Highlights
-
•
Hydroperoxide positional isomers from saturated fatty acid were prepared.
-
•
These hydroperoxide positional isomers were thermally decomposed individually.
-
•
2-, 3-, 4- & 5-positional isomers were involved in 2-alkanones & lactones formation.
-
•
A long-standing hypothesis on saturated fatty acid oxidation was demonstrated.
-
•
These findings contribute to creation of dairy aroma from saturated fatty acids.
Keywords: Saturated fatty acids, Thermal oxidation, Hydroperoxide isomers, 2-Alkanones, Lactones
Abstract
As known for quite a long time now, even saturated fatty acids can be oxidized at high temperatures to produce unique aroma compounds, such as 2-alkanones and lactones. Hydroperoxide positional isomers with a hydroperoxy group at the 2-, 3-, 4-, or 5-position are hypothesized to be responsible for the formation of these aroma components, but this hypothesis has not been verified. For the first time, this study successfully prepared a series of glyceryl trioctanoate hydroperoxide (C8TG;OOH) isomers. The isomers were thermally decomposed, proving that 2-heptanone was selectively formed from C8TG;3-OOH, and γ- and δ-octalactones were mainly formed from C8TG;4- and 5-OOH, respectively. C8TG;2-OOH was also involved in lactone formation, whereas C8TG;6- and 7-OOH were not. This proves the long-standing hypothesis. The mechanism revealed in this work is expected to be useful to create favorable aromas (i.e., 2-alkanones and lactones) from saturated fatty acids.
1. Introduction
In the 1940s industrial field, Farmer, Bloomfield, Sundralingam, and Sutton (1942) studied the oxidation (autoxidation) of unconjugated olefins and proposed that this can be attributed to the hydroperoxide formation through the radical chain reactions initiated at the allylic position of the olefin’s double bond. This theory is widely accepted as a mechanism for the oxidation of unsaturated fatty acids (USFAs) with double bonds, such as oleic, linoleic, and α-linolenic acids, which constitute triacylglycerols in edible oils (Frankel, 1980, Porter et al., 1995, Choe and Min, 2006, Yin et al., 2011). In contrast, saturated fatty acids (SFAs) do not contain double bonds, rendering Farmer’s theory inapplicable. However, it was known that even SFAs can also be oxidized at high temperatures (Stirton et al., 1945, Brodnitz, 1968). Interestingly, unique aromatic decomposition products, such as 2-alkanones (e.g., 2-heptanone, 2-nonanone, and 2-undecanone) and lactones (e.g., γ- and δ-octalactones, decalactones, and dodecalactones), which were not observed in USFA oxidation, have been identified in SFA oxidation (Crossley et al., 1962, Watanabe and Sato, 1970, Selke et al., 1975, Crnjar et al., 1981). This has led to a series of studies focusing on the mechanisms behind the SFA oxidation and the formation pathways of these unique decomposition products, especially from around 1960 to around 1980 (Benton & Wirth, 1953 and other succeeding studies). These important previous studies are summarized in Appendix A, Endres et al., 1962a, Endres et al., 1962b, Ramanathan et al., 1959, Selke et al., 1977, Watanabe and Sato, 1971.
Many researchers at that time assumed that, in the case of SFAs, a hydroperoxy group can be formed at any one of the alkyl carbons, except for the carbonyl carbon and terminal methyl group. They also hypothesized that among these possible hydroperoxide positional isomers, those with a hydroperoxy group at the 2-, 3-, 4-, or 5-position can undergo thermal decomposition to form 2-alkanones and lactones (Crossley et al., 1962, Watanabe and Sato, 1970, Kawada and Yamazaki, 1972, Selke et al., 1975, Jewell and Nawar, 1980, Crnjar et al., 1981). This hypothesis could have been verified if each isomer had been individually prepared and thermally decomposed. However, the technology available at that time was still insufficient for isomer preparation (Brodnitz et al., 1968a, Brodnitz et al., 1968b). Consequently, this hypothesis remains to be verified up to this day.
In recent years, our research group has developed a novel technique for preparing hydroperoxide of USFAs (USFA;OOHs) at the isomer level (Ibusuki et al., 2008, Kato et al., 2015) prior to SFAs. Our technique comprises four steps: 1) preparation of a crude sample containing various USFA;OOH isomers and other oxidation products; 2) after removing the intact (unoxidized) USFA, purifying the USFA;OOH derivative by selectively reacting the USFA;OOH isomers with a vinyl ether compound; 3) deprotecting the USFA;OOH derivative and purifying the USFA;OOH isomer mixture; and 4) isolating individual USFA;OOH isomers and determining the positional information of a hydroperoxy group using the characteristic fragmentation patterns of sodiated isomers observed during liquid chromatography–tandem mass spectrometry (LC–MS/MS) analysis (Kato et al., 2021). As an example, we used this technique to prepare all the hydroperoxide positional isomers of linoleic acid and found new pathways for the formation of specific decomposition products (e.g., acrolein) by thermally decomposing each hydroperoxide isomer (Kato et al., 2022). This technique, which is effective for preparing USFA;OOH positional isomers (Ito et al., 2015, Kato et al., 2018, Miyazaki et al., 2023), is expected to be applicable to the preparation of SFA hydroperoxide (SFA;OOH) isomers.
As mentioned above, SFA;OOH isomers with a hydroperoxy group at the 2-, 3-, 4-, or 5-position are considered to be particularly important. Therefore, in this study, we selected glyceryl trioctanoate (C8TG; Fig. 1A) as a moderate-chain length sample that allowed formation of these isomers and succeeded in the preparation of the desired C8TG;2-, 3-, 4-, and 5-OOH, as well as 6- and 7-OOH, using the technique presented earlier. We elucidated the abovementioned hypothesis, which has remained unproven for decades, by analyzing the thermal decomposition products of each isomer. The study results are expected to significantly advance the technology for producing 2-alkanones and lactones from SFA-rich vegetable oils, such as palm and coconut oils. 2-Alkanones and lactones are aroma compounds found in dairy products (Curioni and Bosset, 2002, Schlutt et al., 2007, Mallia et al., 2008); hence, such a technology has various potential applications, as mentioned in Section 4.
Fig. 1.
(A) Overall preparation steps 1–4 for C8TG;OOH isomers and (B) POV measurement for the crude sample preparation in Step 1.Step 1: A crude sample containing various C8TG;OOH isomers and other oxidation products was prepared by thermal oxidation of C8TG. Step 2: The intact (unoxidized) C8TG was removed, and the C8TG;OOH derivative was purified by selective reaction with a vinyl ether compound (i.e., benzyl isopropenyl ether). Step 3: The C8TG;OOH derivative was deprotected and the C8TG;OOH isomer mixture was purified. Step 4: Individual C8TG;OOH isomers were isolated, and the positional information of the hydroperoxy group was determined through LC–MS/MS analysis. B: The POV data are expressed as mean ± SDs (n = 5).
2. Materials and methods
2.1. Materials and chemicals
Commercially available C8TG with > 98 % purity (Nisshin OilliO Group, Tokyo, Japan) was used in this study. Benzyl isopropenyl ether and pyridinium p-toluenesulfonate (PPTS) were purchased from the Tokyo Chemical Industry (Tokyo, Japan). All the other reagents used were of the highest available grade.
2.2. Preparation steps for the C8TG;OOH positional isomers
We attempted to apply our previously developed preparation technique for UFA;OOH isomers (Ibusuki et al., 2008, Kato et al., 2015) to C8TG (Fig. 1A), and the overall preparation steps were as follows: 1) C8TG was thermally oxidized to obtain a crude sample containing various C8TG;OOH isomers and other oxidation products (this crude sample was obtained while confirming that thermal oxidation indeed progressed by the sweet coconut-like aroma derived from 2-alkanones and lactones); 2) after the removal of the intact C8TG from the crude sample by LC, the C8TG;OOH isomers were selectively reacted with a vinyl ether compound (i.e., benzyl isopropenyl ether); the newly formed C8TG;OOH derivative showed a higher lipophilicity and was easily separated from the other oxidation products on LC; 3) the treatment of the C8TG;OOH derivative with PPTS regenerated the original C8TG;OOH (isomer mixture); and 4) the bonding position of the hydroperoxy group of each isomer was determined by LC–MS/MS after the mixture was separated into individual C8TG;OOH isomers to the greatest extent feasible by LC in the reversed- and normal-phase conditions. We tested various conditions, especially regarding reagent amounts, solvent compositions, reaction times, and LC separation for each step, to determine the method that would allow us to prepare the target of each step’s preparation (e.g., C8TG;OOH derivative or isomer) with the highest yield and purity. The determined method is presented from Section 2.3 to 2.6.
2.3. Preparation step 1: Crude sample preparation by the thermal oxidation of C8TG
C8TG was thermally oxidized to prepare a crude sample containing the maximum possible amounts of C8TG;OOH isomers. Specifically, 50 g of C8TG was placed in a 200 mL glass beaker and heated at 170 °C for 1 h in a mantle heater. Immediately after completion, the obtained crude sample was transferred to a 100 mL screw cap vial filled with nitrogen gas and cooled in ice until below room temperature. The peroxide value (POV) of the crude sample was measured using the AOCS Official Method Cd 8b-90. The crude sample was then weighed and dissolved in methanol to 100 mg/mL concentration.
2.4. Preparation step 2: Removal of the intact C8TG from the crude sample, subsequent derivatization of C8TG;OOH, and fractionation of derivative by LC
The crude sample (900 μL) was injected into a semipreparative 1260 Infinity II LC–MS (6125 LC/MSD, Agilent Technologies, CA, USA) to remove the intact C8TG. A COSMOSIL 5C18-MS-II (10 × 250 mm, 5 μm, Nacalai Tesque, Kyoto, Japan) was used with methanol at a 5.0 mL/min flow rate as a mobile phase. The column temperature was maintained at 40 °C. At the post-column, the eluate was split. One portion (4.999 mL/min flow rate) was sent to the fraction collector. The other portion (0.25 μL/min flow rate) was mixed with methanol/2-propanol (4:1, v/v; containing 0.1 mmol/L sodium acetate) to increase the detection sensitivity, and then sent to the MS. The methanol/2-propanol flow rate was 0.5 mL/min. The semipreparative LC–MS system allowed the fractionation of a sample rich in C8TG;OOH isomers, while monitoring the electrospray ionization MS spectra of the analytes in the m/z range of 100 to 1000. Supplementary Table 2 shows the MS conditions used. The fractionation was repeated for 100 times. The collected fractions were then dried at 25 °C using a rotary evaporator to obtain 206 mg of the sample rich in the C8TG;OOH isomers.
The C8TG;OOH isomers in this sample selectively reacted with a vinyl ether compound (i.e., benzyl isopropenyl ether) to prepare the C8TG;OOH derivative. Specifically, 13 mL acetonitrile containing 2 mmol/L PPTS and 3 mL chloroform were added to 206 mg of the sample. To this solution, 1.25 g (1.3 mL) of benzyl isopropenyl ether was added, stirred with a vortex mixer, and allowed to incubate at 5 °C. After 1 h, the derivatization reaction was stopped by adding 15 mL water and 20 mL methanol. The solution was loaded on a Sep-Pak Vac C18 (35 cc, 10 g, Waters, MA, USA) equilibrated with water. The column was then washed with 40 mL methanol/water (1:1, v/v). The newly formed C8TG;OOH derivative and other remaining oxidation products were eluted with 60 mL ethanol. The eluate was dried, weighed, and made into a 10 mg/mL methanol solution. Subsequently, 900 μL of the solution was injected into a semipreparative LC–MS to separate the C8TG;OOH derivative from the other oxidation products. This fractionation was repeated for 30 times. The collected fractions were then dried to obtain 137 mg of the C8TG;OOH derivative.
2.5. Preparation step 3: Regeneration of the C8TG;OOH isomers by deprotection of the C8TG;OOH derivative
The C8TG;OOH derivative was deprotected under PPTS acidic conditions to regenerate the C8TG;OOH isomer mixture. Specifically, 137 mg of the C8TG;OOH derivative was added with 300 mL methanol containing 2 mmol/L PPTS and allowed to incubate at 5 °C to regenerate C8TG;OOH. After 18 h, the regeneration reaction was stopped by adding 300 mL water. The solution was then loaded on the same Sep-Pak as above equilibrated with water. The column was washed with 40 mL methanol/water (1:1, v/v). The regenerated C8TG;OOH and the remaining unreacted derivative were eluted with 60 mL ethanol. The eluate was dried, weighed, and made into a 100 mg/mL methanol solution. Subsequently, 900 μL of the solution was injected into a semipreparative LC–MS to purify the C8TG;OOH isomer mixture. This purification was repeated for four times. The collected mixture fraction was dried to obtain 104 mg of the C8TG;OOH isomer mixture.
2.6. Preparation step 4: Individual C8TG;OOH isomer isolation and determination of its hydroperoxy group position
The obtained C8TG;OOH isomer mixture was separated under reversed- and normal-phase conditions to isolate the individual C8TG;OOH isomers. For the reversed-phase condition, C8TG;OOH was dissolved in methanol/water (4:1, v/v) to a 10 mg/mL concentration. This solution (900 μL) was injected into a semipreparative LC–MS with the same C18 column as above, but with a different mobile phase (methanol/water (4:1, v/v)) and flow rates (4.998 mL/min to the fraction collector and 2.5 μL/min to the MS). Each separated C8TG;OOH isomer was individually collected. Some coeluted isomers were collected as a mixture. This collection process was repeated for 15 times. Each collected isomer was subjected to the same Sep-Pak as above to remove water and then dried and dissolved in 1 mL hexane. For the isomer purity improvement and for further separation in the case of isomer mixtures, hexane solution (900 μL) was injected into a semipreparative LC–MS under the normal-phase condition (column: Inertsil SIL-100A; 10 × 250 mm, 5 μm, GL Sciences, Tokyo, Japan; mobile phase: hexane/2-propanol (100:0.5, v/v); flow rates: 4.998 mL/min to the fraction collector and 2.5 μL/min to MS; and column temperature: 40 °C). Consequently, each C8TG;OOH isomer was individually isolated. Some isomers were isolated by repeating the isolation procedure several times. Individual C8TG;OOH isomers were dissolved in 10 mL hexane after drying. Their concentrations were measured using a ferrous oxidation–xylenol orange (FOX) 2 assay (Wolff, 1994).
Finally, a portion of the hexane solution (10–200 μL) was diluted with an appropriate amount of methanol and injected into a Nexera X2 LC–MS/MS system (LCMS8050, Shimadzu, Kyoto, Japan) to confirm the purity and structure (i.e., hydroperoxy group position). No columns were used for the analysis. The mobile phase, methanol (flow rate: 0.2 mL/min), was mixed with methanol/2-propanol (4:1, v/v; containing 0.1 mmol/L sodium acetate; flow rate: 0.2 mL/min) to increase the detection sensitivity. It was then directly sent into the MS/MS for the measurement of electrospray ionization MS and product ion MS spectra. The hydroperoxide position of each C8TG;OOH isomer was distinguished by examining the characteristic product ions from the sodiated C8TG;OOH (m/z 525.3, [M + Na]+). Supplementary Table 2 shows the MS/MS conditions used.
2.7. Analysis of the thermal decomposition products of each C8TG;OOH isomer
The hexane solution of each C8TG;OOH isomer was pipetted into a 20 mL headspace screw top vial (Agilent Technologies), such that the amount of each C8TG;OOH isomer was 25 nmol, and hexane was removed by nitrogen gas. The vial caps were closed, and the samples were thermally decomposed under nitrogen atmosphere at 180 °C using a dry block heater. After 10 min, the vials were cooled by air and analyzed for volatile degradation products using a solid-phase microextraction–gas chromatography mass spectrometry (SPME/GC–MS) system. This system comprised a GC (7890B, Agilent Technologies) capable of on-line SPME and an electron ionization MS (Pegasus BT, LECO, MI, USA). DB-WAX (60 m × 0.25 mm × 0.50 µm, Agilent Technologies) was used as the column. Helium was employed as the carrier gas at a 1.0 mL/min flow rate. The column was held at 30 °C for 5 min. The temperature was then increased to 240 °C at 5 °C/min, and then held for 7 min. Component identification was based on a comparison of the retention times and the MS spectra with the standard reagents and MS library. Supplementary Table 3 presents the details of MS and other conditions. In addition to the volatile thermal degradation products, the nonvolatile thermal degradation products and the remaining C8TG;OOH isomers were analyzed by LC–MS/MS as described in Section 2.6.
3. Results and discussion
3.1. Difficulties in the SFA;OOH isomer preparation
As mentioned earlier, even SFAs can be oxidized at high temperatures to generate unique aromatic decomposition products, such as 2-alkanones and lactones, which are not observed in the USFA oxidation. Accordingly, the following hypothetical mechanism was proposed: a hydroperoxy group can be formed at any one of the alkyl carbons in the case of SFAs, and the decomposition of the SFA;OOH isomers with a hydroperoxy group at the 2-, 3-, 4-, or 5-position might generate the previously mentioned volatile degradation products. This hypothesis remained unverified due to the difficulty in preparing the SFA;OOH isomers using the technology available at that time. Therefore, in this study, we selected C8TG as the SFA triacylglycerol and aimed to prepare C8TG;OOH positional isomers, including C8TG;2-, 3-, 4-, and 5-OOH, by applying our technique used to prepare USFA;OOH isomers (Ibusuki et al., 2008, Kato et al., 2015). Please refer to Fig. 1A and the preparation steps described in Section 2.2.
3.2. Preparation step 1: Crude sample preparation by the thermal oxidation of C8TG
The POV is a conventional indicator of the amount of hydroperoxide in a sample. To obtain sufficient amounts of C8TG;OOH isomers including C8TG;2-, 3-, 4-, and 5-OOH, it was considered necessary to prepare a crude sample with a higher POV. No previous reports have yet presented the relationship between the oxidation conditions of C8TG and POV; thus, preliminary experiments were required to determine the oxidation conditions that can maximize the POV. First, 50 g of C8TG was oxidized under the conditions of a fixed 1 h heating time and a temperature that varied in 10 °C increments in 100 °C–200 °C range. After completion, the POV of the samples was measured and the data at each temperature was plotted (Fig. 1B). The results showed that the C8TG oxidation progressed at temperatures above 150 °C, as evidenced by a clear increase in POV. The POV behavior suggested a dominant C8TG;OOH formation from 150 °C to 170 °C and a decomposition occurring above 180 °C. The sweet coconut-like aroma became significant in 180 °C–200 °C temperature range, indicating that C8TG;OOH was actively decomposed to 2-alkanones and lactones. As described above, by oxidizing C8TG under the heating conditions maximizing POV (170 °C, 1 h), a crude sample with a high POV (50 meq/kg) suitable for the C8TG;OOH isomer preparation could be prepared.
3.3. Preparation step 2: Removal of the intact C8TG from the crude sample, subsequent derivatization of C8TG;OOH, and fractionation of derivative by LC
Although a crude sample with a high POV of 50 meq/kg was obtained, C8TG;OOH in the crude sample was estimated to be approximately 1 % based on the calculation from POV. This suggests that a large amount of intact C8TG remained along with other oxidation products. Therefore, the crude sample was injected into a semipreparative LC–MS to remove the intact C8TG. A reversed-phase column was used for separation, and methanol was used as the mobile phase. The analytes were detected with a high sensitivity as sodiated ions (e.g., [M + Na]+) by mixing the split mobile phase with a solution containing 0.1 mmol/L sodium acetate at post-column. Fig. 2A shows that the crude sample was mainly separated into two peaks (i.e., a and b) in the total ion chromatogram (TIC). The MS spectra corresponding to the C8TG;OOH isomers (m/z 525.3), other oxidation products (e.g., C8TG ketone: m/z 507.3; C8TG hydroxide: m/z 509.3), and degradation products (e.g., diacylglycerol: m/z 367.2) were observed on the first eluted peak a at a retention time of approximately 4 min. In contrast, an MS spectrum corresponding to the intact C8TG (m/z 493.4) was solely observed on the later eluted peak b at a retention time of approximately 6 min. The first peak a was fractionated and re-injected; the removal of intact C8TG was confirmed, as intended (Fig. 2A). Peak a was repeatedly fractionated and concentrated to obtain a sample rich in the C8TG;OOH isomers.
Fig. 2.
Intact C8TG removal from the crude sample, C8TG;OOH derivatization (then purification), and C8TG;OOH isomer regeneration.A: A crude sample was analyzed by semipreparative LC–MS, and the MS spectra of peaks a and b were measured. Peak a was fractionated and re-injected. B: After derivatization, the semipreparative LC–MS analysis was performed, and the MS spectra of peaks c and d were measured. Peak d was fractionated and re-injected. C: After deprotection, the semipreparative LC–MS analysis was performed, and the MS spectra of peaks e and f were measured. Peak e was fractionated and re-injected. Red arrows represent the re-injection of fractionated peaks. 2.4, 2.5 present detailed analysis conditions. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The sample still contained other oxidation (e.g., C8TG ketone and C8TG hydroxide) and degradation (e.g., diacylglycerol) products; hence, as the next purification step, all C8TG;OOH isomers were derivatized by reacting with a vinyl ether compound. The retention time shift of the derivative caused by increased lipophilicity was used to separate the C8TG;OOH derivative from the other products. In our previous studies, in which we prepared USFA;OOH isomers (Ibusuki et al., 2008, Kato et al., 2014), we used 2-methoxypropene as a vinyl ether compound. In the present study, we employed benzyl isopropenyl ether to further increase the lipophilicity of the resulting derivative. Consequently, a larger retention time shift was achieved under the reversed-phase conditions (Fig. 2B). That is, after derivatization, C8TG;OOH disappeared in the TIC, and some peaks were instead observed at 5–7 min retention time, of which peak d with a single spectrum of a perketal derivative of C8TG;OOH (m/z 673.4) was clearly observed at 6–7 min retention time. Peak d was fractionated and re-injected; the removal of other components was confirmed, as intended (Fig. 2B). Peak d was repeatedly fractionated and concentrated to obtain the C8TG;OOH derivative.
3.4. Preparation step 3: Regeneration of the C8TG;OOH isomers by the C8TG;OOH derivative deprotection
The C8TG;OOH derivative was deprotected under acidic conditions using PPTS to regenerate the original C8TG;OOH. In our previous studies, we used 2-methoxypropene as a vinyl ether compound and achieved deprotection by reacting with 1.0–1.5 μmol of PPTS per mg of the derivative for 1–12 h (Ibusuki et al., 2008, Kato et al., 2014). In the present study, benzyl isopropenyl ether was utilized, and deprotection hardly proceeded with the abovementioned acidity and reaction time. Therefore, PPTS was increased to 4.4 μmol (per mg of the isomer), and the reaction time was extended to 18 h, and most C8TG;OOH derivatives were deprotected. Consequently, a single clear peak e appeared at 4 min of retention time in the TIC and identified as C8TG;OOH (Fig. 2C). Peak e was collected repeatedly and concentrated to yield a highly purified C8TG;OOH isomer mixture.
3.5. Preparation step 4: Individual C8TG;OOH isomer isolation and determination of its hydroperoxy group position
As mentioned in the Introduction, for SFAs, a hydroperoxy group is speculated to be formed at any one of the alkyl carbons, producing six isomers ranging from C8TG;2-OOH to C8TG;7-OOH. According to the feature of triacylglycerol hydroperoxide, the sn-positional isomers (i.e., 1,2-dioctanoyl-3-hydroperoxyoctanoyl-rac-glycerol and 1,3-dioctanoyl-2-hydroperoxyoctanoyl glycerol) would possibly be separated. Furthermore, because 1,2-dioctanoyl-3-hydroperoxyoctanoyl-rac-glycerol contains two chiral carbons, isomers with diastereomeric relationships might be separated. Thus, we first tested how well the C8TG;OOH isomers could be separated by the addition of water to the mobile phase (methanol). With methanol/water (4:1, v/v), the C8TG;OOH isomer mixture was separated into at least 10 peaks when analyzed in a sensitive selective ion monitoring mode (Fig. 3A). This result suggests the separation of the sn-positional and/or diastereomeric isomers.
Fig. 3.
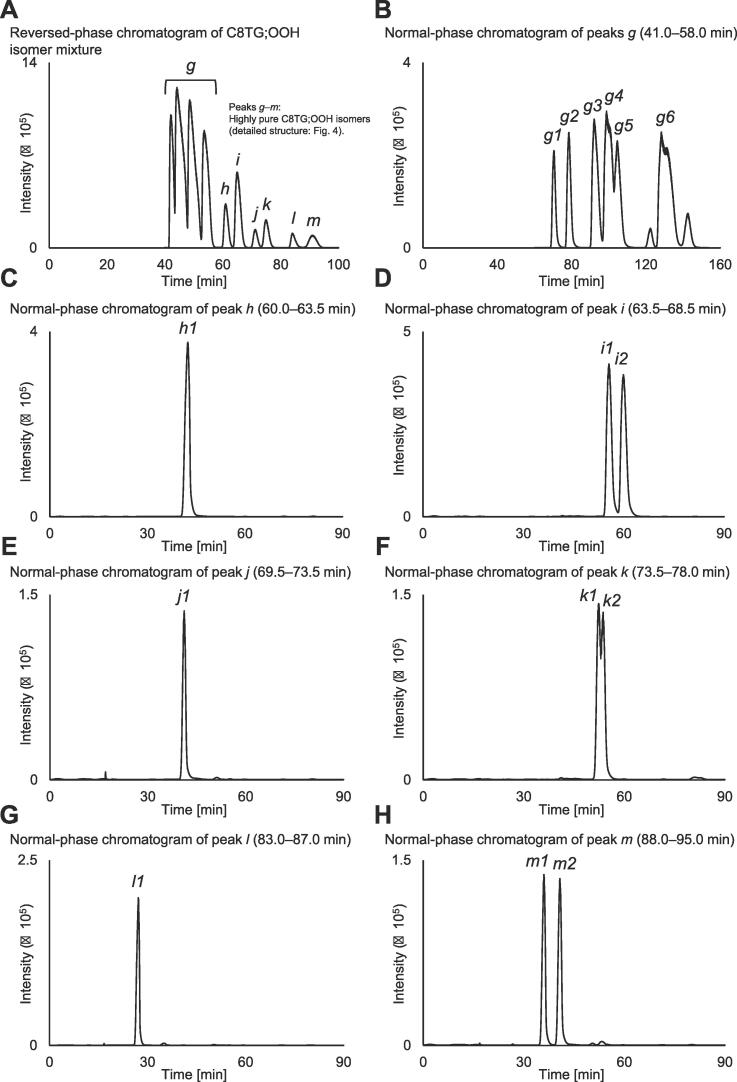
Separation of the C8TG;OOH isomer mixture into individual C8TG;OOH isomers to the greatest extent feasible through LC–MS under reversed- and normal-phase conditions.A: The C8TG;OOH isomer mixture was analyzed by semipreparative LC–MS. Methanol/water (4:1, v/v) was used as mobile phase. The selective ion-monitoring chromatogram of C8TG;OOH (m/z 525.3, [M + Na]+) is shown. B–H: The peaks (i.e., g, h, i, j, k, l, and m) collected under the reversed-phase condition were further separated under the normal-phase condition. Hexane/2-propanol (100:0.5, v/v) was used as the mobile phase. Selective ion monitoring chromatograms of C8TG;OOH (m/z 525.3, [M + Na]+) are shown. Section 2.6 presents the detailed analysis conditions. Please refer to Fig. 4 for the determination of the hydroperoxy group position of each isomer.
The C8TG;OOH isomer mixture was separated into at least 10 peaks with methanol/water (4:1, v/v) (Fig. 3A), so the peaks were fractionated, and the collected isomers were further separated under normal phase conditions in an attempt to separate the isomers as pure as possible. Specifically, using methanol/water (4:1, v/v), the separated peaks (i.e., h, i, j, k, l, and m) eluted after 60 min of retention time were individually collected. Peaks g that eluted from 40 to 60 min were not sufficiently separated; hence, this portion was fractionated together (Fig. 3A). Each peak (i.e., g, h, i, j, k, l, and m) was then injected into a normal-phase, semipreparative LC–MS using hexane/2-propanol (100:0.5, v/v) as the mobile phase. The selective ion monitoring chromatograms in Fig. 3B–H illustrate that some of the isomers collected under reversed-phase conditions were further separated under normal-phase conditions (e.g., peak i into peak i1 and peak i2). Similarly, isomer g obtained as a mixture under reversed-phase conditions was also separated into g1 to g6 under normal-phase conditions. In this study, we obtained six individual isomers, C8TG;2-OOH to C8TG;7-OOH (refer to below for structural analysis). In detail, considering the sn-positional and/or diastereomeric isomers, a total of 15 isomers were prepared. For the reader’s reference, as a feature of the normal-phase condition, C8TG;OOH with a hydroperoxy group on the carbonyl side (i.e., 2-, 3-, or 4-position) was separated into three isomers. Thus, in the case of isomers with such stereostructures, it would be possible to separate their diastereomers.
Finally, each of the 15 isomers was injected into the LC–MS/MS without a column to confirm the purity and determine the hydroperoxy group position. Electrospray ionization MS spectra confirmed that all C8TG;OOH isomers were purified to high purity (Supplementary Fig. S1). Importantly, in the product ion scan of C8TG;OOH (m/z 525.3, [M + Na]+) for each isomer (Fig. 4A–O), fragmentation strictly occurred according to the following rules: A) α-cleavage between the hydroperoxy group-bound carbon and the adjacent carbons (i.e., carbonyl and terminal sides) of the sodiated C8TG;OOH produced the short-chain alkanoyl and oxoalkanoyl fragment ions, respectively; and B) from the former ion, octanoic acid was desorbed (in the case of C8TG;2-OOH, carbon dioxide were further desorbed). The results revealed that the structure of each isomer is indeed as shown in Fig. 4P. The isomers with the same fragmentation pattern would be in an sn-positional and/or diastereomeric isomers. To the best of our knowledge, this is the first case in which SFA;OOH positional isomers were prepared individually. Our technique for preparing USFA;OOH isomers (Ibusuki et al., 2008, Kato et al., 2015, Kato et al., 2021) was also proven to be effective for SFAs.
Fig. 4.
Product ion mass spectra of each C8TG;OOH isomer with m/z 525.3 ([M + Na]+) as the precursor ion.A–O: Each C8TG;OOH isomer (i.e., g1, g2, g3, g4, g5, g6, h1, i1, i2, j1, k1, k2, l1, m1, and m2) was analyzed using LC–MS/MS (without column). P: Characteristic fragments were observed for each isomer. The position of the hydroperoxy group was determined, as shown in this figure. The structures are not written in such a manner that the sn-positional or diastereomeric isomers can be distinguished. Section 2.6 presents the detailed analysis conditions.
3.6. Analysis of the thermal decomposition products derived from each C8TG;OOH isomer
After achieving the isolation of the desired C8TG;2-, 3-, 4-, and 5-OOH as well as 6- and 7-OOH (15 isomers including their sn-positional and/or diastereomeric isomers), we attempted to prove the hypothesis that remained unresolved for decades by analyzing the isomers’ volatile degradation products. We subjected each of the 15 isomers to thermal decomposition at 180 °C (i.e., the temperature at which isomer decomposition was suggested in the preliminary experiments in Section 3.2) and examined whether 2-alkanones and lactones can indeed be formed from C8TG;2-, 3-, 4-, and 5-OOH with SPME/GC–MS. The chromatograms clearly demonstrated that 2-heptanone was selectively formed from C8TG;3-OOH, whereas γ-octalactone and δ-octalactone were mainly produced from C8TG;4-OOH and C8TG;5-OOH, respectively (Fig. 5A). γ-Heptalactone was also produced from C8TG;5-OOH. Small amounts of γ-octalactone, δ-octalactone, γ-heptalactone, and heptanoic acid were formed from C8TG;2-OOH. The 2-alkanones and lactones were rarely formed from C8TG;6-OOH or C8TG;7-OOH (Fig. 5B).
Fig. 5.
Analysis of volatile and nonvolatile thermal degradation products of each C8TG;OOH isomer.A: Each C8TG;OOH isomer was thermally decomposed under nitrogen atmosphere at 180 °C. After 10 min, the volatile degradation products were analyzed by SPME/GC–MS. Component identification was based on a comparison of the retention times and MS spectra with standard reagents, and MS library. B: The peak areas for 2-heptanone, γ-octalactone, δ-octalactone, γ-heptalactone, and heptanoic acid for each isomer are shown. Peak areas are represented by extracting the major fragment ions of each component (i.e., 2-heptanone: m/z 58.06 ± 0.05; γ-heptalactone and γ-octalactone: m/z 85.03 ± 0.05; δ-octalactone: m/z 99.05 ± 0.05; and heptanoic acid: m/z 60.03 ± 0.05). C: Samples after the analysis of the volatile degradation products were diluted with 10 mL methanol and injected into the LC–MS/MS for the analysis of nonvolatile degradation products (Supplementary Fig. 2). The intensities of diacylglycerol (m/z 367.2), C8TG ketone (m/z 507.3), C8TG hydroxide (m/z 509.3), and the remaining C8TG;OOH (m/z 525.3) for each isomer are shown. Our proposed mechanism shown in Fig. 6 was supported by the higher level of diacylglycerol derived from C8TG;2-, 3-, 4-, and 5-OOH, the lower level of C8TG ketone derived from C8TG;2- and 3-OOH, and the lower level of C8TG hydroxide derived from C8TG;4- and 5-OOH. Section 2.7 and Supplementary Tables 2 and 3 present the detailed analysis conditions. All data are expressed as mean ± SDs (n = 3).
Fig. 6 depicts the possible formation mechanisms of 2-alkanones and lactones based on the abovementioned findings. The sample remaining after the SPME analysis was injected into the LC–MS/MS to analyze for nonvolatile degradation products. The trend toward the higher level of diacylglycerol (m/z 367.2) derived from C8TG;2-, 3-, 4-, and 5-OOH, the lower level of C8TG ketone (m/z 507.3) derived from C8TG;2- and 3-OOH, and the lower level of C8TG hydroxide (m/z 509.3) derived from C8TG;4- and 5-OOH were confirmed (Fig. 5C; Supplementary Fig. 2). These results support our proposed mechanism whereby C8TG;2-, 3-, 4-, or 5-OOH is converted to 2-alkanones or lactones via the formation of unstable intermediates (C8TG ketone or C8TG hydroxide) and the subsequent release of diacylglycerol (Fig. 6). This mechanism should be applicable to the oxidation of various SFAs (e.g., SFAs with different chain lengths). This study provides the first strong structural–chemical evidence to prove the long-standing hypothesis. Further investigation of our proposed mechanism (Fig. 6), especially the detailed relationship between the stereostructures (sn-positional and diastereomeric isomers) and aroma compound formation, would be necessary in future work.
Fig. 6.
Possible formation mechanisms of 2-alkanones and lactones from C8TG;OOH.I: C8TG;3-OOH undergoes dehydration, hydrolysis, and decarboxylation to form 2-heptanone. II, III: C8TG;4-(or 5-)OOH undergoes an alkoxy radical, abstracts a hydrogen atom from other molecules, forms 4-(or 5-)hydroxyC8TG, and is cyclized to γ-(or δ-)octalactone. IV: C8TG;2-(or 5-)OOH undergoes an alkoxy radical, abstracts the hydrogen at the 5-(or 2-)position of the same chain, and is cyclized to γ-heptalactone. V, VI: C8TG;2-OOH undergoes an alkoxy radical, abstracts the hydrogen at the 4-(or 5-)position of another chain in the same molecule and is cyclized to γ-(or δ-)octalactone. VII: C8TG;2-OOH undergoes dehydration, hydrolysis, and carbon monoxide is released to heptanoic acid. Further oxidation of heptanoic acid can form 2-alkanones and γ- and δ-lactones with lower molecular weights. DG: diacylglycerol.
4. Conclusion
In this study, SFA;OOH positional isomers (i.e., C8TG;2-, 3-, 4-, 5-, 6-, and 7-OOH) were individually prepared for the first time to elucidate the mechanism by which 2-alkanones and lactones are formed during the thermal oxidation of SFAs. Each isomer was thermally decomposed, and the volatile degradation products were analyzed. The results demonstrated that the formation of 2-alkanones and lactones depends on the isomer structure. That is, C8TG;2-, 3-, 4-, and 5-OOH were involved in the formation of 2-alkanones and lactones, whereas the other isomers were not. Possible formation routes of 2-alkanones and lactones through SFA oxidation were proposed based on the findings. Nonvolatile degradation products, namely diacylglycerol, C8TG ketone, and C8TG hydroxide, were also analyzed, and the results supported our proposed mechanism. To the best of our knowledge, this is the first report of the individual preparation of SFA;OOH positional isomers. We are convinced that our work provides strong structural–chemical evidence to prove the long-standing hypothesis of SFA oxidation.
Finally, the potential applications of this research are described. 2-Alkanones and lactones are important aroma components of dairy products (Curioni and Bosset, 2002, Schlutt et al., 2007, Mallia et al., 2008). In the case of milk fat, heating increases γ- and δ-lactones (Obi et al., 2018). A similar increase has been observed in beef fat (Yoshinaga, Tago, Yoshinaga-Kiriake & Gotoh, 2021). Although the hydroxy fatty acids in milk and beef fat are predicted to be converted to lactones (Yoshinaga et al., 2019), this is the first study to suggest that SFA oxidation may be involved in the formation of such aroma components. Expanding our findings may lead to the development of techniques that can intentionally produce aroma components. For example, vegetable oils containing SFA triacylglycerols with slightly longer chain lengths than C8TG could be used to create the great aroma of animal products such as butter and cheese, which would dramatically expand the range of plant-based foods.
CRediT authorship contribution statement
Kanji Aoyagi: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Shunji Kato: Writing – review & editing, Methodology, Data curation, Conceptualization. Daisuke Isaka: Methodology, Investigation. Yoshinori Sekiguchi: Investigation. Yurika Otoki: Writing – review & editing. Hidetaka Uehara: Supervision, Conceptualization. Kiyotaka Nakagawa: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Ms. Kaori Kato (Central Research Laboratory, Technical Division, The Nisshin OilliO Group, Ltd.) and Ms. Ibuki Kusumoto (Laboratory of Food Function Analysis, Graduate School of Agricultural Science, Tohoku University) for excellent writing assistance. This work was supported in part by KAKENHI (Grant Number 22H02278) of Japan Society for the Promotion of Science, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2023.101074.
Contributor Information
Kanji Aoyagi, Email: k-aoyagi@nisshin-oillio.com.
Shunji Kato, Email: shunji.kato.b5@tohoku.ac.jp.
Daisuke Isaka, Email: d-isaka@nisshin-oillio.com.
Yurika Otoki, Email: yurika.otoki.d7@tohoku.ac.jp.
Hidetaka Uehara, Email: h-uehara@nisshin-oillio.com.
Kiyotaka Nakagawa, Email: kiyotaka.nakagawa.c1@tohoku.ac.jp.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- AOCS Official Method Cd 8b-90 “Peroxide value”. In Official Methods and Recommended Practices of the AOCS (7th edition).
- Benton J.L., Wirth M.M. Position of radical attack during oxidation of long-chain paraffins. Nature. 1953;171:269. doi: 10.1038/171269a0. [DOI] [Google Scholar]
- Brodnitz M.H. Autoxidation of saturated fatty acids. a review. Journal of Agricultural and Food Chemistry. 1968;16(6):994–999. doi: 10.1021/jf60160a001. [DOI] [Google Scholar]
- Brodnitz M.H., Nawar W.W., Fagerson I.S. Autoxidation of saturated fatty acids. I. The initial products of autoxidation of methyl palmitate. Lipids. 1968;3(1):59–64. doi: 10.1007/BF02530970. [DOI] [PubMed] [Google Scholar]
- Brodnitz M.H., Nawar W.W., Fagerson I.S. Autoxidation of saturated fatty acids. II. The determination of the site of hydroperoxide groups in autoxidizing methyl palmitate. Lipids. 1968;3(1):65–71. doi: 10.1007/BF02530971. [DOI] [PubMed] [Google Scholar]
- Choe E., Min D.B. Mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety. 2006;5(4):169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- Crnjar E.D., Witchwoot A., Nawar W.W. Thermal oxidation of a series of saturated triacylglycerols. Journal of Agricultural and Food Chemistry. 1981;29(1):39–42. doi: 10.1021/jf00103a011. [DOI] [Google Scholar]
- Crossley A., Heyes T.D., Hudson B.J.F. The effect of heat on pure triglycerides. Journal of the American Oil Chemists’ Society. 1962;39(1):9–14. doi: 10.1007/BF02633339. [DOI] [Google Scholar]
- Curioni P.M.G., Bosset J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. International Dairy Journal. 2002;12(12):959–984. doi: 10.1016/S0958-6946(02)00124-3. [DOI] [Google Scholar]
- Endres J.G., Bhalerao V.R., Kummerow F.A. Thermal oxidation of synthetic triglycerides I. Composition of oxidized triglycerides. Journal of the American Oil Chemists’ Society. 1962;39(2):118–121. doi: 10.1007/BF02631685. [DOI] [Google Scholar]
- Endres J.G., Bhalerao V.R., Kummerow F.A. Thermal oxidation of synthetic triglycerides. II. Analysis of the volatile condensable and noncondensable phases. Journal of the American Oil Chemists’ Society. 1962;39(3):159–162. doi: 10.1007/BF02632751. [DOI] [Google Scholar]
- Farmer E.H., Bloomfield G.F., Sundralingam A., Sutton D.A. The course and mechanism of autoxidation reactions in olefinic and polyolefinic substances, including rubber. Transactions of the Faraday Society. 1942;38:348–356. doi: 10.1039/TF9423800348. [DOI] [Google Scholar]
- Frankel E.N. Lipid oxidation. Progress in Lipid Research. 1980;19(1–2):1–22. doi: 10.1016/0163-7827(80)90006-5. [DOI] [PubMed] [Google Scholar]
- Ibusuki D., Nakagawa K., Asai A., Oikawa S., Masuda Y., Suzuki T., Miyazawa T. Preparation of pure lipid hydroperoxides. Journal of Lipid Research. 2008;49(12):2668–2677. doi: 10.1194/jlr.D800034-JLR200. [DOI] [PubMed] [Google Scholar]
- Ito J., Mizuochi S., Nakagawa K., Kato S., Miyazawa T. Tandem mass spectrometry analysis of linoleic and arachidonic acid hydroperoxides via promotion of alkali metal adduct formation. Analytical Chemistry. 2015;87(9):4980–4987. doi: 10.1021/acs.analchem.5b00851. [DOI] [PubMed] [Google Scholar]
- Jewell N.E., Nawar W.W. Thermal oxidation of phospholipids 1,2-dipalmitoyl-sn-glycerol-3-phosphoethanolamine. Journal of the American Oil Chemists’ Society. 1980;57(12):398–402. doi: 10.1007/BF02678923. [DOI] [Google Scholar]
- Kato S., Nakagawa K., Suzuki Y., Asai A., Nagao M., Nagashima K., Oikawa S., Miyazawa T. Liquid chromatography–tandem mass spectrometry determination of human plasma 1-palmitoyl-2-hydroperoxyoctadecadienoyl-phosphatidylcholine isomers via promotion of sodium adduct formation. Analytical Biochemistry. 2015;471:51–60. doi: 10.1016/j.ab.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Kato S., Nakagawa K., Suzuki Y., Suzuki K., Mizuochi S., Miyazawa T. Preparation of 13 or 9-hydroperoxy-9Z,11E (9E,11E) or 10E,12Z (10E,12E)-octadecadienoic phosphatidylcholine hydroperoxide. Journal of Oleo Science. 2014;63(5):431–437. doi: 10.5650/jos.ess13225. [DOI] [PubMed] [Google Scholar]
- Kato S., Shimizu N., Hanzawa Y., Otoki Y., Ito J., Kimura F., Takekoshi S., Sakaino M., Sano T., Eitsuka T., Miyazawa T., Nakagawa K. Determination of triacylglycerol oxidation mechanisms in canola oil using liquid chromatography–tandem mass spectrometry. npj Science of Food. 2018;2:article 1. doi: 10.1038/s41538-017-0009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Shimizu N., Ogura Y., Otoki Y., Ito J., Sakaino M., Sano T., Kuwahara S., Takekoshi S., Imagi J., Nakagawa K. Structural analysis of lipid hydroperoxides using mass spectrometry with alkali metals. Journal of the American Society for Mass Spectrometry. 2021;32(9):2399–2409. doi: 10.1021/jasms.1c00039. [DOI] [PubMed] [Google Scholar]
- Kato S., Shimizu N., Otoki Y., Ito J., Sakaino M., Sano T., Takeuchi S., Imagi J., Nakagawa K. Determination of acrolein generation pathways from linoleic acid and linolenic acid: Increment by photo irradiation. npj Science of Food. 2022;6:article 21. doi: 10.1038/s41538-022-00138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada T., Yamazaki M. The study of lauric hard butter. IV. Journal of Japan Oil Chemists’ Society. 1972;21(1):9–12. doi: 10.5650/jos1956.21.9. [DOI] [Google Scholar]
- Mallia S., Escher F., Schlichtherle-Cerny H. Aroma-active compounds of butter: a review. European Food Research and Technology. 2008;226(3):315–325. doi: 10.1007/s00217-006-0555-y. [DOI] [Google Scholar]
- Miyazaki R., Kato S., Otoki Y., Rahmania H., Sakaino M., Takeuchi S., Sato T., Imagi J., Nakagawa K. Elucidation of decomposition pathways of linoleic acid hydroperoxide isomers by GC-MS and LC-MS/MS. Bioscience, Biotechnology, and Biochemistry. 2023;87(2):179–190. doi: 10.1093/bbb/zbac189. [DOI] [PubMed] [Google Scholar]
- Obi J., Yoshinaga K., Tago A., Nagai T., Yoshida A., Beppu F., Gotoh N. Simple quantification of lactones in milk fat by solvent extraction using gas chromatography–mass spectrometry. Journal of Oleo Science. 2018;67(8):941–948. doi: 10.5650/jos.ess18039. [DOI] [PubMed] [Google Scholar]
- Porter N.A., Caldwell S.E., Mills K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30(4):277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- Ramanathan V., Sakuragi T., Kummerow F.A. Thermal oxidation of methyl esters of fatty acids. Journal of the American Oil Chemists’ Society. 1959;36(6):244–248. doi: 10.1007/BF02640068. [DOI] [Google Scholar]
- Schlutt B., Moran N., Schieberle P., Hofmann T. Sensory-directed identification of creaminess-enhancing volatiles and semivolatiles in full-fat cream. Journal of Agricultural and Food Chemistry. 2007;55(23):9634–9645. doi: 10.1021/jf0721545. [DOI] [PubMed] [Google Scholar]
- Selke E., Rohwedder W.K., Dutton H.J. Volatile components from tristearin heated in air. Journal of the American Oil Chemists’ Society. 1975;52(7):232–235. doi: 10.1007/BF02639148. [DOI] [Google Scholar]
- Selke E., Rohwedder W.K., Dutton H.J. Volatile components from triolein heated in air. Journal of the American Oil Chemists’ Society. 1977;54(2):62–67. doi: 10.1007/BF02912391. [DOI] [Google Scholar]
- Stirton A.J., Turer J., Riemenschneider R.W. Oxygen absorption of methyl esters of fat acids, and the effect of antioxidants. Oil Amp: Soap. 1945;22(4):81–83. doi: 10.1007/BF02635538. [DOI] [Google Scholar]
- Watanabe K., Sato Y. Conversion of some saturated fatty acids, aldehydes, and alcohols into γ- and δ-lactones. Agricultural and Biological Chemistry. 1970;34(3):464–472. doi: 10.1271/bbb1961.34.464. [DOI] [Google Scholar]
- Watanabe K., Sato Y. Lactones produced through thermal oxidation of higher fatty acids. Agricultural and Biological Chemistry. 1971;35(2):278–281. doi: 10.1271/bbb1961.35.278. [DOI] [Google Scholar]
- Wolff S.P. Vol. 233. Academic Press; 1994. [18] Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides; pp. 182–189. (Methods in Enzymology). [DOI] [Google Scholar]
- Yin H., Xu L., Porter N.A. Free radical lipid peroxidation: mechanisms and analysis. Chemical Reviews. 2011;111(10):5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K., Obi J., Tago A., Kato Y., Nagai T., Yoshida A., Gotoh N. Analysis of hydroxy triacylglycerol as a lactone precursor in milk fat using liquid chromatography electrospray ionization tandem mass spectrometry. Food Chemistry. 2019;274:298–304. doi: 10.1016/j.foodchem.2018.08.101. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K., Tago A., Yoshinaga-Kiriake A., Gotoh N. Characterization of lactones in Wagyu (Japanese beef) and imported beef by combining solvent extraction and gas chromatography–mass spectrometry. LWT. 2021;135 doi: 10.1016/j.lwt.2020.110015. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.