Abstract
Background
Immunocompromised patients receiving B-cell-depleting therapies are at increased risk of persistent SARS-CoV-2 infection, with many experiencing fatal outcomes. We report a successful outcome in a patient with rheumatoid arthritis (RA) on rituximab diagnosed with COVID-19 in July 2020 with persistent infection for over 245 days.
Results
The patient received numerous treatment courses for persistent COVID-19 infection, including remdesivir, baricitinib, immunoglobulin and high doses of corticosteroids followed by a prolonged taper due to persistent respiratory symptoms and cryptogenic organizing pneumonia. Her clinical course was complicated by Pseudomonas aeruginosa sinusitis with secondary bacteremia, and cytomegalovirus (CMV) viremia and pneumonitis. SARS-CoV-2 positive RNA samples were extracted from two nasopharyngeal swabs and sequenced using targeted amplicon Next-Generation Sequencing which were analyzed for virus evolution over time. Viral sequencing indicated lineage B.1.585.3 SARS-CoV-2 accumulated Spike protein mutations associated with immune evasion and resistance to therapeutics. Upon slowly decreasing the patient's steroids, she had resolution of her symptoms and had a negative nasopharyngeal SARS-CoV-2 PCR and serum CMV PCR in March 2021.
Conclusion
A patient with RA on B-cell depleting therapy developed persistent SARS-CoV-2 infection allowing for virus evolution and had numerous complications, including viral and bacterial co-infections with opportunistic pathogens. Despite intra-host evolution with a more immune evasive SARS-CoV-2 lineage, it was cleared after 245 days with reconstitution of the patient's immune system.
Keywords: COVID-19, Persistent infection, Variant of concern, Virus evolution, Immunocompromised
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has caused more than 768 million reported infections and 6.9 million reported deaths worldwide since it was first recognized in 2019 [1]. Individuals who develop COVID-19 typically have an acute infection that resolves 7–14 days after symptom onset [[2], [3], [4], [5], [6], [7]]. Individuals who are immunocompromised, such as those with cancer, solid organ transplants, human immunodeficiency virus (HIV), and receiving immunomodulators may develop prolonged infection that can be life-threatening. Persistent infections are known to lead to the accumulation of immune escape mutations of concern [8].
Rituximab, a B-cell-depleting therapy, targets the CD20 antigen expressed on the surface of B lymphocytes and decreases the number of B-cells for over 6 months [9]. Rituximab tends to increase one's risk of infection, cause a delay in clearance of some infections, and has been shown to interfere with the development of specific antibodies following influenza, hepatitis B, and SARS-CoV-2 vaccinations [10,11]. Rituximab treatment has been identified as a risk factor for long-term SARS-CoV-2 persistence and associated with severe disease and death [12]. We report on an immunocompromised individual with rheumatoid arthritis (RA), treated with rituximab who developed persistent SARS-CoV-2 infection for 245 days which cleared following numerous hospitalizations which included various treatment courses for COVID-19 and reconstitution of the patient's immune system. Examples of positive outcomes can inform future treatments and improve outcomes among immunocompromised populations. Further research is needed to understand complications associated with persistent SARS-CoV-2 infection, in-host SARS-CoV-2 viral evolution, and optimal standard of care for immunocompromised individuals.
2. Case/case series presentation (Results)
2.1. Clinical report
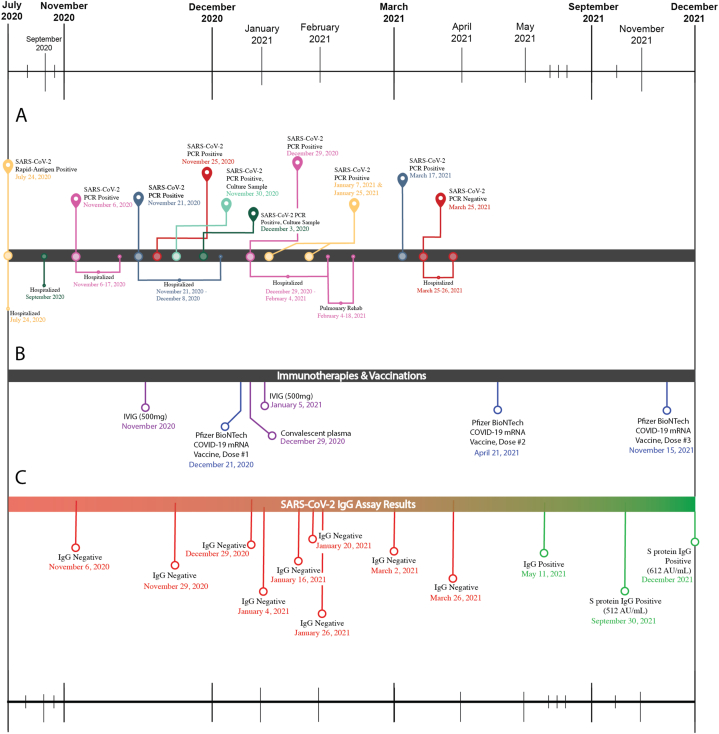
On July 24, 2020, a 64-year-old female with a history of hypertension (HTN), dyslipidemia, diabetes mellitus (DM)-II, anemia, and RA presented with dyspnea, fevers, and cough, and was diagnosed with COVID-19 by rapid antigen test 17 days after receiving her most recent dose of rituximab. She was hospitalized and received a five-day treatment of remdesivir, dexamethasone, intravenous immunoglobulin (IVIG), and antimicrobials for presumed community-acquired pneumonia (CAP). She was discharged home after improvement of her symptoms to complete therapy (Fig. 1). She was readmitted to the hospital in September 2020 for a fever, at which time an extensive work-up, including imaging and bronchoscopy, were unremarkable and was discharged home. She continued to have persistent dyspnea and was admitted to the hospital on November 1, 2020, with fevers and shortness of breath and underwent a bronchoscopy that was negative. She was transferred to Medical University of South Carolina (MUSC) for further care where she was diagnosed with Cryptogenic Organizing Pneumonia (COP) and discharged home on 60 mg prednisone. On November 21, 2020, she was readmitted to the ICU with fevers, dyspnea, and hypoxia and was determined to have persistent SARS-CoV-2 infection in the setting of her immunosuppression. She had a positive nasopharyngeal swab for SARS-CoV-2 real-time reverse transcription polymerase chain reaction (RT-PCR; Cycle number (Cn) = 20.95) and negative SARS-CoV-2 IgG results. She received IVIG (500 mg) on November 24, 2020, and was placed on 125 mg IV methylprednisolone for concern over COP. She was discharged on December 8, 2020, requiring 2 L nasal cannula, 60 mg prednisone, and 50 mg azathioprine.
Fig. 1.
Timelines showing patient disease course, therapies and vaccinations, and results from assessment of antibody-based immune responses to SARS-CoV-2. (A) Immunocompromised patient's clinical course of COVID-19. Maize and purple markers denote SARS-CoV-2 test results. Green denotes a positive viral culture. Downward markers note medical events. (B) Immunotherapies & vaccinations. IVIG (500 mg), convalescent plasma treatment (unknown titer), and vaccination events are shown on the same timescale as A. (C) SARS-CoV-2 IgG assay results. Red markers indicate negative IgG test, green indicate positive IgG test. All IgG tests performed between November 6, 2020, and March 2, 2021, were done using the Abbott ARCHITECT SARS-CoV-2 IgG assay, which detects antibodies directed to a recombinant Nucleocapsid (N) SARS-CoV-2 antigen. IgG tests performed after March 2, 2021, were done using the Liaison SARS-CoV-2 S1/S2 IgG assay (DiaSorin, Italy), which detects IgG antibodies to the spike (S) protein. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
She improved for a few weeks at home and since she was a healthcare worker received her first dose of Pfizer-BioNTech COVID-19 mRNA vaccine on December 21, 2020. However, on December 29, 2020, she developed acute worsening of her symptoms and was readmitted to the hospital, with worsening dyspnea, shortness of breath, and cough, requiring 15 L oxygen. Chest X-ray (CXR) showed bilateral infiltrates (Fig. 2), and nasopharyngeal swab showed a positive SARS-CoV-2 PCR with a Cn = 14.63 and IgG tests remained negative. During her hospitalization, she was also found to have Pseudomonas aeruginosa bacteremia secondary to sinusitis treated with intravenous cefepime and cytomegalovirus (CMV) viremia and presumed pneumonitis treated with intravenous ganciclovir. Due to her critical condition, she was retreated for persistent SARS-CoV-2 with a single dose of convalescent plasma (unknown titer), remdesivir, and 4 mg baricitinib (stopped after two days due to elevated liver function tests (LFTs)). She was also placed on a prednisone taper (Fig. 3) and 100 mg dapsone for Pneumocystis jiroveci pneumonia (PCP) prophylaxis. In early February 2021, she was discharged to pulmonary rehabilitation for two weeks, and then returned home under the care of at-home healthcare workers.
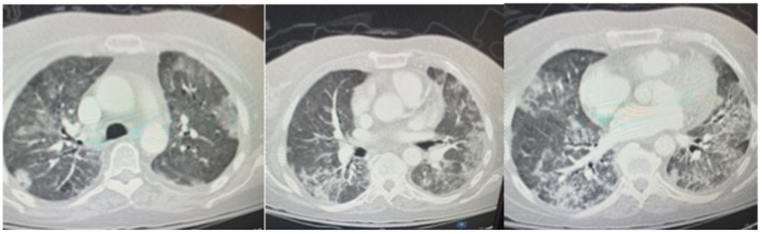
Fig. 2.
The chest computed tomography (CT) scan acquired on December 29, 2020, showed slightly increased small pericardial effusion. No pulmonary embolism was found. Interval improved lung findings compatible with patient's known history of COVID-19 pneumonia
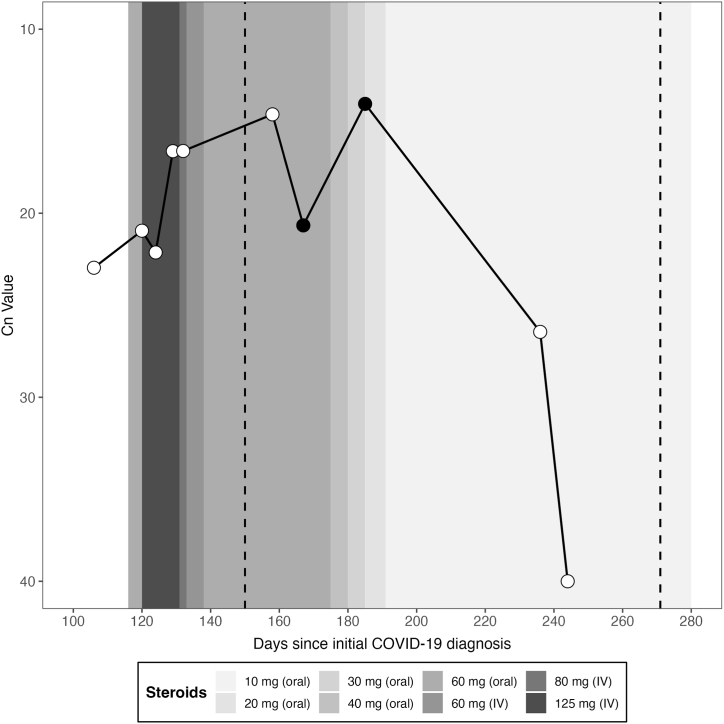
Fig. 3.
Cycle number (Cn) values over immunocompromised patient's clinical course of COVID-19. White points represent Cn values from SARS-CoV-2 rtPCR assays on the indicated day after initial positive SARS-CoV-2 PCR. Black points represent samples that were sequenced. Glucocorticoid steroid treatment (patient received methylprednisolone intravenously (IV) and prednisone orally) is indicated as shaded bars with shading intensity indicating treatment dose. Dashed lines indicate when the patient received her first and second dose of Pfizer BioNTech COVID-19 mRNA vaccine.
On March 17, 2021, she was admitted to the hospital for worsening leukocytosis and thrombocytosis. Chest computed tomography (CT) scan showed significant improvement of the parenchymal opacities previously seen in her lungs. After 245 days of persistent nasopharyngeal SARS-CoV-2 PCR positivity and symptoms in the setting of B-cell depleting therapy, virus eradication was successful – the patient was re-tested in the hospital for SARS-CoV-2 by RT-PCR per hospital protocol and her test came back negative. After work-up for her leukocytosis and thrombocytosis, she was discharged home on March 26, 2021. She received her second and third dose of Pfizer BioNTech COVID-19 mRNA vaccine in April and November 2021, respectively, and developed positive Spike IgG by September 2021 (Fig. 1).
2.2. Viral evolution in the setting of persistent infection
Given the patients prolonged infection with SARS-CoV-2, there was concern for in-host virus evolution in the setting of an immunocompromised host. To evaluate for virus evolution, total RNA was extracted from two nasopharyngeal swabs; viral genomes were then amplified using the ARTIC v4 and v4.1 primer sets as previously described [13]. Amplicons were sequenced using both Illumina and nanopore technologies with the Illumina COVIDSeq Assay and the Oxford Nanopore Ligation Sequencing Kit, respectively. Raw FASTQ files were aligned to the Wuhan Hu-1 reference sequence (MN908947.3) using Bowtie2 v2.4.2, and primers were soft-clipped using SAMtools v1.15.1 [14,15]. Viral genome coverage was assessed with SAMtools, and single nucleotide variants were called with LoFreq v2.1.3.1 [16]. We combined the patient's sequences with all publicly available sequences in GISAID from the patient's home state sampled in January 2021, all B.1.595.3, and the Wuhan reference (Wuhan-Hu-1, NCBI: NC_045512.2), to produce the divergence tree [17]. From GISAID, we conducted quality filter by using complete sequences with full dates and excluding those with low coverage. We removed sequences shorter than 20900 nucleotides or longer than 30100 nucleotides. We aligned the sequences using MAFFT v7.508 [18]. We then masked the 3′ and 5′ regions such that all sequences had defined nucleotides at the ends (no gaps or N's). We built the maximum-likelihood trees using IQ-TREE (v 2.1.4-beta) with the default settings [19].
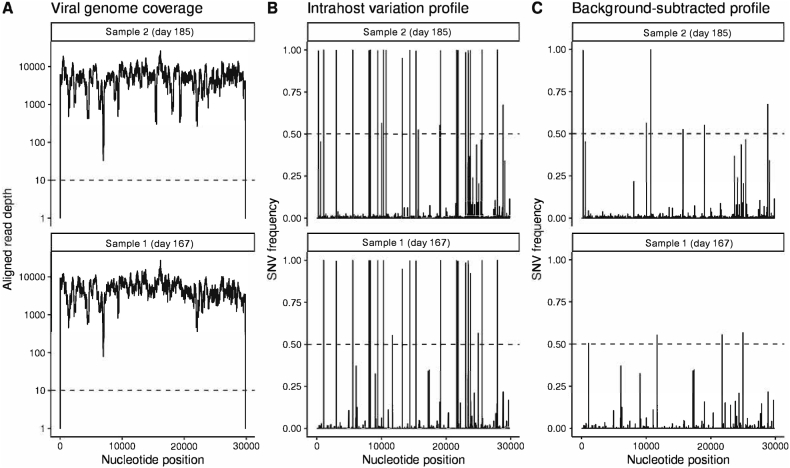
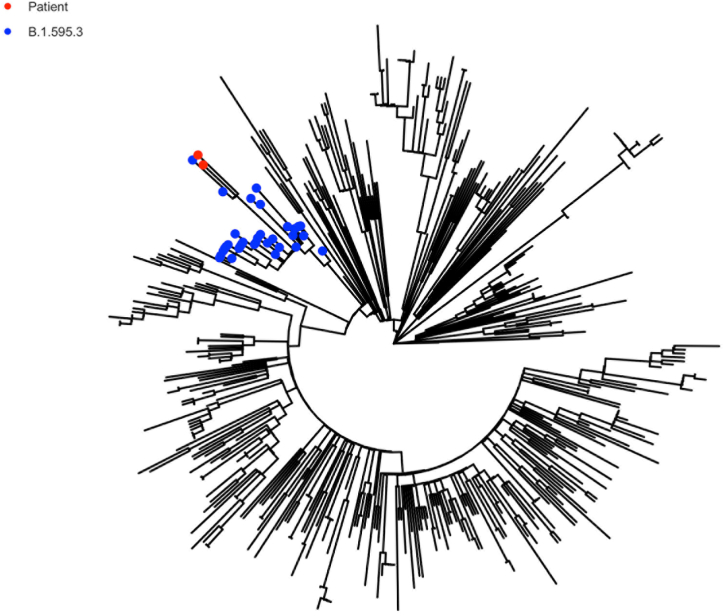
Next-Generation Sequencing (NGS) was performed on two nasopharyngeal swab samples obtained from the patient on day 167 and day 185. Neither of the samples were cultured because of lack of sufficient sample. Initial sequencing and phylogenetic analysis of the resulting consensus (≥50%) genome assigned the virus to the Pangolin lineage B.1.595.3, which was a lineage with a small number of associated genomes that circulated briefly in the patient's home state. The last sequenced instance of B.1.595.3 outside of this individual was detected at the end of June 2020, well prior to day 167 sample acquisition and close to the time point of the initial infection [20]. The viral genome associated with persistent infection showed evidence of significant mutation (Fig. 4), with a phylogenetic tree showing that both samples were a distinct branch from other B.1.595.3 lineage sequences (Fig. 5).
Fig. 4.
Sequencing coverage and variant accumulation in two sequenced samples.
Fig. 5.
Divergence tree showing MUSC genomes in relation to other B.1.595.3 variants. Red points represent the patient's samples and blue points represent other B.1.595.3 samples. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
For this patient, supportive care and weaning of immune suppressive therapy was associated with achieving the clearance of SARS-CoV-2 after persistent infection for 245 days. Though there were many attempted interventions during infection, the effective tapering of steroid treatment and cessation of rituximab therapy were the most instrumental in the patient's disease remission. Previous studies found that in patients infected with novel coronaviruses (SARS, MERS, and SARS-CoV-2), steroid treatment increased the time of viral clearance and viral shedding [[21], [22], [23]]. In our patient, steroid treatment at levels above 30 mg/mL were associated with persistent infection, but when treatment fell below 20 mg/mL, viral load dropped dramatically. There was also a correlation with time since rituximab treatment, which has been found to be associated with increasing levels of functioning B-cells and seroconversion in other rituximab-treated individuals [24,25].
Viral genomic analysis was consistent with the individual being infected once with a viral variant whose transmission was restricted to the east-coast of the US and the last sequenced instance of this variant was at the end of 2020. This suggests a low probability of re-infection. Both of the patient's samples cluster separately and are divergent from all other B.1.595.3 lineage genomes, which indicates a significant period of replication outside of the sampled transmitting population and further supports a single long-term infection (Fig. 5). Based on available viral genomic data, the patient did not appear to transmit the virus to others in the hospital or community during the course of her prolonged infection (Fig. 5).
When sequenced after months of in-host infection, the viral genome showed substantial evolution. Among the 14 SNV differences from the B.1.595.3 lineage, four occurred in the Spike protein. Three, S494P, T22I, and T95A are associated with evasion of antibody neutralization and have been independently observed in variants of concern and virus evolution in other immunocompromised patients [[26], [27], [28]]. These mutations suggest selection for a virus that is more effective at evading adaptive immune responses. These Spike protein mutations were conserved in the second sequenced sample, which notably showed an additional substitution (T224A) in the main viral protease NSP5. This mutation went from a minor variant to near fixation in less than three weeks. T224A has been reported to decrease the potency of boceprevir inhibition of the catalytic domain of the main protease, which is a target for COVID-19 therapeutics [29]. The T224A mutation did not affect this patient's clinical course, as they were not treated with a main protease inhibitor, but rather, the presence of such a mutation is important because the main protease is one of the most characterized SARS-CoV-2 drug targets, and mutations in structures that are important drug targets for SARS-CoV-2 may render existing therapeutics ineffective in its treatment [29]. These results emphasized that the virus adapted in ways that enabled both innate immune evasion and therapeutic evasion.
3.1. Viral clearance associated with successful IgG conversion
The positive outcome in this case is notable and likely reflects that the patient's underlying condition (RA) requiring immunosuppression was not itself lethal, allowing B-cell depletion therapy to be stopped. Laboratory data show varied but significant levels of T cells at the time of elimination of virus but no evidence of B cell reconstitution (below detection level, Supplemental Table 1). This supports the hypothesis that the elimination of virus was not primarily based on a B-cell driven response. The disease course of this patient shows that reconstituting the immune system can clear viruses that have acquired multiple immune evasion mutations in the viral Spike protein. This is a positive contrast to other cases where the acquisition of immune evading mutations was associated with poor outcome. Upon viral clearance, antibody testing showed presence of IgG antibodies against the virus for the first time. This suggests that steroid-mediated immune suppression was having a negative effect on viral clearance long-term. In addition to tapering of steroid treatment, increased time since last rituximab infusion allowed for the expression of an effective adaptive immune response after 245 days of persistent infection. The patient did not seroconvert after receiving a single unit of convalescent plasma (provided by a local blood provider with an unknown titer) on December 29, 2020. At the time the patient received the therapy (i.e., the early phase of the pandemic), convalescent plasma was poorly vetted; in the absence of FDA-approved tests for donated blood products/plasma, there was no verification/validation that the products contained high titers of neutralizing antibodies; donors who self-attested they had COVID were accepted without any standardization of timing following recovery.
3.2. Comparison to other long-term infections
Early reports of long-term infection in immunocompromised individuals have been associated with both significant intrahost genomic variation, the accumulation of immune evasion mutations, and negative outcomes [8,[30], [31], [32], [33]]. Similarly, this patient showed the appearance of antibody-evading mutations similar to those that have arisen in other long-term infections, suggesting that continued immune suppression would have resulted in further virus circulation and increased accumulation of immune evasion mutations. This suggests that the ability to actively manage treatment to decrease immunosuppression enabled the development of an effective polyclonal response, though this cannot be proven.
3.3. Limitations
The patient's successful outcome occurred in the setting of an underlying condition for which B-cell depleting therapy could be safely withdrawn, which is not the case for other patients who have experienced persistent COVID-19 infections. The suggestion that viral clearance was delayed by high dose steroids is consistent with this case, but further studies are needed to understand the role of steroid tapering and association with the clearance of persistent viral infection and symptoms. While our study suggests very significant viral genome mutation during infection, we do not have viral genome sequence at the time of initial infection, which limits a detailed understanding of genomic change over time. Additionally, this case only pertains to a single individual's clinical course with COVID-19 which may not be broadly generalizable to other immunocompromised individuals.
4. Conclusion
This case demonstrates a patient who was immunosuppressed and developed persistent long-term SARS-CoV-2 infection which caused significant morbidity with numerous hospitalizations and co-infections with viral and bacterial opportunistic pathogens. Additionally, in the setting of having persistent infection with SARS-CoV-2 infection she developed significant virus evolution with a more immune evasive variant. Although our patient eventually cleared her infection with reconstitution of her immune system, it is important to understand how to improve management of SARS-CoV-2 in immunosuppressed patient populations to not only optimize patient outcomes but to prevent virus evolution and the potential for new variants to arise.
Ethics statement
The authors confirm that the patient provided informed consent for the publication of their anonymized case details and images.
Data availability statement
The genomic analysis from this study is based on metadata associated with 39 sequences available on GISAID up to January 1, 2021, and accessible at https://doi.org/10.55876/gis8.230415pu (Supplemental Table 2).
CRediT authorship contribution statement
Victoria Overbeck: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Bradford Taylor: Writing – original draft, Visualization, Formal analysis. Jacquelyn Turcinovic: Visualization, Formal analysis, Data curation. Xueting Qiu: Visualization, Formal analysis. Beau Schaeffer: Visualization, Formal analysis. Scott Seitz: Methodology, Formal analysis. Scott Curry: Writing – review & editing, Writing – original draft, Resources, Investigation, Conceptualization. William Hanage: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition, Formal analysis. John H. Connor: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition, Formal analysis. Krutika Kuppalli: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
This work was supported by Massachusetts Consortium on Pathogen Readiness (MassCPR) and the China Evergrande Group. William P. Hanage is a member of Biobot Analytics, Inc. scientific adisory board. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the team at the Medical University of South Carolina who cared for the patient throughout their clinical course of COVID-19. We would also like to thank the Boston University Microarray and Sequencing Resource Core and the MUSC Bioinformatics Core for their assistance with this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e23699.
Contributor Information
John H. Connor, Email: jhconnor@bu.edu.
Krutika Kuppalli, Email: krutika.kuppalli@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/ Available at:
- 2.van Kampen Jja, van de Vijver D.A.M.C., Fraaij P.L.A., et al. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. medRxiv. 2020:2020. doi: 10.1038/s41467-020-20568-4. http://medrxiv.org/content/early/2020/06/09/2020.06.08.20125310.abstract 06.08.20125310. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K., Zhang X., Sun J., et al. Differences of SARS-CoV-2 shedding duration in sputum and nasopharyngeal swab specimens among adult inpatients with COVID-19. Chest. 2020 doi: 10.1016/j.chest.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao A.T., Tong Y.X., Gao C., Zhu L., Zhang Y.J., Zhang S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104346. https://www.sciencedirect.com/science/article/pii/S1386653220300883 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao A.T., Tong Y.X., Zhang S. Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients. Clin. Infect. Dis. 2020;71:2249–2251. doi: 10.1093/cid/ciaa460. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y., Yang M., Shen C., et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv 2020. 2020. http://medrxiv.org/content/early/2020/02/17/2020.02.11.20021493.abstract 02.11.20021493. Available at:
- 7.Zheng S., Fan J., Yu F., et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. https://www.bmj.com/content/bmj/369/bmj.m1443.full.pdf Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borges V., Isidro J., Cunha M., et al. Long-term evolution of SARS-CoV-2 in an immunocompromised patient with non-hodgkin lymphoma. mSphere. 2021;6 doi: 10.1128/mSphere.00244-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galimberti F., McBride J., Cronin M., et al. Evidence-based best practice advice for patients treated with systemic immunosuppressants in relation to COVID-19. Clin. Dermatol. 2020;38:775–780. doi: 10.1016/j.clindermatol.2020.05.003. https://www.sciencedirect.com/science/article/pii/S0738081X20300912 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavakolpour S., Alesaeidi S., Darvishi M., et al. A comprehensive review of rituximab therapy in rheumatoid arthritis patients. Clin. Rheumatol. 2019;38:2977–2994. doi: 10.1007/s10067-019-04699-8. Available at: [DOI] [PubMed] [Google Scholar]
- 11.Magliulo D., Wade S.D., Kyttaris V.C. Immunogenicity of SARS-CoV-2 vaccination in rituximab-treated patients: effect of timing and immunologic parameters. Clin. Immunol. 2022;234 doi: 10.1016/j.clim.2021.108897. https://www.sciencedirect.com/science/article/pii/S1521661621002345 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isnardi C.A., Roberts K., Saurit V., et al. Sociodemographic and clinical factors associated with poor COVID-19 outcomes in patients with rheumatic diseases: data from the SAR-COVID Registry. Clin. Rheumatol. 2022 doi: 10.1007/s10067-022-06393-8. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turcinovic J., Kuhfeldt K., Sullivan M., et al. Linking contact tracing with genomic surveillance to deconvolute SARS-CoV-2 transmission on a university campus. iScience. 2022;25 doi: 10.1016/j.isci.2022.105337. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H., Handsaker B., Wysoker A., et al. The sequence alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilm A., Aw Ppk, Bertrand D., et al. LoFreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res. 2012;40:11189–11201. doi: 10.1093/nar/gks918. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khare S., Gurry C., Freitas L., et al. GISAID's role in pandemic response. China CDC Wkly. 2021;3:1049–1051. doi: 10.46234/ccdcw2021.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katoh K., Misawa K., Kuma K., Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minh B.Q., Schmidt H.A., Chernomor O., et al. IQ-TREE 2: new Models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Toole Á., Hill V., Pybus O.G., et al. Tracking the international spread of SARS-CoV-2 lineages B.1.1.7 and B.1.351/501Y-V2 with grinch. Wellcome Open Res. 2021;6:121. doi: 10.12688/wellcomeopenres.16661.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee N., Allen Chan K.C., Hui D.S., et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J. Clin. Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. https://www.sciencedirect.com/science/article/pii/S1386653204001957 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling Y., Xu S.-B., Lin Y.-X., et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chinese Med J. 2020:133. doi: 10.1097/CM9.0000000000000774. https://journals.lww.com/cmj/Fulltext/2020/05050/Persistence_and_clearance_of_viral_RNA_in_2019.6.aspx Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz K., Jannat-Khah D., Spiera R. B-cell reconstitution is associated with COVID-19 booster vaccine responsiveness in previously seronegative rituximab treated patients. J. Rheumatol. 2022 doi: 10.3899/jrheum.220475. http://www.jrheum.org/content/early/2022/12/09/jrheum.220475.abstract jrheum.220475. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skapenko A., Schulze-Koops H. COVID-19 vaccination in individuals with inflammatory rheumatic diseases. Nat. Rev. Rheumatol. 2022 doi: 10.1038/s41584-022-00892-3. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harari S., Tahor M., Rutsinsky N., et al. Drivers of adaptive evolution during chronic SARS-CoV-2 infections. Nat. Med. 2022;28:1501–1508. doi: 10.1038/s41591-022-01882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bazykin G.A., Stanevich O., Danilenko D., et al. Emergence of Y453F and Δ69-70HV mutations in a lymphoma patient with long-term COVID-19. Virological org. 2021 [Google Scholar]
- 28.Leung W.F., Chorlton S., Tyson J., et al. COVID-19 in an immunocompromised host: persistent shedding of viable SARS-CoV-2 and emergence of multiple mutations: a case report. Int. J. Infect. Dis. 2022;114:178–182. doi: 10.1016/j.ijid.2021.10.045. https://www.sciencedirect.com/science/article/pii/S1201971221008298 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akbulut E. Mutations in main protease of SARS CoV-2 decreased boceprevir affinity. Braz. Arch. Biol. Technol. 2022:64. [Google Scholar]
- 30.Baang J.H., Smith C., Mirabelli C., et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J. Infect. Dis. 2021;223:23–27. doi: 10.1093/infdis/jiaa666. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee C.Y., Shah M.K., Hoyos D., et al. Prolonged SARS-CoV-2 infection in patients with lymphoid Malignancies. Cancer Discov. 2022;12:62–73. doi: 10.1158/2159-8290.CD-21-1033. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monrad I., Sahlertz S.R., Nielsen S.S.F., et al. vol. 8. Open Forum Infectious Diseases; 2021. (Persistent Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Immunocompromised Host Displaying Treatment Induced Viral Evolution). ofab295. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taha Y., Wardle H., Evans A.B., et al. Persistent SARS-CoV-2 infection in patients with secondary antibody deficiency: successful clearance following combination casirivimab and imdevimab (REGN-COV2) monoclonal antibody therapy. Ann. Clin. Microbiol. Antimicrob. 2021;20:85. doi: 10.1186/s12941-021-00491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genomic analysis from this study is based on metadata associated with 39 sequences available on GISAID up to January 1, 2021, and accessible at https://doi.org/10.55876/gis8.230415pu (Supplemental Table 2).