Abstract
Background: Sickle cell disease (SCD) is the most common inherited blood disorder, affecting primarily Black and Hispanic individuals. In 2016, 30-day readmissions incurred 95,445 extra days of hospitalization, $152 million in total hospitalization costs, and $609 million in total hospitalization charges. Objectives: 1) To estimate hospital readmissions within 30 days among patients with SCD in the State of California. 2) Identify the factors associated with readmission within 30 days for SCD patients in California. Methods: We conducted a retrospective observational study of adult SCD patients hospitalized in California between 2005 and 2014. Descriptive statistics and logistic regression models were used to examine significant differences in patient characteristics and their association with hospital readmissions. Results: From 2,728 individual index admissions, 70% presented with single admission, 10% experienced one readmission, and 20% experienced ≥ two readmissions within 30 days. Significant predictors associated with zero vs. one readmission were male gender (OR=1.37, CI: 1.06-1.77), Black ethnicity (OR=3.27, CI: 1.71-6.27) and having Medicare coverage (OR=1.89, CI: 1.30-2.75). Lower likelihood of readmission was found in those with a Charlson Comorbidity index of three or more (OR=0.53, CI: 0.29-0.97). For zero vs. ≥ two readmissions, significant predictors were male gender (OR=1.43, CI: 1.17-1.74), Black ethnicity (OR=6.90, CI: 3.41-13.97), Hispanic ethnicity (OR=2.33, CI: 1.05-5.17), Medicare coverage (OR=3.58, CI: 2.68-4.81) and Medi-Cal coverage (OR=1.70, CI: 1.31-2.20). Lower likelihood for having two or more readmissions were associated with individuals aged 65+ (OR=0.97, CI: 0.96-0.98) and those with self-payment status (OR=0.32, CI: 0.12-0.54). Conclusions: In California, male, Black, and Hispanic patients, as well as those covered by Medicare or Medi-Cal, were found to have an increased risk of hospital readmissions. Redirecting outpatient goals to address these patient populations and risk factors is crucial for reducing readmission rates.
Keywords: Sickle cell disease, health disparity, hospital readmissions, Medi-Cal/Medicare
Introduction
Sickle cell disease (SCD) is the most common inherited blood disorder, affecting approximately 1 in 2500 births and 100,000 individuals in the United States [1]. It is characterized by the presence of abnormal hemoglobin, known as hemoglobin S (HbS), which causes red blood cells to change their shape from a flexible disc to a rigid sickle-like shape. These sickle-shaped cells can become trapped in blood vessels, leading to vaso-occlusion, tissue damage, and pain crises [2,3]. Detection and diagnosis of SCD typically involves a comobination of biochemical and molecular tests [4]. The gold standard and most popular methods, however, included the complete count of blood cells (CBC), hemoglobin (Hb) electrophoresis or high-performance liquid chromatography (HPLC), which can distinguish between normal hemoglobin (HbA), hemoglobin S (HbS), and other abnormal hemoglobin variants [5]. Additionally, genetic testing through DNA analysis is important for precise detection, and can confirm specific genetic mutations responsible for SCD, such as the HbS gene [6].
Current treatment options for SCD aim to alleviate symptoms, manage complications, and improve the patient’s quality of life. The evidence-based recommendation by American Society of Hematology (ASH) for blood transfusion is considering it on an individualized case-by-case bases, considering risk of surgery, complications with prior transfusions, and disease severity to optimize outcomes [7]. For patients with recurrent episodes of ACS, frequent pain, or other complications, ASH suggests, with low certainty, that physicians should consider Hematopoietic stem cell transplantation (HSCT) at an early age, rather than standard of care. Their cautionary recommendation is due to lack of randomized controlled clinical trials for HSCT [8].
In addition to blood transfusion, other management options of SCD involve a combination of pharmacological and non-pharmacological interventions. Key treatment approaches include pain management involving use of analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), and opioids; hydroxyurea to reduce the frequency and severity of pain crises; prophylactic antibiotics to prevent infections in SCD patients, and immunization to protect SCD patients from infection [9-11]. However, the efficacy of these treatments remains variable. More specifically, hydroxyurea has shown significant efficacy in managing SCD [12,13].
Despite advancements in treatment some patients experience better outcomes such as relatively mild disease course, while others experience more frequent complications and hospitalizations, and readmission. In 2004, SCD accounted for 113,000 hospitalizations resulting in $488 million in annual hospitalization costs [14,15]. Hospital readmissions within 30 days have been used as a metric of quality care by the Centers for Medicare & Medicaid Services (CMS) and are considered critical in reducing healthcare-associated costs for individuals with chronic conditions [16-19]. Since October 1, 2012, CMS began penalizing hospitals with excess readmissions [17,19]. Notably, in 2016, 30-day readmissions resulted in 95,445 additional days of hospitalization, costing $152 million in total hospitalization costs, and $609 million in total hospitalization charges [19].
Findings from empirical studies reveal that readmission is the highest in SCD patients who live in socio-economically deprived areas, where access to care is limited to the population most in need [20-24]. Among other factors contributing to increased SCD readmissions, particularly among patients from lower socio-economic status is the number of comorbidities. Specific medical conditions such as asthma, pneumonia, congestive heart failure (CHF) are at higher risk of readmission [19,25].
There is also empirical evidence suggesting disparities in the prevalence, type, severity, and complications, and cost of care related to SCD based on race/ethnicity [26-28]. For instance, among Black newborns in the United States 1 in 365 is affected by SCD compared to approximately 1 in 16,300 in Hispanic newborns [26,29]. Recent research conducted using the National Inpatient Sample (NIS) database, further, underscores the racial discrepancies in SCD-related hospitalizations [26]. The authors found that hospitalization were overwhelmingly represented by Black patients at 93.4% followed by Hispanic patients at 4.8%, and White patients at 1.8% [26]. Moreover, these disparities extended to the clinical manifestations of the disease, with Black patients being more likely to experience sickle cell crises, blindness, and require blood transfusions. In contrast, Hispanic patients had increased odds of mortality compared to Black patients [26].
While it is recognized that SCD patients incur higher care costs due to the acute and chronic complications requiring treatment, there is a limited understanding regarding the readmission rates across different racial/ethnic groups, as well as the association between race/ethnicity and readmission, specifically within the context of a specific State’s healthcare system. In this study, we aim to address these knowledge gaps by aiming to: 1) Estimate hospital readmissions within 30 days among patients with SCD in the State of California. 2) Identify the factors associated with readmission within 30 days for SCD patients in California. By quantifying readmission rates and gaining insight into the various contributing factors, we can better understand the burden of care, overall cost, and the need for interventions to reduce readmissions. Additionally, our findings have the potential to inform state-wide policies, thereby illuminating pathway for improving care management, and patient outcomes [30].
Materials and methods
Study design
We conducted a retrospective observational study using data from the California Department of Health Care Access and Information (HCAI). HCAI includes licensed hospitals comprising general acute care, acute psychiatric, chemical dependency recovery, and psychiatric health facilities. The study data covered the period between 2005 and 2014. We created an analytic dataset by including individuals over 18 years old with a primary or secondary diagnosis of SCD as indicated by the ICD-9-CM code 282.60. Patients who who experienced in-hospital mortality were excluded from the analysis.
We identified potential predictors of 30-day hospital readmissions based on prior research studies [14,31]. These predictors include as age, gender, race/ethnicity, insurance status, and Charlson Comorbidity Index (CCI). The CCI was developed to quantify and account for the cumulative impact of multiple comorbid conditions on a patient’s health status [32]. Each condition is assigned a score based on association with mortality. The higher the CCI score, the higher the predicted risk of mortality or adverse outcomes [32]. The outcome of interest was any instance of readmission within a 30-day. In our study, the initial hospitalization for an individual within our dataset was considered the “index admission”. Subsequent admissions within 30 days of the index admission were classified as readmissions.
To enhance the contextual understanding of the community, we derived additional variables from census data. These variables included the percentage of the population within ZIP codes having a college education, the population below the federal poverty level (FPL), and the percentage with vehicle ownership. This study did not require institutional review board approval or patient consent because no patient data was collected. We used publicly available State data.
Data analysis plan
We assessed predictors of individual readmissions and the number of admissions using descriptive analysis. We dichotomized the outcome into two groups: one readmission versus non-readmission and two or more readmissions versus non-readmission. We modeled the outcomes using logistic regressions, including all potential predictors of 30-day hospital readmissions. The unit of analysis was at the patient visit level.
We reported odds ratios and 95% confidence intervals (CI) from the models. Data analyses were performed with SAS 9.4 (SAS Institute, Cary, NC). The statistical significance was set at p-value < 0.05.
Results
We identified 31,627,239 patient-level admissions from the HCAI database between 2005 and 2014. Among these, 2,934 admissions pertained to individuals over 18 with a primary or secondary diagnosis of SCD. We excluded 206 patients who deceased during hospitalizations, resulting in 2,728 individuals who were admitted and subsequently discharged while still alive.
Sample characteristics
Out of the 2,728 patients who met the inclusion criteria, 60% (n=1640) were female. Age distribution revealed that 64% of the admission cases (n=1,748) were in the 18-44 age category, 26% (n=720) fell within the 45-64 age category, and 10% (n=260) were 65 and older. The majority of patients were Black (n=2182), followed by Hispanic (n=260), White (n=199), and Asian/other (n=87). In terms of insurance status, 59% (n=1696) of patients had either Medicare or Medi-Cal, while 27% (n=732) had private insurance and 14% (n=380) were uninsured or had another source of payment. Regarding patients’ comorbidities, the majority (55%) of patients had a CCI score of 0 (n=1943), with 27% (n=725) having a score of 1, and 18% (n=510) scoring two or higher (Table 1).
Table 1.
Sample characteristic
| Patient Characteristics | n=2,728 (%) | |
|---|---|---|
| Age | 18-44 | 1748 (64.0) |
| 45-64 | 720 (26.0) | |
| 65+ | 260 (10.0) | |
| Gender | Male | 1088 (40.0) |
| Female | 1640 (60.0) | |
| Race/Ethnicity | White | 199 (7.0) |
| Black | 2182 (80.0) | |
| Hispanic | 260 (10.0) | |
| Asian/other | 87 (3.0) | |
| Insurance Status | Medicare | 652 (24.0) |
| Medi-Cal | 964 (35.0) | |
| Private | 732 (27.0) | |
| Uninsured/Self Pay | 259 (10.0) | |
| Other | 121 (4.0) | |
| CCI | 0 | 1943 (55.0) |
| 1 | 725 (27.0) | |
| 2 | 315 (11.0) | |
| 3+ | 195 (7.0) | |
Abbreviation: CCI: Charlson Comorbidity Index.
Readmission patterns
Within our study sample, 1,430 patients were admitted only once during the study period, 483 experienced readmissions beyond 30 days, 275 were readmitted once within 30 days, and 540 encountered two or more readmissions within 30 days following their index admission (Figure 1). Among patients with multiple readmissions within 30 days, the breakdown was: 120 patients had two readmissions, 78 had three, 58 had four, and 284 had five or more (Figure 2).
Figure 1.
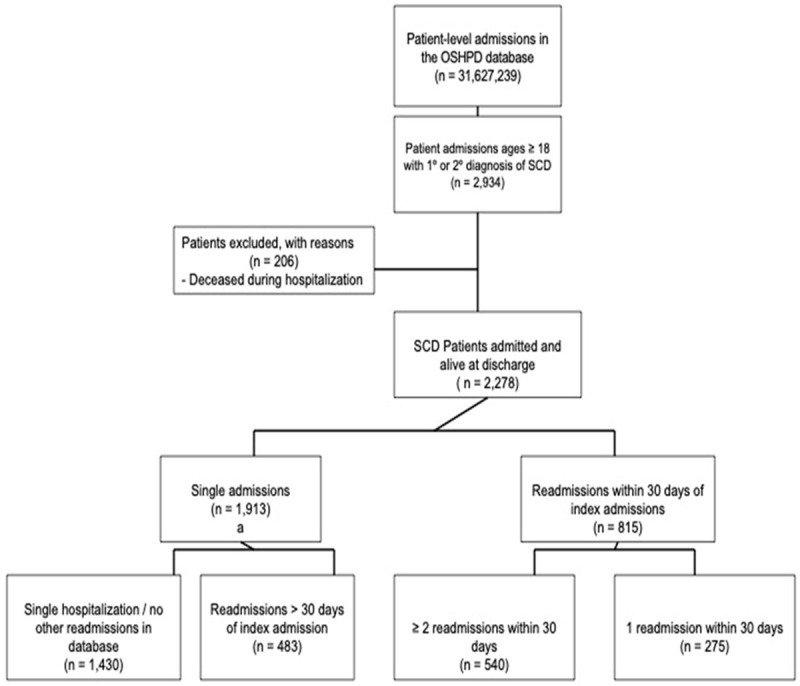
SCD study sample selection flowchart.
Figure 2.
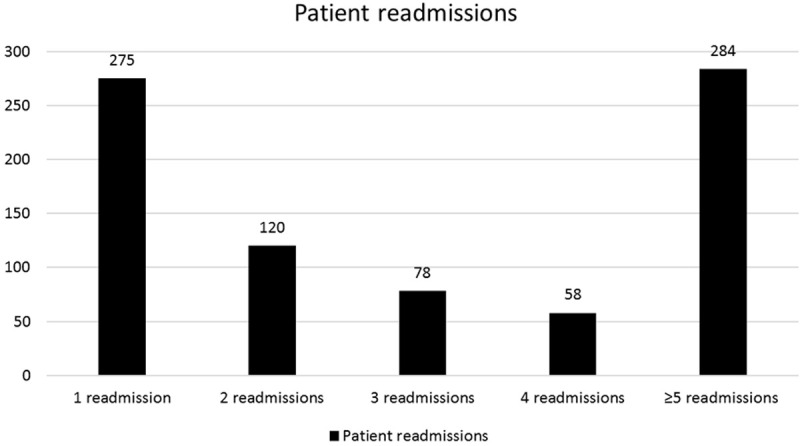
Patters of SCD patient readmissions.
Community characteristics based on the census data
Using census data, we derived three community characteristics: the percentage of those below the FPL, the percentage with a college degree within a neighborhood, and the percentage of those without care ownership (Table 2). Hence, we found that 25% of the patients (n=665) lived in neighborhoods where under 13% had incomes below the FPL, while 26% (n=708) lived in areas where over 24% were below the FPL. Furthermore, 25% of patients (n=671) lived in neighborhoods where under 11% held a college degree, and 27% (n=738) lived in neighborhoods where more than 15% did not own a car (Table 2).
Table 2.
Census variables in quartiles
| Census variables | n=2,728 (%) | |
|---|---|---|
| < 100% below FPL | < 13% | 665 (25.0) |
| 13-19% | 690 (25.0) | |
| 19-24% | 663 (24.0) | |
| ≥ 24% | 708 (26.0) | |
| Percent college degree | < 11% | 671 (25.0) |
| 11-23% | 692 (25.0) | |
| 23-34% | 664 (24.0) | |
| ≥ 34% | 699 (26.0) | |
| Percent without a car | < 6% | 644 (24.0) |
| 6-9% | 690 (25.0) | |
| 10-15% | 654 (24.0) | |
| > 15% | 738 (27.0) | |
Abbreviation: FPL: Federal Poverty Level.
Predictors of readmission
To identify predictors of readmissions within 30 days, we used two logistic regression models (Table 3). Model 1 compared the odds of zero vs. one readmission, and model 2 compared the odds of zero vs. two or more readmissions. Our analysis in model 1 revealed that males had higher odds for readmission (OR=1.37, CI: 1.06-1.77) compared to their female counterparts. Being Black (OR=3.27, CI: 1.71-6.27) and having Medicare (OR=1.89, CI: 1.30-2.75) were associated with higher odds of experiencing single readmission within 30 days compared to patients of other races and those covered by private insurance, respectively. While age and having Medi-Cal coverage and self-pay were not significant predictors, having CCI score of three or higher (OR=0.53, CI: 0.29-0.97) was associated with a lower likelihood of readmission.
Table 3.
Adjusted logistics regression model
| Characteristics | Zero vs. 1 readmission (model 1) | Zero vs. ≥ 2 readmissions (model 2) | ||
|---|---|---|---|---|
|
|
|
|||
| Odds ratio | Confidence interval (95%) | Odds ratio | Confidence interval (95%) | |
| Male | 1.37* | 1.06-1.77 | 1.43* | 1.17-1.74 |
| Age | 0.99 | 0.99-1.00 | 0.97* | 0.96-0.98 |
| Black | 3.27* | 1.71-6.27 | 6.90* | 3.41-13.97 |
| Hispanic | 1.87 | 0.86-4.05 | 2.33* | 1.05-5.17 |
| Medicare | 1.89* | 1.30-2.75 | 3.58* | 2.68-4.81 |
| Medi-Cal | 1.36 | 0.98-1.88 | 1.70* | 1.31-2.20 |
| Self-pay | 0.80 | 0.49-1.32 | 0.32* | 0.12-0.54 |
| CCI 3+ | 0.53* | 0.29-0.97 | 0.96 | 0.61-1.50 |
Statistically significant finding (P < 0.05).
Abbreviation: CCI: Charlson Comorbidity Index.
In model 2, our analysis revealed that males (OR=1.43, CI: 1.17-1.74), Black patients (OR=6.90, CI: 3.41-13.97), and Hispanic patients (OR=2.33, CI: 1.05-5.17) and those covered by Medicare (OR=3.58, CI: 2.68-4.81) or Medi-Cal (OR=1.70, CI: 1.31-2.20) had higher odds of two or more readmissions. In contrast, patients aged 65 and above (OR=0.97, CI: 0.96-0.98) and those with self-payment status (OR=0.32, CI: 0.12-0.54) had lower odds of experiencing two or more readmissions. CCI score was not a significant predictor in this model.
Discussion
Our results revealed that approximately 30% of our sample experienced hospital readmissions within the 30-day timeframe, with 20% experiencing two or more readmissions and an alarming 10% experiencing five or more readmissions during the studied period. Males, those of Black ethnicity, and, patients with Medicare insurance were more likely to have at least one readmission within 30 days of their index admission. Black patients had twice the readmission rates compared to Whites and nearly seven times higher odds of experiencing at least two readmissions; these disparities persisted despite controlling for clinical, demographic, and selected community contextual factors. Our findings support previous studies on readmissions among patients with SCD. However, there were some variations in the study design and scope of the prior studies, such as limited sample size or lack of findings at the state level [14,17,33].
As a social construct, there is a compelling need to further understand the drivers behind these disparities, this requires recognizing the complex interplay of community and individual factors, which our study was unable to capture using NIS database [34,35].
Our findings also highlight patterns among primary payers, where patients on Medicare had elevated odds (1.3 higher), of presenting one readmission but the odds doubled for those with two or more readmissions. It is important to note that Medicare includes not only individuals aged 65 and older but also those with disabilities and end-stage renal disease. This pattern of increased readmissions in patients with Medicare aligns with the existing literature and is not unique to SCD patients [36]. We found that characteristics such as higher comorbidity score, increasing age, and self-payment status were associated with reduced odds of readmissions. These seemingly protective associations could be explained by the possibility of mortality occurring outside the hospital setting or increased follow-up for patients with higher comorbidities [37-39].
Given California’s diverse landscape, engaging local African-American and Hispanic-serving community organizations can enhance efforts to increase awareness about early signs of SCD complications and empower patients and caregivers [40]. This approach acknowledges the unique cultural contexts of these communities and recognizes the importance of tailoring culturally competent, linguistically and literacy-appropriate SCD-related health information and education. Also, establishing a dedicated care coordination team is recommended for patients covered by Med-Cal or Medicare to facilitate hospital-to-outpatient continuity of care [41]. This type of specialized care coordination addresses the complexities that can arise from navigating different healthcare settings and providing patient-centered care to managing SCD. Moreover, SCD patients under Med-Cal or Medicare plans can benefit from medication access programs ensuring uninterrupted access to essential medications [42]. Indeed, uninterrupted access to medication is one of the critical components of effective chronic disease management [43]. In addition, local advocacy groups can guide SCD patients in using state-administered health insurance systems to receive home health services, including skilled nursing care, reducing the risk of readmission. These types of advocacy could empower SCD patients by equipping them with the knowledge to take advantage of all available resources and services. Moreover, comprehensive post-discharge planning with early follow-up care and culturally sensitive collaborative care services for marginalized patients can reduce barriers to care and ensure proper monitoring after discharge, holistic support, and improved health outcomes, as supported by empirical evidence [42,44-46].
Limitations
Our study is subject to several limitations. The Charlson comorbidity index, while useful in predicting 10-year survival rate in cases with multiple comorbidities, may not entirely capture the complexities of illness severity. In addition, it is worth noting that the study excluded pediatric patients, which limits the generalizability of our finding. Previous studies indicate that this population also exhibits high rates of readmissions [33,47,48]. Furthermore, our understanding of patients’ socioeconomic status (SES) was limited by the absence of individual-level data, despite our attempt to estimate SES using census data. Nevertheless, our study offers valuable insights for addressing health disparities and optimizing Med-Cal, since in California substantial SCD hospitalized patient population is covered by Medi-Cal, designed to assist the underprivileged.
Conclusions
Despite advancements in the management of SCD, our findings reveal that a substantial proportion of patients continue to experience readmissions within 30 days of their initial hospitalization. In California, this experience is not limited only to Black patients; it is also prevalent among Hispanic patients, indicating an independently increased risk of hospital readmissions for this population. Also, gender and insurance status, particularly male and Medicare or Medi-Cal coverage, are associated with increased readmission rates. These findings should lead to further efforts to realign outpatient goals and management strategies to address the specific needs of these patient populations and mitigate risk factors. Combining community engagement, specialized care coordination, medication access programs, and culturally sensitive post-discharge planning collectively hold the potential to contribute to reduced readmission rates in this specific patient population.
Disclosure of conflict of interest
None.
Abbreviations
- SCD
Sickle Cell Disease
- CMS
Centers for Medicare & Medicaid Services
- CCI
Charlson Comorbidity Index
- FPL
Federal Poverty Level
- SES
Socioeconomic Status
References
- 1.Strouse J. Chapter 18 - sickle cell disease. In: Aminoff MJ, Boller F, Swaab DF, editors. Handbook of Clinical Neurology. Elsevier; 2016. pp. 311–324. [Google Scholar]
- 2.Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L, Smith WR, Panepinto JA, Weatherall DJ, Costa FF, Vichinsky EP. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. doi: 10.1038/nrdp.2018.10. [DOI] [PubMed] [Google Scholar]
- 3.Arishi WA, Alhadrami HA, Zourob M. Techniques for the detection of sickle cell disease: a review. Micromachines (Basel) 2021;12:519. doi: 10.3390/mi12050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wajcman H, Moradkhani K. Abnormal haemoglobins: detection & characterization. Indian J Med Res. 2011;134:538–546. [PMC free article] [PubMed] [Google Scholar]
- 5.da Fonseca SF, Amorim T, Purificação A, Gonçalves M, Boa-Sorte N. Hemoglobin A2 values in sickle cell disease patients quantified by high performance liquid chromatography and the influence of alpha thalassemia. Rev Bras Hematol Hemoter. 2015;37:296–301. doi: 10.1016/j.bjhh.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark BE, Thein SL. Molecular diagnosis of haemoglobin disorders. Clin Lab Haematol. 2004;26:159–176. doi: 10.1111/j.1365-2257.2004.00607.x. [DOI] [PubMed] [Google Scholar]
- 7.Chou ST, Alsawas M, Fasano RM, Field JJ, Hendrickson JE, Howard J, Kameka M, Kwiatkowski JL, Pirenne F, Shi PA, Stowell SR, Thein SL, Westhoff CM, Wong TE, Akl EA. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv. 2020;4:327–355. doi: 10.1182/bloodadvances.2019001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanter J, Liem RI, Bernaudin F, Bolaños-Meade J, Fitzhugh CD, Hankins JS, Murad MH, Panepinto JA, Rondelli D, Shenoy S, Wagner J, Walters MC, Woolford T, Meerpohl JJ, Tisdale J. American Society of Hematology 2021 guidelines for sickle cell disease: stem cell transplantation. Blood Adv. 2021;5:3668–3689. doi: 10.1182/bloodadvances.2021004394C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunthararajah Y, Vichinsky EP. Chapter 42 - sickle cell disease: clinical features and management. In: Hoffman R, Benz EJ, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, Salama ME, Abutalib SA, editors. Hematology. Seventh edition. Elsevier; 2018. pp. 584–607.pp. e585 [Google Scholar]
- 10.Savage WJ, Buchanan GR, Yawn BP, Afenyi-Annan AN, Ballas SK, Goldsmith JC, Hassell KL, James AH, John-Sowah J, Jordan L, Lottenberg R, Murad MH, Ortiz E, Tanabe PJ, Ware RE, Lanzkron SM. Evidence gaps in the management of sickle cell disease: a summary of needed research. Am J Hematol. 2015;90:273–275. doi: 10.1002/ajh.23945. [DOI] [PubMed] [Google Scholar]
- 11.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390:311–323. doi: 10.1016/S0140-6736(17)30193-9. [DOI] [PubMed] [Google Scholar]
- 12.Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, Witkop C, Bass EB, Segal JB. Systematic review: hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med. 2008;148:939–955. doi: 10.7326/0003-4819-148-12-200806170-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brawley OW, Cornelius LJ, Edwards LR, Gamble VN, Green BL, Inturrisi C, James AH, Laraque D, Mendez M, Montoya CJ, Pollock BH, Robinson L, Scholnik AP, Schori M. National institutes of health consensus development conference statement: hydroxyurea treatment for sickle cell disease. Ann Intern Med. 2008;148:932–938. doi: 10.7326/0003-4819-148-12-200806170-00220. [DOI] [PubMed] [Google Scholar]
- 14.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. doi: 10.1001/jama.2010.378. [DOI] [PubMed] [Google Scholar]
- 15.Steiner CA, Miller JL. Sickle cell disease patients in U.S. hospitals, 2004: Statistical Brief #21. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD, US): Agency for Healthcare Research and Quality; 2006. [PubMed] [Google Scholar]
- 16.Nouraie M, Gordeuk VR. Blood transfusion and 30-day readmission rate in adult patients hospitalized with sickle cell disease crisis. Transfusion. 2015;55:2331–2338. doi: 10.1111/trf.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brodsky MA, Rodeghier M, Sanger M, Byrd J, McClain B, Covert B, Roberts DO, Wilkerson K, DeBaun MR, Kassim AA. Risk factors for 30-day readmission in adults with sickle cell disease. Am J Med. 2017;130:601.e9–601.e15. doi: 10.1016/j.amjmed.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Konda M, Roy AM, Jillella A, Goel A, Sasapu A. Potentially modifiable risk factors for 30-day readmission in adults with sickle cell disease: a national database study. Blood. 2019;134:4857. [Google Scholar]
- 19.Kumar V, Chaudhary N, Achebe MM. Epidemiology and predictors of all-cause 30-day readmission in patients with sickle cell crisis. Sci Rep. 2020;10:2082. doi: 10.1038/s41598-020-58934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.AlJuburi G, Laverty AA, Green SA, Phekoo KJ, Bell D, Majeed A. Socio-economic deprivation and risk of emergency readmission and inpatient mortality in people with sickle cell disease in England: observational study. J Public Health (Oxf) 2013;35:510–517. doi: 10.1093/pubmed/fdt100. [DOI] [PubMed] [Google Scholar]
- 21.Lyratzopoulos G, Havely D, Gemmell I, Cook GA. Factors influencing emergency medical readmission risk in a UK district general hospital: a prospective study. BMC Emerg Med. 2005;5:1. doi: 10.1186/1471-227X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart JT. The inverse care law. Lancet. 1971;1:405–412. doi: 10.1016/s0140-6736(71)92410-x. [DOI] [PubMed] [Google Scholar]
- 23.Saxena S, Eliahoo J, Majeed A. Socioeconomic and ethnic group differences in self reported health status and use of health services by children and young people in England: cross sectional study. BMJ. 2002;325:520. doi: 10.1136/bmj.325.7363.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brumm J, White RS, Arroyo NS, Gaber-Baylis LK, Gupta S, Turnbull ZA, Mehta N. Sickle cell disease is associated with increased morbidity, resource utilization, and readmissions after common abdominal surgeries: a multistate analysis, 2007-2014. J Natl Med Assoc. 2020;112:198–208. doi: 10.1016/j.jnma.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Frei-Jones MJ, Field JJ, DeBaun MR. Risk factors for hospital readmission within 30 days: a new quality measure for children with sickle cell disease. Pediatr Blood Cancer. 2009;52:481–485. doi: 10.1002/pbc.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pokhrel A, Olayemi A, Ogbonda S, Nair K, Wang JC. Racial and ethnic differences in sickle cell disease within the United States: from demographics to outcomes. Eur J Haematol. 2023;110:554–563. doi: 10.1111/ejh.13936. [DOI] [PubMed] [Google Scholar]
- 27.Akingbola TS, Tayo BO, Salako B, Layden JE, Hsu LL, Cooper RS, Gordeuk VR, Saraf SL. Comparison of patients from Nigeria and the USA highlights modifiable risk factors for sickle cell anemia complications. Hemoglobin. 2014;38:236–243. doi: 10.3109/03630269.2014.927363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldwin Z, Jiao B, Basu A, Roth J, Bender MA, Elsisi Z, Johnson KM, Cousin E, Ramsey SD, Devine B. Medical and non-medical costs of sickle cell disease and treatments from a US perspective: a systematic review and landscape analysis. Pharmacoecon Open. 2022;6:469–481. doi: 10.1007/s41669-022-00330-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CDC Centers for Disease Control and Prevention. Data & Statistics on Sickle Cell Disease. 2022 May 2nd, 2022. Available from: https://www.cdc.gov/ncbddd/sicklecell/data.html.
- 30.Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K, Wang Z, Erwin PJ, Sylvester T, Boehmer K, Ting HH, Murad MH, Shippee ND, Montori VM. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174:1095–1107. doi: 10.1001/jamainternmed.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frei-Jones MJ, Field JJ, DeBaun MR. Risk factors for hospital readmission within 30 days: a new quality measure for children with sickle cell disease. Pediatr Blood Cancer. 2009;52:481–485. doi: 10.1002/pbc.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlson ME, Charlson RE, Peterson JC, Marinopoulos SS, Briggs WM, Hollenberg JP. The charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61:1234–1240. doi: 10.1016/j.jclinepi.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Cronin RM, Hankins JS, Byrd J, Pernell BM, Kassim A, Adams-Graves P, Thompson A, Kalinyak K, DeBaun M, Treadwell M. Risk factors for hospitalizations and readmissions among individuals with sickle cell disease: results of a U.S. survey study. Hematology. 2019;24:189–198. doi: 10.1080/16078454.2018.1549801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tewari S, Brousse V, Piel FB, Menzel S, Rees DC. Environmental determinants of severity in sickle cell disease. Haematologica. 2015;100:1108–1116. doi: 10.3324/haematol.2014.120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas R, McGann PT, Beck A, Pfeiffer A, James KM. Characterization of community-based socioeconomic factors, utilization, and adherence in children with sickle cell disease. Blood. 2019;134:4686–4686. [Google Scholar]
- 36.Jiang HJ, Boutwell AE, Maxwell J, Bourgoin A, Regenstein M, Andres E. Understanding patient, provider, and system factors related to medicaid readmissions. Jt Comm J Qual Patient Saf. 2016;42:115–121. doi: 10.1016/s1553-7250(16)42014-3. [DOI] [PubMed] [Google Scholar]
- 37.Cintron-Garcia J, Ajebo G, Kota V, Guddati AK. Mortality trends in sickle cell patients. Am J Blood Res. 2020;10:190–197. [PMC free article] [PubMed] [Google Scholar]
- 38.Ogu UO, Billett HH. Comorbidities in sickle cell disease: adult providers needed! Indian J Med Res. 2018;147:527–529. doi: 10.4103/ijmr.IJMR_1019_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaur K, Faisal A, Kennedy K, Knupp CL, Liles D. Causes of mortality in sickle cell disease patients: a longitudinal review of deceased sickle cell patients. Blood. 2022;140:5440–5441. [Google Scholar]
- 40.Hassell K, Pace B, Wang W, Kulkarni R, Luban N, Johnson CS, Eckman J, Lane P, Woods WG American Society of Pediatric Hematology Oncology. Sickle cell disease summit: from clinical and research disparity to action. Am J Hematol. 2009;84:39–45. doi: 10.1002/ajh.21315. [DOI] [PubMed] [Google Scholar]
- 41.Lewit E, Thirumalai K, Wang CJ. Managed care, hospital characteristics, and inpatient utilization for sickle cell disease patients. J Gen Intern Med. 2018;33:2053–2055. doi: 10.1007/s11606-018-4630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams-Graves P, Bronte-Jordan L. Recent treatment guidelines for managing adult patients with sickle cell disease: challenges in access to care, social issues, and adherence. Expert Rev Hematol. 2016;9:541–552. doi: 10.1080/17474086.2016.1180242. [DOI] [PubMed] [Google Scholar]
- 43.Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000;320:569–572. doi: 10.1136/bmj.320.7234.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanter J, Smith WR, Desai PC, Treadwell M, Andemariam B, Little J, Nugent D, Claster S, Manwani DG, Baker J, Strouse JJ, Osunkwo I, Stewart RW, King A, Shook LM, Roberts JD, Lanzkron S. Building access to care in adult sickle cell disease: defining models of care, essential components, and economic aspects. Blood Adv. 2020;4:3804–3813. doi: 10.1182/bloodadvances.2020001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leschke J, Panepinto JA, Nimmer M, Hoffmann RG, Yan K, Brousseau DC. Outpatient follow-up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58:406–409. doi: 10.1002/pbc.23140. [DOI] [PubMed] [Google Scholar]
- 46.Colombatti R, Montanaro M, Guasti F, Rampazzo P, Meneghetti G, Giordan M, Basso G, Sainati L. Comprehensive care for sickle cell disease immigrant patients: a reproducible model achieving high adherence to minimum standards of care. Pediatr Blood Cancer. 2012;59:1275–1279. doi: 10.1002/pbc.24110. [DOI] [PubMed] [Google Scholar]
- 47.Cortright L, Buckman C, Tumin D, Holder D, Leonard S. Social determinants of health and emergency department use among children with sickle cell disease. J Pediatr Hematol Oncol. 2020;42:e42–e45. doi: 10.1097/MPH.0000000000001669. [DOI] [PubMed] [Google Scholar]
- 48.Dunbar P, Hall M, Gay JC, Hoover C, Markham JL, Bettenhausen JL, Perrin JM, Kuhlthau KA, Crossman M, Garrity B, Berry JG. Hospital readmission of adolescents and young adults with complex chronic disease. JAMA Netw Open. 2019;2:e197613. doi: 10.1001/jamanetworkopen.2019.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]


