Abstract
Geophytes are herbaceous plants that grow anew from underground buds and are excellent models to study storage organ formation. However, molecular studies involving geophytes are constrained due to the presence of a wide spectrum of polysaccharides and polyphenols that contaminate the genomic DNA. At present, several protocols exist for the extraction of genomic DNA from different plant species; however, isolating high-quality DNA from geophytes is challenging. Such challenges are further complexed by longer incubation time and multiple precipitation steps involved in existing DNA isolation methods. To overcome such problems, we aimed to establish a DNA extraction method (SarCTAB) which is an economical, quick, and sustainable way of DNA isolation from geophytes. We improved the traditional CTAB method by optimizing key ingredients such as sarcosine, β-mercaptoethanol, and high molar concentration of sodium chloride (NaCl), which resulted in high concentration and good-quality DNA with lesser polysaccharides, proteins, and polyphenols. This method was evaluated to extract DNA from storage organs of six different geophytes. The SarCTAB method provides an average yield of 1755 ng/µl of high-quality DNA from 100 mg of underground storage tissues with an average standard purity of 1.86 (260/280) and 1.42 (260/230). The isolated genomic DNA performed well with Inter-simple sequence repeat (ISSR) amplification, restriction digestion with EcoRI, and PCR amplification of plant barcode genes viz. matK and rbcL. Also, the cost involved in DNA isolation was low when compared to that with commercially available kits. Overall, SarCTAB method works effectively to isolate high-quality genomic DNA in a cost-effective manner from the underground storage tissues of geophytes, and can be applied for next-generation sequencing, DNA barcoding, and whole genome bisulfite sequencing.
Keywords: Geophytes, DNA isolation, Storage organ, Polysaccharides, CTAB, Sarcosine
Introduction
Geophytic plants resprout from belowground storage organs (bulbs, corms, tuber, and rhizome) following the loss of aboveground parts (Tribble et al. 2021). Geophytes have a dual reproductive system (vegetative propagation and sexual reproduction) that enables them to survive climatic extremes (Khosa et al. 2021). Further, geophytes also accumulate a massive amount of polysaccharides in underground storage organs that enable them to survive seasonal dormancy (Orthen and Wehrmeyer 2004) Storage organs are excellent models to study overall plant morphological diversity and food reserve allocation (Orthen and Wehrmeyer 2004).
The prerequisite of any plant genetic analysis requires intact and high-quality genomic DNA (Tan and Yiap 2009). The purity of genomic DNA is assessed by the absence of contaminants (polysaccharides and polyphenols) and by spectrophotometric absorbance ratio, A260/280 (Kasem et al. 2008). However, such molecular studies underlying storage organs becomes often limiting due to low-quality genomic DNA, contaminated by excessive amounts of polysaccharides and phenols (Kasem et al. 2008). These compounds affect the quality and quantity of extracted DNA rendering it non-amplifiable (Sarwat et al. 2006). Polysaccharides are the commonest contaminant in any geophyte that makes the DNA pellet viscous and sticky (Abdel-Latif and Osman 2017). The presence of polysaccharides in storage tissues inhibits polymerase and restriction enzyme activity (Fang et al. 1992; Pandey et al. 1996). Additionally, polysaccharides contribute to strengthening of cell walls that makes cell wall degradation and DNA extraction difficult.
The extraction of high-quality genomic DNA from geophytes is a time-consuming, costly, and laborious process. Although commercial kits are available, they are expensive and yield inconsistent quality (Von Post et al. 2003). Several manual modifications of cationic detergent, cetyl trimethylammonium bromide (CTAB) protocols, are available based on crops, but work only on few plant species (Abdel-Latif and Osman 2017). The conventional DNA extraction method comprises only CTAB involved in the precipitation of DNA from histone proteins (Irfan et al. 2013). Such traditional methods have limitations when applied for isolating DNA from storage tissues of geophytes.
Our optimized method involves a key ingredient N-lauryl sarcosine or sarcosine along with CTAB (Salzman et al. 1999). Sarcosine is involved in the lysis of cells by membrane disruption and in protein denaturation and dispersion (Mannheim 1990), thus facilitating DNA isolation. This method is rapid and cost-effective when applied to representative species of geophytes such as Gladiolus hortulanus (Gladiolus), Narcissus pseudonarcissus (Daffodil), Crocus sativus (Saffron), Zantedeschia aethiopica (Calla lily), Amaryllis belladonna (Amaryllis), and Solanum tuberosum (Potato). Our sarcosine CTAB (SarCTAB) method provides a typical average yield of 1755 ng/µl of pure-quality genomic DNA, depending upon the milligrams of samples used. The absorbance ratio A260/280 of spectrophotometric measurement falls within a decent purity range of 1.86–2.00 indicating high-quality DNA. Further, the extracted DNA was amenable to ISSR markers and restriction enzymes and showed amplification with plant barcode genes (matK and rbcL) Overall, it is a fast DNA isolation protocol which does not require multiple steps, costly reagents, and long incubation period and thus can be useful for molecular analysis of a wide range of geophytes.
Material and methods
Sample collection and storage
The storage organs of Gladiolus hortulanus, Narcissus pseudonarcissus, Crocus sativus, Zantedeschia aethiopica, Amaryllis belladonna, and Solanum tuberosum include corm, bulbs, rhizomes, and tubers that were collected at the CSIR-Institute of Himalayan Bioresource Technology (CSIR-IHBT), Palampur, HP, India. They were stored at 4 °C to test the applicability of optimized DNA extraction protocol (Table 1; Fig. 1).
Table 1.
List of geophytes with their common and family names tested for different DNA extraction methods
| Species | Common name | Clade(s)–order/family |
|---|---|---|
| Gladiolus grandifloras | Gladiolus | Angiosperm–monocots–Asparagales/Iridaceae |
| Narcissus pseudonarcissus | Daffodil | Angiosperm–monocots–Asparagales/Amaryllidaceae |
| Crocus sativus | Saffron | Angiosperm–monocots–Asparagales/Iridaceae |
| Zantedeschia aethiopica | Calla Lily | Angiosperm–monocots–Alismatales/Araceae |
| Amaryllis belladonna | Amaryllis | Angiosperm–monocots–Asparagales/Amaryllidaceae |
| Solanum tuberosum | Potato | Angiosperm–eudicots–Solanales/Solanaceae |
Fig. 1.

Representative sample geophytes used in this study were procured from the field trials conducted at CSIR-Institute of Himalayan Bioresource Technology, India. Samples: 1—gladiolus, 2—daffodils, 3—saffron, 4—calla lily, 5—amaryllis, 6—potato
Buffers and reagents
Traditional CTAB
The suspension buffer of traditional CTAB comprises EDTA, Tris–HCl, NaCl, sucrose, and 2-β-mercaptoethanol. It requires 100 mg of sample tissue to extract DNA.
Kit A (Illustra Nucleon Phytopure, GE healthcare, UK)
The kit includes the following reagents such as Plant DNA extraction kit reagent 1, reagent 2, and nucleo resin. 100 mg of fresh weight of plant sample is used.
Kit B (DNase Plant Mini kit, Qiagen, USA)
Qiagen kit included Buffer AP1, Buffer P3, Buffer AW1, Buffer AW2, and Buffer AE and columns used were QIAshredder spin column and DNeasy Mini spin column. It also used 100 mg of fresh weight plant tissue for extracting genomic DNA.
SarCTAB
The suspension buffer includes Tris–Cl, EDTA, NaCl, CTAB, mannitol, sarcosine, and 2-β-mercaptoethanol.
Optimization of DNA extraction method protocol (SarCTAB): mini-prep
-
Pre-warm DNA extraction buffer (pH 8) containing working stocks of 0.22 M Tris–Cl (pH 8), 0.02 M EDTA (Sigma-Aldrich, USA) (pH 8), 0.8 M NaCl (SRL, India), 0.8% CTAB (Himedia laboratories, India), 0.14% mannitol, 1% sarcosine (Himedia laboratories, India), and 0.3% 2-β-mercaptoethanol (Sigma-Aldrich, USA) (Fig. 2).
Note: β mercaptoethanol is added after pre-warming to prevent its degradation, mannitol helps in recovery of high molecular weight (MW) DNA, and sarcosine helps in removing proteins and polysaccharides.
-
Grind 100 mg of storage tissue from representative geophytes to fine powder by using pre-chilled mortor and pestle.
Note: The lower the temperature, lower will be the chances of DNA degradation from nuclease activity.
Transfer the finely ground samples to 2 ml centrifuge tubes (Tarsons, India), followed by the addition of 1 ml of pre-warmed extraction buffer and vortex it for 30 s to obtain a homogenous mixture.
-
Incubate the sample mixture at 65 °C for 10 min.
Note: Heating cell lysate with CTAB suspension buffer at 65 °C inhibits enzyme DNase and prevents DNA degradation.
-
Add equal volume of chloroform:isoamyl alcohol (24:1) (v/v) (Sigma-Aldrich, USA) into the sample extract, inverted gently five to ten times, and centrifuged at 12,000g for 20 min at room temperature (RT).
Note: Carefully transfer the upper aqueous phase using wide-bore tips to avoid mechanical damage to DNA and repeat the step until solution is clear.
-
Carefully transfer the supernatant into a fresh 2 ml centrifuge tube, followed by the addition of an equal volume of pre-chilled isopropanol (SRL, India) and incubate at − 20 °C for 20 min to precipitate the DNA.
Note: The longer the chilled incubation, better is the precipitation.
-
To precipitate the total genomic DNA, centrifuge the sample mixture at 12,000g for 20 min and resuspend the pellet in 100 µl of nuclease-free water and divide it into two equal volume (50 µl × 2).
Note: Check the quality of genomic DNA on nanodrop and on agarose gel electrophoresis.
Treat 1/2 of total volume with 2 µl of RNase (10 mg/ml) (Thermo Fisher Scientific) and incubate at 37 °C for 20 min to remove total RNA.
Add nuclease-free water to make a volume up to 100 µl, and to that add an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1) (Sigma-Aldrich, USA) and centrifuge at 12,000g for 10 min.
Total genomic DNA is precipitated by transferring the aqueous phase to a fresh 2 ml microcentrifuge tube, followed by the addition of 50 µl of 3 M of sodium acetate (SRL, India) (pH 5.2) and 2 volumes of absolute ethanol (Himedia, India).
Keep the sample mixture at − 20 °C for 20 min, followed by centrifugation at 12,000g for 10 min at 4 °C.
-
Discard the supernatant and wash the DNA pellet twice with 70% ethanol and air dry.
Note: Make sure that there is no residual ethanol, as it hinders PCR reaction. Avoid overdrying of DNA pellet, as it gets difficult to resuspend.
Finally, resuspend the DNA pellet in 50 µl of TE buffer (1 M Tris–Cl and 0.5 M EDTA, pH 8) and store at − 20 °C.
Fig. 2.
Workflow of optimized DNA isolation protocol (SarCTAB) highlighting the major steps involved in DNA extraction from the underground storage organs of different geophytes
Quantitative and qualitative analysis of isolated DNA
The genomic DNA was quantified by using NanoDrop (Thermo Fisher Scientific) at 260 nm. The genomic DNA purity was determined by observing the ratio at A260/280, while the presence of organic contaminants (polysaccharides and polyphenols) was assessed by calculating the absorbance ratio A260/230 (Wilson and Walker 2005). For quality, extracted DNA was stained with ethidium bromide (10 µg/ml) and loaded on 0.8% agarose (w/v) and subjected to gel electrophoresis at 80 V. After gel electrophoresis, gel was visualized using gel documentation system (iGene IG-618GD, India).
Inter-simple sequence repeat (ISSR) study
The PCR amplification of ISSR2 primer ((GA)8CGAGAGAGAGAGAGAGAC) was carried out using GoTaq green master mix, 2× (Promega, Madison, WI USA) and 10–80 ng of template DNA. ISSR-PCR amplification was carried out for 40 cycles in a thermocycler (Biosystem life technologies) consisting of denaturation at 95 °C for 30 s, annealing at 53 °C for 60 s, and extension at 72 °C for 1 min. The final extension of DNA template was carried out at 72 °C for 7 min. The amplified product was run in 1.5% agarose gel and observed in gel documentation system (iGene IG-618GD, India).
Restriction digestion of isolated DNA samples
1 µg of extracted genomic DNA was treated with EcoRI restriction enzyme and rCut smart buffer (Biolabs, New England) and incubated at 37 °C for 1 h for complete digestion. The digested samples were visualized in 1.5% agarose gel and observed in gel documentation system (iGene IG-618GD, India).
Amplification of plant barcode genes (matK and rbcL)
The PCR amplification of genomic DNA with matK (F: TAATTTACGATCAATTCATTC R: GTTCTAGCACAAGAAAGT) and rbcL (F: ATGTCACCACAAACAGAGACTAAAGC and R: GTAAAATCAAGTCCACCRCG) was carried out using GoTaq green master mix, 2× (Promega, Madison, WI USA) for 40 cycles consisting of denaturation at 95 °C for 30 s, annealing at 53 °C for 60 s, and extension at 72 °C for 1 min. The final extension of DNA template was carried out at 72 °C for 7 min. The amplified fragment for matK (~ 1000 bp) and rbcL (~ 750 bp) was separated in 1.5% agarose gel and observed in gel documentation system (iGene IG-618GD, India).
Results
Genomic DNA isolation
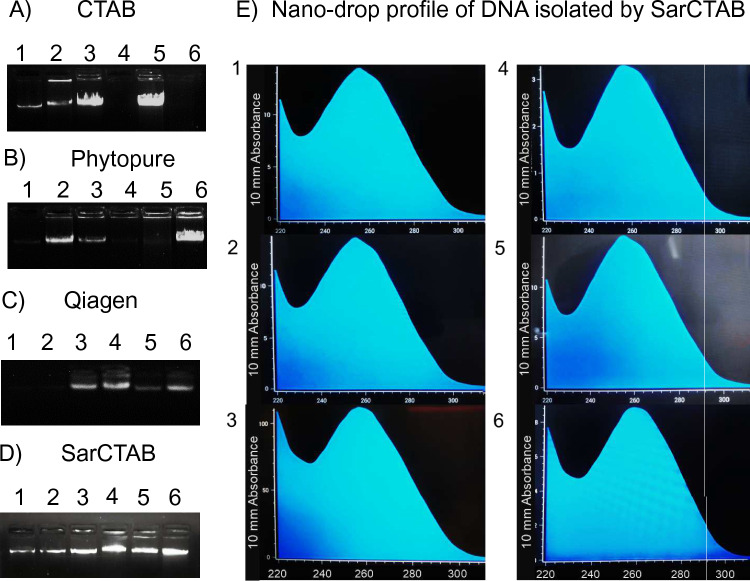
Representative samples of geophytes were isolated using our optimized method, and a clear, distinct band was visible for all the species included in this study as compared to traditional CTAB method and commercial kits such as Illustra Nucleon Phytopure and DNase Plant Mini kit, Qiagen (Fig. 3). Our standardized protocol had no smears or shearing of DNA which substantiated the purity of DNA.
Fig. 3.
A–D Agarose gel electrophoresis showing genomic DNA isolated from sample geophytes by using traditional CTAB, Phytopure, Qiagen, and SarCTAB method resolved under 0.8% agarose gel. E Nanodrop profile measurement of isolated DNA using the SarCTAB method indicating a single characteristic peak at 260 nm. Samples: 1—gladiolus, 2—daffodils, 3—saffron, 4—calla lily, 5—amaryllis, 6—potato
Assessment of DNA quality and purity
The purity of genomic DNA was quantified using the spectrophotometric method. The quantity of the representative samples was compiled and calculated (Fig. 3; Table 2). DNA yield varied across different genera. The DNA extracted varied from 1755 ng/µl per 100 mg of sample used. The purity of isolated DNA ranged from 1.86 to 2.12 for all samples, suggesting that DNA samples were free of polysaccharides and proteins.
Table 2.
Comparative analysis of different methods to extract DNA from geophytes in terms of concentration, purity, time, and cost
| Plant species | DNA extraction method | Elution volume (µl) | Max. DNA yield (ng/µl/100 mg sample) | A260/280 | A260/230 | Time (h) | Cost/sample (US dollars) |
|---|---|---|---|---|---|---|---|
| Gladiolus grandifloras | Traditional CTAB | 50 | 139 | 1.55 | 0.99 | 5 | 2.03 |
| Narcissus pseudonarcissus | 471 | 1.23 | 0.31 | ||||
| Crocus sativus | 174 | 1.73 | 1.35 | ||||
| Zantedeschia aethiopica | 55 | 1.61 | 0.64 | ||||
| Amaryllis belladonna | 213 | 1.71 | 1.01 | ||||
| Solanum tuberosum | 318 | 1.67 | 1.22 | ||||
| Gladiolus grandifloras | Illustra Nucleon Phytopure, GE Healthcare | 50 | 113 | 1.69 | 0.44 | 1.5 | 5.08 |
| Narcissus pseudonarcissus | 559 | 1.69 | 0.33 | ||||
| Crocus sativus | 335 | 1.07 | 0.3 | ||||
| Zantedeschia aethiopica | 224 | 1.44 | 0.36 | ||||
| Amaryllis belladonna | 325 | 1.8 | 0.39 | ||||
| Solanum tuberosum | 142 | 1.92 | 0.69 | ||||
| Gladiolus grandifloras | DNase Plant Mini Kit, Qiagen | 50 | 14 | 1.53 | 1.09 | 1 | 6.72 |
| Narcissus pseudonarcissus | 32 | 1.8 | 4.32 | ||||
| Crocus sativus | 20 | 1.77 | 13.35 | ||||
| Zantedeschia aethiopica | 13 | 1.3 | 1.27 | ||||
| Amaryllis belladonna | 6 | 1.54 | 1.05 | ||||
| Solanum tuberosum | 12 | 1.77 | -6.58 | ||||
| Gladiolus grandifloras | SarCTAB | 50 | 691 | 2.11 | 1.75 | 2 | 2.10 |
| Narcissus pseudonarcissus | 5468 | 1.88 | 1.49 | ||||
| Crocus sativus | 1896 | 1.94 | 1.65 | ||||
| Zantedeschia aethiopica | 455 | 1.86 | 0.86 | ||||
| Amaryllis belladonna | 1331 | 2.12 | 1.07 | ||||
| Solanum tuberosum | 691 | 2.11 | 1.75 |
PCR amplification and DNA digestion analysis
Clear and well-differentiated banding patterns were observed in the ISSR study (Fig. 4) suggesting extracted DNA was in compliance with ISSR study and was reproducible. Similarly, amplification of plant barcode genes (matK and rbcL) was observed around 1000 bp for the isolated samples involved in the study, indicating the integrity of DNA samples that can be used for molecular studies. Further, on testing the DNA sample with restriction enzymes such as EcoRI, we found that pure, intact genomic DNA from geophytes were digested, suggesting the absence of salts or organic contaminants that possibly limit the restriction sites present in the template DNA (Fig. 4).
Fig. 4.
A, B Agarose (1.5%) gel electrophoresis showing PCR amplification of plant barcode genes matK and rbcL by using representative DNA samples indicating successful DNA amplification; C inter-simple sequence repeat (ISSR) pattern in different geophytic DNA samples; D restriction pattern of extracted DNA cleaved by EcoRI with respect to undigested samples (lane 2, lane4, lane 6, lane 8, lane 10, lane 12) indicating the relevance of the method in molecular digestion studies. M: marker; D: digested; UD: undigested. Samples: 1—gladiolus, 2—daffodils, 3—saffron, 4—calla lily, 5—amaryllis, 6—potato
Comparison of the methods used
Traditional CTAB and SarCTAB
The traditional CTAB method included ingredients similar to our optimized protocol except sarcosine and high concentration sodium chloride. However, the optimized protocol has difference in molar concentration of key ingredients, facilitating improved DNA isolation with additive sarcosine.
Kit A (Illustra Nucleon Phytopure)
The tissue requirement was about 100 mg, but this kit protocol involved multiple precipitation steps with no additional reagents that were mentioned in the product booklet. It included only two reagents with nucleon resin in its kit.
Kit B (DNase Plant Mini Kit, Qiagen)
100 mg of sample tissue was used for extraction of genomic DNA. Although it had all the reagents mentioned in the product booklet, it was comparatively expensive and the performance of the kit was not that impressive in terms of yield.
Discussion
The traditional CTAB method has been modified on several instances to reduce contaminants such as phenols, proteins, and polysaccharides that are usually present in underground storage organs (Sahu et al. 2012). Although several published protocols are available, their effectiveness in extracting DNA from a wide spectrum of geophytes falls short. Our standardized protocol works better with underground storage organs and it does not require a long incubation or any other expensive reagents or consumables.
In the present study, we implemented several modifications at various steps to ensure isolation of DNA with better quality and quantity. For plant tissue disruption, pectinase and cellulase was avoided as its inaccurate and non-reprodcucible process. Instead, liquid nitrogen was used to break the cell wall and disrupt cell membrane (Clark 2013). Geophytes are rich in phenolic and polysachharide compounds that interfere with DNA quality. High concentration of 2-β-mercaptoethanol (β-ME, 0.3%) was added to the crude plant extract to remove polyphenols, leaving back clear translucent DNA pellet (Li et al. 2007). Also, β-ME helps to reduce disulfide bonds to denature protein. Removing proteins from nucleic acids ensures nucleic acid integrity. The remaining polysaccharides were removed by using high concentration of salts like sodium acetate (3 M) (Paterson et al. 1993). Sodium acetate with pH 5.2 dissociates into sodium and acetate ions. Further, the sodium ions shield the negative charge of phosphate on sugar-P backbone of DNA and helps in DNA precipitation (Heikrujam et al. 2020). Additional steps of chloroform:isoamyl alcohol removed proteins, cellular debris, and lipids yielding high-quality purified DNA, thereby eliminating the use of phenols that might not be necessary for geophytes (Aboul-Maaty and Oraby 2019). Incubating longer at − 20 °C proved to be better for precipitation of DNA, as isolation of nucleic acid depends on the incubating temperature and time (Michiels et al. 2003). The integrity of genomic DNA was further restored by incorporating the key ingredients. Notably, the percentage of sarcosine used in this protocol not only promotes the lysis of the cell wall matrix of geophytes, but also prevents protein aggregation (Mannheim 1990). The solubility of polysaccharides of geophytes was further enhanced by using 0.8 M NaCl, thereby inhibiting co-precipitation of polysaccharides with genomic DNA (Irfan et al. 2013).
The quantitative estimation of any DNA sample is verified using the spectrophotometric method (NanoDrop). The absorbance profile of NanoDrop gives us an indication about the purity of samples. The absorbance is usually measured at two critical wavelength ratios, A260/280 and A260/230. The 260/280 ratio is used to assess the purity of nucleic acids (DNA and RNA). A ratio of 1.8 generally indicates the purity of extracted DNA. However, the 260/230 ratio ranges from 2.0 to 2.2 that also indicates the purity of DNA in the presence or absence of organic contaminants (salts, phenols, polysaccharides, EDTA) (Wilson and Walker 2005). Our optimized protocol showed a single characterized peak for most of our samples at 260 nm. The 260/280 ratio was between 1.86 and 2.12, indicating insignificant levels of organic contaminants (Pervaiz et al. 2011). However, most of the geophyte DNA samples reproduced using commercial kits (Qiagen, Phytopure) had purity ratio < 1.8. Additionally, the samples when extracted using traditional CTAB protocol had significantly lower 260/230 ratio affecting the DNA quality as observed for maize (Abdel-Latif and Osman 2017). The range of DNA yield obtained using our standardized DNA protocol was between 455 and 5468 ng/µl for 100 mg of sample used. The time-saving commercial kits are mostly dependent on ready to use reagents, thereby reducing operational time and making the process of DNA extraction lot simpler. On the other hand, such kits are not cost-effective, as it includes the cost of spin columns and secondary clean-up reagents. In this context, the cost involved in the preparation of our SarCTAB suspension buffer is around 210 USD for approximately 100 samples, which is much cheaper than commercial kits. On the other hand, the standard cost of Qiagen and Phytopure kit for 50 samples is 336 and 290 USD, respectively (Table 2).
Previously, it has been reported that the shearing of DNA interferes with enzymes (Taq polymerases) and restriction enzymes (EcoRI) during various molecular studies such as PCR, ISSR, restriction digestion, and amplification of barcode genes (Weishing et al. 1995). In the present study, we used 1.5% agarose gel concentration to visualize an intense, high molecular weight DNA from representative samples with no shears or smears. None of the samples showed any significant smears, which usually indicates the degradation of samples (Abdel-Latif and Osman 2017).
Good-quality DNA is very useful for studies such as DNA barcoding, long read, and next-generation and whole genome bisulfite sequencing. The downstream analysis of such genomic studies are threatened by low quantity and pure quality of isolated DNA due to the presence of organic solvents, inhibitors, and contaminants. In a nutshell, DNA quantity and purity and processing cost and time are important factors involved in molecular genomic studies.
Conclusions
In our study, comparative analysis of four DNA extraction methods was undertaken to check the efficacy of each method in contributing to high-quality genomic DNA. Among these, our optimized protocol proved to be better in yielding high-quality and pure DNA that was found amenable to PCR amplification by universal primers and restriction digestion. Interestingly, our results gave a maximum yield of 5468 ng/µl per 100 mg of underground tissue by using an additional lysis reagent, sarcosine. Further, the extracted high-quality DNA pooled by this optimized protocol could be used for whole-genome sequencing and bisulphite sequencing. Therefore, the practical usability of this protocol may be applied to a wide range of geophytes.
Acknowledgements
MD and VR acknowledge the University Grant Commission (UGC) for SRF fellowship; G.Z. acknowledges the support obtained from the projects funded by CSIR project MLP-201, CSIR-FIRST (MLP-178), and DST Start-up Research Grant (SRG), SERB (GAP-294). This manuscript represents CSIR-IHBT publication number 5403.
Authors’ contribution
MD: designed, performed the experiments, analyzed the data, and wrote the original draft; PS: performed the experiments; VR: performed the experiments and tabulated data; BB: provided material; GZ: conceived the idea, edited and finalized the manuscript.
Data availability
All data underlying the results are available as a part of the article, more information can be accessed from corresponding author.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Madhushree Dutta and Paras Sharma are Co-first authors and contributed equally to this work.
References
- Abdel-Latif A, Osman G. Comparison of three genomic DNA extraction methods to obtain high DNA quality from maize. Plant Methods. 2017;13(1):1–9. doi: 10.1186/s13007-016-0152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboul-Maaty NA-F, Oraby HA-S. Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull Natl Res Centre. 2019;43(1):1–10. doi: 10.1186/s42269-019-0066-1. [DOI] [Google Scholar]
- Clark MS. Plant molecular biology—a laboratory manual. Berlin: Springer; 2013. [Google Scholar]
- Fang G, Hammar S, Grumet R. A quick and inexpensive method for removing polysaccharides from plant genomic DNA. Biotechniques. 1992;13(1):52–54. doi: 10.2144/97231bm09. [DOI] [PubMed] [Google Scholar]
- Heikrujam J, Kishor R, Mazumder PB. The chemistry behind plant DNA isolation protocols. Biochem Anal Tools Methods Bio-Mol Stud. 2020;8:131–141. [Google Scholar]
- Irfan M, Ting ZT, Yang W, Chunyu Z, Qing M, Lijun Z, Feng L. Modification of CTAB protocol for maize genomic DNA extraction. Res J Biotechnol. 2013;8(1):41–45. [Google Scholar]
- Kasem S, Rice N, Henry R (2008) DNA extraction from plant tissue. In: Plant genotyping II: SNP technology, p 219
- Khosa J, Bellinazzo F, Kamenetsky Goldstein R, Macknight R, Immink RG. PHOSPHATIDYLETHANOLAMINE-BINDING PROTEINS: the conductors of dual reproduction in plants with vegetative storage organs. J Exp Bot. 2021;72(8):2845–2856. doi: 10.1093/jxb/erab064. [DOI] [PubMed] [Google Scholar]
- Li J, Yang J, Chen D, Zhang X, Tang Z. An optimized mini-preparation method to obtain high-quality genomic DNA from mature leaves of sunflower. Genet Mol Res. 2007;6(4):1064–1071. [PubMed] [Google Scholar]
- Mannheim B. Reagents for molecular biology (catalog) Indianapolis: Boehringer Mannheim Co.; 1990. [Google Scholar]
- Michiels A, Van den Ende W, Tucker M, Van Riet L, Van Laere A. Extraction of high-quality genomic DNA from latex-containing plants. Anal Biochem. 2003;315(1):85–89. doi: 10.1016/S0003-2697(02)00665-6. [DOI] [PubMed] [Google Scholar]
- Orthen B, Wehrmeyer A. Seasonal dynamics of non-structural carbohydrates in bulbs and shoots of the geophyte Galanthus nivalis. Physiol Plant. 2004;120(4):529–536. doi: 10.1111/j.0031-9317.2004.0284.x. [DOI] [PubMed] [Google Scholar]
- Pandey RN, Adams RP, Flournoy LE. Inhibition of random amplified polymorphic DNAs (RAPDs) by plant polysaccharides. Plant Mol Biol Rep. 1996;14(1):17–22. doi: 10.1007/BF02671898. [DOI] [Google Scholar]
- Paterson AH, Brubaker CL, Wendel JF. A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Mol Biol Rep. 1993;11:122–127. doi: 10.1007/BF02670470. [DOI] [Google Scholar]
- Pervaiz Z, Turi N, Khaliq I, Rabbani M, Malik S. Methodology: a modified method for high-quality DNA extraction for molecular analysis in cereal plants. Genet Mol Res GMR. 2011;10(3):1669–1673. doi: 10.4238/vol10-3gmr1346. [DOI] [PubMed] [Google Scholar]
- Sahu SK, Thangaraj M, Kathiresan K (2012) DNA extraction protocol for plants with high levels of secondary metabolites and polysaccharides without using liquid nitrogen and phenol. Int Scholarly Res Notices 2012:205049. 10.5402/2012/205049 [DOI] [PMC free article] [PubMed]
- Salzman R, Fujita T, Zhu-Salzman K, Hasegawa P, Bressan R. An improved RNA isolation method for plant tissues containing high levels of phenolic compounds or carbohydrates. Plant Mol Biol Rep. 1999;17(1):11–17. doi: 10.1023/A:1007520314478. [DOI] [Google Scholar]
- Sarwat M, Singh Negi M, Lakshmikumaran M, Kumar Tyagi A, Das S, Shankar Srivastava P. A standardized protocol for genomic DNA isolation from Terminalia arjuna for genetic diversity analysis. Electron J Biotechnol. 2006;9(1):86–91. doi: 10.2225/vol9-issue1-fulltext-3. [DOI] [Google Scholar]
- Tan SC, Yiap BC. DNA, RNA, and protein extraction: the past and the present. J Biomed Biotechnol. 2009;2009:574398. doi: 10.1155/2009/574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribble CM, Martínez-Gómez J, Howard CC, Males J, Sosa V, Sessa EB, Cellinese N, Specht CD. Get the shovel: morphological and evolutionary complexities of belowground organs in geophytes. Am J Bot. 2021;108(3):372–387. doi: 10.1002/ajb2.1623. [DOI] [PubMed] [Google Scholar]
- Von Post R, Von Post L, Dayteg C, Nilsson M, Forster BP, Tuvesson S. A high-throughput DNA extraction method for barley seed. Euphytica. 2003;130(2):255–260. doi: 10.1023/A:1022863006134. [DOI] [Google Scholar]
- Weishing K, Nybom H, Wolff K, Meyer W (1995) DNA isolation and purification. DNA fingerprinting in plants and fungi, p 2
- Wilson K, Walker J (2005) Molecular biology, bioinformatics and basic techniques. Biochem Mol Biol 166–225
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the results are available as a part of the article, more information can be accessed from corresponding author.