Abstract
Recent advances in microbiome research have informed the potential role of the gut microbiota in the regulation of metabolic, cardiovascular and renal systems and, when altered, in the pathogenesis of various cardiometabolic disorders, including chronic kidney disease (CKD). The improved understanding of gut dysbiosis in cardiometabolic pathologies in turn has led to a vigorous quest for developing therapeutic strategies. These therapeutic strategies aim to investigate whether interventions targeting gut dysbiosis can shift the microbiota towards eubiosis and if these shifts, in turn, translate into improvements in (or prevention of) CKD and its related complications, such as premature cardiovascular disease. Existing evidence suggests that multiple interventions (e.g., plant-based diets, prebiotic, probiotic, and synbiotic supplementation, constipation treatment, fecal microbiota transplantation, and intestinal dialysis) might result in favorable modulation of the gut microbiota in patients with CKD, and thereby potentially contribute to improving clinical outcomes in these patients. In this review, we summarize the current understanding of the characteristics and roles of the gut microbiota in CKD and discuss the potential of emerging gut microbiota-targeted interventions in the management of CKD.
Keywords: cardiovascular disease, chronic kidney disease, constipation, gut microbiota, plant-based diets, nutrition supplements
INTRODUCTION
Chronic kidney disease (CKD) has been a significant global public health problem due to its increasing prevalence and association with poor clinical outcomes, particularly with premature cardiovascular morbidity and mortality.1,2 The accumulation of various gut-derived toxic metabolites (a.k.a. uremic toxins) associated with reduced kidney function has been implicated in the pathogenesis of premature cardiovascular disease in CKD, mediated in part by chronic low-grade systemic inflammation.3 Accumulating evidence indicates that alterations of gut microbiota and intestinal barrier integrity, which are a common phenomenon in patients with CKD, play a key role in the mechanisms underlying complex pathophysiological interactions between the gut, kidney and cardiovascular systems, often referred to as the “gut-kidney-heart axis”.4 Improved understanding of the disease pathologies associated with the gut microbiota has spurred the development of novel gut microbiota-targeted interventions as a means to prevent and treat CKD and its related complications, including premature cardiovascular disease.5 In this review, we provide current evidence on the characteristics and roles of the gut microbiota in patients with CKD. We further discuss potential therapeutic strategies targeting the gut microbiota in the management of CKD that require rigorous investigation in both observational and interventional settings to confirm or refute their benefit.
COMPOSITION OF GUT MICROBIOTA
In the human gastrointestinal tract, there is a complex community of ~30 trillion diverse microorganisms.6 These microorganisms, collectively referred to as the gut microbiota, include bacteria, archaea, fungi, bacteriophages, and eukaryotic viruses, which collectively encode at least 150 times more genes than the human genome alone.7 The numbers of microbes generally increase in the lower gastrointestinal tract, with the colon harboring up to 1012 microorganisms per gram.8 With recent advances in ‘-omics’ technologies, bioinformatics, and modelling approaches, evidence is accumulating that shows significant variations in the overall composition and abundance of the gut microbiota, primarily by two broad classes of factors, namely heritable (e.g., genetic component of the host immune system) and external (e.g., diet, lifestyle, and environmental) factors.9–11 In general, high taxonomic diversity, high microbial gene richness, and stable microbiome functional cores characterize healthy gut microbial communities.12 In metabolically healthy adults, the gut microbiota is generally dominated by bacteria, with >90% of the species belonging to two bacterial phyla, Firmicutes and Bacteroidetes, out of over 50 bacterial phyla found in the environment.13 However, the composition of the gut microbiota can be affected by myriad factors, and currently a healthy human gut microbiota has not been well-defined at any taxonomic resolution.14
GUT DYSBIOSIS IN CKD
A growing body of evidence has indicated that patients with CKD display significant quantitative, compositional and functional alterations of the gut microbiota (a.k.a. gut dysbiosis), frequently characterized by an overgrowth of microbes with pathogenic potential (e.g., Enterobacteriaceae family) and a depletion in commensal or symbiotic microbes (e.g., Lactobacillus, Bifidobacterium, Roseburia, and Faecalibacterium genera).15–18 In previous studies comparing the composition of the gut microbiota between patients with end-stage kidney disease (ESKD) and healthy individuals, the number of Enterobacteriaceae family (especially Escherichia, Klebsiella, and Enterobacter genera), Clostridium perfringens and Enterococci species were significantly higher in patients with ESKD than in healthy individuals.19,20 In another study, researchers compared the composition of the gut microbiota between rats with CKD and control rats and demonstrated a notable decrease in Prevotellaceae and Lactobacillaceae families in the CKD rats.21 Using the stools of 24 patients with ESKD and 12 healthy individuals, the same group of researchers found that there was a significant difference in the abundance of 190 bacterial operational taxonomic units (OTUs) between patients with ESKD and healthy individuals.21 According to a recent meta-analysis of rodent repository data, an increased abundance of certain families from class Clostridia (including Peptostreptococcaceae, Peptococcaceae, Clostridiaceae, and Christenellaceae) was linked to uremia, although no universal trends in microbial population dynamics were found.22 These findings in turn led to an ongoing quest for strategies aimed at restoring symbiotic or commensal gut microbiota as a possible means to improve health outcomes in patients with CKD and ESKD.5
Potential Drivers of Gut Dysbiosis in CKD
The causes of gut dysbiosis in patients with CKD are multifactorial and involve interactions of various etiological factors. One of the major contributing factors to the gut dysbiosis in CKD is a restricted intake of high-fiber diets. Due to the high potassium content of fruits and vegetables, patients with CKD are often advised to restrict the dietary intake of these fiber-rich foods.23 Similarly, the dietary intake of cheese and yogurts is often restricted in patients with CKD because of their high phosphorus content. These dietary habits not only limit the intake of foods with prebiotic and probiotic properties but also lead to reduced gut motility, together contributing to the gut dysbiosis and altered metabolic profiles in patients with CKD.24 Changes in the biochemical environment inside the gut lumen is another important factor affecting the composition of the gut microbiota in CKD. With declining kidney function, metabolic waste products, such as urea, that are normally secreted in the urine accumulate in the blood.25 The accumulated blood urea diffuses into the gut lumen and is then hydrolyzed by bacterial urease to large quantities of ammonia and ammonium hydroxide, which raises luminal pH and alters the gut microbiota.26,27 Other possible etiological factors for the gut dysbiosis in CKD include metabolic acidosis and medication use (e.g., phosphate binders, iron, and antibiotics).28–34
Gut Dysbiosis and Its Consequences in CKD
The selection pressure on the gut microbiota exerted by all the aforementioned factors contributes to the shift of the gut microbiota from a saccharolytic or carbohydrate fermenting bacterial phenotype to a more proteolytic phenotype that generates so-called gut-derived uremic toxins (e.g., p-cresyl sulfate, indoxyl sulfate, phenyl sulfate, and trimethylamine-N-oxide [TMAO]) through amino acid catabolism.35,36 At the same time, the alteration of gut microbiota leads to a reduced production of short-chain fatty acids (SCFAs), such as acetate, butyrate, and propionate, which are bacterial fermentation products of plant-derived carbohydrates primarily by Bacteroidetes and Firmicutes phyla.37 SCFAs are essential energy sources for colonic epithelial cells and have receptor mediated signaling on tight junction proteins, such as claudin-1, occludin, and zonula occludens-1, to regulate the integrity of gut epithelial barrier function.38 They can also protect the gut epithelial barrier by modulating the host immune responses.39 Hence, combined with the increased production of ammonium hydroxide that drives breakdown of the gut epithelial tight junctions,40 the reduced production of SCFAs associated with the gut dysbiosis in CKD contributes to the impairment of intestinal barrier integrity in patients with CKD.41 Of interest, a recent cross-sectional study showed that the relative abundance of Faecalibacterium prausnitzii, a butyrate-producing bacterial species, was markedly reduced in hemodialysis patients with (vs. without) protein-energy wasting (PEW), providing novel insights into the possible involvement of gut dysbiosis in the pathophysiology of PEW in the context of CKD, presumably mediated in part by impaired gut barrier.42 Importantly, the impaired gut barrier integrity, in turn, allows the translocation of gut-derived toxic products into the systemic circulation,20,27,41,43–53 and this so-called “leaky gut” phenomenon has been considered a key pathological mechanism underlying the gut-kidney-heart axis in CKD.4
Gut-Derived Toxic Products and Outcomes in CKD
The gut-derived toxic products that can translocate from the gut into the systemic circulation include bacterial endotoxins (i.e., lipopolysaccharide), uremic toxins (e.g., p-cresyl sulfate, indoxyl sulfate, and TMAO), bacterial DNA fragments, and intact bacteria.20,27,41,43–53 These gut-derived toxic products have been shown to be associated with various adverse clinical outcomes in patients with CKD.54–60
Bacterial endotoxins
Among possible gut-derived products identifiable in the systemic circulation in patients with CKD, bacterial endotoxin, which is a major component of the outer membrane of the cell wall of Gram-negative bacteria (a.k.a. lipopolysaccharide),61 has been most extensively studied for its immunostimulatory and atherogenic properties.62 Circulating bacterial endotoxins bind to toll-like receptor 4 (TLR-4) expressed on various cells, such as endothelial cells, smooth muscle cells, and innate immune cells, and contribute to inflammatory processes.63 The endotoxin-mediated signaling through TLR-4 stimulates interleukin-1 (IL-1) receptor-associated kinase (IRAK) via mutant myeloid differentiation primary response 88 (MyD88) and myeloid differentiation protein 2 (MD2), and the subsequent recruitment of tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) leads to the activation of nuclear factor kappa B (NF-κB) and the release of various proinflammatory cytokines.64 These inflammatory responses can promote procoagulant activity, transformation of macrophages to foam cells, and endothelial cell injury, consequently contributing to the development and progression of atherosclerosis.65
Characteristically, patients with CKD, especially those with ESKD on dialysis, are likely to have a higher chance of being exposed to bacterial endotoxins due to dialysis-related procedures (e.g., through contaminated dialysate, use of peritoneal dialysis catheters or central venous catheters, and needle placement during hemodialysis therapy) and high susceptibility to infections.66,67 However, endogenous endotoxemia of intestinal origin is not a rare complication in patients with CKD and ESKD,49,53,66 although endotoxin can come from body sites other than the gut, such as the mouth.62 In a cross-sectional study examining the levels of endotoxin and bacterial DNA in the blood, gut, and dialysate of hemodialysis patients, researchers demonstrated that plasma endotoxin concentrations were far greater than those in the dialysate, and the bacteria detected in the blood of hemodialysis patients were similar to those in the gut of these patients.53 They also showed that plasma levels of D-lactate, a marker of gut permeability, were higher in hemodialysis patients who had bacterial DNA detected in the blood (along with higher endotoxin levels) compared to those who had no detectable bacterial DNA in the blood (along with lower endotoxin levels).53 In another cross-sectional study of 74 patients with CKD (a mean eGFR of 34 mL/min), researchers showed that endotoxin was detected in the blood of all CKD patients, with its higher levels seen in those with (vs. without) signs of fluid overload.66 Similarly, in a retrospective study examining the levels of circulating bacterial endotoxin across the spectrum of CKD, there was a graded increase in the levels of circulating endotoxin with advancing CKD stages, with the highest levels observed in patients receiving dialysis.68 Additionally, a recent experimental study showed that CKD mice displayed increased serum levels of bacterial endotoxin, along with translocation of living bacteria across impaired gut barrier and systemic inflammation, and the eradication of gut microbiota significantly reduced the serum levels of bacterial endotoxin, prevented bacterial translocation from the gut, and fully reversed all markers of systemic inflammation to the level of nonuremic controls.50 Furthermore, increased circulating endotoxin levels have been reported in patients with heart failure, a disease that, similarly to CKD, is also characterized by fluid overload, systemic congestion and altered gut barrier function.69 These findings strongly suggest that endotoxemia in patients with CKD and ESKD is attributable more (and most likely) to a continuous endotoxin translocation from the gut into the systemic circulation across impaired gut barriers due in part to gut dysbiosis, rather than to exposure to non-gastrointestinal bacterial sources, such as contaminated dialysate. In terms of clinical outcomes associated with circulating endotoxin in CKD, the levels of circulating endotoxin have been shown to be associated with systemic inflammation, markers of malnutrition, cardiac injury, and reduced survival.68 In line with these observations, the associations of endotoxemia with poor survival have been reported in a few epidemiological studies.68,70
Uremic toxins
The phenotypic shift of the gut microbiota (i.e., from saccharolytic to proteolytic bacteria) seen in patients with CKD accelerates the fermentation of dietary protein, particularly animal-derived protein, and contributes the excessive production of precursors of gut-derived toxic metabolites, such as p-cresyl sulfate, indoxyl sulfate, and TMAO.71 p-Cresyl sulfate is a 188-Da protein-bound solute that originates from sulfation of p-cresol, which is a colonic fermentation product of the dietary amino acid phenylalanine and tyrosine.72 Meanwhile, certain intestinal bacteria that have tryptophanase (e.g., Escherichia coli) can convert dietary tryptophan to indole, which is subsequently absorbed into the blood and metabolized by the liver to indoxyl sulfate.73 TMAO is a circulating organic compound derived from the metabolism of dietary choline and L-carnitine into trimethylamine (TMA) by intestinal bacteria which becomes oxidized by the liver.74 Reported microbiota species producing TMA include Deferribacteraceae, Anaeroplasmataceae, Prevotellaceae, Enterobacteriaceae, Anaerococcushydrogenalis, Clostridium asparagiforme, Clostiridium hathewayi, Clostridium sporogenes, Escherichiafergusonii Proteuspenneri, Providencia rettgeri, and Edwardsiella tarda.75–78
As kidney function declines, these gut-derived toxic metabolites progressively accumulate in the blood and exert various deleterious effects on tissues and organs (Figure 1).79 For example, p-cresyl sulfate induces epithelial-to-mesenchymal transition-like changes in mouse proximal renal tubular cells through activation of the renal renin angiotensin aldosterone system (RAAS)/transforming growth factor-beta (TGF-β) pathway, contributing to kidney fibrosis and injury.80 It also induces nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and reactive oxygen species (ROS) production in cardiomyocytes, enhancing their apoptosis.81 TMAO, on the other hand, is shown to induce an alteration of cholesterol and sterol metabolism and promote pro-atherogenic foam cell formation in the arterial wall.75 It is also shown that exposure of platelets to TMAO enhances sub-maximal stimulus-dependent platelet activation from multiple agonists (e.g., thrombin, adenosine diphosphate, and collagen) through augmented intracellular calcium release, leading to platelet hyperreactivity and thrombosis risk.82 In line with these mechanistic observations, epidemiological studies have shown the significant associations of circulating levels of these uremic toxins with adverse clinical outcomes in patients with CKD.55,56,83 For example, elevated levels of TMAO have been independently associated with subsequent risk of cardiovascular events and mortality in patients with CKD,57,84 although this association has not yet been well confirmed in the ESKD population receiving hemodialysis.58,85 In a recent study including both animal diabetic models and a cohort of diabetic patients, phenyl sulfate, another gut microbiota-derived metabolite, was shown to be associated with progression of albuminuria through podocyte damage, and the inhibition of bacterial enzyme responsible for the synthesis of phenol from dietary tyrosine (tyrosine phenol-lyase, which was reported to be expressed only in a minor population of Enterobacteriaceae)86 reduced albuminuria in diabetic mice, suggesting the potential of phenyl sulfate as a future therapeutic target in diabetic kidney disease.36
Figure 1.
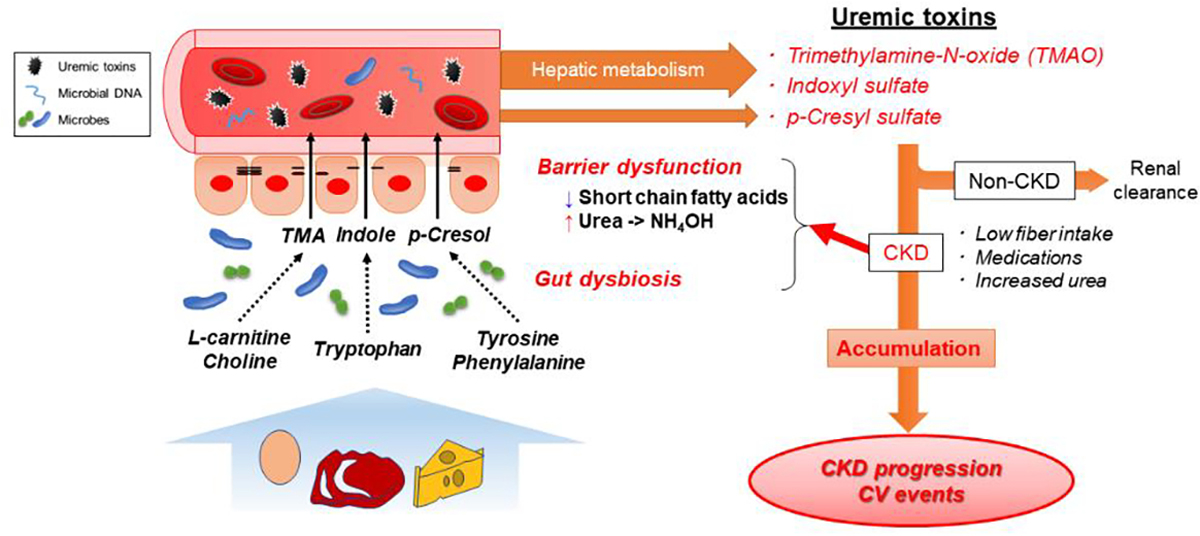
Uremic toxins and their systemic effects in CKD
Abbreviations: CKD = chronic kidney disease; CV = cardiovascular; NH4OH = ammonium hydroxide; TMA = trimethylamine
Microbial DNA fragments
The translocation of microbial DNA fragments (and intact bacteria) from the gut into the systemic circulation in CKD and ESKD has been supported by several studies.20,43,49,52,53 In an experimental study, for example, researchers compared bacterial DNA fragments in the blood, liver, spleen, mesenteric lymph nodes, and gut of uremic rats using an amplification of species-specific fragments of bacterial 16S ribosomal DNA and found identical bacteria both in the extraintestinal sites and in the gut.52 They also showed that plasma high sensitivity C-reactive protein and IL-6 were significantly higher in uremic rats with bacterial DNA in their blood than in those without.52 Among various microbial components, bacterial DNA fragments are the most consistently detectable and easily discerned from human DNA due to their highly conserved unique 16S ribosomal RNA (rRNA) subunit.59 Because of this nature of bacterial DNA fragments, they have recently been recognized as a better quantitative marker of circulating bacterial load compared with bacterial endotoxin which enables the detection of only Gram-negative bacteria.59 Further, emerging evidence suggests the unique pathogenic roles of circulating bacterial DNA in host immune and cardiovascular systems through recognition as pathogen-associated molecular pattern ligands (PAMPs).87–90
Bacterial DNA contains unmethylated cytosine-guanine dinucleotide (CpG) flanked by two purine 5’ and two pyrimidine 3’.91 These structures are recognized by endogenous TLR-9 as a bacterial DNA receptor, which triggers cell signaling pathways including the activation of mitogen-activated protein kinases and nuclear factor kappa B.92 In inflammatory cells such as polymorphonuclear leukocytes, bacterial DNA fragments can exert several biological effects on cellular functions through regulation of chemokine expression, cellular trafficking, adhesion molecules, and phagocyte activity.93 They also rescue polymorphonuclear lymphocytes from constitutive apoptosis and promote the survival of mononuclear cells by inducing IL-6.94
The inflammatory responses induced by bacterial DNA fragments can in turn induce various pathological pathways, including endothelial injury (e.g., through induction of endothelial cell apoptosis), metabolic dysfunction (e.g., through insulin resistance), and cardiac dysfunction (e.g., through suppression of cardiac myocyte contraction).95–97 In fact, in peritoneal dialysis patients without evidence of systemic infection, elevated levels of circulating bacterial DNA have been shown to be independently associated with subsequent risk of cardiovascular events, although the origin of circulating bacterial DNA was undetermined.98 Of interest, the risk of cardiovascular events in this study was more pronounced for bacterial DNA levels than for bacterial endotoxin levels,98 suggesting that circulating bacterial DNA (vs. bacterial endotoxin) could play a more dominant role in the development of cardiovascular disease in patients with ESKD.
Circulating microbiota
The existence of microbial communities in an otherwise classically sterile milieu, such as the blood, is traditionally interpreted as an indication of infection; however, recent advances in amplification and sequencing technologies of microbial DNA have revealed the presence of blood microbial communities (a.k.a. circulating microbiota) in various patient populations without overt infections.60 Evidence is steadily accumulating to suggest the translocation of gut microbiota into the systemic circulation and its roles in kidney and cardiovascular disease pathologies.60,99–105 However, the number of studies reporting the association between compositional alterations of circulating microbiota and clinical outcomes are still extremely limited, and the source of circulating microbiota remains a topic of considerable deliberation.
Gut Dysbiosis and Gastrointestinal Dysmotility in CKD
With advances in microbiome research, studies have uncovered the roles of the commensal or symbiotic gut microbiota in maintaining normal gastrointestinal motility.106,107 The dysbiotic changes in the gut microbiota may result in gastrointestinal dysmotility, which may manifest as constipation, diarrhea, or bloating symptoms.108 In fact, alterations of gut microbiota have been reported in patients with these gastrointestinal manifestations,109 and gut dysbiosis in constipation, for example, has been characterized by a relative decrease in obligate anaerobic bacteria (e.g. Bifidobacterium and Lactobacillus genera) and a parallel increase in microbes with pathogenic potential (e.g., Enterobacteriaceae family).110–112 The pathophysiological mechanisms linking gut dysbiosis and gastrointestinal dysmotility remain to be clarified but may include reduced production of SCFAs,113 increased production of hydrogen sulfide and methane gases,114 and dysregulation of enteric neurons mediated in part by serotonin metabolism106 and by aryl hydrocarbon receptors.107 As an example, the decrease in relative abundance of anaerobic bacteria associated with constipation could result in reduced production of SCFAs that normally stimulate ileal and colonic smooth muscle contractility, thereby contributing to slowing intestinal transit and further worsening of constipation.115 The increase in relative abundance of methanogenic archaea has also be involved in prolonging intestinal transit time through increased methane production.116
Regarding the causal relationship between gut dysbiosis and gut dysmotility in patients with CKD, it is often difficult to determine the causality in these patients who typically share many risk factors for both of these conditions (Figure 2).24 In this context, a recent animal study showed that CKD-related gut dysbiosis was causally linked to suppressed colonic muscle responses,117 suggesting that the gut dysbiosis in CKD may at least in part be causally associated with constipation symptoms in this population. Importantly, the presence of constipation has been shown to be associated with adverse clinical outcomes, such as ESRD, CVD, and mortality, in recent epidemiological studies.118,119
Figure 2.
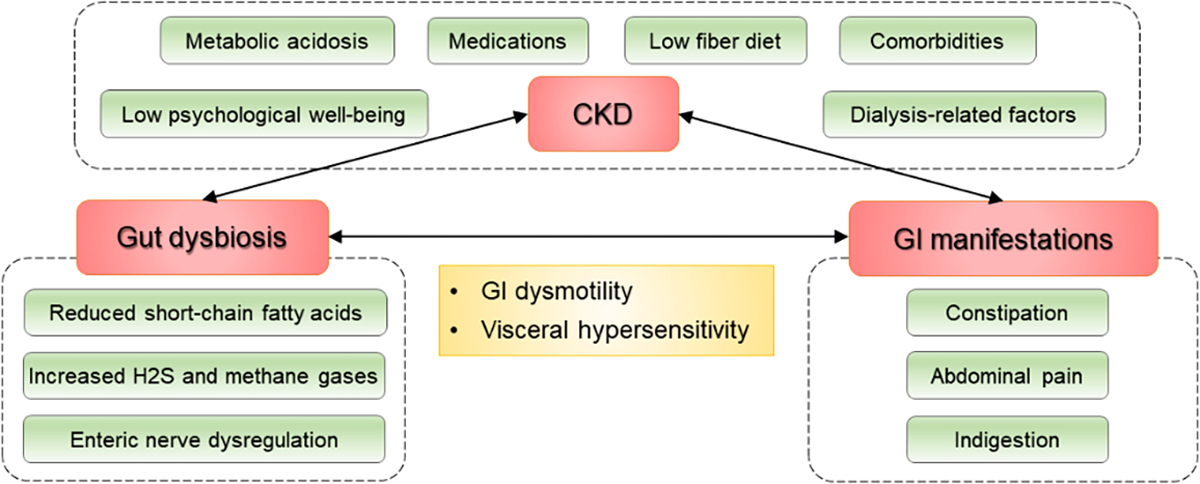
Factors associated with gut dysbiosis and gastrointestinal manifestations in CKD
Abbreviations: CKD = chronic kidney disease; GI = gastrointestinal; H2S = hydrogen sulphide
GUT MICROBIOTA-TARGETED INTERVENTIONS IN CKD
An improved understanding of the involvement of gut dysbiosis in cardiometabolic pathologies has triggered enormous scientific interest and a vigorous quest for the development of novel therapeutic strategies targeting the gut microbiota as a means to prevent and treat CKD and its related complications, such as premature cardiovascular morbidity and mortality.5 Potential strategies include dietary modifications (e.g., plant-based diets)120, dietary supplementation of prebiotics (i.e., nondigestible food ingredients that favorably modifies the composition and/or activity of the gut microbiota),121 probiotics (i.e., live microorganisms that when administered in adequate amounts confer health benefits to the host),122 and synbiotics (i.e., combination of probiotics and prebiotics),123 constipation treatment,24 fecal microbiota transplantation,124 intestinal dialysis, and exercise (Figure 3).125
Figure 3.
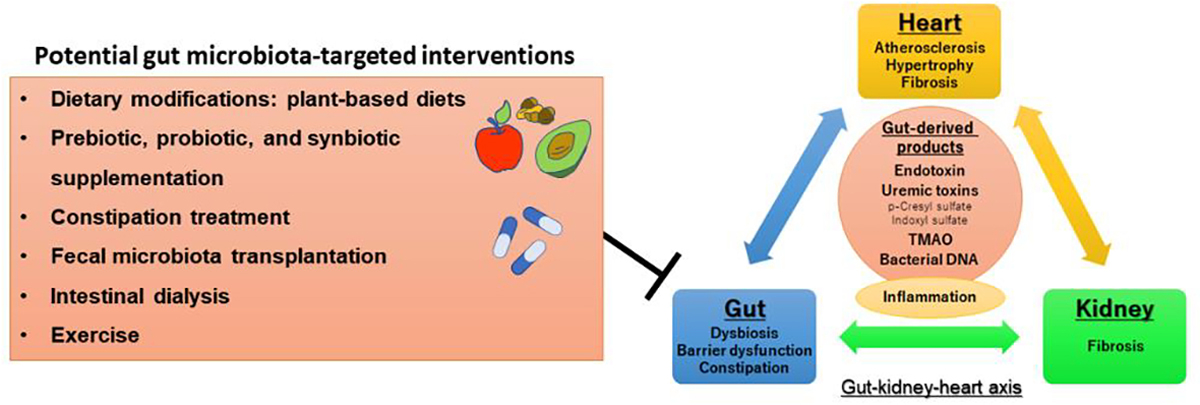
Potential gut microbiota-targeted interventions for the gut-kidney-heart axis
Abbreviation: TMAO = trimethylamine-N-oxide
Part of the figure reprinted with permission from Sumida et al.4
Dietary Modifications: Plant-Based Diets
Plant-based diets focus primarily on plant-dominant products such as seeds, whole grains, legumes, nuts, fruits, and vegetables, while minimizing animal-based products such as fish, meat, eggs, and dairy.126 These diets, often represented by the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets, have gained a wide popularity for the treatment of various lifestyle diseases, including hypertension, diabetes, cardiovascular disease, and CKD.127 In the context of gut microbiota modulation, dietary fibers are a major component of plant-based diets that confer potential health benefits through their favorable influence on the gut microbiota.128
Dietary fibers in edible plants comprise insoluble and soluble carbohydrates (e.g., nonstarch polysaccharides, cellulose, and lignin) and non-digestible resistant starch and oligosaccharides.129 The non-digestible fiber components pass intact through the gastrointestinal tract into the large intestine where they increase viscosity and bulking of the fecal matter, contributing to a faster intestinal transit time.130 In addition, the non-digestible dietary fibers undergo fermentation by the resident anaerobic microbes (mostly by Firmicutes and Bacteroidetes phyla) to form intestinal gaseous products and SCFAs that play an essential role in maintaining the homeostasis of human health (as detailed above).108 In animal studies using CKD rats, dietary intake of foods enriched with amylose resistant starch, which is a fermentable and nondigestible carbohydrate, has been shown to improve gut dysbiosis, attenuate oxidative stress and inflammation, ameliorate metabolic disorders, and even retard the progression of CKD.131,132 Besides fiber components of plant-based diets, fractions of unabsorbed dietary fat can differentially influence the microbial metabolic activities.133 Linoleic acid, which is a polyunsaturated fat that mainly comes from plant sources, is utilized by intestinal bacteria to produce conjugated linoleic acid that has multifaceted beneficial properties, such as anti-inflammatory, anti-adipogenic, and anti-carcinogenic properties.134 Other potential salutary effects of plant-based diets include mitigation of metabolic acidosis and reductions in oxidative stress, uremic toxins and glomerular hyperfiltration.135,136 Meanwhile, animal-based diets that are rich in saturated fats and low in phytochemicals have been shown to shift the composition of gut microbiota toward that favoring a pro-inflammatory state, such as an increase in the abundance and activity of Bilophila wadsworthia, a bacterial species known to contribute to intestinal inflammation.137,138
Currently, the clinical utility of plant-based diets as a gut microbiota-targeted intervention in CKD has been supported by a number of observational studies.139–141 In a recent cross-sectional study of 22 patients on hemodialysis, researchers examined the associations between plant-based diet quality, serum uremic toxins, and gut microbiota profile, and demonstrated that higher (vs. lower) adherence to plant-based diets was significantly associated with lower levels of serum indoxyl sulfate and lower relative abundances of Haemophilus parainfluenzae and genus Haemophilus that were related to elevated serum indoxyl sulfate levels.141 They also showed that an increased intake of food items considered unhealthy, such as animal fats, sweets and desserts, were associated with higher relative abundance of gut bacteria linked to higher serum concentrations of indoxyl sulfate and p-cresyl sulfate.141 In terms of clinical outcomes associated with plant-based diets, a recent meta-analysis of cohort studies has shown that healthy dietary intake of fruits and vegetables was consistently associated with better survival in patients with CKD.142 The association of a healthy dietary pattern with lower risk of incident CKD has also been reported in recent meta-analyses of cohort studies in individuals without preexisting CKD.143,144 Nevertheless, it may be worth noting that a recent large multinational, prospective cohort study (not included in the aforementioned meta-analysis) did not find a significant association of the DASH and Mediterranean diets with cardiovascular or all-cause mortality in patients on hemodialysis.145 These mixed findings from observational studies may be partly due to changes in dietary patterns over time (and possible resultant misclassification). To address the knowledge gap in dietary interventions toward gut microbiota modulation and better outcomes in CKD, well-designed clinical trials will need to use standardized diets with cost considerations, enhance compliance to proposed diets, and better understand the time necessary to induce sustainable change in the gut microbiota.
One of the most frequently stated concerns about fostering plant-based diets in patients with CKD is the perceived risk of hyperkalemia, which however has not been supported by current scientific evidence, presumably due to the enhanced fecal potassium excretion and alkalization associated with the intake of plant-based food sources.146 In a series of clinical trials examining the effect of fruit and vegetable intake on kidney- and cardiovascular-related biochemical parameters in patients with CKD, researchers have demonstrated that an intervention of approximately 2 to 4 cups of fruits and vegetables did not cause hyperkalemia.147–149 A similar finding was also reported in a trial of 22 patients with CKD stages G3–4 who consumed a pure vegan diet for 3 months.150
Prebiotic, Probiotic, and Synbiotic Supplementation
The dietary supplementation of prebiotics, probiotics, and synbiotics has been increasingly recognized as a potential gut microbiota-targeted intervention for patients with CKD. In a recent systematic review and meta-analysis of 16 randomized controlled trials investigating the effects of prebiotic, probiotic, and synbiotic supplementation (ranging in sample size and duration of the individual trials from 9 to 124 patients and 1 to 24 weeks, respectively) on uremic toxins, microbiota profile, and clinical and patient-centered outcomes in patients with CKD, the prebiotic supplementation (consisting respectively of gum arabic, fermentable carbohydrate, hi-maize 260, soluble dietary fiber, and arabinoxylan oligosaccharides) led to a slight but significant reduction in serum urea concentration (mean difference −2.23 mmol/L, 95% CI −3.83 to −0.64, P = 0.006), and the synbiotic supplementation led to significantly higher relative abundances of Bifidobacterium and Lachnospiraceae and a lower abundance of Ruminococcaceae.151 However, these compositional changes in the gut microbiota were investigated in two studies, with the changes in Lachnospiraceae and Ruminococcaceae reported in only one study. It has been suggested that a prebiotic dose of >5 g/day can influence the gut microbiota diversity, while a threshold dose of 15–20 g/day may be required to reduce uremic toxin concentrations.152 Hence, despite possible downstream benefits of these microbial compositional changes (e.g., reduced production of indoxyl sulfate and p-cresyl sulfate),153 their clinical significance need to be confirmed in further studies. More recently, a similar systematic review and meta-analysis from 18 randomized controlled trials in a total of 791 patients on dialysis (ranging in sample size and duration of the individual trials from 15 to 98 patients and 4 to 24 weeks, respectively) reported that probiotic, prebiotic, and synbiotic supplements significantly reduced serum levels of C-reactive protein, IL-6, and indoxyl sulfate and increased serum high-density lipoprotein cholesterol levels.154 It is important to note that the probiotic studies contained various strains and dosages, and this heterogeneity of interventional supplements may still leave a question about the optimal formulation and dosage of these supplements for patients with CKD. Currently, there is insufficient evidence to support the overall treatment effect of prebiotic, probiotic, and synbiotic supplementation on hard clinical outcomes, nor to conclude the superiority of one type of supplementation to another. Well-designed controlled trials are therefore needed to establish an optimal formulation (e.g., strains) and dosages of prebiotic, probiotic, and synbiotic supplementation that effectively and cost-efficiently mitigate the gut dysbiosis and improve its related clinical outcomes in patients with CKD.
Constipation Treatment
Constipation is one of the most common gastrointestinal disorders among patients with CKD, partly because of their sedentary lifestyle, low fiber and fluid intake, multiple comorbidities, and concomitant medications.24 Although constipation is usually perceived as a benign self-limited condition, emerging evidence indicates its independent association with adverse clinical outcomes, such as ESKD, cardiovascular disease, and mortality,118,119 suggesting a greater importance of the adequate management of constipation than previously considered. Potential mechanisms underlying these associations include, among others, an altered gut microbiota (as detailed above), increased accumulation of fecal metabolites (e.g., p-cresyl sulfate), and protein energy wasting associated with constipation,24,155 which appears to make the gut microbiota-targeted interventions a reasonable strategy for the management of constipation in CKD.
In patients with constipation, dietary modification with increased dietary or supplemental fiber intake is traditionally considered a first-line non-pharmacological therapeutic option.156 Although dietary counseling for patients with CKD typically emphasize the restrictions of fiber-rich plant-based diets to prevent hyperkalemia, the aforementioned gastrointestinal and health benefits of plant-based diets may justify consideration of increased intake of these fiber-rich foods as a non-pharmacological approach to the management of constipation in CKD. Nonetheless, pharmacological interventions with laxatives are often required to treat constipation in patients with CKD, particularly in its advanced stages.24,157
Among various types of laxatives currently available for treating constipation, there are a few types of laxative agents (i.e., lactulose, a chloride channel activator [lubiprostone], and a guanylate cyclase C agonist [linaclotide]) that have been shown in animal studies to exert additional therapeutic benefits beyond conventional defecation controls. These include modifications of gut microbiota and intestinal metabolites, improvement of gut barrier integrity, suppression of local inflammatory responses, and even amelioration of CKD progression.158–160 In addition to these favorable effects, the lower cost, wider availability, and more established long-term safety profile of lactulose (vs. two other newer laxatives) might make it a more relevant choice of laxative for the treatment of constipation in the CKD population. In a recent small randomized controlled trial comparing the effect of lactulose (n=16) vs. placebo (n=16) on numbers of fecal Bifidobacterium and Lactobacillus colonies in patients with stage G3 and G4, treatment with lactulose (but not placebo) led to significant increase in numbers of fecal Bifidobacterium and Lactobacillus genera over an 8-week study period.161 However, due to the culture-based assessment of only two bacterial genera and the lack of assessment of uremic toxins and clinical outcomes in this trial, it remains unclear whether lactulose treatment of constipation will confer beneficial effects on the gut microbial community, clinically relevant biochemical parameters, and clinical outcomes in patients with CKD.
Laxative therapy for constipation in CKD may also be beneficial for potassium homeostasis in this population. In patients with CKD whose gut plays an important role in maintaining potassium homeostasis through increased intestinal potassium wasting (as an adaptive mechanism), the slow intestinal transit time and impaction of feces associated with constipation is a likely contributor to the risk of hyperkalemia.24 In a recent observational cohort study of 33,116 patients with advanced CKD, the use (vs. non-use) of laxatives was shown to be independently associated with lower risk of hyperkalemia but not with risk of hypokalemia.162 The finding of this study may be of particular relevance when treating hyperkalemic patients with CKD in the settings of limited availability of potassium-lowering agents or for those refractory to conventional anti-hyperkalemic therapies. Although the risk-benefit profiles of the use of laxatives for potassium management in CKD remain to be clarified, the theoretical safety concern about the use of laxatives, such as progressive loss of kidney function due to drug-induced diarrhea and dehydration, may be alleviated by the reported negligible association of laxative use with change in eGFR in patients with advanced CKD.163 Nevertheless, it may also be important to note that frequent laxative use can lead to undesirable consequences, including lower nutrient absorption.164 The effect of active interventions with laxatives on total body balance of nutrients and electrolytes and their risk-benefit profiles in advanced CKD may deserve future investigations, including clinical trials.
Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT) is a method of transferring the gut microbiota from healthy individuals to patients with gut dysbiosis (typically through an oral administration of encapsulated formulations).165 For years, FMT has been an effective second-line treatment for Clostridium difficile infection, but recently it has been applied to a wide range of diseases including CKD toward the restoration of normal gut homeostasis and the mitigation of heightened risk of the diseases.165 In a recent animal study using mice with adenine-induced CKD, the CKD mice treated with (vs. without) FMT displayed a significant increase in alpha diversity of the gut microbiota, along with significant reduction in p-cresyl sulfate and improvement in glucose tolerance.166 The beneficial effects of FMT on glomerular and tubulointerstitial injuries in diabetic kidney disease have also been reported in a few animal studies.167,168 According to two case reports of patients with membranous nephropathy and IgA nephropathy, FMT therapy was shown to exert favorable effects on kidney function and albuminuria without causing serious adverse events.124,169 In terms of actual procedures of FMT, a case of IgA nephropathy received a total of 40 applications (200 mL daily, 5 days/week) of FMT according to the protocol reported by Paramsothy et al.,170 followed by 57 applications (200 mL daily, 10–15 days/month) over the next 5 months using fresh feces from two healthy young donors.124After the completion of FMT, partial clinical remission of IgA nephropathy was obtained with a 37% decrease in 24-h urinary protein compared to baseline.124 Although there is currently no trial evidence that supports the clinical utility of FMT in the CKD population, the successful clinical application of FMT in treating patients with constipation may support the continued exploration of this therapeutic potential for relevant diseases including CKD,171 with a careful consideration on possible risks of this intervention (e.g., diarrhea, abdominal pain, bacteremia, and fever).172 Future clinical trials are needed to confirm the preliminary findings of FMT and its risk-benefit profiles in patients with CKD.
Intestinal Dialysis
The first recorded application of bowel elimination as a means to treat kidney disease dates back to 40 B.C.173 Until the mid to late 1900s when the modern kidney replacement therapy (e.g., hemodialysis) was introduced, numerous attempts had been made to treat uremic symptoms by utilizing intestinal or colonic lavage/perfusion/irrigation (a.k.a. intestinal dialysis) showing some clinical benefits.174,175 Nowadays, the practice of intestinal dialysis is limited mainly to beauty centers where the procedure is called “colon hydrotherapy” or “colon cleansing”,176 and the application of this therapy has never been approved to treat any diseases, largely due to the lack of sufficient scientific evidence to support the clinical benefits over potential harm. However, the beneficial effects of intestinal dialysis in CKD have been sporadically reported in basic and clinical studies.177–179 In a recent observational study of 178 patients with CKD stages G3-G5, researchers investigated the association of the use of colonic irrigation with CKD progression and showed that the use (vs. non-use) of colonic irrigation was significantly associated with lower risk of CKD progression, which was evident in subgroups of patients with more advanced CKD.179 Of interest, the same group of researchers reported in a follow-up study that the use of colonic irrigation mitigated the CKD-related gut dysbiosis, with species richness in treated patients being more similar to healthy individuals.180 These results may suggest the therapeutic potential of intestinal dialysis as a supplementary therapy to mitigate the risk of CKD progression and its related complications, possibly mediated in part by the gut microbiota modulation. However, given the uncertainty of safety profiles of intestinal dialysis (e.g., possible risks of bowel perforation, nutrient malabsorption, hypokalemia, and massive depletion of beneficial microbes), future larger prospective studies including clinical trials are needed to confirm the safety and efficacy of this therapy in patients with CKD.
Exercise
Accumulating evidence indicates that exercise can enrich gut microbial diversity, enhance the number of beneficial gut microbes, and promote the development of symbiotic or commensal gut bacteria in experimental models and in the non-CKD populations.181,182 In an animal study examining the effect of controlled exercise training on the gut microbiome of obese, non-obese, and hypertensive rats, researchers demonstrated that nonobese and hypertensive rats displayed a different composition of the gut microbiota compared with the obese rats, and exercise enhanced the relative abundance of three genera (Allobaculum, Pseudomonas, and Lactobacillus), with Lactobacillus being the most abundant, while three different genera were shown to be more abundant before exercise training (Streptococcus, Aggregatibacter, and Sutterella).183 In a cross-sectional study of 41 healthy young adults, physical fitness levels were correlated with higher gut microbial diversity regardless of diet.184 The study also showed that fit individuals had the gut microbiota enriched in butyrate-producing taxa, such as Clostridiales, Roseburia, Lachnospiraceae, and Erysipelotrichaceae, resulting in increased production of fecal butyrate, a SCFA associated with improved gut health, suggesting that exercise could be used as an adjuvant therapy for dysbiosis-associated diseases.184 Importantly, despite these observations and putative benefits of exercise on chronic inflammatory diseases through gut microbiota modulation,185 to our knowledge, the effects of exercise on the gut microbiota composition in patients with CKD have not been documented to date. In this context, an ongoing clinical trial (ClinicalTrials.gov identifier: NCT03689569) testing the additive effect of exercise training and high amylose maize resistant starch type 2 supplementation on relevant blood parameters (e.g., indoxyl sulphate and p-cresyl sulfate) may provide novel insights into this field.
CONCLUSIONS
With a growing understanding of the complex involvement of altered gut microbiota in various cardiometabolic pathologies, evidence is accumulating that supports the therapeutic potential of gut microbiota-targeted interventions in the conservative management of CKD. Although many questions and challenges remain to be addressed, given the limited ability of conventional treatments to effectively alleviate the burden of CKD and its complications, perhaps it is now time to further advance our understanding of the risk-benefit profiles of the above discussed gut microbiota-targeted interventions and fully explore their clinical application in patients with CKD through in-depth clinical research, including well-designed small proof-of-principal clinical trials. Concurrently, in addition to existing small-scale interventional studies examining longitudinal changes in the gut microbiota profiles in CKD,153,166,186,187 larger observational studies are necessary to demonstrate whether one-time microbiota assessments can predict clinically meaningful outcomes. These studies, in conjunction, will help identify mechanisms, treatment targets, and the subgroups of patients most likely to benefit from the gut microbiota-targeted interventions.
ACKNOWLEDGEMENTS
CPK is an employee of the US Department of Veterans affairs. Opinions expressed in this paper are those of the authors and do not necessarily represent the opinion of the Department of Veterans Affairs.
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under award number R01DK125586 to KS.
Footnotes
Conflict of Interest
None of the authors have relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.G. B. D. Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73(3S1):A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libetta C, Sepe V, Esposito P, Galli F, Dal Canton A. Oxidative stress and inflammation: Implications in uremia and hemodialysis. Clin Biochem. 2011;44(14–15):1189–1198. [DOI] [PubMed] [Google Scholar]
- 4.Sumida K, Kovesdy CP. The gut-kidney-heart axis in chronic kidney disease. Physiol Int. 2019;106(3):195–206. [DOI] [PubMed] [Google Scholar]
- 5.Sumida K, Lau WL, Kovesdy CP, Kalantar-Zadeh K, Kalantar-Zadeh K. Microbiome modulation as a novel therapeutic approach in chronic kidney disease. Curr Opin Nephrol Hypertens. 2021;30(1):75–84. [DOI] [PubMed] [Google Scholar]
- 6.Abbott A Scientists bust myth that our bodies have more bacteria than human cells. Nature. 2016. [Google Scholar]
- 7.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667–685. [DOI] [PubMed] [Google Scholar]
- 9.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Human Microbiome Project C Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. [DOI] [PubMed] [Google Scholar]
- 15.Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nature Immunology. 2017;18(12):1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14(7):442–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohia S, Vlahou A, Zoidakis J. Microbiome in Chronic Kidney Disease (CKD): An Omics Perspective. Toxins (Basel). 2022;14(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li HB, Xu ML, Xu XD, et al. Faecalibacterium prausnitzii Attenuates CKD via Butyrate-Renal GPR43 Axis. Circ Res. 2022;131(9):e120–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74(2):349–355. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Jiang H, Shi K, Ren Y, Zhang P, Cheng S. Gut bacterial translocation is associated with microinflammation in end-stage renal disease patients. Nephrology (Carlton). 2012;17(8):733–738. [DOI] [PubMed] [Google Scholar]
- 21.Vaziri ND, Wong J, Pahl M, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308–315. [DOI] [PubMed] [Google Scholar]
- 22.Randall DW, Kieswich J, Hoyles L, McCafferty K, Curtis M, Yaqoob MM. Gut Dysbiosis in Experimental Kidney Disease: A Meta-Analysis of Rodent Repository Data. J Am Soc Nephrol. 2023;34(4):533–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12(1):17–31. [DOI] [PubMed] [Google Scholar]
- 24.Sumida K, Yamagata K, Kovesdy CP. Constipation in CKD. Kidney Int Rep. 2020;5(2):121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiner ID, Mitch WE, Sands JM. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clin J Am Soc Nephrol. 2015;10(8):1444–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourke E, Milne MD, Stokes GS. Caecal pH and ammonia in experimental uraemia. Gut. 1966;7(5):558–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang JY. The gastrointestinal tract in uremia. Dig Dis Sci. 1993;38(2):257–268. [DOI] [PubMed] [Google Scholar]
- 28.Kortman GA, Dutilh BE, Maathuis AJ, et al. Microbial Metabolism Shifts Towards an Adverse Profile with Supplementary Iron in the TIM-2 In vitro Model of the Human Colon. Front Microbiol. 2015;6:1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee T, Clavel T, Smirnov K, et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut. 2017;66(5):863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau WL, Vaziri ND, Nunes ACF, et al. The Phosphate Binder Ferric Citrate Alters the Gut Microbiome in Rats with Chronic Kidney Disease. J Pharmacol Exp Ther. 2018;367(3):452–460. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz B, Li H. Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharmaceuticals (Basel). 2018;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro M, Fonseca L, Anjos JS, et al. Oral iron supplementation in patients with chronic kidney disease: Can it be harmful to the gut microbiota? Nutr Clin Pract. 2022;37(1):81–93. [DOI] [PubMed] [Google Scholar]
- 33.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156(Pt 11):3216–3223. [DOI] [PubMed] [Google Scholar]
- 34.Miao YY, Xu CM, Xia M, Zhu HQ, Chen YQ. Relationship between Gut Microbiota and Phosphorus Metabolism in Hemodialysis Patients: A Preliminary Exploration. Chin Med J (Engl). 2018;131(23):2792–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau WL, Savoj J, Nakata MB, Vaziri ND. Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin Sci (Lond). 2018;132(5):509–522. [DOI] [PubMed] [Google Scholar]
- 36.Kikuchi K, Saigusa D, Kanemitsu Y, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nature Communications. 2019;10(1):1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 2016;30(14):1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57(12):3126–3135. [DOI] [PubMed] [Google Scholar]
- 39.van der Beek CM, Dejong CHC, Troost FJ, Masclee AAM, Lenaerts K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr Rev. 2017;75(4):286–305. [DOI] [PubMed] [Google Scholar]
- 40.Lau WL, Vaziri ND. Urea, a true uremic toxin: the empire strikes back. Clin Sci (Lond). 2017;131(1):3–12. [DOI] [PubMed] [Google Scholar]
- 41.Meijers B, Farre R, Dejongh S, Vicario M, Evenepoel P. Intestinal Barrier Function in Chronic Kidney Disease. Toxins (Basel). 2018;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin TY, Hung SC. Association of subjective global assessment of nutritional status with gut microbiota in hemodialysis patients: a case-control study. Nephrol Dial Transplant. 2021;36(6):1104–1111. [DOI] [PubMed] [Google Scholar]
- 43.de Almeida Duarte JB, de Aguilar-Nascimento JE, Nascimento M, Nochi RJ. Bacterial translocation in experimental uremia. Urological Research. 2004;32(4):266–270. [DOI] [PubMed] [Google Scholar]
- 44.Aronov PA, Luo FJ, Plummer NS, et al. Colonic contribution to uremic solutes. J Am Soc Nephrol. 2011;22(9):1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H, Subramanian VS. Disintegration of colonic epithelial tight junction in uremia: a likely cause of CKD-associated inflammation. Nephrol Dial Transplant. 2012;27(7):2686–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaziri ND, Goshtasbi N, Yuan J, et al. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am J Nephrol. 2012;36(5):438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol. 2013;37(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terpstra ML, Singh R, Geerlings SE, Bemelman FJ. Measurement of the intestinal permeability in chronic kidney disease. World J Nephrol. 2016;5(4):378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant. 2016;31(5):737–746. [DOI] [PubMed] [Google Scholar]
- 50.Andersen K, Kesper MS, Marschner JA, et al. Intestinal Dysbiosis, Barrier Dysfunction, and Bacterial Translocation Account for CKD-Related Systemic Inflammation. J Am Soc Nephrol. 2017;28(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dorph S, Oigaard A, Pedersen G, McNair A, Sorensen MB. Gastroduodenal mucosal changes in chronic uremia. Scand J Gastroenterol. 1972;7(7):589–592. [DOI] [PubMed] [Google Scholar]
- 52.Wang F, Zhang P, Jiang H, Cheng S. Gut bacterial translocation contributes to microinflammation in experimental uremia. Dig Dis Sci. 2012;57(11):2856–2862. [DOI] [PubMed] [Google Scholar]
- 53.Shi K, Wang F, Jiang H, et al. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig Dis Sci. 2014;59(9):2109–2117. [DOI] [PubMed] [Google Scholar]
- 54.Wiedermann CJ, Kiechl S, Dunzendorfer S, et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol. 1999;34(7):1975–1981. [DOI] [PubMed] [Google Scholar]
- 55.Meijers BK, Claes K, Bammens B, et al. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol. 2010;5(7):1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stubbs JR, House JA, Ocque AJ, et al. Serum Trimethylamine-N-Oxide is Elevated in CKD and Correlates with Coronary Atherosclerosis Burden. J Am Soc Nephrol. 2016;27(1):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stubbs JR, Stedman MR, Liu S, et al. Trimethylamine N-Oxide and Cardiovascular Outcomes in Patients with End-stage Kidney Disease Receiving Maintenance Hemodialysis. Clin J Am Soc Nephrol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szeto CC, McIntyre CW, Li PK. Circulating Bacterial Fragments as Cardiovascular Risk Factors in CKD. J Am Soc Nephrol. 2018;29(6):1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sumida K, Han Z, Chiu CY, et al. Circulating Microbiota in Cardiometabolic Disease. Front Cell Infect Microbiol. 2022;12:892232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ulevitch RJ, Tobias PS. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol. 1999;11(1):19–22. [DOI] [PubMed] [Google Scholar]
- 62.Bowman JD, Surani S, Horseman MA. Endotoxin, Toll-like Receptor-4, and Atherosclerotic Heart Disease. Curr Cardiol Rev. 2017;13(2):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neal MD, Leaphart C, Levy R, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176(5):3070–3079. [DOI] [PubMed] [Google Scholar]
- 64.Moghimpour Bijani F, Vallejo JG, Rezaei N. Toll-like receptor signaling pathways in cardiovascular diseases: challenges and opportunities. Int Rev Immunol. 2012;31(5):379–395. [DOI] [PubMed] [Google Scholar]
- 65.Cole JE, Georgiou E, Monaco C. The expression and functions of toll-like receptors in atherosclerosis. Mediators Inflamm. 2010;2010:393946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goncalves S, Pecoits-Filho R, Perreto S, et al. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant. 2006;21(10):2788–2794. [DOI] [PubMed] [Google Scholar]
- 67.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McIntyre CW, Harrison LE, Eldehni MT, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(1):133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anker SD, Egerer KR, Volk HD, Kox WJ, Poole-Wilson PA, Coats AJ. Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol. 1997;79(10):1426–1430. [DOI] [PubMed] [Google Scholar]
- 70.Feroze U, Kalantar-Zadeh K, Sterling KA, et al. Examining associations of circulating endotoxin with nutritional status, inflammation, and mortality in hemodialysis patients. J Ren Nutr. 2012;22(3):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hobby GP, Karaduta O, Dusio GF, Singh M, Zybailov BL, Arthur JM. Chronic kidney disease and the gut microbiome. Am J Physiol Renal Physiol. 2019;316(6):F1211–F1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez AW, Recht NS, Hostetter TH, Meyer TW. Removal of P-cresol sulfate by hemodialysis. J Am Soc Nephrol. 2005;16(11):3430–3436. [DOI] [PubMed] [Google Scholar]
- 73.Cummings JH. Fermentation in the human large intestine: evidence and implications for health. Lancet. 1983;1(8335):1206–1209. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. 2015;6(2):e02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109(52):21307–21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu Y, Jameson E, Crosatti M, et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111(11):4268–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25(9):1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One. 2012;7(3):e34026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Han H, Zhu J, Zhu Z, et al. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J Am Heart Assoc. 2015;4(6):e001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu W, Gregory JC, Org E, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165(1):111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(10):1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shafi T, Powe NR, Meyer TW, et al. Trimethylamine N-Oxide and Cardiovascular Events in Hemodialysis Patients. J Am Soc Nephrol. 2017;28(1):321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watkins EB, Phillips RS. Inhibition of tyrosine phenol-lyase from Citrobacter freundii by 2-azatyrosine and 3-azatyrosine. Biochemistry. 2001;40(49):14862–14868. [DOI] [PubMed] [Google Scholar]
- 87.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. [DOI] [PubMed] [Google Scholar]
- 88.Ott SJ, El Mokhtari NE, Musfeldt M, et al. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation. 2006;113(7):929–937. [DOI] [PubMed] [Google Scholar]
- 89.Erridge C The roles of pathogen-associated molecular patterns in atherosclerosis. Trends Cardiovasc Med. 2008;18(2):52–56. [DOI] [PubMed] [Google Scholar]
- 90.Rajaee A, Barnett R, Cheadle WG. Pathogen- and Danger-Associated Molecular Patterns and the Cytokine Response in Sepsis. Surg Infect (Larchmt). 2018;19(2):107–116. [DOI] [PubMed] [Google Scholar]
- 91.Klinman DM, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158(8):3635–3639. [PubMed] [Google Scholar]
- 92.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. [DOI] [PubMed] [Google Scholar]
- 93.El Kebir D, Jozsef L, Filep JG. Neutrophil recognition of bacterial DNA and Toll-like receptor 9-dependent and -independent regulation of neutrophil function. Arch Immunol Ther Exp (Warsz). 2008;56(1):41–53. [DOI] [PubMed] [Google Scholar]
- 94.Navarro MD, Carracedo J, Ramirez R, et al. Bacterial DNA prolongs the survival of inflamed mononuclear cells in haemodialysis patients. Nephrol Dial Transplant. 2007;22(12):3580–3585. [DOI] [PubMed] [Google Scholar]
- 95.Donath MY, Meier DT, Böni-Schnetzler M. Inflammation in the Pathophysiology and Therapy of Cardiometabolic Disease. Endocrine Reviews. 2019;40(4):1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Merino A, Nogueras S, Garcia-Maceira T, et al. Bacterial DNA and endothelial damage in haemodialysis patients. Nephrol Dial Transplant. 2008;23(11):3635–3642. [DOI] [PubMed] [Google Scholar]
- 97.Paladugu B, Kumar A, Parrillo JE, et al. Bacterial DNA and RNA induce rat cardiac myocyte contraction depression in vitro. Shock. 2004;21(4):364–369. [DOI] [PubMed] [Google Scholar]
- 98.Szeto CC, Kwan BC, Chow KM, et al. Circulating bacterial-derived DNA fragment level is a strong predictor of cardiovascular disease in peritoneal dialysis patients. PLoS One. 2015;10(5):e0125162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sato J, Kanazawa A, Ikeda F, et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care. 2014;37(8):2343–2350. [DOI] [PubMed] [Google Scholar]
- 100.Lelouvier B, Servant F, Paisse S, et al. Changes in blood microbiota profiles associated with liver fibrosis in obese patients: A pilot analysis. Hepatology. 2016;64(6):2015–2027. [DOI] [PubMed] [Google Scholar]
- 101.Amar J, Lange C, Payros G, et al. Blood microbiota dysbiosis is associated with the onset of cardiovascular events in a large general population: the D.E.S.I.R. study. PLoS One. 2013;8(1):e54461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rajendhran J, Shankar M, Dinakaran V, Rathinavel A, Gunasekaran P. Contrasting circulating microbiome in cardiovascular disease patients and healthy individuals. Int J Cardiol. 2013;168(5):5118–5120. [DOI] [PubMed] [Google Scholar]
- 103.Amar J, Lelouvier B, Servant F, et al. Blood Microbiota Modification After Myocardial Infarction Depends Upon Low-Density Lipoprotein Cholesterol Levels. J Am Heart Assoc. 2019;8(19):e011797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shah NB, Allegretti AS, Nigwekar SU, et al. Blood Microbiome Profile in CKD : A Pilot Study. Clin J Am Soc Nephrol. 2019;14(5):692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sumida K, Pierre JF, Han Z, et al. Circulating Microbial Signatures and Cardiovascular Death in Patients With ESRD. Kidney Int Rep. 2021;6(10):2617–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Obata Y, Castano A, Boeing S, et al. Neuronal programming by microbiota regulates intestinal physiology. Nature. 2020;578(7794):284–289. [DOI] [PubMed] [Google Scholar]
- 108.Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9(10):577–589. [DOI] [PubMed] [Google Scholar]
- 109.Rajilic-Stojanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141(5):1792–1801. [DOI] [PubMed] [Google Scholar]
- 110.Khalif IL, Quigley EM, Konovitch EA, Maximova ID. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis. 2005;37(11):838–849. [DOI] [PubMed] [Google Scholar]
- 111.Kim SE, Choi SC, Park KS, et al. Change of Fecal Flora and Effectiveness of the Short-term VSL#3 Probiotic Treatment in Patients With Functional Constipation. J Neurogastroenterol Motil. 2015;21(1):111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ohkusa T, Koido S, Nishikawa Y, Sato N. Gut Microbiota and Chronic Constipation: A Review and Update. Front Med (Lausanne). 2019;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lewis SJ, Heaton KW. Increasing butyrate concentration in the distal colon by accelerating intestinal transit. Gut. 1997;41(2):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.El Oufir L, Flourie B, Bruley des Varannes S, et al. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut. 1996;38(6):870–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao Y, Yu YB. Intestinal microbiota and chronic constipation. Springerplus. 2016;5(1):1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoegenauer C, Hammer HF, Mahnert A, Moissl-Eichinger C. Methanogenic archaea in the human gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2022. [DOI] [PubMed] [Google Scholar]
- 117.Nishiyama K, Aono K, Fujimoto Y, et al. Chronic kidney disease after 5/6 nephrectomy disturbs the intestinal microbiota and alters intestinal motility. J Cell Physiol. 2019;234(5):6667–6678. [DOI] [PubMed] [Google Scholar]
- 118.Sumida K, Molnar MZ, Potukuchi PK, et al. Constipation and Incident CKD. J Am Soc Nephrol. 2017;28(4):1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sumida K, Molnar MZ, Potukuchi PK, et al. Constipation and risk of death and cardiovascular events. Atherosclerosis. 2019;281:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Snelson M, Kellow NJ, Coughlan MT. Modulation of the Gut Microbiota by Resistant Starch as a Treatment of Chronic Kidney Diseases: Evidence of Efficacy and Mechanistic Insights. Adv Nutr. 2019;10(2):303–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koppe L, Fouque D. Microbiota and prebiotics modulation of uremic toxin generation. Panminerva Med. 2017;59(2):173–187. [DOI] [PubMed] [Google Scholar]
- 122.Natarajan R, Pechenyak B, Vyas U, et al. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int. 2014;2014:568571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guida B, Germano R, Trio R, et al. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr Metab Cardiovasc Dis. 2014;24(9):1043–1049. [DOI] [PubMed] [Google Scholar]
- 124.Zhao J, Bai M, Yang X, Wang Y, Li R, Sun S. Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: the first case reports. Ren Fail. 2021;43(1):928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sumida K, Lau WL, Kalantar-Zadeh K, Kovesdy CP. Novel intestinal dialysis interventions and microbiome modulation to control uremia. Curr Opin Nephrol Hypertens. 2022;31(1):82–91. [DOI] [PubMed] [Google Scholar]
- 126.Adair KE, Bowden RG. Ameliorating Chronic Kidney Disease Using a Whole Food Plant-Based Diet. Nutrients. 2020;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park YM, Steck SE, Fung TT, et al. Mediterranean diet, Dietary Approaches to Stop Hypertension (DASH) style diet, and metabolic health in U.S. adults. Clin Nutr. 2017;36(5):1301–1309. [DOI] [PubMed] [Google Scholar]
- 128.Zimmer J, Lange B, Frick JS, et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur J Clin Nutr. 2012;66(1):53–60. [DOI] [PubMed] [Google Scholar]
- 129.Chassard C, Lacroix C. Carbohydrates and the human gut microbiota. Curr Opin Clin Nutr Metab Care. 2013;16(4):453–460. [DOI] [PubMed] [Google Scholar]
- 130.Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2(12):1266–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kieffer DA, Piccolo BD, Vaziri ND, et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Renal Physiol. 2016;310(9):F857–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vaziri ND, Liu SM, Lau WL, et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One. 2014;9(12):e114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lam YY, Ha CW, Hoffmann JM, et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity (Silver Spring). 2015;23(7):1429–1439. [DOI] [PubMed] [Google Scholar]
- 134.O’Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152(3):189–205. [DOI] [PubMed] [Google Scholar]
- 135.Carrero JJ, Gonzalez-Ortiz A, Avesani CM, et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat Rev Nephrol. 2020;16(9):525–542. [DOI] [PubMed] [Google Scholar]
- 136.Joshi S, Hashmi S, Shah S, Kalantar-Zadeh K. Plant-based diets for prevention and management of chronic kidney disease. Curr Opin Nephrol Hypertens. 2020;29(1):16–21. [DOI] [PubMed] [Google Scholar]
- 137.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487(7405):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen X, Wei G, Jalili T, et al. The Associations of Plant Protein Intake With All-Cause Mortality in CKD. Am J Kidney Dis. 2016;67(3):423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim H, Caulfield LE, Garcia-Larsen V, et al. Plant-Based Diets and Incident CKD and Kidney Function. Clin J Am Soc Nephrol. 2019;14(5):682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stanford J, Charlton K, Stefoska-Needham A, et al. Associations Among Plant-Based Diet Quality, Uremic Toxins, and Gut Microbiota Profile in Adults Undergoing Hemodialysis Therapy. J Ren Nutr. 2021;31(2):177–188. [DOI] [PubMed] [Google Scholar]
- 142.Kelly JT, Palmer SC, Wai SN, et al. Healthy Dietary Patterns and Risk of Mortality and ESRD in CKD: A Meta-Analysis of Cohort Studies. Clin J Am Soc Nephrol. 2017;12(2):272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bach KE, Kelly JT, Palmer SC, Khalesi S, Strippoli GFM, Campbell KL. Healthy Dietary Patterns and Incidence of CKD. A Meta-Analysis of Cohort Studies. 2019;14(10):1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kelly JT, Su G, Zhang L, et al. Modifiable Lifestyle Factors for Primary Prevention of CKD: A Systematic Review and Meta-Analysis. J Am Soc Nephrol. 2021;32(1):239–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saglimbene VM, Wong G, Craig JC, et al. The Association of Mediterranean and DASH Diets with Mortality in Adults on Hemodialysis: The DIET-HD Multinational Cohort Study. J Am Soc Nephrol. 2018;29(6):1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sumida K, Biruete A, Kistler BM, et al. New Insights into Dietary Approaches to Potassium Management in Chronic Kidney Disease. J Ren Nutr. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8(3):371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goraya N, Simoni J, Jo CH, Wesson DE. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014;86(5):1031–1038. [DOI] [PubMed] [Google Scholar]
- 149.Goraya N, Munoz-Maldonado Y, Simoni J, Wesson DE. Fruit and Vegetable Treatment of Chronic Kidney Disease-Related Metabolic Acidosis Reduces Cardiovascular Risk Better than Sodium Bicarbonate. Am J Nephrol. 2019;49(6):438–448. [DOI] [PubMed] [Google Scholar]
- 150.Barsotti G, Morelli E, Cupisti A, Meola M, Dani L, Giovannetti S. A low-nitrogen low-phosphorus Vegan diet for patients with chronic renal failure. Nephron. 1996;74(2):390–394. [DOI] [PubMed] [Google Scholar]
- 151.McFarlane C, Ramos CI, Johnson DW, Campbell KL. Prebiotic, Probiotic, and Synbiotic Supplementation in Chronic Kidney Disease: A Systematic Review and Meta-analysis. J Ren Nutr. 2019;29(3):209–220. [DOI] [PubMed] [Google Scholar]
- 152.Rossi M, Klein K, Johnson DW, Campbell KL. Pre-, pro-, and synbiotics: do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int J Nephrol. 2012;2012:673631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rossi M, Johnson DW, Morrison M, et al. Synbiotics Easing Renal Failure by Improving Gut Microbiology (SYNERGY): A Randomized Trial. Clin J Am Soc Nephrol. 2016;11(2):223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen C, Wang J, Li J, Zhang W, Ou S. Probiotics, Prebiotics, and Synbiotics for Patients on Dialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Ren Nutr. 2022. [DOI] [PubMed] [Google Scholar]
- 155.Ramos CI, Armani RG, Canziani ME, et al. Bowel Habits and the Association With Uremic Toxins in Non-Dialysis-Dependent Chronic Kidney Disease Patients. J Ren Nutr. 2019. [DOI] [PubMed] [Google Scholar]
- 156.Swier RM, Siebrasse A, Coscia E, Peery AF. Diet in Benign Colonic Disorders: A Narrative Review. Clin Ther. 2022;44(5):657–670. [DOI] [PubMed] [Google Scholar]
- 157.Sumida K, Dashputre AA, Potukuchi PK, et al. Laxative use in patients with advanced chronic kidney disease transitioning to dialysis. Nephrol Dial Transplant. 2021;36(11):2018–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mishima E, Fukuda S, Shima H, et al. Alteration of the Intestinal Environment by Lubiprostone Is Associated with Amelioration of Adenine-Induced CKD. J Am Soc Nephrol. 2015;26(8):1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nanto-Hara F, Kanemitsu Y, Fukuda S, et al. The guanylate cyclase C agonist linaclotide ameliorates the gut-cardio-renal axis in an adenine-induced mouse model of chronic kidney disease. Nephrol Dial Transplant. 2019. [DOI] [PubMed] [Google Scholar]
- 160.Sueyoshi M, Fukunaga M, Mei M, et al. Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats. Clin Exp Nephrol. 2019;23(7):908–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tayebi-Khosroshahi H, Habibzadeh A, Niknafs B, et al. The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial. J Renal Inj Prev. 2016;5(3):162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sumida K, Dashputre AA, Potukuchi PK, et al. Laxative Use and Risk of Dyskalemia in Patients with Advanced CKD Transitioning to Dialysis. J Am Soc Nephrol. 2021;32(4):950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sumida K, Dashputre AA, Potukuchi PK, et al. Laxative Use and Change in Estimated Glomerular Filtration Rate in Patients With Advanced Chronic Kidney Disease. J Ren Nutr. 2021;31(4):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Roerig JL, Steffen KJ, Mitchell JE, Zunker C. Laxative abuse: epidemiology, diagnosis and management. Drugs. 2010;70(12):1487–1503. [DOI] [PubMed] [Google Scholar]
- 165.Bian J, Liebert A, Bicknell B, Chen XM, Huang C, Pollock CA. Faecal Microbiota Transplantation and Chronic Kidney Disease. Nutrients. 2022;14(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Barba C, Soulage CO, Caggiano G, Glorieux G, Fouque D, Koppe L. Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD. Toxins (Basel). 2020;12(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hu ZB, Lu J, Chen PP, et al. Dysbiosis of intestinal microbiota mediates tubulointerstitial injury in diabetic nephropathy via the disruption of cholesterol homeostasis. Theranostics. 2020;10(6):2803–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lu J, Chen PP, Zhang JX, et al. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKalpha activity. Theranostics. 2021;11(10):4728–4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhou G, Zeng J, Peng L, et al. Fecal microbiota transplantation for membranous nephropathy. CEN Case Rep. 2021;10(2):261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389(10075):1218–1228. [DOI] [PubMed] [Google Scholar]
- 171.Tian H, Ge X, Nie Y, et al. Fecal microbiota transplantation in patients with slow-transit constipation: A randomized, clinical trial. PLoS One. 2017;12(2):e0171308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Park SY, Seo GS. Fecal Microbiota Transplantation: Is It Safe? Clin Endosc. 2021;54(2):157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Friedman EA. Bowel as a kidney substitute in renal failure. Am J Kidney Dis. 1996;28(6):943–950. [DOI] [PubMed] [Google Scholar]
- 174.Kolff WJ. New Ways of treating uremia. In: London: J. A. Churchill, Ltd.; 1947. [Google Scholar]
- 175.Pateras VR, Dossetor JB, Gault MH, Helle SJ, Tagushi Y, MacKinnon KJ. The role of intestinal perfusion in the management of chronic uremia. Trans Am Soc Artif Intern Organs. 1964;10:292–297. [PubMed] [Google Scholar]
- 176.Bazzocchi G, Giuberti R. Irrigation, lavage, colonic hydrotherapy: from beauty center to clinic? Tech Coloproctol. 2017;21(1):1–4. [DOI] [PubMed] [Google Scholar]
- 177.Kajbafzadeh AM, Sabetkish N, Sabetkish S. Establishment of colonic dialysis model in uremic rats by right nephrectomy and left partial nephrectomy. J Pediatr Urol. 2018;14(2):159 e151–159 e158. [DOI] [PubMed] [Google Scholar]
- 178.Kajbafzadeh AM, Zeinoddini A, Heidari R, NaserHodjjati H, Tourchi A. A novel alternative for renal replacement therapy: 2-year successful colonic dialysis via a Malone antegrade continent enema stoma. J Pediatr Urol. 2014;10(3):511–514. [DOI] [PubMed] [Google Scholar]
- 179.Dai S, Dai Y, Peng J, Xie X, Ning J. Simplified colonic dialysis with hemodialysis solutions delays the progression of chronic kidney disease. QJM. 2019;112(3):189–196. [DOI] [PubMed] [Google Scholar]
- 180.Li Y, Dai M, Yan J, et al. Colonic dialysis can influence gut flora to protect renal function in patients with pre-dialysis chronic kidney disease. Sci Rep. 2021;11(1):12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Monda V, Villano I, Messina A, et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxid Med Cell Longev. 2017;2017:3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc Sport Sci Rev. 2019;47(2):75–85. [DOI] [PubMed] [Google Scholar]
- 183.Petriz BA, Castro AP, Almeida JA, et al. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genomics. 2014;15(1):511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen J, Guo Y, Gui Y, Xu D. Physical exercise, gut, gut microbiota, and atherosclerotic cardiovascular diseases. Lipids Health Dis. 2018;17(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lambert K, Bird L, Borst AC, et al. Safety and Efficacy of Using Nuts to Improve Bowel Health in Hemodialysis Patients. J Ren Nutr. 2020;30(5):462–469. [DOI] [PubMed] [Google Scholar]
- 187.Genton L, Pruijm M, Teta D, et al. Gut barrier and microbiota changes with glycine and branched-chain amino acid supplementation in chronic haemodialysis patients. J Cachexia Sarcopenia Muscle. 2021;12(6):1527–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]


