Abstract
The fine-tuning of the intricate network of plant growth hormones empowers the balanced responses of plants to environmental and developmental signals. Salicylic acid and jasmonates are emerging as advanced hormones that provide plants with resistance to environmental stresses. Senescence is characterized by coordinated and systematic crosstalk between phytohormones that remodels the biochemical and physiological mechanisms in plants, resulting in cell death. The present investigation examines the role of jasmonates (methyl jasmonate and jasmonic acid) and salicylic acid (SA) in regulating the petal senescence of detached stalks of Cosmos sulphureus. Based on our results, it was revealed that SA and jasmonic acid (JA) at 40 μM and methyl jasmonate (MJ) at 0.75 μM concentration delayed the senescence of detached flowers of C. sulphureus considerably. These growth regulators improved the membrane stability, reinforced the antioxidant enzyme activities and averted the upsurge of hydrogen peroxide (H2O2) content in the petals. Additionally, SA and jasmonates preserved higher content of total phenols, reducing sugars and soluble proteins in the petals, besides impeding the bacterial growth in testing solutions which corroborated with the maximum solution uptake. The elevated soluble protein content was found to be associated with low specific protease activity (SPA) and α-amino acid content in the petal tissues. Our study concluded that SA and jasmonates delayed flower senescence by averting oxidative stress and maintaining the nutritional status of the petals.
Keywords: Jasmonates, Salicylic acid, Cosmos sulphureus, Senescence
Introduction
Plants incessantly monitor their external environment and innate developmental status, precisely fine-tuning the regulatory processes to ensure coordinated growth, differentiation and effective stress responses. This coordination is accomplished by a multifaceted yet highly organized network of diverse signaling molecules (Liu and Timko 2021). Phytohormones are the indispensable set of signaling elicitors that function through a diversity of regulatory mechanisms conferring plasticity to acclimatize with the ever-changing environmental and developmental cues. The jasmonates (linolenic acid oxylipins) serve as plant’s modern-day Swiss Army Knife regulating a plethora of processes in plant cells, including the stimulation of the antioxidant processing system, accrual of sugars, proteins and osmolytes, in addition to maintaining the dynamics of opening and closing of stomata (Baswal et al. 2021). Jasmonates evoke several signal transduction pathways to orchestrate manifold physiological events, which include root inhibition, trichome initiation, anthocyanin accumulation, male fertility, leaf senescence, response to herbivory, pathogens and abiotic stresses (Hu et al. 2017; Huang et al. 2017; Baswal et al. 2021). It is hypothesized that jasmonates, in a conceptual analogy with human adrenaline, generally enhance cellular processes to keep up with augmented metabolic demand during unfavorable periods (Nguyen et al. 2022). These growth regulators do not act in isolation but rather form a complex signaling network alongside other groups of phytohormones like cytokinins, ethylene, gibberellic acid, SA, abscisic acid and auxins. Together, they facilitate the delicate balance between growth and defense mechanisms, thus conferring plant acclimatization to the fluctuating milieus (Wan and Xin 2022). In recent times, there has been notable progress in comprehending the molecular mechanisms involved in jasmonate metabolism, perception, signaling and their crosstalk with other hormones (Howe et al. 2018; Wan and Xin 2022). Such studies have revealed a multifaceted signaling system underlying the coordinated and precisely tuned action of these phytohormones that affect plant behavior. Jasmonates have been shown to augment the postharvest life of fruits like grapes, blackberries, raspberries and apples besides delaying the senescence of various flowers such as roses, gerberas, irises, etc. (Horibe 2022).
Salicylic acid (SA); the precursor of cinnamic acid, is structurally a β-hydroxy phenolic compound consisting of a ring linked to the hydroxyl and carboxyl groups. It is characterized by a few analog compounds such as methyl salicylic acid and acetylsalicylic acid. SA is recognized as the 7th class of plant hormones after the global endorsement of brassinosteroids as the 6th among the other five conventional growth regulators (Ali 2021). SA serves as a critical signaling molecule mediating a spectrum of responses in plants such as nutrient uptake, photosynthesis, secondary metabolite synthesis, abscission reversal, stomatal conductance, flower induction, enzyme activities, pigment accumulation, ethylene biosynthesis and general plant growth (Ali 2021; Hayat et al. 2021; Li et al. 2022a). SA is principally responsible for acquiring systemic resistance against pathogens and abiotic stress (Hayat et al. 2008; Sedaghat et al. 2020; Damalas and Koutroubas 2021). However, the horizon of SA-mediated physiological mechanisms has extended, as several investigations have enumerated the involvement of SA in regulating flower senescence and fruit development (Hayat et al. 2021). Recently, SA has been recognized to affect the postharvest characteristics of fruits, quality maintenance, deterrence of fruit deterioration, pathogen attack and enhancement of fruit life (Ali 2021).
Flower senescence involves a well-coordinated and orderly disassembly of cellular organelles and macromolecules (Ma et al. 2018). The process entails a succession of irrevocable physiological and biochemical events, including oscillations in hormonal levels, loss of osmotic balance, seepage and subsequent depletion of solutes, production of excessive reactive oxygen species (ROS), an upsurge in lipid peroxidation/membrane fluidity, hydrolysis of proteins, nucleic acids and carbohydrates, besides a programmed metabolic transition from nutrient assimilation to nutrient remobilization (Miryeganeh et al. 2021; Sun et al. 2021; Li et al. 2022b). As such, optimization of appropriate postharvest formulations to delay the flower senescence demands critical attention. Recently, jasmonates and SA have been implicated in regulating the postharvest biochemistry and physiology of horticultural and agricultural products (Bagheri and Esna-Ashari 2022; Huang et al. 2022). It has been postulated that senescence in Asteraceae flowers is ethylene-independent (van Doorn and Woltering 2008). Pertinently, oxidative stress, membrane degradation and nutritional starvation are assumed as chief hallmarks of ethylene-insensitive flower senescence (Hunter et al. 2004; Kumar et al. 2021). C. sulphureus Cav., a member of the Asteraceae family, is grown as an ornamental plant, renowned for its vibrant and profuse yellow, orange or red flowers. While it thrives in gardens, landscaping and flower beds, enhancing the realm of ornamental plants, its potential in the cut flower market is constrained by its limited lifespan (Skutnik et al. 2020). This plant like other members of the Asteraceae family is known for its unique inflorescence called the “capitulum” which consists of multiple small flowers arranged in a composite head. Essentially, the capitulum is a pseudanthium, or ‘false’ flower, often referred to as flower heads or capitula, which outwardly mimic single flowers. It consists of an intricately aggregated structure comprising numerous individual flowers, each with its specialized function (Elomaa et al. 2018). The outer flowers are often modified to form colorful ray florets, while the inner flowers typically represent less prominent disc florets. In pursuit of this, the current study focused on examining the effect of jasmonates and SA on different physiological and biochemical parameters such as oxidative stress, membrane integrity, antioxidant machinery and nutritional status that orchestrate senescence in detached ray florets of C. sulphureus.
Materials and methods
Study samples and application of treatments
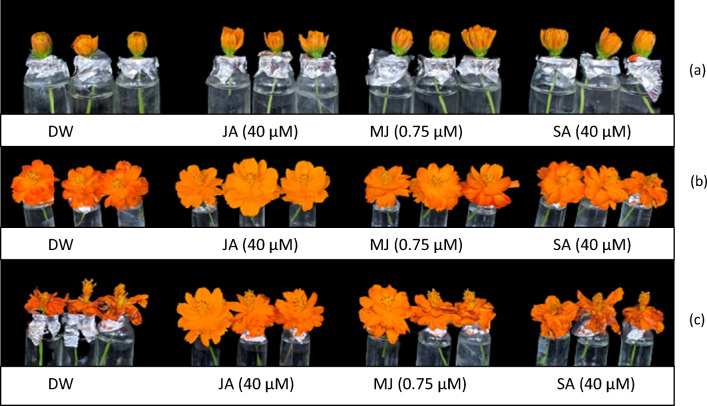
For the current study, healthy and fresh buds of C. sulphureus 1 day before the anthesis stage were carefully collected from the Kashmir University Botanic Garden (KUBG) (Fig. 1a) and subsequently transferred to the laboratory. The flower stems were then trimmed down to a length of 5 cm and grouped randomly into four distinct sets. One set representing the control was held in distilled water (DW). Two sets were supplemented separately with JA and SA at 40 μM and the other set was treated with MJ at 0.75 μM concentration. The day of administration of these treatments was selected as day 0. Each treatment along with the control included 10 replicates. The standardization of these plant growth regulators was accomplished by testing a range of grades of these growth regulators viz., 20, 40 and 60 μM of JA and SA and 0.50, 0.75 and 1.00 μM of MJ (Ahmad and Tahir 2018). Based on the standardizations, it was deduced that JA and SA at 40 μM and MJ at 0.75 μM were optimal formulations that delayed the senescence of detached stalks of C. sulphureus significantly. The investigation was conducted under appropriate conditions, with an average temperature of 25 ± 3 °C, a 12-h light period per day and relative humidity of 66 ± 12%. Effects of these treatments on different physiological and biochemical parameters associated with flower senescence were recorded on day second (D2) and day sixth (D6) of the transfer of flower buds to the respective treatments.
Fig. 1.
Morphological changes in the flowers of C. sulphureus with the progression of flower development from the partially open (a-day 0) through fully open (b-day 2) to the senescent stage (c-day 7). The flowers held in jasmonic acid (JA) and methyl jasmonate (MJ) lasted for 8.5 and 8 days respectively, while those held in salicylic acid (SA) lasted for 7 days as against the flowers held in distilled water (DW) which exhibited an average life of 4 days
Parameters evaluated
Evaluation of flower life
The flower life was taken from day one of the administration of different postharvest treatments till the ornamental value of the flowers was lost.
Evaluation of bacterial density and solution uptake
The bacterial density was determined by recording the optical density (OD) of 1 ml of vase solution taken from each treatment including control at 600 nm using a PC-based UV–Vis spectrophotometer (Systronics) by the method described by Naing et al. (2017) taking E. coli as standard (1 OD = 8 × 108 CFU ml−1). The bacterial density was expressed as CFU ml−1. Solution uptake (ml) was evaluated as the difference between the unutilized volume of solution remaining after the completion of the experiment and the total volume of solution in the vial (Lone et al. 2021).
Assessment of membrane stability index (MSI)
The membrane stability index (MSI) was evaluated by observing the electrical conductivity based on electrolyte seepage in the petal tissues (Sairam 1994). 500 mg of petal tissue was incubated in 25 ml of DW at 25 °C and 100 °C for 30 min and 15 min respectively. The conductivity was detected on the conductivity meter (Elico CM180). The MSI was computed by using the formulae:
The expressions C1 and C2 imply the sample conductivities at 25 °C and 100 °C respectively.
Assessment of hydrogen peroxide (H2O2) content
The H2O2 content was assessed by employing the protocol of Alexieva et al. (2001). 500 mg of petal tissue was macerated in 0.1% (w/v) TCA buffer, followed by centrifugation of homogenate at 12000 g for 15 min 0.5 ml supernatant was taken and added with 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0), followed by the addition of 1 ml of 1 M KI. Finally, the absorbance of the mixture was detected at 390 nm. The H2O2 concentration was determined by a standard curve prepared by using known H2O2 concentration.
Assessment of superoxide dismutase (SOD) activity
The SOD activity was assessed by following Dhindsa et al. (1981) method. The activity was evaluated by determining the inhibition of enzymatic photochemical reduction of nitroblue tetrazolium (NBT). One unit of SOD activity was defined as the concentration of the enzyme that lowers the absorbance of the reaction mixture by 50% as compared to the reaction mixture without the enzyme. The reaction mixture included 50 mM sodium carbonate, 75 µM NBT, 0.1 M ethylenediamine tetraacetic acid (EDTA) and 13 mM methionine in 50 mM phosphate buffer (pH 7.8). The absorbance of the reaction mixture was recorded at 560 nm.The activity was expressed as units min−1 mg−1 protein.
Assessment of catalase (CAT) activity
The CAT activity was determined by employing the Aebi (1984) protocol based on the consumption of hydrogen peroxide (H2O2) in the reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0), enzyme extract (50 µl) and DW making a final volume to 3 ml. The absorbance of the reaction mixture was recorded at 240 nm for 3 min using spectrophotometer. The activity was expressed as µM H2O2 red. min−1 mg−1 protein.
Assessment of ascorbate peroxidase (APX) activity
The APX activity was assayed according to the protocol described by Chen and Asada (1989) which is based on the decrease in absorbance at 290 nm caused due to the oxidation of ascorbate (0.1 mM) in the reaction mixture. The reaction mixture in addition to ascorbate contained potassium phosphate buffer (50 mM) at neutral pH and H2O2 (0.3 mM). The absorbance was recorded at 290 nm for 3 min. The activity was expressed as µmol min−1 mg−1 protein.
Assessment of lipoxygenase (LOX) activity
The LOX activity was determined by the Axelrod et al. (1981) method. To begin the reaction, 10 µl of petal extract was added to the mixture containing Tris–Hydrochloric acid buffer (50 mM) at pH 6.5 and linoleic acid (0.4 mM). The absorbance was recorded at 234 nm for 5 min and activity was expressed as µmol. min−1 mg−1 protein.
Assessment of specific protease activity (SPA)
For assessment of SPA, 1 g of chilled petal tissue was homogenized in 15 ml of pre-chilled phosphate buffer (0.1 M) of pH 6.5 in a chilled pestle and mortar. The mixture was squeezed through a fourfold muslin cloth and centrifuged at 5000 g for 15 min in a refrigerated centrifuge at 5 °C. The supernatant was collected to assess the protease activity by following the protocol of Tayyab and Qamar (1992) with slight modifications. 1 ml of enzyme extract was mixed with 1 ml reaction mixture (0.1% bovine serum albumin in 0.1 M phosphate buffer, pH 6.5). To initiate the reaction, the mixture was incubated at 37 °C for 2 h and was terminated by adding 2 ml of pre-cooled TCA solution with a concentration of 20%. Blanks in which TCA was added before adding the enzyme extract were processed along with each mixture sample. The reaction mixture was centrifuged and subsequently supernatant was taken. Finally, the Lowry et al. (1951) protocol using tyrosine as the standard was employed to estimate the free amino acids (as tyrosine equivalents) by utilizing a suitable volume of the supernatant.
Evaluation of total phenols and reducing sugars
The quantification of phenols was performed by the method as described by Swain and Hillis (1959) using gallic acid as standard. A suitable volume of aliquot from the alcohol-soluble fraction of the tissue extract was diluted to 7 ml with distilled water, followed by the addition of 0.5 ml of Folin-Dennis reagent. After 3 min, 1 ml of saturated solution of sodium carbonate was added and the total volume was made to 10 ml with DW. Absorbance was measured after 30 min at 725 nm.
For the quantification of reducing sugars, Nelson’s (1944) method was employed. An appropriate volume of an aliquot from the alcohol-soluble fraction of the tissue extract was made up to 5 ml with the DW, to which 1 ml of copper (Cu) reagent, (mixture of Cu reagent A and B in the ratio of 50:1) was added. The mixture was heated at 100 °C for 20 min. Samples were removed and allowed to cool down. This was followed by adding 1 ml of arsenomolybdate. The volume was increased to 25 ml by adding DW and absorbance was taken at 520 nm.
Assessment of soluble proteins and α-amino acids
For estimation of soluble proteins, 1 g petal tissue was homogenized in 100 mM phosphate buffer (pH 7.2) comprising 150 mM sodium chloride, 1 mM EDTA, 1% triton X-100, 10% glycerol, 10% PVP and 1 mM dithiothreitol. The mixture was centrifuged at 12000 g at 4OC for 15 min in a pre-cooled refrigerated centrifuge and the supernatant was collected. Following Lowry et al. (1951) method, an appropriate volume of aliquot was used for the quantification of soluble proteins. Rosen’s (1957) protocol was employed for the assessment of α-amino acid content using glycine as standard.
Statistical analysis of the study
The research employed a fully randomized experimental design and Analysis of Variance in SPSS (version 25; Chicago, USA) to compare the average values of the different treatments. To assess the differences between the treatment sets, Duncan’s Multi Range Test was conducted with a significance level of P < 0.05.
Results
Senescence description
The noticeable symptoms of flower senescence demonstrated the loss of turgidity and subsequent wilting of ray florets (Fig. 1). Senescent petals did not exhibit abscission and remained attached to the flower pedicel. The average lifespan of flower (control) after complete opening was found to be 4 days.
Flower life
The flowers supplemented with JA, MJ and SA delayed the flower senescence in C. sulphureus significantly. The maximum life was documented in JA-treated flowers (8.5 days) followed by MJ (8 days) and SA-treated flowers (7 days). The flowers of control (held in DW) registered an average life of 4 days (Fig. 2).
Fig. 2.
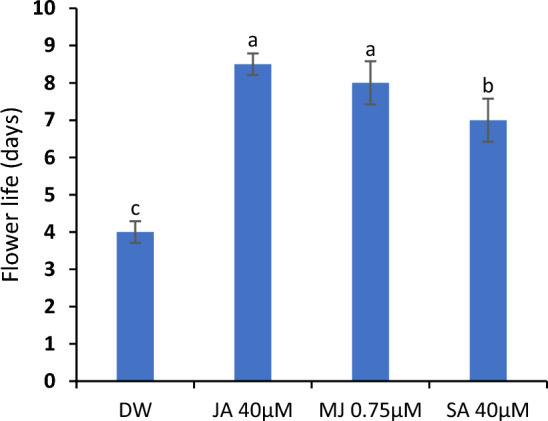
C. sulphureus supplied with distilled water (DW), jasmonic acid (JA 40 μM), methyl jasmonate (MJ 0.75 μM) and salicylic acid (SA 40 μM). Error bars show the ± SE (standard error). The data shows the average value obtained from five replicates. The bars with distinct letters represent the significant differences (P < 0.05)
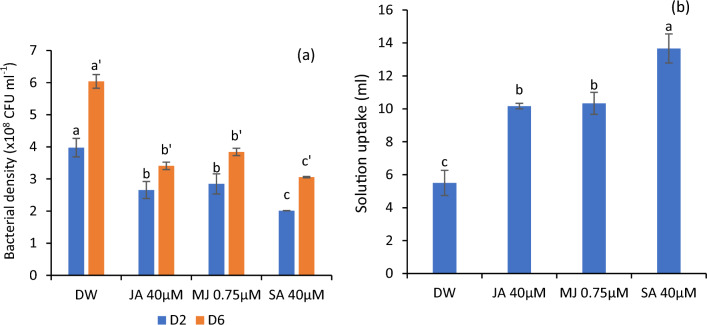
Variations in bacterial density and solution uptake
SA and jasmonates significantly reduced bacterial growth in vial solutions with the highest reduction in SA-treated flowers. Concomitantly, these growth regulators were highly effective in maintaining the efficient solution flow in flowers, as evidenced by the maximum solution uptake. The maximum ascent of the solution was observed in SA-tested flowers followed by jasmonates. On the contrary, the flowers of control registered the highest bacterial growth and the least solution uptake as represented in Fig. 3a, b.
Fig. 3.
Bacterial density in vase solutions (a) and solution uptake (b) in the petals of C. sulphureus supplied with distilled water (DW), jasmonic acid (JA 40 μM), methyl jasmonate (MJ 0.75 μM) and salicylic acid (SA 40 μM). The data shows the average value obtained from three replicates. Error bars indicate the ± SE (standard error). The bars with distinct letters represent the significant differences (P < 0.05). D2 and D6 signify the second and sixth day of the transfer of flower buds to the individual treatments respectively
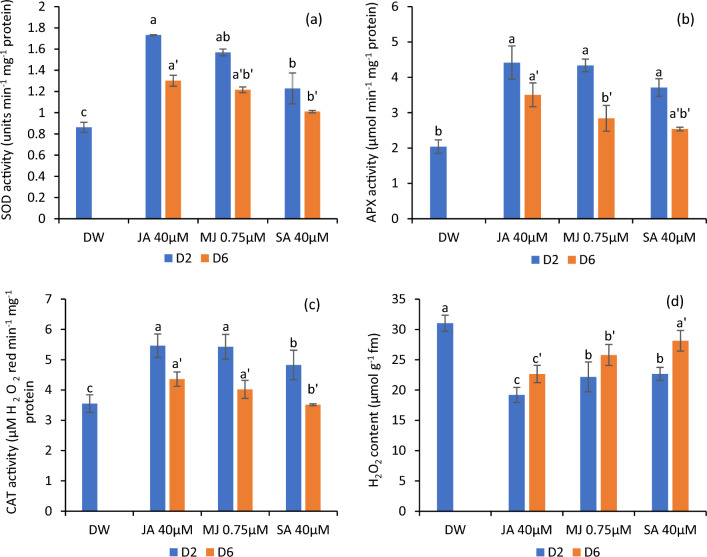
Variations in enzyme activities and H2O2content
The petals administered to jasmonates and SA exhibited upregulated activities of SOD CAT and APX. The maximum activity was registered in jasmonate-treated flowers followed by SA. However, the activities of these enzymes exhibited a profound decline with the progression of flower advancement from day 2 to 6. Intriguingly, the jasmonate and SA-treated petals recorded a considerable reduction in H2O2 levels in comparison to the flowers immersed in DW (control), which registered the highest H2O2 content. Among these treatments, JA was comparatively more efficacious in constraining H2O2 accumulation in comparison to MJ and SA as depicted in Fig. 4a, d.
Fig. 4.
SOD (a) APX (b), CAT (c) activities and H2O2 content (d) in the petals of C. sulphureus supplied with distilled water (DW), jasmonic acid (JA 40 μM), methyl jasmonate (MJ 0.75 μM) and salicylic acid (SA 40 μM). The data shows the average value obtained from three replicates. Error bars indicate the ± SE (standard error). The bars with distinct letters represent the significant differences (P < 0.05). D2 and D6 signify the second and sixth day of the transfer of flower buds to the individual treatments respectively
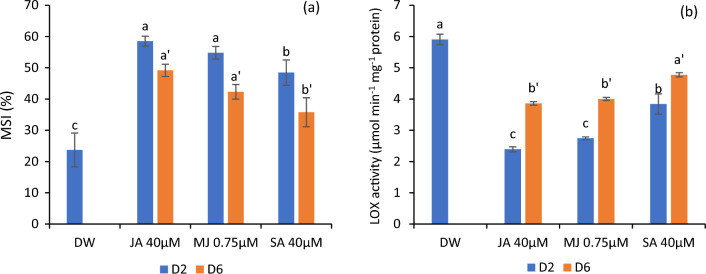
Variations in MSI and LOX activity
The membrane stability assessed as MSI was considerably increased in flowers supplemented with SA, JA and MJ, with maximum index in jasmonate-treated petals. On the contrary, these growth regulators decreased the LOX activity in the petals significantly and the least activity was recorded in jasmonate-treated flowers followed by SA. The highest LOX activity was recorded in the control flowers, which concomitantly registered lower MSI values. The MSI was found to diminish from day second to sixth of the experiment, whereas LOX activity was correspondingly increased as shown in Fig. 5a, b.
Fig. 5.
MSI (a) and LOX activity (b) in the petals of C. sulphureus supplied with distilled water (DW), jasmonic acid (JA 40 μM), methyl jasmonate (MJ 0.75 μM) and salicylic acid (SA 40 μM). The data shows the average value obtained from three replicates. Error bars indicate the ± SE (standard error). The bars with distinct letters represent the significant differences (P < 0.05). D2 and D6 signify the second and sixth day of transfer of the flower buds to the individual treatments respectively
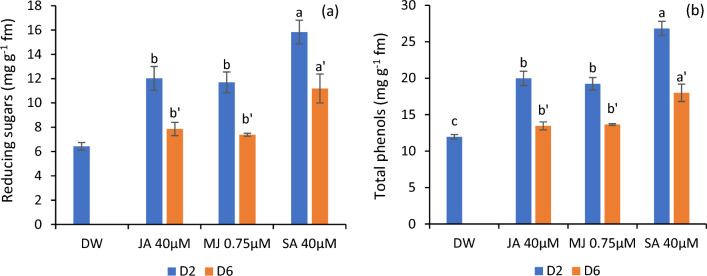
Variations in reducing sugars and total phenols
The flowers supplemented with jasmonates and SA showed an augmented content of reducing sugars when compared to the control, with the highest content in SA-treated flowers. Similar results were obtained in the case of total phenols with maximum content in SA-treated flowers on day 2 and 6 followed by JA and MJ. However, the reducing sugars and total phenolic content were found to decline with flower maturation from day second to sixth of the experiment as shown in Fig. 6a, b.
Fig. 6.
Reducing sugars (a) and total phenols (b) in the petals of C. sulphureus supplied with distilled water (DW), jasmonic acid (JA 40 μM), methyl jasmonate (MJ 0.75 μM) and salicylic acid (SA 40 μM). The data shows the average value obtained from three replicates. Error bars indicate the ± SE (standard error). The bars with distinct letters represent the significant differences (P < 0.05). D2 and D6 signify the second and sixth day of the transfer of flower buds to the individual treatments respectively
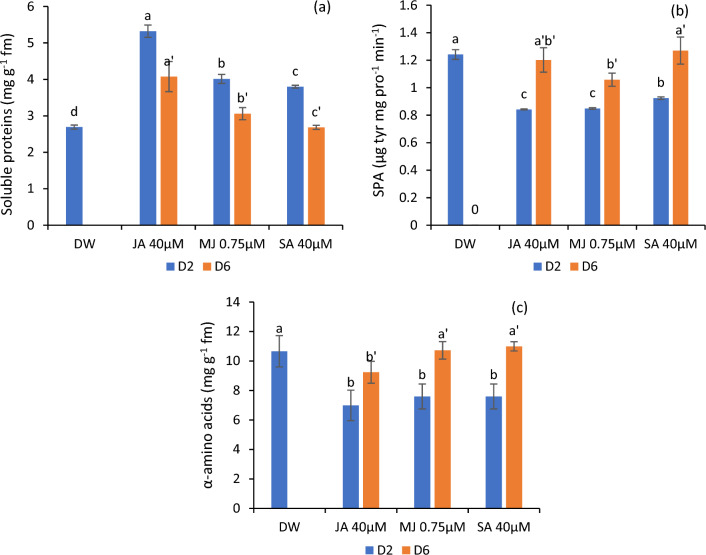
Variations in soluble protein content, α-amino acid content and SPA
In comparison to the control group, the ray florets of flowers tested with JA exhibited a significant increase in soluble proteins, followed by MJ and SA. This elevated content of soluble proteins in the ray florets was found to be associated with a considerable decrease in SPA. The maximum SPA was recorded in the ray florets of flowers held in DW (control) while the minimum activity in jasmonates and SA-treated flowers. The jasmonates and SA-treated petal tissues registered a minimal amount of α-amino acids in comparison to the control (Fig. 7a-c).
Fig. 7.
Soluble proteins (a) SPA (b) and α-amino acid content (c) in the petals of C. sulphureus supplied with distilled water (DW), jasmonic acid (JA 40 μM), methyl jasmonate (MJ 0.75 μM) and salicylic acid (SA 40 μM). The data shows the average value obtained from three replicates. Error bars indicate the ± SE (standard error). The bars with distinct letters represent the significant differences (P < 0.05). D2 and D6 signify the second and sixth day of the transfer of flower buds to the individual treatments respectively
Discussion
SA and jasmonate-mediated signal transduction are particularly known in the context of plant immune responses. These signaling elicitors play a key role in elevating plant tolerance through diverse signaling mechanisms (Arif et al. 2021). The functioning of jasmonates and SA signaling relies on integrated regulatory networks, serving as well-documented instances of hormonal control in plant development. Recent progress indicates that these intricate regulatory networks combine multiple transduction pathways for effectively coordinating plant metabolism and the trade-off between the competing priorities of growth and defense (Huang et al. 2022).
In the present investigation, the average lifespan of detached flowers of C. sulphureus was found to be 4 days as against 5.5 days natural lifespan of flowers when still attached to the plant, signifying a significant variation between the lifespan of intact and cut flowers. Flowers on a plant receive continuous nutrients and benefit from hormonal regulation. The complex microenvironment of a plant contributes to the flower’s overall health. In contrast, cut flowers lose their direct connection with the plant’s vascular system, leading to a loss of nutrient supply. The flowers become susceptible to microbial growth, with bacteria and fungi in the vase solutions hastening the process of senescence. Additionally, cut flowers lack the inherent hormonal regulation thereby affecting the overall hormonal crosstalk during flower senescence (Kondo et al. 2020). As a result, flower longevity is significantly reduced in the cut flowers.
The present work elucidated that SA and jasmonates delayed the flower senescence of detached stalks of C. sulphureus markedly. The enhanced flower longevity of C. sulphureus could be ascribed to the role of these growth regulators in maintaining optimal water conductance in the floral stems by inhibiting bacterial growth. SA has been elucidated to limit the proliferation of bacteria such as S. aureus and E. coli by disrupting their cell walls and membranes (Song et al. 2022; Belay and James Caleb 2022). Microbial growth causes xylem obstruction in flower stems which impedes water conduction in flowers thereby evoking osmotic stress in the florets (Bayat et al. 2017). Furthermore, jasmonates and SA help maintain osmotic balance in plant tissues by accumulating various metabolites, including osmolytes (Ghafari et al. 2020). These results indicate promising applications of these hormones in the postharvest floricultural industry (Song et al. 2022).
Oxidative stress triggered by the loss of synchronization between reactive oxygen species (ROS) and the antioxidant processing system elicits the degradation of membrane lipoproteins and nucleic acids resulting in cell mortality (Raza et al. 2021). On the other hand, a robust defense mechanism exists against oxidative stress in the form of antioxidant enzymes and non-enzymatic processes that catalytically transmute ROS into relatively more stable non-toxic moieties, thus in part restricting the rate of lipid peroxidation (Zhu et al. 2019; Aziz et al. 2020; Ahmad et al. 2021). Senescence can be delayed by the deceleration of peroxidation rates through a robust antioxidant system (Panavas and Rubinstein 1998). SA and jasmonates are known to play an important role in enhancing both enzymatic and non-enzymatic responses to counteract senescence-related processes. Intriguingly, during the current study, these growth regulators markedly amplified the SOD, APX and CAT activities, reducing the H2O2 content in flowers significantly. On the other hand, the activity of LOX, a membrane-bound enzyme was substantially reduced on the administration of these growth regulators. LOX is known to destabilize the membrane integrity by catalyzing the oxidation of fatty acids in plant tissues (Raza et al. 2021). This fortification of the antioxidant system and reduction in LOX activity was in turn associated with high membrane integrity in the floret tissues (Pourzarnegar et al. 2020). Furthermore, it has been observed that SA acts as a potent scavenger of free radicals, effectively preventing the oxidation of cell membranes and reducing the levels of malondialdehyde in gladiolus petals (Rahmani et al. 2015). Such observations have been evidenced in several studies pertaining to the postharvest storage of fruits such as Citrus (Serna-Escolano et al. 2021), Pyrus (Sinha et al. 2022), Prunus (Li et al. 2022a), Ziziphus (Sang et al. 2022), Capsicum (Ge et al. 2020) Dimocarpus (Chen et al. 2020) and pummelo (Huang et al. 2022). The preservation of the postharvest quality of these fruits on the application of jasmonates and SA was associated with high antioxidant enzyme activity and energy metabolism, besides minimal oxidative stress in tissues (Huang et al. 2022). It is noteworthy, that these growth regulators have also been investigated in various flowers like Nicotiana, Consolida and Lilium. Interestingly, these regulators have demonstrated a notable potential to considerably postpone their petal senescence (Nisar et al. 2021; Haq et al. 2022; Liu et al. 2023).
An upsurge of catabolic reactions over anabolic ones is one of the most characteristic signatures of senescence. This shift causes the systematic breakdown of cell organelles, protoplast acidification and tonoplast rupturing, which eventually leads to cell death (van Doorn and Woltering 2008; Wojciechowska et al. 2018). Consistent with this principle, our study showed that several biochemical parameters such as phenols, sugars and soluble proteins were substantially influenced by SA and jasmonates. The introduction of jasmonates and SA as holding solutions diminished carbohydrate deprivation by maintaining high sugar content in C. sulphureus. Pertinently, sugar deprivation is one of the notable signals for flower senescence (van Doorn 2004). Jasmonates have been reported to up-regulate the sucrose phosphate synthase activity thereby augmenting the sucrose level, processed by invertase into reducing sugars (Horibe et al. 2013; Tang et al. 2022). Alternatively, SA may alter respiratory kinetics in petal tissues through uncoupling of the mitochondrial electron transport system which may influence sugar utilization significantly (Norman et al. 2004). Additionally, sugars act as osmoprotectants to maintain osmotic regulation and membrane stability, besides countering ROS (Kaur et al. 2021).
Protein degradation is recognized as the hallmark of petal senescence, serving an essential resolution—resource reallocation (Arora et al. 2007; Shahri and Tahir 2014). This process allocates the valuable components from senescing tissues to the developing sinks of the plant, optimizing resource utilization (Eason et al. 2000; Rogers 2006; Jones, 2013). This demonstrates that the flower senescence is not a chaotic collapse of cellular tissues but a dynamic process coordinated by an intricate network that precisely integrates developmental and environmental cues to elicit the programmed death of organs. Consistent with this notion, our investigation unequivocally confirmed that the tested growth regulators delayed the process of protein degradation by preventing the action of specific proteases known to arrive at the onset of flower senescence (Rogers 2013; Nisar et al. 2021). As such, least SPA was recorded in jasmonate and SA-treated petals as compared to the untreated samples. Conversely, a minimal α-amino acid and high protein content was registered in the petal tissues tested with these phytohormones which could be ascribed to the programmed rescheduling of protein degradation to delay the initiation of senescence. Additionally, the maintenance of high protein content could be accredited to the possible role of these growth regulators in increasing the protein synthesis of the petal tissues. These growth regulators may also trigger the production of some osmoprotectants like proline, which stabilize protein integrity by kosmotropic action (Forlani et al. 2019). Earlier studies imply that the administration of jasmonates and SA retained maximum protein content in the petals of Gerbera, Alstroemeria and Nicotiana which was associated with delayed flower senescence (Shabanian et al. 2018; Ershad Langroudi et al. 2020; Nisar et al. 2021).
Jasmonates and SA are known to stimulate the production of secondary metabolites including phenols in plants (Liu et al. 2023). Phenols, a collection of secondary metabolites benefit the plant tissues by strengthening the anatomical assemblies and averting peroxidation kinetics caused by oxidative stress (Liu et al. 2023). A decline in phenolic content has been correlated with flower senescence (Lone et al. 2021). Phenols act as antioxidants by interacting with diverse free radicals. Due to the hydroxyl (–OH) groups in their chemical structure, phenolic compounds scavenge free radicals and detoxify H2O2. The process by which antioxidants exert their effects can potentially include different pathways, such as single electron transfer, serial proton loss electron transfer, hydrogen atom transfer and the binding of transition metals through chelation (Zeb 2020). SA increases the phenolic content by stimulating the gene expression prerequisite for the phenylpropanoid pathway (Park et al. 2019; Liu et al. 2023). Furthermore, SA has been found to up-regulate the phenylalanine ammonia-lyase activity (Tang et al. 2022), required for the biosynthesis of several defense-related complexes including phenols (Liu et al. 2023). In light of our findings, it may be hypothesized that phenols act as a catalyst for delaying senescence in C. sulphureus by enhancing the antioxidant system. These findings are supported by earlier studies carried out on various flowers such as Calendula, Iris, Consolida and Limonium cut flowers, in which reduction in phenolic content was associated with the petal senescence (Ahmad and Tahir 2018; Lone et al. 2021; Khandan-Mirkohi et al. 2021).
Conclusions and outlook
Until recently, research on SA and jasmonates had primarily focused on their potential involvement in plant development and defense. The study highlights how these signaling molecules play a crucial role in governing flower senescence, a highly genetically regulated process in plant development. Jasmonates and SA delayed the senescence of detached stalks of C. sulphureus by orchestrating various enzymatic and non-enzymatic mechanisms in petals. These novel phytohormones preserved the membrane stability of petals by reducing H2O2 levels and LOX activity, in addition to amplifying the antioxidant enzyme activities in the petals thereby preventing the upsurge in oxidative stress. SA was highly effective in augmenting the soluble proteins, phenolic and sugar content of C. sulphureus followed by JA and MJ, besides maintaining the efficient solution uptake in flowers. These results suggest that SA and jasmonates could be potentially used in postharvest technology to improve the longevity of ornamentals for their cost effectiveness, high efficiency, convenient usage and environmental friendliness features.
Studies are desirable at the genetic level to remarkably expand our knowledge underlying the signal transduction and the crosstalk of these growth regulators with other signaling pathways during flower senescence. Earlier investigations have discovered the role of ABA in the regulation of flower senescence in some ethylene-insensitive flowers. Therefore, future studies would focus on elucidating the possible involvement of SA and jasmonates in antagonizing the ABA action during ethylene-insensitive flower senescence. A thorough understanding of mechanisms enabling crosstalk between these phytohormones can have a considerable impact on trade and commerce in floriculture. Such studies can not only shed light on the complex mechanisms underlying petal senescence but also aid in the development of non-toxic, lucrative methods for enhancing the postharvest appearance of ornamentals.
Acknowledgements
The authors thank Dr. Mohammad Arif Zargar, Assistant Professor, Department of Botany, University of Kashmir for his valuable suggestions throughout this investigation.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahmad SS, Tahir I. Putrescine and jasmonates outplay conventional growth regulators in improving postharvest performance of Iris germanica L. cut scapes. PNAS India Sect B Biol Sci. 2018;88(1):391–402. [Google Scholar]
- Ahmad P, Raja V, Ashraf M, Wijaya L, Bajguz A, Alyemeni MN. Jasmonic acid (JA) and gibberellic acid (GA3) mitigated Cd-toxicity in chickpea plants through restricted cd uptake and oxidative stress management. Sci Rep. 2021;11(1):19768. doi: 10.1038/s41598-021-98753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexieva V, Sergiev I, Mapelli S, Karanov E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001;24(12):1337–1344. doi: 10.1046/j.1365-3040.2001.00778.x. [DOI] [Google Scholar]
- Ali B (2021) Role of salicylic acid in pre-and post-harvest attributes in horticulture. Salicylic Acid-A Versatile Plant Growth Regul 47–64
- Arif Y, Singh P, Siddiqui H, Hayat S (2021) Interplay between salicylates and jasmonates under stress. Salicylic Acid-A Versatile Plant Growth Regul 153–173
- Arora A, Singh VP, Sindhu SS, Rao DN, Voleti SR. Oxidative stress mechanisms during flower senescence. Plant Stress. 2007;1(2):157–172. [Google Scholar]
- Axelrod B, Cheesbrough TM, Laakso S (1981) Lipoxygenase from soybeans: EC 1.13. 11.12 Linoleate: oxygen oxidoreductase. In: Methods in enzymology. Academic Press, vol 71, pp 441–451
- Aziz S, Younis A, Jaskani MJ, Ahmad R. Effect of PGRs on antioxidant activity and phytochemical in delay senescence of lily cut flowers. Agronomy. 2020;10(11):1704. doi: 10.3390/agronomy10111704. [DOI] [Google Scholar]
- Bagheri M, Esna-Ashari M. Effects of postharvest methyl jasmonate treatment on persimmon quality during cold storage. Sci Hortic. 2022;294:110756. doi: 10.1016/j.scienta.2021.110756. [DOI] [Google Scholar]
- Baswal AK, Dhaliwal HS, Singh Z, Mahajan BVC. Post-harvest application of methyl Jasmonate, 1-methylcyclopropene and salicylic acid elevates health-promoting compounds in cold-stored ‘Kinnow’ Mandarin (Citrus nobilis Lour x C. deliciosa Tenora) Fruit. Int J Fruit Sci. 2021;21(1):147–157. doi: 10.1080/15538362.2020.1860865. [DOI] [Google Scholar]
- Bayat H, Aminifard MH. Salicylic acid treatment extends the vase life of five commercial cut flowers. Electron J Biol. 2017;13(1):67–72. [Google Scholar]
- Belay ZA, James Caleb O. Role of integrated omics in unravelling fruit stress and defence responses during postharvest: a review. Food Chem Mol Sci. 2022;5:100118. doi: 10.1016/j.fochms.2022.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GX, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30(7):987–998. [Google Scholar]
- Chen Y, Sun J, Lin H, Lin M, Lin Y, Wang H, et al. Salicylic acid treatment suppresses Phomopsis longanae chi-induced disease development of postharvest longan fruit by modulating membrane lipid metabolism. Postharvest Biol Tech. 2020;164:111168. doi: 10.1016/j.postharvbio.2020.111168. [DOI] [Google Scholar]
- Damalas CA, Koutroubas SD (2021) Foliar applications of salicylic acid for improving crop tolerance to drought stress: a review. Salicyl Acid-A Versatile Plant Growth Regul 65–76
- Dhindsa RS, Plumb-Dhindsa PAMELA, Thorpe TA. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot. 1981;32(1):93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- Eason JR, Johnston JW, de Vré L. Reversal of glyphosate inhibition of Sandersonia aurantiaca flower senescence with aromatic amino acids. Postharvest Biol Technol. 2000;18(1):81–84. doi: 10.1016/S0925-5214(99)00058-7. [DOI] [Google Scholar]
- Elomaa P, Zhao Y, Zhang T (2018) Flower heads in Asteraceae—recruitment of conserved developmental regulators to control the flower-like inflorescence architecture. Hortic Res 5 [DOI] [PMC free article] [PubMed]
- Ershad Langroudi M, Hashemabadi D, Kalatejari S, Asadpour L. Effects of pre-and postharvest applications of salicylic acid on the vase life of cut Alstroemeria flowers (Alstroemeria hybrida) J Hortic Postharvest Res. 2020;3(1):115–124. [Google Scholar]
- Forlani G, Trovato M, Funck D, Signorelli S. Osmoprotectant-mediated abiotic stress tolerance in plants. Cham: Springer; 2019. Regulation of proline accumulation and its molecular and physiological functions in stress defence; pp. 73–97. [Google Scholar]
- Ge W, Zhao Y, Kong X, Sun H, Luo M, Yao M, Wei B, Ji S. Combining salicylic acid and trisodium phosphate alleviates chilling injury in bell pepper (Capsicum annuum L.) through enhancing fatty-acid desaturation efficiency and water retention. Food Chem. 2020;327:127057. doi: 10.1016/j.foodchem.2020.127057. [DOI] [PubMed] [Google Scholar]
- Ghafari H, Hassanpour H, Jafari M, Besharat S. Physiological, biochemical and gene-expressional responses to water deficit in apple subjected to partial root-zone drying (PRD) Plant Physiol Biochem. 2020;148:333–346. doi: 10.1016/j.plaphy.2020.01.034. [DOI] [PubMed] [Google Scholar]
- Haq A, Lone ML, Farooq S, Parveen S, Altaf F, Tahir I, Kaushik P, El-Serehy HA. Efficacy of salicylic acid in modulating physiological andbiochemical mechanisms to improve postharvest longevity in cut spikes of Consolida ajacis (L.) Schur. Saudi J Biol Sci. 2022;29(2):713–720. doi: 10.1016/j.sjbs.2021.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat S, Hasan SA, Fariduddin Q, Ahmad A. Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J Plant Interact. 2008;3(4):297–304. doi: 10.1080/17429140802320797. [DOI] [Google Scholar]
- Hayat S, Siddiqui H, Damalas CA, editors. Salicylic acid-a versatile plant growth regulator. Heidelberg: Springer International Publishing; 2021. [Google Scholar]
- Horibe T, Yamaki S, Yamada K. Effects of auxin and methyl jasmonate on cut rose petal growth through activation of acid invertase. Postharvest Biol Technol. 2013;86:195–200. doi: 10.1016/j.postharvbio.2013.06.033. [DOI] [Google Scholar]
- Horibe T (2022) Post-harvest physiology of cut flowers: use of methyl jasmonate as a quality-retention agent. Jasmonates Brassinosteroids Plants 199–204
- Howe GA, Major IT, Koo AJ. Modularity in jasmonate signaling for multistress resilience. Annu Rev Plant Biol. 2018;69:387–415. doi: 10.1146/annurev-arplant-042817-040047. [DOI] [PubMed] [Google Scholar]
- Hu Y, Jiang Y, Han X, Wang H, Pan J, Yu D. Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones. J Exp Bot. 2017;68(6):1361–1369. doi: 10.1093/jxb/erx004. [DOI] [PubMed] [Google Scholar]
- Huang H, Liu B, Liu L, Song S. Jasmonate action in plant growth and development. J Exp Bot. 2017;68(6):1349–1359. doi: 10.1093/jxb/erw495. [DOI] [PubMed] [Google Scholar]
- Huang Q, Huang L, Chen J, Zhang Y, Kai W, Chen C. Maintenance of postharvest storability and overall quality of ‘Jinshayou’pummelo fruit by salicylic acid treatment. Front Plant Sci. 2022;13:1086375. doi: 10.3389/fpls.2022.1086375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DA, Ferrante A, Vernieri P, Reid MS. Role of abscisic acid in perianth senescence of daffodil (Narcissus pseudonarcissus “Dutch Master”) Physiol Plant. 2004;121(2):313–321. doi: 10.1111/j.0031-9317.2004.0311.x. [DOI] [PubMed] [Google Scholar]
- Jones ML. Mineral nutrient remobilization during corolla senescence in ethylene-sensitive and-insensitive flowers. AoB Plants. 2013;5:plt023. doi: 10.1093/aobpla/plt023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Manna M, Thakur T, Gautam V, Salvi P. Imperative role of sugar signaling and transport during drought stress responses in plants. Physiol Plant. 2021;171(4):833–848. doi: 10.1111/ppl.13364. [DOI] [PubMed] [Google Scholar]
- Khandan-Mirkohi A, Pirgazi R, Taheri MR, Ajdanian L, Babaei M, Jozay M, Hesari M. Effects of salicylic acid and humic material preharvest treatments on postharvest physiological properties of statice cut flowers. Sci Hortic. 2021;283:110009. doi: 10.1016/j.scienta.2021.110009. [DOI] [Google Scholar]
- Kondo M, Nakajima T, Shibuya K, Ichimura K. Comparison of petal senescence between cut and intact carnation flowers using potted plants. Sci Hortic. 2020;272:109564. doi: 10.1016/j.scienta.2020.109564. [DOI] [Google Scholar]
- Kumar N, Tokas J, Raghavendra M, Singal HR. Impact of exogenous salicylic acid treatment on the cell wall metabolism and ripening process in postharvest tomato fruit stored at ambient temperature. Int J Food Sci Tech. 2021;56:2961–2972. doi: 10.1111/ijfs.14936. [DOI] [Google Scholar]
- Li Y, He H, Hou Y, Kelimu A, Wu F, Zhao Y, et al. Salicylic acid treatment delays apricot (Prunus armeniaca L.) fruit softening by inhibiting ethylene biosynthesis and cell wall degradation. Sci Hortic. 2022;300:111061. doi: 10.1016/j.scienta.2022.1110. [DOI] [Google Scholar]
- Li K, Zhang C, Cao J, Qu G. Characteristics of histological alterations and hormone-variations in floral tissues of edible daylily (Hemerocallis citrina) buds during postharvest senescence. Postharvest Biol Technol. 2022;193:112054. doi: 10.1016/j.postharvbio.2022.112054. [DOI] [Google Scholar]
- Liu H, Timko MP. Jasmonic acid signaling and molecular crosstalk with other phytohormones. Int J Mol Sci. 2021;22(6):2914. doi: 10.3390/ijms22062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tang Y, Zhang W, Liang R, Luo K, Jiang X, Yang P, Leifeng X, Ming J. Postharvest methyl jasmonate treatment enhanced biological activity by promoting phenylpropanoid metabolic pathways in Lilium brownii var. viridulum. Sci Hortic. 2023;308:111551. doi: 10.1016/j.scienta.2022.111551. [DOI] [Google Scholar]
- Lone ML, Haq AU, Farooq S, Altaf F, Tahir I. Nitric oxide effectively curtails neck bending and mitigates senescence in isolated flowers of Calendula officinalis L. Physiol Mol Biol Plants. 2021;27:835–845. doi: 10.1007/s12298-021-00969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Ma N, Ma C, Liu Y, Shahid MO, Wang C, Gao J. Petal senescence: a hormone view. J Exp Bot. 2018;69(4):719–732. doi: 10.1093/jxb/ery009. [DOI] [PubMed] [Google Scholar]
- Miryeganeh M. Senescence: the compromised time of death that plants may call on themselves. Genes. 2021;12(2):143. doi: 10.3390/genes12020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naing AH, Lee K, Kim KO, Ai TN, Kim CK. Involvement of sodium nitroprusside (SNP)in the mechanism that delays stem bending of different gerbera cultivars. Front Plant Sci. 2017;8:2045. doi: 10.3389/fpls.2017.02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153(2):375–380. doi: 10.1016/S0021-9258(18)71980-7. [DOI] [Google Scholar]
- Nguyen TH, Goossens A, Lacchini E. Jasmonate: a hormone of primary importance for plant metabolism. Curr Opin Plant Biol. 2022;67:102197. doi: 10.1016/j.pbi.2022.102197. [DOI] [PubMed] [Google Scholar]
- Nisar S, Dar RA, Tahir I. Salicylic acid retards senescence and makes flowers last longer in Nicotiana plumbaginifolia (Viv) Plant Physiol Rep. 2021;26(1):128–136. doi: 10.1007/s40502-021-00569-1. [DOI] [Google Scholar]
- Norman C, Howell KA, Millar AH, Whelan JM, Day DA. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol. 2004;134(1):492–501. doi: 10.1104/pp.103.031039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panavas T, Rubinstein B. Oxidative events during programmed cell death of daylily (Hemerocallis hybrid) petals. Plant Sci. 1998;133(2):125–138. doi: 10.1016/S0168-9452(98)00034-X. [DOI] [Google Scholar]
- Park CH, Yeo HJ, Park YE, Chun SW, Chung YS, Lee SY, Park SU. Influence of chitosan, salicylic acid and jasmonic acid on phenylpropanoid accumulation in germinated buckwheat (Fagopyrum esculentum Moench) Foods. 2019;8(5):153. doi: 10.3390/foods8050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourzarnegar F, Hashemabadi D, Kaviani B. Cerium nitrate and salicylic acid on vase life, lipid peroxidation, and antioxidant enzymes activity in cut lisianthus flowers. Ornament Hortic. 2020;26(4):658–669. doi: 10.1590/2447-536x.v26i4.2227. [DOI] [Google Scholar]
- Rahmani I, Ahmadi N, Ghanati F, Sadeghi M. Effects of salicylic acid applied pre-or post-transport on post-harvest characteristics and antioxidant enzyme activity of gladiolus cut flower spikes. N Z J Crop Hortic Sci. 2015;43(4):294–305. doi: 10.1080/01140671.2015.1096799. [DOI] [Google Scholar]
- Raza A, Charagh S, Zahid Z, Mubarik MS, Javed R, Siddiqui MH, Hasanuzzaman M. Jasmonic acid: a key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep. 2021;40(8):1513–1541. doi: 10.1007/s00299-020-02614-z. [DOI] [PubMed] [Google Scholar]
- Rogers HJ. Programmed cell death in floral organs: How and why do flowers die? Ann Bot. 2006;97(3):309–315. doi: 10.1093/aob/mcj051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HJ. From models to ornamentals: How is flower senescence regulated? Plant Mol Biol. 2013;82:563–574. doi: 10.1007/s11103-012-9968-0. [DOI] [PubMed] [Google Scholar]
- Rosen H. A modified ninhydrin colorimetric analysis for amino acids. Arch Biochem Biophys. 1957;67(1):10–15. doi: 10.1016/0003-9861(57)90241-2. [DOI] [PubMed] [Google Scholar]
- Sairam RK. Effect of moisture-stress on physiological activities of two contrasting wheat genotypes. Indian J Exp Biol. 1994;32:594–594. [Google Scholar]
- Sang Y, Liu Y, Tang Y, Yang W, Guo M, Chen G. Transcriptome sequencing reveals mechanism of improved antioxidant capacity and maintained postharvest quality of winter jujube during cold storage after salicylic acid treatment. Postharvest Biol Technol. 2022;189:111929. doi: 10.1016/j.postharvbio.2022.111929. [DOI] [Google Scholar]
- Sedaghat M, Sarvestani ZT, Emam Y, Bidgoli AM, Sorooshzadeh A. Foliar-applied GR24 and salicylic acid enhanced wheat drought tolerance. Russ J Plant Physiol. 2020;67:733–739. doi: 10.1134/S1021443720040159. [DOI] [Google Scholar]
- Serna-Escolano V, Martínez-Romero D, Giménez MJ, Serrano M, García-Martínez S, Valero D, Valverde JM, Zapata PJ. Enhancing antioxidant systems by preharvest treatments with methyl jasmonate and salicylic acid leads to maintain lemon quality during cold storage. Food Chemistry. 2021;338:128044. doi: 10.1016/j.foodchem.2020.128044. [DOI] [PubMed] [Google Scholar]
- Shabanian S, Esfahani MN, Karamian R, Tran LSP. Physiological and biochemical modifications by postharvest treatment with sodium nitroprusside extend vase life of cut flowers of two gerbera cultivars. Postharvest Biol Technol. 2018;137:1–8. doi: 10.1016/j.postharvbio.2017.11.009. [DOI] [Google Scholar]
- Shahri W, Tahir I. Flower senescence: some molecular aspects. Planta. 2014;239(2):277–297. doi: 10.1007/s00425-013-1984-z. [DOI] [PubMed] [Google Scholar]
- Sinha A, Gill PP, Jawandha SK, Kaur P, Grewal SK. Salicylic acid enriched beeswax coatings suppress fruit softening in pears by modulation of cell wall degrading enzymes under different storage conditions. Food Packag Shelf. 2022;32:100821. doi: 10.1016/j.fpsl.2022.100821. [DOI] [Google Scholar]
- Skutnik E, Jędrzejuk A, Rabiza-Świder J, Rochala-Wojciechowska J, Latkowska M, Łukaszewska A. Nano-silver as a novel biocide for control of senescence in garden cosmos. Sci Rep. 2020;10(1):1–9. doi: 10.1038/s41598-020-67098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Li R, Zhang Q, He S, Wang Y. Antibacterial effect and possible mechanism of salicylic acid microcapsules against Escherichia coli and Staphylococcus aureus. Int J Environ Res Public Health. 2022;19(19):12761. doi: 10.3390/ijerph191912761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Qin M, Yu Q, Huang Z, Xiao Y, Li Y, Ma N, Gao J. Molecular understanding of postharvest flower opening and senescence. Mol Hortic. 2021;1(1):7. doi: 10.1186/s43897-021-00015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10(1):63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Tang T, Zhou H, Wang L, Zhao J, Ma L, Ling J, Li G, Huang W, Li P, Zhang Y. Post-harvest application of methyl jasmonate or prohydrojasmon affects color development and anthocyanins biosynthesis in peach by regulation of sucrose metabolism. Front Nutr. 2022;9:871467. doi: 10.3389/fnut.2022.871467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayyab S, Qamar S. A look into enzyme kinetics: some introductory experiments. Biochem Educ. 1992;20(2):116–118. doi: 10.1016/0307-4412(92)90122-3. [DOI] [Google Scholar]
- van Doorn WG. Is petal senescence due to sugar starvation? Plant Physiol. 2004;134(1):35–42. doi: 10.1104/pp.103.033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ. Physiology and molecular biology of petal senescence. J Exp Bot. 2008;59(3):453–480. doi: 10.1093/jxb/erm356. [DOI] [PubMed] [Google Scholar]
- Wan S, Xin XF (2022) Regulation and integration of plant jasmonate signaling: a comparative view of monocot and dicot. J Genet Genom [DOI] [PubMed]
- Wojciechowska N, Sobieszczuk-Nowicka E, Bagniewska-Zadworna A. Plant organ senescence–regulation by manifold pathways. Plant Biol. 2018;20(2):167–181. doi: 10.1111/plb.12672. [DOI] [PubMed] [Google Scholar]
- Zeb A. Concept, mechanism, and applications of phenolic antioxidants in foods. J Food Biochem. 2020;44(9):e13394. doi: 10.1111/jfbc.13394. [DOI] [PubMed] [Google Scholar]
- Zhu G, Yin J, Guo X, Chen X, Zhi W, Liu J, Wang Y, Lu H, Jiao X, Zhou G. Gibberellic acid amended antioxidant enzyme and osmotic regulation to improve salt tolerance of okra at early growth stage. Int J Agric Biol. 2019;22(2):270–276. [Google Scholar]