Highlights
-
•
Disruption of vaccination coverage as a result of the COVID-19 pandemic.
-
•
Increased the number of laboratory-confirmed measles cases in 2022.
-
•
Laboratory-confirmed measles cases were significantly high in the unvaccinated population.
-
•
The measles-containing vaccine coverage of 88% was observed in 2022.
Keywords: Measles, Outbreaks, Tanzania
Abstract
Objectives
Tanzania observed a gradual increase in the number of measles cases since 2019 with a large outbreak recorded during 2022. This study describes the trend of measles in Tanzania over a 5-year period from 2018-2022.
Methods
This was a descriptive study conducted using routine measles case-based surveillance system including 195 councils of the United Republic of Tanzania.
Results
Between 2018 and 2022 there were 12,253 measles cases reported. Out of 10,691 (87.25%) samples tested by enzyme-linked immunosorbent assay, 903 (8.4%) were measles immunoglobulin M positive. The highest number of laboratory-confirmed measles cases was in 2022 (64.8%), followed by 2020 (13.8%), and 2019 (13.5%). Out of 1279 unvaccinated cases, 213 (16.7%) were laboratory-confirmed measles cases compared to 77/723 (10.6%) who were partially vaccinated and 71/1121 (6.3%) who were fully vaccinated (P < 0.001). Children aged between 1-4 years constituted the most confirmed measles cases after laboratory testing, followed by those aged 5-9 years. There was a notable increase in the number of laboratory-confirmed measles cases in children <1 year and 10-14 years during 2022 compared to previous years. The vaccination coverage of the first dose of measles-containing vaccine (MCV1) was maintained >90% since 2013 while MCV2 increased gradually reaching 88% in 2022.
Conclusions
Accumulation of susceptible children to measles due to suboptimal measles vaccination coverage over the years has resulted in an increase in the number of laboratory-confirmed measles cases in Tanzania with more cases recorded during the COVID-19 pandemic. Strengthening surveillance, routine immunization, and targeted strategies are key to achieving the immunity levels required to interrupt measles outbreaks.
Introduction
Vaccines provide a safe and cost-effective solution to vaccine-preventable infectious diseases. However, vaccine-preventable infectious diseases still pose a serious public health threat, especially in the world's poor regions [1]. In sub-Saharan Africa, this burden is further aggravated by the occurrence of concurrent epidemics such as COVID-19, Ebola virus disease, monkeypox, and measles overstretching the already weak public health system [2]. In addition to human conflicts, natural disasters, and vaccine hesitancy, the COVID-19 pandemic has disrupted routine immunization services leaving millions of children under-vaccinated or unvaccinated against vaccine-preventable diseases as evidenced by the decline in the number of administered doses of diphtheria-pertussis-tetanus-containing vaccine and the first dose of measles virus-containing vaccine (MCV) [3,4]. This large increase in unvaccinated populations especially in lower and middle-income countries is a huge setback to the global goal of elimination and eradication of vaccine-preventable diseases (VPDs), such as poliomyelitis and measles [5].
Of the VPDs, measles is the most contagious viral disease, with the highest reproduction number. The disease is preventable through the administration of two doses of MCV [5]. Vaccination coverage of at least 95% of the target population with two doses of MCV is required to achieve herd immunity against measles [6]. This is achieved through routine immunization and implementation of supplemental immunization activities (SIAs) at regular intervals determined by the epidemic cycle of measles. SIAs provide an opportunity for the first dose of measles to children previously unvaccinated while strengthening the immunity of those previously vaccinated with the first dose of measles [7].
Tanzania has been implementing measles SIAs as part of elimination strategies since 1999. During 1999 and 2000, the country conducted subnational catch-up campaigns targeting children 5-9 years in high-risk districts (37 districts in 1999, 54 high-risk districts in 2000).
Subnational catch-up measles SIAs were also conducted in 2001 and 2002 covering children ages 9 months-14 years in 34 high-risk districts, 7-14 years in 88 high-risk districts, and 9 months-14 years in five high-risk districts. In 2005, a country-wide measles follow-up campaign for all children 9 months-5 years was conducted. Subsequently, national measles SIAs, both catch-up and follow campaigns in different age groups have been routinely conducted every 3 years up to 2014. The last SIA was conducted in October 2019 covering all children 9 months-5 years [8].
In addition to achieving herd immunity, strengthening measles surveillance is critical to monitoring and evaluating emerging patterns and trends of the disease [9]. Nevertheless, measles vaccination is hindered by vaccine hesitancy, pandemic occurrences, weak vaccine supply chain, misinformation, immigration caused by prolonged humanitarian crises, inadequate human resources, and host factors [10], [11], [12]. These factors have hindered the realization of the 2011 World Health Organization (WHO) African Region's (AFRO) resolution for measles elimination by 2020 [6]. Protracted and widespread measles outbreaks have been reported in countries neighboring Tanzania, particularly Burundi and the Democratic Republic of the Congo (DRC) [13,14].
The Expanded Program on Immunizations (EPI) in Tanzania was established in 1975 by the introduction of polio and measles vaccines and tetanus toxoid vaccine. Tanzania introduced a second dose of measles into routine immunization and switched to the use of the measles-rubella (MR) vaccine in 2014. In 2015, EPI was transformed into the Immunization and Vaccine Development (IVD) Programme to strengthen routine immunization services. At the moment, a total of 13 antigens are included in the routine immunization program for the prevention of measles, rubella/congenital rubella syndrome, poliomyelitis, cervical cancer, pneumonia, tetanus, diphtheria, pertussis, hepatitis B, tuberculosis, rotavirus diarrhea, and meningitis. The United Republic of Tanzania established case-based measles surveillance (MSS) in 2006 using the infrastructure available for polio surveillance and in accordance with the WHO AFRO guidelines [15]. By 2019, the measles vaccination coverage had reached more than 90%. However, the 2021 WHO/United Nations International Children's Emergency Fund Estimates of National Immunization Coverage (WUENIC) reported the first and second measles virus-containing vaccination coverage of 76% and 62%, respectively, with an estimated million susceptible children. The decreased measles vaccination coverage indicates an increase in the number of partially vaccinated and unvaccinated children population putting Tanzania at risk of measles outbreaks. This study was designed to describe the trend of measles in the country by reviewing case-based surveillance data over a 5-year period from 2018-2022; the period just before THE COVID-19 pandemic (2018 and 2019), and during the pandemic (2020, 2021, and 2022).
Methods
Study area, design, duration, and population
This is a descriptive study conducted using routine MSS including 195 councils of the United Republic of Tanzania between 2018 and 2022. Surveillance was conducted in accordance with the WHO Measles Outbreak Guide (2022) case definitions and case classifications.
Data source
Data were obtained from MSS of the United Republic of Tanzania from 2018-2022. Case investigation forms were completed, and blood specimens were collected and processed to obtain sera that were transported in reverse cold chain to the National Public Health Laboratory (ISO15189:2012 Accredited) in Dar es Salaam for subsequent measles confirmatory testing. Measles-specific immunoglobulin M (IgM) antibodies were detected using enzyme-linked immunosorbent assay (ELISA) (EuroimmunⓇ, Lübeck, Germany, Catalog No. EL2610-9601-4M), according to the manufacturer's instructions. The 2018-2022 MR vaccination coverage was obtained from the Tanzania Vaccine Information Management System and from the WUENIC (https://immunizationdata.who.int/pages/profiles/tza.html).
Data analysis
Data were obtained from case investigation forms which captured information including location, demographic data, clinical symptoms and history, and vaccination status. Laboratory testing results were entered into the corresponding case investigation forms and a final case classification was determined in accordance with the WHO Measles Outbreak Guide (2022) case definitions and case classifications [16]. All data were entered into the MSS database at the IVD Programme of the Ministry of Health using Microsoft Access (Microsoft Corporation, Washington, USA). Demographic, clinical, and case classification data were summarized using proportions over time, location, age, and target population. Outbreak trends were summarized by plotting measles cases over time (per month and annually). The non-MR febrile rash illness (FRI) rates were determined at a target of two cases per 100,000 population while the incidence of measles cases at a target of one case per 1,000,000 population. Maps showing confirmed measles cases by reporting councils per year were developed using ArcGIS Version 10.5.1 (Esri, Redlands, CA).
Ethical considerations
This surveillance was undertaken as part of the routine MSS of the Tanzania Ministry of Health. As private health information was solicited by clinicians, the risk of a breach of confidentiality of private health information to non-study personnel or appropriate public health authorities was minimized by storing clinical investigation forms in accordance with standard clinical practice. The electronic MSS database was decoded and contained only the unique identifiers and aggregated data without the personal identifiers. Unique identifiers included three-letter codes for country, region, and district, plus two last digits for the year of onset and a case serial number beginning with 0001, for example, TAN-DSM-ILA-22-0039 for Tanzania, Dar es Salaam region, Ilala district, year 2022 and subject serial number 39. Permission to publish surveillance data was sought from the joint Catholic University of Health and Allied Sciences/Bugando Medical Center Research Ethics and Review Committee with reference number: Ref. No. BU/36/DRI/009/Vol I.
Results
The trend of measles cases
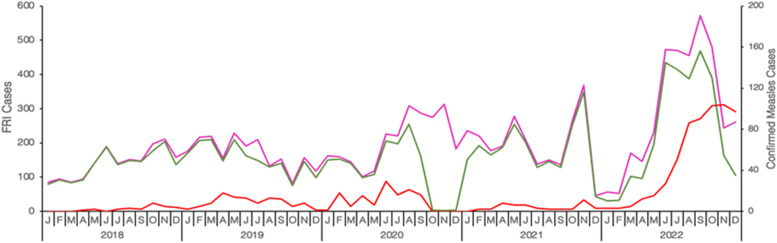
A total of 12,253 subjects with FRI were reported between January 2018 and December 2022, of which 2340 (19.0%) cases were laboratory confirmed, epidemiologically linked, and clinically compatible. There was slight male predominance among the reported cases (51.6%). The highest number of reported cases were aged 1-4 years (68.4%) followed by 5-9 years (18.2%). Of 12,253 samples from reported cases with FRI; 10,691 (87.25%) samples were tested by ELISA for confirmation of measles, and 903 (8.4%) were confirmed to be measles IgM positive. The highest number of laboratory-confirmed measles cases was recorded in 2022 (64.8%), followed by 2020 (13.8%), and 2019 (13.5%). Although the number of laboratory-confirmed measles cases fluctuated, a consistent increase was notable from 2018 through 2020 with a decline during 2021. A surge of confirmed cases was observed starting from April 2022 (Figure 1). A measles outbreak in Tanzania was confirmed in July 2022, as per the WHO Measles Outbreak Guide (2022). Transmission of measles was observed throughout the year with peaks observed biannually in April and July except for 2022 when the peak extended from April through December.
Figure 1.
Trends of reported fever and rash illness (FRI) cases and confirmed measles cases from 2018-2022. The number of reported FRI cases (measles suspected cases) is indicated in pink, the actual number of FRI cases that were tested for measles confirmation in the laboratory is indicated in green, and the number of confirmed measles cases is indicated in red. The number of reported and tested FRI cases is plotted in the left ordinate (y-axis) while the number of confirmed measles cases is plotted in the right ordinate (y-axis).
Recent measles outbreak
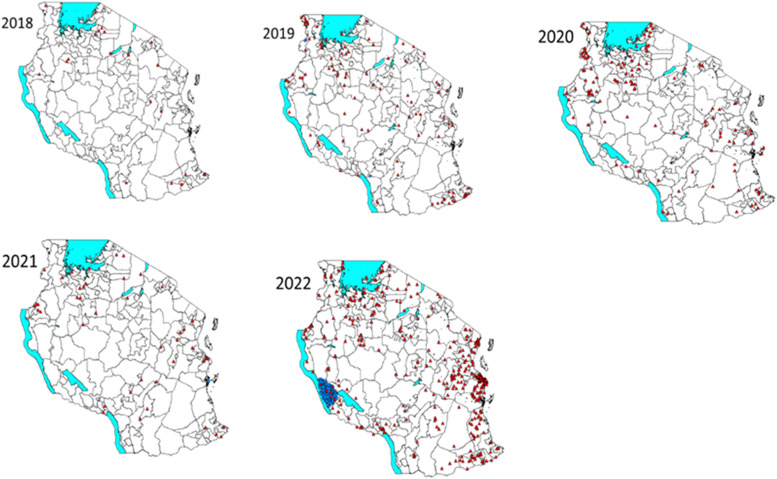
From April 2022, Tanzania observed a surge of confirmed cases of measles and confirmed the first outbreak in July 2022. Cumulatively, 3861 suspected measles cases were tested, resulting in 596 laboratory-confirmed measles cases with a positivity rate of 15.4%. The most affected age group were children <5 years and 7-15 years age groups. The outbreak was first confirmed in Dar es Salaam and then spread to involve Tanga and Pwani, which are all along the coast (Figure 2). The outbreak involved adjacent districts in some regions. In Zanzibar, the measles outbreak was also confirmed in two districts in Unguja and three districts in Pemba with 20 laboratory-confirmed cases. Majority of cases, 65% were children <5 years of age. Only 3 of 20 (15%) laboratory-confirmed cases were vaccinated.
Figure 2.
Geographical distribution of laboratory-confirmed and epidemiologically-linked cases, January 2018-December 2022. The laboratory-confirmed measles cases are indicated by red dots; the epidemiologically linked cases are indicated by blue dots. Boundaries between councils are indicated by grey lines.
Incidence rate of measles cases
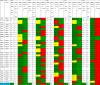
Measles incidence was reported per 1,000,000 population (1:1,000,000) of measles-confirmed cases including laboratory confirmed, epidemiologically linked, and clinically compatible. The target indicator was <1 case per 1,000,000 population. During the 5-year period, the number of regions with measles incidence >1 varied with the highest number recorded in 2022. A total of 6, 16, 18, 7, and 27 regions had an incidence rate of measles cases per million population >1 in 2018, 2019, 2020, 2021, and 2022, respectively. Overall, the incidence rate for measles per 1,000,000 population for the 5 years was 0.5. The incidence rate was low between 2018 and 2021 and increased to 10.1 per million population in 2022 (Table 1). Data indicates the evidence of sporadic measles cases annually with western, northern, and southern parts of the country recording a significant number of cases except during 2022 when a high number of cases were recorded in the coastal regions.
Table 1.
Measles incidence rates in different regions and National level during 2018-2022.
 |
Age and vaccination status of confirmed measles cases
Tanzania provides routine immunization for measles using a combined MR vaccine to infants at the age of 9 (MR1) and 18 months (MR2). Nevertheless, vaccination is given to children who miss the recommended MR1 and MR2 schedules during encounters or periodically through catch vaccinations. The number of eligible infant populations for MR1 and MR2 vaccination from 2018-2022 is shown in Table 1. The MR1 vaccination administrative coverage at the national level for the 5 years was >90% while that of MR2 ranged between 78-98% (Table 2). Catch-up vaccinations and periodic intensification of routine immunization led to a significant increase in coverage during 2022. Although immunization performance regained pre-COVID-19 pandemic performance levels in 2022, the large immunity gap caused by the pandemic led to persistent measles transmission.
Table 2.
National administrative Coverage of first (MR1) at 9 months of age and second measles-rubella vaccine (MR2) at 18 months of age in Tanzania, 2018-2022.
| Year | Population <1 Year Current Year (MR1 Target) | Population <1 Year Last Year (MR2 Target) | MR1 |
MR2 |
||
|---|---|---|---|---|---|---|
| Vaccinated | % Coverage | Vaccinated | % Coverage | |||
| 2018 | 2,013,744 | 1,929,690 | 2,114,133 | 100 | 1,612,087 | 84 |
| 2019 | 2,061,343 | 2,013,744 | 2,104,972 | 102 | 1,780,440 | 88 |
| 2020 | 2,116,379 | 2,061,343 | 2,119,442 | 100 | 1,703,809 | 83 |
| 2021 | 2,176,804 | 2,116,379 | 1,999,571 | 91 | 1,649,797 | 78 |
| 2022 | 2,213,094 | 2,176,804 | 2,423,315 | 110 | 2,134,651 | 98 |
The WUENIC data indicates vaccination coverages below target for both MR1 and MR2 with the lowest values (77% for MR1 while 62% for MR2) recorded during 2021, the peak year for the COVID-19 pandemic with intense vaccination response and surveillance. The post-campaign coverage survey for the October 2019 campaign was conducted shortly after the campaign. At the national level, coverage of MR was at 88.2%, with MR coverage varying across regions from 69.0% in Katavi to 100% in Kusini Unguja.
Of the 903 confirmed measles cases, 378 (41.9%) were vaccinated with at least one dose, 368 (40.7%) were unvaccinated, and the vaccination status of 157 (17.4%) individuals was unreported. The available data that segregated confirmed cases by the vaccination status of the cases with laboratory results showed that out of 1279 unvaccinated cases, 213 (16.7%) were laboratory confirmed compared to 77/723 (10.6%) who were partially vaccinated and 71/1121 (6.3%) who were fully vaccinated (P < 0.001).
Demographic characteristics of suspected and confirmed measles cases
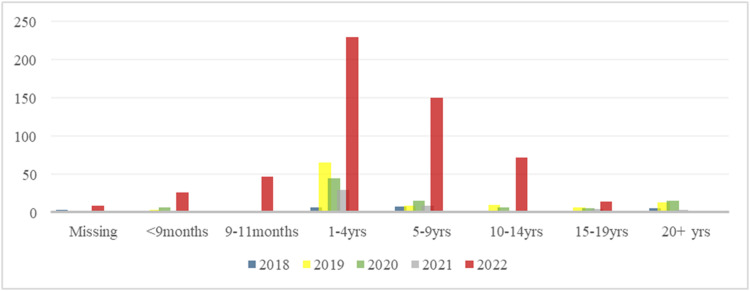
Children aged 1-4 years constituted most of the confirmed measles cases after laboratory testing, followed by those aged 5-9 years. There was a notable increase in the number of laboratory-confirmed cases in children <1 year and 10-14 years during 2022 compared to previous years (Figure 3).
Figure 3.
Age group distribution of laboratory-confirmed measles cases in 2018-2022.
Discussion
This study documents the first large measles outbreak in the year 2022 since the establishment of the National Measles Surveillance System in the United Republic of Tanzania. The cases were more in the regions along the coast of the Indian Ocean and the laboratory-confirmed cases were significantly more among unvaccinated children. Measles is a vaccine-preventable disease requiring herd immunity reached by having a coverage of >95% with two doses of MCV at all levels, that is, facility, council, regional, and national levels [17]. The possible reasons for the outbreaks could be an accumulation of susceptible individuals who missed routine vaccination, hence increasing the number of zero-dose and partially vaccinated children. Another possible explanation could be the existence of pockets of low vaccination coverage in some population groups due to lack of access to health services, for example, migrants from DRC in the Nkasi district and the displaced population in the Kaliua district [18,19].
Although immunization performance regained pre-COVID-19 pandemic performance levels in 2022, the large immunity gap caused by the pandemic led to increased measles transmission. During 2022 the country faced sporadic measles outbreaks in all 26 regions in Tanzania mainland and two regions from Zanzibar Island. The 2022 measles outbreak was likely due to the accumulation of susceptible children who were not vaccinated. Among the factors contributing to low vaccination rates was the COVID-19 pandemic that negatively impacted immunization activities as a result of the cancellation of mobile and outreach services, hence suboptimal surveillance performance. Also, due to the COVID-19 pandemic, there were cancellations of flights which led to the interruption of vaccine supply. Similar observations have been reported in other countries, whereby the COVID-19 pandemic has been linked with low vaccination coverage [3,20]. In addition, the lack of strong provider recommendations to vaccinate during the patient encounter might lead to missed opportunities for those presenting beyond the age for scheduled vaccination in Tanzania, hence the increasing number of susceptible children in the community. Despite the efforts made by the Tanzanian government through the Ministry of Health, more targeted monitoring of vaccination performance at all levels is highly recommended in Tanzania to prevent future outbreaks. Large measles outbreaks were also reported previously in western Uganda and DRC despite the existence of a well-established surveillance system and vaccination program among children <5 years of age [18,21].
In the present study, it was observed that the outbreak occurred mostly in coastal regions compared to other regions and the peak of cases occurred around July and August 2022 when the outbreak was declared by the Ministry of Health. A possible explanation could be high population density and movements in these regions that might be linked to increased susceptible individuals in the community that resulted in the outbreak. The first evidence of a laboratory-confirmed measles outbreak was in Dar es Salaam region. It should be noted that Dar es Salaam is one of the regions with a high population density and a high influx of people from all regions. The region is the main port of entry connecting all regions through the port and airport [22]. Increased movements in densely populated areas have been linked to the geographical spread of the virus elsewhere [23], [24], [25]. Regarding the high number of cases in July and August 2022, the possible explanation could be due to the fact that these months in Tanzania are considered the dry season in a big geographical area which has been associated with the accelerated spread of the virus elsewhere [26,27]. Further studies to establish seasonality are warranted in Tanzania.
In this study, it was observed that a high proportion of unvaccinated and partially vaccinated children (single dose) were laboratory confirmed to have measles. Similarly, a hospital-based study documented severe morbidity due to measles in unvaccinated individuals in Tanzania [28]. This can be explained by the fact that low vaccination coverage usually results in the accumulation of susceptible populations over time which can lead to outbreaks. This is indicative that a single dose of MCV is not enough to confer long-lasting immunity, hence, more emphasis on completing the two scheduled doses is of great importance to prevent future outbreaks. Insufficient immunity after a single dose has been linked to the presence of maternal antibodies that dampen the vaccine response [29]. There is a paramount need to emphasize the timely provision of two doses of MR to all children across the country to prevent future measles outbreaks, as well as rubella, which is also endemic in Tanzania.
This study observed that the most affected group were children aged between 9 and 59 months and case fatality rate (CFR) was found to be 1.0% which is comparable to previous studies in Congo-DRC and Philadelphia that reported CFR of 1.4% and 1.2%, respectively [21,30]. CFR reported in the present study is lower than that reported in a previous study in Niger [31]. A possible explanation could be the underreporting of measles-related deaths in the community as most of the deaths were recorded from the healthcare facilities.
Limitation
We are not sure that all cases who attended the health facilities were registered in the surveillance system and perhaps, due to low accessibility to health services, some cases did not attend health facilities.
Conclusion and recommendations
Measles is mainly a disease of childhood in Tanzania affecting mostly the unvaccinated, however, other age groups remain susceptible. The trend of MCV2 coverage between 2018-2021 was <95%, the coverage that is required to interrupt measles transmission. Lowest coverages during the COVID-19 pandemic due to interrupted routine immunization services and vaccine supply resulted in increased cases of laboratory-confirmed cases in 2022, the largest outbreak since the introduction of MCV in Tanzania. Targeted strategies are required to attain ≥95% coverage for both MCV1 and MCV2 to be able to interrupt measles transmission through enhancing vaccine uptake by reaching every child, monitoring vaccine quality, and storage to ensure efficacy and surveillance system to ensure timely detection and reporting.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
Funding
This work was funded as part of the routine measles-rubella surveillance supported by the Ministry of Health and the World Health Organization.
Acknowledgments
This work was made possible due to the close cooperation of mother and guardians of children in the country, and also the support of stakeholders from the national to vaccination center levels. All are highly acknowledged. We also thank the technical staff at the National laboratory who analyzed the samples.
This work was made possible due to close collaboration between surveillance focal persons at the Health facilities, District, Regional, the community of care givers and the support of stakeholders from national to vaccination center levels. The authors are grateful to Ministry of Health, Tanzania, and the WHO for supporting this surveillance.
Author contributions
FM, MMM, GM, OM, and SEM conceived the idea, FM, RM, HN, VM, FK, DM, BN, CN, BK, WM, AM, FT, AM, NM, BO, DM, EK, and DK participated in specimen/data collection. BK, KT, MK, and MB did laboratory analysis of the samples. FM, MMM, DM, and SEM did data analysis; FM, MM, GM, OM, MB, DM, FSK ENN, MMS, DN, RK, AI, AB, FH, EK, JJC, PK, FK, FT, DM, AS, BM, JM, RM, MM, HN, BK, KT, MK, WM, and SEM did data interpretation. FM, DM, BM, JM, and DM wrote the first draft of the manuscript; GM, RK, OM, FSK, MMM, and SEM did a critical review of the manuscript. All authors approved the final version of the manuscript.
Institutional review board statement
Permission to publish surveillance data was sought from the joint Catholic University of Health and Allied Sciences, Bugando Medical Center Research Ethics and Review Committee.
Data availability statement
All information has been included in the manuscript.
Informed consent statement
Not applicable, the data used in this report was part of the routine national measles-rubella case-based surveillance.
References
- 1.Ngwa CH, Doungtsop B-CK, Bihnwi R, Ngo NV, Yang NM. Burden of vaccine-preventable diseases, trends in vaccine coverage and current challenges in the implementation of the expanded program on immunization: a situation analysis of Cameroon. Hum Vaccin Immunother. 2022;18 doi: 10.1080/21645515.2021.1939620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shet A, Carr K, Danovaro-Holliday MC, Sodha SV, Prosperi C, Wunderlich J, et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: evidence of disruption and recovery from 170 countries and territories. Lancet Glob Health. 2022;10:e186–e194. doi: 10.1016/S2214-109X(21)00512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Causey K, Fullman N, Sorensen RJD, Galles NC, Zheng P, Aravkin A, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modelling study. Lancet. 2021;398:522–534. doi: 10.1016/S2214-109X(21)00512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guglielmi G. Pandemic drives largest drop in childhood vaccinations in 30 years. Nature. 2022;608:253. doi: 10.1038/d41586-022-02051-w. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesan P. Worrying global decline in measles immunisation. Lancet Microbe. 2022;3:e9. doi: 10.1016/S2666-5247(21)00335-9. [DOI] [PubMed] [Google Scholar]
- 6.Dixon MG, Ferrari M, Antoni S, Li X, Portnoy A, Lambert B, et al. Progress toward regional measles elimination—worldwide, 2000–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1563–1569. doi: 10.15585/mmwr.mm7045a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masresha B, Luce R, Shibeshi M, Katsande R, Fall A, Okeibunor J, et al. Status of measles elimination in eleven countries with high routine immunisation coverage in the WHO African region. J Immunol Sci. 2018;(Suppl):140–144. doi: 10.29245/2578-3009/2018/si.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed N, Simba D, Mphuru A, Lyimo D, Kyesi F. Lessons learned in the implementation of supplementary immunization activity (SIA) field guidelines for injectable vaccines – Experiences from Tanzania. Vaccine. 2020;38:7741–7746. doi: 10.1016/j.vaccine.2020.10.050. [DOI] [PubMed] [Google Scholar]
- 9.Minta AA, Ferrari M, Antoni S, Portnoy A, Sbarra A, Lambert B, et al. Progress toward regional measles elimination—worldwide, 2000–2021. MMWR Morb Mortal Wkly Rep. 2022;71:1489–1495. doi: 10.15585/mmwr.mm7147a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colinet G, Rossignol J, Peetermans J. A study of the stability of a bivalent measles-mumps vaccine. J Biol Stand. 1982;10:341–346. doi: 10.1016/S0092-1157(82)80011-5. [DOI] [PubMed] [Google Scholar]
- 11.Galazka A, Milstein J, Zaffran M. Thermostability of vaccine, WHO document WHO/GPV/98.07. Geneva: World Health Organization, 1998.
- 12.Mulholland K. Measles and pertussis in developing countries with good vaccine coverage. Lancet. 1995;345:305–307. doi: 10.1016/s0140-6736(95)90282-1. [DOI] [PubMed] [Google Scholar]
- 13.Sodjinou VD, Mengouo MN, Douba A, Tanifum P, Yapi MD, Kuzanwa KR, et al. Epidemiological characteristics of a protracted and widespread measles outbreak in the Democratic Republic of Congo, 2018 –2020. Pan Afr Med J. 2022;42:282. doi: 10.11604/pamj.2022.42.282.34410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagcchi S. COVID-19 and measles: double trouble for Burundi. Lancet Microbe. 2020;1:e65. doi: 10.1016/S2666-5247(20)30040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masresha BG, Dixon MG, Kriss JL, Katsande R, Shibeshi ME, Luce R, et al. Progress toward measles elimination—African Region, 2013–2016. MMWR Morb Mortal Wkly Rep. 2017;66:436–443. doi: 10.15585/mmwr.mm6617a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization . African Regional guidelines for measles and rubella surveillance-Draft version April 2015. World Health Organization; Geneva: 2015. African regional guidelines for measles and rubella surveillance. [Google Scholar]
- 17.Nic Lochlainn LMN, de Gier B, van der Maas N, van Binnendijk R, Strebel PM, Goodman T, et al. Effect of measles vaccination in infants younger than 9 months on the immune response to subsequent measles vaccine doses: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19:1246–1254. doi: 10.1016/S1473-3099(19)30396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walekhwa AW, Ntaro M, Kawungezi PC, Achangwa C, Muhindo R, Baguma E, et al. Measles outbreak in Western Uganda: a case-control study. BMC Infect Dis. 2021;21:596. doi: 10.1186/s12879-021-06213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouadio IK, Kamigaki T, Oshitani H. Measles outbreaks in displaced populations: a review of transmission, morbidity and mortality associated factors. BMC Int Health Hum Rights. 2010;10:5. doi: 10.1186/1472-698X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma M, Singh SK, Sharma L, Dwiwedi MK, Agarwal D, Gupta GK, et al. Magnitude and causes of routine immunization disruptions during COVID-19 pandemic in developing countries. J Family Med Prim Care. 2021;10:3991–3997. doi: 10.4103/jfmpc.jfmpc_1102_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grout L, Minetti A, Hurtado N, François G, Fermon F, Chatelain A, et al. Measles in Democratic Republic of Congo: an outbreak description from Katanga, 2010–2011. BMC Infect Dis. 2013;13:232. doi: 10.1186/1471-2334-13-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanzania Ministry of Health, Community Development, Gender, Elderly and Children . Dar es Salaam: National Bureau of Statistics (NBS); 2016. Tanzania demographic and health survey and malaria: indicator survey (TDHS-MIS) [Google Scholar]
- 23.Qin S, Ding Y, Yan R, He H., Measles in Zhejiang China, 2004–2017: population density and proportion of floating populations effects on measles epidemic. Health Secur. 2019;17:193–199. doi: 10.1089/hs.2019.0011. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikura H. Relation between measles incidence and population size under the advanced vaccine program. Jpn J Infect Dis. 2012;65:88–91. doi: 10.7883/yoken.65.88. [DOI] [PubMed] [Google Scholar]
- 25.Yaméogo KR, Perry RT, Yaméogo A, Kambiré C, Kondé MK, Nshimirimana D, et al. Migration as a risk factor for measles after a mass vaccination campaign, Burkina Faso, 2002. Int J Epidemiol. 2005;34:556–564. doi: 10.1093/ije/dyi001. [DOI] [PubMed] [Google Scholar]
- 26.Ferrari MJ, Grais RF, Bharti N, Conlan AJ, Bjørnstad ON, Wolfson LJ, et al. The dynamics of measles in sub-Saharan Africa. Nature. 2008;451:679–684. doi: 10.1038/nature06509. [DOI] [PubMed] [Google Scholar]
- 27.Musa TH, Kambo RI, Ahmed AA, Musa HH. Epidemiology of measles cases in south Darfur State, Sudan, 2011–2015. Biomed Environ Sci. 2017;30:917–921. doi: 10.3967/bes2017.123. [DOI] [PubMed] [Google Scholar]
- 28.Mnyika KS, Akim C. Epidemiology of measles in Tanzania: a hospital-based survey of measles morbidity and mortality. East Afr J Pub Health. 2005;2:24. doi: 10.4314/eajph.v2i2.38960. [DOI] [Google Scholar]
- 29.Metcalf CJE, Klepac P, Ferrari M, Grais RF, Djibo A, Grenfell BT. Modelling the first dose of measles vaccination: the role of maternal immunity, demographic factors, and delivery systems. Epidemiol Infect. 2011;139:265–274. doi: 10.1017/S0950268810001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodgers DV, Gindler JS, Atkinson WL, Markowitz LE. High attack rates and case fatality during a measles outbreak in groups with religious exemption to vaccination. Pediatr Infect Dis J. 1993;12:288–292. doi: 10.1097/00006454-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Nandy R, Handzel T, Zaneidou M, Biey J, Coddy RZ, Perry R, et al. Case-fatality rate during a measles outbreak in eastern Niger in 2003. Clin Infect Dis. 2006;42:322–328. doi: 10.1086/499240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All information has been included in the manuscript.