Abstract
Background
Pneumothorax is a common complication induced by computed tomography (CT)-guided percutaneous needle biopsy, with a frequency of 17–40.4%. It remains debatable how to predict and prevent the occurrence of post-biopsy pneumothorax. In a real-world setting, we investigated the characteristics associated with pneumothorax in primary lung nodule biopsy.
Methods
This clinical registry cohort study recorded patients with newly diagnosed pulmonary nodules from 10 medical centers from April 2021 to April 2022, and the data were input into the electronic data capture (EDC) system. The eligibility criteria for participants included being within the age range of 18 to 80 years and expressing a willingness to undergo percutaneous puncture biopsy, among other requirements. Conversely, the exclusion criteria included an inability to cooperate throughout the biopsy process and the emergence of new health issues during the study duration resulting in attendance delays, among other factors. This study collected data from 924 patients, out of which 593 were included after exclusion. The essential characteristics, imaging features of pulmonary nodules, and technical factors associated with percutaneous biopsy were recorded. T-tests or one-way analysis of variance (ANOVA) were performed for continuous variables and Pearson’s χ2 test, likelihood ratio, or Fisher’s exact test were applied for categorical variables for comparison as appropriate, followed by multivariate logistic regression.
Results
The overall incidence of pneumothorax was 13.0% (77/593), among which timely pneumothorax was 10.3% (61/593), delayed pneumothorax was 2.7% (16/593), and the rate of chest tube placement was 3.4% (20/593). There was no significant difference in the incidence of pneumothorax in a needle size range of 16–19 G (P=0.129), but the incidence of pneumothorax was lower with 17 G needles than with 18 G. An increased morbidity of pneumothorax was correlated with age (P=0.003), emphysema (P=0.006), and operation time (P=0.002). There was no significant increase in the incidence of pneumothorax between 1 or 2 passes through the pleura (P=0.062). However, multiple pleural passes (3 times) increased the chances of pneumothorax significantly (P=0.022). These risk factors have a certain clinical value in predicting the incidence of post-biopsy pneumothorax, and the area under the curve (AUC) was 0.749.
Conclusions
The most common post-biopsy complication, pneumothorax, was managed conservatively in most cases. A maximum of two pleural passes does not increase the incidence of pneumothorax, and the 17 G needle is more suitable for percutaneous biopsy of pulmonary nodules in the real world.
Keywords: Lung nodule, biopsy, pneumothorax, computed tomography (CT)-guided
Introduction
Lung nodules are detected in approximately 30% of chest computed tomography (CT) scans, and at least 95% are benign (1). Radiologically, these nodules are often classified into three categories: pure ground glass, part-solid, and solid nodules. Although CT scan is commonly used to evaluate pulmonary nodules, it may not always provide a definitive diagnosis, especially for nodules with atypical imaging features. In such cases, percutaneous CT-guided lung nodule biopsy (PCLNB) is necessary to accurately identify the nodule. This biopsy technique has high sensitivity, specificity, and accuracy, making it the most important diagnostic tool for lung nodules (2,3). Currently, CT guidance is the most widely used method for percutaneous lung nodule biopsy, but ultrasound, fluoroscopy, and cone-beam CT are also used in certain cases for their real-time capabilities (4,5).
Percutaneous lung nodule biopsy can lead to various complications, including pneumothorax, hemothorax, hemoptysis, and air embolism. These complications are influenced by factors such as the size of the biopsy needle, the utilization of a coaxial technique, and the cooperation of the patient. Pneumothorax, with a frequency of 17–40.4% (6-8), is the most prevalent complication of PCLNB. Numerous prior studies evaluating the risk factors for post-biopsy pneumothorax have presented conflicting results, particularly regarding the influence of non-coaxial and coaxial techniques, lesion size, lesion location, and so on (9-13). These studies, however, did not investigate the correlation between pneumothorax and the numbers of pass through the pleura performed with 16–19 G needles, as well as the subtypes of emphysema such as centrilobular emphysema, panlobular emphysema, para-septal emphysemas, and para-scar emphysemas (6,9-15).
There is currently no specific guideline or consensus on pulmonary nodule biopsy techniques, with the British Thoracic Society (16) or multidisciplinary experts in China on radio-guided lung biopsy (17) serving as the primary sources of information. As a result, developing an appropriate pulmonary nodule biopsy method based on real-world data is advantageous for the implementation and promotion of PCLNB, as well as the management of complications. Measures that can be taken to help prevent the development of complication of pneumothorax might enhance thoracic surgeons’ or patients’ confidence in preoperative biopsy; after all, preoperative biopsy has been demonstrated to decrease the occurrence of benign lesions after surgery (2,18).
This observational study aimed to evaluate the frequency of pneumothorax after PCLNB and assess possible factors associated with pneumothorax. We present this article in accordance with the STROBE reporting checklist (available at https://qims.amegroups.com/article/view/10.21037/qims-23-891/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Army Medical University (IRB No. KY2021084). All participating hospitals were informed and agreed to the study. Informed consent was provided by all individual participants.
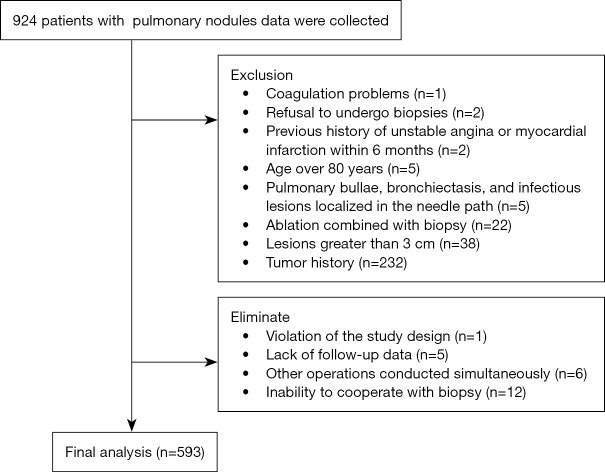
This prospective study recorded data from patients with pulmonary nodules from 10 medical centers between April 2021 and April 2022, including seven university hospitals and three city- or provincial-level tertiary hospitals. The clinical data, imaging, biopsy procedures, and complications of patients undergoing PCLNB were collected and inputted into the electronic data capture (EDC) system. The data of 924 patients were recorded, out of which 593 patients were included in the analysis based on the inclusion and exclusion criteria (Figure 1). The preparation before the operation, equipment selection, and operation procedures of each center were carried out according to the consensus of Chinese experts (17). All CT-guided biopsies were performed with the patient breathing freely and by practitioners with over 3 years of experience in CT-guided percutaneous biopsy.
Figure 1.
Flowchart showing the inclusion and exclusion criteria for the 593 patients who underwent percutaneous CT-guided pulmonary nodule biopsy. CT, computed tomography.
Inclusion and exclusion criteria
The study involved patients who underwent lung nodule biopsy at various research centers and met the biopsy evaluation criteria set by Chinese experts (17). The exclusion criteria were as follows: (I) patients who were below 18 or above 80 years of age; (II) patients who refused percutaneous puncture biopsy; (III) patients who underwent biopsy combined with ablation; (IV) patients who had lesions after local treatment such as ablation and stereotactic radiotherapy; (V) patients who had obvious bullae, bronchiectasis, or infectious lesions in the biopsy path; (VI) irreversible bleeding tendencies or coagulation dysfunction; (VII) severe chronic obstructive pulmonary disease (COPD) or a history of asthma; (VIII) a history of malignant tumors or pathologically diagnosed malignant lesions; (IX) unstable angina or myocardial infarction within 6 months; (X) severe mental illness, pregnancy, or lactation; (XI) suspected echinococcosis or vascular malformation; and (XII) severe cardiopulmonary insufficiency. Further elimination criteria were as follows: (I) inability to cooperate with biopsy; (II) development of new health problems during the study period leading to attendance delays; (III) lack of follow-up data; (IV) other operations conducted simultaneously; and (V) patients who no longer participated or were unwilling to cooperate with the study design after their participation.
Indicators and definitions
Basic patient characteristics were collected including whether they were an inpatient or outpatient, age, multidisciplinary treatment (yes vs. no), sex, smoking (yes vs. no), diabetes (yes vs. no), and hypertension (>140/90 vs. ≤140/90 mmHg). A previous study demonstrated that pulmonary function test variables were not associated with the development of pneumothorax (10); however, Ohno et al.’s research presents contrasting conclusions (19). This study only documented the presence of emphysema in the lobe where the biopsy was performed. Emphysema was characterized by areas of low attenuation on CT (15). The Eastern Cooperative Oncology Group (ECOG) rating scale is as follows: 0: fully active, able to carry on all pre-disease performance without restrictions; 1: restricted in physically strenuous activity but ambulatory and able to carry out light work; 2: ambulatory and capable of all self-care but unable to carry out any work activities; physically mobile for more than 50% of waking hours; 3: capable of only limited self-care; confined to bed or chair more than 50% of waking hours; 4: completely disabled; cannot carry out any self-care; totally confined to the bed or chair; 5: dead.
Lesion factors included the maximum diameter of the target lesions in the standard lung window CT axial view [window plane =−600 Hounsfield units (HU), window width =1,500] to determine the size of the lesions, and lobe (upper and right middle lobe vs. lower). In this study, the areas of pulmonary nodules were broadly classified into three categories according to the location of the lesions. Peripheral nodules were defined as lesions <2 cm from the parietal pleura, hilar nodules were defined as lesions <2 cm from the hilum, and intermediate nodules were located between the peripheral and hilar nodules. The consolidation to tumor ratio was also recorded (predominantly ground glass vs. predominant consolidation) (20).
Technical factors included coaxial or non-coaxial, semi-automatic or automatic, preoperative breathing training (yes or no), patients were asked to perform the rhythmic breathing exercises preoperatively to reduce pain and the impact of changes in surgical position on the rhythm of breathing movements, needle patch sealing (yes or no), and operation time was the difference between the scan time of the first image and the scan time of the last picture. Depending on the location of the lesion, the patient was operated on in the supine, prone (right or left) lateral position to ensure the safest and shortest path to the lesion. However, this may not be the most straightforward way to move lesions. The length of the puncture, defined as the length of aerated lung parenchyma crossed by a biopsy needle from the pleura to the lesion, was recorded. Needles varied in diameter from 16 to 19 G and were divided into two groups (16–17 vs. 18–19 G). The passing of the needle through the visceral pleura or oblique fissure was recorded as one and two pleural passes, respectively. It is optimal to minimize pleural injury during lung nodule biopsy. However, when the lung nodule is located adjacent to the interlobar fissure and the path of puncture within the same lung lobe is obstructed or carries high risk, an alternative approach through a different lung lobe may be necessary to biopsy the nodule adjacent to the interlobar fissure. In such cases, the procedure may result in three pleural passes. If a non-coaxial needle is used for the second sampling, it has the potential to result in six pleural passes.
Pneumothorax
During the procedure and post-procedural CT scans, the presence of air densities in the pleural cavity indicated pneumothorax. Pneumothorax detected within 4 hours after biopsy was classified as timely, whereas pneumothorax detected from 4 hours after the procedure until the next day on chest X-ray was classified as delayed. The principle of chest tube placement is that when the lung is compressed by 30% or more, there is evidence of persistent air leak or dyspnea, even when the lung is compressed by less than 30%.
Statistical analysis
All data were described as mean ± standard deviation for continuous variables and numerical values (percentages) for categorical variables. Data were subjected to univariate analysis using t-tests or one-way analysis of variance (ANOVA) for continuous variables and Pearson’s χ2 test, likelihood ratio, or Fisher’s exact test for categorical variables for comparison. In our study, the incidence of immediate and delayed pneumothorax was low, and few positive dependent variables could influence the results of the multivariate analysis, so we performed immediate and delayed pneumothorax for multi-factor analysis. All reported P values are 2-sided and have not been adjusted for multiple testing, and P<0.05 was considered indicative of a statistically significant difference. Statistical analysis was performed by using the software SPSS 26.0 (IBM Corp., Armonk, NY, USA).
Results
A total of 593 patients were included in this study (Figure 1); 50.6% (300/593) were male, 49.4% (293/593) were female, the mean age was 59.26±11.30 years, 31.7% (188/593) were outpatients, and 13.8% (82/593) of were evaluated by a multidisciplinary team before the biopsy. ECOG evaluation and proportion were as follows: 0 grade comprised 84.5% (501/593), 1 grade comprised 14.5% (86/593), 2 grade comprised 0.8% (5/593), and 3 grade comprised 0.2% (1/593). Follow-up duration of pulmonary nodules: 63.6% (377/593) of ≤30 days, 11.5% (68/593) of 30–60 days, 25.0% (148/593) of >60 days. Procedures were performed without breathing training in 82.0% (486/593) of patients, and breathing training was performed in 18.0% (107/593) of patients before procedures. Patients with diabetes accounted for 10.8% (64/593), and 39.5% (234/593) of the patients had history of hypertension (140/90 mmHg). The overall incidence of post-biopsy pneumothorax was 13.0% (77/593), of which the incidence of immediate onset of pneumothorax was 79.2% (61/77), that of delayed pneumothorax was 20.8% (16/77), and 26.0% (20/77) of patients required chest tube placement. The incidence of subcutaneous pneumatosis was 1.0% (6/593). Some 45.7% (271/593) were coaxial, 54.3% (322/593) were non-coaxial, 1 pleural access was performed in 82.8% (491/593) of cases, 2 pleural accesses were performed in 14.5% (86/593) of cases, and 3 pleural accesses were performed in 2.7% of cases (16/593). Needle size, nodule size, nodule location, and subtypes of emphysema comparison between groups are shown in Tables 1,2.
Table 1. Univariate analysis of pneumothorax.
| Variable | Pneumothorax | P value | |
|---|---|---|---|
| No | Yes | ||
| Hospitalization | |||
| Outpatient | 163 (86.7) | 25 (13.3) | 0.877 |
| Inpatient | 353 (82.7) | 52 (12.8) | |
| MDT | |||
| No | 449 (87.9) | 62 (12.1) | 0.123 |
| Yes | 67 (81.7) | 15 (18.3) | |
| Age (years) | 58.53±11.45 | 64.11±8.88 | <0.0001 |
| Sex | |||
| Male | 255 (85.0) | 45 (15.0) | 0.140 |
| Female | 261 (89.1) | 32 (10.9) | |
| Smoke | |||
| No | 343 (88.2) | 46 (11.8) | 0.246 |
| Yes | 173 (84.8) | 31 (15.2) | |
| Diabetes | |||
| No | 466 (87.9) | 64 (12.1) | 0.056 |
| Yes | 50 (79.4) | 13 (20.6) | |
| Hypertension | |||
| No | 312 (86.9) | 47 (13.1) | 0.923 |
| Yes | 204 (87.2) | 30 (12.8) | |
| Emphysema | |||
| No | 457 (89.1) | 56 (10.9) | <0.0001 |
| Yes | 59 (73.8) | 21 (26.3) | |
| Lobe | |||
| Upper | 264 (84.9) | 47 (15.1) | 0.105 |
| Lower | 252 (89.4) | 30 (10.6) | |
| Location | |||
| Peripheral | 381 (85.6) | 64 (14.4) | 0.242 |
| Hilar | 46 (92.0) | 4 (8.0) | |
| Intermediate | 89 (90.8) | 9 (9.2) | |
| CTR | |||
| ≤50% | 125 (91.2) | 12 (8.8) | 0.093 |
| >50% | 391 (85.7) | 65 (14.3) | |
| Breathing training | |||
| No | 420 (86.4) | 66 (13.6) | 0.358 |
| Yes | 96 (89.7) | 11 (10.3) | |
| Operation time (min) | 16.41±8.63 | 20.04±13.01 | 0.001 |
| Length of puncture (cm) | 3.79±4.47 | 3.58±2.26 | 0.687 |
| Size of needles | |||
| 16 or 17 G | 371 (88.8) | 47 (11.2) | 0.051 |
| 18 or 19 G | 145 (82.9) | 30 (17.1) | |
| Manner of cutting | |||
| Semi-automatic | 285 (85.3) | 49 (14.7) | 0.165 |
| Automatic | 231 (89.2) | 28 (10.8) | |
| Coaxial technical | |||
| Non-coaxial | 272 (84.5) | 50 (15.5) | 0.045 |
| Coaxial | 244 (90.0) | 27 (10.0) | |
Data are presented as n (%) and mean ± standard deviation. MDT, multidisciplinary treatment; CTR, consolidation to tumor ratio.
Table 2. Univariate analysis of multiple categorical variables of pneumothorax.
| Variables | Pneumothorax | P value | |
|---|---|---|---|
| No | Yes | ||
| Needle size | |||
| 16 G | 151 (86.3) | 24 (13.7) | 0.129 |
| 17 G | 220 (90.5) | 23 (9.5) | |
| 18 G | 121 (82.3) | 26 (17.7) | |
| 19 G | 24 (85.7) | 4 (14.3) | |
| Nodule size (D) | |||
| D ≤1 cm | 78 (91.8) | 7 (8.2) | 0.139 |
| 1 cm < D ≤2 cm | 245 (88.1) | 33 (11.9) | |
| 2 cm < D ≤3 cm | 193 (83.9) | 37 (16.1) | |
| Pleural passes | |||
| 1 | 429 (87.4) | 62 (12.6) | 0.084 |
| 2 | 76 (88.4) | 10 (11.6) | |
| ≥3 | 11 (68.8) | 5 (31.3) | |
| Location | |||
| Peripheral | 381 (85.6) | 64 (14.4) | 0.215 |
| Intermediate | 89 (90.8) | 9 (9.2) | |
| Hilar | 46 (92.0) | 4 (8.0) | |
| Emphysema | |||
| No | 457 (89.1) | 56 (10.9) | 0.007 |
| Centrilobular | 29 (74.4) | 10 (25.6) | |
| Panlobular | 8 (66.7) | 4 (33.3) | |
| Para-septal | 19 (73.1) | 7 (26.9) | |
| Para-scar | 3 (100.0) | 0 | |
| Position | |||
| Supine | 204 (86.4) | 32 (13.6) | 0.572 |
| Prone | 211 (88.7) | 27 (11.3) | |
| Lateral (right or left) | 101 (84.9) | 18 (15.1) | |
Data are presented as n (%). D, diameter.
The overall rate of pneumothorax had no significant relationship with 16–19 G (P=0.129), but 17 G had a lower incidence of pneumothorax than 18 G. In 1 pleural pass, the incidence of pneumothorax was higher with 18 G than it was with 16 and 17 G. The incidence of pneumothorax after 2 pleural punctures with 16 and 18 G was similar. The incidence of pneumothorax with 16, 17, and 19 G was similar after three pleural passes (Table 3). The operation time of the nodule size ≤1, 1–2, and 2–3 cm was 20.48±12.88, 15.91±8.27, and 16.72±8.88 min, respectively, and the difference was statistically significant among the three groups (Welch =4.810, P=0.009). The operative time was longer in the size ≤1 cm group than in the size of 1–2 and 2–3 cm groups (P=0.008, 0.043), and the operative time was similar between the size of 1–2 and 2–3 cm groups (P=0.641), but the occurrence of pneumothorax in size of ≤1 cm group was similar with that in other groups.
Table 3. The relationship between different needle size, pleural passes and pneumothorax.
| Pleural passes | Pneumothorax | 16 G, n (%) | 17 G, n (%) | 18 G, n (%) | 19 G, n (%) | P value |
|---|---|---|---|---|---|---|
| 1 | No | 137 (86.7) | 217 (91.2) | 54 (75.0) | 21 (91.3) | 0.004 |
| Yes | 21 (13.3) | 21 (8.8) | 18 (25.0) | 2 (8.7) | ||
| 2 | No | 9 (81.8) | – | 67 (89.3) | – | 0.493 |
| Yes | 2 (18.2) | – | 8 (10.7) | – | ||
| 3 | No | 5 (83.3) | 3 (60.0) | – | 3 (60.0) | 0.604 |
| Yes | 1 (16.7) | 2 (40.0) | – | 2 (40.0) | ||
| Total | No | 151 (86.3) | 220 (90.5) | 121 (82.3) | 24 (85.7) | 0.129 |
| Yes | 24 (13.7) | 23 (9.5) | 26 (17.7) | 4 (14.3) |
The incidence of pulmonary hemorrhage (defined as bleeding >2 cm along the needle tract, including hemoptysis or bleeding seeping into other lobes) was found to be 27.2% (161/593) in this study. Additionally, the occurrence rate of pleural reaction was 0.7% (4/593), chest wall hematoma was 0.2% (1/593), and air embolism was 0.2% (1/593) among the study population.
Univariate analysis of pneumothorax
Univariate analysis (Tables 1,2) demonstrated that pneumothorax was more likely to occur in age (P<0.0001), emphysema (P<0.0001), operation time (P=0.001), and non-coaxial (P=0.045). Other patients-, lesion-, and technique-related variables were not significantly associated with the occurrence of pneumothorax. Based on procedures and previous literature, the occurrence of pneumothorax depends on a number of factors, including needle size (P=0.129), nodule size (P=0.139), nodule location (P=0.215), number of pleural passes (P=0.084), and length of puncture (P=0.687).
Multivariate analysis of pneumothorax
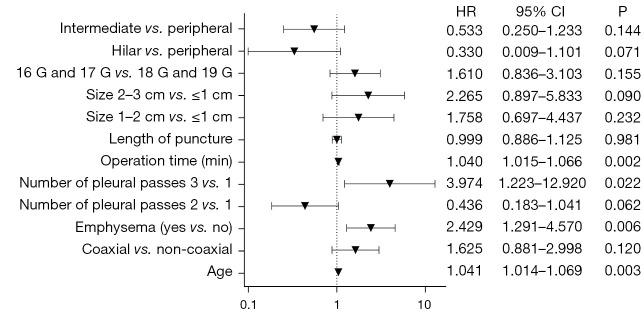
Multivariate analysis results are shown in Figure 2. The risk of development of pneumothorax in patients with emphysema was 2.429 times that of patients without emphysema [hazard ratio (HR) =2.429; 95% confidence interval (CI): 1.291–4.570]. Prolonged operation time (HR =1.040; 95% CI: 1.015–1.066) and age as a continuous variable were also revealed as pneumothorax risk factors (HR =1.041; 95% CI: 1.014–1.069). The risk of pneumothorax was significantly increased after 3 passes through pleura (HR =3.974; 95% CI: 1.223–12.920). Other variables were not significantly associated with pneumothorax. These factors had a certain clinical value in predicting pneumothorax, and the area under the curve (AUC) was 0.749 (95% CI: 0.692–0.806) (Figure 3).
Figure 2.
The results of multivariate analysis showed that age, emphysema, prolonged operation time and more than two pleural perforations were risk factors for pneumothorax. HR, hazard ratio; CI, confidence interval.
Figure 3.
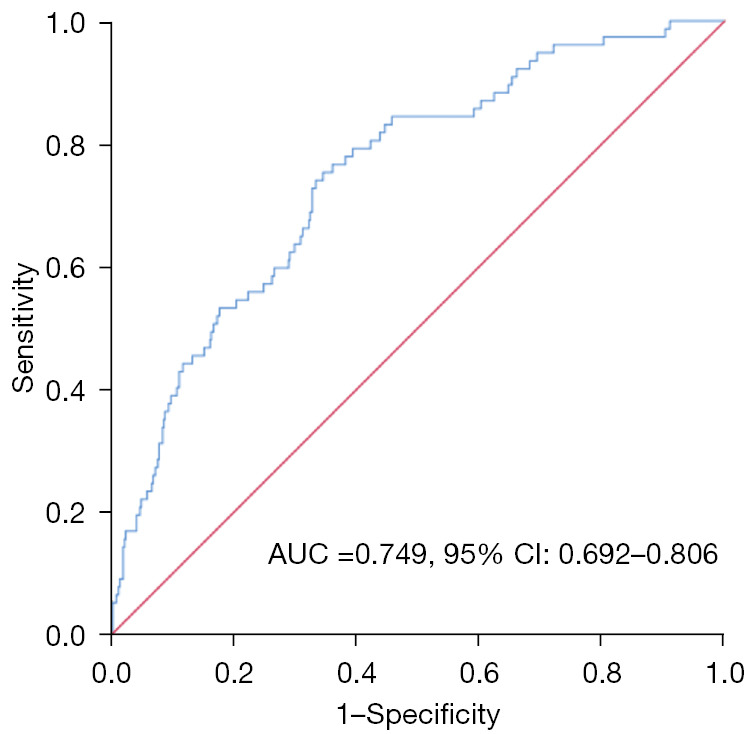
The probability of pneumothorax for each patient was calculated, and the ROC curve was drawn based on the likelihood. AUC =0.749 (95% CI: 0.692–0.806). AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic.
Discussion
This observation is the first prospective, multicenter, real-world study to evaluate variables associated with pneumothorax after PCLNB.
In this study, the diagnostic criteria of pneumothorax included continuous air leaking, difficulty in breathing, or lungs compressed by more than 30%. Among 77 of 593 patients (13.0%) who developed pneumothorax, 10.3% (61/593) had developed an immediate pneumothorax and 2.7% (16/593) had developed delayed pneumothorax; 3.4% (20/593) of them required a chest tube placement. These results are consistent with those reported by Khan et al. (6). Nevertheless, previous retrospective studies have shown a higher incidence of pneumothorax (17–40.4%) and intubation (3–17.4%) (4,6-8,21,22). Najafi et al. (23) proposed a method to reduce the rate of pneumothorax by positioning the patient biopsy side down, needle removal during expiration, autologous blood patch sealing, rapid rollover, and pleural patching, also known as “PEARL”. The occurrence of pneumothorax in the PEARL group was 16 of 100 (16%), less than that in the control group (37 of 100, 37%) (P=0.001), but there was no significant difference in the incidence of pneumothorax compared with our group 12.9% (χ2=0.056, P=0.841). This lack of difference may be attributed to the characteristics of the patients enrolled in the study. None of the patients underwent pleural patching, needle removal during expiration, rapid rollover, or positioning biopsy side down in our observation. No correlation was detected between pneumothorax and patient’s position (Table 2) (χ2=1.119, P=0.572). Treatment with autologous blood, physiological saline, gelatin, gelatin sponge, and thrombin for needle patch sealing failed to lower the incidence of pneumothorax in patients with emphysema [5.1% (30/593) (χ2=0.379, P=0.538)]. As a result, we recommend caution when using the PEARL approach to reduce the incidence of pneumothorax in patients with lung nodules biopsy.
The incidence of pneumothorax was 15.3% (25/163) among outpatients, with just 3 cases (1.8%) requiring chest tube placement, which was lower than that reported in a previous study (24). These findings serve as a guide for clinicians performing PCLNB in outpatients with pulmonary nodules. In addition to patients with pulmonary nodules who matched the inclusion criteria, these findings benefitted from the unified, standardized procedure of percutaneous transthoracic needle biopsy conducted by Chinese experts (17).
No significant difference in the occurrence of pneumothorax was observed in the needle size range of 16 to 19 G. The comparison between groups showed that the incidence of pneumothorax in needle size of 17 G was lower than that in 18 G group, and multivariate analysis showed that the 16–17 G needle was not a risk factor for pneumothorax compared with the 18–19 G. These results are different from the retrospective study, which argued that needles larger than 18 G are considered a risk factor of pneumothorax, and asserted that the use of a smaller needle produces a substantially decreased in the rate of pneumothorax (8,9,25). The number of passes through the pleura is considered an important factor that may have been overlooked in their analysis.
It is advantageous to obtain more tissue samples for subsequent diagnosis, such as electron microscopy, immunohistochemistry, gene sequencing, and analysis of tumor markers. Larger needle size, coaxial technique, and the number of non-coaxial passes through the pleura can increase the volume of samples. Our data showed no increase in the risk of pneumothorax with the non-coaxial approach, and the results were consistent with the study by Nour-Eldin et al. (13). In 1 pleural pass, the rate of pneumothorax with 18 G is higher than that with 16 and 17 G. The incidence of pneumothorax after two pleural punctures with 16 and 18 G was similar. The incidence of pneumothorax with 16, 17, and 19 G was similar after three pleural passes. The number of passes needed per procedure has not been defined, and there are also studies suggesting that the number of passes is significantly associated with the development of pneumothorax (7,26,27). The multivariate analysis of this study showed that one or two pleural access did not increase the risk of pneumothorax, but three passes significantly increased the risk. Theoretically, an increase in the number of pleural passes should reasonably lead to a higher risk of pneumothorax. However, a larger sample size is necessary to validate whether these statistically significant findings have clinical significance. Regardless of the needle size used, the most important puncture strategy is to limit the number of pleural passes.
This study confirms that emphysema is a major risk factor for pneumothorax. The incidence of emphysema was higher in different types of emphysema than in non-emphysema in the operable lobe, and the results were supported by previous studies (7,13,21,28), which did not analyze subtypes of emphysema. Many previous studies did not include emphysema or did not report it as a risk factor (6,10-12,14,25,29). Chest CT scan can describe emphysema subtypes, and emphysema subtypes were substantially linked with post-biopsy pneumothorax. Although our study found that the incidence of pneumothorax was similar in different subtypes of emphysema, we also need to pay attention to the preoperative differential diagnosis of emphysema, which is conducive to the prediction of post-biopsy pneumothorax. In addition, the incidence of emphysema increases with age. Our research suggests that age as a continuous variable is a significant risk factor for pneumothorax, inconsistent with previous studies (14,25,26).
Another important factor is the operation time, which is an important indicator of the surgical team’s overall cooperation ability and the surgeon’s skill level. Similar to our findings, Khan et al. (6) considered that prolonged puncture time was associated with higher rates of pneumothorax, and the lesion diameters of most of their patients were larger than 3 cm. It is generally believed that the smaller the pulmonary nodule, the more frequently the needle is adjusted during the procedure, therefore prolonging the operation time and leading to a greater chance of pneumothorax. This study has also confirmed the validity of this theory, showing that every additional minute was associated with a 1.04-fold increase in the risk of pneumothorax. With the increased duration of the retention of the puncture needle in the pleura, the crevasse expands more in size because of breathing movement, resulting in a postoperative pneumothorax. There was no significant difference in operation time among different regions of pulmonary nodules (χ2=3.122, P=0.210), but the size of the lesions provides an opposite result. Therefore, the nodules’ size deserves the operator’s attention, and the ideal needle trajectory should be carefully determined from the CT image to reduce the operation time. Several other factors can lead to prolonged procedure time. We recommend that operators should follow the standardized guidelines similar to those unanimous recommendations by Chinese experts in this study.
In this observational study, the mean follow-up time for pulmonary nodules was 111.48±258.02 days, and the patients with pulmonary nodules had adequate follow-up before undergoing PCLNB. The patient’s ECOG scores were 0.17±0.4, and none of them had previously received systemic therapy. These factors did not affect the incidence of pneumothorax. However, adequate follow-up time and better ECOG could improve the PCLNB operator confidence, especially in patients with predominantly ground glass nodules. Our study has identified several common and important risk factors for PCLNB to help predict the development of complications and promote the clinical application to PCLNB. Other factors reported in the literature were excluded, including length of puncture, nodule size, needle size from 16 to 19 G, and nodule location.
Certainly, this study had limitations. Firstly, it was a prospective observational study collecting data from a multicenter; this was not a randomized controlled trial. Secondly, the incidence of immediate and delayed pneumothorax was low, and the few positive dependent variables could influence the results of the multivariate analysis. Thirdly, there was a different physician in each department during the study period, which can lead to a large variation in operation time. Finally, our study design did not analyze the different CT guidance devices; these differences may have influenced the analysis results.
Conclusions
PCLNB remains a minimally invasive procedure with a low complication rate. Age, emphysema subtypes, operation time, and the number of passes through the pleura were found to be significant predictors of post-biopsy pneumothorax in this study, and the 17 G needle is more suitable for percutaneous biopsy of pulmonary nodules in the real world. Furthermore, a unified technical approach is more favorable to the management of complications and a standardized PCLNB procedure is necessary.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Army Medical University (IRB No. KY2021084). All participating hospitals were informed and agreed to the study. Informed consent was provided by all individual participants.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://qims.amegroups.com/article/view/10.21037/qims-23-891/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-23-891/coif). The authors have no conflicts of interest to declare.
References
- 1.Mazzone PJ, Lam L. Evaluating the Patient With a Pulmonary Nodule: A Review. JAMA 2022;327:264-73. 10.1001/jama.2021.24287 [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Zhang Q, Bai L, Li TY, He C, Ma QL, Li LS, Huang XQ, Qian GS. Assessment of the cancer risk factors of solitary pulmonary nodules. Oncotarget 2017;8:29318-27. 10.18632/oncotarget.16426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He C, Yu H, Li C, Zhang X, Huang Z, Liu M, Tong L, Zhu J, Wu W, Huang X. Recurrence and disease-free survival outcomes after computed tomography-guided needle biopsy in stage IA non-small cell lung cancer patients in China: a propensity score matching analysis. Quant Imaging Med Surg 2021;11:3472-80. 10.21037/qims-20-931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon SH, Park CM, Lee KH, Lim KY, Suh YJ, Im DJ, Hur J, Han DH, Kang MJ, Choo JY, Kim C, Kim JI, Hong H. Analysis of Complications of Percutaneous Transthoracic Needle Biopsy Using CT-Guidance Modalities In a Multicenter Cohort of 10568 Biopsies. Korean J Radiol 2019;20:323-31. 10.3348/kjr.2018.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren Q, Zhou Y, Yan M, Zheng C, Zhou G, Xia X. Imaging-guided percutaneous transthoracic needle biopsy of nodules in the lung base: fluoroscopy CT versus cone-beam CT. Clin Radiol 2022;77:e394-9. 10.1016/j.crad.2022.02.005 [DOI] [PubMed] [Google Scholar]
- 6.Khan MF, Straub R, Moghaddam SR, Maataoui A, Gurung J, Wagner TO, Ackermann H, Thalhammer A, Vogl TJ, Jacobi V. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol 2008;18:1356-63. 10.1007/s00330-008-0893-1 [DOI] [PubMed] [Google Scholar]
- 7.Cox JE, Chiles C, McManus CM, Aquino SL, Choplin RH. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology 1999;212:165-8. 10.1148/radiology.212.1.r99jl33165 [DOI] [PubMed] [Google Scholar]
- 8.Kuban JD, Tam AL, Huang SY, Ensor JE, Philip AS, Chen GJ, Ahrar J, Murthy R, Avritscher R, Madoff DC, Mahvash A, Ahrar K, Wallace MJ, Nachiappan AC, Gupta S. The Effect of Needle Gauge on the Risk of Pneumothorax and Chest Tube Placement After Percutaneous Computed Tomographic (CT)-Guided Lung Biopsy. Cardiovasc Intervent Radiol 2015;38:1595-602. 10.1007/s00270-015-1097-0 [DOI] [PubMed] [Google Scholar]
- 9.Huang MD, Weng HH, Hsu SL, Hsu LS, Lin WM, Chen CW, Tsai YH. Accuracy and complications of CT-guided pulmonary core biopsy in small nodules: a single-center experience. Cancer Imaging 2019;19:51. 10.1186/s40644-019-0240-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laurent F, Michel P, Latrabe V, Tunon de Lara M, Marthan R. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: incidence and risk factors. AJR Am J Roentgenol 1999;172:1049-53. 10.2214/ajr.172.4.10587145 [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Du Y, Yang HF, Yu JH, Xu XX. CT-guided percutaneous core needle biopsy for small (≤20 mm) pulmonary lesions. Clin Radiol 2013;68:e43-8. 10.1016/j.crad.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 12.Niu XK, Bhetuwal A, Yang HF. CT-guided core needle biopsy of pleural lesions: evaluating diagnostic yield and associated complications. Korean J Radiol 2015;16:206-12. 10.3348/kjr.2015.16.1.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nour-Eldin NE, Alsubhi M, Emam A, Lehnert T, Beeres M, Jacobi V, Gruber-Rouh T, Scholtz JE, Vogl TJ, Naguib NN. Pneumothorax Complicating Coaxial and Non-coaxial CT-Guided Lung Biopsy: Comparative Analysis of Determining Risk Factors and Management of Pneumothorax in a Retrospective Review of 650 Patients. Cardiovasc Intervent Radiol 2016;39:261-70. 10.1007/s00270-015-1167-3 [DOI] [PubMed] [Google Scholar]
- 14.Nour-Eldin NE, Alsubhi M, Naguib NN, Lehnert T, Emam A, Beeres M, Bodelle B, Koitka K, Vogl TJ, Jacobi V. Risk factor analysis of pulmonary hemorrhage complicating CT-guided lung biopsy in coaxial and non-coaxial core biopsy techniques in 650 patients. Eur J Radiol 2014;83:1945-52. 10.1016/j.ejrad.2014.06.023 [DOI] [PubMed] [Google Scholar]
- 15.Litmanovich D, Boiselle PM, Bankier AA. CT of pulmonary emphysema--current status, challenges, and future directions. Eur Radiol 2009;19:537-51. 10.1007/s00330-008-1186-4 [DOI] [PubMed] [Google Scholar]
- 16.Manhire A, Charig M, Clelland C, Gleeson F, Miller R, Moss H, Pointon K, Richardson C, Sawicka E; . Guidelines for radiologically guided lung biopsy. Thorax 2003;58:920-36. 10.1136/thorax.58.11.920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Z, Shi H, Li W, Lin D, Wang C, Liu C, et al. Chinese multidisciplinary expert consensus: Guidelines on percutaneous transthoracic needle biopsy. Thorac Cancer 2018;9:1530-43. 10.1111/1759-7714.12849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barta JA, Henschke CI, Flores RM, Yip R, Yankelevitz DF, Powell CA. Lung Cancer Diagnosis by Fine Needle Aspiration Is Associated With Reduction in Resection of Nonmalignant Lung Nodules. Ann Thorac Surg 2017;103:1795-801. 10.1016/j.athoracsur.2016.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohno Y, Hatabu H, Takenaka D, Higashino T, Watanabe H, Ohbayashi C, Sugimura K. CT-guided transthoracic needle aspiration biopsy of small (< or = 20 mm) solitary pulmonary nodules. AJR Am J Roentgenol 2003;180:1665-9. 10.2214/ajr.180.6.1801665 [DOI] [PubMed] [Google Scholar]
- 20.Matsunaga T, Suzuki K, Takamochi K, Oh S. What is the radiological definition of part-solid tumour in lung cancer?†. Eur J Cardiothorac Surg 2017;51:242-7. 10.1093/ejcts/ezw344 [DOI] [PubMed] [Google Scholar]
- 21.Lim WH, Park CM, Yoon SH, Lim HJ, Hwang EJ, Lee JH, Goo JM. Time-dependent analysis of incidence, risk factors and clinical significance of pneumothorax after percutaneous lung biopsy. Eur Radiol 2018;28:1328-37. 10.1007/s00330-017-5058-7 [DOI] [PubMed] [Google Scholar]
- 22.Westcott JL, Rao N, Colley DP. Transthoracic needle biopsy of small pulmonary nodules. Radiology 1997;202:97-103. 10.1148/radiology.202.1.8988197 [DOI] [PubMed] [Google Scholar]
- 23.Najafi A, Al Ahmar M, Bonnet B, Delpla A, Kobe A, Madani K, Roux C, Deschamps F, de Baère T, Tselikas L. The PEARL Approach for CT-guided Lung Biopsy: Assessment of Complication Rate. Radiology 2022;302:473-80. 10.1148/radiol.2021210360 [DOI] [PubMed] [Google Scholar]
- 24.Poe RH, Kallay MC. Transthoracic needle biopsy of lung in nonhospitalized patients. Chest 1987;92:676-8. 10.1378/chest.92.4.676 [DOI] [PubMed] [Google Scholar]
- 25.Geraghty PR, Kee ST, McFarlane G, Razavi MK, Sze DY, Dake MD. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology 2003;229:475-81. 10.1148/radiol.2291020499 [DOI] [PubMed] [Google Scholar]
- 26.Boskovic T, Stanic J, Pena-Karan S, Zarogoulidis P, Drevelegas K, Katsikogiannis N, Machairiotis N, Mpakas A, Tsakiridis K, Kesisis G, Tsiouda T, Kougioumtzi I, Arikas S, Zarogoulidis K. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-107. 10.3978/j.issn.2072-1439.2013.12.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halloush RA, Khasawneh FA, Saleh HA, Soubani AO, Piskorowski TJ, Al-Abbadi MA. Fine needle aspiration cytology of lung lesions: a clinicopathological and cytopathological review of 150 cases with emphasis on the relation between the number of passes and the incidence of pneumothorax. Cytopathology 2007;18:44-51. 10.1111/j.1365-2303.2007.00410.x [DOI] [PubMed] [Google Scholar]
- 28.O'Neill AC, McCarthy C, Ridge CA, Mitchell P, Hanrahan E, Butler M, Keane MP, Dodd JD. Rapid needle-out patient-rollover time after percutaneous CT-guided transthoracic biopsy of lung nodules: effect on pneumothorax rate. Radiology 2012;262:314-9. 10.1148/radiol.11103506 [DOI] [PubMed] [Google Scholar]
- 29.Drumm O, Joyce EA, de Blacam C, Gleeson T, Kavanagh J, McCarthy E, McDermott R, Beddy P. CT-guided Lung Biopsy: Effect of Biopsy-side Down Position on Pneumothorax and Chest Tube Placement. Radiology 2019;292:190-6. 10.1148/radiol.2019182321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as