Abstract
Vitamin D gene polymorphisms are known to be associated with asthma and allergic rhinitis in children. However, the genetic association of the same in South Indian children with above condition is still unknown. The present study was designed with the objective to analyze the association of polymorphisms of vitamin D receptor gene (VDR) (rs797532, rs154410, rs2258470, rs731236) and transport gene of vitamin D (rs7041) in children with asthma and allergic rhinitis in South India. Children (1–18years) presenting with symptoms suggestive of asthma and allergic rhinitis to hospital based outpatient department, in Vellore, South India were recruited as cases and children presenting with minor illness without respiratory complaints were enrolled as controls during January 2018 to September 2021. Polymorphisms were genotyped using tetra-arms PCR. Significant increase in levels of absolute eosinophil counts and serum IgE levels with decrease in vitamin D levels was seen among the cases. Significant association between levels of vitamin D and serum IgE was also observed. Analysis of polymorphisms showed that, in comparison to homozygous major allele the odds of having heterozygous (OR0.55 (0.3, 0.99) and homozygous minor form (OR0.52 (0.28, 0.97) of rs7975232, homozygous minor (OR 0.51 (0.34, 0.76)) and alternate allele (OR 0.7 (0.53, 0.93)) of rs154410 and homozygous minor form (OR 0.57 (0.37, 0.88) of rs731236 was significantly lesser among the cases. Genotypic model of rs154410 (p0.023) and allele form of rs7041 (p 0.041) were significantly associated with vitamin D levels however no association of gene blocks with cases was seen in haplotype analysis. There was an apparent gene pool difference noted in comparative analysis between Indian studies. The study is the first in south India to analyze levels of serum IgE, Vitamin D levels, association of VDR polymorphisms, and rs7041 in children with asthma.
Keywords: Asthma and allergic rhinitis, Vitamin pathway-related genes, Polymorphisms, Indian children
Highlights of the present study
-
•
Study showed association of VDR polymorphisms in control group compared to children with asthma and AR.
-
•
This is the first study to analyze the association of rs7041 polymorphism in children with asthma or AR in India.
-
•
The association of rs1544410 to controls is unique to the South Indian population.
-
•
Association of gene polymorphisms of vitamin D in chidlren with asthma or AR show interregional variations in India
-
•
High Serum levels of IgE and AEC, low levels of vitamin D are noticed in children with asthma and AR
1. Introduction
India has a huge burden of asthma and allergic rhinitis, with incidence ranging from 5 to 6%for asthma and 11–24.4 % for allergic rhinitis [1,2]. It is known that occurrence of asthma and allergic rhinitis (AR) is due to a complex interplay and non-linear variable relationship between genetic and environmental factors [3]. Among them, vitamin D and the ubiquitous nature of its receptors is known to influence the functions of Th1 and Th2 cytokines and hence the development of atopic disorders [4]. India also has a high burden of Vitamin D deficiency in children, which may affect the prevalence of atopic diseases [5,6]. Several studies have established an association of polymorphisms of the vitamin D receptor (VDR) gene and certain metabolic and transport genes of vitamin D. These studies have shown variations in association based on ethnicity and geographical location [[7], [8], [9]].
Among the genes associated with Vitamin D, VDR gene polymorphisms is known to alter the vitamin D function and regulate gene expressions which are concerned with the inflammation in the airway epithelium [10]. VDR gene is located on chromosome 12q13. The polymorphisms of the same are rs7975232, rs1544410, rs2228570, and rs731236 and have been extensively studied in various population. Also, among several genes of Vitamin D, the transport gene (GC) of vitamin D has been studied in several respiratory disorders. GC encodes for the vitamin D binding protein (VDBP) which binds the vitamin D metabolites. The VDBP is known to be associated with macrophage activation and neutrophil chemotaxis and hence has a role in immunomodulatory effect in the lung. GC gene is located in chromosome 4q13 and has 13 exons. There are two common functional SNPs in exon 11 of GC – rs4588 and rs7041. Both the SNPs have been associated with binding affinity for 25OHD [11,12]. However, the role of the VDR gene and GC gene polymorphisms in Indian children with asthma and AR has been scant especially from South India.
Hence, the present study was undertaken within an aim to analyze the association of the genetic polymorphisms of VDR gene and rs7041 on asthma and AR in South Indian children under 18 years of age. (Fig. 1).
Fig. 1.
Genes involved in Vitamin D metabolic pathway.
2. Materials and methods
2.1. Materials and methods
A prospective case control study was designed with an aim to study the association of polymorphisms of vitamin D receptor (VDR) gene and transport gene (GC) of vitamin D in children with asthma and/or allergic rhinitis. The Institutional ethics committee of Nalam Medical Centre and Hospital and VIT University, Vellore, Tamilnadu approved the study protocol. (No. NMCHE0005). The study included children under 18 who attended the pediatric outpatient department of the hospital for the first time with symptoms suggestive of asthma or allergic rhinitis, or both from January 2018 to September 2020. We excluded children with chronic respiratory diseases like cystic fibrosis or congenital heart diseases from the study. The control group included children who came for minor illness that did not include any respiratory, chronic or congenital illnesses. The baseline demographic characteristics of the study population like age, anthropometry, symptoms and severity of asthma, vaccination status, and family history of asthma and tobacco exposure was collected using standard questionnaire. Informed consent was obtained from the parents of the cases and control groups.
Analysis of serum IgE and serum vitamin D (25 hydroxy vitamin D) was estimated using protein binding immunoassay with electrochemiluminescence (ECLIA) detection after internal validation. (CobasE411 Roche, Austria). Absolute Eosinophil count (AEC) was measured using flowcytometry analysis by integrated analyzer. (Mindray 6- part BC-6000 Hematology analyzer, Mumbai, India). All children in the case group underwent allergy skin testing to confirm atopy using fixed allergen panels that included dust mites, Prosopis julifora, Parthenium hysterophus, cockroach, alternaria, cat dander, milk, egg white, peanut, chicken, and fish. The allergens were obtained from Creative Diagnostics Medicare private limited, No14, F- Type Market, Sector 7, Navi Mumbai, India 400,703. Sensitization for even one antigen was taken as confirmation of atopic nature.
In order to grade the severity of asthma, children above the age of 5 who could perform the pulmonary function testing (PFT) was assessed for their lung functions using spirometry (Easy One Air, NDD medical technologies, Canada) and in those who could not perform the same peak flow meter was used. Child who could not perform PFT especially those under 5 years of age were assessed for asthma severity using GINA grading, which included the number of days’ children had day-time symptoms, night awakenings, needed reliever medications for symptoms more than twice a week, and activity limitation due to asthma [13].
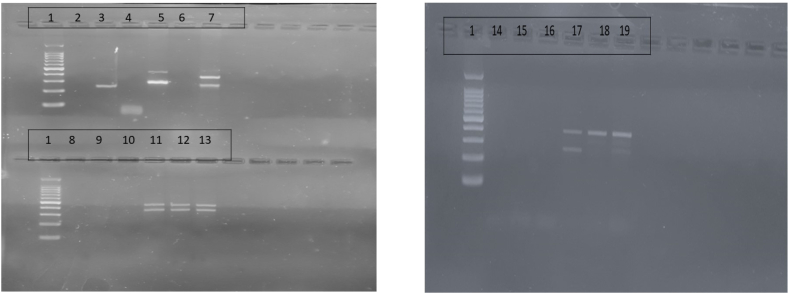
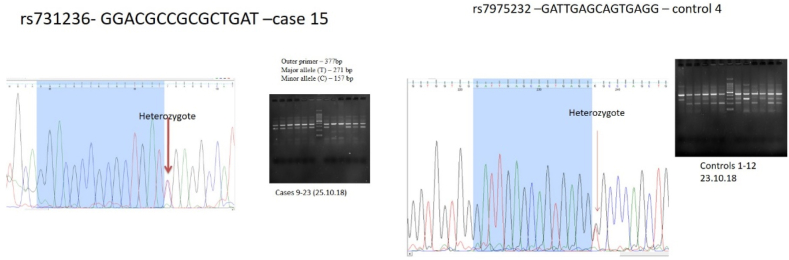
Genotypic analysis was undertaken using tetra arms PCR after standardization. Machel Nagel Nucleospin Mini Kit was used for DNA extraction. The outer and inner primers for single nuclear polymorphisms (SNPs) rs7975232, rs1544410, rs2228570 and rs731236 of Vitamin D receptor (VDR) gene, and rs7041 of transport gene GC were designed (Supplement Table 1). Further, tetra arms PCR was carried as per protocol given in Fig. 2. The polymorphisms were finally analyzed using Bio-Rad EZ gel system (Fig. 3). Confirmation of the identified polymorphism variant was done with Sanger sequencing with a representative sample of 25 for all five polymorphisms both for cases and controls. (Representative image of the Sanger sequence given in Fig. 4).
Fig. 2.
Genotypic Analysis protocol using Tetra-Arms PCR.
Fig. 3.
The images of the polymorphisms analyzed using tetra arms PCR as seen in the agarose gel
Legend - 1-100bp ladder, 2 – rs7041 Negative control (NC) (T/G), 3- rs7041 Positive control (PC) (T/G), 4 – rs7975232 NC (G/T), 5- rs7975232 PC(G/T), 6 - rs731236 NC(T/C), 7 – rs731236 PC(T/C), 8 – rs1544410 NC(G/A),9 - rs1544410 NC(G/C), 10-rs1544410 NC(G/T), 11- rs1544410 PC (G/A), 12 - rs1544410 PC(G/C), 13 - rs1544410 PC(G/T), 14 – rs2228570 NC(T/C), 15 - rs2228570 NC(T/A), 16 - rs2228570 NC(T/G), 17 - rs2228570 PC(T/C), 18 - rs2228570 PC(T/A), 19 - rs2228570 PC(T/G).
Fig. 4.
Representative image of the Sanger sequencing done for confirmation of the identified variant.
2.2. Statistical analysis
Sample size was calculated using “n Master 2.0 software” and number in each group was estimated using a two-sided significance test with 90 % power and a 5 % significance level of the study. Based on the study done in adjacent geographical location by Sharif et al. assuming 17 % difference in the levels of vitamin D between the study groups, an estimated sample size was obtained in each of the study group [14]. However, expecting an attrition rate of 10 %, two hundred children were selected in each group.
The data was then analyzed using R software version 4.1.1 [15]. Independent sample t-test or Mann-Whitney U – test was used for the continuous measurements to compare the baseline characteristics after checking the normality assumption. The chi-square test or Fischer's exact test was used for the categorical observations based on expected frequency. The SNPs were analyzed using the chi-square test and odd's ratio was computed as a measure of logistic regression. The different patient characteristics were compared within the cases based on the severity of asthma, and post hoc analysis was carried out. Bonferroni correction method was applied to adjust alpha error and p-values computed accordingly. Hardy-Weinberg equilibrium (HWE) analysis was performed to compare the SNPs' between cases and controls. Haplotype analysis was done using the R Studio and the library “haplo.stats” was used to assess the association of gene blocks with cases and controls. P-value was considered significant at 5 % level of significance for all comparisons.
3. Results
3.1. Baseline demographic characteristics of the population studied
Two hundred seven cases and two hundred controls were selected for the present study. The results of the baseline characteristics are given in Table 1. The mean age of the cases was 7 years compared to 4 years among the controls. There was no statistical difference in anthropometric characteristics or neonatal admissions between the study groups. The incidence of atopic dermatitis, vaccination history for influenza, and family history showed significant association in the cases compared to control (p < 0.001). Atopic dermatitis was more in cases (21 (10 %)) compared to controls (10 (5 %). Among the cases, only 43 (21 %) were vaccinated against influenza compared to 69 (35 %) of controls. Most of the children in the case group (99 (48 %) had significant family history compared to controls (30 (15 %). Among the case group, the incidence of asthma was higher in grandparents (29 %) in comparison to both the parents (14 %) and siblings (10 %).
Table 1.
Results of the demographic characteristics and lab parameters in the study population.
| PARAMETERS | GROUP |
P- value | |
|---|---|---|---|
| CASES (N = 207) | CONTROLS (N = 200) | ||
| AGE | 7 (4,11) | 4 [2,7] | <0.001 |
| GENDER | |||
| Male | 132 (64 %) | 118 (59 %) | 0.323 |
| Female | 75 (36 %) | 82 (41 %) | |
| Height Z score | −0.01 ± 1.27 | 0.08 ± 1.16 | 0.635 |
| Weight Z score | 0 ± 1.54 | 0.17 ± 2.21 | 0.876 |
| BMI Z score | 0 ± 1.11 | 0 ± 1.47 | 0.805 |
| Neonatal admission (y) | 30 (14 %) | 18 (9 %) | 0.086 |
| Atopic dermatitis(y) | 21 (10 %) | 10 (5 %) | 0.050 |
| Flu vaccinations | 43 (21 %) | 69 (35 %) | 0.002 |
| Family history of asthma | 99 (48 %) | 30 (15 %) | <0.001 |
| Grandparents with h/o asthma | 60 (29 %) | 22 (11 %) | <0.001 |
| Father with h/0 asthma | 29 (14 %) | 6 (3 %) | <0.001 |
| Mother with h/0 asthma | 28 (14 %) | 2 (1 %) | <0.001 |
| Siblings with h/0 asthma | 21 (10 %) | 2 (1 %) | <0.001 |
| Mean AEC | 464.8 ± 342.5 | 179.8 ± 180.1 | <0.001 |
| Absolute eosinophils count | |||
| Normal | 91 (44 %) | 174 (87 %) | <0.001 |
| Abnormal | 116 (56 %) | 25 (13 %) | |
| Mean serum IgE level | 887.3 ± 1118.4 | 422.3 ± 572.5 | <0.001 |
| Serum IgE | |||
| Normal | 59 (29 %) | 103 (52 %) | <0.001 |
| Abnormal | 148 (71 %) | 97 (49 %) | |
| Mean Vitamin D levels | 17.2 ± 6.7 | 20.2 ± 8.8 | <0.001 |
| Vitamin D | |||
| Deficient | 160 (77 %) | 126 (63 %) | <0.002 |
| Insufficient | 36 (17 %) | 52 (26 %) | |
| Normal | 11 (5 %0 | 14 (7 %) | |
| High | 0 (0 %) | 8 (4 %) | |
| Atopic nature | 188 (90.8 %) | Not applicable | |
| Severity of asthma | |||
| Well Controlled | 14 (6.76 %) | Not applicable | |
| Partly controlled | 145 (70 %) | Not applicable | |
| Uncontrolled | 48 (23.19 %) | Not applicable | |
3.2. Lab parameters analyzed
The levels of serum IgE and AEC was higher in cases compared to controls and the difference was statistically significant. The mean level of Vitamin D in cases was 17.2 ± 6.7 ng/ml which was lower when compared to controls (20.2 ± 8.8 ng/ml) and the difference was statistically significant (p < 0.001). (Table 1).
3.3. Atopic status and level of control of asthma
Among the 207 cases who underwent allergy skin testing 188 children showed sensitization to tested allergens. While 139 children had demonstrated multiple sensitization, 29 had sensitization to two antigens and 20 had mono sensitization. Among the cases, 120 children could perform PFTs and based on their FEV1 levels severity was graded. Using clinical criteria and PFT wherever appropriate, 148 (70 %) children had party-controlled asthma, 48 (23.19 %) had uncontrolled and 14 (6.76 %) had well-controlled asthma.
3.4. Correlation between serum IgE levels and vitamin D
The relationship between serum IgE levels and Vitamin D levels were further analyzed and it was found that there was a significant relationship between the two parameters. Hence, it is possible that when used together both of the parameters can be used as biomarkers in children with asthma (Table 2).
Table 2.
Relationship between serum IgE levels and Vitamin D.
| VITAMIN D | IGE |
P-VALUE | |
|---|---|---|---|
| NORMAL (N = 324) | ABNORMAL (N = 490) | ||
| DEFICIENT | 200 (61.7 %) | 372 (75.9 %) | <0.001 |
| INSUFFICIENT | 86 (26.5 %) | 90 (18.4 %) | |
| NORMAL | 24 (7.4 %) | 26 (5.3 %) | |
| HIGH | 14 (4.3 %) | 2 (0.4 %) | |
3.5. Analysis of the biochemical parameters with level of control of asthma in cases
Serum IgE and absolute eosinophil counts showed significant association with level of control of asthma. The levels were significantly abnormal as the severity of asthma increased. AEC levels were significantly higher even in children with well controlled asthma in comparison to controls. The association with level of control of asthma was not seen with serum Vitamin D levels and there was no statistical association between them (Table 3).
Table 3.
Comparison of biochemical parameters and level of control of asthma.
| Parameters | Controls | Level of control in cases |
Control Vs well controlled€ | Control Vs partly controlled€ | Control Vs not controlled€ | ||
|---|---|---|---|---|---|---|---|
| Well controlled | Partly controlled | Not controlled | |||||
| Vitamin D levels | |||||||
| Deficient | 126 (63 %) | 11 (79 %) | 107 (74 %) | 42 (88 %) | 0.999 | 0.415 | 0.013 |
| Insufficient | 52 (26 %) | 2 (14 %) | 28 (19 %) | 6 (13 %) | 0.999 | 0.999 | 0.567 |
| Normal | 14 (7 %) | 1 (7 %) | 10 (7 %) | 0 (0 %) | 0.999 | 0.999 | 0.71 |
| High | 8 (4 %) | 0 (0 %) | 0 (0 %) | 0 (0 %) | 0.999 | 0.178 | 0.999 |
| Serum IgE | |||||||
| Normal | 103 (52 %) | 3 (21 %) | 43 (30 %) | 13 (27 %) | 0.178 | <0.001 | 0.014 |
| Abnormal | 97 (49 %) | 11 (79 %) | 102 (70 %) | 35 (73 %) | 0.178 | <0.001 | 0.014 |
| Absolute Eosinophil Count | |||||||
| Normal | 174 (87 %) | 7 (50 %) | 61 (42 %) | 23 (48 %) | 0.001 | <0.001 | <0.001 |
| Abnormal | 25 (13 %) | 7 (50 %) | 84 (58 %) | 25 (52 %) | 0.001 | <0.001 | <0.001 |
3.6. Analysis of associations of polymorphisms of genes in the vitamin D metabolic pathway
3.6 a - Results of the analysis of HWE for the study population indicated no significant level for the SNPs in control groups (p > 0.05) except in rs7975232. The frequency distributions of the SNPs in cases and controls determined by chi-square tests for the polymorphisms showed that the frequency of genotypes tested did not deviate from the expectations predicted (Supplement Table 2).
3.6 b –Results of the association of polymorphisms in study group: The following findings were observed in the study. In comparison with homozygous major the heterozygote and homozygous minor polymorphism of rs7975232, minor allele and homozygous minor form of rs1544410, homozygous minor form of rs731236 were significantly less in cases and hence these forms of polymorphisms could be protective in our population. There was no significant association of the allelic forms of the polymorphisms with cases or controls except for the minor allele of rs1544410 (Table 4). However, the above significance was not present when the multivariate analysis was done by adjusting the significant clinical baseline parameters noted in Table 2. The polymorphisms did not show any significant association with level of control of asthma and the biochemical parameters (Supplement Tables 3 and 4). When the clinical characters were compared, there was significant association of males in control group for rs2228570 and in cases for rs7041 (Supplement Table 3).
Table 4.
Association of polymorphisms in the study groups.
| Polymorphisms | Group |
OR (95%CI) | p- value | |
|---|---|---|---|---|
| Cases n (%) | Control n (%) | |||
| rs7975232 | ||||
| GG | 37 (18 %) | 23 (12 %) | REF | |
| GT | 104 (50 %) | 110 (55 %) | 0.55 (0.3,0.99) | 0.045 |
| TT | 61 (29 %) | 66 (33 %) | 0.52 (0.28,0.97) | 0.041 |
| G | 180 (43 %) | 154 (39 %) | REF | |
| T | 224 (54 %) | 244 (61 %) | 0.79 (0.59,1.04) | 0.093 |
| rs154410 | ||||
| GG | 57 (28 %) | 45 (23 %) | REF | |
| GA | 99 (48 %) | 85 (43 %) | 0.88 (0.63,1.25) | 0.481 |
| AA | 42 (20 %) | 60 (30 %) | 0.51 (0.34,0.76) | 0.001 |
| G | 215 (52 %) | 173 (43 %) | REF | |
| A | 181 (44 %) | 207 (52 %) | 0.7 (0.53,0.93) | 0.015 |
| rs 2,228,570 | ||||
| TT | 16 (8 %) | 17 (9 %) | REF | |
| TC | 83 (40 %) | 81 (41 %) | 1.08 (0.51,2.30) | 0.824 |
| CC | 102 (49 %) | 96 (48 %) | 1.12 (0.54,2.35) | 0.747 |
| T | 115 (28 %) | 115 (29 %) | REF | |
| C | 287 (69 %) | 273 (68 %) | 1.05 (0.77,1.42) | 0.750 |
| rs731236 | ||||
| TT | 98 (47 %) | 86 (43 %) | REF | |
| CT | 77 (37 %) | 79 (40 %) | 0.84 (0.62,1.13) | 0.248 |
| CC | 23 (11 %) | 32 (16 %) | 0.57 (0.37,0.88) | 0.012 |
| T | 275 (66 %) | 249 (62 %) | REF | |
| C | 121 (29 %) | 145 (36 %) | 0.76 (0.56,1.02) | 0.064 |
| rs7041 | ||||
| TT | 30 (14 %) | 37 (19 %) | REF | |
| GT | 99 (48 %) | 100 (50 %) | 1.22 (0.70,2.12) | 0.481 |
| GG | 72 (35 %) | 58 (29 %) | 1.53 (0.84,2.76) | 0.159 |
| T | 159 (38 %) | 174 (44 %) | REF | |
| G | 243 (59 %) | 216 (54 %) | 1.23 (0.93,1.63) | 0.149 |
4. 6 c -associations of polymorphisms with vitamin D levels
When the levels of Vitamin D where compared with the polymorphisms, there was association of genotypic forms of rs1544410 and allelic for of rs7041 with the levels of vitamin D. However, none of the other SNPs showed any association with Vitamin D levels (Table 5).
Table 5.
Association of polymorphisms with Vitamin D levels among the cases.
| SNP | Vitamin D |
Significance |
|||
|---|---|---|---|---|---|
| Deficient | Insufficient | Normal | High | ||
| rs7975232 | |||||
| GG | 47 (16.6 %) | 12 (14.0 %) | 1 (4.2 %) | 0 (0.0 %) | 0.350 |
| GT | 154 (54.4 %) | 41 (47.7 %) | 14 (58.3 %) | 5 (62.5 %) | |
| TT | 82 (29.0 %) | 33 (38.4 %) | 9 (37.5 %) | 3 (37.5 %) | |
| G | 248 (43.8 %) | 65 (37.8 %) | 16 (33.3 %) | 5 (31.3 %) | 0.240 |
| T | 318 (56.2 %) | 107 (62.2 %) | 32 (66.7 %) | 11 (68.8 %) | |
| rs1544410 | |||||
| GG | 76 (27.8 %) | 24 (28.9 %) | 2 (8.0 %) | 0 (0.0 %) | 0.023 |
| AG | 63 (23.1 %) | 28 (33.7 %) | 10 (40.0 %) | 1 (14.3 %) | |
| AA | 134 (49.1 %) | 31 (37.3 %) | 13 (52.0 %) | 6 (85.7 %) | |
| G | 286 (52.4 %) | 79 (47.6 %) | 17 (34.0 %) | 6 (42.9 %) | 0.071 |
| A | 260 (47.6 %) | 87 (52.4 %) | 33 (66.0 %) | 8 (57.1 %) | |
| rs2228570 | |||||
| CC | 143 (51.4 %) | 42 (50.0 %) | 10 (40.0 %) | 3 (37.5 %) | 0.820 |
| TC | 110 (39.6 %) | 37 (44.0 %) | 13 (52.0 %) | 4 (50.0 %) | |
| TT | 25 (9.0 %) | 5 (6.0 %) | 2 (8.0 %) | 1 (12.5 %) | |
| C | 396 (71.2 %) | 121 (72.0 %) | 33 (66.0 %) | 10 (62.5 %) | 0.740 |
| T | 160 (28.8 %) | 47 (28.0 %) | 17 (34.0 %) | 6 (37.5 %) | |
| rs731236 | |||||
| CC | 130 (46.9 %) | 38 (44.7 %) | 11 (44.0 %) | 5 (62.5 %) | 0.280 |
| TC | 116 (41.9 %) | 29 (34.1 %) | 9 (36.0 %) | 2 (25.0 %) | |
| TT | 31 (11.2 %) | 18 (21.2 %) | 5 (20.0 %) | 1 (12.5 %) | |
| T | 376 (67.9 %) | 105 (61.8 %) | 31 (62.0 %) | 12 (75.0 %) | 0.370 |
| C | 178 (32.1 %) | 65 (38.2 %) | 19 (38.0 %) | 4 (25.0 %) | |
| rs7041 | |||||
| GG | 81 (29.0 %) | 33 (38.8 %) | 13 (54.2 %) | 3 (37.5 %) | 0.140 |
| GT | 148 (53.0 %) | 37 (43.5 %) | 10 (41.7 %) | 4 (50.0 %) | |
| TT | 50 (17.9 %) | 15 (17.6 %) | 1 (4.2 %) | 1 (12.5 %) | |
| G | 310 (55.6 %) | 103 (60.6 %) | 36 (75.0 %) | 10 (62.5 %) | 0.054 |
| T | 248 (44.4 %) | 67 (39.4 %) | 12 (25.0 %) | 6 (37.5 %) | |
4.1. Haplotype analysis
Haplotype analysis was carried out to study the association of different gene block with cases & controls. The frequency of the haplotypes was analyzed among 31 haplotypes analyzed, GGGCT was the most common haplotype with 14 % frequency however none of the haplotypes showed any association with asthma (Table 6).
Table 6.
Haplotypes occurring with at least 5 % frequency and association with asthma.
| Haplotype | Frequency of occurrence | OR (95%CI) | P -value |
|---|---|---|---|
| GGGCT | 0.1439 | 1 (ref Haplotype) | |
| GGGTT | 0.0722 | 0.51 (0.20–1.31) | 0.1628 |
| GGTCT | 0.1170 | 0.71 (0.33–1.54) | 0.3834 |
| TAGCC | 0.1209 | 0.86 (0.44–1.69) | 0.6609 |
| TAGCT | 0.0779 | 0.96 (0.40–2.27) | 0.9230 |
| TATCC | 0.0749 | 1.82 (0.79–4.17) | 0.1578 |
| genoH.rare | 0.3932 | 0.86 (0.50–1.46) | 0.5707 |
5. Discussion
Asthma is a major cause of morbidity in children and association of asthma and allergic rhinitis with vitamin D levels has been established in previous literatures. VDR is an intracellular receptor and is expressed in majority of immune cells and the active metabolite of vitamin D is an important immune modulator contributing to several diseases including asthma. The interaction between vitamin D and immune system is influenced by polymorphisms of genes involved in the vitamin D metabolic pathway and has been investigated in different studies in other countries [16]. The present study was an attempt to analyze the association of polymorphisms of VDR and GC gene in South Indian population as studies have been sparse. Further association of serum IgE, vitamin D and AEC levels in children with asthma and to the polymorphisms were also analyzed.
In the present study significant association of rs7975232 in heterozygous (OR 0.55 (0.3, 0.99) and homozygous form (OR 0.52 (0.28, 0.97), rs1544410 in minor allele (OR0.7 (0.53, 0.93) and heterozygous form (0.51 (0.34, 0.76) and minor allele of rs731236 (0.57 (0.37, 0.88) was noted and they were found to be less in cases and hence protective.
Comparative analysis of the association of polymorphisms with studies done across the world has been summarized in Table 7. The analysis shows significant interregional variation even within India with the study from North India by Awasthi et al. showing association of rs7975232 with cases and no association of rs1544410 [8]. There was also age related variation with the present study showing no association of rs2228570 in children in contrast to the association seen in controls in adults in the study done by Rajaram et al. from South India [17]. The results of the present study showed similarity to several Asian studies [7,[18], [19], [20]]. In contrast the North Indian population showed greater similarity to the studies done in European population [[21], [22], [23], [24], [25]]. The present study did not show any association of the polymorphisms with severity of asthma similar to studies from Asia in contrast to studies done by Awasthi et al. and Iordanidou et al. [8,17,21,23]. While our haplotype analysis did not reveal any association with asthma, Awasthi et al. had found association of VDR haplotype polymorphisms, confirming the interregional difference [8].
Table 7.
Comparison of occurrence of polymorphisms among various countries.
| SNPs | Genotype | No of studies | Result of meta-analysis- Overall | Asia | Europe | Awasthi (North India) | Adult (Jipmer) | Present Study |
|---|---|---|---|---|---|---|---|---|
| rs7975232 | Major allele | 7 | 0.81 (0.71,0.91) 0.381 |
– | 0.81 (0.71,0.91) | Ref | – | Ref |
| Minor allele | 7 | 1.21 (1.07,1,37) 0.350 | – | 1.21 (1.07,1.37) | 0.88 (0.26–2.98) 0.849 | – | 0.79 (0.59,1.04) 0.093 |
|
| Homozygous major | 11 | 0.83 (0.70,0.98) 0.011 | 1.17 (0.59,2.33) 0.001 | 0.83 (0.70,0.98) | Ref | – | Ref | |
| Heterozygous | 11 | 1.08 (0.93,1.25) 0.000 | 1.07 (0.76,1.51) 0.000 | 1.08 (0.93,1,25) | 1.83 (1.01–3.32) 0.046 | – | 0.55 (0.3,0.99) 0.045 |
|
| Homozygous minor | 11 | 0.91 (0.77,1.09) 0.000 | 0.61 (0.44,0.83) 0.000 | 0.91 (0.77,1.09) | 1.74 (0.85–3.53) 0.12 | – | 0.52 (0.28,0.97) 0.041 |
|
| rs1544410 | Major allele | 3 | 1.10 (0.90.1.34) 0.177 | – | 0.93 (0.71,1.22) | Ref | – | Ref |
| Minor allele | 3 | 0.91 (0.75,1,11) | – | 1.07 (0.82,1.40) | 0.20 (0.02–1.64) 0.135 | – | 0.7 (0.53,0.93) 0.001 |
|
| Homozygous major | 6 | 1.25 (0.87,1.74) 0.038 | 2.07 (0.46,9.21) 0.038 | 0.77 (0.48,1.26) | Ref | – | Ref | |
| Heterozygous | 6 | 1.35 (1.07,1.71) 0.000 | 3.36 (2.12,5.32) 0.003 | 1.18 (0.81,1.72) | 1.02 (0.51–2.03) 0.950 | – | 0.51 (0.34,0.76) 0.001 |
|
| Homozygous minor | 6 | 1.35 (1.07,1.71) 0.000 | 4.40 (3.18,6.09) 0.000 | 0.98 (0.06,1.47) | 1.80 (0.60–5.40) 0.292 | – | 0.88 (0.63,1.25) 0.481 |
|
| rs731236 | Major allele | 7 | 1.22 (1.08,1.38) 0.000 | – | 1.32 (0.96,1.81) | Ref | – | Ref |
| Minor allele | 7 | 1.13 (1.01,1.27) 0.001 | – | 0.76 (0.55,1.04) | 6.17 (0.77–49.57) 0.087 | – | 0.76 (0.56,1.02) 0.064 |
|
| Homozygous major | 10 | 0.73 (0.58,0.92) 0.001 | 0 | 0.93 (0.55,1.23) | 1.77 (0.71–4.37) 0.215 | – | ref | |
| Heterozygous | 10 | 1.08 (0.93,1.26) 0.000 | 1.08 (0.57,2.03) 0.000 | 0.82 (0.55,1.23) | 1.81 (0.86–3.80) 0.116 | – | 0.84 (0.62,1.13) 0.248 |
|
| Homozygous minor | 10 | 0.99 (0.86,1.14) 0.000 | 0.68 (0.51,0.92) 0.000 | 1.61 (0.98,2.64) | Ref | – | 0.57 (0.37,0.88) 0.012 | |
| rs2225870 | Major allele | 6 | 1.34 (1.17,1.52) 0.000 | – | 0.99 (0.80,1.23) | Ref | – | 1.05 (0.77,1.42) 0.750 |
| Minor allele | 6 | 0.89 (0.78,1.02) 0.052 | – | 1.01 (0.82,1.25) | 1.48 (0.42–5.16) 0.535 | – | Ref | |
| Homozygous major | 10 | 0.96 (0.80,1.16) 0.000 | 0.44 (0.26,0.75) 0.000 | 0.92 (0.68,1.24) | Ref | – | 1.12 (0.54,2.35) 0.747 |
|
| Heterozygous | 10 | 0.99 (0.85,1.14) 0.000 | 0.61 (0.42,0.88) 0.000 | 1.17 (0.87,1.56) | 1.28 (0.71–2.30) 0.396 | – | 1.08 (0.51,2.30) 0.824 |
|
| Homozygous minor | 10 | 1.07 (0.90,1.27) 0.000 | 1.45 (0.94,2,23) 0.000 | 0.87 (0.58,1.31) | 1.83 (0.89–3.76) 0.099 | 0.022 | Ref |
The interregional variations seen in the present study can be explained by the differential expression of the VDR gene. VDR influences the biological activity of vitamin D, and the four SNPs discussed above can alter the mRNA stability. Among these rs2228570 polymorphisms alter the ATG start codon in VDR and changes in rs154410 modulates the expression of VDR protein whereas rs7975232 and rs731236 influences the mRNA stability. Further rs154410, rs7975232, and rs731236 are silent codon alterations and their association with other functional SNPs can alter the expression of VDR target gene [25]. This probably explains the protective role of rs7975232, rs1544410. And rs731236 in our ethnic population probably due to dietary factors and lifestyle influencing the expression of the VDR gene in the presence of polymorphism [9,25].
Similarity of association of polymorphisms in South India to Asian studies and North Indian studies to European population can be explained by the hypothesis analyzed in the studies by Ali et al. (2014) and Moorjani et al. (2013) who have found two major genetic lineages - the Indo- Aryan and Dravidian lineage among the Indian population contributed by the gene-flow from Europe to North India. Hence, polymorphism association with children with asthma is variable and very specific for geographical region and ethnicity.
The present study is the first to analyze rs7041 in Indian population though no significant association was noted. There has been varying degree of association of the above polymorphism in children with asthma in several studies as discussed in Table 8 [19,[25], [26], [27]].
Table 8.
Comparison of occurrence of rs7041 among various population.
| rs7041 | OR (CI) P value/Inference – Present study | Fawzy et al. (Egypt) | Leung et al. (Hongkong) | Nawas- Nazario (Canada) |
|---|---|---|---|---|
| GG + GT | REF | ref | ||
| TT (DOMINANT) | 1.34 (0.79,2.27) 0.277 NS | 2.30 (1.25–4.23) 0.007 Significant |
0.87 (0.69,1.09) 0.219 NS |
p-0.02 wild type in additive associated with disease |
| GG + TT | REF | |||
| GT (OVERDOMINANT) | 1.09 (0.74,1.61) 0.661 NS |
0.85 (0.71,1,01) 0.067 NS |
||
| GG | REF | 10.7 (4.26–26.9) 0.0001 significant | ||
| GT + TT (RECESSIVE) | 0.77 (0.5,1.16)0.211 NS |
Ref | 0.67 (0.45,1.00) 0.049 Significant |
Protective - significant |
| Allelic model | ||||
| G | 1.23 (0.93,1.63) 0.149 | 3.08 (2.03–4.69) 0.0001 Significant |
||
| T | ref | ref | ||
The low levels of vitamin D seen in the present study has been similar to studies done in India and has also been observed in a meta-analysis [28,29]. However, the present study was the first in India to analyze the association of Vitamin D levels to the VDR polymorphisms. The association of rs1544410 and rs7041 was observed in the present study which is in contrast to studies done by Papadopoulou et al. This apparent difference in associations can be attributed to altered VDR functionality affected by vitamin D levels which leads to altered signaling [24].
Though levels of serum IgE and AEC were significantly higher in cases and also showed an increasing trend in the mean levels when the severity of asthma increased, the polymorphisms did not show any relationship to the same. The association of serum IgE levels and AEC in children with asthma has been similar to several studies in India, however, the present study was the first to analyze the same in relation to the VDR and GC gene polymorphisms [[30], [31], [32], [33], [34], [35]].
6. Conclusion
In conclusion, this study showed significant correlation of biochemical parameters in children with asthma and association of polymorphisms with Vitamin D gene.
This is the first study to be undertaken in South India on VDR polymorphisms and perhaps the first to look into the association of rs7041 in Indian children. Three polymorphisms, rs7975232, rs731236, and rs1544410 were found to be protective. The association of rs1544410 appears to be unique for the studied South Indian population. rs154410, and rs7041 were also associated with vitamin D levels. None of the polymorphisms showed any association with the severity of asthma. The present study also reflected the apparent gene pool difference within India probably due to the difference in genetic lineage. Further population research on these polymorphisms can add to knowledge and insight and aid in our country's intervention strategies with vitamin D.
Our study was also perhaps the first in India to analyze the association of serum IgE levels, AEC and vitamin D to the above polymorphisms in India in children.
The main limitation of the present study has been the limited sample size.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
CRediT authorship contribution statement
Narmada Ashok: Writing - original draft, Validation, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Radha Saraswathy: Writing - review & editing, Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
1. Department of Genetic Diagnostics, Anderson Diagnostic Labs, Chennai, for analysis.
2. Mr. John Michael, Statistician, St. John's Medical College, and Hospital, Bangalore, for statistical analysis.
3. Dr. Ponnuraja, Head of Department of Statistics, Indian Council of Medical Research for Haplotype analysis.
3. Dr. Nedunchezian, Professor,Mehta Hospitals, Chennai for critical inputs.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23673.
Contributor Information
Narmada Ashok, Email: dr.narmadhaashok@gmail.com.
Radha Saraswathy, Email: radhasaraswathy@vit.ac.in.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Krishna M.T., Mahesh P.A., Vedanthan P., Moitra S., Mehta V., Christopher D.J. An appraisal of allergic disorders in India and an urgent call for action. World Allergy Organization Journal. 2020 doi: 10.1016/j.waojou.2020.100446. https://www.worldallergyorganizationjournal.org/article/S1939-4551(20)30349-5/fulltext Jul 1[cited 2021 Oct 24];13(7). Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh S., Salvi S., Awasthi S., Singh N., Barne M.S., Singh M., et al. Time trends in prevalence of symptoms of asthma in Indian children: ISAAC and GAN studies. Eur. Respir. J. 2020 https://erj.ersjournals.com/content/56/suppl_64/200 Sep 7[cited 2021 Oct 25];56(suppl 64). Available from: [Google Scholar]
- 3.Thomsen S.F. Genetics of asthma: an introduction for the clinician. Eur Clin Respir J. 2015 Jan 16:2. doi: 10.3402/ecrj.v2.24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bantz S.K., Zhu Z., Zheng T. The role of vitamin D in pediatric asthma. Ann Pediatr Child Health. 2015;3(1):1032. [PMC free article] [PubMed] [Google Scholar]
- 5.Balasubramanian S., Dhanalakshmi K., Amperayani S. Vitamin D deficiency in childhood-a review of current guidelines on diagnosis and management. Indian Pediatr. 2013 Jul;50(7):669–675. doi: 10.1007/s13312-013-0200-3. [DOI] [PubMed] [Google Scholar]
- 6.Surve S, Chauhan S, Amdekar Y, Joshi B.Vitamin D Deficiency in Children: an Update on its Prevalence, Therapeutics and Knowledge Gaps. :8..
- 7.Ashok N., Kirubakaran R., Saraswathy R. Association of vitamin D gene polymorphisms in children with asthma - a systematic review. Heliyon. 2020 Sep 1;6(9) doi: 10.1016/j.heliyon.2020.e04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awasthi N., Awasthi S., Pandey S., Gupta S. Association of vitamin D receptor gene polymorphisms in North Indian children with asthma: a case-control study. Int J Mol Epidemiol Genet. 2021 Apr 15;12(2):24–34. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y., Li S. Meta-analysis of vitamin D receptor gene polymorphisms in childhood asthma. Frontiers in Pediatrics. 2022 doi: 10.3389/fped.2022.843691. https://www.frontiersin.org/articles/10.3389/fped.2022.843691 cited 2022 Sep 3];10. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramadan A., Sallam S.F., Elsheikh M.S., Ishak S.R., Abdelsayed M.G.R., Salah M., et al. VDR gene expression in asthmatic children patients in relation to vitamin D status and supplementation. Gene Reports. 2019 Jun;15 [Google Scholar]
- 11.Chishimba L., Thickett D.R., Stockley R.A., Wood A.M. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax. 2010 May 1;65(5):456–462. doi: 10.1136/thx.2009.128793. [DOI] [PubMed] [Google Scholar]
- 12.Li F., Jiang L., Willis-Owen S.A., Zhang Y., Gao J. Vitamin D binding protein variants associate with asthma susceptibility in the Chinese han population. BMC Med. Genet. 2011 Aug 3;12:103. doi: 10.1186/1471-2350-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.GINA strategy Management of asthma | Independent professional body guideline | Guidelines. 2021. https://www.guidelines.co.uk/respiratory/gina-strategy-2021-management-of-asthma/455344.article [Internet]. [cited 2022 Jan 30]. Available from.
- 14.Sharif A., Haddad Kashani H., Sharif M.R. Association of 25-hydroxy vitamin D with asthma and its severity in children: a case-control study. Clin. Mol. Allergy. 2020;18:7. doi: 10.1186/s12948-020-00122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2021. R: A Language and Environment for Statistical ## Computing.https://www.R-project.org/https://www.R-project.org/ [Google Scholar]
- 16.Mohammadi A., Khanbabaei H., Nasiri-Kalmarzi R., Khademi F., Jafari M., Tajik N. Vitamin D receptor ApaI (rs7975232), BsmI (rs1544410), Fok 1 (rs2228570), and TaqI (rs731236) gene polymorphisms and susceptibility to pulmonary tuberculosis in an Iranian population: a systematic review and meta-analysis. J. Microbiol. Immunol. Infect. 2020;53(6):827–835. doi: 10.1016/j.jmii.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Rajaram M., Selvarajan S., Neelamegan R., Kamalanathan S., Gunaseelan V., Xavier A.S., et al. Effects of genetic polymorphisms in Vitamin D metabolic pathway on Vitamin D level and asthma control in South Indian patients with bronchial asthma. Lung India. 2019;36(6):483–491. doi: 10.4103/lungindia.lungindia_23_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou C., Zhu X., Chang X. Correlation of vitamin D receptor with bronchial asthma in children. Exp. Ther. Med. 2018 Mar 1;15(3):2773–2776. doi: 10.3892/etm.2018.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munkhbayarlakh S., Kao H.F., Hou Y.I., Tuvshintur N., Bayar-Ulzii B., Narantsetseg L., et al. Vitamin D plasma concentration and vitamin D receptor genetic variants confer risk of asthma: a comparison study of Taiwanese and Mongolian populations. World Allergy Organ J. 2019 Nov 1;12(11) doi: 10.1016/j.waojou.2019.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung T.F., Wang S.S., Tang M.F., shan Kong AP., Sy H.Y., Hon K.L., et al. Childhood asthma and spirometric indices are associated with polymorphic markers of two vitamin D 25-hydroxylase genes. Pediatr. Allergy Immunol. 2015 Jun;26(4):375–382. doi: 10.1111/pai.12392. [DOI] [PubMed] [Google Scholar]
- 21.Iordanidou M., Paraskakis E., Giannakopoulou E., Tavridou A., Gentile G., Borro M., et al. Vitamin D receptor ApaI a allele is associated with better childhood asthma control and improvement in ability for daily activities. OMICS A J. Integr. Biol. 2014 Nov;18(11):673–681. doi: 10.1089/omi.2014.0023. [DOI] [PubMed] [Google Scholar]
- 22.Kilic M., Ecin S., Taskin E., Sen A., Kara M. The vitamin D receptor gene polymorphisms in asthmatic children: a case-control study. Pediatr Allergy Immunol Pulmonol. 2019 Jun 1;32(2):63–69. doi: 10.1089/ped.2018.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maalmi H., Sassi F.H., Berraies A., Ammar J., Hamzaoui K., Hamzaoui A. Association of vitamin D receptor gene polymorphisms with susceptibility to asthma in Tunisian children: a case control study. Hum. Immunol. 2013 Feb;74(2):234–240. doi: 10.1016/j.humimm.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulou A., Kouis P., Middleton N., Kolokotroni O., Karpathios T., Nicolaidou P., et al. Association of vitamin D receptor gene polymorphisms and vitamin D levels with asthma and atopy in Cypriot adolescents: a case–control study. Multidisciplinary Respiratory Medicine. 2015 Sep 4;10(1):26. doi: 10.1186/s40248-015-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makoui M.H., Imani D., Motallebnezhad M., Azimi M., Razi B. Vitamin D receptor gene polymorphism and susceptibility to asthma. Ann. Allergy Asthma Immunol. 2020 Jan;124(1):57–69. doi: 10.1016/j.anai.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Fawzy M.S., Elgazzaz M.G., Ibrahim A., Hussein M.H., Khashana M.S., Toraih E.A. Association of group‐specific component exon 11 polymorphisms with bronchial asthma in children and adolescents. Scand. J. Immunol. 2018 Dec 12 doi: 10.1111/sji.12740. [DOI] [PubMed] [Google Scholar]
- 27.Navas-Nazario A., Li F., Shabanova V., Weiss P., Cole D.E., Carpenter T.O., et al. Effect of vitamin D binding protein (DBP) genotype on the development of asthma in children. Ann. Allergy Asthma Immunol. 2014 Jun;112(6):519–524. doi: 10.1016/j.anai.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anand V., Yadev I., Bindusha S. Severity of asthma and Vitamin D status in children: a case–control study in a tertiary care center. Indian J. Allergy Asthma Immunol. 2020;34(2):103. [Google Scholar]
- 29.Jat K.R., Khairwa A. Vitamin D and asthma in children: a systematic review and meta-analysis of observational studies. Lung India. 2017;34(4):355–363. doi: 10.4103/0970-2113.209227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandran CNM, Kiran GS, Babu KR, Buchineni M.Serum IgE Levels as a Marker of Disease Activity in Childhood Asthma - A Cross Sectional Study..
- 31.Begum A G.S., N R N.R.K. A study on correlation of serum IgE levels with diagnosis and severity of asthma in children. International Journal of Contemporary Pediatrics. 2018 Oct 22;5(6):2240–2243. [Google Scholar]
- 32.Rathoria E., Bansal U., Gupta A., Gupta N.B., Ahuja R., Rathoria R. Study of serum IgE levels in childhood asthma in Barabanki region, India. Int J Contemp Pediatr. 2018 Aug 24;5(5):1755. [Google Scholar]
- 33.Chaudhary G.S., Kumar A., Shashtri M., Chaudhary V. Comparison of total serum immunoglobulin E and absolute eosinophil count levels among asthmatic and non-asthmatic children. Indian J. Child Health. 2017 Sep 26;4(3):345–347. [Google Scholar]
- 34.Lama M., Chatterjee M., Chaudhuri T.K. Total serum immunoglobulin E in children with asthma. Indian J. Clin. Biochem. 2013 Apr;28(2):197–200. doi: 10.1007/s12291-012-0247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trivedi P.P., Patel A.H. Serum immunoglobulin E and absolute eosinophil count as markers of severity in childhood asthma. International Journal of Contemporary Pediatrics. 2020 Jan 23;7(2):413–418. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.