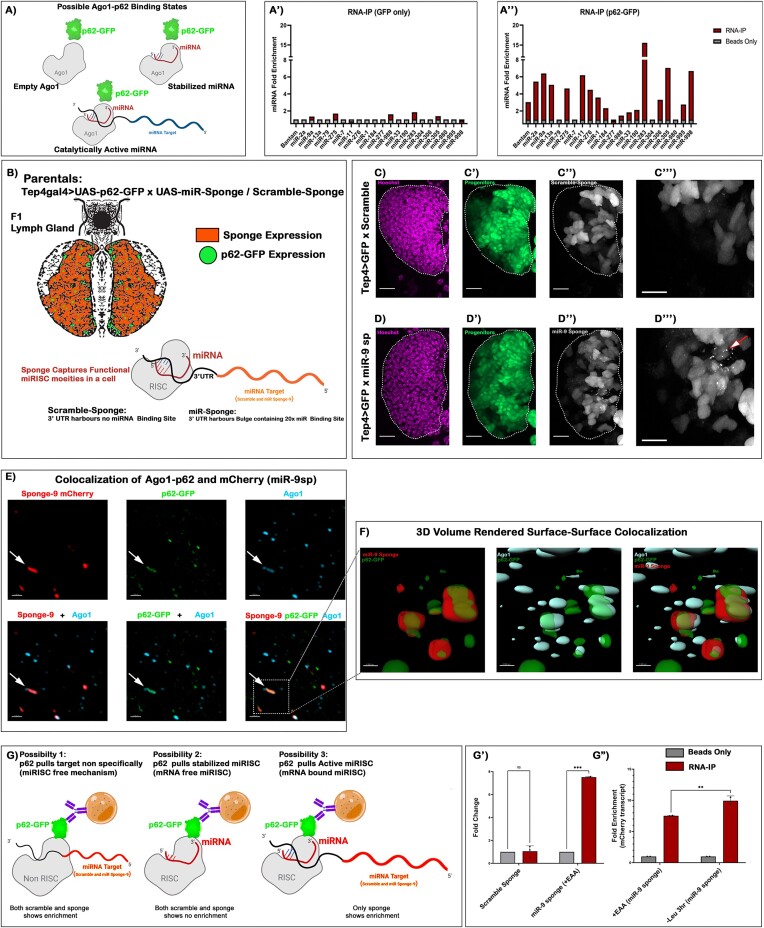
Ref(2)p/p62 in Drosophila is known to interact with Ago-1 physically. The same study also reports that the selective binding of Ref(2)p/p62 is not involved in an empty-Ago-1 turnover (28). Despite an affirmative targeting of Argonaute by selective autophagy receptor (p62) and its ability to alter miRNA function, a context through which a cell can target miRNA-loaded Argonaute still needs to be addressed. First and foremost, we wanted to evaluate the leucine-responsive binding of Ref(2)p/p62 to a miRNA-loaded Ago-1 (catalytically active miRISC). A p62-GFP construct was expressed using ubiquitous Gal4 (Da-Gal4), and RNA Immuno-precipitation (RNA-IP) was performed with anti-GFP as the bait, and LG-specific miRNAs were screened as its prey (Scheme in Figure 4A). Interestingly, a differential enrichment of various specific miRNAs in p62-bound beads was evidenced (Figure 4A'–A''). These findings elucidate an unappreciated ability of p62 to sequester mature miRNAs.
Figure 4.
Leucine-autophagy axis can target miRNA–mRNA complex in Drosophila blood progenitors. (A) Schematic representation of possible Ago1-Ref(2)p/p62 binding states. (A'–A″) Levels of miRNA enrichment following RNA-IP using anti-GFP as bait and LG-specific miRNAs were screened as its prey. (A') RNA-IP in Da-Gal4 > GFP background demonstrates a negligible enrichment of LG- specific miRNAs. (A'') Depicts differential enrichment of various specific miRNAs in Da-Gal4 > p62-GFP background. (B) Schematic representation of the experimental setup enabling progenitor specific assay of p62 interacting with miRNA sponge transcripts. (C–C''') Progenitor-specific expression (Tep4 > GFP, Green) of scramble sponge. (D–D''') Progenitor-specific expression (Tep4 > GFP, green) of miR-9 sponge, arrow marks the formation of mCherry punctae upon miR-9 sponge expression. (E) Super-resolution microscopy-based analysis of miRNA-9 sponge (mCherry) punctae with dual labelling with p62-GFP and anti-Ago-1 antibody. miR-9 sponge marked in red, p62-GFP in green and Ago-1 in cyan. (F) 3D volume rendering to evaluate signal's surface analysis to address object-based co-localization of miRNA-9 sponge punctae with p62 and Ago-1. (G) Schematic representation of various possibilities of RNA-IP upon expressing miR-9 sponge construct specifically in the LG progenitors using Tep4 > p62-GFP. (G') qPCR analysis of the levels of mCherry transcript enriched after performing RNA-IP from LG progenitors upon expressing scramble (Tep4Gal4; UAS-p62-GFP, UAS-scramble sponge) and miR-9 sponge (Tep4Gal4; UAS-p62-GFP, UAS-miR-9 sponge). (G'') Fold enrichment of mCherry transcript (Tep4Gal4; UAS-p62-GFP, UAS-miR-9sponge) after 3 h pulse of leucine deprivation resulting in 10-fold increase w.r.t. control food (+EAA) having a 7.5-fold enrichment. Scale, 20 μm in all images. Individual dots represent ‘n’ of the sample. Two-way ANOVA was performed for grouped analyses. One-way ANOVA was performed for individual multiple comparisons. Error bar: standard deviation (SD). Data are mean ± SD. *P< 0.033, **P< 0.002 and ***P< 0.001. See also Supplementary Figure S4.
Our next goal was to identify whether the mature miRNAs bound to Ref(2)p/p62 are indeed part of the active miRISC. Through a qPCR-based differential expression analysis, we found that some miRNAs in LG that interact with Ref(2)p/p62 exhibited downregulation in response to leucine deficiency (Supplementary Figure S4A). However, some miRNA demonstrated upregulation in both Ref(2)p interacting and non-interacting fractions. Interestingly, the downregulation of miRNAs was restricted only to the Ref(2)p interacting fractions. Among them, dme-miRNA-9 demonstrated a measurable downregulation upon leucine deprivation.
To investigate whether the downregulation of miRNA-9 through Ref(2)p/p62 is targeted in its RISC-loaded state, we modified our approach by coupling the RNA-IP method with the miRNA sponge technology (Figure 4B). The rationale for performing RNA-IP with miRNA-9 sponge construct lies in its strategic design. The sponge transcript incorporates multiple binding sites against a specific miRNA onto its 3′ UTR. The association between a microRNA (miRNA) and its corresponding sponge construct is contingent upon the assembly of the functional miRNA-induced silencing complex (miRISC) over the modified miRNA binding site. This tailored methodology provides a meticulous means to decipher a protein's ability to interact with a functional miRISC. Therefore, by employing the miRNA-9 sponge construct, we aimed to address Ref2p/p62’s propensity to interact with a leucine responsive miRNA engaged to its cognate target.
On microscopy-based analysis, a negligible change in the overall size of the LG upon miRNA-9 sponge expression was noted (Supplementary Figure S4B), depicting that deprivation of singular miRNA is not enough to generate a phenotype. However, characterization at a single-cell resolution demonstrated a heightened occurrence of discernible sponge mCherry punctae compared to its scramble (Figure 4C–D'''). We speculated that these puncta observed were a part of the ongoing mCherry translation targeted by Ref(2)p/p62 for selective autophagy. Employing super-resolution microscopy followed by a 3D-volume rendering-based surface-surface co-localization, it was evident that some of these mCherry sponge puncta not only colocalized with p62 but these sponge puncta were also bound to Ago1 while it was being targeted by Ref(2)p/p62 (Figure 4E, F).
To further provide quantitative molecular evidence towards Ref(2)p/p62 targeting miRNA–mRNA hybrid upon leucine deprivation, we performed an RNA-IP experiment by co-expressing p62-GFP and miRNA-9 sponge in a progenitor-dependent manner. In the control condition, p62-GFP bound beads exhibited a 7.5-fold enrichment of sponge transcript. However, this enrichment increased up to 10-folds upon a 3 h leucine deprivation (Figure 4G-G''), demonstrating that the targeting of the sponge transcript by p62 happens with a viable miRNA binding site. Collectively these results suggest that the p62 can bind miRNA not only in its stabilized form (miRNA-RISC) but also in its catalytically active form (miRNA–mRNA RISC) upon leucine deprivation within blood progenitors.
Human blood progenitors exhibit leucine-responsive proliferation similar to Drosophila
Our next aim was to investigate whether this nutrient-responsive feed identified from Drosophila blood progenitors is also conserved in human hematopoietic progenitors. Hence, we cultured a panel of human bone marrow-derived progenitors and FACS sorted CD34+ CD10-Lin– population to specifically analyze myeloid biased hematopoietic stem-progenitor cells (HSPC) (Supplementary Figure S5A-A'). Additionally, we also used CD34+ immature hematopoietic progenitor-like AML cell line termed ‘KG1’ for further analysis of miRNAs in our study (77,78).
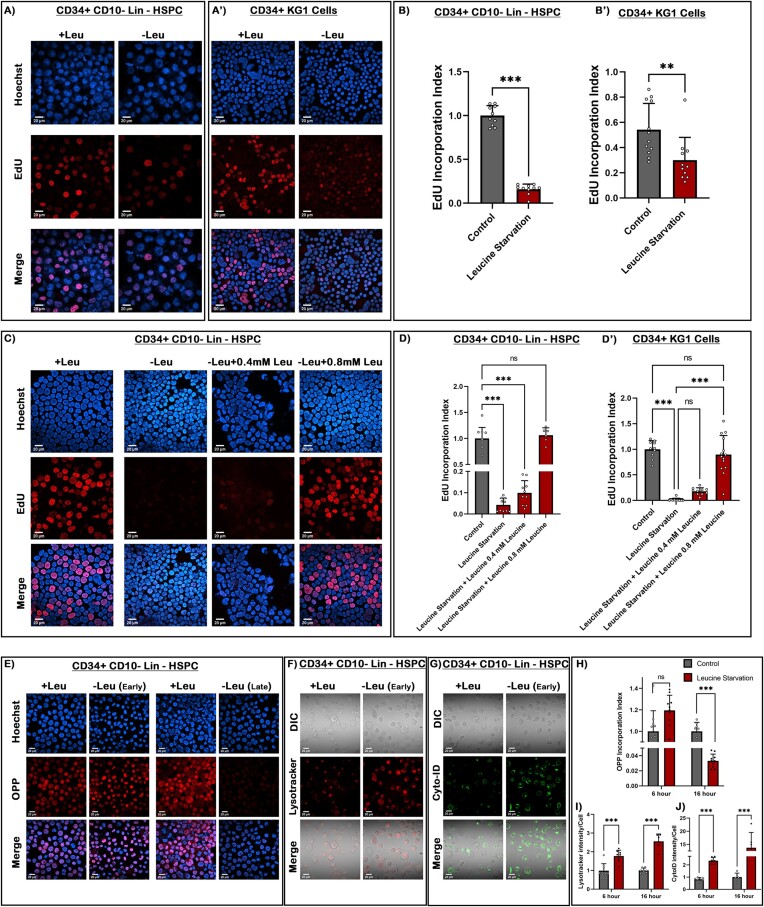
To investigate the effect of leucine on these cells, we cultured them in a leucine deficient media and assayed their proliferation rate through EdU incorporation and FACS-based analysis. A significant decrease was observed in the proliferation rate (Figure 5A–B') and the S-phase of the cell cycle (Supplementary Figure S5B, C).
Figure 5.
Human blood progenitors exhibit leucine responsive proliferation similar to Drosophila. (A–A') EdU incorporation analysis of CD34+ CD10-Lin- myeloid biased HSPC in undeprived and leucine-deficient media. (B and B') Quantitative analysis in the proliferation rate of CD34+ CD10-Lin- HSPCs and KG1 cells. (C–D') Dose-dependent recovery in the proliferation rate of CD34+ CD10-Lin– HSPC upon increasing concentration of leucine in leucine deprived media. (D–D') Quantitative analysis of the recovery in proliferation index of CD34+ CD10-Lin- HSPCs and KG1 cells. (E) OPP incorporation rate analysis of CD34+ CD10-Lin-myeloid biased HSPC in early stages (6 h) of leucine deprivation versus late stages (16 h). (F) Lysotracker staining of acidic autolysosome upon leucine starvation at an early time point in HSPCs. (G) Cyto-ID staining of autophagosomes in response to leucine starvation at early time point in HSPCs. (H) Quantitative analysis of OPP incorporation rate at 6 and 16 h of leucine starvation condition in HSPCs. (I) Quantitative measurement of increased acidic vesicles (Lysotracker) in HSPCs. (J) Quantitative measurement of autophagosomes (Cyto-ID) in HSPCs. Scale, 20 μm in all images. Individual dots represent ‘n’ of the sample. Two-way ANOVA was performed for grouped analyses. One-way ANOVA was performed for individual multiple comparisons. Error bar: standard deviation (SD). Data are mean ± SD. *P< 0.033, **P< 0.002 and ***P< 0.001. See also Supplementary Figure S5.
Similar to Drosophila blood progenitors (Figure 2C and Supplementary Figure S2A–A'), the proliferation rate of these cells exhibited a dose-dependent recovery following an incrementally increasing treatment of leucine (Figure 5C–D'), validating a significant anti-proliferative effect of leucine starvation on cell cycle progression. As seen in the findings conducted on the effects of leucine on human HSPC (79,80), our results also demonstrate that a decrease in the availability of leucine results in a consequent inhibition in the proliferation of human blood cells like CD34+ HSPC and progenitor-like AML cell lines.
Additionally, we demonstrated in Drosophila blood progenitors, upon leucine deprivation, the rate of global protein synthesis does not exhibit any immediate changes. We observe a similar trend in human HSPC and myeloid progenitor-like cell lines (visualized by the OPP incorporation Figure 5E and H, Supplementary Figure S5D, E). This observation implies that despite the restriction imposed on the external source of leucine, other regulatory mechanisms (like autophagy) governing a cell's internal amino acid availability might exhibit differential sensitivities to leucine restriction. Only as leucine scarcity persists, protein synthesis processes experience a noticeable slowdown.
However, a dramatic increase in the number of acidic vacuoles or autophagolysosomes was immediately evident in HSPCs and myeloid progenitor-like cell lines, respectively (visualized by Lysotracker Red staining) (Figure 5F and I, Supplementary Figure S5F). Furthermore, labelling the cells with CytoID (a marker specific for autophagy) demonstrated a drastic increase in lysosomal vesicles as a response to autophagy initiation (Figure 5G and J, Supplementary Figure S5G). These findings signify that acute leucine starvation triggers rapid and robust autophagy response without immediately affecting the levels of proteins synthesis in both human and Drosophila blood progenitors.
Leucine-based proliferation of human blood progenitors also targets autophagy-dependent miRNA turnover
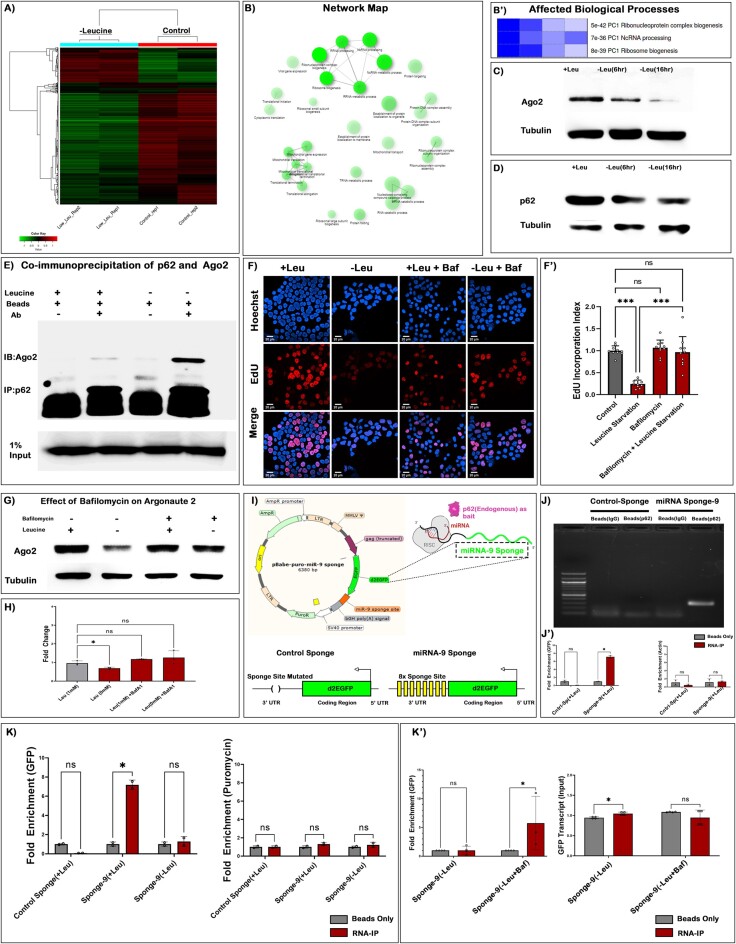
To gain a deeper insight to the global transcriptomic changes in HSPCs. We performed a high-throughput transcriptomic analysis following a 6 h leucine deprivation. This investigation revealed that a large number of genes are dysregulated (Figure 6A, Supplementary Figure S6A, B). Post-analysis, we could attribute a gene ontological function of dysregulated genes observed in CD34+ HSPCs during the early hours (6 h) of leucine deprivation. Comparing differentially expressed genes in 6 h leucine-deprived HSPC to those in the control group revealed that non-coding processing pathways, ribonucleoprotein biogenesis pathway, ribosome biogenesis pathways, etc. were among the earliest responding and most significantly affected biological processes (Figure 6B–B'). Multiple transcripts involved in cell cycle, and lysosomal processing, also demonstrated dysregulation in the absence of leucine within HSPCs (Supplementary Figure S6C, D). These results obtained from high-throughput RNA-sequencing were further validated using RT-qPCR analysis (Supplementary Figure S6E).
Figure 6.
Leucine-based proliferation of human blood progenitors also targets autophagy-dependent miRNA turnover. (A) Heatmap representing high throughput transcriptomic analysis at 6 h pulse of leucine starvation in CD34+ CD10-Lin– HPSCs. (B) Network map depicting pathway characterization of the differentially expressing genes in CD34+ CD10-Lin– HSPC as a response to leucine deprivation. (B') GO analysis of the most significantly affected biological process in CD34+ CD10-Lin- HSPCs as a response to leucine deprivation. (C) Western blot depicting reduction in Ago2 protein upon early and late response to leucine starvation. (D) Levels of p62/SQSTM1 show a similar reduction as in (C). (E) Co-immunoprecipitation depicting physical interaction between p62 and Ago2. The blot depicts a basal level of Ago2 interaction that increases upon leucine deprivation (Low exposure of the same blot is in Supplementary Figure S6G). (F) EdU incorporation analysis upon leucine starvation and simultaneous treatment with BafilomycinA1 (BafA1). (F') Quantitative analysis on the proliferation rate upon leucine deprivation and BafA1 treatment. (G) Levels of Ago-2 upon leucine deprivation and BafA1 treatment detected by western blotting. (H) Quantitative analysis of Ago2 protein levels upon leucine deprivation and BafA1 treatment. (I) Schematic representing miRNA-9 sponge construct used for RNA-IP (above). Destabilized GFP transcript with deleted sponge sites (served as Control-Sponge) and destabilized GFP transcript with 8x sponge sites (miRNA-9 Sponge) (below). (J) Agarose gel demonstrates a qualitative assessment of miRNA-9 sponge enrichment upon p62-RNA-IP. (J') A qPCR-based analysis of Control-sponge and miRNA-9 sponge enrichment upon p62-RNA-IP and assessment of an endogenous transcript (actin) within the pulled fraction. (K) qPCR analysis of RNA-IP done with p62 as bait using Control-sponge and miRNA-9 sponge construct upon leucine deprivation (left). qPCR analysis of puromycin transcript acts as a negative control (right). (K') qPCR analysis of RNA-IP done with p62 on miRNA-9 sponge construct upon leucine deprivation and BafA1 treatment during leucine deprivation (left). A qPCR analysis of GFP in the Input fraction of the same (right). Scale, 20 μm in all images. Individual dots represent ‘n’ of the sample. Multiple t-test with Welch correction was performed for RNA-IP analysis. One-way ANOVA was performed for individual multiple comparisons. Error bar: standard deviation (SD). Data are mean ± SD. *P< 0.033, **P< 0.002 and ***P< 0.001.
Thus, these results implicate the existence of a complex leucine-responsive axis that generates an autophagy-based integrated stress response affecting the overall growth of blood progenitors.
The next objective of our investigation was to determine whether the alteration of leucine levels directly influences the rate of Argonaute turnover. Therefore, we focussed explicitly on the turnover kinetics of Argonaute-2 (a functional homologue of Drosophila Ago1) upon leucine deprivation. Immuno-blotting of Argonaute-2 was performed in a time-kinetic fashion which conclusively demonstrated a gradual reduction in the levels of Ago2 protein upon leucine deprivation (Figure 6C). A similar response to leucine deprivation was not observed when we investigated the involvement of a different Argonaute protein (Argonaute-1) (Supplementary Figure S6F). To ascertain whether an identical axis akin to Drosophila also exists in the human blood progenitors, we measured the levels of Sqstm-1 (p62) upon leucine starvation. We found a similar time-dependent decrease in the levels of p62 (Figure 6D, Supplementary Figure S7A–A'). However, the interaction of p62 and Ago2 is not documented in a human context. Using co-immunoprecipitation, a basal level of physical interaction of p62 could be detected with Ago2 under normal physiological conditions (Figure 6E). Upon leucine deprivation, this interaction between Ago2 and p62 increases (Figure 6E, Supplementary Figure S6G–G'). A specific enrichment of similar but separate protein like Argonaute1 was not observed upon leucine deficiency (Supplementary Figure S6H). Additionally, upon treatment with a different essential amino acid (valine), no enrichment in Ago2 was observed, as evident upon leucine deficiency (Supplementary Figure S6I). These results demonstrate that Drosophila and human blood progenitors, in response to leucine deprivation, specifically sequester the slicer Argonaute for degradation in a p62-dependent manner.
To address that the decrease in the levels of Ago2 is a consequence of autophagy-based degradation, we utilized a pharmacological inhibitor of macro-autophagy, Bafilomycin A1 (BafA1) (81). Interestingly, BafA1 treatment was very efficacious in recovering the proliferation defects in HSPCs induced by leucine starvation (Figure 6F–F'). Additionally, at the protein level, a similar rescue in the expression of Ago2 was evident in the same experimental setup (Figure 6G, H). These findings strongly suggest that the activation of autophagy upon leucine deprivation targets Argonaute2.
Taking a cue from our Drosophila data and based on the fact that miR-9 is an evolutionarily well-conserved miRNA (82), we analyzed the levels of miRNA-9 on leucine deprivation. A sharp decrease in the levels of miRNA-9 was evidenced within 2 h of leucine deprivation (Supplementary Figure S7B). It was again found that this decrease in miRNA-9 expression resulted due to autophagy, as inhibiting the autophagy flux in the cells with BafA1 resulted in the rescue of miRNA-9 level (Supplementary Figure S7B), resembling the same recovery pattern observed in the cases of p62 and Ago2.
Scanning through multiple miRNA target databases (miRbase, TargetScan Fly, TargetScan-Human etc), we analyzed the expression profile of several miRNA-9 targets upon leucine deprivation (Supplementary Figure S7C, D). It was found that Oct1/ Pou2F1 and Onecut are two candidates that responds to leucine deprivation. Interestingly, Oct1 showed an immediate response similar to that of miRNA-9. Oct1 is a known mediator of hematopoietic stress response in blood progenitors and is a target mediating proliferative effect in hematopoietic malignancies (83). A two-fold decrease in the expression of Oct1 (∼6 sites) was evidenced upon 2 h of leucine deprivation. However, transcripts with scanty or no miRNA binding sites did not exhibit any change (Supplementary Figure S7E). We argued if the reduction in the levels of Oct1 is due to miRNA -9 targeted autophagy, then blocking autophagy through BafA1 treatment should restore its level. Indeed, BafA1 treatment resulted in a complete rescue in the expression levels of Oct1 upon leucine deprivation (Supplementary Figure S7E').
However, endogenous transcripts often feature a multitude of diverse miRNA binding sites in their regulatory domains. Furthermore, endogenous transcripts may also be associated with RNA-binding proteins (RBPs) other than miRISC, which could be susceptible to regulation by p62. In recognition of such intricacies and to mitigate potential misinterpretation, we used a validated miRNA-9 sponge construct (pBabe-puro-miR-9 sponge addgene #25040) to ascertain the involvement of leucine-mediated autophagy of mRNA bound miRISC (Figure 6I) in human AML cell lines. To ensure a reliable interpretation of the miRNA-9 sponge construct, we specifically excised the miRNA-9 binding sites from the construct, resulting in a ‘Control sponge’ devoid of these regulatory elements (Figure 6I). While a specific enrichment of miRNA-9 sponge was found in the endogenous p62 fraction, the ‘Control-sponge’ construct did not demonstrate any enrichment (Figure 6J–J'). Therefore, this implicated that miRNA-based recognition is crucial for p62-dependent regulation of the cognate mRNA.
Additionally, upon leucine deprivation, the enrichment of the sponge transcript within the p62 fraction was promptly relinquished (Figure 6K). This reduction in the enrichment could be rescued by subjecting the cells to treatment with bafilomycin A1 (Figure 6K').
These observations depict an autophagy-dependent miRNA degradation along with its cognate targets, as a rapid response to leucine deprivation experienced by blood progenitors across taxa.
Discussion
In animal cells, it is identified that the most predominant mode of miRNA-based inhibition is characterized by stalling an active translation apparatus engaged on its cognate mRNA target (84,85). Although, there are substantial evidences that demonstrate miRNAs can cause mRNA degradation upon high complementarity of miRNA to its target (86–91). Nevertheless, an extensive proportion of miRNAs target their respective mRNAs with partial complementarity leading to translational inhibition rather than degradation (8,12). Despite these observations, studies have demonstrated that the exogenous introduction of miRNAs into Hela cells can reduce the number of transcripts with potential miRNA binding sites (88). Furthermore, in C. elegans, it is known that let-7 and lin-4 miRNAs can also decrease its target mRNA levels (86). These reports substantiate evidences for mechanisms in animal cells that can lead to a miRNA-directed reduction in the mRNA target (92). Despite the comprehension of miRNA driven mRNA degradation, reports on cellular signals and mechanisms orchestrating miRNA mediated mRNA degradation remains elusive. Furthermore, the direct engagement of the autophagy machinery in discerning and degrading miRNA–mRNA hybrids remains unexplored in the field of miRNA biology.
We identified one such evolutionarily conserved mechanism in blood progenitors wherein a decrease in extracellular leucine availability can lead to an autophagy-based degradation of miRNA bound mRNAs. This nutrient-derived signal selectively traffics miRISC into an autolysosomal compartment encompassing the cognate mRNA target, that is actively engaged by the miRNA. The prevailing comprehension of miRNA binding to its mRNA target leads to translational inhibition and is inherently reversible in nature. However, the mechanisms proposed in this study depict the feasibility of miRNA-targeted mRNA into an irreversible path towards autophagy, suggesting a more potent mode of miRNA-based inhibition.
The modality of miRNA-based inhibition through translational repression confines its regulation predominantly to protein-coding genes. This perspective undermines the potential of miRNAs to regulate transcripts such as non-coding RNAs (ncRNAs) that lack translation potential. Despite, multiple reports demonstrating the binding of miRNA to ncRNAs, these investigations predominantly link non-coding RNAs as a regulator of miRNA expression (93–97).The mechanism proposed in this study, where the miRNA–target interaction is recognized by ‘Autophagy’ leading to its degradation, can now provide an added dimension to miRNA as regulators of not only mRNAs but also cell biologically important ncRNAs, which do not harbor any translational apparatus.
Recently, mRNAs have also been reported in a cell's autophagosome (22,23). However, elucidating the precise mechanism underpinning the targeted selection and trafficking of distinct RNA species—both coding and non-coding—into autophagosomes is an evolving context in cellular autophagy. Our present investigation, demonstrates a pivotal role of p62 as one of the key proteins orchestrating the transit of miRNA–mRNA-loaded Argonaute into autolysosmal degradation. Our results show that there is a differential interaction of miRNAs with that of p62. Argonaute is the principal mediator of the miRNA-induced silencing complex (miRISC). It acts as the scaffold onto which miRNAs are assembled, thereby enabling precise and directed recognition of their respective RNA targets. Therefore, we postulate that the physical interaction between p62 and an Argonaute loaded with miRNA and its target mRNA is more likely to be indirect rather than a direct binding event. Considering the intricate composition of miRISC as a multifaceted assembly of proteins and enzymes, it is plausible that specific constituents within the miRISC assembly contribute to the selective recognition of p62, facilitating the differential targeting of miRNA-loaded RISC assemblies.
Leucine-miRISC-Autophagy axis addressed in this study is essential for the growth of Drosophila blood progenitor and human HSPCs. The persistence of this axis between Drosophila and humans demonstrates that this axis has been negatively selected to remain similar to the last common ancestor of Drosophila and humans. The resulting conservation of this feed in the taxa also attributes to its indispensability in regulating a developmentally important context of hematopoiesis. Studies demonstrate that hematopoietic cells are amongst the most highly proliferative cells in the body of an organism, with myeloid progenitors showing the most rapid turnover rate among all other blood cell types (98). Several amino acids have been reported to be essential for proper hematopoiesis in humans. Valine, an essential and branched-chain amino acid (ratio metrically with leucine and isoleucine), is vital for HSC proliferation. Additionally, it was found that leucine is completely indispensable for the proliferation of hematopoietic progenitors (79,80). In conjunction with our findings and reports from human HSPCs, we can infer that leucine's availability is crucial for proliferating blood cells in taxa ranging from invertebrates to vertebrates. Although leucine is a known signal which can feed to the mTOR pathway via RAG proteins, it has been found that amino acid sensors to mTORC1 like Rag proteins, respond differentially and in a dispensable manner within subsets of hematopoietic progenitors (99). The indispensable need for leucine with a differential function of RAG proteins hints that human blood harbors mechanisms other than the traditional leucine/amino acid-sensing pathway known to us. The leucine-miRISC-autophagy axis depicts the existence of several yet-to-be-identified cellular cues for regulating a catalytically active miRISC, and its connection to diverse biological phenomena would be an exciting finding in the future.
Supplementary Material
Acknowledgements
We thank Dr David Van Vactor. Dr Irene Miguel-Aliaga, Dr U Banerjee, Dr Geetanjali Chawla, Dr Richa Ricky and Dr. Jishy Varghese for reagents. We thank all members of the two laboratories for their valuable input. We thank IISER Mohali's Confocal Facility and FACS Facility, the Bloomington Drosophila Stock Center at Indiana University and Kyoto Stock Center flies. Models ‘Created with BioRender.com’.
Contributor Information
Sushmit Ghosh, Developmental Genetic Laboratory, 140306 Punjab, India; Department of Biological Sciences, Indian Institute of Science Education and Research Mohali (IISER Mohali), SAS Nagar, Knowledge City, Sector 81, Manauli P.O., 140306 Punjab, India.
Sreemoyee Chakraborti, Developmental Genetic Laboratory, 140306 Punjab, India; Department of Biological Sciences, Indian Institute of Science Education and Research Mohali (IISER Mohali), SAS Nagar, Knowledge City, Sector 81, Manauli P.O., 140306 Punjab, India.
Devki Devi, Developmental Genetic Laboratory, 140306 Punjab, India; Department of Biological Sciences, Indian Institute of Science Education and Research Mohali (IISER Mohali), SAS Nagar, Knowledge City, Sector 81, Manauli P.O., 140306 Punjab, India.
Rajesh Sahu, Developmental Genetic Laboratory, 140306 Punjab, India; Department of Biological Sciences, Indian Institute of Science Education and Research Mohali (IISER Mohali), SAS Nagar, Knowledge City, Sector 81, Manauli P.O., 140306 Punjab, India.
Sudip Mandal, Molecular, Cell and Developmental Biology Laboratory,140306 Punjab, India; Department of Biological Sciences, Indian Institute of Science Education and Research Mohali (IISER Mohali), SAS Nagar, Knowledge City, Sector 81, Manauli P.O., 140306 Punjab, India.
Lolitika Mandal, Developmental Genetic Laboratory, 140306 Punjab, India; Department of Biological Sciences, Indian Institute of Science Education and Research Mohali (IISER Mohali), SAS Nagar, Knowledge City, Sector 81, Manauli P.O., 140306 Punjab, India.
Data availability
All transcriptomic data underlying this article are available in the Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/ and can be accessed under GSE224749. Data related to to q-PCR, immunoblots and Gel Micrographs are available in Figshare at https://doi.org/10.6084/m9.figshare.24080409.v1, https://doi.org/10.6084/m9.figshare.24052974.v1 and https://doi.org/10.6084/m9.figshare.24061428.v1. All other data will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary Data are available at NAR Online.
Funding
Council of Scientific & Industrial Research (CSIR), Govt. of India (to S.G. and Devki); KVPY Scholarship (to R.S.); DBT postdoctoral award [DBT-RA/2021/Jan/N/22 to S.C.]; Institutional support and DST-SERB Grant [CRG/2020/000511 to S.M.]; DBT Wellcome-Trust India Alliance Senior Fellowship [IA/S/17/1/503100] and DST-SERB Power Grant [SPG/2021/000122 to L.M.]. Funding for open access charge: Indian Institute of Science Education and Research Mohali (IISER Mohali).
Conflict of interest statement. None declared.
References
- 1. Lau N.C., Lim L.P., Weinstein E.G., Bartel D.P.. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001; 294:858–862. [DOI] [PubMed] [Google Scholar]
- 2. Lee R.C., Ambros V.. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001; 294:862–864. [DOI] [PubMed] [Google Scholar]
- 3. Lee Y., Kim M., Han J., Yeom K.H., Lee S., Baek S.H., Kim V.N.. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004; 23:4051–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee R.C., Feinbaum R.L., Ambros V.. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993; 75:843–854. [DOI] [PubMed] [Google Scholar]
- 5. Wightman B., Ha I., Ruvkun G.. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993; 75:855–862. [DOI] [PubMed] [Google Scholar]
- 6. Olsen P.H., Ambros V.. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev. Biol. 1999; 216:671–680. [DOI] [PubMed] [Google Scholar]
- 7. Reinhart B.J., Slack F.J., Basson M., Pasquinelli A.E., Bettinger J.C., Rougvie A.E., Horvitz H.R., Ruvkun G.. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000; 403:901–906. [DOI] [PubMed] [Google Scholar]
- 8. Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009; 136:215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jonas S., Izaurralde E.. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015; 16:421–433. [DOI] [PubMed] [Google Scholar]
- 10. Brenner J.L., Jasiewicz K.L., Fahley A.F., Kemp B.J., Abbott A.L.. Loss of individual microRNAs causes mutant phenotypes in sensitized genetic backgrounds in C. elegans. Curr. Biol. 2010; 20:1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carthew R.W. Gene regulation by microRNAs. Curr. Opin. Genet. Dev. 2006; 16:203–208. [DOI] [PubMed] [Google Scholar]
- 12. Bartel D.P. Metazoan MicroRNAs. Cell. 2018; 173:20–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chawla G., Sokol N.S.. MicroRNAs in Drosophila development. Int. Rev. Cell Mol. Biol. 2011; 286:1–65. [DOI] [PubMed] [Google Scholar]
- 14. Han B.W., Hung J.H., Weng Z., Zamore P.D., Ameres S.L.. The 3′-to-5′ exoribonuclease Nibbler shapes the 3′ ends of microRNAs bound to Drosophila Argonaute1. Curr. Biol. 2011; 21:1878–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu N., Abe M., Sabin L.R., Hendriks G.J., Naqvi A.S., Yu Z., Cherry S., Bonini N.M.. The exoribonuclease Nibbler controls 3′ end processing of microRNAs in Drosophila. Curr. Biol. 2011; 21:1888–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chatterjee S., Grosshans H.. Active turnover modulates mature microRNA activity in Caenorhabditis elegans. Nature. 2009; 461:546–549. [DOI] [PubMed] [Google Scholar]
- 17. Kai Z.S., Pasquinelli A.E.. MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nat. Struct. Mol. Biol. 2010; 17:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rüegger S., Großhans H.. MicroRNA turnover: when, how, and why. Trends Biochem. Sci. 2012; 37:436–446. [DOI] [PubMed] [Google Scholar]
- 19. Shen B., Goodman H.M.. Uridine addition after MicroRNA-directed cleavage. Science. 2004; 306:997–997. [DOI] [PubMed] [Google Scholar]
- 20. Liu J., Carmell M.A., Rivas F.V., Marsden C.G., Thomson J.M., Song J.J., Hammond S.M., Joshua-Tor L., Hannon G.J.. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004; 305:1437–1441. [DOI] [PubMed] [Google Scholar]
- 21. Han J., Mendell J.T.. MicroRNA turnover: a tale of tailing, trimming, and targets. Trends Biochem. Sci. 2023; 48:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Makino S., Kawamata T., Iwasaki S., Ohsumi Y.. Selectivity of mRNA degradation by autophagy in yeast. Nat. Commun. 2021; 12:2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hwang H.J., Ha H., Lee B.S., Kim B.H., Song H.K., Kim Y.K.. LC3B is an RNA-binding protein to trigger rapid mRNA degradation during autophagy. Nat. Commun. 2022; 13:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kobayashi H., Shoji K., Kiyokawa K., Negishi L., Tomari Y.. Iruka eliminates dysfunctional argonaute by selective ubiquitination of its empty State. Mol. Cell. 2019; 73:119–129. [DOI] [PubMed] [Google Scholar]
- 25. Zhang L., Ding L., Cheung T.H., Dong M.Q., Chen J., Sewell A.K., Liu X., Yates J.R. 3rd, Han M.. Systematic identification of C. elegans miRISC proteins, miRNAs, and mRNA targets by their interactions with GW182 proteins AIN-1 and AIN-2. Mol. Cell. 2007; 28:598–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang P., Zhang H.. Autophagy modulates miRNA-mediated gene silencing and selectively degrades AIN-1/GW182 in C. elegans. EMBO Rep. 2013; 14:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Allen E.A., Baehrecke E.H.. Autophagy in animal development. Cell Death Differ. 2020; 27:903–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kobayashi H., Shoji K., Kiyokawa K., Negishi L., Tomari Y.. VCP machinery mediates autophagic degradation of empty argonaute. Cell Rep. 2019; 28:1144–1153. [DOI] [PubMed] [Google Scholar]
- 29. Kobayashi H., Tomari Y.. Identification of an AGO (Argonaute) protein as a prey of TER94/VCP. Autophagy. 2020; 16:190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gibbings D., Mostowy S., Jay F., Schwab Y., Cossart P., Voinnet O.. Correction: corrigendum: selective autophagy degrades DICER and AGO2 and regulates miRNA activity. Nat. Cell Biol. 2015; 17:1088–1088. [DOI] [PubMed] [Google Scholar]
- 31. Ameres S.L., Horwich M.D., Hung J.H., Xu J., Ghildiyal M., Weng Z., Zamore P.D.. Target RNA-directed trimming and tailing of small silencing RNAs. Science. 2010; 328:1534–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baccarini A., Chauhan H., Gardner T.J., Jayaprakash A.D., Sachidanandam R., Brown B.D.. Kinetic analysis reveals the fate of a microRNA following target regulation in mammalian cells. Curr. Biol. 2011; 21:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen C., Ridzon D.A., Broomer A.J., Zhou Z., Lee D.H., Nguyen J.T., Barbisin M., Xu N.L., Mahuvakar V.R., Andersen M.R.et al.. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005; 33:e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kramer M.F. Stem-loop RT-qPCR for miRNAs. Curr. Protoc. Mol. Biol. 2011; Chapter 15:Unit 15.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Varkonyi-Gasic E., Wu R., Wood M., Walton E.F., Hellens R.P.. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods. 2007; 3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ge S.X., Son E.W., Yao R.. iDEP: an integrated web application for differential expression and pathway analysis of RNA-seq data. BMC Bioinf. 2018; 19:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klionsky D.J., Abdel-Aziz A.K., Abdelfatah S., Abdellatif M., Abdoli A., Abel S., Abeliovich H., Abildgaard M.H., Abudu Y.P., Acevedo-Arozena A.et al.. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1. Autophagy. 2021; 17:1–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Banerjee U., Girard J.R., Goins L.M., Spratford C.M.. Drosophila as a genetic model for hematopoiesis. Genetics. 2019; 211:367–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jung S.H., Evans C.J., Uemura C., Banerjee U.. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005; 132:2521–2533. [DOI] [PubMed] [Google Scholar]
- 40. Mandal L., Banerjee U., Hartenstein V.. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat. Genet. 2004; 36:1019–1023. [DOI] [PubMed] [Google Scholar]
- 41. Evans C.J., Hartenstein V., Banerjee U.. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell. 2003; 5:673–690. [DOI] [PubMed] [Google Scholar]
- 42. Cho B., Yoon S.H., Lee D., Koranteng F., Tattikota S.G., Cha N., Shin M., Do H., Hu Y., Oh S.Y.et al.. Single-cell transcriptome maps of myeloid blood cell lineages in Drosophila. Nat. Commun. 2020; 11:4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Girard J.R., Goins L.M., Vuu D.M., Sharpley M.S., Spratford C.M., Mantri S.R., Banerjee U.. Paths and pathways that generate cell-type heterogeneity and developmental progression in hematopoiesis. eLife. 2021; 10:e67516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reichholf B., Herzog V.A., Fasching N., Manzenreither R.A., Sowemimo I., Ameres S.L.. Time-resolved small RNA sequencing unravels the molecular principles of MicroRNA homeostasis. Mol. Cell. 2019; 75:756–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zeb Q., Wang C., Shafiq S., Liu L.. Barh D., Azevedo V.. Single-Cell Omics. 2019; Academic Press; 101–135. [Google Scholar]
- 46. Hwang B., Lee J.H., Bang D.. Single-cell RNA sequencing technologies and bioinformaticspipelines. Exp. Mol. Med. 2018; 50:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nichterwitz S., Chen G., Aguila Benitez J., Yilmaz M., Storvall H., Cao M., Sandberg R., Deng Q., Hedlund E.. Laser capture microscopy coupled with Smart-seq2 for precise spatial transcriptomic profiling. Nat. Commun. 2016; 7:12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krzemień J., Dubois L., Makki R., Meister M., Vincent A., Crozatier M.. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007; 446:325–328. [DOI] [PubMed] [Google Scholar]
- 49. Goto A., Kadowaki T., Kitagawa Y.. Drosophila hemolectin gene is expressed in embryonic and larval hemocytes and its knock down causes bleeding defects11Two hml-GAL4 lines (w1118; P{w + mc = GAL4-Hml} 5-6 and w1118; P{w + mc = GAL4-Hml}6-4) and one homozygote of d-hml-GAL4, UAS-gfpnlacZ, UAS-gfp(S65T)/d-hml-GAL4, UAS-gfpnlacZ, UAS-gfp(S65T) (w1118; P{w + mc = GAL4-Hml} 6-4 P{w + mc = UAS-GFP::lacZ.nls} 15.1) transgenic lines are available from the Bloomington Stock Center with the stock numbers of 6395, 6396, and 6397, respectively. Dev. Biol. 2003; 264:582–591. [DOI] [PubMed] [Google Scholar]
- 50. Vigoreaux J.O., Saide J.D., Pardue M.L.. Structurally differentDrosophila striated muscles utilize distinct variants of Z-band-associated proteins. J. Muscle Res. Cell Motil. 1991; 12:340–354. [DOI] [PubMed] [Google Scholar]
- 51. Saide J.D., Chin-Bow S., Hogan-Sheldon J., Busquets-Turner L., Vigoreaux J.O., Valgeirsdottir K., Pardue M.L.. Characterization of components of Z-bands in the fibrillar flight muscle of Drosophilamelanogaster. J. Cell Biol. 1989; 109:2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yannoni Y.M., White K.. Association of the neuron-specific RNA binding domain-containing protein ELAV with the coiled body in Drosophila neurons. Chromosoma. 1997; 105:332–341. [DOI] [PubMed] [Google Scholar]
- 53. Campos A.R., Rosen D.R., Robinow S.N., White K.. Molecular analysis of the locus elav in Drosophila melanogaster: a gene whose embryonic expression is neural specific. EMBO J. 1987; 6:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pérez-Hidalgo L., Moreno S.. Nutrients control cell size. Cell Cycle. 2016; 15:1655–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lloyd A.C. The regulation of cell size. Cell. 2013; 154:1194–1205. [DOI] [PubMed] [Google Scholar]
- 56. Björklund M. Cell size homeostasis: metabolic control of growth and cell division. Biochim. Biophys. Acta. Mol. Cell Res. 2019; 1866:409–417. [DOI] [PubMed] [Google Scholar]
- 57. Palm W., Thompson C.B.. Nutrient acquisition strategies of mammalian cells. Nature. 2017; 546:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hosios A.M., Hecht V.C., Danai L.V., Johnson M.O., Rathmell J.C., Steinhauser M.L., Manalis S.R., Vander Heiden M.G.. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell. 2016; 36:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shim J., Mukherjee T., Banerjee U.. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat. Cell Biol. 2012; 14:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dragojlovic-Munther M., Martinez-Agosto J.A.. Multifaceted roles of PTEN and TSC orchestrate growth and differentiation of Drosophila blood progenitors. Development. 2012; 139:3752–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Benmimoun B., Polesello C., Waltzer L., Haenlin M.. Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development. 2012; 139:1713–1717. [DOI] [PubMed] [Google Scholar]
- 62. Hutvagner G., Simard M.J.. Argonaute proteins: key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008; 9:22–32. [DOI] [PubMed] [Google Scholar]
- 63. Reynolds B., Roversi P., Laynes R., Kazi S., Boyd C.A., Goberdhan D.C.. Drosophila expresses a CD98 transporter with an evolutionarily conserved structure and amino acid-transport properties. Biochem. J. 2009; 420:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martin J.F., Hersperger E., Simcox A., Shearn A.. minidiscs encodes a putative amino acid transporter subunit required non-autonomously for imaginal cell proliferation. Mech. Dev. 2000; 92:155–167. [DOI] [PubMed] [Google Scholar]
- 65. Han J.M., Jeong S.J., Park M.C., Kim G., Kwon N.H., Kim H.K., Ha S.H., Ryu S.H., Kim S.. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012; 149:410–424. [DOI] [PubMed] [Google Scholar]
- 66. Kim S., Yoon I., Son J., Park J., Kim K., Lee J.H., Park S.Y., Kang B.S., Han J.M., Hwang K.Y.et al.. Leucine-sensing mechanism of leucyl-tRNA synthetase 1 for mTORC1 activation. Cell Rep. 2021; 35:109031. [DOI] [PubMed] [Google Scholar]
- 67. Liu G.Y., Sabatini D.M.. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020; 21:183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Saxton R.A., Knockenhauer K.E., Wolfson R.L., Chantranupong L., Pacold M.E., Wang T., Schwartz T.U., Sabatini D.M.. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science. 2016; 351:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dodd K.M., Tee A.R.. Leucine and mTORC1: a complex relationship. Am. J. Physiol. Endocrinol. Metab. 2012; 302:E1329–E1342. [DOI] [PubMed] [Google Scholar]
- 70. Gu X., Jouandin P., Lalgudi P.V., Binari R., Valenstein M.L., Reid M.A., Allen A.E., Kamitaki N., Locasale J.W., Perrimon N.et al.. Sestrin mediates detection of and adaptation to low-leucine diets in Drosophila. Nature. 2022; 608:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Scott R.C., Schuldiner O., Neufeld T.P.. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell. 2004; 7:167–178. [DOI] [PubMed] [Google Scholar]
- 72. Zhou J., Tan S.H., Nicolas V., Bauvy C., Yang N.D., Zhang J., Xue Y., Codogno P., Shen H.M.. Activation of lysosomal function in the course of autophagy via mTORC1 suppression and autophagosome-lysosome fusion. Cell Res. 2013; 23:508–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Neufeld T.P. TOR-dependent control of autophagy: biting the hand that feeds. Curr. Opin. Cell Biol. 2010; 22:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scott R.C., Juhász G., Neufeld T.P.. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr. Biol. 2007; 17:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mauvezin C., Ayala C., Braden C.R., Kim J., Neufeld T.P.. Assays to monitor autophagy in Drosophila. Methods. 2014; 68:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chang Y.Y., Neufeld T.P.. An Atg1/Atg13 complex with multiple roles in TOR-mediated autophagy regulation. Mol. Biol. Cell. 2009; 20:2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Francis K., Lee G.M., Palsson B.O.. Characterization of the KG1a cell line for use in a cell migration based screening assay. Biotechnol. Bioprocess Eng. 2002; 7:178–184. [Google Scholar]
- 78. AbuSamra D.B., Aleisa F.A., Al-Amoodi A.S., Jalal Ahmed H.M., Chin C.J., Abuelela A.F., Bergam P., Sougrat R., Merzaban J.S.. Not just a marker: CD34 on human hematopoietic stem/progenitor cells dominates vascular selectin binding along with CD44. Blood Adv. 2017; 1:2799–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Taya Y., Ota Y., Wilkinson A.C., Kanazawa A., Watarai H., Kasai M., Nakauchi H., Yamazaki S.. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science. 2016; 354:1152–1155. [DOI] [PubMed] [Google Scholar]
- 80. Wilkinson A.C., Morita M., Nakauchi H., Yamazaki S.. Branched-chain amino acid depletion conditions bone marrow for hematopoietic stem cell transplantation avoiding amino acid imbalance-associated toxicity. Exp. Hematol. 2018; 63:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mauvezin C., Neufeld T.P.. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy. 2015; 11:1437–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yuva-Aydemir Y., Simkin A., Gascon E., Gao F.B.. MicroRNA-9: functional evolution of a conserved small regulatory RNA. RNA Biol. 2011; 8:557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jafek J.L., Shakya A., Tai P.Y., Ibarra A., Kim H., Maddox J., Chumley J., Spangrude G.J., Miles R.R., Kelley T.W.et al.. Transcription factor Oct1 protects against hematopoietic stress and promotes acute myeloid leukemia. Exp. Hematol. 2019; 76:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brennecke J., Hipfner D.R., Stark A., Russell R.B., Cohen S.M.. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003; 113:25–36. [DOI] [PubMed] [Google Scholar]
- 85. Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004; 116:281–297. [DOI] [PubMed] [Google Scholar]
- 86. Bagga S., Bracht J., Hunter S., Massirer K., Holtz J., Eachus R., Pasquinelli A.E.. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005; 122:553–563. [DOI] [PubMed] [Google Scholar]
- 87. Hutvágner G., Zamore P.D.. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002; 297:2056–2060. [DOI] [PubMed] [Google Scholar]
- 88. Lim L.P., Lau N.C., Garrett-Engele P., Grimson A., Schelter J.M., Castle J., Bartel D.P., Linsley P.S., Johnson J.M.. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005; 433:769–773. [DOI] [PubMed] [Google Scholar]
- 89. Newbury S.F., Mühlemann O., Stoecklin G.. Turnover in the Alps: an mRNA perspective. Workshops on mechanisms and regulation of mRNA turnover. EMBO Rep. 2006; 7:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wu L., Fan J., Belasco J.G.. MicroRNAs direct rapid deadenylation of mRNA. Proc. Natl. Acad. Sci. U.S.A. 2006; 103:4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yekta S., Shih I.H., Bartel D.P.. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004; 304:594–596. [DOI] [PubMed] [Google Scholar]
- 92. Valencia-Sanchez M.A., Liu J., Hannon G.J., Parker R.. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006; 20:515–524. [DOI] [PubMed] [Google Scholar]
- 93. Kingston E.R., Blodgett L.W., Bartel D.P.. Endogenous transcripts direct microRNA degradation in Drosophila, and this targeted degradation is required for proper embryonic development. Mol. Cell. 2022; 82:3872–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bak R.O., Mikkelsen J.G.. miRNA sponges: soaking up miRNAs for regulation of gene expression. Wiley Interdiscipl. Rev.: RNA. 2014; 5:317–333. [DOI] [PubMed] [Google Scholar]
- 95. Denzler R., Agarwal V., Stefano J., Bartel D.P., Stoffel M.. Assessing the ceRNA hypothesis with quantitative measurements of miRNA and target abundance. Mol. Cell. 2014; 54:766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang J., Liu X., Wu H., Ni P., Gu Z., Qiao Y., Chen N., Sun F., Fan Q.. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010; 38:5366–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P.. A ceRNA hypothesis: the Rosetta stone of a hidden RNA language?. Cell. 2011; 146:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Foudi A., Hochedlinger K., Van Buren D., Schindler J.W., Jaenisch R., Carey V., Hock H.. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat. Biotechnol. 2009; 27:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kalaitzidis D., Lee D., Efeyan A., Kfoury Y., Nayyar N., Sykes D.B., Mercier F.E., Papazian A., Baryawno N., Victora G.D.et al.. Amino acid-insensitive mTORC1 regulation enables nutritional stress resilience in hematopoietic stem cells. J. Clin. Invest. 2017; 127:1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All transcriptomic data underlying this article are available in the Gene Expression Omnibus at https://www.ncbi.nlm.nih.gov/geo/ and can be accessed under GSE224749. Data related to to q-PCR, immunoblots and Gel Micrographs are available in Figshare at https://doi.org/10.6084/m9.figshare.24080409.v1, https://doi.org/10.6084/m9.figshare.24052974.v1 and https://doi.org/10.6084/m9.figshare.24061428.v1. All other data will be shared on reasonable request to the corresponding author.