Abstract
Diabetes has affected nearly half a billion people worldwide. According to current guidelines, glycemic control is essential to mitigate diabetic complications. The antihyperglycemic effects of various chemically synthesized nanoparticles have been reported in animal models. However, their impact on humans has not been previously reported. This study was conducted to biosynthesize and assess the antihyperglycemic property of silica nanoparticles (SiO2-NPs) since they are non-toxic and biocompatible. SiO2-NPs biosynthesized using the endophytic fungus Fusarium oxysporum. In this collaborative study, 26 people, either hyperglycemic or euglycemic, diagnosed at the Endocrinology Outpatients, according to the American Diabetes Association, USA, were recruited. Silica nanoparticles were characterized and assessed for in vitro antihyperglycemic property using blood samples. Particle size distribution based on TEM images confirms that the average size of silica nanoparticle is 25 nm and is monodispersed in nature. The XRD pattern shows that only one broad peak at 2θ = 220 corresponds to the plane (101) of silica nanoparticles. UV Visible spectra show the λmax at 270 nm, peaks in FTIR at 1536 cm−1, 1640 cm−1, and 3420 cm−1 for the protein cap. The mean blood glucose was 120.2 mg/dL in the ‘SiO2-NP untreated’ group and decreased to 97.24 mg/dL in the ‘SiO2-NP treated’ group. A paired t-test (P-value < 0.0001) indicates a strong relationship between antihyperglycemia and silica NP. In our study, it has been observed that the biosynthesized silica nanoparticles using the endophytic fungus Fusarium oxysporum show antihyperglycemic property in vitro.
Graphical Abstract
Keywords: Diabetes, Silica Nanoparticle, Hyperglycemia, Blood Glucose
Introduction
Type 2 diabetes mellitus (T2DM) is the most predominant form of diabetes worldwide, accounting for more than 90% of cases globally. According to the International Diabetes Federation, 463 million people had diabetes across the globe in 2019, and by 2045 this could reach up to 700 million [1]. Diabetes has a huge financial impact, and the complications it causes eventually lead to a declining quality of life and a shorter life span [2]. Diabetes is characterized by insufficient or absolute lack of insulin secretion, insulin resistance, altered fuel metabolism, and the development of diabetes-specific macrovascular and microvascular complications [3]. As these complications are related to the severity and duration of hyperglycemic exposure, the primary goal of therapy is glycemic control [4]. Nanomedicine has recently been attempted to treat diabetes, and significant results are obtained from in vivo and in vitro studies [5]. Biosynthesized nanoparticles are being extensively used in biomedical research due to their small size, water dispersibility, high stability, and biocompatibility [6–9].
The antihyperglycemic effects of different nanoparticles have been evaluated in the recent past. Alloxan-induced Wistar albino rats treated with gold nanoparticles (AuNPs) had lower levels of blood glucose [10]. Gold NP was administered to these rats for 28 consecutive days and showed a significant decline in plasma glucose, cholesterol, and triglycerides. Gold NP also increases plasma insulin levels through its protein tyrosine phosphatase 1B inhibitory activity (PTP1B), a negative regulator of the insulin signaling pathway [11]. Ali Alkaladi and colleagues examined the anti-diabetic properties of zinc oxide NPs and silver NPs in diabetic rats given STZ. They discovered a notable decline in blood glucose levels, elevated insulin and insulin receptor expression, and upregulation of the GLUT-2 and glucokinase genes [12]. The administration of Cu-NP also reduces the blood glucose of broiler chicken when compared to CuSO4 and the control groups. NF-KB and TNFα mRNA expression in broiler chicken hepatocytes were measured after treatment with Cu-NP to evaluate the immune response. Cu-NP treatment had no effect on the expression of these genes when compared to the control group [13]. Superparamagnetic iron oxide nanoparticles (SPIONs) lower blood glucose and lipids by interacting with the mRNA expression of the genes related to glucose and lipid metabolism. Many of the genes involved in the pathogenesis of diabetic complications were suppressed following the administration of SPIONs. Genes associated with phagocytosis, such as SEC16B, were highly expressed in human primary adipocytes, on the other hand. According to these findings, there is a possibility that SPIONs could have an antidiabetic effect that is comparable to metformin or even better, warranting further study to explore the therapeutic potential [14, 15].
Silica is one of the most abundant minerals found in nature. In medical science, it is widely used for osteoporotic bone implants as silica ion [16], dental implants as calcium silicate [17], scaffolds, and dermatology. Silica helps maintain glycoprotein stability and integrity associated with collagen [18], and it also boosts the immune system and reduces the risk of atherosclerosis. Silica is an essential component for the proper growth and development of plants. Silica also increases the resistance to fungi and plays a significant role in the reproduction of plants [19]. Recently it has been used in the biomedical field as a nanosized form due to its high thermal stability, biocompatibility, and enormous surface flexibility. Silica nanoparticle (SiO2-NP) has various biomedical applications like tissue regeneration, drug delivery [20], cleaning of oral biofilm on dental implants [21], dermatology, diagnostic and therapeutic devices (ophthalmological and bio-glasses, scaffolds) [22–25]. Amorphous-SiO2-NP seems nontoxic [26], is recognized as safe by the United States Food and Drug Administration (FDA), and can be used as oral delivery ingredients. It has recently been widely applied as an additive in cosmetics, food, and oral drugs [27]. The United States National Cancer Institute (NCI) has also confirmed that most engineered NPs are far less toxic than products containing a higher magnitude of silica, such as household cleaning products, dandruff remedies, and insecticides. Moreover, silica is easily eliminated through urine, preventing its accumulation in the kidney, in contrast to crystalline silica [28]. Very little study has been done on the antidiabetic or antihyperglycemic effect of silica NPs. In this present study, biosynthesized silica NPs are characterized and biotransformed by protein capping to yield water-dispersible SiO2-NPs, which the animal and human circulation readily absorb; therefore, they have been used to evaluate their in vitro antihyperglycemic effect. To the best of our knowledge, this study is the first that explores the in vitro glucose-lowering effect of silica NPs using human blood samples.
Materials and Methods
Characterization of Silica Nanoparticles
Biosynthesized silica nanoparticles were characterized using some well-recognized techniques like Transmission Electron Microscopy (TEM), X-ray powder diffraction (XRD), and Selected Area (electron) Diffraction (SAED), Fourier-Transform Infrared Spectroscopy (FTIR), and Ultraviolet–Visible Spectrophotometry (UV–Vis).
Transmission Electron Microscopy (TEM)
The shape, size, and particle size distribution of SiO2-NPs were investigated using Transmission Electron Microscopy (TEM), JEM-JEOL, JAPAN, which was performed under magnification of 100,000X. The carbon‐coated copper TEM grid was made by placing an aqueous solution containing nanoparticles onto the grid with subsequent evaporation of water molecules by heating. The selected Area (Electron) Diffraction (SAED) pattern of SiO2-NP was also captured during TEM imaging at an angular magnification of 0.001 mm per degree and beam operating potential of 200 kV.
X-Ray Powder Diffraction (XRD)
X-ray powder diffraction (XRD) measurement of the lyophilized powder sample of silica nanoparticles was carried out using an X-ray powder diffractometer (Rigaku, Tokyo, Japan) to investigate the crystal structure and morphology of the biotransformed silica nanoparticles. The diffraction pattern was recorded in the 2θ range of 10° to 80° (step size 0.02°) and a time of 5 s per step at 30 kV voltage with 15 mA current. The XRD analysis used monochromatic high-intensity CuKα radiation (λ = 1.54060 A0).
Ultraviolet–Visible Spectrophotometry (UV–Vis)
Most extensively, UV-Visible Spectroscopy is used to study the surface adsorption and optical properties of the nanoparticles. The double beam Cary 5000 UV-Vis-NIR spectrophotometer (Agilent, USA) was operated at a resolution of 1 nm for the spectrometric analysis of the silica nanoparticles fabricated using biological root (thus, naturally protein capped) to confirm the capping of protein molecules on the surface of silica nanoparticles. The spectrum of the as-synthesized aqueous nanoparticle solution was recorded in the 200–800 nm wavelength range against water (used as a blank).
Fourier Transform Infrared Spectroscopic Analysis (FT-IR)
FT-IR analysis used to verify the presence of characteristic metal–oxygen bond vibration and the amide bands of capping proteins in biosynthesized metal oxide nanoparticles. The Perkin Elmer (USA) Fourier transform infrared spectrophotometer was used for FT-IR measurements of biotransformed SiO2-NPs in the spectral range of 400–4000 cm−1. A thin plate of KBr containing SiO2-NPs was used for the measurement, while a pure KBr plate was used to correct for background effects.
Selection Criteria for Subjects
Our study is an in vitro study conducted by the Rajiv Gandhi Centre for Diabetes & Endocrinology, J N Medical College, AMU, Aligarh, jointly with the Interdisciplinary Nanotechnology Centre, AMU, Aligarh. 26 subjects were studied after obtaining informed consent and institutional ethical approval (D.No.263/FM/IEC). Twenty-six subjects, irrespective of age, were recruited for this study and were either hyperglycemic (15.38%) or euglycemic (84.62%). Diagnostic criteria were as per the American Diabetes Association, USA.
Collection and Processing of Blood Sample
A total of 1 mL of peripheral blood was collected aseptically (from a cubital vein) from outdoor patients of Rajiv Gandhi Centre for Diabetes and Endocrinology, J N Medical College, AMU, Aligarh. The blood samples were collected and divided into two separate EDTA vacutainers (0.5 mL each) immediately following the standard protocol. 50 μl PBS (without SiO2-NP) was added to the 1st part of the blood sample. The other 50 μl SiO2-NP (dissolved in PBS) was added to the 2nd part of the same blood sample. Both the EDTA vials were incubated for 2 h at room temperature.
Assessment of Blood Glucose by the O-Toluidine Method (Colorimetric)
Blood proteins are precipitated with trichloroacetic acid (TCA) to obtain a crystal-clear protein free filtrate (PFF) solution. The PFF obtained contains glucose and other small biomolecules. Glucose reacts with O-toluidine reagent (in acetic acid) in presence of heat and gives rise to a blue-green N-glycosylamine derivative. The intensity of this blue-green color is proportional to the amount of glucose present in the medium. Reagents: (a) Ortho-toluidine reagent: 90 mL of O-toluidine was added to 5gm thiourea, diluted up to 1 L with glacial acetic acid, stored in a brown bottle, and kept at 4 °C until used; (b) 10% Trichloroacetic acid (TCA): 10gm of TCA was dissolved in distilled water and make the volume up to 100 mL; (c) Glucose standard solution (0.1 mg/mL): 10 mg of glucose was dissolved in 50 mL of distilled water in a 100 mL volumetric flask. To this, 30 mL of 10% TCA was added and made up the volume up to 100 mL with distilled water; (d) Blank solution: 30 mL of 10% TCA was diluted to 100 mL (with distilled water); (e) Silica nanoparticle: 20 μg of silica nanoparticle (particle size < 100 nm) dissolved in phosphate glucose buffer (PBS); (f) Phosphate glucose buffer (PBS): 8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4, 10 g D-glucose were dissolved in 800 mL of distilled water and made up the volume to 1L.
| 1st Part [EDTA vacutainer with PBS] | 2nd Part [EDTA vacutainer with SiO2-NP dissolved in PBS] | |
|---|---|---|
| Blood | 0.5 mL | 0.5 mL |
| Phosphate glucose buffer (PBS) | 50μL | 00μL |
| SiO2-NP (dissolved in PBS) | 00μL | 50μL |
Procedure: Preparation of Protein-Free Filtrate (PFF): 3 mL of distilled water and 0.5 mL of blood were taken in TARSONS conical tubes and mixed appropriately. 1.5 mL of 10% TCA was added, thoroughly mixed, and allowed to stand for 10 min before filtering. Whatman 42 filter paper has been used to obtain protein-free filtrate (PFF). Development of color: 1 mL PFF from 1st part and 1 mL PFF from 2nd part of each sample is transferred to a separate glass tube. 1 mL of glucose standard solution and 1 mL of blank solution were added to separate glass tubes. 5 mL of O-toluidine was added to all these tubes and mixed thoroughly. The tubes were kept in a boiling water bath for 10 min, cooled, and the optical density was measured using a spectrophotometer (mySPEC, avantor, USA) at a wavelength of 620 nm.
| PFF from 1st Part | PFF from 2nd Part | |
|---|---|---|
| Protein Free Filtrate (PFF) | 1 mL | 1 mL |
| O-Toluidine | 5 mL | 5 mL |
| Kept in a boiling water bath for 10 min, cooled under tap water |
The concentration of blood glucose was measured using the formula:
Statistical Analysis
Data were analyzed using GraphPad Prism 8.0 (GraphPad Software, California, USA), and Origin 5.0 has been used to represent nanoparticles characterization-related graphs. All data were normally distributed and were confirmed by Anderson–Darling and Kolmogorov–Smirnov test. The data were expressed as a mean with a 95% confidence interval (CI). A comparison between the groups was made using a paired t-test and a value of p < 0.05 was considered a statistically significant difference.
Results
Images captured by transmission electron microscopy (TEM) confirmed that the silica particles were indeed nanoscale (Fig. 1). The average size of the silica nanoparticles was found to be 25 nm by plotting the particle size distribution histogram (Fig. 2a) from the TEM images. The obtained silica nanoparticles are monodispersed in nature, as demonstrated by transmission electron microscopy (TEM). The X-ray powder diffraction pattern of silica nanoparticles is displayed in Fig. 2b. The pattern exhibits only one broad peak at 2θ = 220 corresponds to the plane (101) of silica nanoparticles.[29]. The broadening of the peak indicates its amorphous nature, confirmed by the SAED pattern. UV Visible spectra (Fig. 2c) and FTIR spectra (Fig. 2d) of silica nanoparticles were recorded to ensure protein capping onto the surface of it. UV Visible spectra show the absorption maxima at 270 nm due to the absorption of protein molecules, whereas the peaks observed in FTIR at 1536 cm−1, 1640 cm−1, and 3420 cm−1 for the stretching vibration of Amide-II, Amide-I, and N–H or O–H of capped protein molecules, respectively. The peaks appeared at 795 cm−1, 960 cm−1, and 1093 cm−1, corresponding to Si–O symmetric bond vibration, Si–OH, and Si–O asymmetric bond vibration, respectively, which are the characteristic peaks of silica nanoparticle[30].
Fig. 1.
Transmission electron microscopy (TEM) images of monodisperse silica nanoparticles a, b and c showing shape, size, and particle size distribution. Selected area (electron) diffraction pattern of silica nanoparticles d showing its amorphous nature
Fig. 2.
a Particle size distribution curve of monodispersed Silica nanoparticles to obtain average particle size (25 nm). b XRD spectra of Silica nanoparticles showing amorphous nature. c UV–Visible spectra of Silica nanoparticles. d FTIR spectra of Silica nanoparticles
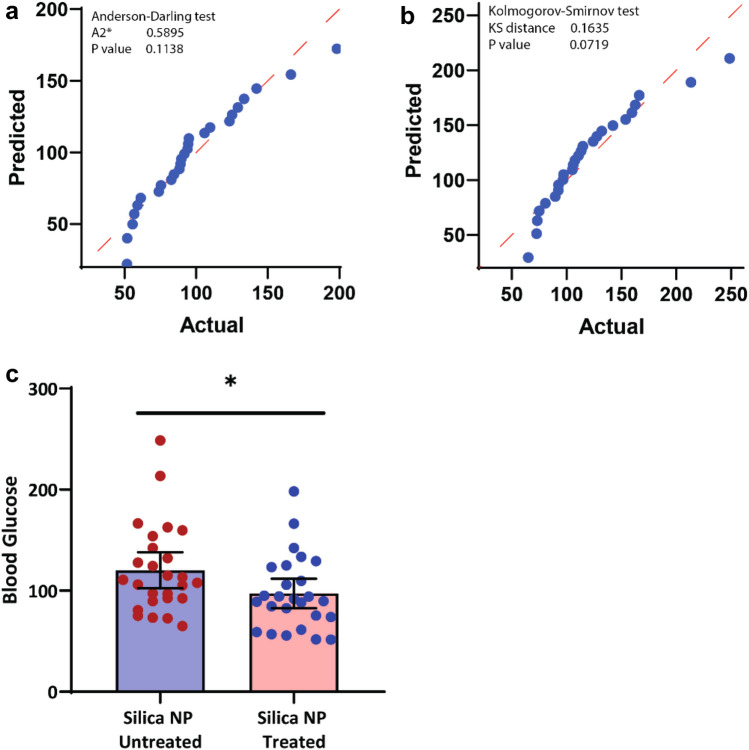
The starting baseline mean blood glucose remained the same as we used the same blood sample before the treatment with PBS and PBS + NP. The glucose concentration of the blood samples that were treated (incubated with PBS and NP dissolved in PBS) was estimated by measuring the optical density and plotted the values in the graph (Fig. 3c). Table 1 shows that the average (mean) blood glucose value is 120.2 mg/dL in the ‘SiO2-NP untreated’ group, whereas this value has been decreased to 97.24 mg/dL in the ‘SiO2-NP treated’ group. The maximum blood glucose value in the ‘SiO2-NP untreated’ group is 248.7 mg/dl, while in the ‘SiO2-NP treated’ group, it is 198.3 mg/dL. Anderson–Darling and Kolmogorov–Smirnov test were used to ensure the distribution of both the groups (treated and untreated) and found normally distributed (Fig. 3a, b). The parametric paired-samples t-test was used to compare the two groups. P-value < 0.0001 indicates that the silica nanoparticle is strongly associated with lowering the blood glucose level when evaluated in vitro (Table 2).
Fig. 3.
a Normal QQ plot of `silica NP treated group’ showing its values are normally distributed and is confirmed by Anderson–Darling test. b Normal QQ plot of `silica NP untreated group’ confirmed its normality by Kolmogorov-Smirnovtest. c Distribution of individual values among `silica NP treated’ & `untreated groups’. Mean with 95% CI are considered to show the difference
Table 1.
Descriptive Statistics of ‘NP untreated’ and ‘NP treated’ data set
| SiO2-NP untreated n = 26 |
SiO2-NP treated n = 26 |
|
|---|---|---|
| Minimum Blood Glucose Value (mg/dL) | 65.02 | 51.66 |
| 25% Percentile | 91.75 | 70.77 |
| Median | 109.3 | 90.68 |
| 75% Percentile | 145.2 | 123.7 |
| Maximum Blood Glucose Value (mg/dL) | 248.7 | 198.3 |
| Mean | 120.2 | 97.24 |
| Std. Deviation | 43.79 | 36.28 |
| Std. Error of Mean | 8.588 | 7.114 |
| Lower 95% CI | 102.5 | 82.59 |
| Upper 95% CI | 137.9 | 111.9 |
Table 2.
Paired samples t-test for comparing “SiO2-NP untreated” and “SiO2-NP treated” groups
| Paired Differences | t | df | P-value (2-tailed) | ||||
|---|---|---|---|---|---|---|---|
| Mean | S.D | S.E mean | 95% Confidence Interval of the Difference | ||||
| Lower | Upper | ||||||
| -22.96 | 21.44 | 4.204 | − 31.62 | − 14.30 | 5 | 25 | < 0.0001 |
Discussion
The prevalence of diabetes has reached an alarming rate, and it has emerged as one of the fastest-growing health emergencies in the twenty-first century. According to the International Diabetes Federation, recently 74% increase has been reported in South-East Asia[1]. Chronic hyperglycemia is associated with microvascular & macrovascular complications and ultimately leads to reduced life quality. So the primary goal of therapy for the treatment of diabetes is glycemic control[4].
As mentioned before, numerous studies have been performed to assess the antihyperglycemic effects of various nanoparticles. Most inorganic nanoparticles are chemically synthesized in a very hostile environment. These techniques have a number of negative impacts on the environment, including being costly, toxic, inconvenient, and yielding larger particles that agglomerate due to a lack of capping agents and rupture RBC membranes. Therefore, the biological protocol is followed to synthesize nanomaterials under ambient conditions. These biofabricated NPs come with the added benefit of being naturally protein capped, which increases water dispersibility, high stability, and superior shelf life. In this study, the size of the silica NP has been chosen to be less than 100 nm (25 nm) since greater than 100 nm (∼600 nm) particle size can cause intense membrane deformity leading to hemolysis [31]. The antihyperglycemic effect of silica NP might be due to the increased activity of GLUT1 in RBC or increased translocation of GLUT4 in WBC [32], which leads to the increased influx of glucose into the cell and subsequently utilized by glycolysis. In addition, silica NP may bind to the tyrosine kinase domain of the insulin receptor, triggering downstream signaling that promotes glycolysis. The antihyperglycemic property may also be achieved by triggering enzymes of the glycolytic pathway (Fig. 4).
Fig. 4.

The probable sites where the silica nanoparticle can act to exert its antihyperglycemic effect by modulating glucose metabolism
As silica nanoparticles are excellent in drug delivery and targeting, the antihyperglycemic property might also be due to the glucose delivery to the specific receptors (GLUT1, GLUT4) [33].
All the mechanisms mentioned above might be the probable causes of the antihyperglycemic property of silica nanoparticle. Further studies are needed to explore the precise mechanism by which silica nanoparticles exert an antihyperglycemic effect.
Conclusion
Our results demonstrated that the functionalized biotransformed silica nanoparticles synthesized using endophytic fungus Fusarium oxysporum have provided antihyperglycemic property when studied in vitro on human blood samples. It appears that silica NP have an antihyperglycemic effect, as blood glucose levels were reduced in nearly all samples after treatment. Signaling mechanisms by which silica nanoparticle exerts an antihyperglycemic effect can be unveiled if subjected to further studies.
In this present study, it may be concluded that the biosynthesized silica nanoparticles could explore opportunities for developing antidiabetic nanomedicine and its possible therapeutic potential in hyperglycemic conditions for the management of diabetes in the coming future.
Acknowledgements
We acknowledge the Department of Biotechnology (DBT), Government of India, for setting up a Centre of Excellence (COE, BT/PR1-3584/COE/34/29/2015) at the Interdisciplinary Nanotechnology Centre (INC), Aligarh Muslim University, AMU, Aligarh, UP-202002, India.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guariguata L, Whiting D, Weil C, Unwin N. The international diabetes federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res Clin Pract. 2011;94(3):322–332. doi: 10.1016/j.diabres.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Gregg EW. The changing tides of the type 2 diabetes epidemicd smooth sailing or troubled waters ahead? Kelly West Award Lecture 2016. Diabetes Care. 2017;40(10):1289–1297. doi: 10.2337/dci16-0055. [DOI] [PubMed] [Google Scholar]
- 3.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, et al. Diabetes and mortality following acute coronary syndromes. J Am Med Assoc. 2007;298(7):765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 4.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 5.Shao T, Yuan P, Zhu L, Xu H, Li X, He S, et al. Carbon nanoparticles inhibit α-glucosidase activity and induce a hypoglycemic effect in diabetic mice. Molecules. 2019;24(18):3257. doi: 10.3390/molecules24183257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bansal V, Rautaray D, Ahmad A, Sastry M. Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J Mater Chem. 2004;14(22):3303–3305. doi: 10.1039/b407904c. [DOI] [Google Scholar]
- 7.Ahmad A, Mukherjee P, Mandal D, Senapati S, Khan MI, Kumar R, et al. Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus Fusarium Oxysporum. J Am Chem Soc. 2002;124(41):12108–12109. doi: 10.1021/ja027296o. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad A, Mukherjee P, Senapati S, Mandal D, Khan MI, Kumar R, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surfaces B Biointerfaces. 2003;28(4):313–318. doi: 10.1016/S0927-7765(02)00174-1. [DOI] [Google Scholar]
- 9.Rautaray D, Sanyal A, Adyanthaya SD, Ahmad A, Sastry M. Biological synthesis of strontium carbonate crystals using the fungus Fusarium oxysporum. Langmuir. 2004;20(16):6827–6833. doi: 10.1021/la049244d. [DOI] [PubMed] [Google Scholar]
- 10.Karthick V, Kumar VG, Dhas TS, Singaravelu G, Sadiq AM, Govindaraju K. Effect of biologically synthesized gold nanoparticles on alloxan-induced diabetic rats-An in vivo approach. Colloids Surfaces B Biointerfaces. 2014;122:505–511. doi: 10.1016/j.colsurfb.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Venkatachalam M, Govindaraju K, Mohamed Sadiq A, Tamilselvan S, Ganesh Kumar V, Singaravelu G. Functionalization of gold nanoparticles as antidiabetic nanomaterial. Spectrochim Acta Part A Mol Biomol Spectrosc. 2013;15(2):2015–2023. doi: 10.1016/j.saa.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 12.Alkaladi A, Abdelazim AM, Afifi M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int J Mol Sci. 2014; [DOI] [PMC free article] [PubMed]
- 13.Scott A, Vadalasetty KP, Łukasiewicz M, Jaworski S, Wierzbicki M, Chwalibog A, et al. Effect of different levels of copper nanoparticles and copper sulphate on performance, metabolism and blood biochemical profiles in broiler chicken. J Anim Physiol Anim Nutr (Berl). 2018;102(1):e364–e373. doi: 10.1111/jpn.12754. [DOI] [PubMed] [Google Scholar]
- 14.Sharifi S, Daghighi S, Motazacker MM, Badlou B, Sanjabi B, Akbarkhanzadeh A, et al. Superparamagnetic iron oxide nanoparticles alter expression of obesity and T2D-associated risk genes in human adipocytes. Sci Rep. 2013;3(1):1–12. doi: 10.1038/srep02173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali LMA, Shaker SA, Pinol R, Millan A, Hanafy MY, Helmy MH, et al. Effect of superparamagnetic iron oxide nanoparticles on glucose homeostasis on type 2 diabetes experimental model. Life Sci. 2020;245:117361. doi: 10.1016/j.lfs.2020.117361. [DOI] [PubMed] [Google Scholar]
- 16.Chandran S, John A. Osseointegration of osteoporotic bone implants: Role of stem cells, Silica and Strontium—a concise review. J Clin Orthop Trauma. 2019;10:S32–S36. doi: 10.1016/j.jcot.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi S, Wu J, Xu Y, Zhang Y, Wang R, Li K, et al. Chemical stability and antimicrobial activity of plasma-sprayed cerium oxide-incorporated calcium silicate coating in dental implants. Implant Dent. 2019;28(6):564–570. doi: 10.1097/ID.0000000000000937. [DOI] [PubMed] [Google Scholar]
- 18.Heinemann S, Coradin T, Desimone MF. Bio-inspired silica-collagen materials: Applications and perspectives in the medical field. Biomater Sci. 2013;1(7):688–702. doi: 10.1039/c3bm00014a. [DOI] [PubMed] [Google Scholar]
- 19.Epstein E. Silicon. Annu Rev Plant Biol. 1999;50:641. doi: 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- 20.Huang PK, Lin SX, Tsai MJ, Leong MK, Lin SR, Kankala RK, et al. Encapsulation of 16-hydroxycleroda-3,13-dine-16,15-olide in mesoporous silica nanoparticles as a natural dipeptidyl peptidase-4 inhibitor potentiated hypoglycemia in diabetic mice. Nanomaterials. 2017;7(5):112. doi: 10.3390/nano7050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Hashedi AA, Laurenti M, Amine Mezour M, Basiri T, Touazine H, Jahazi M, et al. Advanced inorganic nanocomposite for decontaminating titanium dental implants. J Biomed Mater Res - Part B Appl Biomater. 2019;107(3):761–772. doi: 10.1002/jbm.b.34170. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Fang L, Zhang Y, Zhu H, Cao S. Silica-Based Scaffolds: Fabrication, Synthesis and Properties. Front Biomat Des, Synth Strat Biocompat Polym Scaffolds Biomed Appl. 2014;1:305. doi: 10.2174/9781608058761114010014. [DOI] [Google Scholar]
- 23.Navarro M, Serra T. Biomimetic mineralization of ceramics and glasses. In Biomineralization and Biomaterials: Fundamentals and Applications. (2016).
- 24.Mendoza-Novelo B, González-García G, Mata-Mata JL, Castellano LE, Cuéllar-Mata P, Ávila EE. A biological scaffold filled with silica and simultaneously crosslinked with polyurethane. Mater Lett. 2013;106:369–372. doi: 10.1016/j.matlet.2013.05.088. [DOI] [Google Scholar]
- 25.Brunner TJ, Stark WJ, Boccaccini AR. Nanoscale Bioactive Silicate Glasses in Biomedical Applications. In Nanotechnologies for the Life Sciences (2010).
- 26.Zhang H, Dunphy DR, Jiang X, Meng H, Sun B, Tarn D, et al. Processing pathway dependence of amorphous silica nanoparticle toxicity: colloidal vs pyrolytic. J Am Chem Soc. 2012;134(38):15790–15804. doi: 10.1021/ja304907c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasaai MR. Nanosized particles of silica and its derivatives for applications in various branches of food and nutrition sectors. J Nanotechnol. (2015)
- 28.Croissant JG, Fatieiev Y, Khashab NM. Degradability and clearance of silicon, organosilica, silsesquioxane, silica mixed oxide, and mesoporous silica nanoparticles. Adv Mater. 2017;29(9):1604634. doi: 10.1002/adma.201604634. [DOI] [PubMed] [Google Scholar]
- 29.Vinoda BM, Vinuth M, Bodke Y, Manjanna J. Photocatalytic degradation of toxic methyl red dye using silica nanoparticles synthesized from rice husk ash. J Environ Anal Toxicol. 2015;5(336):2161–2525. [Google Scholar]
- 30.Bansal V, Rautaray D, Bharde A, Ahire K, Sanyal A, Ahmad A, et al. Fungus-mediated biosynthesis of silica and titania particles. J Mater Chem. 2005;15(26):2583–2589. doi: 10.1039/b503008k. [DOI] [Google Scholar]
- 31.Zhao Y, Sun X, Zhang G, Trewyn BG, Slowing II, Lin VSY. Interaction of mesoporous silica nanoparticles with human red blood cell membranes: size and surface effects. ACS Nano. 2011;5(2):1366–1375. doi: 10.1021/nn103077k. [DOI] [PubMed] [Google Scholar]
- 32.Maratou E, Dimitriadis G, Kollias A, Boutati E, Lambadiari V, Mitrou P, Raptis SA. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Invest. 2007;37(4):282–290. doi: 10.1111/j.1365-2362.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Lin Y, Di D, Zhang X, Wang D, Zhao Q, et al. Gold nanoparticle-gated mesoporous silica as redox-triggered drug delivery for chemo-photothermal synergistic therapy. J Colloid Interface Sci. 2017;508:323–331. doi: 10.1016/j.jcis.2017.08.050. [DOI] [PubMed] [Google Scholar]