Abstract
Microporous annealed particle (MAP) hydrogels are an attractive platform for engineering biomaterials with controlled heterogeneity. Here, we introduce a microfluidic method to create physicochemical gradients within poly(ethylene glycol) based MAP hydrogels. By combining microfluidic mixing and droplet generator modules, microgels with varying properties were produced by adjusting the relative flow rates between two precursor solutions and collected layer-by-layer in a syringe. Subsequently, the microgels were injected out of the syringe and then annealed with thiol-ene click chemistry. Fluorescence intensity measurements of constructs annealed in vitro and after mock implantation into a tissue defect showed that a continuous gradient profile was achieved and maintained after injection, indicating utility for in situ hydrogel formation. The effects of physicochemical property gradients on human mesenchymal stem cells (hMSCs) were also studied. Microgel stiffness was studied first, and the hMSCs exhibited increased spreading and proliferation as stiffness increased along the gradient. Microgel degradability was also studied, revealing a critical degradability threshold above which the hMSCs spread robustly and below which they were isolated and exhibited reduced spreading. This method of generating spatial gradients in MAP hydrogels could be further used to gain new insights into cell-material interactions, which could be leveraged for tissue engineering applications.
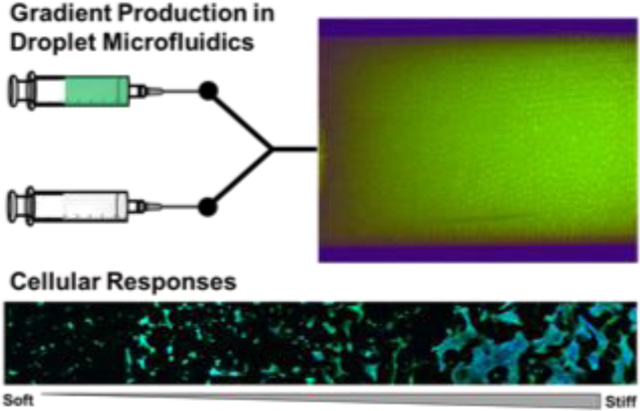
A new droplet microfluidic approach to obtain microporous annealed particle hydrogels with physicochemical gradients is presented. Gradient formation is achieved by precisely controlling the mixing of two precursor solutions, and the gradient can be maintained after injection. This approach can be leveraged to produce new materials for tissue repair and to gain unique insights on cell-material interactions.
Keywords: hydrogels, microgels, gradient, microfluidics, cell-material interactions
Graphical Abstract
1. Introduction
The extracellular matrix (ECM) consists of hundreds of proteins and glycoproteins and presents a complex milieu of biochemical and physical cues that guide cellular activities during tissue development and regeneration.[1] Developing biomaterials that mimic the ECM is of great interest in the tissue engineering field in order to better understand cell-material interactions and orchestrate regeneration and repair. Hydrogels are considered to be ECM mimetic and have been widely utilized as injectable biomaterial systems for stem cell delivery.[2] However, while their bulk physicochemical properties can be engineered to regulate cellular responses, most injectable hydrogel platforms are crosslinked in situ directly from homogeneous precursor solutions. Recent advances in 3D printing and photolithography have made it possible to manufacture hydrogels with spatially varying physicochemical properties.[3] However, hydrogels manufactured through these techniques lack injectability since chemical crosslinking is required to prevent mixing of the heterogeneous regions and, thus, lock in the spatial patterning.[4]
Recently, assembling individual microgels into 3D tissue engineering scaffolds, termed as microporous annealed particle (MAP) hydrogels, has emerged as a promising new approach to fabricating hydrogels for tissue engineering. Injectability is one important feature of MAP hydrogels since the microgels can be injected and secondary crosslinking can take place in situ.[5] In addition, MAP hydrogels inherently possess a highly interconnected microporous structure, which permits enhanced cell spreading and proliferation compared to conventional nanoporous hydrogels.[5,a] This approach is also uniquely suited to form hydrogel scaffolds with heterogeneous properties, as the modular nature of the microgel assembly process allows the incorporation of multiple microgel formulations within a single construct.[6] For example, Mealy et al. recently demonstrated the assembly of two distinct microgels with random distribution through cyclodextrin and adamantane guest–host interactions and achieved multiplexing of molecule release and degradation profiles of the MAP hydrogels.[7] They also showed that the materials could be injected into rat myocardial infarcts due to the microscale size of the microgels and shear-thinning behavior. In addition, Darling et al. described zone patterning of microgels distinguished by different fluorescent labeling and showed that these microgel divisions could be largely maintained after injection into murine models of wound healing and stroke.[8] However, regeneration often involves more complex presentations of microenvironmental cues, such as physicochemical gradients.[9] While the zone patterning approach can in theory be used to manually assemble different batches of microgels with varying physicochemical properties, the resulting gradients would be coarse and discrete in nature. Therefore, an approach to fabricate continuous gradients in MAP hydrogels in a precise and controllable fashion is highly desired.
Producing MAP hydrogels with physicochemical gradients is non-trivial and presents two significant technical challenges. The first challenge is the requirement for a variable precursor solution input to allow the fabrication of microgels with varying properties over time. This requirement eliminates microgel synthesis methods such as electrospraying and emulsification, which produce batches of microgels with homogenous properties.[10] Alternatively, microfluidic techniques, which are often used to produce microgel building blocks for MAP hydrogels,[5,a, 6–7, 11] are suitable as they can generate droplets in a simple microfluidic channel one at a time and precursor solutions can be mixed at varying ratios within the microfluidic channel without manual pipetting steps.[12] While microfluidic techniques have previously been used to generate gradients in hydrogel slabs,[13] microfluidics has not been previously applied to the production of gradients in MAP hydrogels due to the second technical challenge, which is the requirement to spatially localize the microgels to desired regions after their synthesis.
Herein we report a method to create gradients in MAP hydrogels using a microfluidic droplet generator equipped with a microfluidic mixing module, thiol-ene click chemistry, and layered packing into a collection device. Importantly, the microfluidic system permitted mixing of two distinct precursor solutions at programmed ratios to precisely modulate the composition of the microgels over time, whereas the collection and packing strategy maintained their positions until annealing could be performed. Initial experiments were performed using fluorophore-labelled microgels, and the effects of layer thickness on gradient patterning was studied. Subsequently, the extrusion of the microgels and maintenance of the gradient after annealing via secondary thiol-ene photopolymerization was studied in vitro and in a mock implantation study. Lastly, MAP hydrogel scaffolds with gradients in microgel stiffness and degradability were produced to study cell-material interactions with human mesenchymal stem cells (hMSCs).
2. Results and Discussion
2.1. Microgel production via microfluidics
A microfluidic droplet generator with a mixing module that is capable of altering mixing ratios of precursor solution was used to generate the microgel building blocks for the MAP hydrogels (Figure 1a). The Y-shaped microfluidic mixing module had two inlets through which two different gel precursor solutions with different properties were flown at varying flow rate ratios, which were controlled by programming the flow rates of the two solutions over time through two independently controllable syringe pumps. By changing the flow rate ratios, a stream of mixed solutions having different properties could be generated and the total flow rate kept constant. The microgel solutions composed of the two different precursor solutions were flown into a T-junction droplet generator module where the mixed precursor solutions were pinched off by the continuous oil phase to generate discrete water-in-oil emulsion droplets. A winding channel created chaotic advection within the droplets to accelerate the mixing of gel solutions within the droplets.[14] The droplets were then flown into a tubing and photopolymerized downstream, followed by collecting the polymerized microgels layer-by-layer in a syringe, which could then be dispensed as needed.
Figure 1.
Microgel synthesis using a microfluidic device with a Y-shaped mixing module and a T-junction droplet generator module. a) Schematic of the microfluidic device-based microgel production procedure. Right side photograph showing examples of microgel patterning using this method. The scale bar is 5 mm. b) Fluorescent images of non-fluorescent and fluorescent gel solutions merging together with varying ratios in the Y-shaped mixing module. c) Fluorescent images showing that the two gel solutions were mixed completely within 12 seconds after droplet formation. d) Size distribution of microgels synthesized from 5 kDa PEG-norbornene and PEG-dithiol.
The microgel precursor solutions contained four-arm poly(ethylene glycol) (PEG)—norbornene, bi-functional thiol crosslinker, photoinitiator, and mono-thiol cell-adhesive peptide ligand. Thus, the microgels were synthesized via thiol-ene click chemistry. This synthetic approach enables facile tuning of the physicochemical properties of the resultant microgels simply by adjusting the specific composition of the precursor solution, which was exploited for various gradient designs (Table S1, Supporting Information). Additional advantages of thiol-norbornene click chemistry are its oxygen insensitivity, which is important because oxygen inhibition can be a challenge in microfluidics,[15] fast reaction kinetics to achieve crosslinking in the outlet tubing,[16] and the step-growth nature of the reaction. The latter feature was leveraged to ensure that the microgels would contain unreacted norbornene groups and could be annealed with a bis-thiol linker and a secondary thiol-ene photopolymerization after gel particle generation, similar to our previous work.[10,a] Compared to other microgel assembly chemistries, the use of thiol-ene photopolymerization for annealing here is important because the reaction can be spatiotemporally controlled to prevent premature assembly of the packed microgels and, thus, maintain their injectability.[17] Thiol-ene chemistry also provides cytocompatible conditions for cell incorporation.[10,a]
We first characterized the droplet generation from the mixture of two precursor solutions by using fluorescein-containing (FITC gel, 100 μM) and fluorophore-free precursor solutions (blank gel) (Figure 1b and Video S1, Supporting Information). By keeping the total flow rate of those solutions constant, varying the relative flow rates of these two gel solutions resulted in different FITC concentrations in the droplets, and the flow rates were programmed so that alternating layers of packed microgels (alternating FITC-containing and non-fluorescent microgel layers) were generated within the collection syringe (Figure 1a, right image). Complete mixing of the two solutions within the droplets was achieved within 12.5 seconds due to accelerated diffusion in the winding channel (Figure 1c), which was critical to achieving uniform physicochemical properties within a single microgel droplet. Crosslinked microgels were monodispersed with an average diameter of 355 ± 9 μm (CV = 2.5 %) (Figure 1d). If different microgel sizes are desired for specific applications, the dimensions of the microchannel can be simply modified to obtain different microgel sizes as needed.
Fluorinated oil with non-ionic surfactant was used as the continuous oil phase. This aspect is notable since the oil must be removed after microgel preparation for biomedical applications, but microgel washing often requires rigorous agitation, which would disturb the microgel packing and patterning. However, fluorinated oil is a desirable choice in this application due to its high volatility, as it can be removed without washing steps. Previous work utilized the volatility of fluorinated oil to achieve hexagonal packing of microfluidic hydrogel particles.[18] Here, we show that this oil can be completely removed by evaporation at room temperature (Figure S1, Supporting Information) and, thus, the patterned microgel array was not disturbed during the oil removal process. In addition, fluorinated oil and non-ionic surfactant systems have exhibited the best biocompatibility to date in droplet microfluidics.[19] Therefore, this system also provides opportunities to form cell-laden microgel arrays, which could be leveraged for patterning of different cell types in other future applications.
2.2. Microgel patterning and gradient profiles
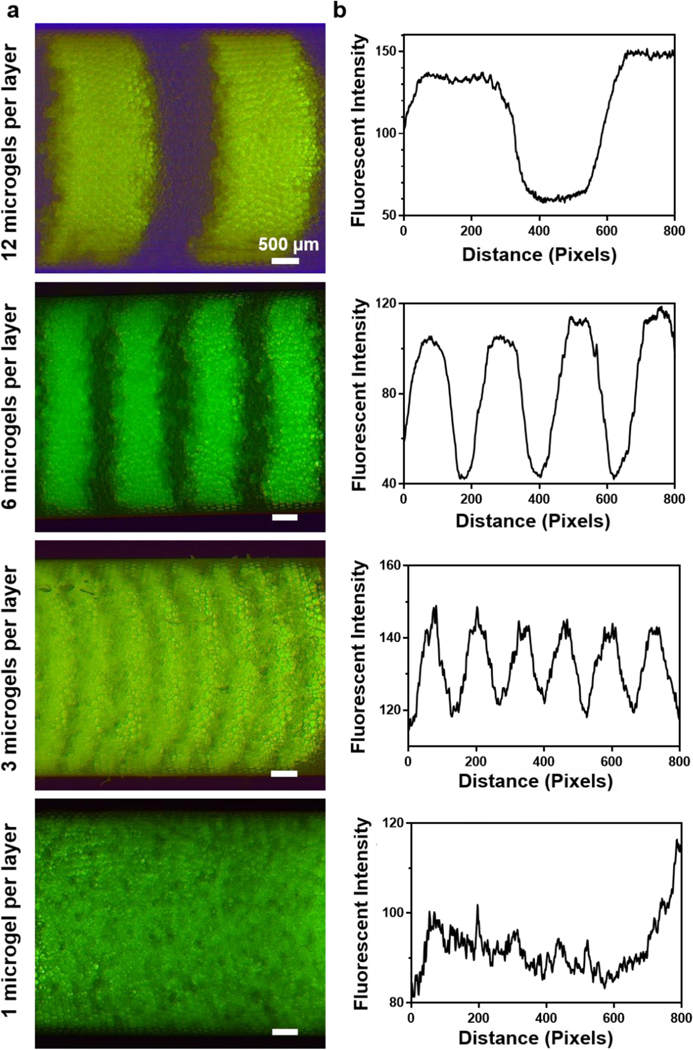
To characterize the resolution of microgel packing and the resulting microgel gradient profiles, further testing was conducted by altering the FITC concentration in the microgels over time. As crosslinked microgels came out from the outlet tubing, they were packed layer-by-layer into a 1 mL syringe pre-filled with fluorinated oil. Since fluorinated oil has a higher density than aqueous gel phase, microgels would float and spread to occupy the oil surface. As more gel droplets come into this collection syringe, the next layer will form on top of the first layer. To characterize the accuracy of microgel packing, we first investigated the packing of microgels by creating alternating layers of fluorescent and non-fluorescent microgels by switching the precursor solution between 100 % FITC gel and 100 % blank gel. Here, microgels having a diameter of 355 μm were generated at a rate of 3.7 droplets/s (total flow rate of two precursor solutions: 140 μl/h , carrier oil flow rate: 350 μl/h). The packed layer thickness, which is defined by the number of microgels per layer and controllable by the duration of microgel collection time for the given microgel generation speed, was varied to be 1, 3, 6, and 12 microgels per layer. Considering the syringe inner area of 17.94 mm2, this means that generating a monolayer of microgels would take 80 s. Distinguishable layers of microgels were identified in the 3, 6, and 12 microgels per layer groups, with the layer thickness approximately matching the expected values based on the microgel size and generation time (Figure 2). However, we did not observe distinguishable layers in the 1 microgel per layer group, which was attributed to microgel spreading and the nature of spherical packing. It is notable that the switching of precursor solution input can be completed almost instantaneously and, thus, will not affect the gradient composition (Video S2, Supporting Information).
Figure 2.
Layer-by-layer packed microgels in a syringe with alternating fluorescent and non-fluorescent microgel layers. a) Fluorescent images of packed microgels with 1, 3, 6, and 12 microgels per layer. b) Fluorescent profiles of packed microgels with 1, 3, 6, and 12 microgels per layer.
Whereas the aforementioned results were for binary patterns, creating physicochemical gradients requires that the flow rate of one gel solution undergo step increases while that of the other gel solution undergoes step decreases (Figure S2, Supporting Information). The duration of each step was then determined to adjust the thickness of each gradient layer. We then studied the fluorescent gradient profiles of packed microgels with 1, 3, 6, and 12 microgels per layer (Figure 3). Continuous gradient profiles were observed when the layer thickness was 1, 3 or 6 microgels, which also correlated well to the standard curve of FITC gel precursor solutions (Figure S3, Supporting Information). Importantly, the microgel spreading at layer boundaries shown in the alternating layer packing smooths the gradient and facilitates the generation of a precise fluorescent microgel gradient with minimum layer thickness. However, a step gradient profile was shown in the 12 microgels per layer group. All groups had a similar range of fluorescence intensity, indicating the accuracy of microgel packing. Overall, these results indicate that our microfluidic method can generate continuous gradients as desired and, thus, provide a foundation for producing physicochemical property gradients by simply altering the precursor solutions during the microgel generation process.
Figure 3.
Packed microgels in a syringe with fluorescent gradient. a) Fluorescent images of microgel gradients with 1, 3, 6, and 12 microgels per layer. b) Fluorescent profiles of microgel gradients with 1, 3, 6, and 12 microgels per layer.
2.3. Annealing and mock implantation of gradient MAP hydrogel scaffolds
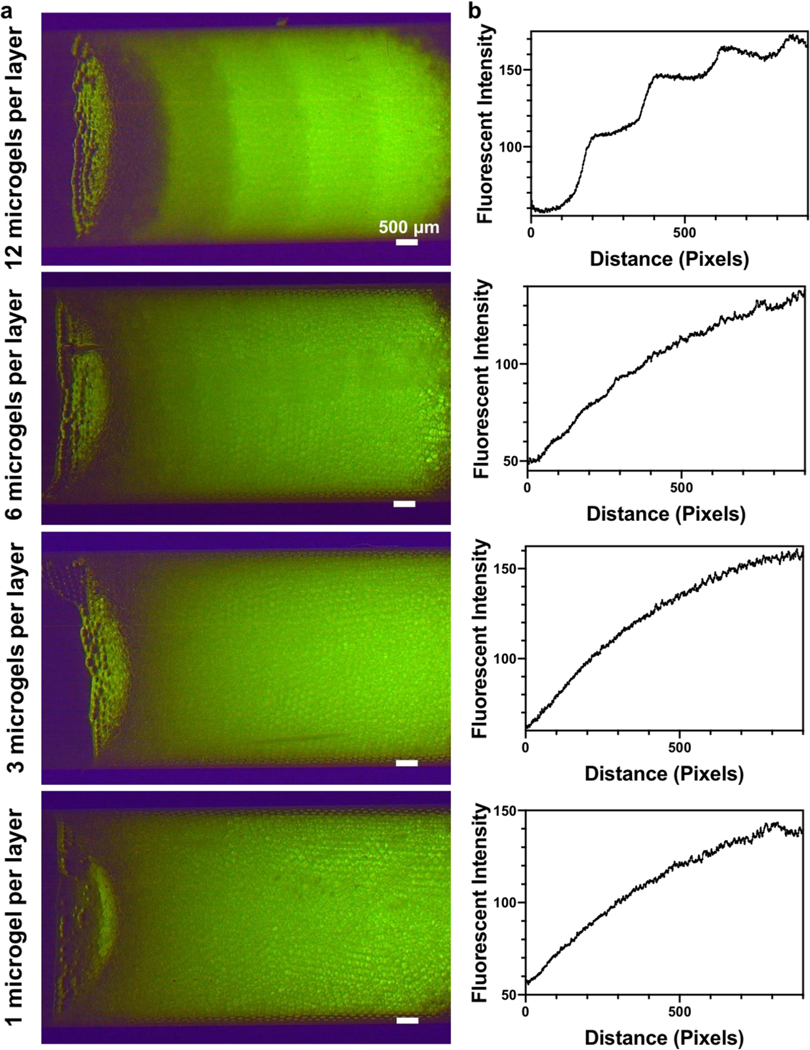
Next, we tested if gradients could be maintained after the microgels are injected out of the syringe and annealed into MAP hydrogels. Prior to injection, the surrounding oil in the syringe was allowed to evaporate to facilitate stable packing of microgels. Next, the packed microgels in the syringe were injected into a rectangular silicone mold (Figure 4). After injection, fluorescence microscopy revealed that the continuous fluorescent intensity profile was maintained (Figure 4b), suggesting excellent injectability of microgel gradients and limited movement of the microgels during the injection process. PEG-dithiol linker and photoinitiator were then added on top of the gradient to anneal the microgels via thiol-ene click chemistry.
Figure 4.
Extrusion of the microgel gradients. a) Schematic illustrating microgel extrusion into a 3 mm rectangular mold and microgel annealing into scaffolds. b) Fluorescent image of an extruded gradient MAP scaffold with 6 microgels per layer and quantification of fluorescent intensity throughout the scaffolds. c) Image showing gradient MAP scaffolds injected into a mouse femoral defect.
Importantly, we show that these gradient MAP hydrogels can be implanted into a tissue defect, using mock implantation into a mouse femoral defect as an example (Figure 4c). The packed microgel scaffold was injected from the syringe into the femoral defect and the fluorescent intensity gradient was mostly maintained within the implanted microgels. The annealing process was performed in situ after implantation with UV irradiation (10 mM/cm2 for 3 mins). This example demonstrates the possibility of applying gradient or patterned MAP scaffolds into tissue defects. Future applications include MAP hydrogels with stiffness gradients that can permit durotaxis to promote rapid migration of endogenous cells into the defect center to accelerate tissue healing. Alternatively, gradient MAP hydrogels exhibiting a gradual and smooth transition from osteogenic to chondrogenic cues could be engineered to direct the behavior of stem and progenitor cells and facilitate repair of osteochondral defects.
2.4. Insight on cell-material interactions from gradient MAP hydrogels
Cell-material interactions were also investigated using the 6 microgels per layer gradient MAP hydrogels by seeding hMSCs during the annealing process. MAP hydrogels with stiffness gradients from Young’s modulus of 9.8 to 29.2 kPa were achieved by using hydrogel macromers with varying molecular weights (from 5 kDa to 20 kDa PEG-norbornene) in the two gel precursor solution inlets (Figure 5). After 2 days of culture, confocal fluorescence microscopy images showed the hMSCs exhibited greater spreading and proliferation with increasing stiffness throughout the scaffold (Figure 5c and d). Moreover, the increases were continuous, which is similar to what is seen in 2D cultures.[20] Importantly, this result corroborates our prior finding that hMSC behavior in MAP hydrogels follows trends observed in 2D rather than conventional 3D hydrogel cultures,[10,a] which we attribute to the more permissive microporous cellular microenvironment. Future work could leverage similar gradients to better understand the effects of microgel stiffness on cell mechanotransduction and hMSC lineage-specific behavior in MAP hydrogels.
Figure 5.
hMSC proliferation and spreading in MAP scaffolds having a stiffness gradient. a) Z-projection image of hMSCs cultured within the stiffness gradient scaffolds. Green represents F-actin and blue represents nuclei. b) Young’s moduli of the MAP scaffolds from the two precursor solutions. Quantification of c) average cell number per μL and d) average cell volume in each region.
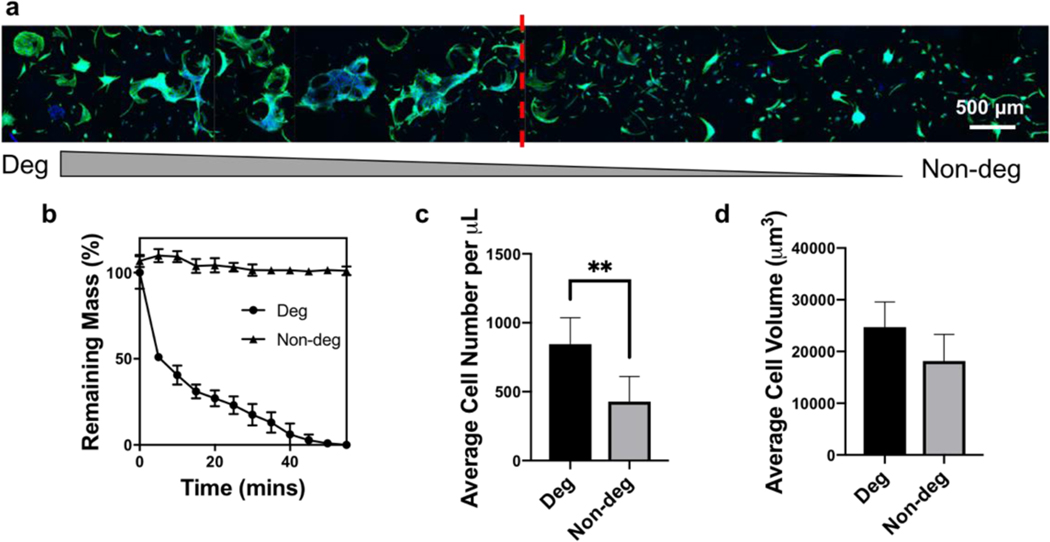
Next, MAP hydrogels with a degradability gradient from 100% to 0% degradability were produced by using matrix metalloproteinase degradable KCGPQGIWGQCK and non-degradable PEG-dithiol crosslinkers in the two gel precursor solution inlets (Figure 6). However, instead of continuous spreading and proliferation trends throughout the scaffold, there was a critical reverse gelation point in the degradability gradient scaffolds. Above this point (i.e., left side of the image in Figure 6a), hMSCs spread robustly and formed a cellular network around the microgels, whereas cells were isolated into individual divisions with less spreading below this point (i.e., right side of the image in Figure 6a). It appears that the ratio of degradable linker needs to be higher than the critical reverse gelation point for cells to fully degrade the surrounding gel and spread better. Interestingly, the fact that the cells either spread better or not suggests that there may not be a benefit to using microgels with 100% degradable crosslinker.
Figure 6.
hMSC proliferation and spreading in MAP scaffolds with a degradability gradient. a) Z-projection image of hMSCs cultured within the degradability gradient scaffolds. Green represents F-actin and blue represents nuclei. b) Degradation curves of the MAP scaffolds prepared from the two precursor solutions. Quantification of c) average cell number per μL and d) average cell volume in degradable and non-degradable regions. **p < 0.01, one-way ANOVA.
Overall, the effects of various physicochemical properties on cellular responses in MAP hydrogels is of high interest for the design of these materials, with key parameters being microgel size, stiffness, linker concentration, and RGD concentration.[10] However, these studies have all been carried out in MAP hydrogels with discrete parameters. In contrast, the gradient MAP hydrogels developed here can present continuous physicochemical values to provide more information on cell-material interactions compared to testing discrete values, for example cells exhibit differential spreading trends in the degradability and stiffness gradients here, making it a powerful platform to screen cell-material interactions.
3. Conclusion
We developed a microfluidic approach combining a microfluidic mixer module and a droplet generator module to create gradient MAP hydrogel scaffolds with changing physiochemical profiles within the scaffolds. This approach was successfully utilized to create MAP hydrogels with continuous physicochemical gradient. The injectability and suitability for implantation of gradient MAP hydrogels were demonstrated by mock implantation in which the scaffolds were directly injected into a mouse femoral defect. In addition, the ability to create gradients in MAP hydrogels can be leveraged to gain unique insights into cell-material interactions, which was demonstrated by showing that hMSCs exhibited differential spreading trends in the degradability and stiffness gradient scaffolds. In summary, the modular and high-accuracy nature of the MAP hydrogel generation method developed here has the potential to be utilized broadly in tissue engineering and regenerative medicine. Future work could include generation of gradient MAP scaffolds in a more complex manner with this microfluidic method, such as two-way gradients, to screen the interplay of two physicochemical cues on cell-material interactions for example.
4. Experimental Section
Preparation of microfluidic device:
The microfluidic device was made of polydimethylsiloxane (PDMS) by standard soft lithography.[21] Master molds were fabricated on a 4-inch silicon wafer by a photolithographic technique using a negative photoresistor (SU8 2075, MicoChem). Microfluidic devices were molded from master molds by pouring degassed PDMS (Sylgard 184, Dow, elastomer: crosslinker = 10:1) and cured at 85 °C for 1 h. PDMS devices were then placed onto a glass slide coated with PDMS (elastomer: crosslinker = 20:1) and bonded together at 85 °C overnight. The Y-shaped mixing module allowed mixing of two different gel solutions at different ratios.[22] Microgel droplets were generated at a T-junction where the oil phase broke off the mixed gel solution into droplets. A winding channel was used to create chaotic advection in droplets to accelerate the mixing of gel solutions within droplets.[14] The channel height of the microfluidic device was 150 μm, with the oil phase channel width being 200 μm and the Y-shaped mixing module channel width being 160 μm.
Generation of microgel through microfluidics :
PEG thiol-ene based gel precursor solutions consisted of four-arm PEG-norbornene (synthesized from four-arm PEG-hydroxyl as previously reported)[23], bi-functional thiol crosslinker, lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP, synthesized as previously described)[24] photoinitiator, and CGRGDS (prepared via Fmoc solid phase peptide synthesis). The physicochemical properties of the microfluidically generated microgels were tuned by adjusting the gel composition.
Fluorescent intensity gradients were achieved using non-fluorescent and SAMSA-fluorescein (100 μM, Invitrogen) labelled microgels (note: SAMSA-fluorescein possesses a thiol and is conjugated to the PEG). The stiffness gradient was achieved by using 5 kDa and 20 kDa PEG-norbornene. The degradability gradient was achieved by using PEG-dithiol (3,400 Da, Laysan Bio.) and KCGPQGIWGQCK (AAPPtec) crosslinkers.
Syringe pumps (Pico-plus, Harvard Apparatus) were used to control volumetric flow rates of all input streams through a LabView program (National Instruments). The total flow rate of the two gel solutions into the microfluidic device was 140 μl/h, and the flow rate of fluorinated oil (Novec 7500, 3M) containing 2% fluorosurfactant (Pico-surf 1, Dolomite) was 350 μl/h. The droplet generation speed was 13,320 droplets/h. The generated droplets were photocrosslinked into microgels downstream in the outlet tubing (25 mW/cm2, 72 s, 365 nm, Lumen Dynamics Omnicure S2000 Series) and collected into a 1 mL syringe. A hole was punched at the bottom of the syringe to remove excessive oil during microgel collection. 100 μL of microgels having gradients in their physiochemical compositions were collected in the syringe, with another 100 μL of buffer microgels (i.e., no gradient profile) collected at the bottom to fill the dead space during injection. The fluorinated oil was allowed to evaporate from the packed microgels for 2 days at room temperature to achieve stable packing before use.
Annealing into gradient MAP scaffolds:
The packed microgel gradients were slowly injected into a rectangular shaped silicone mold with a width of 3 mm, which was approximately the same width as the inner diameter of the 1 mL syringe. The injected microgels were stored overnight for complete evaporation and removal of the fluorinated oil. 4 μL 20 wt% PEG-dithiol and 1.5 μL 100 mM LAP were added onto the microgels to anneal (10 mW/cm2, 3 mins, 365 nm) them into gradient MAP scaffolds. The scaffolds were then allowed to swell in 1X phosphate buffered saline until reaching equilibrium.
Characterization:
Mixing of the two gel solutions and microgel droplet formation were observed using an upright microscope (Eclipse LV 100D, Nikon) with a high-speed camera (C11440, Hamamastu). Swollen microgels were imaged using a light microscope, and their sizes were measured using the ImageJ software (NIH). A stereomicroscope (Stemi 508, Zeiss) with 0.5X objective was used to image the entire gradient scaffold in fluorescence field. The fluorescent intensity was quantified using the ImageJ software. The Young’s moduli of MAP hydrogels prepared from the hydrogel precursor solutions were measured by atomic force microscopy (Dimension Icon, Bruker) with a SiO2 colloidal probe (5 μm diameter, spring constants 0.6 N/m; Novascan). The degradation curves of MAP hydrogels prepared from hydrogel solutions were obtained by immersing samples in a 0.2 mg/mL collagenase B (Sigma) solution at 37 °C and weighing the remaining mass every 15 minutes.
Mock implantation study:
Freshly euthanized C57BL/6 mice were provided by the Animal Resource Sharing Program of the Comparative Medicine Program at Texas A&M University. The mice were used to demonstrate the implantation of microgel gradients into a mouse critical-sized femoral defect model, as previously described.[25] In brief, the skin was incised along the longitudinal axis of the femur and the intermuscular boundary was dissected to expose the femur. A segment was cut from the mouse femur using a fine micro-drill (Braintree Scientific) fitted with a fine diamond-grit coated cutting wheel (Strauss Diamond). The microgel gradients were injected into the bone space and then the microgels were assembled via in situ photopolymerization (10 mW/cm2, 3 mins, 365 nm) with the addition of 4 μL 20 wt% PEG-dithiol and 1.5 μL 100 mM LAP. A stereomicroscope (Stemi 508, Zeiss) with a 0.5X objective was then used to image the gradient MAP scaffolds.
Cell seeding:
hMSCs were acquired from the Institute of Regenerative Medicine at Texas A&M University and cultured in α-Minimal essential medium (Gibco) supplemented with 20% v/v fetal bovine serum (FBS, Atlanta Biologicals), 2 mM GlutaMAX (Gibco), 50 U/mL penicillin (Gibco), and 50 μg/mL streptomycin (Gibco) at 5% CO2 and 37 °C in a humidified environment. hMSCs were used up to Passage 5. hMSC suspensions were mixed with 4 μL 20 wt% PEG-dithiol and 1.5 μL 100 mM LAP, and the mixture was seeded throughout the gradient scaffold during the annealing process. For 100 μL scaffolds, 500,000 cells were seeded. hMSCs were cultured within the stiffness gradient scaffolds for 2 days and the degradability gradient scaffolds for 5 days.
Immunostaining and imaging:
Samples were fixed using 4% formaldehyde for 15 min at room temperature. Cytoskeletal staining was performed using rhodamine phalloidin (1:40, Invitrogen) with counter staining of 4′,6-diamidino-2-phenylindole (DAPI) (1:1000, Jackson ImmunoResearch). Samples were imaged using a confocal microscope (FV1000, Olympus) with 200 μm Z-stack throughout the scaffolds. The images were analyzed using the ImageJ software. For cell number quantification, the 3D Objects Counter plugin was used to count the number of nuclei based on DAPI staining. The Voxel Counter plugin was used to measure the total cell volume in a z-stack based on phalloidin staining after thresholding. The average cell volume was then calculated by dividing the total cell volume by cell number.
Supplementary Material
Acknowledgements
S. X. and J. D. contributed equally to this work. The authors thank Dr. Akhilesh Gaharwar for the use of stereomicroscopy and Eoin McNeill for assistance with the mouse femoral defect mock implantation. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH) under Award Number R21 AR071625 to D. A.. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The research was also partially supported by the Army Research Office (ARO) grant W911NF-19-1-0290 to A. H..
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Shangjing Xin, Department of Biomedical Engineering, Texas A&M University, College Station, TX, 77843 USA.
Dr. Jing Dai, Department of Electrical and Computer Engineering, Texas A&M University, College Station, TX, 77843 USA
Carl A. Gregory, Department of Molecular and Cellular Medicine, Institute for Regenerative Medicine Texas A&M Health Science Center, College Station, TX, 77807 USA
Arum Han, Department of Biomedical Engineering, Texas A&M University, College Station, TX, 77843 USA Department of Electrical and Computer Engineering, Texas A&M University, College Station, TX, 77843 USA.
Daniel L. Alge, Department of Biomedical Engineering, Texas A&M University, College Station, TX, 77843 USA Department of Materials Science and Engineering, Texas A&M University, College Station, TX, 77843 USA.
References
- [1] a).Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Umansky KB, Yifa O, Kain D, Rajchman D, Leach J, Bassat DR, Nature 2017, 547, 179; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Daley WP, Peters SB, Larsen M, Journal of cell science 2008, 121, 255; [DOI] [PubMed] [Google Scholar]; c) Stanton AE, Tong X, Yang F, Acta biomaterialia 2019, 96, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2] a).Madl CM, Heilshorn SC, Blau HM, Nature 2018, 557, 335; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dong Y, Rodrigues M, Li X, Kwon SH, Kosaric N, Khong S, Gao Y, Wang W, Gurtner GC, Advanced Functional Materials 2017, 27, 1606619; [Google Scholar]; c) Ballios BG, Cooke MJ, van der Kooy D, Shoichet MS, Biomaterials 2010, 31, 2555; [DOI] [PubMed] [Google Scholar]; d)Hong KH, Kim YM, Song SC, Advanced Science 2019, 6, 1900597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3] a).Vega SL, Kwon MY, Song KH, Wang C, Mauck RL, Han L, Burdick JA, Nature communications 2018, 9, 614; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gaharwar AK, Arpanaei A, Andresen TL, Dolatshahi-Pirouz A, Advanced Materials 2016, 28, 771; [DOI] [PubMed] [Google Scholar]; c) Gao F, Xu Z, Liang Q, Liu B, Li H, Wu Y, Zhang Y, Lin Z, Wu M, Ruan C, Advanced Functional Materials 2018, 28, 1706644. [Google Scholar]
- [4] a).Ouyang L, Highley CB, Sun W, Burdick JA, Advanced materials 2017, 29, 1604983; [DOI] [PubMed] [Google Scholar]; b) Moroni L, Burdick JA, Highley C, Lee SJ, Morimoto Y, Takeuchi S, Yoo JJ, Nature Reviews Materials 2018, 3, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5] a).Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T, Nature materials 2015, 14, 737; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Nih LR, Sideris E, Carmichael ST, Segura T, Advanced Materials 2017, 29, 1606471; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Xin S, Chimene D, Garza JE, Gaharwar AK, Alge DL, Biomaterials science 2019, 7, 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de Rutte JM, Koh J, Di Carlo D, Advanced Functional Materials 2019, 29, 1900071. [Google Scholar]
- [7].Mealy JE, Chung JJ, Jeong HH, Issadore D, Lee D, Atluri P, Burdick JA, Advanced Materials 2018, 30, 1705912. [DOI] [PubMed] [Google Scholar]
- [8].Darling NJ, Sideris E, Hamada N, Carmichael ST, Segura T, Advanced Science 2018, 5, 1801046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9] a).Radhakrishnan J, Manigandan A, Chinnaswamy P, Subramanian A, Sethuraman S, Biomaterials 2018, 162, 82; [DOI] [PubMed] [Google Scholar]; b) Li P, Markson JS, Wang S, Chen S, Vachharajani V, Elowitz MB, Science 2018, 360, 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10] a).Xin S, Wyman OM, Alge DL, Advanced healthcare materials 2018, 7, 1800160; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Caldwell AS, Campbell GT, Shekiro KM, Anseth KS, Advanced healthcare materials 2017, 6, 1700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Headen DM, García JR, García AJ, Microsystems & Nanoengineering 2018, 4, 17076. [Google Scholar]
- [12].Lin F, Saadi W, Rhee SW, Wang S-J, Mittal S, Jeon NL, Lab on a Chip 2004, 4, 164. [DOI] [PubMed] [Google Scholar]
- [13] a).Burdick JA, Khademhosseini A, Langer R, Langmuir 2004, 20, 5153; [DOI] [PubMed] [Google Scholar]; b) Jeon O, Alt DS, Linderman SW, Alsberg E, Advanced materials 2013, 25, 6366; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Pedron S, Becka E, Harley BA, Advanced Materials 2015, 27, 1567. [DOI] [PubMed] [Google Scholar]
- [14].Song H, Tice JD, Ismagilov RF, Angewandte Chemie International Edition 2003, 42, 768. [DOI] [PubMed] [Google Scholar]
- [15].Xia B, Krutkramelis K, Oakey J, Biomacromolecules 2016, 17, 2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hoyle CE, Bowman CN, Angewandte Chemie International Edition 2010, 49, 1540. [DOI] [PubMed] [Google Scholar]
- [17].Qin XH, Wang X, Rottmar M, Nelson BJ, Maniura-Weber K, Advanced Materials 2018, 30, 1705564. [DOI] [PubMed] [Google Scholar]
- [18].Du H, Cont A, Steinacher M, Amstad E, Langmuir 2018, 34, 3459. [DOI] [PubMed] [Google Scholar]
- [19] a).Choi C-H, Wang H, Lee H, Kim JH, Zhang L, Mao A, Mooney DJ, Weitz DA, Lab on a Chip 2016, 16, 1549; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wagner O, Thiele J, Weinhart M, Mazutis L, Weitz DA, Huck WT, Haag R, Lab on a Chip 2016, 16, 65. [DOI] [PubMed] [Google Scholar]
- [20].Hadden WJ, Young JL, Holle AW, McFetridge ML, Kim DY, Wijesinghe P, Taylor-Weiner H, Wen JH, Lee AR, Bieback K, Proceedings of the National Academy of Sciences 2017, 114, 5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21] a).Unger MA, Chou H-P, Thorsen T, Scherer A, Quake SR, Science 2000, 288, 113; [DOI] [PubMed] [Google Scholar]; b) Dai J, Suh SJ, Hamon M, Hong JW, Biotechnology journal 2015, 10, 1783; [DOI] [PubMed] [Google Scholar]; c) Dai J, Kim HS, Guzman AR, Shim W-B, Han A, RSC Advances 2016, 6, 20516. [Google Scholar]
- [22].Kim J, Taylor D, Agrawal N, Wang H, Kim H, Han A, Rege K, Jayaraman A, Lab on a Chip 2012, 12, 1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jivan F, Yegappan R, Pearce H, Carrow JK, McShane M, Gaharwar AK, Alge DL, Biomacromolecules 2016, 17, 3516. [DOI] [PubMed] [Google Scholar]
- [24].Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS, Biomaterials 2009, 30, 6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clough BH, McCarley MR, Gregory CA, JoVE (Journal of Visualized Experiments) 2015, 97, e52368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.