Summary
During their vegetative growth plants reiteratively produces leaves, buds and internodes at the apical end of the shoot. The identity of these organs changes as the shoot develops. Some traits change gradually, but others change in a coordinated fashion, allowing shoot development to be divided into discrete juvenile and adult phases. The transition between these phases is called vegetative phase change. Historically, vegetative phase change has been studied because it is thought to be associated with an increase in reproductive competence. However, this is not true for all species; indeed, heterochronic variation in the timing of vegetative phase change and flowering has made important contributions to plant evolution. In this review, we describe the molecular mechanism of vegetative phase change, how the timing of this process is controlled by endogenous and environmental factors, and its ecological and evolutionary significance.
Introduction
A plant shoot develops by producing leaves, buds, and internodes at regular intervals at the shoot apex. Each of these organs is morphologically or physiologically distinct from the organs produced earlier in development1,2, which raises the question of how this reiterative process is modified over time to generate morphological and physiological diversity. This question has been studied extensively in both vertebrates and short germ band insects, which also develop by producing organs/segments sequentially at one end of their body axis. In these animals, segmental identity is thought to be specified by the interaction between a segmentation clock and a morphogen gradient.3 Here we discuss the temporal regulation of organ identity during the vegetative phase of shoot development.
Some traits change gradually as a shoot develops, whereas others change abruptly at predictable times in shoot development and, thus, at specific positions on the shoot. This latter phenomenon was first recognized by Hildebrand4 and Goebel5, who used it to divide vegetative development into juvenile and adult phases; the transition between these phases is called “vegetative phase change”. As the shoot develops it also acquires the ability produce structures involved in sexual reproduction (e.g., flowers or cones). Whether it actually produces these structures depends on a complex interaction between many internal and environmental factors.6 Because most plants produce reproductive structures after the transition to the adult vegetative phase, vegetative phase change and the transition from a reproductively-incompetent to a competent phase of shoot development have frequently been considered part of a single program of shoot maturation. As a result, the terms “juvenile” and “adult” are most often used to refer to, respectively, shoots bearing only leaves (vegetative shoots) and shoots that produce reproductive structures such as flowers or cones (reproductive shoots).7 However, the length of time between the onset of the adult vegetative phase and the production of reproductive structures varies tremendously within8,9 and between species. Many trees flower years after the transition to the adult vegetative phase, whereas others—specifically, some species of Acacia10, Eucalyptus11, and many woody species native to New Zealand12–remain permanently juvenile and flower in this condition. These observations suggest that vegetative phase change and the acquisition of reproductive competence are separate, interacting processes, rather than part of a single program of shoot maturation. Unfortunately, very little is known about the regulation of reproductive competence, compared to the regulation of floral induction, which has been extensively investigated.13 This review will therefore focus primarily on the mechanism of vegetative phase change. We will first describe some of the key features of vegetative phase change and then discuss the role of MIR156 and SPL genes in this process and how these genes are regulated.
The phenomenon of vegetative phase change
For many years, vegetative phase change was studied primarily in the vine, Hedera helix (English ivy), and in Acacia, Eucalyptus, and woody species native to New Zealand because these species undergo particularly dramatic changes in leaf and shoot morphology during development.2,14,15 More recent studies have been conducted primarily with short-lived herbaceous plants such as Arabidopsis, maize, and rice because of the advantages these species offer for molecular genetic analysis. Despite the significant difference in the length of their life cycles, many aspects of vegetative phase change are quite similar in these two groups of plants.
In woody plants, phase-specific traits have traditionally been distinguished from other traits that change during shoot development by their stability.16 For example, lateral buds produced during the juvenile phase continue to produce juvenile traits even after the primary shoot has transitioned to the adult phase. Similarly, adult shoots of woody plants rarely revert to producing juvenile traits, even when grafted to a juvenile root stock. This is in contrast to other age-related traits in woody plants (e.g. growth rate), which can be reversed by grafting a shoot tip to a root stock, or by rooting the shoot tip.17 With the discovery of genes that regulate vegetative phase change it is now possible to distinguish phase-specific from age-related traits by whether a trait is affected by variation in the expression of these genes in mutant or transgenic plants. As will be described in more detail later in this review, this approach has revealed the tremendous diversity of phase-specific traits, and has facilitated research on the function of these traits in plant biology.
Phase-specific traits vary between species but commonly include the shape and anatomy of the leaf blade, the relative lengths of the leaf sheath/petiole and leaf blade, branching patterns, the capacity for adventitious root production, the presence of epicuticular wax, anthocyanin, trichomes, thorns and other specialized cells and organs, the rate of photosynthesis, and the degree or type of disease and insect resistance.2,5,14,18 Most species of Acacia, for example, initially produce compound leaves, but then switch during vegetative phase change to producing a simple, vertically oriented leaf known as a phyllode (Figure 1A). In contrast, the ant-acacia, Vachellia collinsii, only produces compound leaves.19 However, in this species, leaves produced early in shoot development are subtended by small stipules and either lack or have a very small extrafloral nectary, whereas the leaves produced later in development have large thorns, one or more large extrafloral nectaries, and produce Beltian bodies (nutrient-rich structures that serve as food for the ants that live in association with these trees) at the tips of their leaflets (Figure 1B). Juvenile and adult leaves in maize are similar in shape but differ in many epidermal traits (Figure 1C). Specifically, juvenile maize leaves are covered with epicuticular wax, and have bulbous epidermal cells with a thin cuticle and weakly interdigitated cell walls that stain purple with toluidine blue. Adult leaves lack epicuticular wax but possess trichomes and bulliform cells; their epidermal cells are relatively flat and have a thick cuticle and tightly interdigitated cell walls that stain aqua with toluidine blue.20 Juvenile maize leaves also have a lower rate of photosynthesis than adult leaves21,22 and are more susceptible to common rust and European corn borer.23 In Arabidopsis, juvenile leaves lack trichomes on their abaxial (lower) surface and are rounder and less serrated24–26 than adult leaves, and have smaller palisade cells27, a less complex vascular system28,29 and more complex transfer cells30 than these leaves (Figure 1D, 2A). They also have a lower rate of photosynthesis21 and are more susceptible to Pseudomonas syringae31 and insect larvae32 than adult leaves.
Figure 1: Species-specific patterns of vegetative phase change.
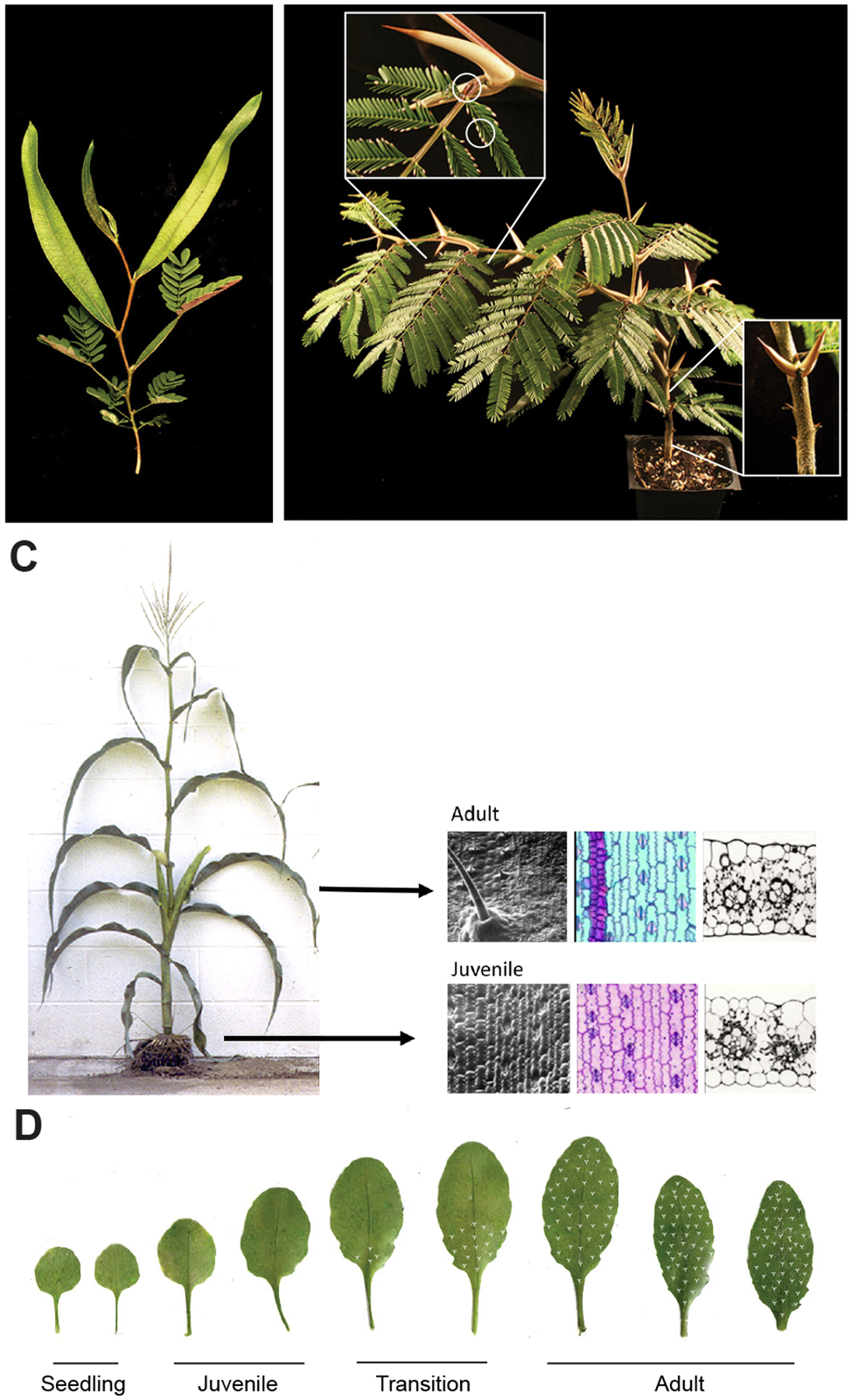
A) Acacia saligna is typical of over 1,000 species of Acacia in Australia. These species initially produce compound leaves, and then produce leaves with a vertically expanded base and a compound tip before finally producing a simple, vertically oriented leaf known as a phyllode.
B) Vachellia collinsii initially produces compound leaves with a small extra floral nectary (EFN) on the petiole and small stipules. At the 5–10th node, it begins to produce leaves with a large EFN, large thorn-like stipules, and Beltian Bodies (BB) on the tips of leaflets.19 The juvenile leaves of this plant have abscised, but their small stipules are still visible.
C) Juvenile and adult leaves in maize are distinguished by a variety of epidermal traits including the presence or absence of epidermal hairs and bulliform cells, the staining of the cell wall with Toluidine blue, cuticle thickness, and the shape of the lateral and outer cell walls of pavement cells.
D) Rosette leaves of the Columbia accession of Arabidopsis grown under long day conditions. As described in the text, the morphology of these leaves and the distribution of abaxial trichomes (shown schematically as white marks) allows shoot development to be divided into the 4 phases shown here.
Figure 2: miR156 is expressed in a similar pattern in a short-lived annual and in a perennial tree.
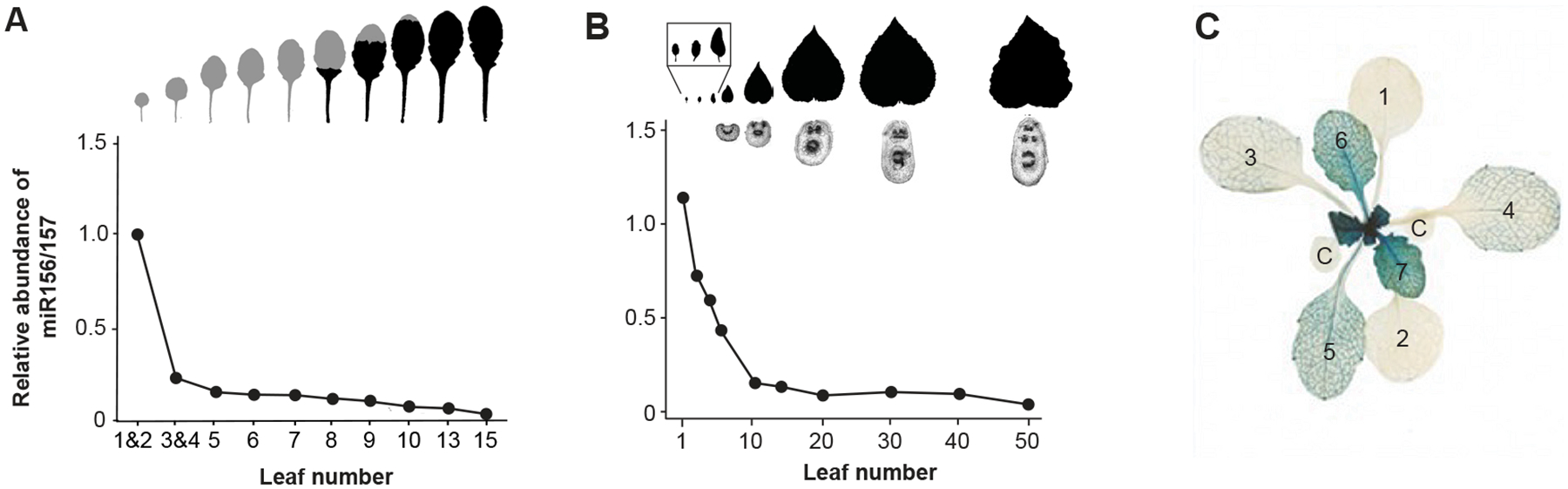
A) The relative level of miR156 in successive rosette leaves of Arabidopsis plants (Columbia) grown in short days to delay flowering. Modified with permission from Fig. 2 in He et al.38
B) The relative level of miR156 in successive leaves of seed-grown plants of the aspen Populus tremula x alba. A cross section of the petiole is shown below the leaf blade to illustrate its change in shape (round to eliptical) and vascular pattern. Modified with permission from Fig. 1 in Lawrence et al.60
C) Arabidopsis plant transformed with a genomic SPL9-GUS construct. There is no visible GUS activity in the cotyledons (C) and in leaves 1&2, consistent with the very high level of miR156 in these organs. GUS activity increases in successive leaves starting with leaf 3. Modified with permission from Fig. 2 in Xu et al.51
Although it is common to divide vegetative development into a juvenile and an adult phase, it is more accurate to divide this process into 4 phases—a brief “seedling” phase, a juvenile phase that is similar to the seedling phase but is distinct from this phase and longer in duration, a transition phase in which leaves are divided into a distal juvenile domain and a proximal adult domain, and an adult phase (Figure 1D).29,33–35 In maize, for example, the first two leaves express all of the epidermal traits characteristic of juvenile leaves but are morphologically and anatomically distinct from juvenile leaves20 and also have a different pattern of gene expression.36 Transition leaves possess juvenile epidermal traits, such as epicuticular wax, at the leaf tip and along the margins of the leaf, and adult epidermal traits at the base of the leaf; adult leaves possess these traits throughout the length of the leaf blade.20 Similarly, the first two leaves in Arabidopsis have all of the traits of juvenile leaves but are smaller, rounder, and less responsive to GA than juvenile leaves25 and have a characteristic pattern of gene expression.37,38 Transition leaves in Arabidopsis have abaxial trichomes at the base of the leaf, but not at the tip, and adult leaves have abaxial trichomes along their entire abaxial surface (Figure 1D). As we will discuss later in greater detail, the apical-basal distribution of juvenile and adult traits in transition leaves indicates that leaf identity is determined relatively late in leaf development.39
The molecular mechanism of vegetative phase change
Regulation of vegetative phase change by the miR156/SPL module
The first insight into genetic mechanism of vegetative phase change came from 3 spontaneous dominant mutations in maize--Teopod1, Teopod2 and Corngrass/Teopod3—whose pleiotropic phenotype was found to reflect the prolonged expression of traits normally restricted to the first nodes of the shoot40. This result revealed that maize possesses juvenile and adult vegetative phases, and that the transition between these phases is under genetic control. It opened the way to subsequent genetic analyses of vegetative phase change in both maize20,41–48 and Arabidopsis25,49–51 and to the discovery that the microRNA miR156 is the master regulator of this transition.52–57
In Arabidopsis38, maize36,54, rice58,59, Populus tremula x alba60 and Vachellia collinsi19, miR156 is expressed at very high levels in the first 1–5 leaves produced after germination, and at a much lower and slowly declining level in subsequent leaves. In Arabidopsis, for example, the level of miR156 drops 70% from leaf 1 to leaf 3, and then another 10–20% from leaf 3 to leaf 15 (Figure 2A).38,61 A similar pattern is observed in the tree, Populus tremula x alba, where a major change in miR156 expression occurs between leaves 1 and 5 (Figure 2B).60 This pattern is reflected in the expression of the targets of miR156, which accumulate at very low levels in the first few leaves of the shoot, and at increasingly higher levels in later leaves. In Arabidopsis, for example, reporter constructs for all 10 of the genes directly repressed by miR156 are not detectable in cotyledons and leaves 1 and 2, and only begin to be visible starting with leaf 3 or later (Figure 2C).37 This expression pattern explains the observation that leaves 1 and 2 are much smaller and qualitatively different from other juvenile leaves, and that leaves produced later in shoot growth display gradual variation in traits such as leaf shape, complexity of leaf veination, the number of leaf serrations, and the density of abaxial and adaxial trichomes.25,26,28,29 It also explains why these first two leaves are developmentally more robust than later leaves.25,62
Exactly how this expression pattern is achieved is still unclear. The abundance of the primary transcripts of MIR156A and MIR156C in Arabidopsis demonstrate that these genes are transcribed more strongly in juvenile leaves than in adult leaves, and is consistent with the amount of mature miR156 in these leaves.38,63,64 On the other hand, experiments in maize41,44, Arabidopsis65 and potato66, indicate that miR156 is diffusible between organs, raising the possibility that at least some of the miR156 in more apical leaves could have come from juvenile leaves with higher levels of miR156. Although there is good evidence that the timing of vegetative phase change is regulated by a change in the transcription of miR156 genes64,67,68, factors that affect the diffusion of miR156 could also be important regulators of this process.
miR156, and the closely related miRNAs, miR157 and miR529, act by repressing the expression of an ancient plant-specific family of transcription factors, known as SQUAMOSA PROMOTER BINDING PROTEINS (SBP/SPL).69–71 As their name indicates, SBP family members were first identified by their ability to bind to the promoter of the snapdragon floral identity gene, SQUAMOSA, the orthologue of APETALA1 (AP1) in Arabidopsis.72 SBP-LIKE (SPL) genes were subsequently identified in Arabidopsis73,74, the alga Chlamydomonas reinhardtii75, in the moss Physcomitrium patens76,77, and in all other surveyed land plants.78–80 In Arabidopsis, miR156/157 regulate the expression of 10 SPL genes37,70,79 through a combination of transcript cleavage81,82 and translational repression83,84, with translational repression being the dominant mechanism.38 A recent genetic analysis suggests the cellular site of translational repression by miR156 may differ from that of other miRNAs.85
Some of the effects of the miR156/SPL module are mediated by miR172. In Arabidopsis, miR172 has a complex expression pattern that depends both on inputs from the miR156/SPL module as well as genes involved in leaf expansion and floral induction.61,86,87 The AP2-like transcription factors targeted by miR172 are important repressors of flowering time, and the function of the miR172/AP2-like module has been studied primarily from this perspective.61,86–90 These transcription factors also promote juvenile epidermal traits and repress adult epidermal traits in both Arabidopsis53,61,91,92 and maize.45,47,48
Vegetative phase change and flowering
It is often assumed that the acquisition of reproductive competence is dependent on vegetative phase change, although there is very little evidence for this in trees93, where flowering occurs long after vegetatively phase change, and some species remain permanently juvenile and flower in this condition.10,11 Analyses of natural variation in vegetative phase change and flowering in both woody9 and herbaceous species8,94 has revealed that these processes are largely independent under floral inductive conditions, indicating that evolution can manipulate this relationship to adapt species to specific ecological conditions.
It was initially proposed that the developmental increase in reproductive competence in Arabidopsis is regulated by miR156/SPL through its effect on miR172 (the “age-dependent pathway”) because miR172 is downstream of miR156/SPL and has a major effect on flowering time.53 However, subsequent studies showed that under environmental conditions conducive to flowering, variation in the level of miR156 has almost no effect on the sensitivity of plants to a floral inductive stimulus61 and is not correlated with variation in flowering time in different natural accessions.8 These results indicate that the age-dependent increase in reproductive competence in Arabidopsis is not regulated by the vegetative phase change pathway in plants that are sensitive to photoperiodic induction.
The effect of the miR156/SPL module on flowering can be difficult to determine in some species because SPL genes have a significant effect on the rate of leaf initiation and on the expression of genes (e.g. AP1, LFY) involved in the floral meristem identity transition (i.e. the transformation of vegetative buds into flowers or flower-bearing shoots during the development of the inflorescence). Consequently, leaf number—which is commonly used as a proxy for flowering time—is a poor predictor of flowering time in plants with elevated or reduced levels of miR156 or SPL proteins. The Tp2 mutant of maize, for example, has an elevated level of miR156 due to the over-expression of Zma-miR156h.56,57 This mutant has an increased number of leaves and a small unbranched tassel with only a few flowers, but does not exhibit a major delay in its sensitivity to a floral inductive photoperiodic stimulus, and terminates shoot development at same time as wild type plants43; the “leafy” phenotype of this mutant is attributable to a delay in the floral meristem identity transition, not to a delay in the acquisition of reproductive competence. Similarly, in wheat95 and snapdragon96, over-expression of miR156 or suppression of SPL gene expression does not significantly delay the timing of the transition from a vegetative to an inflorescence growth pattern, although these genotypes produce an increased number of leaves because of an accelerated rate of leaf initiation and/or a delay in the floral meristem identity transition.
In contrast, over-expression of miR156 delays floral induction in tobacco97, alfalfa98 and switchgrass99, and silencing of PhSBP1 and PhSBP2 delays floral induction in petunia.100 In switchgrass and alfalfa, floral induction was only delayed significantly in transgenic lines that had extremely high levels of miR156. Lines with lower levels of miR156 had aberrant vegetative morphology but flowered normally. This result suggests that in these species miR156 may inhibit flowering during the seedling stage of development, when miR156 is present at very high levels, but have relatively little effect on flowering during the juvenile phase. To determine if reproductive competence (i.e., the ability to respond to floral inductive stimuli) is regulated by the same mechanism as vegetative phase change, it is important to know if varying the level of miR156 within its normal range has an effect on flowering time, and this is not usually examined. It is clear that the miR156/SPL module has the potential to regulate reproductive competence in some species and in some conditions, but it is unlikely to be a major contributor to variation in flowering time in all plants.
Genetic analysis of the function of SBP/SPL genes
The phenotype of plants over-expressing miR156—that is, plants with reduced expression of miR156-regulated SBP/SPL genes--has been characterized in many taxa, including Arabidopsis32,52,53,101–104, maize23,40,54, rice59,105,106, wheat95, tobacco97,107, alfalfa98, Populus55,60, switchgrass108,109 and tomato.110,111 In addition to the prolonged expression of juvenile morphological traits and juvenile patterns of photosynthesis, disease resistance, and insect resistance, this phenotype typically includes an increase in the rate of leaf initiation, an increase in shoot branching and lateral root production, and a variety of floral and fruit defects, including the transformation of floral buds into leafy shoots. The appearance of these phase-specific traits may not be completely coordinated, and some traits are more sensitive to environmental conditions or experimental manipulation than others8,19,112. This result would be explained if the many SPL genes regulated by miR156 were functionally distinct and had different sensitivities to miR156, were regulated differently at a transcriptional level, or interacted with unique sets of RNAs or proteins. In this case, developmental or environmentally-induced variation in the level of miR156 could affect some SPL genes more than others, resulting in a lack of coordination between different phase-specific traits. As described below, research in Arabidopsis and a few other species has shown that SPL genes are indeed functionally differentiated and have different transcription patterns, but whether they are differentially sensitive to miR156 remains to be determined.
The function of individual SPL genes has most often been inferred from the phenotype of plants expressing a miR156-resistant version of the gene under the regulation of the endogenous or a constitutive promoter. However, this approach can produce misleading information because it causes genes to be expressed at unusually high levels at inappropriate times and in inappropriate tissues. Methods that reduce or eliminate SPL gene expression (e.g. over-expression of miR156 or loss-of-function mutations in SPL genes) provide more reliable information about gene function, so our discussion will be limited to studies that have employed this approach. The phenotypes of loss-of-function mutations and the expression patterns of miR156-insensitive reporters for the 10 miR156-targeted SPL genes in Arabidopsis reveal that these genes can be divided into several distinct groups.37 SPL9 and SPL13 are strongly transcribed and have similar and quite significant effects on most aspects of vegetative phase change, demonstrating that they are the major regulators of vegetative phase change and have shared functions in this process. SPL15 is transcribed at a lower level than SPL9 and SPL13 and has a smaller effect on vegetative phase change than these genes, but has larger effect on floral induction, particularly under short days.37,113,114 SPL2, SPL10 and SPL11 are expressed at a relatively low level and have a relatively small effect on morphological aspects of vegetative phase change37,53,115, but are exclusively responsible for mediating adult resistance to Pseudomonas syringae.31 The function of SPL6 is unclear because mutations in this gene have no obvious morphological phenotype and although they have been reported to affect disease resistance116, this result has not been confirmed.31 SPL3, and its paralogues SPL4 and SPL5, were initially thought to be important for floral induction because over-expression of SPL3 causes early flowering.73 However, this phenotype was later shown to be an artefact of over-expression because plants mutant for all three of these genes flower at the same time as wild type plants.37 The inflorescence phenotype of this triple mutant37, and an analysis of the direct targets of SPL3117, demonstrate that SPL3/4/5 promote the floral meristem identity transition, a process that occurs after floral induction and is regulated by AP1 and LFY, which are direct targets of SPL3,. SPL3 is strongly transcribed during the adult vegetative phase, but whether it has a function in vegetative development is unknown because spl3 mutants have no obvious morphological phenotype.37
Three functionally redundant SBP/SPL genes in maize—tasselsheath4 (tsh4), unbranched2 (ub2) and unbranched3 (ub3)—control many aspects of lateral organ development. Loss-of-function mutations in tsh4 cause the development of bracts in the inflorescence118, while plants triply mutant for tsh4, ub2 and ub3 have numerous tillers, an unbranched leafy inflorescence, and flower at the same time as wild type but produce more leaves than normal because they have an elevated rate of leaf initiation.119 This phenotype is very similar to that of Corngrass, Teopod1, and Teopod240, all of which cause the overexpression of miR15654,56,57. Whether tsh4, ub2 and ub3 regulate leaf traits associated with vegetative phase change is unknown because these traits were not examined in these studies. The rice gene, OsSPL14—also known as Wealthy Farmer’s Panicle (WFP)120, and Ideal Plant Architecture (IPA)121—is closely related to tsh4, ub2 and ub3, and has a similar function. Like these maize genes, loss-of-function mutations in OsSPL14 dramatically increase tillering and produce a leafy inflorescence.122,123 Another SPL gene involved in the development of the maize inflorescence is Teosinte glume architecture1 (Tga1). Tga1 regulates the development of the glumes surrounding the maize kernel and, along with its upstream regulator Teosinte branched1124, was an important target of selection during maize domestication.125
The snapdragon genes, AmSBP1, the tomato gene, SlCNR, and the petunia genes, PhCNR, PhSBP1 and PhSBP2, are in the same clade as SPL3, SPL4 and SPL5 in Arabidopsis.79,100 Viral-induced gene silencing of AmSBP1 produces a leafy inflorescence, a phenotype that is similar—albeit much more extreme—to the phenotype of loss-of-function mutations in SPL3, SPL4 and SPL5.96 In petunia, viral-induced silencing of PhSB1 delays inflorescence initiation, flower production, and leaf initiation, whereas silencing of PhSBP2 had no effect on the timing of inflorescence initiation, but delays flower production.100 In contrast, silencing of SlCNR delays fruit ripening, but has no obvious effects on flowering time or inflorescence development.126,127 These results suggest that members of this clade primarily regulate floral meristem identity and fruit development, although they may regulate floral initiation in some species.
How do SBP/SPL genes promote adult vegetative traits?
In many species, a change in leaf shape is the most obvious indicator of vegetative phase change. This change can be quite dramatic, as in Acacia (Figure 1A), but most often involves an increase in leaf serration or leaf lobing, or a change in the angle of the leaf base or the shape or length of the petiole or leaf sheath. In Arabidopsis, for example, the first few leaves of the rosette have long, thin petioles, and have a round leaf blade with few serrations. Leaves produced later in shoot development become increasingly serrated and more elongated, their petioles become thicker and shorter, and the boundary between the leaf blade and petiole becomes less distinct (Figure 1D, 2A).26 The increase in leaf serration is regulated, in part, by post-translational interactions between SPL proteins and two families of transcription factors that regulate leaf serration, CUC and TCP128. CUC proteins promote serration, but are prevented from doing so in juvenile leaves by their interaction with TCP proteins. This interaction is disrupted in adult leaves by SPL9, which binds to TCP4, thus preventing it from interacting with CUC proteins (Figure 3).
Figure 3: The mechanisms by which phase-specific traits are regulated by miR156/miR157.
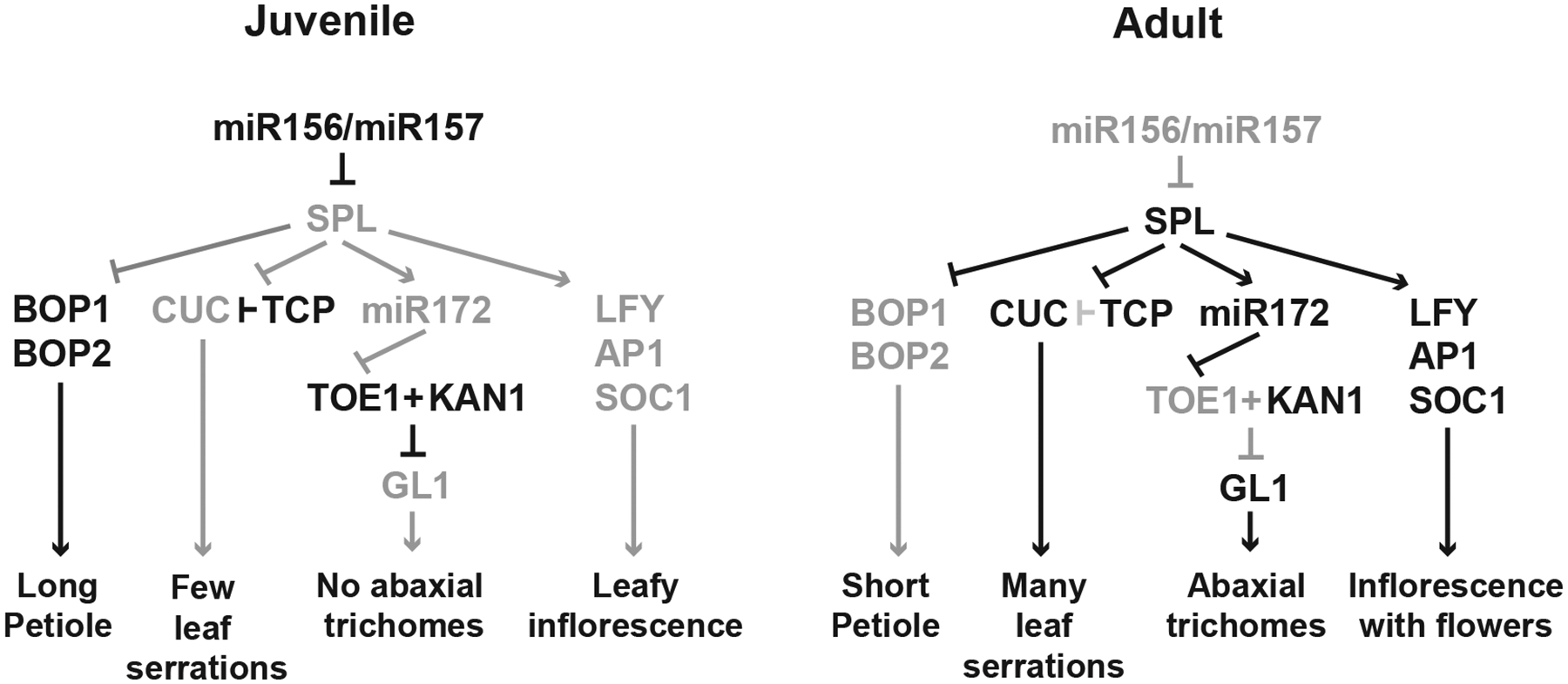
Active genes/functions are represented by black lines and black text, and inactive genes and functions are represented by gray lines and text. The result of these genetic interactions is provided at the bottom of the figure.
The change in the length and shape of the leaf blade and petiole is regulated by BOP1 and BOP2 (Figure 3).129 In juvenile leaves, these transcription factors promote petiole development by repressing the expansion of the leaf blade. In adult leaves their transcription is repressed by SPL9 and SPL13, which leads to the extension of the leaf blade onto the petiole and produces the acute leaf base and relatively short petiole characteristic of adult leaves. The basal expansion of adult leaf blades is facilitated by enhanced anisotropic cell growth, which is associated with increased SPL activity.130 This mechanism also operates in rice. In rice, juvenile leaves have a long leaf sheath and very short leaf blade, whereas adult leaves have a long leaf blade and a relatively short leaf sheath. This difference is regulated by OsBOP genes, which promote the development of the leaf sheath and repress the development of leaf blade.131 As in Arabidopsis, these genes are expressed at high levels in juvenile leaves, but are repressed in adult leaves by SPL transcription factors, allowing for the development of the leaf blade.
The distribution of trichomes on the leaf blade is regulated by SPL genes in part through their effect on miR172 expression. SPL9 and SPL15 promote the transcription of MIR172B53,113,132, and thus contribute to the relatively high level of miR172 in adult leaves compared to juvenile leaves.61 This expression pattern is expected to produce higher levels of AP2-like transcription factors in juvenile leaves than in adult leaves. One of these transcription factors is TOE1. TOE1 interacts with the transcription factor, KANADI1--which is expressed in abaxial leaf cells—to repress the transcription of the trichome regulator, GLABRA1, on this surface of the leaf.91–92 As TOE1 is expected to be present at higher levels in juvenile leaves than in adult leaves, this would explain the absence of abaxial trichomes on juvenile leaves (Figure 3). However, SPL regulation of miR172 cannot be the only explanation for the effect of SPL genes on abaxial trichome production because the trichome phenotype of plants with elevated SPL levels is only partially suppressed by mutations that nearly eliminate miR172 expression.61 This result suggests that SPL genes also regulate this phenotype independently of miR172.
In maize40 and rice106, elongated branches known as tillers are only produced by juvenile nodes. It is unknown if the capacity for branch outgrowth is also a juvenile trait in species that do not branch excessively, but this may be the case because constitutive over-expression of miR156 produces excessive branching in every species in which this trait has been engineered. This result suggests that SPL proteins repress branch outgrowth during the adult phase, when miR156 levels are low. The mechanism by which they do this has been elucidated in both rice123,133 and wheat.95 In both species, SPL proteins repress branching by promoting the transcription of orthologues of the maize branching repressor TEOSINTE BRANCHED1. The activity of these SPL proteins is also regulated by the hormone strigolactone through its effect on the repressor, D53.95,133 In the absence of strigolactone, SPL proteins are inactivated by their association with D53, which enables branch outgrowth. D53 is degraded in the presence of strigolactone, allowing SPL proteins to promote the transcription of TB1 and thus repress branch outgrowth.
Epigenetic regulation of miR156 expression
In 1962, Brink made the prescient suggestion that vegetative phase change is regulated by “self-perpetuating accessory materials” he termed “parachromatin”.16 He also suggested that these materials were responsible for stable developmental states in many other organisms, including animals. This paper was published before the discovery of transcription factors and other forms of gene regulation in eukaryotes, and so was largely ignored. It is therefore remarkable that he was essentially correct. Based largely on work in Arabidopsis, it is now clear that epigenetic factors play a central role in coordinating the temporal decline in miR156 expression, and therefore the timing of vegetative phase change (Figure 4). Core to this epigenetic timing mechanism is the balance between active (e.g. H3K27ac) and repressive (e.g. H3K27me3) histone marks at MIR156 loci. Levels of H3K27ac are determined by the activity of histone acetyl, and histone deacetyl, transferases.134 On the other hand, H3K27me3 levels are mediated by the activity of the transcriptional repressor complexes POLYCOMB REPRESSIVE COMPLEX 2 (PRC2) and PRC1. PRC2 contains a methyltransferase and directly deposits H3K27me3, whereas PRC1 represses gene expression via H2A ubiquitination and also promotes PRC2 activity at a subset of its targets.135
Figure 4: Model for the epigenetic regulation of MIR156 transcription in Arabidopsis.
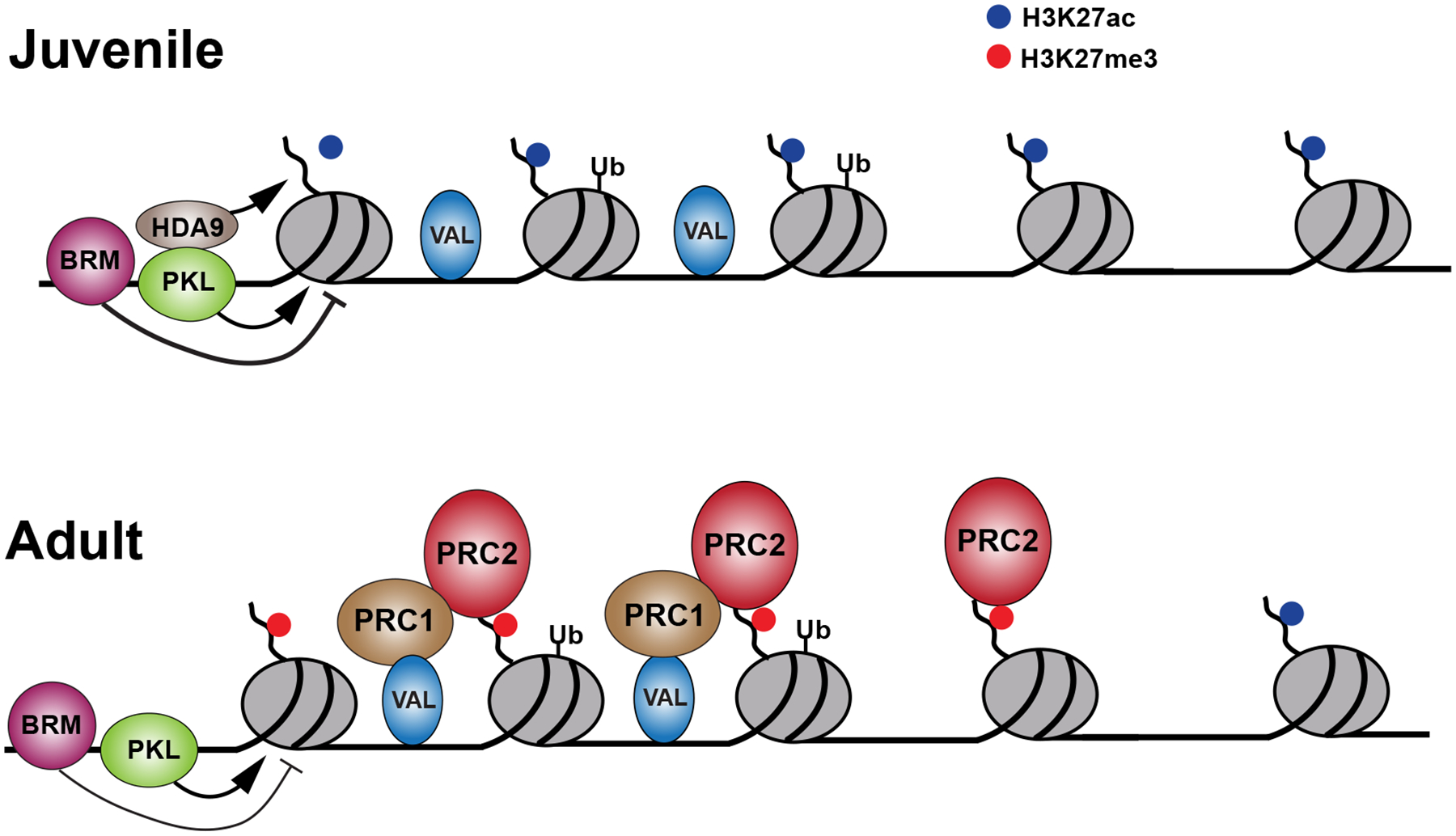
This model combines data from studies of MIR156A and MIR156C, which are regulated by slightly different epigenetic mechanisms that have not been completely elucidated. MIR156 transcription is regulated by nucleosome remodeling and histone modifications. During the juvenile phase, the chromatin remodeler, Brahma (BRM), repositions the +2 and −1 nucleosome to promote transcription, while PKL prevents repositioning of the +1 nucleosome. It is unclear whether BRM remains at MIR156 genes during the adult phase, but in any case, PKL appears to have a more significant effect at this stage. PKL represses transcription both by inhibiting nucleosome repositioning and through its association with histone deacetylases, most importantly, HDA9. The loss of the active mark, H3K27ac, is associated with an increase in the repressive mark, H3K27me3, which is deposited by PRC2. Binding of PRC2 to MIR156 is promoted by PRC1 but can also occur independently of PRC1. In addition to promoting the activity of PRC2, PRC1 may represses MIR156 transcription via H2A ubiquitination. The transcription factor, VAL1, is present at MIR156 throughout development and binds to the BMI1A component of PRC1, facilitating its association with MIR156. However, VAL1 is not responsible for the temporal regulation of PRC1 or PRC2 activity.
During vegetative development, H3K27me3 marks are gradually deposited in place of H3K27ac, leading to the transcriptional repression of MIR156A/C and the transition to adult development.64 Binding of PRC2 to MIR156A/C increases during the period when the transcription of these genes is down-regulated, and loss-of-function mutations in the PRC2 methyltransferases SWINGER (SWN) delay vegetative phase change, elevate miR156 accumulation and reduce H3K27me3 levels at MIR156A/C – with subtly different effects on MIR156A and MIR156C.64 PRC1 is important for PRC2 activity at MIR156A/C as loss of the PRC1 recruitment factors VIVIPAROUS1 ABI3-LIKE1 (VAL1) and VAL2, and of PRC1 components themselves, increases the overall level of miR156 and reduces H3K27me3 deposition (predominantly at MIR156C).136,137 In addition to its role in recruiting PRC2, the H2A ubiquitination function of PRC1 also appears to be important in the repression of MIR156A/C.68,136 Loss of the PRC accessory protein LIKE-HETEROCHROMATIN 1 (LHP1) in rice leads to elevated transcription of MIR156 loci concomitant with reduced H3K27me3138, suggesting PRC-mediated silencing of MIR156 is conserved across flowering plants.
H3K27ac removal is largely determined by HISTONE DEACETYLASE 9 (HDA9).68,139 The rate of both H3K27ac removal by HDA9, and H3K27me3 deposition by PRC2, is promoted by the CHD3 chromatin remodeler PICKLE (PKL). Genetic analyses have shown that pkl enhances the hda9 and swn delayed vegetative phase change phenotypes, with a far stronger effect of the respective double mutant on MIR156A/C expression than either single mutant.64,68 PKL therefore plays a critical role in coordinating the temporal exchange of histone modifications during vegetative phase change, at least in part through its effects on nucleosome occupancy.64 In animals, the PKL homolog Mi2β/CHD4 forms part of the nucleosome remodeling and deacetylation (NuRD) complex, which facilitates PRC2-recruitment via precursory H3K27ac removal.140 Whether or not an equivalent NuRD complex functions in plants has been somewhat controversial.141 However, the recent discovery that PKL physically interacts with HDA6 and HDA968, and functions in both H3K27 deacetylation and methylation64, strongly suggests that plants possess a NuRD-like complex.
The repressive effects of PKL and PRC2 are antagonized by chromatin remodeling complexes that promote MIR156A/C expression. The SWI2/SNF2 chromatin remodeling ATPase BRAHMA (BRM) reduces nucleosome occupancy and H3K27me3 levels at MIR156A, and loss of function brm mutants exhibit early phase change.142 Likewise, the SWR1 chromatin remodeling complex promotes MIR156A/C expression through maintenance of the H2A.Z histone variant and deposition of the positive H3K4me3 histone mark.143,144
Despite recent insights from epigenetic studies, how the timing of the deposition of H3K27me3 at MIR156A/C is controlled remains to be determined. Based on the maintenance of a MIR156C reporter construct in quiescent cells relative to actively dividing ones, and chemical inhibition of the cell cycle, it has recently been proposed that the deposition of H3K27me3 is triggered by cell division.139 Although this model would provide a parsimonious explanation for how miR156 activity decreases with age, it conflicts with the observed rates of phase change in mutants with slower25,65 and faster85 rates of cell division. Furthermore, a cell division-dependent decline in miR156 expression within cells of the SAM is not consistent with the almost permanently quiescent nature of some SAM cells145, nor is cell division-dependent repression of miR156 expression consistent with the re-initiation of miR156 expression during leaf development105, reproductive development67 or culture-induced rejuvenation.39
How other genetic factors found to regulate miR156 expression might interact with a putative cell division model is unclear. For example, the transcription factors AGL15/18, MYB33, ABI3/5, FUS3 and NF-YB8/NF-YA8146–151 all promote miR156 expression whereas PLT2, PNY/PNF, the CDK8 mediator complex and the barley histone acetyltransferase MND8 all repress miR156 expression.152–156 Which MIR156 loci these factors regulate, and whether they do so in the context of vegetative phase change, is not always apparent. An intriguing additional layer of genetic regulation at MIR156A/C was revealed by the discovery that micropeptides encoded by open reading frames upstream of the miRNA hairpin initiate self-activating loops that promote expression of the primary miRNA transcripts.157–159
The likely complexity of the phase change clock is highlighted by the remarkable robustness of the temporal expression of MIR156A/C despite extensive attempts to genetically disrupt it. In almost all cases, loss of function mutations in regulators of MIR156A/C lead to changes in the overall level of miR156, rather than a change in the rate of its decline.64,68,137,138,142,144 Consistent with its role in both H3K27 deacetylation and trimethylation, genetic combinations featuring pkl appear to have the strongest reduction in the rate of decline, as pkl swn and pkl hda9 mutant plants maintain a relatively consistent level of MIR156C expression.64,68 Importantly, MIR156A expression—while increased overall—still declines in a temporal pattern in these plants. Despite their recent duplication and sequence similarity, MIR156A and MIR156C are regulated by only partially overlapping mechanisms. This divergence likely reinforces the rigid timing mechanism of phase change and may provide an excellent model for the study of molecular evolution.
Ultimately, the phase change clock needs to be reset between generations. Although the nature of this clock is still unknown, a cis-regulatory region that is conserved between MIR156A/C and is required for their embryonic re-activation has recently been identified and may provide some clues.67 Genetic and biochemical assays suggest the embryogenesis regulator LEAFY COTYLEDON 2 (LEC2) binds this region and is critical for resetting MIR156A/C expression. How LEC2 and the closely related VAL1/2, which bind the same DNA motif and have opposing effects on the timing of vegetative phase change137, coordinate activation vs repression of MIR156A/C remains to be determined.
How is the timing of vegetative phase change regulated?
Although there is good evidence that vegetative phase change is the result of the transcriptional repression of miR156 and miR157 genes by epigenetic factors, this does not answer the question of how the timing of this process is regulated. The endogenous and exogenous factors responsible for variation in leaf morphology during shoot development (heteroblasty) were of considerable interest in the first half of the 20th century1, but these studies were never framed in terms of the more general process of vegetative phase change because it was assumed that these factors acted specifically on leaf morphogenesis, not on the developmental phase of the shoot as a whole. More recent studies have shown that at least some of these factors (e.g. defoliation, sugar, low light intensity) affect the expression of miR156/miR157 or the transcription of SPL genes, indicating that the literature on heteroblasty is a good source of inspiration for research on the mechanism of vegetative phase change. Linking these factors to changes in the epigenetic landscape of miR156 and miR157 genes remains a challenge, however.
Role of the shoot apical meristem
Whether the shoot apical meristem (SAM) controls the timing of vegetative phase change autonomously, or simply responds to external cues, is a classic question in plant biology.160,161 It was traditionally thought that the juvenile-to-adult transition is regulated within the SAM162, but more recent work has demonstrated that pre-existing leaves promote both the initiation and the maintenance of the adult phase. For example, below a threshold number of leaf primordia, maize apex explants will rejuvenate during culture, suggesting that a leaf-derived signal is actively required to maintain apices in the adult phase.39,163 In apices cultured with 6 leaf primordia, the existing primordia underwent rejuvenation while the SAM remained in the adult phase39, demonstrating that leaf identity in maize does not require a change in the state of the SAM. Leaf ablation experiments in Arabidopsis have demonstrated that the transition to the adult phase is promoted by a leaf-derived signal that represses the expression of miR156.164 Other experiments showed that this signal is diffusible and suggested that it might be glucose or another carbohydrate.63,165 Additionally, cytokinin regulates the timing of abaxial trichome production in leaves, not in the SAM.166 On the other hand, the observation that mutant plants with defective SAMs produce adult leaves precociously25,65,167 demonstrates that the SAM is not an entirely neutral player. Importantly, the phase change phenotypes of these mutants, and plants with reduced miR156 activity, can be partly rescued by expression of miR156 specifically within the SAM.65 Together, these results suggest that vegetative phase change is regulated by a complex interaction between leaves and the SAM, with the SAM being important for the specification of juvenile identity very early in development, whereas later shoot identity is regulated primarily by leaves.
Regulation of vegetative phase change by sugar
As noted earlier in this review, ablation studies indicate that leaves produce a stable diffusible factor that represses miR156164, and subsequent studies have suggested this factor might be sugar. One attractive idea is that the “phase change clock” is the increase in the amount of sugar produced by the shoot as its photosynthetic capacity increases as a result of an increase leaf number or leaf expansion. Given the increased energetic cost of producing adult leaves22, carbohydrate-based signaling represents an attractive way to synchronize vegetative phase change with nutritional state, and the striking effect of low light conditions on the timing of vegetative phase change (described below) supports this idea.168
Glucose, fructose and sucrose all suppress MIR156A/C expression, in part through the glucose sensor HEXOKINASE1.63,165 The sucrose level in a plant influences the timing of vegetative phase change via the signaling metabolite trehalose-6-phosphate, which is synthesized by the enzyme TREHALOSE PHOSPHATE SYNTHASE1 (TPS1). Loss of TPS1 function leads to delayed phase change and elevated MIR156A/C expression.169,170 The sugar sensor SUCROSE NON-FERMENTING1 RELATED-KINASE (SnRK1) likely functions downstream of TPS1, as inhibition of SnRK1 activity suppresses the increased miR156 accumulation of tps1.171 Intriguingly, the master integrator of nutrient status TARGET OF RAPAMYCIN (TOR) kinase has recently been shown to regulate PRC2 activity through phosphorylation of the core PRC2 component FIE172, suggesting a potential mechanism for how plant energy status coordinates the timing of phase change.
Hormonal regulation of vegetative phase change
Plant hormones were found to regulate the timing of vegetative phase change long before the molecular mechanism was elucidated.173 Gibberellic acid (GA) accelerates or delays phase change in woody species and accelerates vegetative phase change in both Arabidopsis and maize.25,46 The miR156 pathway operates at least partly downstream of GA as constitutive expression of miR156 and PKL are both epistatic to the effects of GA signaling.174,175 However, SPL9 and SPL15 have been shown to physically interact with GA-sensitive DELLA proteins113,174,176, suggesting that the regulation of vegetative phase change by GA is multi-layered. More recently, jasmonic acid (JA) has been shown to delay phase change by promoting the expression of miR156 in rice and maize.36,177 SPL proteins also interact with JA signaling factors32.
Interactions between plant hormone networks and the miR156/SPL pathway appear to occur more frequently at the protein level as SPL proteins physically interact with brassinosteroid178,179, strigolactone123,180, ABA181 and cytokinin182 signaling components. SPL proteins and cytokinin signaling also function complementarily to promote miR172 expression.166 The observation that hormones act downstream of miR156/miR157, as well as the absence of evidence linking developmental changes in the level of these hormones with vegetative phase change, suggest that plant hormones mainly affect the manifestation of vegetative phase change, rather than its timing.
Regulation of vegetative phase change by environmental conditions
The control of developmental timing is intrinsically robust but also sensitive to environmental conditions. In many species the timing of vegetative phase change is delayed by low light intensity.1,19,168,183 The discovery that juvenile leaves are photosynthetically more efficient under low light intensity than adult leaves21,22 provides a physiological explanation for this delay, and its molecular basis is suggested by the observation that low light intensity increases the abundance of miR156 in Arabidopsis and Vachellia collinsii.19,168 In the case of Arabidopsis, the delay is independent of phytochrome and cryptochrome signaling and is associated with a reduction in H3K27me3 at MIR156C, although miR156-independent regulation of SPL gene expression is also a contributing factor.168 The effects of low light are at least in part mediated through nutritional status, as exogenous sucrose partly suppresses the low light phenotype. Shade-adapted species photosynthesize efficiently under low light intensity throughout their life and have many morphological traits (the “shade tolerance syndrome”) typical of juvenile shoots.184 It may be that the phenotype of these plants reflects the extended expression of the juvenile phase resulting from either elevated levels of miR156 or the reduced transcription (e.g. via mutation) of one or more SPL genes. Levels of miR156 are also regulated by soil nutrient conditions. Consistent with the idea that vegetative phase change is delayed until conditions are favorable, phosphate and nitrogen deficiency induce miR156 accumulation.143,185
Although phytochrome signaling does not play a role in the response to light intensity, it does regulate the miR156/SPL module in response to light quality. Under far-red enriched light, Arabidopsis undergoes a “shade-avoidance syndrome”, which is distinct from the shade tolerance syndrome induced by overall low light levels168. As part of the shade-avoidance syndrome, petioles lengthen, branching is inhibited and plants flower earlier. Constitutive expression of miR156 suppresses the shade-avoidance syndrome, which is induced by the PHYTOCHROME INTERACTING FACTOR (PIF) family of transcription factors. As PIF family members repress miR156 expression, it has been suggested that PIFs promote the shade-avoidance response via miR156.186 However, the MIR156 loci that PIF proteins regulate (i.e. MIR156B/D/E/F/H) have limited roles in Arabidopsis development38,67, making the significance of the interaction between these proteins and MIR156 loci unclear. PIFs187, and the phytochrome signaling components FHY3 and FAR1180,188, directly interact with SPL proteins, suggesting multi-layered regulation of the miR156/SPL network by light quality. This regulatory complexity is highlighted by the finding that UV-B wavelengths elevate miR156 levels via inhibition of H3K27me3 deposition.189
Vegetative phase change is also sensitive to temperature and drought. Arabidopsis grown at 16°C exhibits delayed phase change and increased levels of miR156 relative to plants grown at 23°C190,191. Interestingly, the elevated level of miR156 in 16°C plants is regulated post-transcriptionally as pri-MIR156A transcript levels are lower in these plants than in plants grown at 23°C.192 Extremely high temperatures also increase miR156 levels, and this may lead to increased tolerance to heat stress.193 Similarly, miR156 expression is induced by drought while over-expression of miR156 promotes drought tolerance.194 Downstream of miR156, SPLs limit plant responses to increased temperature by reducing sensitivity to auxin.191,195 As temperature and shade are both mediated by PIF signaling, SPL-dependent auxin sensitivity may also contribute to the potential role of miR156 in the shade-avoidance response.
We have focused here on the regulation of miR156. It is important to emphasize that the timing of vegetative phase change can also be affected by factors that affect the transcription196–198 or activity128,178,199–202 of the SPL proteins regulated by miR156. The relative importance for vegetative phase change of these mechanisms vs. post-transcriptional regulation of SPL transcripts by miR156 remains to be determined.
The evolution and ecological significance of vegetative phase change
The ancestral functions of the miR156/SPL module
SBP/SPL genes can be traced back to the algae whereas miR156—one of the most ancient and highly conserved miRNAs in plants—emerged when plants first colonized land.76,203 As the ancestral land plants looked very different from the flowering plants in which miR156/SPL function has mainly been studied, identifying the ancestral function of miR156 and its role in the establishment of land plants is a key area of research. Studies of two experimentally tractable bryophyte models, the moss, Physcometrium (Physcomitrella) patens, and the liverwort, Marchantia polymorpha—whose lineage diverged from the rest of the plant kingdom approximately 500 million years ago204,205—have started to yield answers.
Unlike flowering plants, these organisms spend most of their life cycle as haploid gametophytes, and the functions of the miR156/SPL module have been investigated during this phase of the life cycle. Early moss development consists of a 2D filamentous growth phase, during which gametophores – leafy shoots derived from a single apical meristem cell – subsequently initiate. The growth of gametophores terminates following the production of reproductive organs which, after fertilization, nurture the diploid sporophyte. Gametophores produce morphologically different leaves along their axis, which have been interpreted as juvenile and adult forms206. In Tortula, the timing of the transition between these two leaf types differs between species, and some species initiate reproductive development having only produced juvenile leaves206. In Physcomitrium, miR156 levels increase slightly during the filamentous-to-gametophore transition, and then decrease dramatically as the gametophore develops; consistent with this pattern, SPL/SBP gene expression decreases and then increases dramatically during gametophore maturation207. Transgenic lines with reduced levels of miR156 produce gametophores much less frequently than wild type, whereas Ppsbp3 mutants produce more gametophores than normal208, indicating that PpSBP3 represses meristem development. Whether this module also plays a role in the juvenile-to-adult transition during gametophore development is unknown because the phenotype of the gametophores in these lines was not characterized.
In contrast to mosses, gametophytes of M. polymorpha grow as a branching thallus from which umbrella-like gametangia arise. M. polymorpha has 4 SPL/SBP genes, one of which, MpSPL2, is orthologous to the miR156-regulated genes in higher plants. MpSPL2 is regulated by miR529c, a miRNA closely related to miR156.209. Loss of miR529c or over-expression of MpSPL2 causes the production of gametangia in the absence of an inductive far red light stimulus; loss of MpSPL2 has minor effects on gametangia morphology and the timing of gametangia formation, but does not affect gamete production or function.210 These results suggest that MpSPL2 promotes, but is not required for the transition to reproductive development. A second SPL/SBP gene, MpSPL1, is regulated by the liverwort-specific miRNA, Mpo-MR13, and affects thallus branching by promoting meristem dormancy.211
The functions of the miR156/SPL module in the gametophyte of bryophytes closely resemble their functions in the sporophytes of angiosperms. For example, the role of PpSBP3 in suppressing the development of apical initials that give rise to the gametophore is similar to the function of SPL genes in Arabidopsis, which suppress the growth of the shoot apical meristem.65 Although the function of the miR156/SPL module during gametophore development is unknown, the complementary temporal expression patterns of these genes is equivalent to their expression during shoot development in flowering plants, so it would not be surprising if they regulated the juvenile-to-adult transition during this phase of moss development. The role of MpSPL2 in gametangia development in Marchantia is similar to the roles of SPL genes in the regulation of flowering time and floral meristem identity. Although MpSPL1 is not in the same clade as miR156-regulated SPL family members, it has an analogous function in branch development as these genes. It is clear, therefore, that a role for miR156 in the regulation of critical developmental processes, including developmental identity and meristematic growth, is widely conserved across land plants. Furthermore, miR156 likely regulated phenomena equivalent to vegetative phase change in the ancestral land plant. Whether or not miR156 regulated development in both the gametophyte and sporophyte phases of early land plants, or if its sporophytic role in angiosperms was co-opted from ancestral gametophytic genetic networks212, is a topic for future research.
The evolution of vegetative phase change in higher plants
Plants face different challenges at different times in their development and must evolve temporally specific morphological and physiological adaptations to these conditions. Much of the biomass on earth consists of perennial species—specifically trees and shrubs--that spend most of their life in the vegetative phase of development. These species must contend with a wide range of changing environmental conditions during their lifespan, and phase-specific traits likely evolved in response to these conditions. The striking conservation of juvenile leaf morphology in Acacia compared to the enormous diversity in adult leaf morphology in this genus10, as well as genetic analysis in Arabidopsis62, indicate that the juvenile phase is ecologically and evolutionarily more robust than the adult phase. We suspect that this is because there is a conserved set of phase-specific traits that contributes to the survival of juvenile plants and is therefore under strong selection. Identifying these traits, as well as the traits shared by adult shoots, is an important topic for future research.
Variation in the timing of vegetative phase change can be an important mechanism for the evolution of adaptive traits because the timing of vegetative phase change can vary relative to the timing of floral initiation. This means that variation in the timing of different phase-specific traits, or of vegetative phase change as a whole, can enable plants to adapt to specific environmental conditions without altering the entire life history of a plant. For example, neotenous species of Acacia are found specifically in the southeast and southwest corners of Australia, which have a cooler and wetter climates than the rest of the subcontinent, where most phyllodinous species are found10. Similarly, precocious ecotypes of Arabidopsis are most often found in regions with significant seasonal variation and range in temperature8. In the Azores, neotenous accessions of Cardamine hirsuta are found predominantly in regions with warm, wet summers94, which is similar to the climate in which neotenous species of Acacia are found. Interestingly, these neotenous accessions flower earlier than accessions with a relatively short juvenile phase. The vegetative phenotype of these neotenous accessions of C. hirsuta is attributable to a loss-of-function mutation in SPL9.94 In Eucalyptus globulus, natural variation in the timing of vegetative phase change was correlated with variation in the abundance of miR156 and mapped to miR156.5.213 These and other results discussed in this review suggest that the miR156/SPL module has been the target of natural selection many times during evolution, and could be a major contributor to morphological and physiological diversity within the plant kingdom.
Concluding Remarks
The timing of vegetative phase change can be modulated by a change in the rate of decline in miR156/miR157 during shoot growth or by an overall increase or decrease in the abundance of these miRNAs. Factors that affect the rate of decline represent components of a developmental clock, the identity of which is still unknown. As discussed here, this clock could be the increase in the photosynthetic capacity of the shoot, given that conditions that reduce this capacity (defoliation, low light intensity, photosynthetic mutations) delay this transition. It is also possible that there are two clocks—one that measures the changing physiology of the shoot, and a second that is independent of this input. Whatever the case, these clocks are expected to affect the abundance of H3K25me3, given that this modification is required for the down-regulation of miR156 genes.64 An obvious possibility is that the deposition of this mark is controlled by factors that target chromatin modifying complexes to these genes at a particular time in shoot development. However, this leaves open the question of how the temporal expression of these targeting factors is regulated. We favor the hypothesis that the decline in the transcription of miR156/miR157 genes is the result of a difference in the constitutive rate at which H3K27ac and H3K27me3 or other activating and repressive chromatin modifications are added to these genes. This rate might be mostly dependent on intrinsic differences in the activity of the complexes that deposit these marks, and may have an amplification mechanism (e.g. H3K27me3 promotes the addition of more H3K27me3). Testing this hypothesis will require sophisticated measurements of these marks in the SAM and leaf primordia, combined with mathematical modelling based on these parameters. With the increasing interest in vegetative phase change, answers to these and other questions about this important process may not be long in coming.
Acknowledgments
We are grateful for the comments of Aman Husbands, Doris Wagner, and Mingli Xu on this manuscript, and for the contributions of Jianfei Zhao and Erica Lawrence to the figures. Research in the authors’ laboratories is supported by a grant from the National Institutes of Health R01 GM51893 to RSP and by a Royal Society University Research Fellowship (URF/221069) to JPF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- 1.Allsopp A (1967). Heteroblastic development in vascular plants. Adv. Morphol 6, 127–171. [DOI] [PubMed] [Google Scholar]
- 2.Doorenbos J (1965). Juvenile and adult phases in woody plants. Encyl. Plant Physiol 15, 1222–1235. [Google Scholar]
- 3.Diaz-Cuadros M, Pourquie O, and El-Sherif E (2021). Patterning with clocks and genetic cascades: Segmentation and regionalization of vertebrate versus insect body plans. PLoS Genet 17, e1009812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hildebrand F (1875). Ueber die Jungendzustände solcher Pflanzen, welche im Alter vom vegetativen Charakter ihrer Verwandten abweichen. Flora 21, 321–330. [Google Scholar]
- 5.Goebel K (1900). Organography of plants Part I. General organography. (English translation by I. B. Balfour) (Clarendon Press; ). [Google Scholar]
- 6.Cho LH, Yoon J, and An G (2017). The control of flowering time by environmental factors. Plant J 90, 708–719. [DOI] [PubMed] [Google Scholar]
- 7.Matsoukas IG (2014). Attainment of reproductive competence, phase transition, and quantification of juvenility in mutant genetic screens. Frontiers in Plant Science 5, 10.3389/fpls.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doody E, Zha Y, He J, and Poethig RS (2022). The genetic basis of natural variation in the timing of vegetative phase change in Arabidopsis thaliana. Development 149. 10.1242/dev.200321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan GJ, Potts BM, and Wiltshire RJE (1999). Strong, independent, quantitative genetic control of the timing of vegetative phase change and first flowering in Eucalyptus globulus ssp globulus (Tasmanian Blue Gum). Heredity 83, 179–187. [DOI] [PubMed] [Google Scholar]
- 10.Renner MAM, Foster CSP, Miller JT, and Murphy DJ (2021). Phyllodes and bipinnate leaves of Acacia exhibit contemporary continental-scale environmental correlation and evolutionary transition-rate heterogeneity. Aust Sys Bot 34, 595–608. [Google Scholar]
- 11.Wiltshire RJE, Potts BM, and Reid JB (1998). Genetic control of reproductive and vegetative phase change in the Eucalyptus risdonii E-tenuiramis complex. Aust J Bot 46, 45–63. [Google Scholar]
- 12.Godley EJ (1985). Paths to maturity. New Zealand J. Bot 23, 687–706. [Google Scholar]
- 13.Freytes SN, Canelo M, and Cerdan PD (2021). Regulation of flowering time: when and where? Curr Opin Plant Biol 63, 102049. [DOI] [PubMed] [Google Scholar]
- 14.Hackett WP (1985). Juvenility, maturation and rejuvenation in woody plants. Hort. Rev 7, 109–155. [Google Scholar]
- 15.Horrell BA, Jameson PE, and Bannister PB (1990). Growth regulation and phase change in some New Zealand heteroblastic plants. New Zealand J Bot 28, 187–193. [Google Scholar]
- 16.Brink RA (1962). Phase change in higher plants and somatic cell heredity. Quart. Rev. Biol 37, 1–22. [Google Scholar]
- 17.Greenwood MS (1995). Juvenility and maturation in conifers: current concepts. Tree Physiol. 15, 433–438. [DOI] [PubMed] [Google Scholar]
- 18.Poethig RS (2013). Vegetative phase change and shoot maturation in plants. Curr Top Dev Biol 105, 125–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leichty AR, and Poethig RS (2019). Development and evolution of age-dependent defenses in ant-acacias. Proc Natl Acad Sci U S A 116, 15596–15601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bongard-Pierce DK, Evans MMS, and Poethig RS (1996). Heteroblastic features of leaf anatomy in maize and their genetic regulation. Intl J Plant Sci 157, 331–340. [Google Scholar]
- 21.Lawrence EH, Springer CJ, Helliker BR, and Poethig RS (2021). miR156-mediated changes in leaf composition lead to altered photosynthetic traits during vegetative phase change. New Phytol 231, 1008–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence EH, Springer CJ, Helliker BR, and Poethig RS (2022). The carbon economics of vegetative phase change. Plant Cell Environ 45, 1286–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abedon BG, and Tracy WF (1996). Corngrass1 of maize (Zea mays L) delays development of adult plant resistance to common rust (Puccinia sorghi Schw) and European corn borer (Ostrinia nubilalis hubner). J Hered 87, 219–223. [Google Scholar]
- 24.Chien JC, and Sussex IM (1996). Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by Gibberellins and photoperiod in Arabidopsis thaliana (L) Heynh. Plant Physiol 111, 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telfer A, Bollman KM, and Poethig RS (1997). Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development 124, 645–654. [DOI] [PubMed] [Google Scholar]
- 26.Willmann MR, and Poethig RS (2011). The effect of the floral repressor FLC on the timing and progression of vegetative phase change in Arabidopsis. Development 138, 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Usami T, Horiguchi G, Yano S, and Tsukaya H (2009). The more and smaller cells mutants of Arabidopsis thaliana identify novel roles for SQUAMOSA PROMOTER BINDING PROTEIN-LIKE genes in the control of heteroblasty. Development 136, 955–964. [DOI] [PubMed] [Google Scholar]
- 28.Candela H, Martinez-Laborda A, and Micol JL (1999). Venation pattern formation in Arabidopsis thaliana vegetative leaves. Dev Biol 205, 205–216. [DOI] [PubMed] [Google Scholar]
- 29.Steynen QJ, Bolokoski DA, and Schultz EA (2001). Alteration in flowering time causes accelerated or decelerated progression through Arabidopsis vegetative phases. Can J of Bot 79, 657–665. [Google Scholar]
- 30.Nguyen ST, Greaves T, and McCurdy DW (2017). Heteroblastic development of transfer cells Is controlled by the microRNA miR156/SPL module. Plant Physiol 173, 1676–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu L, Qi P, Peper A, Kong F, Yao Y, and Yang L (2023). Distinct function of SPL genes in age-related resistance in Arabidopsis. PLoS Pathog 19, e1011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao YB, Liu YQ, Chen DY, Chen FY, Fang X, Hong GJ, Wang LJ, Wang JW, and Chen XY (2017). Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat Commun 8, 13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould KS (1993). Leaf heteroblasty in Pseudopanax crassifolius: Functional significance of leaf morphology and anatomy. Ann. Bot 71, 61–70. [Google Scholar]
- 34.Day JS, Gould KS, and Jameson PE (1997). Vegetative architecture of Elaeocarpus hookerianus. Transition from juvenile to adult. Ann. Bot 79, 617–624. [Google Scholar]
- 35.James SA, and Bell DT (2001). Leaf morphological and anatomical characteristics of heteroblastic Eucalyptus globulus ssp globulus (Myrtaceae). Aust J Bot 49, 259–269. [Google Scholar]
- 36.Beydler B, Osadchuk K, Cheng CL, Manak JR, and Irish EE (2016). The juvenile phase of maize sees upregulation of stress-response genes and Is extended by exogenous jasmonic acid. Plant Physiol 171, 2648–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu M, Hu T, Zhao J, Park MY, Earley KW, Wu G, Yang L, and Poethig RS (2016). Developmental functions of miR156-regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes in Arabidopsis thaliana. PLoS Genet 12, e1006263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He J, Xu M, Willmann MR, McCormick K, Hu T, Yang L, Starker CG, Voytas DF, Meyers BC, and Poethig RS (2018). Threshold-dependent repression of SPL gene expression by miR156/miR157 controls vegetative phase change in Arabidopsis thaliana. PLoS Genet 14, e1007337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orkwiszewski JA, and Poethig RS (2000). Phase identity of the maize leaf is determined after leaf initiation. Proc Natl Acad Sci U S A 97, 10631–10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poethig RS (1988). Heterochronic mutations affecting shoot development in maize. Genetics 119, 959–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poethig RS (1988). A non-cell-autonomous mutation regulating juvenility in maize. Nature 336, 82–83. [Google Scholar]
- 42.Dudley M, and Poethig RS (1991). The effect of a heterochronic mutation, Teopod2, on the cell lineage of the maize shoot. Development 111, 733–739. [DOI] [PubMed] [Google Scholar]
- 43.Bassiri A, Irish EE, and Poethig RS (1992). Heterochronic effects of Teopod 2 on the growth and photosensitivity of the maize shoot. Plant Cell 4, 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudley M, and Poethig RS (1993). The heterochronic Teopod1 and Teopod2 mutations of maize are expressed non-cell-autonomously. Genetics 133, 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Evans MM, Passas HJ, and Poethig RS (1994). Heterochronic effects of glossy15 mutations on epidermal cell identity in maize. Development 120, 1971–1981. [DOI] [PubMed] [Google Scholar]
- 46.Evans MM, and Poethig RS (1995). Gibberellins promote vegetative phase change and reproductive maturity in maize. Plant Physiol 108, 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moose SP, and Sisco PH (1994). Glossy15 controls the epidermal juvenile-to-adult phase transition in maize. Plant Cell 6, 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moose SP, and Sisco PH (1996). Glossy15, an APETALA2-like gene from maize that regulates leaf epidermal cell identity. Genes Dev 10, 3018–3027. [DOI] [PubMed] [Google Scholar]
- 49.Bollman KM, Aukerman MJ, Park MY, Hunter C, Berardini TZ, and Poethig RS (2003). HASTY, the Arabidopsis ortholog of Exportin 5/MSN5, regulates phase change and morphogenesis. Development 130, 1493–1504. [DOI] [PubMed] [Google Scholar]
- 50.Berardini TZ, Bollman K, Sun H, and Poethig RS (2001). Regulation of vegetative phase change in Arabidopsis thaliana by cyclophilin 40. Science 291, 2405–2407. [DOI] [PubMed] [Google Scholar]
- 51.Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, and Poethig RS (2005). Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci U S A 102, 3691–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu G, and Poethig RS (2006). Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133, 3539–3547. 10.1242/dev.02521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu G, Park MY, Conway SR, Wang JW, Weigel D, and Poethig RS (2009). The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chuck G, Cigan AM, Saeteurn K, and Hake S (2007). The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nature Genetics 39, 544–549. [DOI] [PubMed] [Google Scholar]
- 55.Wang J-W, Park MY, Wang L-J, Koo Y, Chen X-Y, Weigel D, and Poethig RS (2011). MiRNA control of vegetative phase change in tees. Plos Genetics 7, e1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauer M, Zhao J, Park M, Khangura R, Dilkes BP, and Poethig RS (2023). Identification of the Teopod1, Teopod2, and Early Phase Change genes in maize. G3. 10.1093/g3journal/jkad179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Wang X, Wei J, Miao X, Shang X, and Li L (2023). Genetic mapping and functional analysis of a classical tassel branch number mutant Tp2 in maize. Front Plant Sci 14, 1183697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka N, Itoh H, Sentoku N, Kojima M, Sakakibara H, Izawa T, Itoh J, and Nagato Y (2011). The COP1 ortholog PPS regulates the juvenile-adult and vegetative-reproductive phase changes in rice. Plant Cell 23, 2143–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie K, Wu C, and Xiong L (2006). Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol 142, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawrence EH, Leichty AR, Doody EE, Ma C, Strauss SH, and Poethig RS (2021). Vegetative phase change in Populus tremula x alba. New Phytol 231, 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J, Doody E, and Poethig RS (2023). Reproductive competence is regulated independently of vegetative phase change in Arabidopsis thaliana. Curr Biol 33, 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lawrence-Paul EH, Poethig RS, and Lasky JR (2023). Vegetative phase change causes age-dependent changes in phenotypic plasticity. New Phytol 240, 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang L, Xu M, Koo Y, He J, and Poethig RS (2013). Sugar promotes vegetative phase change in Arabidopsis thaliana by repressing the expression of MIR156A and MIR156C. eLife 2, e00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu M, Hu T, Smith MR, and Poethig RS (2016). Epigenetic regulation of vegetative phase change in Arabidopsis. Plant Cell 28, 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fouracre JP, and Poethig RS (2019). Role for the shoot apical meristem in the specification of juvenile leaf identity in Arabidopsis. Proc Natl Acad Sci U S A 116, 10168–10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhogale S, Mahajan AS, Natarajan B, Rajabhoj M, Thulasiram HV, and Banerjee AK (2014). MicroRNA156: a potential graft-transmissible microRNA that modulates plant architecture and tuberization in Solanum tuberosum ssp. andigena. Plant Physiol 164, 1011–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao J, Zhang K, Cheng YJ, Yu S, Shang GD, Wang FX, Wu LY, Xu ZG, Mai YX, Zhao XY, et al. (2022). A robust mechanism for resetting juvenility during each generation in Arabidopsis. Nat Plants 8, 257–268. [DOI] [PubMed] [Google Scholar]
- 68.Hu T, Manuela D, Hinsch V, and Xu M (2022). PICKLE associates with HISTONE DEACETYLASE9 to mediate vegetative phase change in Arabidopsis. New Phytol 235, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, and Bartel DP (2002). Prediction of plant microRNA targets. Cell 110, 513–520. [DOI] [PubMed] [Google Scholar]
- 70.Gandikota M, Birkenbihl RP, Hohmann S, Cardon GH, Saedler H, and Huijser P (2007). The miRNA156/157 recognition element in the 3’ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J 49, 683–693. [DOI] [PubMed] [Google Scholar]
- 71.Ling LZ, and Zhang SD (2012). Exploring the evolutionary differences of SBP-box genes targeted by miR156 and miR529 in plants. Genetica 140, 317–324. [DOI] [PubMed] [Google Scholar]
- 72.Klein J, Saedler H, and Huijser P (1996). A new family of DNA binding proteins includes putative transcriptional regulators of the Antirrhinum majus floral meristem identity gene SQUAMOSA. Mol Gen Genet 250, 7–16. [DOI] [PubMed] [Google Scholar]
- 73.Cardon GH, Hohmann S, Nettesheim K, Saedler H, and Huijser P (1997). Functional analysis of the Arabidopsis thaliana SBP-box gene SPL3: a novel gene involved in the floral transition. Plant J 12, 367–377. [DOI] [PubMed] [Google Scholar]
- 74.Cardon G, Hohmann S, Klein J, Nettesheim K, Saedler H, and Huijser P (1999). Molecular characterisation of the Arabidopsis SBP-box genes. Gene 237, 91–104. [DOI] [PubMed] [Google Scholar]
- 75.Kropat J, Tottey S, Birkenbihl RP, Depege N, Huijser P, and Merchant S (2005). A regulator of nutritional copper signaling in Chlamydomonas is an SBP domain protein that recognizes the GTAC core of copper response element. Proc Natl Acad Sci U S A 102, 18730–18735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arazi T, Talmor-Neiman M, Stav R, Riese M, Huijser P, and Baulcombe DC (2005). Cloning and characterization of micro-RNAs from moss. Plant J 43, 837–848. [DOI] [PubMed] [Google Scholar]
- 77.Riese M, Hohmann S, Saedler H, Munster T, and Huijser P (2007). Comparative analysis of the SBP-box gene families in P. patens and seed plants. Gene 401, 28–37. [DOI] [PubMed] [Google Scholar]
- 78.Guo AY, Zhu QH, Gu X, Ge S, Yang J, and Luo J (2008). Genome-wide identification and evolutionary analysis of the plant specific SBP-box transcription factor family. Gene 418, 1–8. [DOI] [PubMed] [Google Scholar]
- 79.Preston JC, and Hileman LC (2013). Functional evolution in the plant SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) gene family. Front Plant Sci 4, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang SD, Ling LZ, and Yi TS (2015). Evolution and divergence of SBP-box genes in land plants. BMC Genomics 16, 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arribas-Hernandez L, Kielpinski LJ, and Brodersen P (2016). mRNA decay of most Arabidopsis miRNA targets requires slicer activity of AGO1. Plant Physiol 171, 2620–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ronemus M, Vaughn MW, and Martienssen RA (2006). MicroRNA-targeted and small interfering RNA-mediated mRNA degradation is regulated by Argonaute, Dicer, and RNA-dependent RNA polymerase in Arabidopsis. Plant Cell 18, 1559–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth L, and Voinnet O (2008). Widespread translational inhibition by plant miRNAs and siRNAs. Science 320, 1185–1190. [DOI] [PubMed] [Google Scholar]
- 84.Yang L, Wu G, and Poethig RS (2012). Mutations in the GW-repeat protein SUO reveal a developmental function for microRNA-mediated translational repression in Arabidopsis. Proc Natl Acad Sci U S A 109, 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fouracre JP, Chen VJ, and Poethig RS (2020). ALTERED MERISTEM PROGRAM1 regulates leaf identity independently of miR156-mediated translational repression. Development 147, 10.1242/dev.186874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmid M, Uhlenhaut NH, Godard F, Demar M, Bressan R, Weigel D, and Lohmann JU (2003). Dissection of floral induction pathways using global expression analysis. Development 130, 6001–6012. [DOI] [PubMed] [Google Scholar]
- 87.Jung JH, Seo YH, Seo PJ, Reyes JL, Yun J, Chua NH, and Park CM (2007). The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 19, 2736–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aukerman MJ, and Sakai H (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15, 2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lian H, Wang L, Ma N, Zhou CM, Han L, Zhang TQ, and Wang JW (2021). Redundant and specific roles of individual MIR172 genes in plant development. PLoS Biol 19, e3001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Maoileidigh DS, van Driel AD, Singh A, Sang Q, Le Bec N, Vincent C, de Olalla EBG, Vayssieres A, Romera Branchat M, Severing E, et al. (2021). Systematic analyses of the MIR172 family members of Arabidopsis define their distinct roles in regulation of APETALA2 during floral transition. PLoS Biol 19, e3001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu Y, Qian Z, Zhou B, and Wu G (2019). Age-dependent heteroblastic development of leaf hairs in Arabidopsis. New Phytol 224, 741–748. [DOI] [PubMed] [Google Scholar]
- 92.Wang L, Zhou CM, Mai YX, Li LZ, Gao J, Shang GD, Lian H, Han L, Zhang TQ, Tang HB, et al. (2019). A spatiotemporally regulated transcriptional complex underlies heteroblastic development of leaf hairs in Arabidopsis thaliana. EMBO J 38, 10.15252/embj.2018100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bond BJ (2000). Age-related changes in photosynthesis of woody plants. Trends Plant Sci 5, 349–353. [DOI] [PubMed] [Google Scholar]
- 94.Baumgarten L, Pieper B, Song B, Mane S, Lempe J, Lamb J, Cooke EL, Srivastava R, Strutt S, Zanko D, et al. (2023). Pan-European study of genotypes and phenotypes in the Arabidopsis relative Cardamine hirsuta reveals how adaptation, demography, and development shape diversity patterns. PLoS Biol 21, e3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu J, Cheng X, Liu P, and Sun J (2017). miR156-Targeted SBP-Box transcription factors Interact with DWARF53 to regulate TEOSINTE BRANCHED1 and BARREN STALK1 expression in bread wheat. Plant Physiol 174, 1931–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Preston JC, and Hileman LC (2010). SQUAMOSA-PROMOTER BINDING PROTEIN 1 initiates flowering in Antirrhinum majus through the activation of meristem identity genes. Plant J 62, 704–712. [DOI] [PubMed] [Google Scholar]
- 97.Feng S, Xu Y, Guo C, Zheng J, Zhou B, Zhang Y, Ding Y, Zhang L, Zhu Z, Wang H, and Wu G (2016). Modulation of miR156 to identify traits associated with vegetative phase change in tobacco (Nicotiana tabacum). J Exp Bot 67, 1493–1504. [DOI] [PubMed] [Google Scholar]
- 98.Aung B, Gruber MY, Amyot L, Omari K, Bertrand A, and Hannoufa A (2015). MicroRNA156 as a promising tool for alfalfa improvement. Plant Biotechnol J 13, 779–790. [DOI] [PubMed] [Google Scholar]
- 99.Johnson CR, Millwood RJ, Tang Y, Gou J, Sykes RW, Turner GB, Davis MF, Sang Y, Wang ZY, and Stewart CN Jr. (2017). Field-grown miR156 transgenic switchgrass reproduction, yield, global gene expression analysis, and bioconfinement. Biotechnol Biofuels 10, 255. 10.1186/s13068-017-0939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Preston JC, Jorgensen SA, Orozco R, and Hileman LC (2016). Paralogous SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes differentially regulate leaf initiation and reproductive phase change in petunia. Planta 243, 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, and Weigel D (2005). Specific effects of microRNAs on the plant transcriptome. Dev Cell 8, 517–527. [DOI] [PubMed] [Google Scholar]
- 102.Wang J-W, Schwab R, Czech B, Mica E, and Weigel D (2008). Dual effects of miR156-targeted SPL genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell 20, 1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu N, Niu QW, Ng KH, and Chua NH (2015). The role of miR156/SPLs modules in Arabidopsis lateral root development. Plant J 83, 673–685. [DOI] [PubMed] [Google Scholar]
- 104.Lawrence EH, Springer CJ, Helliker BR, and Poethig RS (2021). MicroRNA156-mediated changes in leaf composition lead to altered photosynthetic traits during vegetative phase change. New Phytol 231, 1008–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie K, Shen J, Hou X, Yao J, Li X, Xiao J, and Xiong L (2012). Gradual increase of miR156 regulates temporal expression changes of numerous genes during leaf development in rice. Plant Physiology 158, 1382–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang L, Sun S, Jin J, Fu D, Yang X, Weng X, Xu C, Li X, Xiao J, and Zhang Q (2015). Coordinated regulation of vegetative and reproductive branching in rice. Proc Natl Acad Sci U S A 112, 15504–15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang T, Wang J, and Zhou C (2015). The role of miR156 in developmental transitions in Nicotiana tabacum. Sci China Life Sci 58, 253–260. [DOI] [PubMed] [Google Scholar]
- 108.Fu C, Sunkar R, Zhou C, Shen H, Zhang JY, Matts J, Wolf J, Mann DG, Stewart CN Jr., Tang Y, and Wang ZY (2012). Overexpression of miR156 in switchgrass (Panicum virgatum L.) results in various morphological alterations and leads to improved biomass production. Plant Biotech J 10, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baxter HL, Mazarei M, Dumitrache A, Natzke JM, Rodriguez M Jr., Gou J, Fu C, Sykes RW, Turner GB, Davis MF, et al. (2018). Transgenic miR156 switchgrass in the field: growth, recalcitrance and rust susceptibility. Plant Biotechnol J 16, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang X, Zou Z, Zhang J, Zhang Y, Han Q, Hu T, Xu X, Liu H, Li H, and Ye Z (2011). Over-expression of sly-miR156a in tomato results in multiple vegetative and reproductive trait alterations and partial phenocopy of the sft mutant. FEBS Lett 585, 435–439. [DOI] [PubMed] [Google Scholar]
- 111.Ferreira e Silva GF, Silva EM, Azevedo Mda S, Guivin MA, Ramiro DA, Figueiredo CR, Carrer H, Peres LE, and Nogueira FT (2014). microRNA156-targeted SPL/SBP box transcription factors regulate tomato ovary and fruit development. Plant J 78, 604–618. [DOI] [PubMed] [Google Scholar]
- 112.Hackett WP, and Murray JR (1997). Approaches to understanding maturation or phase change. In Biotechnology of Ornamental Plants, Geneve RL, Preece JE, and Merkle SA, eds. (CAB International; ), pp. 73–86. [Google Scholar]
- 113.Hyun Y, Richter R, Vincent C, Martinez-Gallegos R, Porri A, and Coupland G (2016). Multi-layered regulation of SPL15 and cooperation with SOC1 integrate endogenous flowering pathways at the Arabidopsis shoot meristem. Dev Cell 37, 254–266. [DOI] [PubMed] [Google Scholar]
- 114.Hyun Y, Vincent C, Tilmes V, Bergonzi S, Kiefer C, Richter R, Martinez-Gallegos R, Severing E, and Coupland G (2019). A regulatory circuit conferring varied flowering response to cold in annual and perennial plants. Science 363, 409–412. [DOI] [PubMed] [Google Scholar]
- 115.Schwarz S, Grande AV, Bujdoso N, Saedler H, and Huijser P (2008). The microRNA regulated SBP-box genes SPL9 and SPL15 control shoot maturation in Arabidopsis. Plant Mol Biol 67, 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Padmanabhan MS, Ma S, Burch-Smith TM, Czymmek K, Huijser P, and Dinesh-Kumar SP (2013). Novel positive regulatory role for the SPL6 transcription factor in the N TIR-NB-LRR receptor-mediated plant innate immunity. PLoS Pathog 9, e1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamaguchi A, Wu M-F, Yang L, Wu G, Poethig RS, and Wagner D (2009). The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Developmental Cell 17, 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chuck G, Whipple C, Jackson D, and Hake S (2010). The maize SBP-box transcription factor encoded by tasselsheath4 regulates bract development and the establishment of meristem boundaries. Development 137, 1585–1585. [DOI] [PubMed] [Google Scholar]
- 119.Chuck GS, Brown PJ, Meeley R, and Hake S (2014). Maize SBP-box transcription factors unbranched2 and unbranched3 affect yield traits by regulating the rate of lateral primordia initiation. Proc Natl Acad Sci U S A 111, 18775–18780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, Matsuoka M, Kitano H, and Ashikari M (2010). OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet 42, 545–549. [DOI] [PubMed] [Google Scholar]
- 121.Jiao Y, Wang Y, Xue D, Wang J, Yan M, Liu G, Dong G, Zeng D, Lu Z, Zhu X, et al. (2010). Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet 42, 541–544. [DOI] [PubMed] [Google Scholar]
- 122.Li M, Li X, Zhou Z, Wu P, Fang M, Pan X, Lin Q, Luo W, Wu G, and Li H (2016). Reassessment of the four yield-related genes Gn1a, DEP1, GS3, and IPA1 in rice using a CRISPR/Cas9 System. Front Plant Sci 7, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Song XG, Lu ZF, Yu H, Shao GN, Xiong JS, Meng XB, Jing YH, Liu GF, Xiong GS, Duan JB, et al. (2017). IPA1 functions as a downstream transcription factor repressed by D53 in strigolactone signaling in rice. Cell Research 27, 1128–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Studer AJ, Wang H, and Doebley JF (2017). Selection during maize domestication targeted a gene network controlling plant and inflorescence architecture. Genetics 207, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang H, Nussbaum-Wagler T, Li B, Zhao Q, Vigouroux Y, Faller M, Bomblies K, Lukens L, and Doebley JF (2005). The origin of the naked grains of maize. Nature 436, 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Manning K, Tor M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, and Seymour GB (2006). A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38, 948–952. [DOI] [PubMed] [Google Scholar]
- 127.Chen W, Kong J, Lai T, Manning K, Wu C, Wang Y, Qin C, Li B, Yu Z, Zhang X, et al. (2015). Tuning LeSPL-CNR expression by SlymiR157 affects tomato fruit ripening. Sci Rep 5, 7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rubio-Somoza I, Zhou CM, Confraria A, Martinho C, von Born P, Baena-Gonzalez E, Wang JW, and Weigel D (2014). Temporal control of leaf complexity by miRNA-regulated licensing of protein complexes. Curr Biol 24, 2714–2719. [DOI] [PubMed] [Google Scholar]
- 129.Hu T, Manuela D, and Xu M (2023). SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 9 and 13 repress BLADE-ON-PETIOLE 1 and 2 directly to promote adult leaf morphology in Arabidopsis. J Exp Bot 74, 1926–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tang HB, Wang J, Wang L, Shang GD, Xu ZG, Mai YX, Liu YT, Zhang TQ, and Wang JW (2023). Anisotropic cell growth at the leaf base promotes age-related changes in leaf shape in Arabidopsis thaliana. Plant Cell 35, 1386–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Toriba T, Tokunaga H, Shiga T, Nie F, Naramoto S, Honda E, Tanaka K, Taji T, Itoh JI, and Kyozuka J (2019). BLADE-ON-PETIOLE genes temporally and developmentally regulate the sheath to blade ratio of rice leaves. Nat Commun 10, 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J-W, Czech B, and Weigel D (2009). miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138, 738–749. [DOI] [PubMed] [Google Scholar]
- 133.Lu Z, Yu H, Xiong G, Wang J, Jiao Y, Liu G, Jing Y, Meng X, Hu X, Qian Q, et al. (2013). Genome-wide binding analysis of the transcription activator ideal plant architecture1 reveals a complex network regulating rice plant architecture. Plant Cell 25, 3743–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen X, Ding AB, and Zhong X (2020). Functions and mechanisms of plant histone deacetylases. Sci China Life Sci 63, 206–216. [DOI] [PubMed] [Google Scholar]
- 135.Baile F, Gomez-Zambrano A, and Calonje M (2022). Roles of Polycomb complexes in regulating gene expression and chromatin structure in plants. Plant Commun 3, 100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pico S, Ortiz-Marchena MI, Merini W, and Calonje M (2015). Deciphering the role of POLYCOMB REPRESSIVE COMPLEX1 variants in regulating the acquisition of flowering competence in Arabidopsis. Plant Physiol 168, 1286–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fouracre JP, He J, Chen VJ, Sidoli S, and Poethig RS (2021). VAL genes regulate vegetative phase change via miR156-dependent and independent mechanisms. PLoS Genet 17, e1009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cui Y, Cheng J, Ruan S, Qi P, Liu W, Bian H, Ye L, Zhang Y, Hu J, and Dong G (2020). The heterochronic gene Oryza sativa LIKE HETEROCHROMATIN PROTEIN 1 modulates miR156b/c/i/e levels. J Int Plant Biol 62, 1839–1852. [DOI] [PubMed] [Google Scholar]
- 139.Cheng YJ, Shang GD, Xu ZG, Yu S, Wu LY, Zhai D, Tian SL, Gao J, Wang L, and Wang JW (2021). Cell division in the shoot apical meristem is a trigger for miR156 decline and vegetative phase transition in Arabidopsis. Proc Natl Acad Sci U S A 118. 10.1073/pnas.2115667118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reynolds N, Salmon-Divon M, Dvinge H, Hynes-Allen A, Balasooriya G, Leaford D, Behrens A, Bertone P, and Hendrich B (2012). NuRD-mediated deacetylation of H3K27 facilitates recruitment of Polycomb Repressive Complex 2 to direct gene repression. EMBO J 31, 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ho KK, Zhang H, Golden BL, and Ogas J (2013). PICKLE is a CHD subfamily II ATP-dependent chromatin remodeling factor. Biochim Biophys Acta 1829, 199–210. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu Y, Guo C, Zhou B, Li C, Wang H, Zheng B, Ding H, Zhu Z, Peragine A, Cui Y, et al. (2016). Regulation of vegetative phase change by SWI2/SNF2 chromatin remodeling ATPase BRAHMA. Plant Physiol 172, 2416–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Choi K, Kim J, Muller SY, Oh M, Underwood C, Henderson I, and Lee I (2016). Regulation of microRNA-mediated developmental changes by the SWR1 chromatin remodeling complex. Plant Physiol 171, 1128–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu M, Leichty AR, Hu T, and Poethig RS (2018). H2A.Z promotes the transcription of MIR156A and MIR156C in Arabidopsis by facilitating the deposition of H3K4me3. Development 145, 10.1242/dev.152868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Watson JM, Platzer A, Kazda A, Akimcheva S, Valuchova S, Nizhynska V, Nordborg M, and Riha K (2016). Germline replications and somatic mutation accumulation are independent of vegetative life span in Arabidopsis. Proc Natl Acad Sci U S A 113, 12226–12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Serivichyaswat P, Ryu HS, Kim W, Kim S, Chung KS, Kim JJ, and Ahn JH (2015). Expression of the floral repressor miRNA156 is positively regulated by the AGAMOUS-like proteins AGL15 and AGL18. Mol Cells 38, 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo C, Xu Y, Shi M, Lai Y, Wu X, Wang H, Zhu Z, Poethig RS, and Wu G (2017). Repression of miR156 by miR159 regulates the timing of the juvenile-to-adult transition in Arabidopsis. Plant Cell 29, 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wei Q, Ma C, Xu Y, Wang T, Chen Y, Lu J, Zhang L, Jiang CZ, Hong B, and Gao J (2017). Control of Chrysanthemum flowering through integration with an aging pathway. Nat Commun 8, 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao H, Lin K, Ma L, Chen Q, Gan S, and Li G (2020). Arabidopsis NUCLEAR FACTOR Y A8 inhibits the juvenile-to-adult transition by activating transcription of MIR156s. J Exp Bot 71, 4890–4902. 1 [DOI] [PubMed] [Google Scholar]
- 150.Tian R, Wang F, Zheng Q, Niza V, Downie AB, and Perry SE (2020). Direct and indirect targets of the Arabidopsis seed transcription factor ABSCISIC ACID INSENSITIVE3. Plant J 103, 1679–1694. [DOI] [PubMed] [Google Scholar]
- 151.Wang F, and Perry SE (2013). Identification of direct targets of FUSCA3, a key regulator of Arabidopsis seed development. Plant Physiol 161, 1251–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Laskowski MJ, Tiley HC, Fang Y, Epstein A, Fu Y, Ramos R, Drummond TJ, Heidstra R, Bhakhri P, Baskin TI, and Leyser O (2022). The miR156 juvenility factor and PLETHORA 2 form a regulatory network and influence timing of meristem growth and lateral root emergence. Development 149, 10.1242/dev.199871. [DOI] [PubMed] [Google Scholar]
- 153.Lal S, Pacis LB, and Smith HM (2011). Regulation of the SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE genes/microRNA156 module by the homeodomain proteins PENNYWISE and POUND-FOOLISH in Arabidopsis. Mol Plant 4, 1123–1132. [DOI] [PubMed] [Google Scholar]
- 154.Stewart Gillmor C, Silva-Ortega CO, Willmann MR, Buendia-Monreal M, and Poethig RS (2014). The Arabidopsis Mediator CDK8 module genes CCT (MED12) and GCT (MED13) are global regulators of developmental phase transitions. Development 141, 4580–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Buendia-Monreal M, and Gillmor CS (2017). Convergent repression of miR156 by sugar and the CDK8 module of Arabidopsis Mediator. Dev Biol 423, 19–23. [DOI] [PubMed] [Google Scholar]
- 156.Walla A, Wilma van Esse G, Kirschner GK, Guo G, Brunje A, Finkemeier I, Simon R, and von Korff M (2020). An Acyl-CoA N-Acyltransferase regulates meristem phase change and plant architecture in barley. Plant Physiol 183, 1088–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lauressergues D, Ormancey M, Guillotin B, San Clemente H, Camborde L, Duboe C, Tourneur S, Charpentier P, Barozet A, Jauneau A, et al. (2022). Characterization of plant microRNA-encoded peptides (miPEPs) reveals molecular mechanisms from the translation to activity and specificity. Cell Rep 38, 110339. [DOI] [PubMed] [Google Scholar]
- 158.Lauressergues D, Couzigou JM, Clemente HS, Martinez Y, Dunand C, Becard G, and Combier JP (2015). Primary transcripts of microRNAs encode regulatory peptides. Nature 520, 90–93. [DOI] [PubMed] [Google Scholar]
- 159.Erokhina TN, Ryazantsev DY, Samokhvalova LV, Mozhaev AA, Orsa AN, Zavriev SK, and Morozov SY (2021). Activity of chemically synthesized peptide encoded by the miR156A precursor and conserved in the Brassicaceae family plants. Biochemistry (Mosc) 86, 551–562. [DOI] [PubMed] [Google Scholar]
- 160.Sussex I (1964). The permanence of meristems: Developmental organizers or reactors to exogenous stimuli? In Meristems and Differentiation, Brookhaven Symposia in Biology, (Brookhaven National Laboratory; ), pp. 1–12. [Google Scholar]
- 161.Fouracre JP, and Poethig RS (2020). Lonely at the top? Regulation of shoot apical meristem activity by intrinsic and extrinsic factors. Curr Opin Plant Biol 58, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wardlaw CW (1965). The morphogenetic rôle of apical meristems: fundamental aspects (illustrated by means of the shoot apical meristem). In Differentiation and Development / Differenzierung und Entwicklung, Ruhland W, ed. (Springer; ), pp. 443–451. [Google Scholar]
- 163.Irish EE, and Karlen S (1998). Restoration of juvenility in maize shoots by meristem culture. Intl. J. Plant Sci 159, 695–701. [Google Scholar]
- 164.Yang L, Conway SR, and Poethig RS (2011). Vegetative phase change is mediated by a leaf-derived signal that represses the transcription of miR156. Development 138, 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yu S, Cao L, Zhou CM, Zhang TQ, Lian H, Sun Y, Wu J, Huang J, Wang G, and Wang JW (2013). Sugar is an endogenous cue for juvenile-to-adult phase transition in plants. eLife 2, e00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Werner S, Bartrina I, and Schmulling T (2021). Cytokinin regulates vegetative phase change in Arabidopsis thaliana through the miR172/TOE1-TOE2 module. Nat Commun 12, 5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hamada S, Onouchi H, Tanaka H, Kudo M, Liu YG, Shibata D, MacHida C, and Machida Y (2000). Mutations in the WUSCHEL gene of Arabidopsis thaliana result in the development of shoots without juvenile leaves. Plant J 24, 91–101. [DOI] [PubMed] [Google Scholar]
- 168.Xu M, Hu T, and Poethig RS (2021). Low light intensity delays vegetative phase change. Plant Physiol 187, 1177–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, and Schmid M (2013). Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339, 704–707. [DOI] [PubMed] [Google Scholar]
- 170.Ponnu J, Schlereth A, Zacharaki V, Dzialo MA, Abel C, Feil R, Schmid M, and Wahl V (2020). The trehalose 6-phosphate pathway impacts vegetative phase change in Arabidopsis thaliana. Plant J 104, 768–780. [DOI] [PubMed] [Google Scholar]
- 171.Zacharaki V, Ponnu J, Crepin N, Langenecker T, Hagmann J, Skorzinski N, Musialak-Lange M, Wahl V, Rolland F, and Schmid M (2022). Impaired KIN10 function restores developmental defects in the Arabidopsis trehalose 6-phosphate synthase1 (tps1) mutant. New Phytol 235, 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ye R, Wang M, Du H, Chhajed S, Koh J, Liu KH, Shin J, Wu Y, Shi L, Xu L, et al. (2022). Glucose-driven TOR-FIE-PRC2 signalling controls plant development. Nature 609, 986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zimmerman RH, Hackett WP, and Pharis RP (1985). Hormonal aspects of phase change and precocious flowering. In Hormonal Regulation of Development III. Encyclopedia of Plant Physiology,, Pharis RP, and Reid DM, eds. (Springer; ), pp. 79–115. [Google Scholar]
- 174.Yu S, Galvao VC, Zhang YC, Horrer D, Zhang TQ, Hao YH, Feng YQ, Wang S, Schmid M, and Wang JW (2012). Gibberellin regulates the Arabidopsis floral transition through miR156-targeted SQUAMOSA promoter binding-like transcription factors. Plant Cell 24, 3320–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park J, Oh DH, Dassanayake M, Nguyen KT, Ogas J, Choi G, and Sun TP (2017). Gibberellin signaling requires chromatin remodeler PICKLE to promote vegetative growth and phase transitions. Plant Physiol 173, 1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yamaguchi N, Winter CM, Wu MF, Kanno Y, Yamaguchi A, Seo M, and Wagner D (2014). Gibberellin acts positively then negatively to control onset of flower formation in Arabidopsis. Science 344, 638–641. [DOI] [PubMed] [Google Scholar]
- 177.Hibara K, Isono M, Mimura M, Sentoku N, Kojima M, Sakakibara H, Kitomi Y, Yoshikawa T, Itoh J, and Nagato Y (2016). Jasmonate regulates juvenile-to-adult phase transition in rice. Development 143, 3407–3416. [DOI] [PubMed] [Google Scholar]
- 178.Zhou B, Luo Q, Shen Y, Wei L, Song X, Liao H, Ni L, Shen T, Du X, Han J, et al. (2023). Coordinated regulation of vegetative phase change by brassinosteroids and the age pathway in Arabidopsis. Nat Commun 14, 2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang L, Yu P, Lyu J, Hu Y, Han C, Bai MY, and Fan M (2021). BZR1 physically interacts with SPL9 to regulate the vegetative phase change and cell elongation in Arabidopsis. Int J Mol Sci 22, 10.3390/ijms221910415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xie Y, Liu Y, Ma M, Zhou Q, Zhao Y, Zhao B, Wang B, Wei H, and Wang H (2020). Arabidopsis FHY3 and FAR1 integrate light and strigolactone signaling to regulate branching. Nat Commun 11, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dong H, Yan S, Jing Y, Yang R, Zhang Y, Zhou Y, Zhu Y, and Sun J (2021). MIR156-Targeted SPL9 Is phosphorylated by SnRK2s and interacts with ABI5 to enhance ABA responses in Arabidopsis. Front Plant Sci 12, 708573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang TQ, Lian H, Tang H, Dolezal K, Zhou CM, Yu S, Chen JH, Chen Q, Liu H, Ljung K, and Wang JW (2015). An intrinsic microRNA timer regulates progressive decline in shoot regenerative capacity in plants. Plant Cell 27, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cameron RJ (1970). Light intensity and the growth of Eucalyptus seedlings. Aust. J. Bot 18, 29–43. [Google Scholar]
- 184.Valladares F, and Niinemets U (2008). Shade tolerance, a key plant feature of complex nature and consequences. Ann Rev Ecol Syst 39, 237–257. [Google Scholar]
- 185.Hsieh LC, Lin SI, Shih AC, Chen JW, Lin WY, Tseng CY, Li WH, and Chiou TJ (2009). Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 151, 2120–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Xie Y, Liu Y, Wang H, Ma X, Wang B, Wu G, and Wang H (2017). Phytochrome-interacting factors directly suppress MIR156 expression to enhance shade-avoidance syndrome in Arabidopsis. Nat Commun 8, 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang L, He G, Li Y, Yang Z, Liu T, Xie X, Kong X, and Sun J (2022). PIL transcription factors directly interact with SPLs and repress tillering/branching in plants. New Phytol 233, 1414–1425. [DOI] [PubMed] [Google Scholar]
- 188.Xie Y, Zhou Q, Zhao Y, Li Q, Liu Y, Ma M, Wang B, Shen R, Zheng Z, and Wang H (2020). FHY3 and FAR1 integrate light signals with the miR156-SPL module-mediated aging pathway to regulate Arabidopsis Ffowering. Mol Plant 13, 483–498. [DOI] [PubMed] [Google Scholar]
- 189.Dotto M, Gomez MS, Soto MS, and Casati P (2018). UV-B radiation delays flowering time through changes in the PRC2 complex activity and miR156 levels in Arabidopsis thaliana. Plant Cell Environ 41, 1394–1406. [DOI] [PubMed] [Google Scholar]
- 190.Lee H, Yoo SJ, Lee JH, Kim W, Yoo SK, Fitzgerald H, Carrington JC, and Ahn JH (2010). Genetic framework for flowering-time regulation by ambient temperature-responsive miRNAs in Arabidopsis. Nucleic Acids Res 38, 3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kim JJ, Lee JH, Kim W, Jung HS, Huijser P, and Ahn JH (2012). The microRNA156-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3 module regulates ambient temperature-responsive flowering via FLOWERING LOCUS T in Arabidopsis. Plant Physiol 159, 461–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim W, Kim HE, Jun AR, Jung MG, Jin S, Lee JH, and Ahn JH (2016). Structural determinants of miR156a precursor processing in temperature-responsive flowering in Arabidopsis. J Exp Bot 67, 4659–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stief A, Altmann S, Hoffmann K, Pant BD, Scheible WR, and Baurle I (2014). Arabidopsis miR156 regulates tolerance to recurring environmental stress through SPL transcription factors. Plant Cell 26, 1792–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cui LG, Shan JX, Shi M, Gao JP, and Lin HX (2014). The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J 80, 1108–1117. [DOI] [PubMed] [Google Scholar]
- 195.Sang Q, Fan L, Liu T, Qiu Y, Du J, Mo B, Chen M, and Chen X (2023). MicroRNA156 conditions auxin sensitivity to enable growth plasticity in response to environmental changes in Arabidopsis. Nat Commun 14, 1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rahimi A, Karami O, Balazadeh S, and Offringa R (2022). miR156-independent repression of the ageing pathway by longevity-promoting AHL proteins in Arabidopsis. New Phytol 235, 2424–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li J, Wang Z, Hu Y, Cao Y, and Ma L (2017). Polycomb Group Proteins RING1A and RING1B regulate the vegetative phase transition in Arabidopsis. Front Plant Sci 8, 867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kim JY, Oh JE, Noh YS, and Noh B (2015). Epigenetic control of juvenile-to-adult phase transition by the Arabidopsis SAGA-like complex. Plant J 83, 537–545. [DOI] [PubMed] [Google Scholar]
- 199.Wang J, Yu H, Xiong G, Lu Z, Jiao Y, Meng X, Liu G, Chen X, Wang Y, and Li J (2017). Tissue-specific ubiquitination by IPA1 INTERACTING PROTEIN1 modulates IPA1 protein levels to regulate plant architecture in rice. Plant Cell 29, 697–707. 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang J, Zhou L, Shi H, Chern M, Yu H, Yi H, He M, Yin J, Zhu X, Li Y, et al. (2018). A single transcription factor promotes both yield and immunity in rice. Science 361, 1026–1028. [DOI] [PubMed] [Google Scholar]
- 201.Li RJ, Li LM, Liu XL, Kim JC, Jenks MA, and Lu S (2019). Diurnal regulation of plant epidermal wax synthesis through antagonistic roles of the transcription factors SPL9 and DEWAX. Plant Cell 31, 2711–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gou JY, Felippes FF, Liu CJ, Weigel D, and Wang JW (2011). Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 23, 1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Molnar A, Schwach F, Studholme DJ, Thuenemann EC, and Baulcombe DC (2007). miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature 447, 1126–1129. [DOI] [PubMed] [Google Scholar]
- 204.Bowman JL (2022). The liverwort Marchantia polymorpha, a model for all ages. Curr Top Dev Biol 147, 1–32. [DOI] [PubMed] [Google Scholar]
- 205.Rensing SA, Goffinet B, Meyberg R, Wu SZ, and Bezanilla M (2020). The moss Physcomitrium (Physcomitrella) patens: a model organism for non-seed plants. Plant Cell 32, 1361–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mishler BD (1986). Ontogeny and phylogeny in Tortula (Musci, Pottiaceae). Syst Bot 11, 189–208. [Google Scholar]
- 207.Alonso-Peral MM, Sun C, and Millar AA (2012). MicroRNA159 can act as a switch or tuning microRNA independently of its abundance in Arabidopsis. PLoS One 7, e34751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cho SH, Coruh C, and Axtell MJ (2012). miR156 and miR390 regulate tasiRNA accumulation and developmental timing in Physcomitrella patens. Plant Cell 24, 4837–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tsuzuki M, Nishihama R, Ishizaki K, Kurihara Y, Matsui M, Bowman JL, Kohchi T, Hamada T, and Watanabe Y (2016). Profiling and characterization of small RNAs in the liverwort, Marchantia polymorpha, belonging to the first diverged land plants. Plant Cell Physiol 57, 359–372. [DOI] [PubMed] [Google Scholar]
- 210.Tsuzuki M, Futagami K, Shimamura M, Inoue C, Kunimoto K, Oogami T, Tomita Y, Inoue K, Kohchi T, Yamaoka S, et al. (2019). An early arising role of the microRNA156/529-SPL module in reproductive development revealed by the liverwort Marchantia polymorpha. Curr Biol 29, 3307–3314 [DOI] [PubMed] [Google Scholar]
- 211.Streubel S, Deiber S, Rotzer J, Mosiolek M, Jandrasits K , and Dolan L (2023). Meristem dormancy in Marchantia polymorpha is regulated by a liverwort-specific miRNA and a clade III SPL gene. Curr Biol 33, 660–674 [DOI] [PubMed] [Google Scholar]
- 212.Fouracre JP and Harrison CJ (2022) How was apical growth regulated in the ancestral land plant? Insights from the development of non-seed plants. Plant Physiol. 190, 100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hudson CJ, Freeman JS, Jones RC, Potts BM, Wong MM, Weller JL, Hecht VF, Poethig RS, and Vaillancourt RE (2014). Genetic control of heterochrony in Eucalyptus globulus. G3 4, 1235–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]


