Abstract
A clinical case of a 19-year-old male patient with pharmacoresistant seizures occurring following parieto-occipital tumor-resection at age 6 is described. Seizure surgery work-up included prolonged video EEG monitoring and head CT without contrast. Seizure focus was localized to the left temporal lobe, and we felt that the patient was an excellent candidate for seizure surgery. The patient underwent a left frontotemporal craniotomy for removal of the seizure focus with intraoperative electrocorticography (ECoG) conducted pre and post resection. ECoG recordings pre- and post-resection confirmed resolution of seizure generation. Imaging obtained immediately postoperatively showed complete resection of the residual tumor with no evidence of recurrence in follow-ups. A year after the surgery the patient is seizure-free but remains on seizure medication. With the patient's consent the excised epileptogenic tissue was used for ex-vivo research studies. The microelectrode recordings confirmed epileptiform activity in the excised tissue incubated in excitatory artificial cerebrospinal fluid. The epileptiform activity in the epileptogenic tissue was suppressed by addition of KRM–II–81, a novel α2/3 subtype preferring GABAA receptor (GABAAR) potentiator with previously demonstrated antiepileptic efficacy in multiple animal models of epilepsy and with reduced potential for CNS side-effects compared to classical benzodiazepine GABAAR potentiators. These findings support the proposition that KRM–II–81 might reduce seizure burden in pharmacoresistant patients.
Keywords: Seizure, Pharmacoresistant, GABAA receptor, GABA-PAM, Subtype preferring
Abbreviations
- AED
antiepileptic drugs
- CSF
cerebrospinal fluid
- CT
computer tomography
- ECoG
electrocorticogram
- EEG
electroencephalogram
- FLAIR
fluid-attenuated inversion recovery
- GABAAR
GABAA receptor
- LFP
local field potential
- PAM
peripheral allosteric modulator
1. Introduction
Pharmacoresistant epilepsy occurs in 30 % of the epileptic patient population [[1], [2], [3]] where about 50 % of cases emerge before age five [4,5]. Lack of seizure control in these patients, typically taking multiple daily antiseizure medications, risks the health and well-being of this patient population. Seizures in some of these patients can be treated successfully by surgery to remove the seizure focus [6]. It is thus recognized that the discovery of improved antiseizure medications is critical to best-practice patient care [7].
At present, the cause(s) of pharmacoresistant epilepsy are not fully understood. However, a prevailing hypothesis is the development of tolerance to the anticonvulsant effects of antiseizure medications, a phenomenon that has been well described for some antiepileptic drugs (AEDs) [8]. A new GABAA receptor (GABAAR) potentiator (GABAkine), KRM–II–81, is being developed as an antiepileptic agent due to its reduced liability for sedation and abuse and its efficacy in multiple animal models of pharmacoresistant epilepsy [9]. Importantly, KRM–II–81, has been shown to be resistant to tolerance development [10,9] which limits the chronic use of classic benzodiazepines such as diazepam. The present case report describes the successful resection of a left temporal lobe seizure focus in a 19-year-old male patient that had exhibited progressively worsening pharmacoresistant epilepsy from age 6. Freshly resected cortical tissue from this patient was used to demonstrate the suppression of epileptic bursting by KRM–II–81. The present case report provides a one-year seizure-free follow-up on post resection of the left temporal seizure focus. Electrophysiological data from the epileptic tissue support the idea that KRM–II–81 might reduce seizure burden in pharmacoresistant patients.
2. Case presentation
The patient presented in 2009 (age 6 years) with a large left parieto-occipital tumor, obstructive hydrocephalus, and seizure disorder. Initial MRI study showed a massive heterogeneous cystic and solid mass centered within the atria of the lateral ventricle. There was a cystic extension into the pineal region and into the region of the quadrigeminal cisterns as well as a prominence of the left lateral ventricle without transependymal cerebrospinal fluid flow. The abnormal parenchyma was extending in the left medial temporal lobe and the ependyma of the left lateral ventricle possibly suggesting an invasive etiology. The preoperative EEG was normal and the electrocorticography (ECoG) was not needed. The patient underwent a near total resection of the tumor and ventriculoperitoneal shunt placement, which was performed successfully, and the patient recovered completely. A small section of tumor however extended into the medial aspect of the temporal lobe and was not possible to resect with our surgical approach. In addition, the histological evaluation of resected tumor revealed juvenile pilocytic astrocytoma with unusually elevated proliferative activity as well as scattered mitotic activity. For these reasons the patient received post-operative chemotherapy and was monitored with serial MRI studies. The images showed a non-enhancing hyperintense FLAIR mass in the left medial temporal lobe which remained stable in size. The patient, however, began having seizures one year after his surgery. Initially, good seizure control was achieved with Keppra and carbamazepine, but seizures worsened subsequently and became refractory to medications. Diazepam, a GABAAR potentiator, was prescribed as needed for prolonged seizures. The patient also began having headaches that were not related to shunt malfunction or his tumor but rather to his frequent seizures.
Despite the seizures, the patient was reluctant to undergo additional surgery. In 2018, he underwent a phase-I work-up to determine if he was a candidate for seizure surgery. The work-up included prolonged video EEG monitoring for localization of his seizure focus. There were repetitive epileptiform discharges and focal subclinical seizures arising from the left temporal region (T3) during sleep. The patient also showed interictal epileptiform discharges arising in the left mid temporal region (T3). These data suggested that the patient had a left temporal seizure focus and that the abnormal tissue in the left temporal lobe was likely the cause; however, the patient was still reluctant to undergo surgery. Consequently, he continued to take 2 antiseizure medications and was still having frequent seizures - at least 1 to 2 seizures per week lasting roughly 3–4 min per seizure. Sometimes these episodes were associated with difficulty breathing. He also continued to have headaches.
In June of 2021, he had a generalized tonic-clonic seizure that required emergency room assistance. A head MRI without contrast showed no tumor progression, and a 3rd medication, Zonisamide, was prescribed to help with seizure control.
Despite adding the 3rd medication, his seizures became progressively more frequent and more intense, and the patient decided to undergo seizure surgery. A seizure surgery work-up included MRI with and without contrast, which confirmed no change in tumor size. Functional MRI for localization of eloquent cortex could not be performed due to presence of shunt and PET was not needed. The video EEG was repeated after temporary suspension of antiepileptic medications. During the three-day period we recorded frequent left frontotemporal spike-wave discharges and three focal onset seizures that originated from his left anterior temporal lobe. The video EEG confirmed the left medial temporal lobe tumor and the adjacent temporal lobe area as the focus of patient's frequent seizures thus eliminating the need for additional stereoEEG.
In August 2021 the patient underwent a left frontotemporal craniotomy for removal of residual tumor and surrounding seizure focus in the left temporal lobe. Intraoperative ECoG was performed pre- and post-resection, which confirmed resolution of seizure generation (Fig. 1).
Fig. 1.
ECoG tracings pre and post resection of the left temporal lobe seizure focus. A. Pre-resection: A combination of spike and wave epileptiform discharges are seen with superimposed fast (beta frequency) activity over G2-G3 and G3-G4. B. This second pre-resection ECoG screen shot more clearly shows the spike and wave activity without the superimposed fast activity. C. Post-resection, there is a normal mixture of frequencies, primarily alpha and beta, without the overriding fast activity observed prominently prior to resection.
Imaging obtained immediately postoperatively showed complete resection of the tumor and subsequent imaging has shown no evidence of recurrence. At one year post surgery, the patient is doing well and has had no seizures or headaches post resection. At present, he is still on seizure medication.
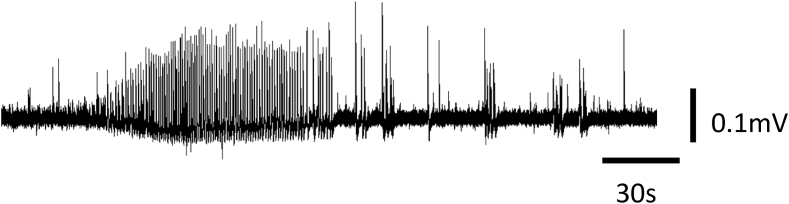
Prior to surgery, the patient gave consent for use of some of the epileptogenic tissue for research purposes. An in vitro electrophysiological study of the cortical tissue harvested from the patient was conducted immediately post resection. The tissue was oxygenated and transported to the laboratory in cold artificial CSF. Tissue slices were prepared as previously described and placed on microelectrode arrays of 60 electrodes [11]. Local field potentials (LFPs) with sharp positive peaks exceeding the threshold set at 3 standard deviations of the signal were marked (spikes), and the time of the maximum excursion was recorded as the time of that LFP. Epileptiform bursts were defined as clusters of uniform spikes occurring at a frequency >1 Hz and lasting more than 30s (Fig. 2).
Fig. 2.
Epileptiform burst of local field potentials recorded from left temporal lobe seizure focus brain tissue freshly excised from the patient. The recording was obtained in control solution (5 mM K+, 0 mM Mg2+ and 30 μM 4-AP). Bursts spread through a large area of the slice and were simultaneously recorded in multiple electrodes. Six such bursts were recorded in the 1 h control period.
Slices of resected human cortical tissue are typically not active in artificial CSF alone [12] as was the case in the current study. By bathing the slice in 30 μM 4-aminopyridine (4-AP) and reduced Mg2+, however, we evoked field potentials in all active channels of the array (n = 57). Three channels failed due to electrode malfunction and were excluded. We first recorded activity of the slice for 1 h in 30 μM 4-AP and reduced Mg2+ to establish a baseline. The average firing rate was 1.3 ± 0.03 Hz (all values are presented as Mean ± SEM) and the average spike amplitude was 30.7 ± 0.6 μV (Fig. 3).
Fig. 3.
KRM–II–81 (30 μM) had no effect on spike amplitude in patient epileptic slice. Average spike amplitude was measured during control conditions (Control), in the presence of 30 μM KRM–II–81 (KRM–II–81), and after the wash of KRM–II–81 (Wash). Mean ± SEM (n = 57). Differences were not significant (p > 0.05, one-way ANOVA, followed by Dunnett's Multiple Comparison test).
In addition to spiking there were epileptiform bursts of spiking activity which occurred at a frequency of 0.002 Hz (6 per hour) and lasted 96.1 ± 7.6 s. Epileptiform bursts were characterized by rapid firing of uniform LFPs (tonic-like) and were followed by firing of small clusters of LFPs (clonic-like) (Fig. 2).
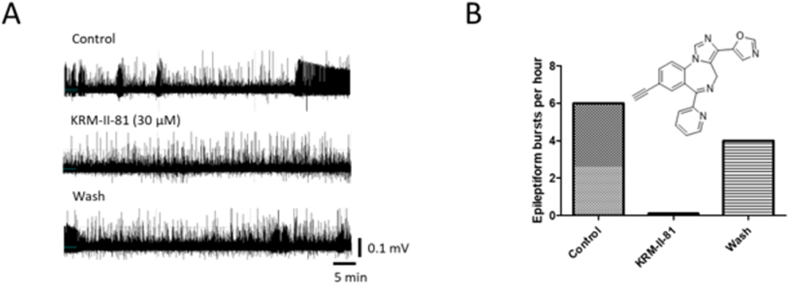
Next, we added KRM–II–81 (30 μM) and recorded activity for another hour, still in the presence of 30 μM 4-AP. There was a gradual increase in firing rate (1.62 ± 0.03 Hz; p < 0.05) with spike amplitude unchanged (30.4 ± 0.2 μV; p > 0.05) (Fig. 3). The gradual increase in firing rate continued during the 1 h wash of KRM–II–81 with the average firing rate increasing to 1.75 ± 0.04 Hz (p < 0.05) while the spike amplitude remained unchanged (30.3 ± 0.2 μV; p > 0.05). KRM–II–81 produced a complete cessation of epileptiform bursting activity during the 1 h incubation, which partially recovered during the 1 h wash period (Fig. 4).
Fig. 4.
Dampening effects of KRM–II–81 on epileptiform bursting in a slice of left temporal lobe focal brain tissue resected from the patient. A. Data were collected for 1 h under control conditions (Control), in the presence of 30 μM KRM–II–81 (KRM–II–81 (30 μM)), and after the wash out of KRM–II–81 (Wash). B. Summary data for the epileptiform burst activity under 1 h control, KRM–II–81, and wash out conditions. KRM–II–81 completely suppressed epileptic bursting activity in the slice.
3. Discussion
The presented case report illustrates successful surgical treatment of a patient with pharmacoresistant epilepsy. The patient underwent parieto-occipital tumor resection at the age of 6 years and suffered from progressively increasing seizure burden post-operatively. The seizures were resistant to pharmacotherapy with up to three antiepileptic medications at maximum tolerated doses. In addition, a GABAkine, diazepam, was prescribed for acute use with prolonged seizures, as the development of tolerance to diazepam precludes its chronic use. Resection of the identified seizure focus in the left temporal lobe resulted in immediate and enduring cessation of seizures with ongoing medication over this one-year follow-up.
New GABAkines are being developed for epilepsy [13] and the neuroactive steroid, ganaxolone, was recently approved for treatment of seizures in pediatric CDKL5 patients [14]. Another GABAkine, darigabat, has demonstrated efficacy in photosensitive epileptic patients and is currently in clinical development [13]. KRM–II–81, is a selective potentiator of α2/3-containing GABAARs and is one of the latest compounds to enter development for seizure control where it has broad efficacy across a range of animal models of epilepsy [[9], [15]] with enhanced efficacy and reduced side effect burden compared to nonselective GABAkines [[13], [16], [17], [18]]. The tissue finding in our patient where KRM–II–81 completely suppressed epileptic bursting in freshly-transected epileptic focal tissue is consistent with our previous report on KRM–II–81 suppressing the hyper-excitation network of rat cortical neurons invitro, without altering spontaneous neuronal activity [15].
The current data on suppression of epileptiform activity in slices of pharmacoresistant brain tissue are also consistent with our previous studies on rodent models of pharmacoresistance. KRM–II–81 dampens seizures in a 6 Hz corneal stimulation model in mice, the mesial temporal lobe model in mice, the lamotrigine-insensitive kindling model in rats, and a kainate-induced chronic epilepsy model in rats. KRM–II–81 was active in all of these pharmacoresistance models and in many cases produced greater efficacy than several standard-of-care antiseizure medicines [9]. The relative lack of sedation and tolerance development with KRM–II–81 [10,[9], [15]] also bodes well for a potential new drug for pharmacoresistant epilepsy [8].
While animal models of pharmacoresistant epilepsy afford a good predictive basis for human translatability, the present report from epileptic tissue of a patient resistant to multiple antiseizure medications further strengthens the case for progressing KRM–II–81 as a potential improved therapy for pharmacoresistant patients.
Data availability
The case report data are not publicly available due to patient privacy concerns.
CRediT authorship contribution statement
Jodi L. Smith: Conceptualization, Data curation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Jeremy Wertz: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Arnold Lippa: Methodology, Resources, Supervision, Writing - review & editing. Xingjie Ping: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Xiaoming Jin: Conceptualization, Methodology, Supervision, Writing - review & editing. James M. Cook: Resources, Supervision, Writing - review & editing. Jeffrey M. Witkin: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing - original draft, Writing - review & editing. Rok Cerne: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of competing interest
James Cook is a patent holder for KRM–II–81. Arnold Lippa, Rok Cerne, and Jeffrey Witkin are associated with RespireRx Pharmaceuticals Inc that holds the license agreement for KRM–II–81.
Acknowledgements
We thank the Henry and Nellie Pence Foundation and the Lucas family for generously supporting this research. We are also grateful for excellent clinical support from Heather Cero and Emily Vance.
References
- 1.Banerjee J., Chandra S.P., Kurwale N., Tripathi M. Epileptogenic networks and drug-resistant epilepsy: present and future perspectives of epilepsy research-Utility for the epileptologist and the epilepsy surgeon. Ann. Indian Acad. Neurol. 2014;17:S134–S140. doi: 10.4103/0972-2327.128688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marson A.G., Al-Kharusi A.M., Alwaidh M., Appleton R., Baker G.A., Chadwick D.W., et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–1015. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinha S., Siddiqui K.A. Definition of intractable epilepsy. Neurosciences. 2011;16:3–9. [PubMed] [Google Scholar]
- 4.Hauser W.A. Epidemiology of epilepsy in children. Neurosurg. Clin. 1995;6:419–429. [PubMed] [Google Scholar]
- 5.Hauser W.A. Seizure disorders: the changes with age. Epilepsia. 1992;33(Suppl 4):S6–S14. doi: 10.1111/j.1528-1157.1992.tb06222.x. [DOI] [PubMed] [Google Scholar]
- 6.Adelson P.D. Temporal lobectomy in children with intractable seizures. Pediatr. Neurosurg. 2001;34:268–277. doi: 10.1159/000056036. [DOI] [PubMed] [Google Scholar]
- 7.Witkin J.M., Golani L., Smith J.L. eighth ed. John Wiley and Sons, Inc.; Hoboken, NJ: 2021. New and Emerging Antiepileptic Drugs. Burger's Medicinal Chemistry, Drug Discovery and Development. [Google Scholar]
- 8.Löscher W., Schmidt D. Experimental and clinical evidence for loss of effect (tolerance) during prolonged treatment with antiepileptic drugs. Epilepsia. 2006;47:1253–1284. doi: 10.1111/j.1528-1167.2006.00607.x. [DOI] [PubMed] [Google Scholar]
- 9.Witkin J.M., Li G., Golani L.K., Xiong W., Smith J.L., Ping X., et al. The positive allosteric modulator of α2/3-containing GABAA receptors, KRM-II-81, is active in pharmaco-resistant models of epilepsy and reduces hyperexcitability after traumatic brain injury. J. Pharmacol. Exp. Therapeut. 2020;372:83–94. doi: 10.1124/jpet.119.260968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biggerstaff A., Kivell B., Smith J.L., Mian M.Y., Golani L.K., Rashid F., et al. The α2,3-selective potentiators of GABAA receptors, KRM-II-81 and MP-III-80, produce anxiolytic-like effects and block chemotherapy-induced hyperalgesia in mice without tolerance development. Pharmacol. Biochem. Behav. 2020;196 doi: 10.1016/j.pbb.2020.172996. [DOI] [PubMed] [Google Scholar]
- 11.Zwart R., Sher E., Ping X., Jin X., Sims J.R., Chappell A.S., et al. Perampanel, an antagonist of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, for the treatment of epilepsy: studies in human epileptic brain and nonepileptic brain and in rodent models. J. Pharmacol. Exp. Therapeut. 2014;351:124–133. doi: 10.1124/jpet.114.212779. [DOI] [PubMed] [Google Scholar]
- 12.Hobbs J.P., Smith J.L., Beggs J.M. Aberrant neuronal avalanches in cortical tissue removed from juvenile epilepsy patients. J. Clin. Neurophysiol. 2010;27:380–386. doi: 10.1097/WNP.0b013e3181fdf8d3. [DOI] [PubMed] [Google Scholar]
- 13.Witkin J.M., Lippa A., Smith J.L., Jin X., Ping X., Biggerstaff A., et al. The imidazodiazepine, KRM-II-81: an example of a newly emerging generation of GABAkines for neurological and psychiatric disorders. Pharmacol. Biochem. Behav. 2022 doi: 10.1016/j.pbb.2021.173321. [DOI] [PubMed] [Google Scholar]
- 14.Knight E.M.P., Amin S., Bahi-Buisson N., Benke T.A., Cross J.H., Demarest S.T., et al. Safety and efficacy of ganaxolone in patients with CDKL5 deficiency disorder: results from the double-blind phase of a randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2022;21:417–427. doi: 10.1016/S1474-4422(22)00077-1. [DOI] [PubMed] [Google Scholar]
- 15.Witkin J.M., Smith J.L., Ping X., Gleason S.D., Poe M.M., Li G., et al. Bioisosteres of ethyl 8-ethynyl-6-(pyridin-2-yl)-4H-benzo[f]imidazo [1,5-a][1,4]diazepine-3-carboxylate (HZ-166) as novel alpha 2,3 selective potentiators of GABAA receptors: improved bioavailability enhances anticonvulsant efficacy. Neuropharmacology. 2018;137:332–343. doi: 10.1016/j.neuropharm.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Cerne R., Lippa A., Poe M.M., Smith J.L., Jin X., Ping X., et al. GABAkines - advances in the discovery, development, and commercialization of positive allosteric modulators of GABAA receptors. Pharmacol. Ther. 2021 doi: 10.1016/j.pharmthera.2021.108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poe M.M., Methuku K.R., Li G., Verma A.R., Teske K.A., Stafford D.C., et al. Synthesis and characterization of a novel gamma-aminobutyric acid type A (GABAA) receptor ligand that combines outstanding metabolic stability, pharmacokinetics, and anxiolytic efficacy. J. Med. Chem. 2016;59:10800–10806. doi: 10.1021/acs.jmedchem.6b01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witkin J.M., Cerne R., Davis P.G., Freeman K.B., do Carmo J.M., Rowlett J.K., et al. The α2,3-selective potentiator of GABAA receptors, KRM-II-81, reduces nociceptive-associated behaviors induced by formalin and spinal nerve ligation in rats. Pharmacol. Biochem. Behav. 2019;180:22–31. doi: 10.1016/j.pbb.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The case report data are not publicly available due to patient privacy concerns.