Summary
Nowadays, Western diets and lifestyle lead to an increasing occurrence of chronic gut inflammation that represents an emerging health concern with still a lack of successful therapies. Fermented foods, and their associated lactic acid bacteria, have recently regained popularity for their probiotic potential including the maintenance of gut homeostasis by modulating the immune and inflammatory response. Our study aims to investigate the crosstalk between the food-borne strain Lactiplantibacillus plantarum C9O4 and intestinal epithelial cells in an in vitro inflammation model. Cytokines profile shows the ability of C9O4 to significantly reduce levels of IL-2, IL-5, IL-6, and IFN-γ. Proteomic functional analysis reveals an immunoregulatory role of C9O4, able to revert the detrimental effects of IFN-γ through the JAK/STAT pathway in inflamed intestinal cells. These results suggest a promising therapeutic role of fermented food-associated microbes for the management of gastrointestinal inflammatory diseases. Data are available via ProteomeXchange with identifier PXD042175.
Subject areas: Gastroenterology, Molecular biology, Food chemistry
Graphical abstract
Highlights
-
•
Inflammation activates pathways involved in gastrointestinal diseases via IRF in NCM460
-
•
Lpb. plantarum C9O4 has a protective role by reducing level of inflammatory biomarkers
-
•
STAT1 has a crucial role in C9O4 mechanism of action in reverting inflammation
-
•
C9O4 plays an immunoregulatory role by stimulating the IFN-γ mediated JAK/STAT pathway
Gastroenterology; Molecular biology; Food chemistry
Introduction
The human gastrointestinal (GI) tract is a complex environment, and its health status is modulated by the strict host-microbes dialog that interplays between commensal bacteria and the intestinal epithelium. Commensal microbes, as well as bacteria associated to fermented foods and/or probiotics, can play a crucial role to maintain the gut immune homeostasis by modulating the immune and inflammatory response.1 Nowadays, the interaction of beneficial microbes with the human GI system is emerging as a promising field of research to face the health concern related to the increasing occurrence of chronic gut inflammations, such as inflammatory bowel disease (IBD). IBD are chronic relapsing intestinal disorders, characterized by a dysregulated balance between pro- and anti-inflammatory processes in the intestinal tract, followed by invasion of immune cells to the intestinal mucosa that leads to chronic inflammation.2
Despite many research advances, standard therapies (anti-inflammatory or immunosuppressive drugs) are not satisfactory. The lack of therapeutic options in IBD is a serious issue and lactic acid bacteria (LAB), the most common bacteria found in fermented foods and used in probiotic preparations, have emerged as novel strategies to ameliorate intestinal disorders.1,3 The potential benefits of LAB are associated with a reduced risk of developing chronic diseases and with several other beneficial activities, such as modulating the immune system.4 Among LAB, Lactiplantibacillus (Lpb.) plantarum is a flexible species that could be found as a natural inhabitant both in several fermented foods and in the human gastrointestinal tract, from which some strains have already been characterized as probiotics.5 Pre-clinical studies on the effects of Lpb. plantarum for the prevention and management of IBD have been deeply reported by Le and Yang.6
The gut-microbiota variability7 and the modulation of immune response of intestinal lymphoid and epithelial cells are speculated as the main mechanisms of action,4,6 even though the exact role of Lpb. plantarum in IBD is still not well elucidated. It has been reported that some Lpb. plantarum strains are able to interact with the intestinal ecosystem by downregulating inflammatory cascade in intestinal chronic inflammation and also by reducing the production of pro-inflammatory cytokines, alleviating thus dextran sulfate sodium (DSS)-induced colitis in mice.8,9
The modulation of pro-inflammatory cytokines could be related to the balance between Th1 and Th2 cells regulated by Lpb. plantarum that lead to the differential production of several cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)- 1β, IL-6, IL-10, IL-12, and interferon-gamma (IFN-γ).6
Moreover, the inhibition of apoptosis by Lpb. plantarum has been speculated as another mechanism of action, for instance by blocking cyclooxygenase-2 (COX-2) in Th1, or by inhibiting the apoptosis of intestinal epithelial cells,10 as well as improving intestinal barrier function via upregulation of tight junction proteins (i.e., ZO-1 and occludins) and downregulation of apoptotic-related proteins.11
To date, the precise mechanism of action of the host-microbe dialog is still unclear, even though it is well established the strain-dependent nature of this interaction, highlighting that the strain selection and the evaluation of the effects of individual strains is of paramount importance, as requested by international guidelines for probiotics selection.12 In our previous studies, we have characterized and selected the food-associated Lpb. plantarum C9O4 strain for some probiotic properties.13,14 Indeed, we have underlined the ability of this strain to display immunoregulatory effects15 as well as to ameliorate inflammation and related oxidative stress by triggering IL17/IL23 axis, a key cytokines pathway involved in the pathogenesis of chronic inflammation such as IBD.16 Based on these findings, the aim of this study was to deepen the immunomodulatory properties of Lpb. plantarum C9O4 in an in vitro intestinal inflammation model of normal human colon mucosa cell (NCM460) treated with an inflammatory stimuli by evaluating, through a proteomic approach, the differential proteins expression, the alterations of molecular functions and the biological processes including the modulation of pro- and anti-inflammatory cytokines release.
Results
Proteomic profile of NCM460 cells
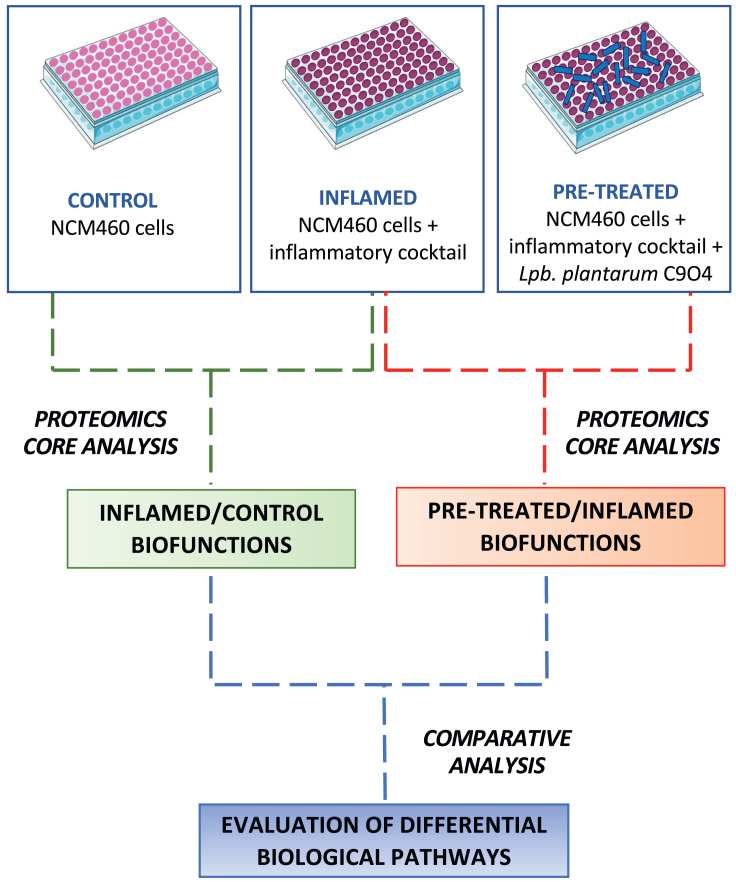
We investigated intracellular proteomic changes in NCM460 cells grown under conventional conditions, exposed to an inflammatory stimulus or inflamed after the pre-treatment with live Lpb. plantarum C9O4 cells. In particular, we performed two different comparisons. Firstly, as a proof-of-concept, inflamed cells were compared to control ones in order to verify and identify the phenotypic modulation response of the cell to the inflammatory stimuli used. Then, we performed a proteomics investigation on pre-treated-inflamed cells with a Lpb. plantarum strain in order to evaluate the probable molecular mechanisms involved in the protective effect induced by the strain (Figure 1).
Figure 1.
Workflow scheme of the experimental proteomics strategy
It highlights that two functional analyses (INFLAMED Vs. CONTROL and PRE-TREATED Vs. INFLAMED) performed by Ingenuity Pathway Software (IPA, Qiagen) were compared as comparison analysis for evaluating the different biological pathways between inflamed condition and pre-treatment with Lpb. plantarum C9O4.
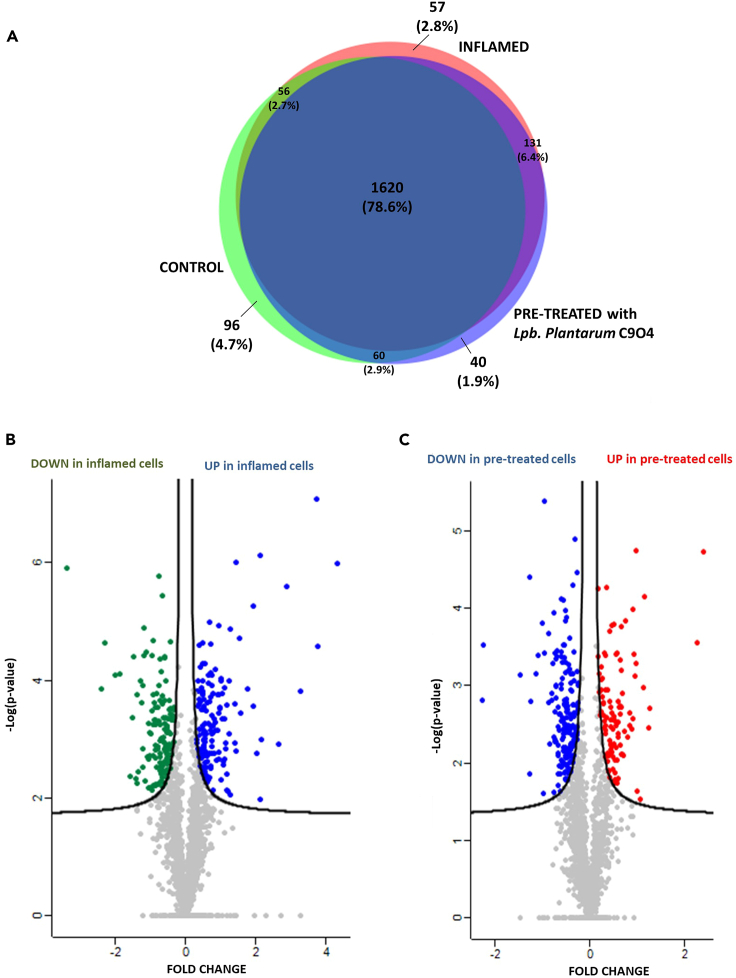
As a result, 1,832, 1,864, and 1,851 proteins were identified and quantified in control, inflamed, and pre-treated cells, respectively (Figure 2A). Among them, 78.6% of proteins (1,620) were present in the three conditions, while 96, 57, and 40 were uniquely identified in control (4.7%), inflamed (2.8%), and pre-treated (1.9%), respectively. The list of the quantified proteins is reported in Table S1.
Figure 2.
Global proteomic profile of NCM460 cells under three different treatment conditions
(A) Venn diagram of unique and shared proteins identified across all three conditions.
(B) Volcano plot of the differential proteins expressed in NCM460 cells subjected to an inflammatory stimulus and control ones. Proteins not significantly differential are represented in gray; while in blue and in green are represented the proteins significantly upregulated and downregulated in inflamed cells, respectively.
(C) Volcano plot of the differential proteins expressed in NCM460 cells pre-treated with Lpb. plantarum C9O4 and inflamed ones. Proteins not significantly differential are represented in gray; while in red and in blue are represented the proteins significantly upregulated and downregulated in inflamed cells, respectively. Proteins are plotted based on fold change and -Log (p value) using a false discovery rate (FDR) of 0.01 and an S0 of 0.05 for both comparisons.
The statistical comparison of the global proteomics expression on the three experimental conditions was reported in the volcano plots of Figure 2 (panel B for inflamed vs. control cells and panel C for cells pre-treated with Lpb. plantarum C9O4 compared to those subjected to inflammation). We demonstrated that cytokine cocktail used as inflammatory stimulus influenced the cellular processes of NCM460 cells. In fact, among the detected and quantified proteins, 302 proteins were significantly regulated (p < 0.05) of which 147 were downregulated and 155 upregulated, as shown by green and blue dots, respectively (Figure 2B).
The second comparison highlighted 276 proteins significantly regulated (p < 0.05), of which 173 were downregulated (blue dots) and 103 upregulated (red dots) in pre-treated cells (Figure 2C). The list of differential proteins is reported in Table S1 sheets “VP”.
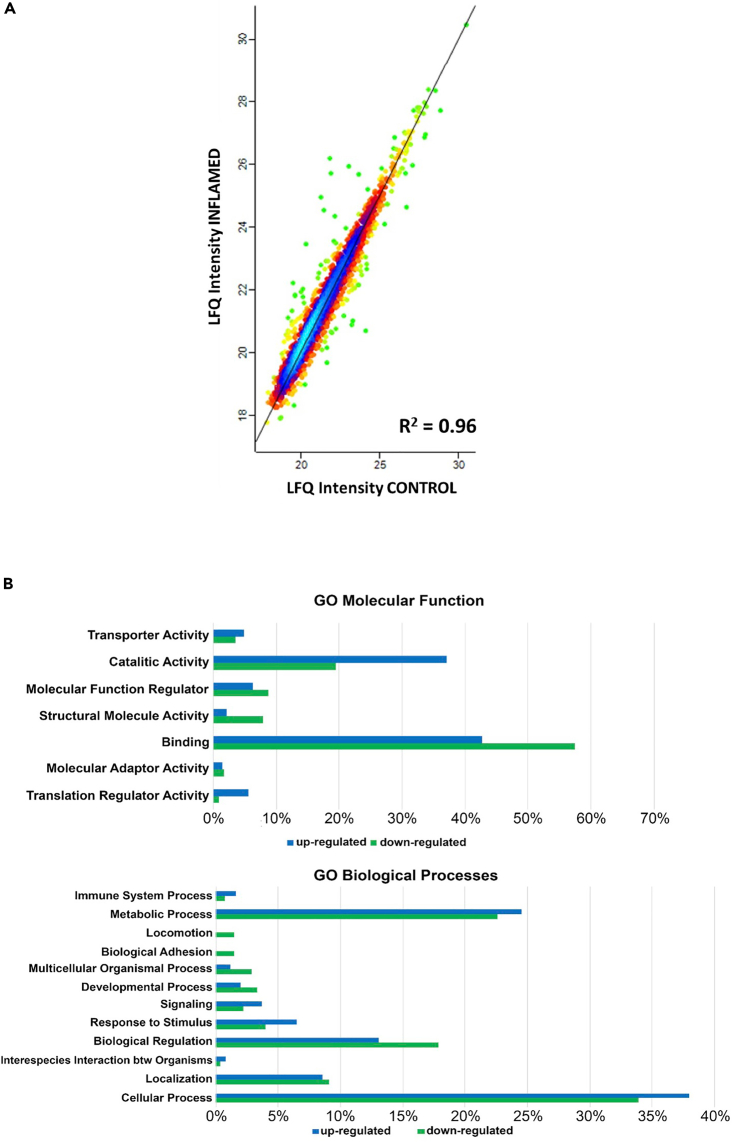
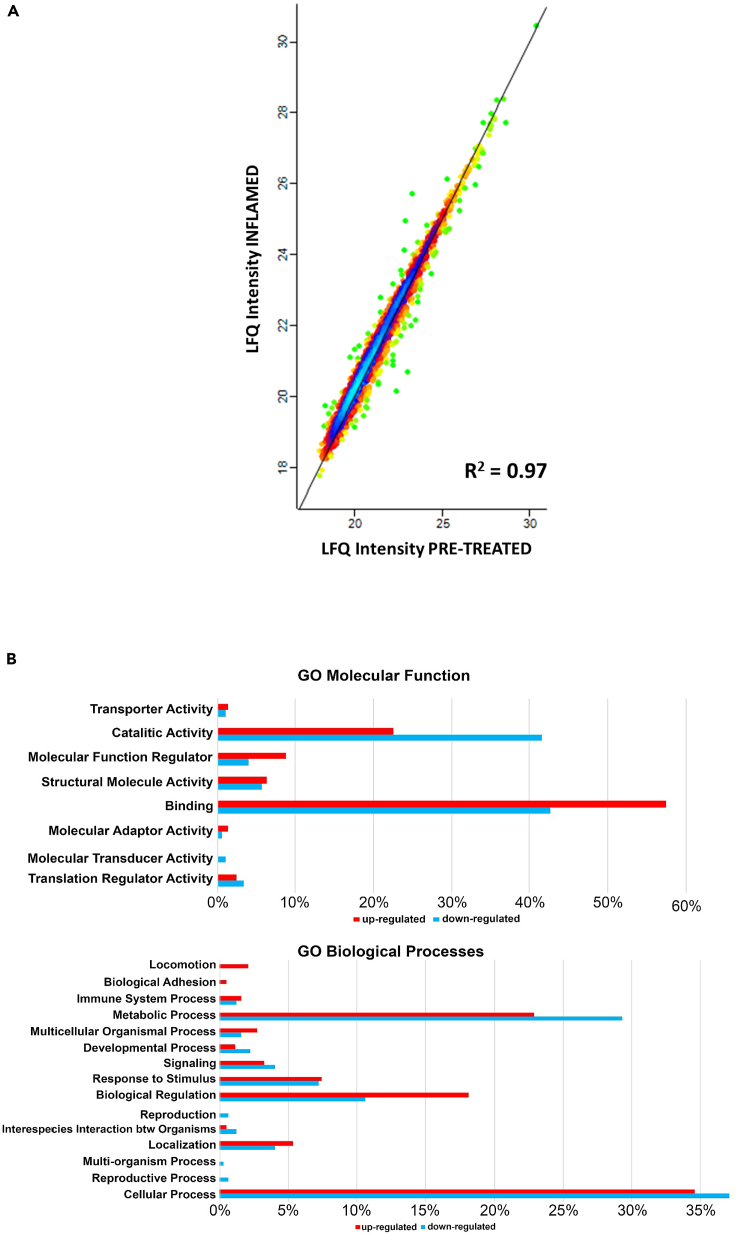
To globally analyze the differentially expressed proteins, we used the PANTHER GO database that allowed us to classify and group these proteins into two major categories: molecular function and biological processes (Figures 3B and 4B), comparing the percentage of proteins significantly regulated. As shown in the Figure 3B, the majority of the proteins significantly up- or downregulated under the inflamed status were involved in catalytic and binding activities; in particular, under the biological processes category, most proteomic changes were observed in metabolic processes, cellular processes, and biological regulation. Accordingly, the inflammatory status led to inhibition of proteins involved in biological regulation, locomotion, or adhesion, however, there was a significative upregulation of proteins involved in immune system process and response to the stimulus in pre-treated cells (Figure 4B).
Figure 3.
Proteomics analysis of NCM460 cells: INFLAMED Vs. CONTROL
(A) Protein expression correlation is reported as density plot with a Pearson correlation of 0.96.
(B) Gene Ontology (GO) reclassification of significantly expressed proteins in NCM460 inflamed cells using PANTHER database for molecular function and biological process.
Figure 4.
Proteomics analysis of NCM460 cells: PRE-TREATED with Lpb. plantarum C9O4 Vs. INFLAMED
(A) Protein expression correlation is reported as density plot with a Pearson correlation of 0.97.
(B) Gene ontology (GO) reclassification of significantly expressed proteins in NCM460 cells pre-treated with Lpb. plantarum C9O4 using PANTHER database for molecular function and biological process.
Inflammatory stimulus activated pathways involved in gastrointestinal diseases via interferon regulatory factors in NCM460 cells
Quantitative proteomics data obtained with MaxQuant software were used for functional analysis using Ingenuity Pathway Analysis (IPA tool, Qiagen). “Comparison analysis” was used to highlight the biological processes underlying the identified protein patterns of NCM460 cells. Actually, downstream analysis corroborated a significant inflammation in NCM460 cells exposed to inflammatory cocktail compared to control ones. Generically, protein signature indicated that the inflammatory stimulus clearly activated general pathways involved in gastrointestinal diseases (i.e., liver cancer p value = 1.36 × 10−14, Z score = 2.16, data not shown).
Moreover, we predicted the cascade of upstream transcriptional regulators that can explain the observed gene expression changes in inflamed cells. Among them, the most significant upregulated upstream found were interferon regulatory factors (IRF) gene, IRF7 (p value = 6.57 × 10−4, Z score = 4.656) and IRF3 (p value = 9.81 × 10−3, Z score = 4.549) together with interferon γ (IFN-γ gene p value = 4.66 × 10−7, Z score = 4.478), tumor necrosis factor (TNF gene -value = 6.57 × 10−4, Z score = 3.95), and interleukin 1β (IL-1β, p value = 0.2, Z score = 4.96), as proof-of-concept that inflammation has occurred because IFNγ, TNF, and IL-1β have been used in the cytokines inflammatory cocktail. Table 1 lists the main genes predicted as upstream regulators with their Z score activation values as comparison analysis of the two distinct functional analyses performed by IPA software.
Table 1.
List of the main differential upstream regulators of the two comparisons
| Upstream Regulators | Description | INFLAMED/ CONTROL |
PRE-TREATED/ INFLAMED |
|---|---|---|---|
| IFNA2 | Interferon alpha-2 | 5.245 | −3.018 |
| IRF3 | Interferon regulatory factor 3 | 4.549 | −3.229 |
| Interferon alpha | Interferon alpha-7 | 5.314 | −2.153 |
| IRF1 | Interferon regulatory factor 1 | 4.004 | −3.243 |
| IRF7 | Interferon regulatory factor 7 | 4.656 | −2.55 |
| IFNG | Interferon gamma | 4.478 | −2.391 |
| STAT1 | Signal transducer and activator of transcription 1-alpha/beta | 4.412 | −1.347 |
| TNF | Tumor necrosis factor | 3.946 | −1.444 |
| Ifn gamma | Tyrosine-protein kinase JAK2 | 3.354 | −1.418 |
| IRF5 | Interferon regulatory factor 5 | 2.818 | −1.707 |
| OSM | Oncostatin-M | 3.528 | −0.829 |
| IFN type 1 | Signal transducer and activator of transcription 1-alpha/beta | 2.586 | −0.917 |
| IL1B | Interleukin-1 beta | 4.96 | 0 |
| IL12 | Interleukin-12 receptor subunit beta-1 | 2.4 | 0 |
Table reports the main genes involved in inflammatory response with their predictive z scores (positive values: activation, z score ≥2.00; negative values: inhibition, z-score ≤ −2.00). The further investigated upstream regulators are highlighted in bold.
Lpb. plantarum C9O4 pre-treatment reverses the effects of the inflammatory cocktail in inflamed NCM460 cells
On the contrary, in the comparison pre-treated/inflamed cells IRF genes were strongly inhibited, i.e., IRF7 gene (p value = 7.81 × 10−5, Z score = −2.55), IRF3 gene (p value = 0.00367, Z score = −3.229) together with IFN-γ (p value = 6.97 × 10−8, Z score = −2.391), TNF (p value = 2.72 × 10−4, Z score = −1.44). Between the common inhibited upstream regulators, the most significant are shown in Table 1 comparing their modulation with the previous comparison (inflamed/control). Figure 5A shows the mechanistic network of IFNγ, a member of the type II interferon class secreted by cells of both the innate and adaptive immune systems. It is indirectly linked to TNF and IRF1 and IRF7. Instead, IRF3 was both quantified as downregulated protein in cell models (expression fold change = −1.123, -log(p value) = 2.96, see Figure 2B and Table S1) and predicted as inhibited upstream in pre-treated cells compared to inflamed ones. Since IRF complex translocates to the nucleus and activates the transcription of INFα and INFβ, as well as other INF-induced genes, our proteomics data demonstrate that Lpb. plantarum C9O4 interacts with inflamed NCM460 cells by modulating inflammatory processes.
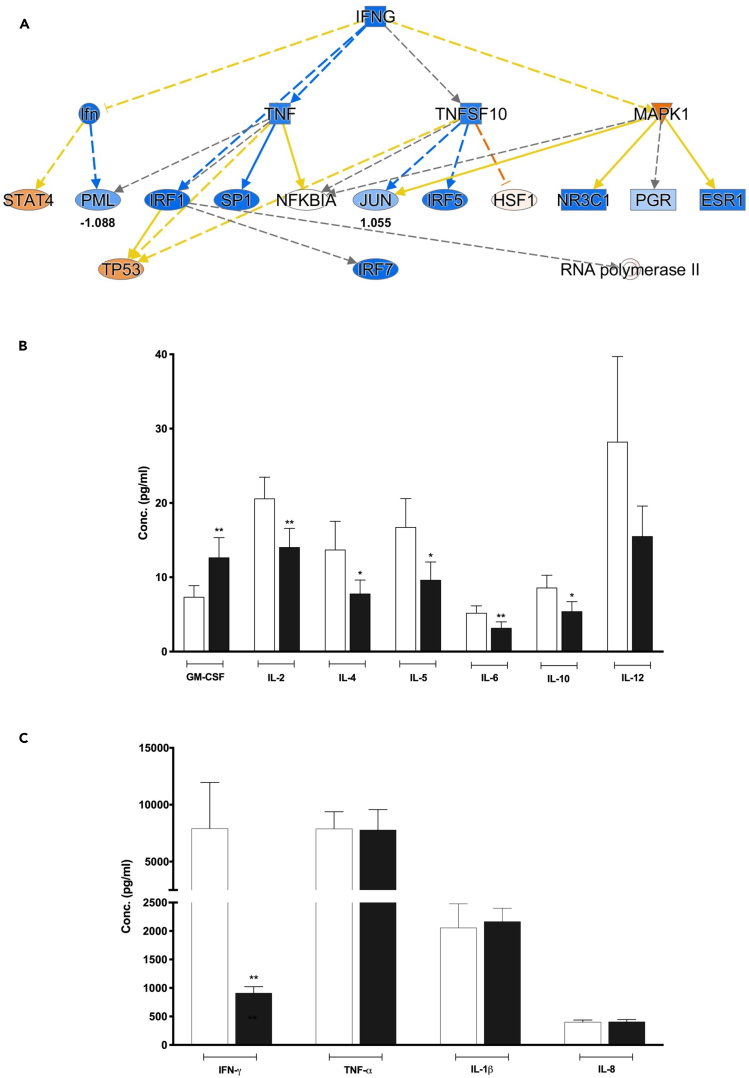
Figure 5.
IFN-gamma networks and cytokines modulation in Lpb. plantarum pre-treated inflamed NCM40 cells
(A) IFN-gamma mechanistic networks. Upstream regulator analysis of IPA software highlights that IFN-gamma is significantly inhibited in pre-treated cells compared to inflamed ones. Colors represent predicted activation (orange) or inhibition (blue), and their intensity is correlated proportionally to the significance of the predicted activation or inhibition. Lines represent the predicted direct (solid lines) or indirect (dotted lines) relationship among the genes. Color of the lines represent the activation (orange), inhibition (blue), inconsistent (yellow) and not predicted (gray) relationships. Finally, the type of the molecule is represented by a different shape, being square forms for cytokine, ellipse forms for transcription regulators, rectangular forms for ligand-dependent nuclear receptors and double circles form for groups or complexes.
(B and C) Lpb. plantarum C9O4 modulation of a set of cytokines release on inflamed NCM460 cells. Values are expressed as mean ± SEM ∗∗p < 0.005 and ∗p < 0.05 versus inflamed control release (ELISA).
In this contest, we highlighted the overall ability of Lpb. plantarum C9O4 to modulate pro- and anti-inflammatory cytokines in the inflamed cell model. Indeed, pre-treatment with Lpb. plantarum C9O4 had a protective role on inflammation since it was able to significantly reduce levels of interleukins, i.e., IL-2, IL-5, IL-6, and IFN-γ (Figure 5B e 5C); interestingly a similar, but not significant, trend was also observed in IL-12 content (Figure 5B) corroborated by proteomics data with no modulation of IL-12 (Z score not predicted, see Table 1) in cells pre-treated with Lpb. plantarum.
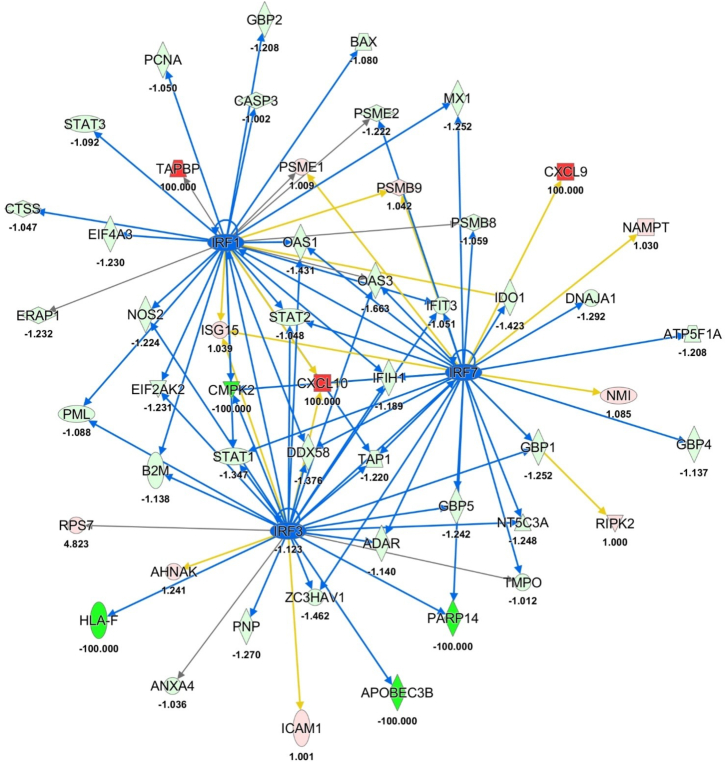
Moreover, between quantified proteins signal transducer and activator of transcription 1 (STAT1) seemed to have a notable role in the reverting the inflammatory effects in NCM460 Lpb. plantarum C9O4 pre-treated cells because it was found significantly downregulated in these cell models (expression fold change = −1.347, -log(p value) = 2.46, see Figure 2C and Table S1). On the contrary, it was significantly upregulated in NCM460 cells subjected to inflammatory stimulus with an expression fold change of 2.14 and a log(p value) of 6.13 (see Figure 2B; Table S1). In addition, it was directly connected to the significative predict inhibition of IRF1, IRF3, and IRF7 as upstream regulators in pre-treated cellular model (Figure 6). As reported in Table 1, STAT1 was counted among the most significant upstream regulators predicted by IPA tool too. In particular, it showed the same trend that is the strong activation of STAT1 in the inflamed cells compared to control ones (p value = 0.11, Z score = 4.20), and it was found unmodulated in the second comparison (pre-treated/inflamed) with a p value of 0.06 and Z score of −1.35, indicating a strong modulation of the immune system after the incubation with the Lpb. plantarum strain that mirrored the capability of reversing the effects of inflammation.
Figure 6.
The interaction network of IRF1, IRF3, and IRF7
In the figure is depicted the interaction network of IRF1, IRF3, and IRF7 as inhibited upstream regulator in Lpb. plantarum C9O4 pre-treated cells compared to inflamed ones. Lines represent the predicted direct (solid lines) relationship among the genes and quantified proteins. Color of the lines represent the inhibition (blue), inconsistent (yellow), and not prediction (gray). Instead, green and red shapes show the down-expressed and the up-expressed proteins, respectively, with their expression fold change.
Discussion
In recent years there has been an increasing number of studies that evaluate the potential role of fermented food-associated strains as potential probiotics.4,17,18,19,20 In particular, we have previously demonstrated that Lpb. plantarum strains can protect intestinal epithelial cells from oxidant stress by modulating reactive oxygen species and IL-23/IL-17 axis.16 In this regard, the strain C9O4, isolated from table olives and previously tested for its ability to interact with human epithelial cells,14 had a positive role in decreasing the release of both cytokines under inflammation conditions. However, despite the evidence of a positive regulation of the inflammatory response, underlying molecular mechanisms of this host-microbe dialog remain unclear. Using a proteomic approach, this study aimed to determine whether Lpb. plantarum C9O4 could modulate the deleterious effects of a pro-inflammatory cocktail of cytokines on intestinal epithelial cells and to identify the possible mechanisms responsible for this modulation.
Our data demonstrated that Lpb. plantarum C9O4 pre-treatment reverses the effects of the inflammatory cocktail consisting of IFN-γ, TNF-α, and IL-1β. Co-incubation with bacteria alters the global protein profile of the host intestinal cells after the inflammation insult, suggesting that there is an active communication between cells-bacteria that significantly changes their biological and molecular functions. In comparison with inflamed cells, those that were also treated with Lpb. plantarum C9O4 showed a protein expression profile mainly characterized by a reduction of the inflammation markers, including those used as inflammation insult, IFN-γ and TNF-α (Table 1).
The reduction in the expression of IFN-γ could be a very interesting probiotic feature, since its role has been proved in decreasing intestinal barrier function by reducing the localization and the expression of tight junction proteins, such as occludin and zonulin-1 (ZO-1).21,22,23 In this context, we should recall that positive effects of some LAB attenuating the effects of IFN-γ have been previously demonstrated. In particular, protein metabolites secreted by Lacticaseiobacillus rhamnosus strain GG (LGG) attenuates barrier dysfunction evoked by IFN-γ in human enteroids.24
IFN-γ and TNF-α effects are subject to complex cross-regulatory networks that evoke the activation and release of specific kinases, cytokines, and cofactors, and in turn, is negatively regulated secondary to induced expression of various suppressor of cytokine signaling (SOCS) proteins. These latter contribute to the constitutive regulation of cytokine responses in a variety of cell types. In particular, the intracellular signaling pathways induced by IFN-γ rely on the activation of the signal transducer factors STAT1 and STAT3. JAK-STAT signaling has been identified as a key mediator in the immune regulatory processes.25,26 IFN-γ activates the pathway through its receptors IFNGR1 and IFNGR2 which, in turn and in association with JAK1 and JAK2, lead to the phosphorylation of STAT1. Once STAT1 is phosphorylated, it is translocated to the nucleus and binds IFN-γ activation site (GAS) elements, initiating the transcription of IFN-γ induced genes and activating the immune response.25 Proteomic overview of the first comparison (inflamed/control) revealed that the cytokine cocktail upregulated proteins involved in inflammation, being especially important interferon related genes, which result to be significantly downregulated after the pre-treatment with the Lpb. plantarum strain. The upstream regulator analysis of the different proteomic profiles points to IRF7, IRF3, and STAT1 as the main drivers of such modulation (Figure 6). Previous studies have already showed the ability of some strains to revert the deleterious effects of IFN-γ on epithelial cells by modulating JAK/STAT pathway.27,28,29 In particular, a combination of Lacticaseibacillus rhamnosus GG and Streptococcus thermophilus have antagonistic effects against IFN- γ by inhibiting phosphorylation of SOCS3 and STAT3,30,31 and a similar behavior has been also reported for the probiotic mixture VSL#3.32,33 Lately, other probiotics species have been recognized as potential dietary supplements for the reduction of inflammation associated with chronic intestinal diseases by modulating JAK/STAT and NF-κB pathways.34,35,36 Moreover, proteomic results showed a significant upregulation of many pro-inflammatory cytokines in inflamed cells, such as TNF-α and IFN-γ, whose synergistic action could promote and trigger apoptotic processes, as reported by Li et al.37 It has been identified JAK1/2 as the sole kinase drivers of the intestinal epithelial cell death triggered by IFN-γ/TNF-α,38 possibly as a result of chronic STAT1 and NF-kB signaling. These increased levels of STAT1 phosphorylated and active have been also detected in inflamed mucosa of patients affected by chronic disease.39,40,41 However, it is worth highlighting that the concomitant exposure of intestinal cells to inflammation stimulus and Lpb. plantarum C9O4 reverts the inflammatory processes; thus, this event, together with the reduction in the release of pro-inflammatory cytokines, makes the Lpb. plantarum strains a suitable candidate to be considered as a probiotic.
Based on our results, Lpb. plantarum C9O4 plays an immunoregulatory role by stimulating the IFN-γ mediated JAK/STAT pathway, resulting in downregulation of interferon-stimulating genes (ISG). Preliminary experiments (data not shown) encourage us to hypothesize that Lpb. plantarum C9O4 might be decreasing the phosphorylation of the transcription factor STAT-1, whereas not interfering on the phosphorylation of ERK. However, as already pointed out in literature, modulation of the immune system by ingested bacteria appears to be a complex and multifactorial process, in which the direct contact with cells through the Toll-like receptors or the release of bioactive compounds might act synergistically. Due to the importance of the JAK/STAT pathway, inhibitors of this pathway might come to constitute an innovative therapeutic agent for the management of gastrointestinal inflammatory diseases.
Limitations of the study
Our study elucidates a protective role on inflammation by food-associated Lpb. plantarum C9O4 via significantly reducing levels of inflammation biomarkers which in turn could contribute to reverting the apoptosis process in an intestinal cell model, through the IFN-γ mediated JAK/STAT pathway that seems to be the major immunomodulatory mechanism of action. However, IFN-γ is subject to a complex cross-regulatory network involving the release of many specific kinases, cytokines, and cofactors that remains still not well elucidated within the host-microbe dialog. Although preliminary experimental data lead to hypothesize that Lpb. plantarum C9O4 might be decreasing the phosphorylation of STAT-1, but not of ERK, we expect to confirm this aspect at molecular level in future studies.
Due to the relevance of the JAK/STAT pathway as a therapeutic target, it could be of paramount importance to verify the protective role of Lpb. plantarum C9O4 in in vivo studies, in order to confirm the suitability of this selected strain as an alternative dietary strategy for the management of chronic intestinal inflammatory diseases.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Lactiplantibacillus plantarum C9O4 | Isolated from table olives (Prete et al.113) University of Teramo | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| MRS broth ISO 15214 | Liofilchem srl, Roseto degli Abruzzi, Italy | Cat#610025 |
| Phosphate Buffered Saline (PBS) | Sigma-Aldrich, Saint Louis, MO, United States | Cat#P4417 |
| Penicillin/Streptomycin 100X | Corning, NY, United States | Cat#3002-CI |
| Fetal Bovine Serum (FBS) | Corning, NY, United States | Cat#35-010-CV |
| M3:BaseF™medium | INCELL Corporation, LLC, San Antonio, TX, United States | Cat#M300F-500 |
| Non-essential Amino Acids 100x Solution | Euroclone, Pero, Italy | Cat#ECB3054D |
| Recombinant Human TNF-alpha | R&D System, Minneapolis, Minnesota, United States | Cat#210-TA-020 |
| Recombinant Human IL-1 beta/IL-1F2 | R&D System, Minneapolis, Minnesota, United States | Cat#201-LB-005 |
| Recombinant Human IFN-γ | R&D System, Minneapolis, Minnesota, United States | Cat#285-IF-100 |
| Critical commercial assays | ||
| LUNARIS™Technology multiplex protein analysis | AYOXXA Biosystem GmbH | https://www.ayoxxa.com |
| Deposited data | ||
| mass spectrometry proteomics data | This paper | Database: ProteomeXchange Consortium via the PRIDE partner repository; dataset identifier PXD04217546 |
| Experimental models: Cell lines | ||
| NCM460 | INCELL Corporation, LLC, San Antonio, TX, United States | RRID: CVCL_0460 |
| Software and algorithms | ||
| MaxQuant version 1.6.10.50 | Max-Planck Institute for Biochemistry, Martinsried, Germany | RRID:SCR_014485 https://www.maxquant.org/ |
| PERSEUS | MaxPlank Institute | RRID:SCR_015753 https://maxquant.net/perseus/ |
| UniProt database | The UniProt consortium | RRID:SCR_002380 http://www.uniprot.org/ |
| Ingenuity Pathway Analysis | Qiagen, Hilden, Germany | RRID:SCR_008653tt http://www.ingenuity.com/products/pathways_analysis.html |
| PANTHER CLASSIFICATION SYSTEM | Mi et al.42 | RRID:SCR_004869 http://www.pantherdb.org/about.jsp |
| PRIDE PROTEOMICS IDENTIFICATIONS DATABASE | Perez-Riverol Y et al.43 | RRID:SCR_003411 https://www.ebi.ac.uk/pride/ |
| Prism 8.3 software | GraphPad Software Inc., La Jolla, CA, United States | RRID:SCR_002798 http://www.graphpad.com/ |
| Other | ||
| Costar® 24-well Clear TC-treated Multiple Well Plates | Corning, NY, United States | Cat#3527 |
| Costar® 6-well Clear TC-treated Multiple Well Plates | Corning, NY, United States | Cat#3506 |
| UltiMate™3000 UPLC | Thermo Fisher Scientific, Waltham, MA, USA | N/A |
| Orbitrap Fusion™ Tribrid™ | Thermo Fisher Scientific, Waltham, MA, USA | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Prof. Aldo Corsetti (acorsetti@unite.it).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD04217546.
-
•
This paper does not report original code.
-
•
Any additional information required to re-analyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
Bacterial strain
Lpb. plantarum strain C9O4 was isolated from table olives and belongs to our laboratory collection at the University of Teramo. The strain investigated in this study has been used in previous studies, characterized for several properties, and selected for its antioxidant and anti-inflammatory properties.16 Lpb. plantarum C9O4 cells were routinely grown under microaerophilic conditions at 37°C in de Man, Rogosa and Sharp (MRS) broth (Liofilchem srl, Roseto degli Abruzzi, Italy) to obtain overnight cultures. Then, the inoculum concentration (109 CFU/ml) was checked turbidimetrically by measuring optical density at 600 nm. Subsequently, MRS broth was removed by centrifugation (13000 x g, 10 min, 4°C), microbial cells were washed twice in the same volume with sterile PBS and finally resuspended in sterile PBS at 109 CFU/ml before each experiment, according to Prete et al. 2020.16
Intestinal inflammation model
NCM460 cells (INCELL Corporation, LLC, San Antonio, TX, United States), originally derived from the normal colon mucosa of a 68-year old Hispanic male,44 were grown in INCELL’s enriched M3Base medium supplemented with 1% (v/v) Penicillin/Streptomycin 100X (Corning, NY, United States), 1 % (v/v) Non-Essential Amino Acids 100 X solution (Euroclone, Pero, Italy), and 10 % (v/v) heated inactivated Faetal Bovine Serum (FBS; Corning, NY, United States). Cells were grown in culture dishes at 37°C in a 5 % CO2 atmosphere and seeded at the P9 passage (1.5x105 cells/well in 24-well plate (Corning, NY, United States) for cytokine detection and 3x106 cells/well in 6-well plate (Corning, NY, United States) for proteomic analysis) 24 hours prior the co-incubation conditions, for letting them reach confluence.
Once confluent monolayers were achieved, medium was removed, and cells were exposed to Lpb. plantarum strains as pre-treatment for 4 hours (∼100 bacteria/cell), as reported in previous studies.14,16 After the incubation, unbound strains were removed with 2 washing steps with sterile PBS. Subsequently, inflammation was induced for 24 hours using an inflammatory cocktail consisting of 10 ng/ml TNF-α, 5 ng/ml IL-1β, and 10 ng/ml IFN- γ14. Supernatants were collected for cytokines analysis and cells were recovered to perform proteomic analysis.
Method details
Cytokines modulation
Evaluation of the cytokines’ modulation on inflamed cells with and without co-incubation with Lpb. plantarum C9O4 was performed through the high-throughput method for multiplex protein analysis (LUNARIS Technology, AYOXXA Biosystem GmbH), as previously described in Prete et al.16
Proteomics and bioinformatics analyses
We investigated the proteome profile of NCM460 cells by comparing the protein expression of control cells (negative control) and cells pre-treated with Lpb. plantarum C9O4 with those exposed to an inflammatory stimulus (inflamed). In particular, we performed proteomics analysis on one biological replicate for each different treatment. All samples were prepared for Filter Aided Sample Preparation (FASP) method in order to digest 50 μg of proteins for each treatment. For label free proteomics analyses, each cell treatments were analysed in triplicate (technical replicates) by using the UltiMateTM 3000 UPLC (Thermo Fisher Scientific, Waltham, MA, USA) chromatographic system coupled to the Orbitrap FusionTM TribridTM (Thermo Fisher Scientific) mass spectrometer, according to the LC-MS/MS parameters specifications reported in our previous works.45,46 Proteomics raw file were processed using the MaxQuant version 1.6.10.50 (Max-Planck Institute for Biochemistry, Martinsried, Germany) matching spectra against the UniProt database (released 2018_04, taxonomy Homo sapiens, 20,874 entries). LFQ Intensity values were used to quantify protein abundance in each sample whenever the protein was quantified in at least two analytical replicates for each cell treatment. All processing parameters are described in Damiani et al.47 Protein expression variability was evaluated by density plot with Pearson correlation (R2) values of mean LFQ intensities value-transformed to log2 scale and reported as density plots (Figures 3 and 4). PANTHER Classification System was used for Gene Ontology protein reclassifications (Panel B of Figures 3 and 4).
Finally, protein ratios (inflamed cells/negative control and pre-treated cells/inflamed cells) were used for “Core Analysis” performed through Ingenuity Pathway Analysis (IPA software, Qiagen, Hilden, Germany), which is able to predict the activation (z-scores ≥ 2.0) or inhibition (z-scores ≤ −2.0) of transcriptional regulators or downstreams for the loaded protein dataset thanks to all published literature citations stored in the IPA system. “Comparison Analysis” was used to further compare the functional information related to upstream regulators and diseases and biofunctions. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD04217546.
Quantification and statistical analysis
Cytokines results are reported as mean ± SD of triplicate experiments. Data were analyzed by means of Prism 8.3 software (GraphPad Software Inc., La Jolla, CA, United States) using unpaired Student’s t-test. A level of p < 0.05 was considered statistically significant.
Acknowledgments
The authors gratefully acknowledge the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie (grant agreement 713714 ESR 07 to N.G.G. and A.C.), the Autism Research Institute (grant 2021 to N.B.) and Fondazione CARISPAQ (grant 2022) for the financial support.
Author contributions
Conceptualization, A.C., N.B., and P.D.B.; Methodology, M.C.C. and R.P.; Investigation, M.C.C., R.P., F.D.M., and N.G.G.; Validation, A.C., N.B., and P.D.B.; Data curation, M.C.C. and R.P.; Writing – original draft, M.C.C., R.P., and N.G.G.; Writing – review & editing, M.C.C., R.P., and G.S.; Visualization, M.C.C. and R.P.; Funding Acquisition, N.B., A.C., and N.G.G; Resource, A.C., N.B., and P.D.B.; Supervision, A.C., N.B., and P.D.B.
Declaration of interests
The authors declare no competing interests.
Published: November 17, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108481.
Supplemental information
References
- 1.Cristofori F., Dargenio V.N., Dargenio C., Miniello V.L., Barone M., Francavilla R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.578386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mentella M.C., Scaldaferri F., Pizzoferrato M., Gasbarrini A., Miggiano G.A.D. Nutrition, IBD and Gut Microbiota: A Review. Nutrients. 2020;12:944. doi: 10.3390/nu12040944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guandalini S., Sansotta N. Probiotics in the Treatment of Inflammatory Bowel Disease. Adv. Exp. Med. Biol. 2019;1125:101–107. doi: 10.1007/5584_2018_319. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Gonzalez N., Battista N., Prete R., Corsetti A. Health-Promoting Role of Lactiplantibacillus plantarum Isolated from Fermented Foods. Microorganisms. 2021;9:349. doi: 10.3390/microorganisms9020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corsetti A., Prete R., Garcia-Gonzalez N. In: Reference Module in Food Science. Lee A., editor. Elsevier; 2018. Lactic Acid Bacteria: Lactobacillus spp.: Lactobacillus plantarum; pp. 1–9. [Google Scholar]
- 6.Le B., Yang S.H. Efficacy of Lactobacillus plantarum in prevention of inflammatory bowel disease. Toxicol Rep. 2018;5:314–317. doi: 10.1016/j.toxrep.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scaldaferri F., Gerardi V., Lopetuso L.R., Del Zompo F., Mangiola F., Boškoski I., Bruno G., Petito V., Laterza L., Cammarota G., et al. Gut microbial flora, prebiotics, and probiotics in IBD: their current usage and utility. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/435268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka A., Kanmura S., Morinaga Y., Kawabata K., Arima S., Sasaki F., Nasu Y., Tanoue S., Hashimoto S., Takeshita M., et al. Oral administration of Lactobacillus plantarum 06CC2 prevents experimental colitis in mice via an anti-inflammatory response. Mol. Med. Rep. 2020;21:1181–1191. doi: 10.3892/mmr.2020.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vetuschi A., Battista N., Pompili S., Cappariello A., Prete R., Taticchi A., Selvaggini R., Latella G G., Corsetti A., Sferra R. The antiinflammatory and antifibrotic effect of olive phenols and Lactiplantibacillus plantarum IMC513 in dextran sodium sulfate-induced chronic colitis. Nutrition. 2022;94 doi: 10.1016/j.nut.2021.111511. [DOI] [PubMed] [Google Scholar]
- 10.Ahrne S., Hagslatt M.L.J. Effect of lactobacilli on paracellular permeability in the gut. Nutrients. 2011;3:104–117. doi: 10.3390/nu3010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu X., Wei M., Yang D., Wu X., Wei H., Xu F. Lactiplantibacillus plantarum Strain FLPL05 Promotes Longevity in Mice by Improving Intestinal Barrier. Probiotics Antimicrob. Proteins. 2023;15:1193–1205. doi: 10.1007/s12602-022-09933-5. [DOI] [PubMed] [Google Scholar]
- 12.Binda S., Hill C., Johansen E., Obis D., Pot B., Sanders M.E., Tremblay A., Ouwehand A.C. Criteria to Qualify Microorganisms as "Probiotic" in Foods and Dietary Supplements. Front. Microbiol. 2020;11:1662. doi: 10.3389/fmicb.2020.01662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prete R., Tofalo R., Federici E., Ciarrocchi A., Cenci G., Corsetti A. Food-Associated Lactobacillus plantarum and Yeasts Inhibit the Genotoxic Effect of 4-Nitroquinoline-1-Oxide. Front. Microbiol. 2017;8:2349. doi: 10.3389/fmicb.2017.02349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Gonzalez N., Prete R., Battista N., Corsetti A. Adhesion Properties of Food-Associated Lactobacillus plantarum Strains on Human Intestinal Epithelial Cells and Modulation of IL-8 Release. Front. Microbiol. 2018;9:2392. doi: 10.3389/fmicb.2018.02392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Gonzalez N., Nuñez-Sanchez M.A., Villoria Recio M., Battista N., Gahan C.G.M., Corsetti A. Immunomodulation of J774A.1 Murine Macrophages by. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.557143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prete R., Garcia-Gonzalez N., Di Mattia C.D., Corsetti A., Battista N. Food-borne Lactiplantibacillus plantarum protect normal intestinal cells against inflammation by modulating reactive oxygen species and IL-23/IL-17 axis. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-73201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prete R., Long S.L., Joyce S.A., Corsetti A. Genotypic and phenotypic characterization of food-associated Lactobacillus plantarum isolates for potential probiotic activities. FEMS Microbiol. Lett. 2020;367:fnaa076. doi: 10.1093/femsle/fnaa076. [DOI] [PubMed] [Google Scholar]
- 18.Roobab U., Batool Z., Manzoor M.F., Shabbir M.A., Khan M.R., Aadil R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020;32:17–28. [Google Scholar]
- 19.Marco M.L., Heeney D., Binda S., Cifelli C.J., Cotter P.D., Foligné B., Gänzle M., Kort R., Pasin G., Pihlanto A., et al. Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Marco M.L., Sanders M.E., Gänzle M., Arrieta M.C., Cotter P.D., De Vuyst L., Hill C., Holzapfel W., Lebeer S., Merenstein D., et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021;18:196–208. doi: 10.1038/s41575-020-00390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madara J.L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J. Clin. Invest. 1989;83:724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Sadi R., Boivin M., Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bardenbacher M., Ruder B., Britzen-Laurent N., Schmid B., Waldner M., Naschberger E., Scharl M., Müller W., Günther C., Becker C., et al. Permeability analyses and three dimensional imaging of interferon gamma-induced barrier disintegration in intestinal organoids. Stem Cell Res. 2019;35 doi: 10.1016/j.scr.2019.101383. [DOI] [PubMed] [Google Scholar]
- 24.Han X., Lee A., Huang S., Gao J., Spence J.R., Owyang C. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut Microb. 2019;10:59–76. doi: 10.1080/19490976.2018.1479625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen K.L., Brockwell N.K., Parker B.S. JAK-STAT Signaling: A Double-Edged Sword of Immune Regulation and Cancer Progression. Cancers. 2019;11 doi: 10.3390/cancers11122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lei H., Crawford M.S., McCole D.F. JAK-STAT Pathway Regulation of Intestinal Permeability: Pathogenic Roles and Therapeutic Opportunities in Inflammatory Bowel Disease. Pharmaceuticals. 2021;14:840. doi: 10.3390/ph14090840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plaza-Diaz J., Gomez-Llorente C., Fontana L., Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J. Gastroenterol. 2014;20:15632–15649. doi: 10.3748/wjg.v20.i42.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sang L.X., Chang B., Wang B.Y., Liu W.X., Jiang M. Live and heat-killed probiotic: effects on chronic experimental colitis induced by dextran sulfate sodium (DSS) in rats. Int. J. Clin. Exp. Med. 2015;8:20072–20078. [PMC free article] [PubMed] [Google Scholar]
- 29.Llewellyn A., Foey A. Probiotic Modulation of Innate Cell Pathogen Sensing and Signaling Events. Nutrients. 2017;9:1156. doi: 10.3390/nu9101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latvala S., Miettinen M., Kekkonen R.A., Korpela R., Julkunen I. Lactobacillus rhamnosus GG and Streptococcus thermophilus induce suppressor of cytokine signalling 3 (SOCS3) gene expression directly and indirectly via interleukin-10 in human primary macrophages. Clin. Exp. Immunol. 2011;165:94–103. doi: 10.1111/j.1365-2249.2011.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayoc-Becerra A., Krishnan M., Fan S., Jimenez J., Hernandez R., Gibson K., Preciado R., Butt G., McCole D.F. The JAK-Inhibitor Tofacitinib Rescues Human Intestinal Epithelial Cells and Colonoids from Cytokine-Induced Barrier Dysfunction. Inflamm. Bowel Dis. 2020;26:407–422. doi: 10.1093/ibd/izz266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Do E.J., Hwang S.W., Kim S.Y., Ryu Y.M., Cho E.A., Chung E.J., Park S., Lee H.J., Byeon J.S., Ye B.D., et al. Suppression of colitis-associated carcinogenesis through modulation of IL-6/STAT3 pathway by balsalazide and VSL#3. J. Gastroenterol. Hepatol. 2016;31:1453–1461. doi: 10.1111/jgh.13280. [DOI] [PubMed] [Google Scholar]
- 33.Cheng F.S., Pan D., Chang B., Jiang M., Sang L.X. Probiotic mixture VSL#3: An overview of basic and clinical studies in chronic diseases. World J. Clin. Cases. 2020;8:1361–1384. doi: 10.12998/wjcc.v8.i8.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aghamohammad S., Sepehr A., Miri S.T., Najafi S., Pourshafie M.R., Rohani M. Anti-inflammatory and immunomodulatory effects of Lactobacillus spp. as a preservative and therapeutic agent for IBD control. Immun. Inflamm. Dis. 2022;10:e635. doi: 10.1002/iid3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aghamohammad S., Sepehr A., Miri S.T., Najafi S., Rohani M., Pourshafiea M.R. The effects of the probiotic cocktail on modulation of the NF-kB and JAK/STAT signaling pathways involved in the inflammatory response in bowel disease model. BMC Immunol. 2022;23:8. doi: 10.1186/s12865-022-00484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun M., Liu Y., Song Y., Gao Y., Zhao F., Luo Y., Qian F., Mu G., Tuo Y. The ameliorative effect of Lactobacillus plantarum-12 on DSS-induced murine colitis. Food Funct. 2020;11:5205–5222. doi: 10.1039/d0fo00007h. [DOI] [PubMed] [Google Scholar]
- 37.Li X., Shang B., Li Y.N., Shi Y., Shao C. IFNγ and TNFα synergistically induce apoptosis of mesenchymal stem/stromal cells via the induction of nitric oxide. Stem Cell Res. Ther. 2019;10:18. doi: 10.1186/s13287-018-1102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woznicki J.A., Saini N., Flood P., Rajaram S., Lee C.M., Stamou P., Skowyra A., Bustamante-Garrido M., Regazzoni K., Crawford N., et al. TNF-α synergises with IFN-γ to induce caspase-8-JAK1/2-STAT1-dependent death of intestinal epithelial cells. Cell Death Dis. 2021;12:864. doi: 10.1038/s41419-021-04151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schreiber S., Rosenstiel P., Hampe J., Nikolaus S., Groessner B., Schottelius A., Kühbacher T., Hämling J., Fölsch U.R., Seegert D. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut. 2002;51:379–385. doi: 10.1136/gut.51.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stolzer I., Dressel A., Chiriac M.T., Neurath M.F., Günther C. An IFN-STAT Axis Augments Tissue Damage and Inflammation in a Mouse Model of Crohn's Disease. Front. Med. 2021;8 doi: 10.3389/fmed.2021.644244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y.L., Chen M., Zhu H., Zhuo M.X., Chen P., Mao Y.J., Li L.Y., Zhao Q., Wu M., Ye M. STAT1 epigenetically regulates LCP2 and TNFAIP2 by recruiting EP300 to contribute to the pathogenesis of inflammatory bowel disease. Clin. Epigenetics. 2021;13:127. doi: 10.1186/s13148-021-01101-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mi H., Muruganujan A., Thomas P.D. PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 2013;41:D377–D386. doi: 10.1093/nar/gks1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Riverol Y., Bai J., Bandla C., García-Seisdedos D., Hewapathirana S., Kamatchinathan S., Kundu D.J., Prakash A., Frericks-Zipper A., Eisenacher M., et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–D552. doi: 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moyer M.P., Manzano L.A., Merriman R.L., Stauffer J.S., Tanzer L.R. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell. Dev. Biol. Anim. 1996;32:315–317. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- 45.Madonna R., Moscato S., Polizzi E., Pieragostino D., Cufaro M.C., Del Boccio P., Bianchi F., De Caterina R., Mattii L. Connexin 43 and Connexin 26 Involvement in the Ponatinib-Induced Cardiomyopathy: Sex-Related Differences in a Murine Model. Int. J. Mol. Sci. 2021;22:5815. doi: 10.3390/ijms22115815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potenza F., Cufaro M.C., Di Biase L., Panella V., Di Campli A., Ruggieri A.G., Dufrusine B., Restelli E., Pietrangelo L., Protasi F., et al. Proteomic Analysis of Marinesco-Sjogren Syndrome Fibroblasts Indicates Pro-Survival Metabolic Adaptation to SIL1 Loss. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222212449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damiani V., Cufaro M.C., Fucito M., Dufrusine B., Rossi C., Del Boccio P., Federici L., Turco M.C., Sallese M., Pieragostino D., De Laurenzi V. Proteomics Approach Highlights Early Changes in Human Fibroblasts-Pancreatic Ductal Adenocarcinoma Cells Crosstalk. Cells. 2022;11:1160. doi: 10.3390/cells11071160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD04217546.
-
•
This paper does not report original code.
-
•
Any additional information required to re-analyze the data reported in this paper is available from the lead contact upon request.