ABSTRACT
The function of Vasohibin-1 (VASH1) in human cancer has not been thoroughly or comprehensively examined. Here, we identified the tumor suppressor part of VASH1 across cancers, including epithelial ovarian tumors. Our study carefully contrasted the expression of VASH1 in pancancer and nontumorous tissues in a public database to explore its regulatory role in clinical prognosis, diagnosis, tumor purity, and immune cell infiltration. Next, we explored the antitumor mechanism of VASH1 through drug sensitivity, functional enrichment, and phenotypic experiments in ovarian cancer. Research suggests that the expression of VASH1 in neoplastic tissues is lower than that in normal tissues. VASH1 affects the OS and RFS of several tumor types. In addition, VASH1 expression resulted in a high OS and RFS in the diagnosis of tumor and nontumor tissues and negatively regulated tumor purity. Moreover, VASH1 controls the tumor microenvironment by regulating immunocyte infiltration. In ovarian cancer, VASH1 can serve as a biomarker to estimate the efficacy of chemotherapy. Functional enrichment analysis suggests that VASH1 plays a tumor suppressor role by regulating the extracellular matrix receptor pathway. VASH1 inhibition of the malignant phenotype of ovarian cancer cells was further confirmed by in vivo experiments. These results indicate that VASH1 acts as a cancer-inhibiting factor and potential therapeutic target in ovarian cancer.
KEYWORDS: VASH1, ovarian cancer, suppressor, tumor purity, immune cell infiltration
Introduction
Ovarian cancer is a major cause of death in patients with gynecological malignancies. Epithelial ovarian cancer is the most common type of cancer and has a very low five-year survival rate.1 Although rapid progress has been made in tumor cytoreductive surgery, cisplatin-based chemotherapy, targeted drugs, and immunotherapy, the clinical results in ovarian cancer patients remain unsatisfactory because most patients have local progression and extensive distant metastases when they are diagnosed.2 Although most ovarian cancer patients respond to treatment at the beginning, nearly three-quarters of patients relapse within three years.3
Watanabe et al. first reported and named VASH1 in 2004.4 VASH1 is a member of the angiostatin family of proteins. Inhibition of tumor angiogenesis has been shown to be an effective therapeutic target for cancer patients. To date, diverse proangiogenic factors and angiogenesis inhibitors have been used clinically.5 Research has confirmed that endothelial cells express VASH1, which can inhibit migration, proliferation, and angiogenesis by controlling the biological function of endothelial cells.6–8 VASH1 is not limited to endothelial cells; at the same time, it is also expressed in human tumor cells.9,10 Research has shown that VASH1 plays a major role in a variety of malignant tumors, including prostate cancer, upper urinary tract urothelial carcinomas, lung cancer, and cervical tumors.11–16 Studies have shown that VASH1 exerts antitumor effects by suppressing vascularization in the TME.17,18
Nevertheless, the role of VASH1 in human pancancer has not yet been comprehensively investigated. Therefore, in our research, we first analyzed the distinct expression of VASH1 in different tumors and then explored the predictive value of VASH1 in clinical prognosis and diagnosis. We also studied the effects of VASH1 on tumor purity and immune cell infiltration. In an effort to further probe the relationship between VASH1 expression and biological significance, the research finally focused on ovarian cancer, exploring drug sensitivity, functional enrichment analysis, and regulation of the malignant phenotype. The outcome of this study implied the antitumor effect of VASH1 in pancancer, confirmed by bioinformatics analysis combining experiments, and provided evidence for new targets to inhibit the progression of ovarian cancer.
Materials & methods
Gepia
Gene Expression Profiling Interactive Analysis (http://gepia.cancer-pku.cn/) is a communal database for normal and tumor gene expression profiling, including RNA sequencing expression data of over 9700 tumor specimens and over 8500 normal samples. VASH1 expression levels in pancancer and nontumorous tissues were compared.
TNMplot database
The TNMplot database (http://www.tnmplot.com) is a online tool for gene expression level in normal, metastatic, and tumor tissues. We used this method to explore the expression level of VASH1 in diverse tumor and normal tissues.
TIMER database
TIMER (https://cistrome.shiny apps.io/timer/) utilizes RNA-seq expression profile data to detect the infiltration degree of multiple immune cells in neoplastic tissues. The DiffExp module allowed users to study the differential expression of VASH1 in all TCGA tumors and adjacent normal tissues.
Kaplan–Meier Plotter
The K–M Plotter (http://kmplot.com/analysis/) is a survival analysis website with complete and authoritative data, which was used to analyze overall survival and relapse-free survival.
LinkedOmics
We examined the link between VASH1 expression and the purity of multiple tumors using the LinkedOmics website (http://www.linkedomics.org/login.php). This website was also used to perform GO and KEGG analyses of genes related to VASH1 expression in ovarian cancer.
ROC plotter
The study used ROC plotter (http://www.rocplot.org) to probe VASH1 expression and drug response using transcriptome-level data from patients with breast, ovarian, and colorectal cancer, and glioblastoma.
Cell lines and cell culture
All ovarian cancer cell lines (A2780 RRID:CVCL_0134, Caov-3 RRID: CVCL_0203, OVCAR-3 RRID: CVCL_0465, and SK-OV-3 RRID: CVCL_0532) and normal epithelial cell lines (IOSE80, RRID: CVCL_5546) were purchased from Procell Life Technology Co., Ltd. (Wuhan, China) with supporting cell line authentication. The cells were cultured in RPMI 1640 medium (Gibco Company, New York, USA) containing ten percent fetal bovine serum, 100 µg/mL streptomycin, and 100 IU/mL penicillin in a 37°C incubator with 5% CO2.
Cell transfection
Prior to transfection, the cells were grown to cover 60% of the culture flasks. Each flask was cultured with approximately 5 ml serum-free medium, 5 μl Polyplus-Transfection ® (JetPRIME, USA), and 100 pmol pcDNA-VASH1 (General biol, China) or si-VASH1 (General biol, China). After 8 h, the medium was replaced with complete medium.
Quantitative real-time PCR
RT‒qPCR was performed to analyze VASH1 expression in the samples. RNA was purified using the Total RNA Kit (Omega), and a total of 1,000 μg RNA was reverse transcribed into cDNA using the RT‒PCR Transcriptor First Strand cDNA Synthesis Kit (Roche). qPCR was performed to detect the mRNA expression level using FastStart Universal SYBR Green Master (ROX) (Roche) with the Applied Biosystems StepOnePlus™ Real-Time PCR system (Thermo Fisher Scientific, Inc., USA). Human β-actin was used as an internal reference to determine mRNA expression. The expression levels were calculated using the 2‑ΔΔCq method.
Western blot analysis
Integral proteins were collected using RIPA buffer (BiYunTian, China). A bicinchoninic acid (BCA) kit (Biotech, China) was used to determine protein concentrations. A total of 20 μg of protein was separated using 12.5% SDS‒PAGE and transferred onto a PVDF membrane (Bio-Rad). After blocking with five percent skim milk for one hour at ambient temperature, the membrane was subsequently incubated in a solution of diluted rabbit polyclonal antibody to VASH1 (ABclonal, A6148, 1:1000).
Proliferation assay
A total of 3 × 103 cells were cultivated in 96-well plates, and the culture solution was replaced with fresh RPMI 1640 every day. The Cell Counting Kit-8 (BiYunTian, China) was used to test cell proliferation after 24, 48, and 72 h of incubation.
Colony formation assay
The cells were seeded into a 6-well plate at a density of 500 cells/well. At 37°C and 5% CO2, the cells were cultured for 2 weeks, and the solution was replaced with fresh RPMI1640 every 2 days. The culture was terminated when visible colonies were terminated. The cells were then washed with PBS and fixed with 4% paraformaldehyde for 15 min at room temperature. Finally, the cells were stained with 0.1% crystal violet (Solarbio, China) at room temperature for 20 min, and the staining solution was removed by washing. The colonies were photographed using a digital camera.
Wounding healing assay
Cells were seeded in 6-well plates and cultured until they covered the monolayer. A (yellow) pipette tip was used to draw a straight scratch to form a wound. Healing was observed under a microscope at 12 and 24 h, and a series of photographs were taken to document the process.
Statistical analysis
Each experiment was repeated independently at least three times. The statistical analysis in this study was carried out automatically using an online database. P values were calculated using an unpaired Student’s t test. P value < 0.05.
Results
Variant expression of VASH1 in human pancancer
We explored the differential expression of VASH1 in distinct carcinomas by using multiple databases. Comprehensive analysis of the GEPIA, TNMplot, and TIMER databases revealed that VASH1 expression levels were lower in ACC, LUAD, LUSC, OV, and UCEC than in normal tissues. The expression of VASH1 was higher in CHOL, ESCA, KIRC, KIRP, LIHC, SKCM, and THCA (Figure 1). The results revealed an opposite trend for VASH1 in different types of tumors.
Figure 1.
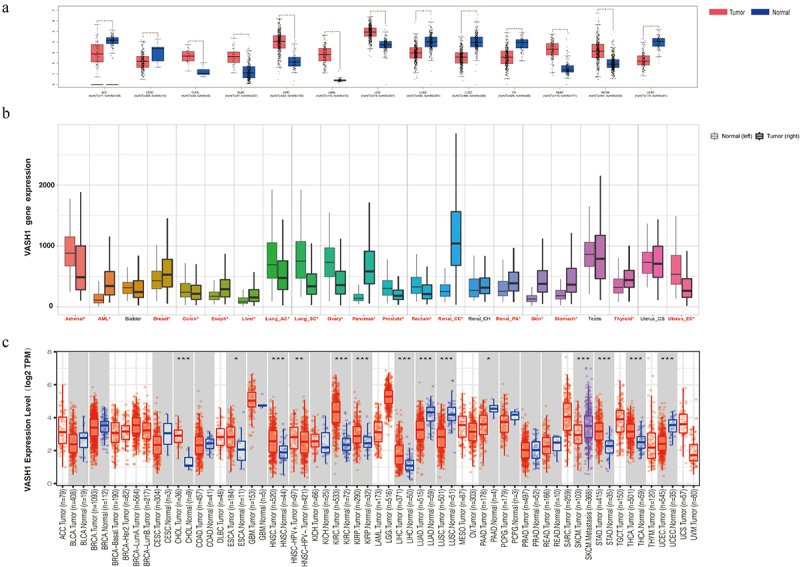
VASH1 expression level in human pan‐cancer and normal tissue. (a) GEPIA database, (b) TNMplot database, (c) TIMER database.
Effect of VASH1 expression on clinical prognosis
K-M plotter was used to predict the clinical prognosis associated with VASH1. As shown in Figure 2–e, patients with a high level of VASH1 mRNA had better overall survival in HNCS (HR = 0.65, P = 2.5e-03), KIRC (HR = 0.54, P = 5.6e-04), LUAD (HR = 0.67, P = 9.5e-03), PAAD (HR = 0.4, P = 6.5e-06), and READ (HR = 0.45, P = 4.0e-02). Similarly, patients with high levels of VASH1 mRNA had better RFS outcomes in LUSC (HR = 0.45, P = 2.4e-02), PAAD (HR = 0.30, P = 4.0e-02), OV (HR = 0.67, P = 4.0e-02), and UCEC (HR = 0.51, P = 1.3e-02) (Figure 2f–i).
Figure 2.
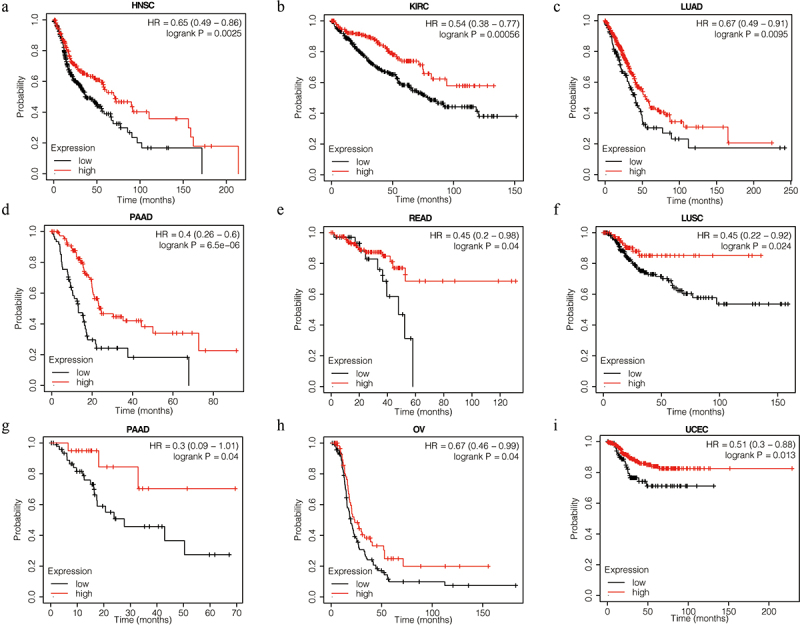
VASH1 expression is associated with OS and RFS. High expression of VASH1 correlates with better OS in HNSC (a), KIRC (b), LUAD (c), PAAD (d), and READ (e). High expression of VASH1 correlates with better RFS in LUSC (f), PAAD (g), OV (h), and UCEC (i). OS, overall survival; RFS, relapse-free survival; HNCS, head and neck squamous cell carcinoma; KIRC, kidney renal clear cell carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PAAD, pancreatic adenocarcinoma; READ, rectum adenocarcinoma; OV, ovarian serous cystadenocarcinoma; UCEC, uterine corpus endometrial carcinoma.
To further investigate the function of VASH1 in clinical diagnosis, we generated ROC curves for different tumors. The results showed that it could be used as a beneficial biomarker for the clinical diagnosis of CHOL (AUC = 0.991, CI = 0.970–1.000), KIRC (AUC = 0.933, CI = 0.908–0.959), LIHC (AUC = 0.749, CI = 0.683–0.815), LUAD (AUC = 0.854, CI = 0.804–0.904), LUSC (AUC = 0.911, CI = 0.870–0.953), OV (AUC = 0.883, CI = 0.847–0.918), STAD (AUC = 0.869, CI = 0.814–0.925), THCA (AUC = 0.783, CI = 0.716–0.850), and UCEC (AUC = 0.880, CI = 0.836–0.925) (Figure 3). These findings indicated that VASH1 is a valuable biomarker for clinical guidance.
Figure 3.
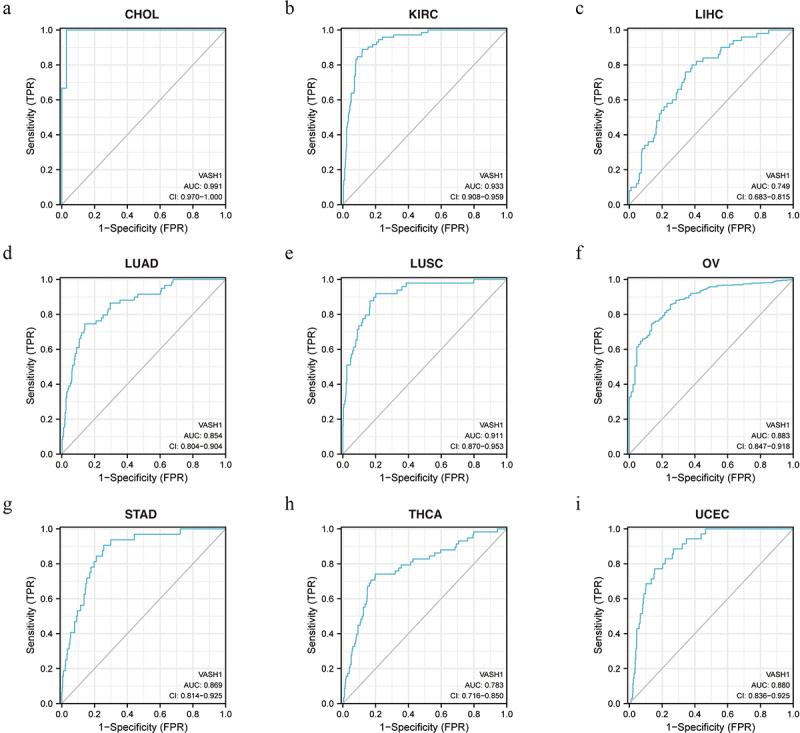
The function of VASH1 in clinical diagnosis. VASH1 could be used as a beneficial biomarker for clinical diagnosis in CHOL (a), KIRC (b), LIHC (c), LUAD (d), LUSC (e), OV (f), STAD (g), THCA (h) and UCEC (i). CHOL, cholangiocarcinoma; KIRC, renal clear cell carcinoma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; STAD, stomach adenocarcinoma; THCA, thyroid carcinoma; UCEC, uterine corpus endometrial carcinoma.
VASH1 negatively affected the purity of tumors
The LinkedOmics website was used to study the association between VASH1 expression and tumor purity. The results indicated that high expression of VASH1 led to a decrease in tumor purity in CESC (Spearman-Correlation= −0.405), COAD (Spearman-Correlation= −0.606), KIRC (Spearman-Correlation= −0.366), LGG (Spearman-Correlation= −0.139), LUAD (Spearman-Correlation= −0.482), LUSC (Spearman-Correlation= −0.493), OV (Spearman-Correlation= −0.355), PRAD (Spearman-Correlation= −0.458), and UCEC (Spearman-Correlation= −0.300), which verified that VASH1 was inversely related to tumor purity (Figure 4).
Figure 4.
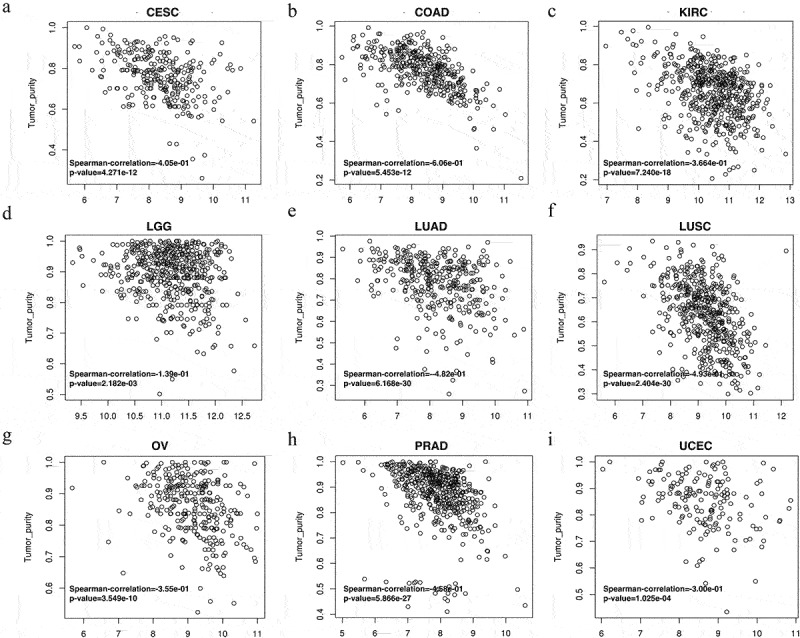
High expression of VASH1 led to a decrease in tumor purity in human cancer. VASH1 inversely affected tumor purity in CESC (a), COAD (b), KIRC (c), LGG (d), LUAD (e), LUSC (f), OV (g), PRAD (h), and UCEC (i). CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; COAD, colon adenocarcinoma; KIRC, kidney renal clear cell carcinoma; LGG, brain lower grade glioma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; PRAD, prostate adenocarcinoma; UCEC, uterine corpus endometrial carcinoma. All p < .05.
VASH1 impacted immune cell infiltration
In recent years, tumor immunocyte infiltration has gradually become a research focus, and tumor immunotherapy has achieved high performance. VASH1 was relevant to immune cell infiltration in numerous types of tumors. We defined a strong correlation coefficient greater than 0.4 as a strong correlation. As shown in Figure 5, VASH1 expression was positively correlated with Tem, macrophages, TFH, and iDC in CESC; iDC, NK cells, Tem, pDC, and macrophages in LUAD; Tem, macrophages, NK cells, iDC, TFH, and pDC in LUSC; Tem, NK cells, and iDC in OV; iDC, macrophages, DC, eosinophils, T cells, B cells, and mast cells in PCPG; aDC, cytotoxic cells, neutrophils, macrophages, T cells, Th1 cells, Treg, and B cells in SARC; Th1 cells, iDC, Tregs, NK CD56dim cells, T cells, and neutrophils in SKCM; and Tem in UCEC. By integrating the types of immune cells infiltrated in various tumors, it was found that VASH1 mainly affected the infiltration degree of iDCs, Tems, macrophages, and NK cells.
Figure 5.
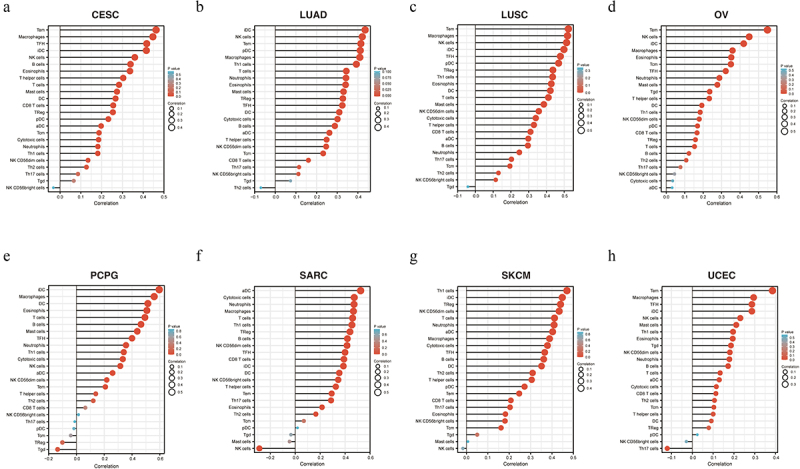
VASH1 was associated with immune cell infiltration in numerous kinds of tumors, including CESC (a), LUAD (b), LUSC (c), OV (d), PCPG (e), SARC (f), SKCM (g), and UCEC (h). CESC, cervical squamous cell carcinoma and endocervical adenocarcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; OV, ovarian serous cystadenocarcinoma; PCPG, pheochromocytoma and paraganglioma; SARC, sarcoma; SKCM, skin cutaneous melanoma; UCEC, uterine corpus endometrial carcinoma.
The effect of VASH1 expression on drug therapy responsiveness in ovarian cancer
After comprehensively understanding the role of VASH1 across cancers, we focused on its function in ovarian cancer. According to ROC plotter database analysis, patients with high VASH1 expression were more sensitive to multiple first- and second-line chemotherapy drugs for ovarian cancer. VASH1 could serve as a highly reliable detection biomarker for estimating the efficacy of chemotherapy, including cisplatin (AUC = 0.975, p = 0e + 00), epirubicin (AUC = 0.926, p = 1.3e-10), etoposide (AUC = 0.787, p = 1.7e-03), docetaxel (AUC = 0.851, p = 8.8e-06), gemcitabine (AUC = 0.870, p = 2.1e-06), olaparib (AUC = 0.740, p = 9.7e-03), topotecan (AUC = 0.889, p = 7.9e-07), vinblastine (AUC = 0.748, p = 1.9e-02), and vincristine (AUC = 0.773, p = 2.1e-02) (Figure 6).
Figure 6.
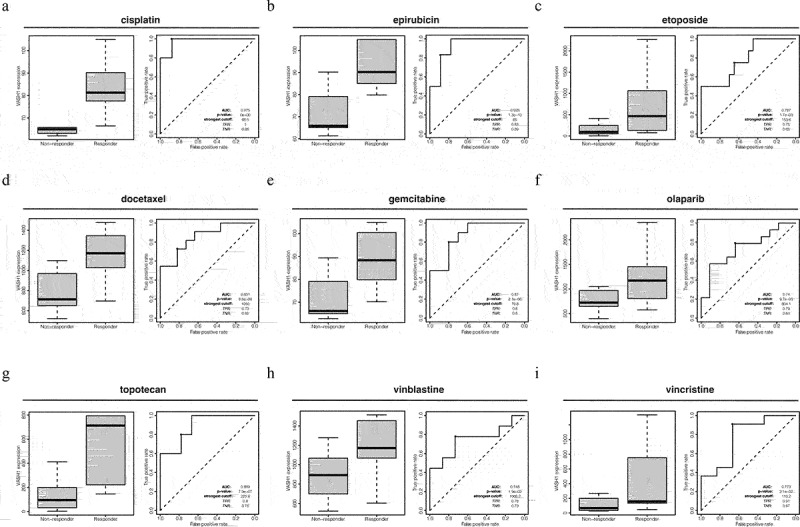
High VASH1 expression resulted in a higher sensitivity to multiple drugs in ovarian cancer.
GO and KEGG enrichment analysis of VASH1 in ovarian cancer
We identified differentially expressed genes correlated with VASH1 in ovarian cancer and concentrated on the potential biological functions of these genes. GO function enrichment analysis showed that functions focused on translational initiation, mitochondrial gene expression, and extracellular structure organization for BP; mitochondrial protein complex, respiratory chain, and collagen trimer for CC; structural constituent of ribosome; rRNA binding; and extracellular matrix structural constituent of MF. KEGG enrichment analysis revealed that the genes were mainly involved with the ribosome, oxidative phosphorylation, and malaria. We also found that genes related to extracellular matrix receptor interaction were coexpressed with VASH1, including ITGA4 (Spearman-correlation = 0.63, p = 0), ITGA5 (Spearman-correlation = 0.59, p = 0), VWF (Spearman-correlation = 0.54, p = 0), COL6A2 (Spearman-correlation = 0.51, p = 0), FN1 (Spearman-correlation = 0.47, p = 0), LAMB1 (Spearman-correlation = 0.48, p = 0), and LAMA4 (Spearman-correlation = 0.4, p = 0) (Figure 7).
Figure 7.
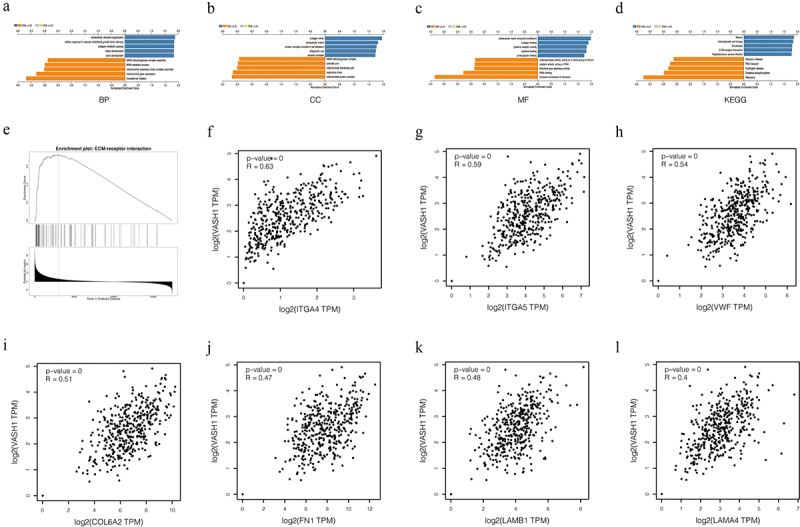
VASH1 plays important biological functions in ovarian cancer. BP (a), CC (b), MF (c), KEGG (d), ECM-receptor interaction (e). The correlation between VASH1 and coexpressed genes (f-l).
VASH1 inhibited the malignant phenotype of ovarian cancer cells
To further explore the specific role of VASH1 in the development of ovarian cancer, we examined the expression of VASH1 in normal ovarian epithelial cells (IOSE80) and ovarian cancer cells (A2780, OVCAR-3, Caov-3, and SKOV3). Compared with IOSE80 cells, the expression of VASH1 was decreased in cancer cells (Figure 8a). We then constructed VASH1 overexpression and knockdown cell lines and verified their transfection efficiency by qRT‒PCR and western blotting (Figure 8b, c). The reduction in VASH1 expression significantly enhanced proliferation and colony formation, and VASH1 overexpression inhibited cell proliferation (Figure 8d, e). Using the wounding healing assay, we examined the effect of VASH1 on cell migration ability. Compared to the control group, remarkable wound healing was observed at 12 and 24 h after VASH1 expression was downregulated. Conversely, overexpression of VASH1 inhibited wound healing ability (Figure 8f).
Figure 8.
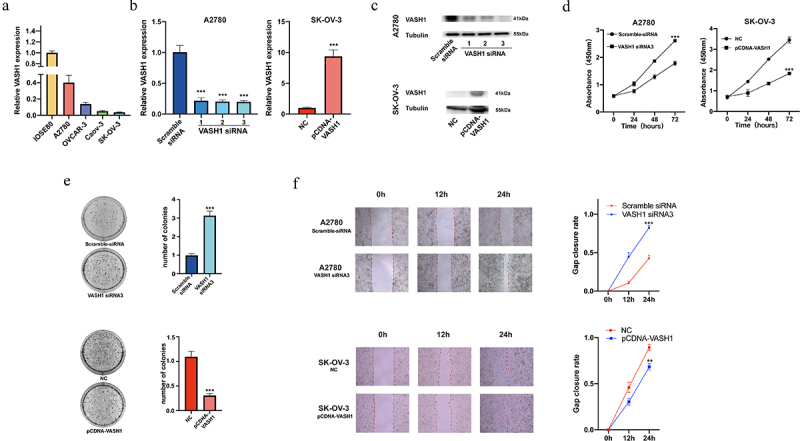
VASH1 could restrain the malignant phenotype of ovarian cancer cells. VASH1 expression was compared among ovarian cancer cell lines and normal ovarian epithelial cells (a). The level of VASH1 expression was determined after transfection (b, c), proliferation assay (d), colony formation (e), and wounding healing assay (f) with VASH1 overexpression and knockdown.
Discussion
VASH1 has been shown to be related to the relapse and progression of various tumors by inhibiting angiogenesis.19–21 This has become the focus of tumor research. However, the molecular mechanism of VASH1 has not yet been completely clarified.
Our results showed that the expression of VASH1 in various tumors was significantly different from that in normal tissues. However, VASH1 was consistently associated with better OS and RFS in pancancer patients. Several studies have confirmed that the expression level of VASH1 is related to the prognosis of many solid tumors. Zhao et al.22 showed that high expression of VASH1 was associated with a more satisfactory prognosis of renal cell carcinoma by inhibiting tumor angiogenesis.23 VASH1 overexpression represses tumor growth and promotes apoptosis by inhibiting the G0/G1 cell cycle. At the same time, an increase in VASH1 enhances tumor sensitivity to chemotherapy.24 In colon cancer, overexpression of VASH1 remarkably reduces growth, movement, and clonogenic capacity.25 In ovarian cancer, VASH1 inhibits the expression of IGF-1 and inhibits angiogenesis.17 This further suggests that VASH1 may act as an antitumor agent through diverse mechanisms. According to the ROC curve results, VASH1 is also of high value in the prediction and diagnosis of general cancer, with high sensitivity and a low false-positive rate.
Tumor tissue is a complex mixture, including immune cells, interstitial cells, stromal cells, and other nontumor cells in tumor tissue, which commonly affect patient outcomes.26,27 The proportion of tumor cells is known as tumor purity.28 Our data showed that the expression of VASH1 inversely affected tumor purity, which meant that we could reduce tumor purity and regulate the infiltration of immune cells in the TME by increasing VASH1 expression. In recent years, immunotherapy has become an effective method for treating malignant tumors. Generally, chemotherapy, endocrine therapy, and targeted therapy target only tumor cells. Unlike previous biological therapies, it is a new way of regulating or enhancing the immune response of the body to tumor cells by using appropriate methods to achieve a therapeutic effect. Immunotherapy can kill tumors by strengthening the autoimmune system. Therefore, immunotherapy has become a new breakthrough in the domain of tumor therapy after surgery, chemotherapy, radiotherapy, endocrine therapy, and targeted treatment.29,30 We found that VASH1 was mainly associated with iDC, macrophage, and NK cell infiltration. This mixture is considered to play a vital role in tumor growth, disease progression, and drug resistance. It has been reported that dendritic cells (DCs) account for a small part of the tumor microenvironment and are an important antitumor component with the ability to promote T-cell immunity and immunotherapeutic response.31 DCs play a central role in regulating the balance between CD8+ T-cell immunity and tumor antigen tolerance. Cross-initiation is the process by which DCs activate CD8+ T cells through cross-presentation of foreign antigens and plays a key role in the generation of antitumor CD8+ T-cell immunity.32 NK cells can control tumor growth by directly interacting with cancer cells and other immune cells. The presence of NK cells in the tumor microenvironment may lead to a good prognosis. The infiltration of NK cells and CD8+ T cells in the TME of patients with colorectal cancer was positively correlated with prolonged survival. In gastric and esophageal cancers, the proportion of infiltrating CD56dim NK cells gradually decreases with disease progression.33
Ovarian cancer is a gynecological malignant tumor with the highest mortality rate. Tumor cytoreductive surgery is the main treatment, followed by adjuvant chemotherapy.34 The 5-year survival rate of women diagnosed with high-grade serous ovarian cancer is between 35% and 40% because 15% − 25% of patients have primary treatment resistance, while most of the remaining women develop chemotherapeutic resistance.35 Hence, there is an urgent need to improve the sensitivity of ovarian cancer patients to therapeutic drugs. Studies have confirmed that VASH1 could affect drug sensitivity through different pathways. Shuji Mikami et al. found that the VASH1 density in metastatic clear-cell renal cell carcinoma treated with sunitinib was significantly higher than that in untreated ones, indicating that VASH1 may be related to endothelial cell resistance to sunitinib treatment.36 In osteosarcoma cells, it was identified that VASH1 was able to inhibit adriamycin resistance through regulation of the AKT signal pathway.37 In addition, miR‐335‐5p promotes insulin resistance by activating the TGF‐β pathway and inhibiting VASH1 expression.38 In our study, it was found that in this study increased expression of VASH1 could improve the responsiveness of ovarian cancer to many drugs, including first- and second-line chemotherapy drugs and PARP inhibitors commonly used in the clinic. This suggests that increasing the expression level of VASH1 may reverse drug resistance in patients. In this study, GSEA outcomes also showed that the expression of VASH1 was positively correlated with the ECM receptor interaction pathway in ovarian cancer, including genes related to integrin, collagen, fibronectin, and laminins. ECM receptors are the main regulatory pathways that control various cellular processes, including survival, proliferation, migration, invasion, DNA damage, signal transduction, and repair.39 Downregulation of ECM composition and structure is related to carcinogenesis and cancer progression.40,41 Therefore, we suspect that the upregulation of VASH1 is positively correlated with the ECM receptor to inhibit ovarian cancer metastasis and that VASH1 may be a possible therapeutic target. We further verified the bioinformatic analysis results of the VASH1 antitumor effect through in vitro experiments. Overexpression of VASH1 reduces cell proliferation, cloning, and migration in ovarian cancer cell lines.
Conclusions
This study revealed the antitumor effect of VASH1 across cancers. VASH1 is a candidate prognostic and diagnostic marker for many types of cancers and can be used to evaluate the infiltration of immune cells into malignant tissues. Thus, VASH1 may contribute to the comprehensive prevention and treatment of ovarian cancer as a potential therapeutic target.
Abbreviations
- VASH1
Vasohibin-1
- OS
overall survival
- RFS
recurrence free survival
- TME
tumor microenvironment
- GEPIA
Gene Expression Profiling Interactive Analysis
- TCGA
The cancer genome atlas
- GO
gene ontology
- KEGG
Kyoto encyclopedia of genes and genomes
- ACC
adrenocortical carcinoma
- LUAD
lung adenocarcinoma
- LUSC
lung squamous cell carcinoma
- OV
ovarian serous cystadenocarcinoma
- UCEC
uterine corpus endometrial carcinoma
- CHOL
cholangiocarcinoma
- ESCA
esophageal carcinoma
- KIRC
kidney renal clear cell carcinoma
- KIRP
kidney renal papillary cell carcinoma
- LIHC
liver hepatocellular carcinoma
- SKCM
skin cutaneous melanoma
- THCA
thyroid carcinoma
- HNCS
head and neck squamous cell carcinoma
- PAAD
pancreatic adenocarcinoma
- READ
rectal adenocarcinoma
- STAD
stomach adenocarcinoma
- CESC
endocervical adenocarcinoma
- COAD
colon adenocarcinoma
- LGG
brain lower grade glioma
- PCPG
pheochromocytoma and paraganglioma
- SARC
sarcoma
- Tem
effector memory T Cell
- TFH
follicular helper T cell
- DC
dendritic cell
- iDC
interdigitating dendritic cell
- NK
natural killer cell
- pDC
plasmacytoid dendritic cell
- Adc
active dendritic cell
- BP
biological process
- CC
cell component
- MF
molecular function
- ITGA4
integrin, alpha 4
- ITGA5
integrin, alpha 5
- VWF
von Willebrand factor
- COL6A2
collagen, type VI, alpha 2
- FN1
fibronectin 1
- LAMB1
laminin, beta 1
- LAMA4
laminin, alpha 4
- IGF-1
insulin-like growth factor 1
- PARP
poly ADP-ribose polymerase
- GSEA
Gene Set EnrichmentAnalysis
- ECM
extracellular matrix.
Acknowledgments
This work was supported by the following research funds: the National Natural Science Foundation of China (Grant Numbers 81872507 and 82173238), the Natural Science Foundation of Heilongjiang Province (ZD2020H007), Nn10 Plan (Grant Numbers Nn10py2017-01), and First Affiliated Hospital of Harbin Medical University (2023M19).
Biographies
Yan Li is studying for her doctorate from Harbin Medical University and currently serves as an attending physician. She is mainly dedicated to research on ovarian cancer.
Liang Meng has a master's degree. She is mainly dedicated to research in virology.
Ge Lou graduated from Harbin Medical University in 1989 and currently serves as the Director of Gynecology at Harbin Medical University Cancer Hospital. He studied at Georgetown University Medical Center in the United States from 1999 to 2000.He is skilled in the diagnosis and treatment of ovarian cancer, cervical cancer, and endometrial cancer.
Funding Statement
The work was supported by the National Natural Science Foundation of China [82173238]; National Natural Science Foundation of China [81872507]; First Affiliated Hospital of Harbin Medical University [2023M19].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
L.G. provided the funds and designed the overall ideas and research methods for the project. L.Y. was responsible for analysis, organizing the data, and writing the original draft. M.L. was responsible for reviewing and editing the manuscript. All authors have read and approved the final manuscript.
References
- 1.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL.. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–897. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280–304. doi: 10.3322/caac.21559. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Chen LM, Cristea M, DeRosa M, Eisenhauer EL, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(2):191–226. doi: 10.6004/jnccn.2021.0007. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K, Hasegawa Y, Yamashita H, Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, et al. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest. 2004;114(7):898–907. doi: 10.1172/JCI200421152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 6.Kern J, Steurer M, Gastl G, Gunsilius E, Untergasser G. Vasohibin inhibits angiogenic sprouting in vitro and supports vascular maturation processes in vivo. BMC Cancer. 2009;9(1):284. doi: 10.1186/1471-2407-9-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato Y. The vasohibin family. Pharmaceutic (Basel). 2010;3(2):433–440. doi: 10.3390/ph3020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horie S, Suzuki Y, Kobayashi M, Kadonosono T, Kondoh S, Kodama T, Sato Y. Distinctive role of vasohibin-1A and its splicing variant vasohibin-1B in tumor angiogenesis. Cancer Gene Ther. 2016;23(5):133–141. doi: 10.1038/cgt.2016.13. [DOI] [PubMed] [Google Scholar]
- 9.Kimura H, Miyashita H, Suzuki Y, Kobayashi M, Watanabe K, Sonoda H, Ohta H, Fujiwara T, Shimosegawa T, Sato Y. Distinctive localization and opposed roles of vasohibin-1 and vasohibin-2 in the regulation of angiogenesis. Blood. 2009;113(19):4810–4818. doi: 10.1182/blood-2008-07-170316. [DOI] [PubMed] [Google Scholar]
- 10.Shen Z, Seppanen H, Kauttu T, Vainionpaa S, Ye Y, Wang S, Mustonen H, Puolakkainen P. Vasohibin-1 expression is regulated by transforming growth factor-beta/bone morphogenic protein signaling pathway between tumor-associated macrophages and pancreatic cancer cells. J Interferon Cytokine Res. 2013;33(8):428–433. doi: 10.1089/jir.2012.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosaka T, Miyazaki Y, Miyajima A, Mikami S, Hayashi Y, Tanaka N, Nagata H, Kikuchi E, Nakagawa K, Okada Y, et al. The prognostic significance of vasohibin-1 expression in patients with prostate cancer. Br J Cancer. 2013;108(10):2123–2129. doi: 10.1038/bjc.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazaki Y, Kosaka T, Mikami S, Kikuchi E, Tanaka N, Maeda T, Ishida M, Miyajima A, Nakagawa K, Okada Y, et al. The prognostic significance of vasohibin-1 expression in patients with upper urinary tract urothelial carcinoma. Clin Cancer Res. 2012;18(15):4145–4153. doi: 10.1158/1078-0432.CCR-12-0073. [DOI] [PubMed] [Google Scholar]
- 13.Yoshinaga K, Ito K, Moriya T, Nagase S, Takano T, Niikura H, Sasano H, Yaegashi N, Sato Y. Roles of intrinsic angiogenesis inhibitor, vasohibin, in cervical carcinomas. Cancer Sci. 2011;102(2):446–451. doi: 10.1111/j.1349-7006.2010.01812.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang T, Yu TT, Zhang DM, Hou XM, Liu XJ, Zhao D, Shan L. Vasohibin-1 expression detected by immunohistochemistry correlates with prognosis in non-small cell lung cancer. Med Oncol. 2014;31(5):963. doi: 10.1007/s12032-014-0963-y. [DOI] [PubMed] [Google Scholar]
- 15.Zhou CF, Ma J, Huang L, Yi HY, Zhang YM, Wu XG, Yan RM, Liang L, Zhong M, Yu YH, et al. Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1. Oncogene. 2019;38(8):1256–1268. doi: 10.1038/s41388-018-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto M, Ozawa S, Ninomiya Y, Koyanagi K, Oguma J, Kazuno A, Hara H, Yatabe K, Kajiwara H, Nakamura N, et al. Plasma vasohibin-1 and vasohibin-2 are useful biomarkers in patients with esophageal squamous cell carcinoma. Esophagus: Offici J Japan Esophageal Soc. 2020;17(3):289–297. doi: 10.1007/s10388-020-00719-8. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi Y, Saga Y, Koyanagi T, Takei Y, Machida S, Taneichi A, Mizukami H, Sato Y, Matsubara S, Fujiwara H. Vasohibin-1 expression inhibits advancement of ovarian cancer producing various angiogenic factors. Cancer Sci. 2016;107(5):629–637. doi: 10.1111/cas.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi Y, Saga Y, Koyanagi T, Takei Y, Machida S, Taneichi A, Mizukami H, Sato Y, Matsubara S, Fujiwara H. The angiogenesis regulator vasohibin-1 inhibits ovarian cancer growth and peritoneal dissemination and prolongs host survival. Int J Oncol. 2015;47(6):2057–2063. doi: 10.3892/ijo.2015.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue C, Miki Y, Saito-Koyama R, Kobayashi K, Seyama K, Okada Y, Sasano H. Vasohibin-1 and -2 in pulmonary lymphangioleiomyomatosis (LAM) cells associated with angiogenic and prognostic factors. Pathol Res Pract. 2022;230:153758. doi: 10.1016/j.prp.2022.153758. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Z, Zhang Y, Chen X, Wu P, Chen D. Long noncoding RNA RBMS3-AS3 acts as a microRNA-4534 sponge to inhibit the progression of prostate cancer by upregulating VASH1. Gene Ther. 2020;27(3–4):143–156. doi: 10.1038/s41434-019-0108-1. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Deng Q, Lv Z, Ling Y, Hou X, Chen Z, Dinglin X, Ma S, Li D, Wu Y, et al. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol Cancer. 2019;18(1):181. doi: 10.1186/s12943-019-1108-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Zhao G, Na R, Li L, Xiao H, Ding N, Sun Y, Han R. Vasohibin-1 inhibits angiogenesis and suppresses tumor growth in renal cell carcinoma. Oncol Rep. 2017;38(2):1021–1028. doi: 10.3892/or.2017.5746. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Hu Y, Qi S, Luo X, Yu H. Structural basis of tubulin detyrosination by vasohibins. Nature Structural & Molecular Biology. 2019;26(7):583–591. doi: 10.1038/s41594-019-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikami S, Oya M, Kosaka T, Mizuno R, Miyazaki Y, Sato Y, Okada Y. Increased vasohibin-1 expression is associated with metastasis and poor prognosis of renal cell carcinoma patients. Lab Invest. 2017;97(7):854–862. doi: 10.1038/labinvest.2017.26. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y. Double-face of vasohibin-1 for the maintenance of vascular homeostasis and healthy longevity. J Atheroscler Thromb. 2018;25(6):461–466. doi: 10.5551/jat.43398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Y, Xiong Z, Cao H, Li C, Wanggou S, Li X. Multi-dimensional omics characterization in glioblastoma identifies the purity-associated pattern and prognostic gene signatures. Cancer Cell Int. 2020;20(1):37. doi: 10.1186/s12935-020-1116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiam-Galvez K, Allen B, Spitzer M. Systemic immunity in cancer, Nature reviews. Nat Rev Cancer. 2021;21(6):345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riley R, June C, Langer R, Mitchell M. Delivery technologies for cancer immunotherapy, Nature reviews. Drug Discovery. 2019;18(3):175–196. doi: 10.1038/s41573-018-0006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerhard GM, Bill R, Messemaker M, Klein AM, Pittet MJ. Tumor-infiltrating dendritic cell states are conserved across solid human cancers. J Exp Med. 2021;218(1). doi: 10.1084/jem.20200264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu C, Jiang A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front Immunol. 2018;9:3059. doi: 10.3389/fimmu.2018.03059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cozar B, Greppi M, Carpentier S, Narni-Mancinelli E, Chiossone L, Vivier E. Tumor-infiltrating Natural killer cells. Cancer Discov. 2021;11(1):34–44. doi: 10.1158/2159-8290.CD-20-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuroki L, Guntupalli SR. Treatment of epithelial ovarian cancer. BMJ. 2020;371:m3773. doi: 10.1136/bmj.m3773. [DOI] [PubMed] [Google Scholar]
- 35.Christie EL, Bowtell DDL. Acquired chemotherapy resistance in ovarian cancer. Ann Oncol. 2017;28(suppl_8):viii13–viii15. doi: 10.1093/annonc/mdx446. [DOI] [PubMed] [Google Scholar]
- 36.Mikami S, Oya M, Kosaka T, Mizuno R, Miyazaki Y, Sato Y, Okada Y. Increased vasohibin-1 expression is associated with metastasis and poor prognosis of renal cell carcinoma patients. Lab Invest. 2017;97(7):854–862. doi: 10.1038/labinvest.2017.26. [DOI] [PubMed] [Google Scholar]
- 37.Huang W, Ren Y, Liu H. Vasohibin 1 inhibits Adriamycin resistance in osteosarcoma cells via the protein kinase B signaling pathway. Oncol Lett. 2018;15(4):5983–5988. doi: 10.3892/ol.2018.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang X, Qin Q. miR-335-5p induces insulin resistance and pancreatic islet β-cell secretion in gestational diabetes mellitus mice through VASH1-mediated TGF-β signaling pathway. J Cell Physiol. 2019;234(5):6654–6666. doi: 10.1002/jcp.27406. [DOI] [PubMed] [Google Scholar]
- 39.Serafim RB, da Silva P, Cardoso C, Di Cristofaro LFM, Netto RP, de Almeida R, Navegante G, Storti CB, de Sousa JF, de Souza FC, et al. Expression Profiling of glioblastoma cell lines reveals novel extracellular matrix-receptor genes correlated with the responsiveness of glioma patients to ionizing radiation. Front Oncol. 2021;11:668090. doi: 10.3389/fonc.2021.668090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo Y, Ren C, Huang W, Yang W, Bao Y. Oncogenic ACSM1 in prostate cancer is through metabolic and extracellular matrix-receptor interaction signaling pathways. Am J Cancer Res. 2022;12(4):1824–1842. [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CX, Luo KJ, Yang JP, Huang YC, Cardenas ER, Nicholson BJ, Jiang JX. Connexins and cAMP Cross-talk in cancer progression and metastasis. Cancers Basel. 2020;13(1):58. doi: 10.3390/cancers13010058. [DOI] [PMC free article] [PubMed] [Google Scholar]


