Abstract
Cerebrovascular events may attribute to the fragmentation of a cardiac tumor. Due to the small number of reported cases of large vascular occlusion-acute ischemic stroke (LVO-AIS) associated with atrial myxoma, current guidelines still follow the principle of intravenous thrombolysis priority, even if LVO-AIS patients are eligible for mechanical thrombectomy, and have not recommended the timing of cardiac surgery or preoperative anticoagulation and antithrombotic therapy. Surgical removal is the definitive therapy for cardiac myxomas, especially for left-sided myxomas. With this case, we aim to demonstrate the main challenges that clinicians may encounter when dealing with patients with AIS secondary to cardiac myxoma: the difficulties with clinical diagnosis, strategies for reperfusion therapy, and therapeutic management of cardiac myxoma.
Keywords: Cardiac myxoma, Cardiovascular event, Large vascular occlusion-acute ischemic stroke (LVO-AIS), Mechanical thrombectomy (MT), Case report
1. Introduction
Cardioembolic stroke accounts for 20 % of all acute ischemic stroke (AIS) cases, of which cardiac myxoma only accounts for 0.5 %, with females in their 50s being at the greatest risk [1,2]. About 75 % of cardiac myxomas originate in the left atrium; hence, systemic embolism is particularly frequent, especially that of the cerebral arteries [3]. A stroke due to cardioembolism is generally severe and life-threatening. Therefore, urgent vascular recanalization and cardiac myxoma resection are required after diagnosis.
2. Case presentation
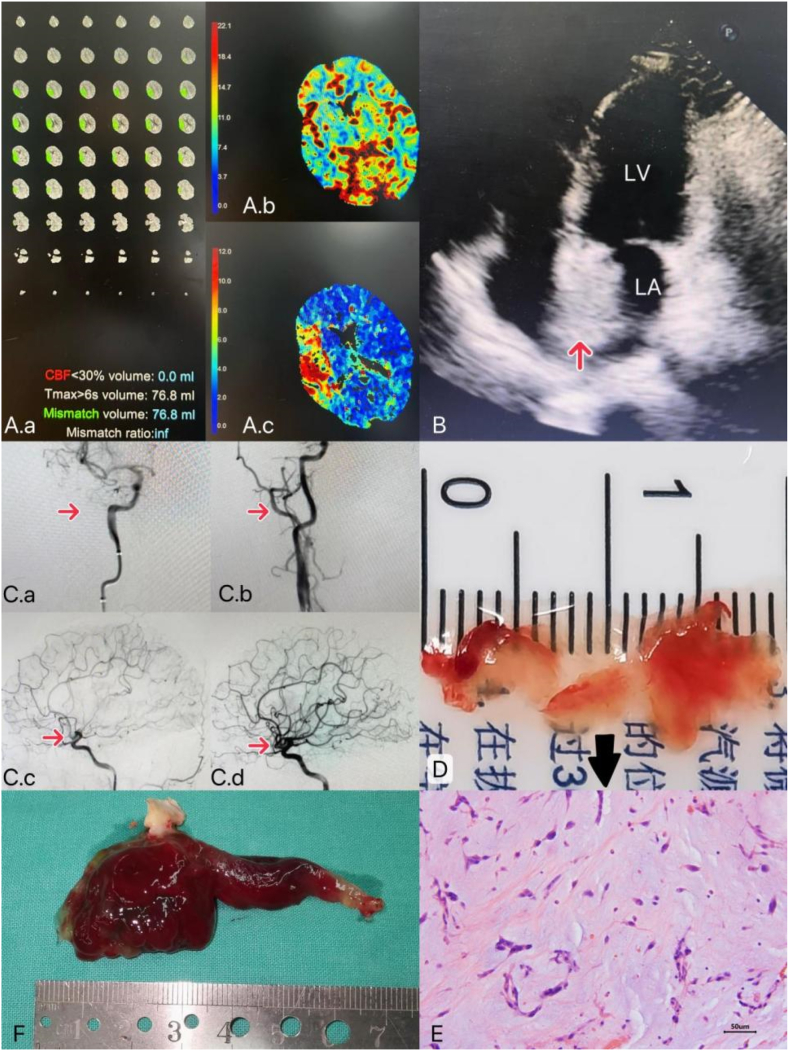
A previously healthy 55-year-old woman without known medical problems presented to our hospital with sudden weakness in the left limbs, slurred speech, and drooling for 4 h. Her consciousness became altered on the way to the hospital. Left facial paresis, grade 0 and grade 3 myodynamia of the left and right limbs, respectively, and positive left Babinski sign were found on physical exam. The National Institutes of Health Stroke Scale (NIHSS) score was 14. Brain computed tomography (CT) angiography confirmed the diagnosis of large vascular occlusion-acute ischemic stroke (LVO-AIS) with total infarction of the anterior circulation. Fast-processing of ischemic stroke (F-STROKE) suggested an extremely low ischemic core volume (ICV) and large penumbra volume (PV), which could predict that mechanical thrombectomy (MT) would be the most beneficial treatment (Fig. 1A). Furthermore, a characteristic “tumor plop” was identified during cardiac auscultation at the early diastolic mitral region. Transthoracic echocardiography revealed a solid mass of 38 mm × 25 mm in the left atrium (Fig. 1B). She underwent MT 4.5 hours after the stroke onset without intravenous thrombolysis (IVT) (Fig. 1C). Afterward, her neurological symptoms significantly improved. NIHSS score was changed to 4. The embolus removed from the intracranial artery was not a regular blood clot but a gelatinous one (Fig. 1D). Moreover, the pathological assessment indicated that it was a part of myxoma (Fig. 1E). She had underwent a successful left atrial myxoma resection and atrial septal repair within seven days of hospitalization (Fig. 1F). Due to non-thrombotic LVO-AIS, only a low dose of low molecular weight heparin sodium (100 UI/kg/day) was administered preoperatively, reducing the amount of extravasated blood exudation by approximately 400 mL after cardiopulmonary bypass. She had an unremarkable postoperative course and was discharged home eight days after the surgery. There was no symptomatic intracranial hemorrhage (sICH) during the two-month follow-up.
Fig. 1.
(A) F-STROKE demonstration. (A.a) hypoperfusion volume: 76.8 mL, infarction core volume: 0 mL, mismatch volume: 76.8 mL. (A.b) Cerebral blood flow (CBF), blood flow scales from slow (dark blue) to fast (deep red), decreased blood flow from right cerebral artery obstruction is indicated by green and blue areas. (A.c) T max (time to peak) represents the time for blood to fill the vessels. The higher the number (the redder the color), the more severe the cerebral ischemia. (B) Transthoracic echocardiography shows a 38 × 25-mm pedicled solid mass in the left atrium attaching to the atrial septum. Arrow indicates myxoma cordis in the left atrium. LA, left atrium; RA, right atrium. (C) Cerebral angiography before and after MT. (C.a, C.c) Before: complete occlusion was observed at the bifurcation of the M1 segment of the right middle cerebral artery. The right anterior cerebral artery partially compensates for blood flow to the brain region supplied by the right middle cerebral artery. (C.b, C.d) After: the occluded artery was recanalized. (D) A gelatinous embolus under the naked eye. (E) Histopathology of the gelatinous embolus 200 × , showing abundant mucinous spindle cells, characteristic acidic mucopolysaccharide matrix, and embedded polygons, consistent with cardiac myxoma. (F) A rare bifurcated myxoma. Its bigger part is in the left atrium facing the mitral valve direction, while the other portion is heading into the left atrial appendage. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
There were two major difficulties in this case, make strategies for reperfusion therapy and investigate the cause of cerebral embolism, especially the former.
Effective reperfusion therapy includes IVT and MT. Alteplase IVT administered within 4.5 hours since stroke onset had improved body function at 3–6 months [4]. However, MT can be performed in stroke specialty centers with anterior circulated stroke based on imaging features within 6–24 hours of stroke onset [5]. This case demonstrated that MT alone could achieve a good clinical outcome for LVO-AIS caused by left atrial myxoma, because the decision time of reperfusion therapy was out of the critical IVT time window (4.5 h). Additionally, the patient had no underlying diseases or vascular risk factors. Meanwhile, F-stroke indicated that MT would provide more net clinical benefit for this patient [6]. Measurements of acute ICV and PV have been considered as effective predictors for clinical outcome in patients receiving MT [7,8]. It is well evidenced that MT is more beneficial in patients with small ICV (<70 mL), large PV (>15 mL), and a mismatch ratio of <1.8 between total hypoperfusion and ICV [9]. MT may be superior to intravenous thrombolysis by minimizing the risk of hemorrhage in the setting of atrial myxoma emboli [10]. Indeed, the patient who underwent MT without IVT showed marked improvement in neurological signs and symptoms within seven days and did not develop sICH. Therefore, it may be reasonable to support the implementation of a rapid and direct MT-alone strategy instead of bridging therapy in patients with AIS-LVO.
Although cardiac myxoma should be resected as soon as possible to avoid systemic embolism that endangers life, current guidelines have not recommended the timing of cardiac surgery or related preoperative anticoagulation and antithrombotic therapy due to the small number of reported cases of atrial myxoma-associated AIS. Prolonged time interval between stroke and tumor excision surgery is significantly associated with stroke recurrence [10]. On account of the most spontaneous hemorrhagic transformation after AIS occurs within seven days of symptom onset [11]. Hence, this patient underwent cardiac surgery seven days after recanalization with MT and appeared to be operable and safe to operate.
IVT prior to MT is referred to as bridging therapy recommended for most patients for whom appropriate to both types of reperfusion therapy [12]. Patients with LVO-AIS should receive IVT immediately if eligible, even if MT is considered [13]. MT should then begin as soon as possible [14]. Every 10 minutes of earlier MT treatment resulted in 39 days of disability-free life [15]. Treatment of LVO-AIS caused by embolic atrial myxoma is controversial, largely because the embolus could be composed of the tumor itself, adhered thrombotic material or a combination of both [16]. The potential disadvantages of IVT initiation include delayed MT, increased risk of sICH, and migration to the distal part of the vessel beyond the reach of MT after the partial dissolution of large vessel thrombosis [17]. Controversially, IVT or bridging therapy may temporarily recirculate vessels because the embolus may consist of a tumor, adherent thrombus, or a combination of both, yet the risk of ICH is high, especially considering fatal sICH after cardiopulmonary bypass [16]. At the same time, there is increasing evidence that MT alone may not be inferior to the clinical outcome achieved by bridging therapy [[18], [19], [20]]. An updated meta-analysis in more than 2000 patients with acute ischemic stroke showed that sICH occurred in 5 % of patients after IVT, and cerebral micro-bleeds presence on pretreatment MRI scans is associated with an approximate doubling of the risk of sICH following IVT [21]. Another meta-analysis reported 8.6 % of sICH after bridging therapy [12]. Accordingly, it is sometimes reasonable to avoid the use of both medical and mechanical treatments in favor of a single strategy of rapid and direct MT for AIS-LVO patients.
Although, MT has represented important progress in the treatment of LVO-AIS in recent years, which can significantly improve the prognosis [14,15]. However, the current guidelines still follow the principle of IVT priority, and bridging therapy is currently superior to direct thrombectomy in patients with anterior circulation ischemic stroke [13,22,23].
TOAST classification for ischemic stroke has been widely used with good inter-observer consistency. The five TOAST subtypes of ischemic stroke are: large artery atherosclerosis, cardioembolism, small vessel occlusion, stroke of other determined etiology, and stroke of undetermined etiology [24]. Patient in this case was a manual laborer with good lifestyle, and without traditional vascular risk factors such as hypertension, hyperlipidemia, atherosclerosis, etc. Careful questioning of the patient and her husband revealed that she had never previously had headaches, dizzines, or sudden loss of consciousness after strenuous exercise, amaurosis fugax, aphasia or dysarthria, hemianopsia, loss of partial sensation, amnesia, or confusion. On the basis of detailed history taking, the patient's family history included hypertension only in her father, and no other family history was identified. We should beware of cardioaortic sources of cerebral embolism, such as atrial fibrillation, valvular heart disease, myxoma, etc. Therefore, emergent echocardiography should be completed prior to treatment, providing basis for MT. Transthoracic echocardiography is often recommended to plan secondary stroke management, providing diagnosis evidence of cardiogenic stroke [25,26]. It's not only wide availability, non-invasive, but also giving information about the cardiac myxoma such as myxoma location, pedicle location, size, activity form [27]. In this case, the myxoma showed a rare bifurcated shape. The echocardiography revealed only the portion of myxoma in the left atrium facing the mitral valve. In fact, a portion of the myxoma protruded into the left atrial appendage, making the excision procedure extra difficult. Consequently, preoperative transesophageal echocardiography (TEE) would improve the success rate of this rare bifurcated myxoma excision.
A delayed cerebral aneurysm may appear or continue to grow despite the surgical removal of the atrial myxoma [28]. Aneurysms could be found before the diagnosis, at the same time as cardiac myxoma, or even 25 years after resection of the atrial mass, the mean time to diagnoses was 4.5 years [29]. Therefore, long-term follow-up of patients with cardiac myxomas for possible cooccurrence of cerebral aneurysms is also very important [30]. Cerebral aneurysm was not found before MT and 14 days after the heart surgery in this case. We will review cerebral angiography on her annually for early detection and intervention.
4. Conclusion
In summary, we herein presented a case of LVO-AIS secondary to the left atrial myxoma that achieved good clinical outcome with MT, reducing the risk of sICH before and after the heart surgery. According to this case, we strongly suggest that echocardiography should be completed in an emergency room to exclude cardioaortic sources of cerebral embolism in patients with no vascular risk factors, providing a basis for reperfusion therapy. We also found that left atrial myxoma could be excised within seven days after MT, along with administering preoperative low-dose heparin. Additionally, preoperative TEE can improve the success rate of myxoma excision surgery because of the different shapes that cardiac myxoma can present with.
Funding
This work was supported by Clinical Key Specialty Construction Foundation of Shanghai (no. shslczdzk04701). The funding source has no role in the design of the study and will not have any role during the execution, analyses, interpretation of the data, or decision to submit results.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
This case was reviewed and approved by the Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine affiliated to Shanghai Traditional Chinese Medicine. The patient had provided her written informed consent to participate in this case study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
CRediT authorship contribution statement
Xindi Wu: Writing – review & editing, Writing – original draft, Project administration, Methodology. Tongyu Chen: Supervision, Resources, Project administration. Yan Han: Resources, Project administration, Data curation. Ke Wang: Validation, Supervision. Jia Zhou: Visualization, Validation, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the patient who participated in this study and the staff from the Departments of Cardiothoracic Surgery and Neurology.
References
- 1.Yuan S.M., Humuruola G. Stroke of a cardiac myxoma origin. Rev. Bras. Cir. Cardiovasc. 2015;30:225–234. doi: 10.5935/1678-9741.20150022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferro J.M. Cardioembolic stroke: an update. Lancet Neurol. 2003;2:177–188. doi: 10.1016/s1474-4422(03)00324-7. [DOI] [PubMed] [Google Scholar]
- 3.K. R. Cardiac myxomas. N. Engl. J. Med. 1995;333:1610–1617. doi: 10.1056/NEJM199512143332407. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakaran S., Ruff I., Bernstein R.A. Acute stroke intervention: a systematic review. JAMA. 2015;313:1451–1462. doi: 10.1001/jama.2015.3058. [DOI] [PubMed] [Google Scholar]
- 5.Nogueira R.G., Jadhav A.P., Haussen D.C., Bonafe A., Budzik R.F., Bhuva P., et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N. Engl. J. Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 6.Shi Z., Li J., Zhao M., Zhang M., Wang T., Chen L., et al. Baseline cerebral ischemic core quantified by different automatic software and its predictive value for clinical outcome. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.608799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bivard A., Levi C., Krishnamurthy V., McElduff P., Miteff F., Spratt N.J., et al. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain. 2015;138:1919–1931. doi: 10.1093/brain/awv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiehler J., Thomalla G., Bernhardt M., Kniep H., Berlis A., Dorn F., et al. Eraser. Stroke. 2019;50:1275–1278. doi: 10.1161/STROKEAHA.119.024858. [DOI] [PubMed] [Google Scholar]
- 9.Wannamaker R., Guinand T., Menon B.K., Demchuk A., Goyal M., Frei D., et al. Computed tomographic perfusion predicts poor outcomes in a randomized trial of endovascular therapy. Stroke. 2018;49:1426–1433. doi: 10.1161/STROKEAHA.117.019806. [DOI] [PubMed] [Google Scholar]
- 10.Rosário M., Fonseca A.C., Sotero F.D., Ferro J.M. Neurological complications of cardiac tumors. Curr. Neurol. Neurosci. Rep. 2019:15. doi: 10.1007/s11910-019-0931-1. 26,19(4) [DOI] [PubMed] [Google Scholar]
- 11.Liu G.Z., Hu R., Peng D.T., G. B. o. C. M. A. Geriatric Neurology Group and s. Writing Group of Chinese expert consensus on diagnosis of cardiogenic. Consensus on diagnosis and treatment of hemorrhagic transformation after acute ischemic stroke in China 2019. Chin. J. Neurol. 2019;4:252–265. doi: 10.1097/CM9.0000000000001217. [DOI] [Google Scholar]
- 12.Mazighi M., Meseguer E., Labreuche J., Amarenco P. Bridging therapy in acute ischemic stroke: a systematic review and meta-analysis. Stroke. 2012;43:1302–1308. doi: 10.1161/STROKEAHA.111.635029. [DOI] [PubMed] [Google Scholar]
- 13.Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 14.Jahan R., Saver J.L., Schwamm L.H., Fonarow G.C., Liang L., Matsouaka R.A., et al. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA. 2019;322:252–263. doi: 10.1001/jama.2019.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunz W.G., Hunink M.G., Almekhlafi M.A., Menon B.K., Saver J.L., Dippel D.W.J., et al. Public health and cost consequences of time delays to thrombectomy for acute ischemic stroke. Neurology. 2020;95:e2465–e2475. doi: 10.1212/WNL.0000000000010867. [DOI] [PubMed] [Google Scholar]
- 16.da Silva I.R., de Freitas G.R. Is it safe to proceed with thrombolytic therapy for acute ischemic stroke in a patient with cardiac myxoma? Case report and review of the literature. Eur. Neurol. 2012;68:185–186. doi: 10.1159/000340019. [DOI] [PubMed] [Google Scholar]
- 17.Saver Jl O.A. Intravenous thrombolysis before endovascular thrombectomy for acute ischemic stroke. JAMA. 2021;325:229–231. doi: 10.1001/jama.2020.22388. [DOI] [PubMed] [Google Scholar]
- 18.Yang P., Zhang Y., Zhang L., Zhang Y., Treurniet K.M., Chen W., et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N. Engl. J. Med. 2020;382:1981–1993. doi: 10.1056/NEJMoa2001123. [DOI] [PubMed] [Google Scholar]
- 19.Zi W., Qiu Z., Li F., Sang H., Wu D., Luo W., et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021;325:234–243. doi: 10.1001/jama.2020.23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki K., Matsumaru Y., Takeuchi M., Morimoto M., Kanazawa R., Takayama Y., et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021;325:244–253. doi: 10.1001/jama.2020.23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charidimou A., Shoamanesh A., Wilson D., Gang Q., Fox Z., Jäger H.R., et al. Cerebral microbleeds and postthrombolysis intracerebral hemorrhage risk Updated meta-analysis. Neurology. 2015;85 doi: 10.1212/WNL.0000000000001923. 927-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chinese Society of Neurology Chinese stroke society, neurovascular intervention group of Chinese society of Neurology. Chinese guidelines for the endovascular treatment of acute ischemic stroke 2022. Chin. J. Neurol. 2022;55:565–580. doi: 10.1161/STR.0000000000000211. [DOI] [Google Scholar]
- 23.Turc G., Tsivgoulis G., Audebert H.J., Boogaarts H., Bhogal P., De Marchis G.M., et al. European Stroke Organisation (ESO)-European Society for Minimally Invasive Neurological Therapy (ESMINT) expedited recommendation on indication for intravenous thrombolysis before mechanical thrombectomy in patients with acute ischemic stroke and anterior circulation large vessel occlusion. J. Neurointerventional Surg. 2022;14(3):209. doi: 10.1136/neurintsurg-2021-018589. [DOI] [PubMed] [Google Scholar]
- 24.Adams H.P., Jr., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 25.Qin L., Xu X., Ding L., Li Z., Li J. Identifying diagnosis evidence of cardiogenic stroke from Chinese echocardiograph reports. BMC Med. Inf. Decis. Making. 2020;20:126. doi: 10.1186/s12911-020-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fralick M., Goldberg N., Rohailla S., Guo Y., Burke M.J., Lapointe-Shaw L., et al. Value of routine echocardiography in the management of stroke. CMAJ (Can. Med. Assoc. J.) 2019;191:E853–E859. doi: 10.1503/cmaj.190111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyebally S., Chen D., Bhattacharyya S., Mughrabi A., Hussain Z., Manisty C., et al. Cardiac tumors: JACC CardioOncology state-of-the-art review. JACC CardioOncol. 2020;2:293–311. doi: 10.1016/j.jaccao.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Q., Zhang X., Wu P., Wang M., Zhou Y., Feng Y. Multiple intracranial aneurysms followed left atrial myxoma: case report and literature review. J. Thorac. Dis. 2013;5(6):E227–E231. doi: 10.3978/j.issn.2072-1439.2013.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chojdak-Łukasiewicz J., Budrewicz S., Waliszewska-Prosół M. Cerebral aneurysms caused by atrial myxoma-A systematic review of the literature. J. Personalized Med. 2022:8. doi: 10.3390/jpm13010008. 21;13(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ku L., Cheng Y., Ma X. A rare left atrial myxoma associated with multiple intracranial aneurysms. J. Card. Surg. 2022;37(8):2414–2415. doi: 10.1111/jocs.16621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.