Abstract
The development of skin substitutes aims to replace, mimic, or improve the functions of human skin, regenerate damaged skin tissue, and replace or enhance skin function. This includes artificial skin, scaffolds or devices designed for treatment, imitation, or improvement of skin function in wounds and injuries. Therefore, tremendous efforts have been made to develop functional skin substitutes. However, there is still few reports systematically discuss the relationship between the advanced function and design requirements. In this paper, we review the classification, functions, and design requirements of artificial skin or skin substitutes. Different manufacturing strategies for skin substitutes such as hydrogels, 3D/4D printing, electrospinning, microfluidics are summarized. This review also introduces currently available skin substitutes in clinical trials and on the market and the related regulatory requirements. Finally, the prospects and challenges of skin substitutes in the field of tissue engineering are discussed.
Keywords: Skin substitutes, Artificial skin, Design strategy, Manufacturing methods, Skin regeneration
Graphical abstract
1. Introduction
The skin is vulnerable to frequent damages. Minor superficial skin injuries are capable of healing by epithelialization without any particular treatment. Severe injuries may delay recovery time and cause a permanent loss of skin function or life-threatening. Skin diseases are the fourth most common non-fatal disease, affecting one-third of the global population [1]. Caring for skin diseases requires time, medical care, and financial investment, placing a heavy burden on governments [2]. Skin diseases also have a significant impact on people's mental health, which is an urgent problem that needs to be addressed [3]. Therefore, clinical intervention is critical for wound healing. The most effective strategy is to use an artificial skin substitute, such as a wound dressing or skin graft, as a temporary matrix to facilitate the healing process [4].
Treating wounds has always been a challenge for humans. Suitable skin substitutes are necessary for skin tissue engineering, so it is important for to choose appropriate materials and study their characteristics in skin tissue engineering [5]. With advances in technology, researchers began to explore various materials and preparation methods, gradually expanding the application of skin substitutes to a wider range of clinical fields.
In addition to basic protective functions such as water retention, antibacterial, and adhesion, skin substitutes are also designed for advanced functions such as mechanical strength, scar resistance, and intelligent skin to simulate normal skin. Cells are incorporated in skin substitutes for secreting proteins, cytokines, and growth factors used for wound healing. Different cells are selected to simulate the epidermis, dermis, and dermo-epidermal composite to simulate normal skin structure. More and more multifunctional skin substitutes are being developed.
Time doesn't cure everything, but skin substitutes is making an effort to achieve this. In this review, the function and design requirements of artificial skin substitutes are summarized, and the development status of artificial skin with different functions is classified and introduced. Additionally, representative manufacturing methods and research results to realize the advanced functions are discussed. Although many biologically engineered methods, such as decellularization and cell-containing scaffolds, have been explored to treat wounds, many of these methods are still in the preclinical stage. This review also introduced the current research progress on clinical and commercial products and the related regulatory requirements (Fig. 1).
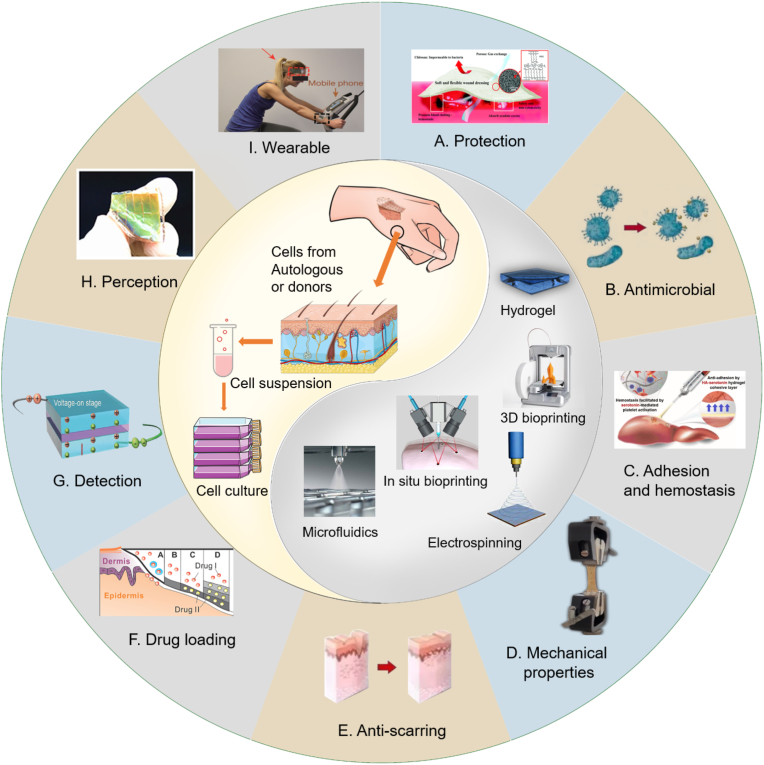
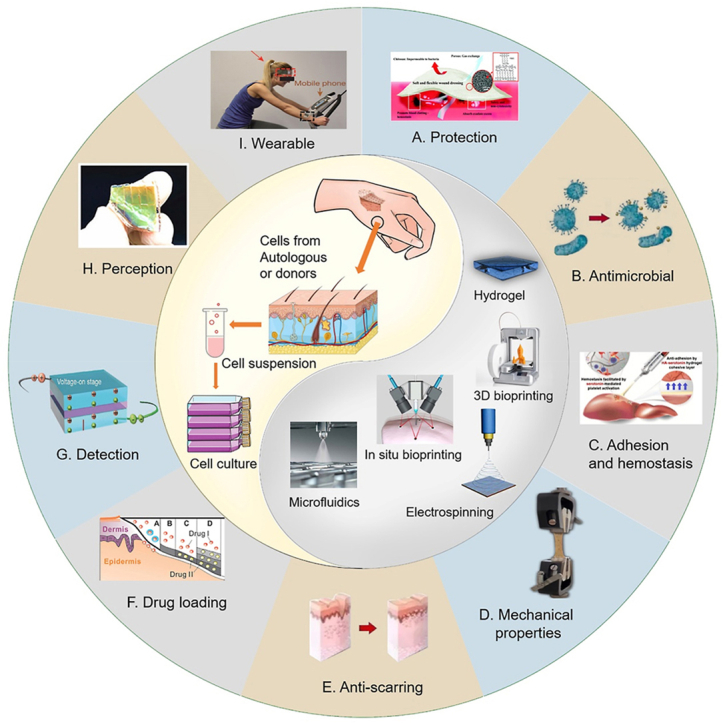
Fig. 1.
Scheme of the properties for skin substitutes. Skin substitutes can be prepared from autologous or allogeneic cell cultures for wound coverage and treatment. Technologies such as hydrogels, 3D/4D bioprinting, in-situ bioprinting, electrospinning, or microfluidics can be employed to manufacture these substitutes. Skin substitutes are characterized by their properties of providing (A) protection [6], (B) antibacterial activity, (C) adhesion and hemostasis [7], (D) mechanical strength, (E) anti-scarring ability, (F) drug loading [8], (G) detection [9], (H) perception [10], and (I) wearability [11].
2. Wounds and skin substitutes
2.1. Skin and wound management
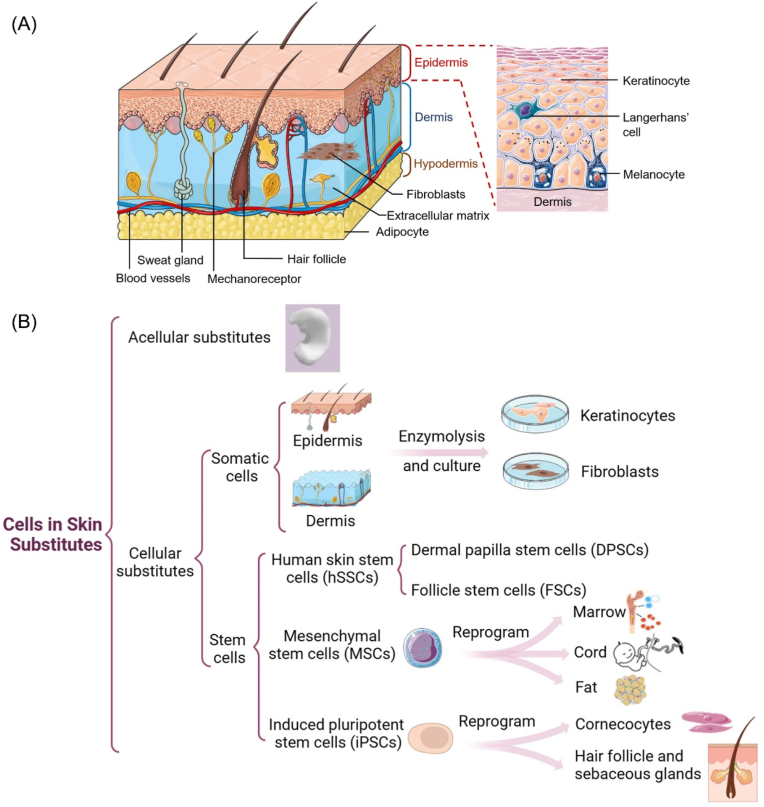
Human skin (Fig. 2A) consists of several types of cells distributed across the skin layers, including the epidermis, dermis, and subcutaneous tissue [12]. The epidermis, which is attached to the dermis's extracellular matrix with a thickness of 10–30 μm, is primarily composed of keratinocytes and other cells, such as Melanocytes, Langerhans cells, and Merkel cells [13]. Keratinocytes can form numerous tight junctions between cells and are capable of unlimited proliferation [14]. Melanocytes are responsible for producing and transferring melanin to the Keratinocytes and aesthetically harmonize the color [15]. Langerhans cells are involved in antigen presentation during the immune process, while Merkel cells are responsible for tactile responses [16].
Fig. 2.
Human skin structure and skin substitutes. A. The structure of human skin comprises three main layers, including epidermis (keratinocytes, melanocytes, Langerhans cells), dermis (with fibroblasts and ECM components), and the hypodermis. B. Cell types in skin substitutes. The skin substitutes can be divided into acellular substitutes and cellular substitutes classified by their composition.
The dermis connects the epidermis and the subcutaneous tissue, mainly consisting of fibroblasts and extracellular matrix (ECM). Fibroblasts are the primary cellular components of the dermis, capable of supporting the formation of ECM by secreting proteins, cytokines, and growth factors used for wound healing. Fibroblasts can also migrate to the wound site and differentiate into myofibroblasts, making them attractive candidates as carrier cells for designing skin substitutes [17]. The ECM represents the largest component of skin and contains a complex collection of fibrous structural proteins (mainly collagen) and polysaccharides. The ECM provides dermal skin with strength and elasticity, allowing the cells to be suspended and form 3D structures [18,19]. Besides, the dermis contains abundant blood vessels to create a microvascular network, angiogenesis are important to skin regeneration as vascular defect can hinder the supply of nutrition, oxygen, and growth factors [20,21]. Hypodermis is mainly composed of adipose tissue cells and are the primary storage for triglyceride fat and will be involved in the regeneration of the skin and skin accessories [22,23]. The three layers of skin work together to create an effective barrier against the external environment while allowing the transmission of sensory information, playing a vital role in maintaining the body's internal balance [24].
2.2. Wounds and skin substitutes
The wound can be divided into acute wound and chronic wound based on the time needed of wound healing. Acute wound healing follows the four processes of hemostasis, proliferation, maturation, and remodeling. The total healing time is typically about 8–12 weeks. However, if not treated properly, acute wounds can become chronic wounds, leading to long-term inflammation. Chronic wounds usually damage the inner lining of the skin and are associated with delayed or inadequate healing. In diabetic foot ulcer, the healing process is interrupted and replaced by a persistent state of inflammation and long-term microcirculatory deficiencies [25].
Moreover, diabetic wounds or burn wounds are vulnerable to bacteria and other pathogens due to impaired integrity and immune responses, resulting increased reactive oxygen species (ROS) production and worsening of the inflammatory response [26]. Severe burns can also impair organ function and lead to multiple organ dysfunction syndrome. The recruited inflammatory cells at the wound produce massive amounts of ROS, which result in oxidative stress [27]. Inflammatory factors such as tumor necrosis factor α (TNF-α) and interleukins 6, 8, and 1 β (IL-6, IL-8, and IL-1β) are over produced and will increase thus prevent transition to the proliferative phase and disrupts the processes of angiogenesis, ECM remodeling, and re-epithelialization for tissue repair [26].
Wound dressings play a crucial role in the healing of complex acute and chronic wounds by absorbing wound exudate, maintaining a moist environment, and promoting accelerated wound healing. Furthermore, wound dressings can provide controlled release and spatio-temporal transmission of drugs or growth factors during wound healing to regulate cell proliferation and differentiation [28]. However, passive dressings such as gauze can only provide coverage and absorb exudates from the wound surface. These dressings may also attach to newly formed granulation tissue and cause pain when removed.
Although auto transplantation is considered the gold standard for skin regeneration, it has some disadvantages, such as inadequate skin supply, secondary damage, and unsuitability for diabetic patients. Additionally, there is a shortage of donors and a risk of hyperplastic scars or keloids for donors. Allotransplantation and xenotransplantation both carry the risk of virus transmission and immune rejection, which can affect the survival rate of transplanted skin [29].
With the development in tissue engineering and biomaterial manufacturing, a variety of advanced artificial skin substitutes have been developed and are providing clinical solutions for patients who need tissue replacement, ultimately improving their quality of life [30]. In this review, skin substitutes are defined as a material or device that can be applied to a patient's damaged skin, with the aim of replacing, imitating, or improving any skin function. This encompasses a diverse range of wound dressings that can be placed at the wound site to temporarily or permanently replace the skin's functionality, including acellular skin substitutes, such as acellular matrix, as well as skin substitutes, which are materials and scaffolds specifically designed for wound management.
2.3. Cells in skin substitutes
The category of skin substitutes can be divided into several types, including acellular substitutes and cellular substitutes classified by their composition. In terms of skin structure, skin substitutes can be applied as epidermal, dermal, or dermo-epidermal (composite). Based on the type of biomaterials used, skin substitutes can be classified into biological (autologous, allogeneic, xenogeneic) or synthetic (biodegradable, non-biodegradable) materials [31].
Acellular substitutes are tissues that have been decellularized from cadaveric dermis, allogenes, or animals. Acellular skin substitutes have intact ECM components and can retain the primary functional structure of normal dermal tissue [32]. They are frequently utilized in a variety of pathologies, such as diabetic foot ulcer, rare skin conditions, and plastic surgery [33,34]. However, acellular skin substitutes have limitations, including poor barrier function and the risk of disease transmission. Moreover, the sterilization process such as irradiation, may damage or change the structural components of skin substitutes [35]. In addition to mammalian acellular grafts, acellular fish skin (AFS) xenografts can be used to treat superficial and partial-thickness burns [36]. AFS contains collagen, fibrin, proteoglycans and glycosaminoglycans, and offers advantages of accelerating wound healing, reducing treatment costs, and low immunogenicity [37].
Xenografts implants sometimes will be recognized by the host as “foreign bodies,” triggering a complex signaling cascade known as the foreign body response (FBR) during wound healing [38]. The FBR involves four stages: protein adsorption, acute inflammation, chronic inflammation, and collagen encapsulation. Skin grafts typically trigger an acute inflammatory reaction in the host within a few hours of transplantation, causing inflammatory cells such as neutrophils and monocytes to migrate to the implantation site, release proinflammatory cytokines, recruit and activate macrophages, and trigger a cascade immune response [39]. The fibroblasts that migrate to the wound continually deposit collagen fibers around the implants, resulting in a highly fibrotic structure in the outer layer of the implant, which is the phrase of chronic inflammation [40]. While FBR is the natural protective mechanism of the body, both chronic inflammation and acute inflammation will bring strong discomfort to patients, greatly affecting the function of implanted materials. Therefore, immune regulation design is necessary to reduce the inflammatory reaction of skin substitutes in order to alleviate immune rejection and improve the biocompatibility of skin substitutes.
The inclusion of cells within the skin substitutes will promote the regeneration of natural skin through the secretion of growth factors. Cell-based skin substitutes typically contain somatic cells or stem cells (Fig. 2B). Among the human somatic cells, keratinocytes and fibroblasts are the most commonly cells used to fabricate cellular skin substitutes. Many other skin cells such as melanocytes and adipocytes are also included to create functional skin substitutes. Keratinocytes are essential for the re-epithelialization process and provide barriers against the skin and environment. Monteiro et al. developed a polyelectrolyte multilayer film with hyaluronic acid and poly-l-lysine by spray-assisted layer-by-layer assemble with keratinocytes attached and proliferated on the coated porous scaffolds. This epidermal–dermal scaffold can promote cell adhesion and regeneration for full-thickness skin defects [41]. Fibroblasts are the most abundant cells in the dermis and synthesize collagen and differentiate into myofibroblast phenotype during the wound closure process [8]. The synthesis and deposition of collagen and other ECM components will lead to the formation of the granulation tissue, which is a symbol during wound healing. Skin pigmentation is also crucial for the recovery of skin function. Melanocytes are responsible for regulating skin color and can restore the pigmentation of skin substitutes. The combination of keratinocytes with in skin substitute can result in the color that close to the natural epidermis. This is essential for addressing aesthetic concerns and promoting the mental well-being of patients after healing [42]. Adipocytes, which are located around hair follicles, play a crucial role in fibroblast migration and participate in the regeneration of skin appendages during wound healing [23].
Skin cells are a good source of autologous cells as they can be directly used, but their applications are limited due to the limited availability of donor cells and potential damage to the donation sites. Stem cells offer a promising alternative cellular source for the fabrication of artificial skin substitutes. The most commonly studied stem cells in wound healing include human skin stem cells (hSSCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs) [43].
hSSCs, which are extracted from the skin, have similar differentiation ability to other tissue-derived stem cells [44]. hSSCs can be isolated from different parts of skin such as dermal papilla or hair follicles, named dermal papilla stem cells (DPSCs) and follicle stem cells (FSCs), respectively [45]. MSCs are found in various tissues, including human bone marrow-derived MSCs, human umbilical cord Wharton's jelly-derived MSCs, and human adipose tissue-derived MSCs, respectively [46]. MSCs has the ability to differentiate into cells of epidermal and dermal lineages and contribute to tissue regeneration by producing growth factors, cytokines, and chemokines [47]. Moreover, MSCs can be activated to initiate immunomodulatory functions by producing immunoregulatory factors, and thereby regulating inflammation and reduce inflammation-related scar formation [48]. iPSCs are stem cells generated from the reprogramming process of individual somatic cells and have similar characteristics to embryonic stem cells on morphology, self-renewal, and differentiation capacity [49]. iPSCs can be extracted from fibroblasts or cord blood mononuclear cells and specialized differentiation into fibroblasts and keratinocytes, respectively [50]. Bilousova's group reported the differentiation of mouse iPSCs into a keratinocyte lineage, and the keratinocyte iPSCs were able to regenerate the epidermis, hair follicles, and sebaceous glands in a graft assay in vivo [51]. iPSCs have also been reported to differentiate into other cell types, such as melanocytes, sensory neurons and Schwann cells [52].
Immune cells, such as neutrophils, macrophages, and mast cells, play essential roles in the wound healing process [53]. Therefore, incorporating human immune cells into skin substitutes may provide opportunities to enhance regeneration potential and develop more complex models of skin substitutes.
2.4. Skin substitutes simulate different skin structure
The skin substitutes can be classified anatomically as epidermal, dermal, or composite substitutes based on their structure and intended application.
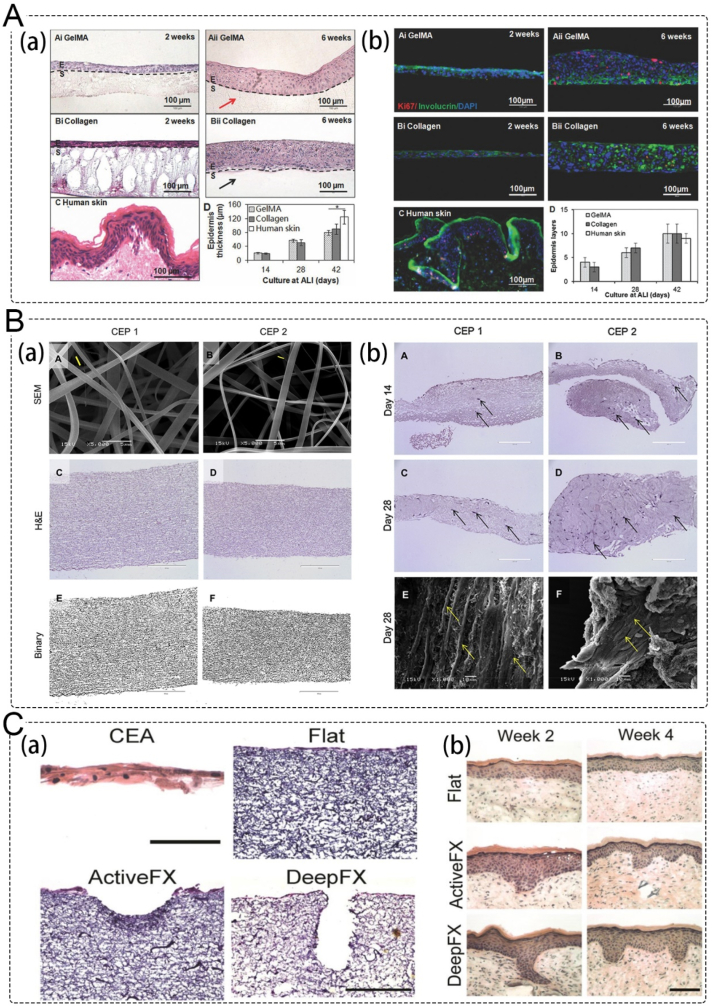
Epidermal substitutes typically consist of a single layer of autologous keratinocytes isolated from a donor and expanded in vitro. Although cultured epidermal autografts have the advantage of autologous graft compatibility, they also have some drawbacks such as high cost, short shelf life, and poor infection tolerance [54]. To overcome these limitations, Zhao et al. developed a HaCaT cells-encapsulated photo-crosslinking gelatin methyl acrylamide (GelMA) hydrogel (Fig. 3A) that can prolong degradation in collagenase solution, reduce water loss, and promote epidermal reconstruction [55].
Fig. 3.
Application of epidermal substitutes and dermal substitutes. A. Photocrosslinkable gelatin hydrogel for epidermal substitutes; (a) H&E stained sections of reconstructed epidermis on GelMA and control collagen scaffolds; (b) expression of proteins of reconstructed epidermis on hydrogel scaffolds [55]. Copyright 2016, Wiley. B. Electropsun collagen–elastin–PCL scaffolds as dermal substitutes; (a) SEM, H&E, and binary images of cross-linked CEP 1 and CEP 2 scaffolds; (b) H&E staining and SEM images of HDFs proliferation and infiltration on CEP 1 and CEP 2 scaffolds [58]. Copyright 2019, Elsevier. C. Skin substitutes mimic the dermal-epidermal junction; (a) H&E of CEAs (at in vitro culture day 20), and flat electrospun collagen dermal templates; (b) H&E stained cryosections of CEA-dermal template composite grafts 2 and 4 weeks post-grafting, demonstrating development of rete ridges when CEAs were grafted [67]. Copyright 2020, Mary Ann Liebert, Inc.
The dermis, which constitutes the bulk of the skin, provides strength and nourishment to the epidermis. Loss of dermal tissue due to wounds can result in the loss of fibroblasts and delay the wound healing process [56]. Autologous skin transplantation or dermal substitutes are often used to treat severe burns, and one key factor in the development of dermal substitutes is the regeneration of scaffold elasticity. For example, a sodium alginate/gelatin composite dermal substitute scaffold was fabricated using a motor-assisted 3D extrusion system and showed good cell proliferation results [57]. Chong et al. developed a triple-polymer scaffold composed of collagen, elastin, and polycaprolactone (CEP) with enhanced mechanical properties and elasticity. The electrospun CEP scaffolds were observed to promote keratinocyte and fibroblast proliferation, tissue integration, and early-stage angiogenesis after subcutaneous implantation (Fig. 3B) [58].
Dermo-epidermal composite skin substitutes refer to skin substitutes that include both epidermal and dermal components. Lin et al. developed a tri-layered chitosan-based scaffold that replicates the structure of full-thickness skin. They used a compact layer on top to simulate the epidermis, a thin film to simulate the basement membrane at the dermal-epidermal junction, and a porous layer to simulate the dermis, allowing for dermal cell proliferation [59]. OrCel (Orate International, Inc., USA) is an FDA-approved substitute that is synthesized by culturing allogeneic neonatal keratinocytes and fibroblasts in a type I bovine collagen porous sponge. This bi-layered composite allograft can produce an array of growth factors such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), Fibroblast growth factor (FGF), keratinocyte growth factor-1, and transforming growth factor-α, as well as other cytokines, which are favorable for host cell proliferation, migration, and wound healing [60]. Peramo et al. developed a 3D tissue structure containing epidermal skin, vermillion, and oral mucosa in a continuous layer. The mucocutaneous equivalent was fabricated using full-thickness human skin and oral mucosa explants to simulate the morphological features of the lips. This technique can be used as a general basis for other mucocutaneous areas, such as the anus and vagina [61].
The dermis and epidermis play an essential role in protection, and the meeting point of these two layers is known as the dermal-epidermal junction (DEJ) [62]. In native skin, the DEJ structure is corrugated, forming rete ridges (50–400 μm in width) in the epidermis projecting more deeply into the dermis and generating dermal papillae (50–200 μm in depth) [63]. DEJ can maintenance of structural integrity and control of the cellular microenvironment, which are essential for the appropriate keratinocytes functioning within these areas and plays a pivotal role in dermal-epidermal homeostasis and adhesion.
However, the interface between the epithelial layer and the underlying connective tissue in many skin substitutes are planar, causing weak adhesion between these layers [64]. So, mimicking the topographical and anatomical attributes of human skin mimic the skin microstructure is very important. It has been reported that after seeding keratinocytes onto decellularized dermis and reconstituting an epidermis, the dermal topography collapses and finally creates false rete ridges [65]. Ramos-Rodriguez et al. introduced complexity within electrospun membranes to mimic the morphology of the rete ridges in the skin [66]. Malara et al. developed a dermal template with stable dermal papillae through electrospinning collagen and seeding with human dermal fibroblasts using a combination of electrospinning and laser structuring (Fig. 3C). The ridged templates resulted in rete ridge was observed to formation in 2 weeks after grafting [67].
3. Functions of skin substitutes
3.1. Protective function as dressings
3.1.1. Water retention
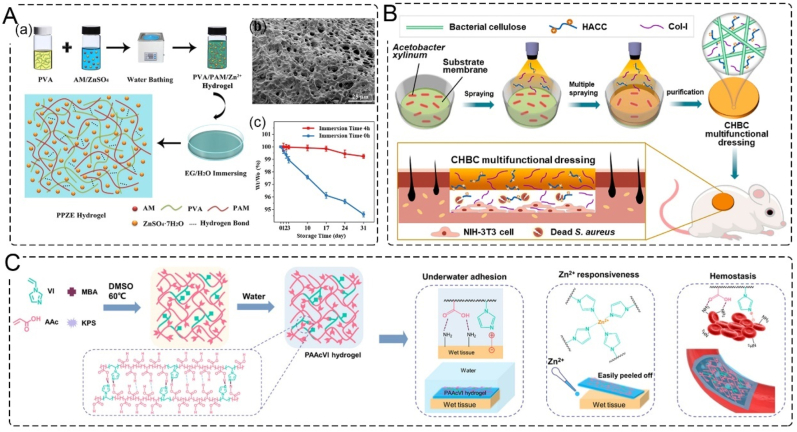
Based on the concept of moist healing, which suggests that moisture benefits cells activities and promotes wound healing [68], water retention is an essential feature of wound dressing. While traditional wound dressings, such as gauze and cotton, insulate the injured tissue from the external environment, developing a wound dressing with good water retention properties is crucial. To enhance the freezing tolerance and moisture retention ability of organohydrogels, various agents can be added, such as ionic liquids, inorganic salts, zwitterionic osmolytes, and polyhydric alcohols [69]. For instance, Peng et al. developed a double network organohydrogel by incorporating ZnSO4 into a polyvinyl alcohol-polyacrylamide (PPZE) hydrogel in a mixed solvent of ethylene glycol and water. After storage at ambient temperature for 31 days, the organohydrogel exhibited a moisture retaining rate of above 99.3 % (Fig. 4A) [70].
Fig. 4.
Skin substitutes with water retention, antibacterial, adhesion, and hemostasis. A. Moisture retention PPZE hydrogel; (a) schematic illustration on the synthesis and structure of PPZE hydrogel; (b) SEM image of PPZE hydrogel; (c) effect of EG immersion on the weight-retention of PPZE-2 hydrogel with the storage time [70]. Copyright 2022, Elsevier. B. Schematic illustration of the preparation of a CHBC functional dressing via a novel membrane–liquid interface culture and the process of treating infected wounds in vivo [81]. Copyright 2022, Elsevier. C. Schematic illustration of PAAcVI hydrogel with tough underwater adhesion, Zn2+ responsiveness, and efficient hemostasis [84]. Copyright 2022, American Chemical Society.
Polysaccharide-based wound dressings are effective strategies in the field of wound care, as they combine an ECM-like structure with excellent biomimicry. Chitosan, alginate [2], hyaluronic acid, konjac glucomannan [71] are common polysaccharides used in wound dressing. Hyaluronic acid and lactose-modified chitosan nanofibrous wound dressings were prepared by electrospinning, and their moisturizing ability helped to accelerate wound closure and have anti-scarring potential [72].
3.1.2. Antibacterial
Chronic wound infection is a serious problem during the healing process, as the skin cannot undergo self-repair under continuous bacterial infection. Antibiotics are a preferred strategy for the clinical treatment of infected wounds, and many antimicrobial drugs, such as tetracycline [73], are encapsulated in wound dressings. Inorganic metal nanoparticles, including silver [74], gold [75], zinc [76], photothermal therapy based on metal materials [77], carbon-based materials [78], nanozymes [79], or metal–organic frameworks (MOFs) [80], have been extensively studied. However, bacterial resistance caused by antibiotics and the potential for long-term retention of inorganic metal antimicrobials call for more effective antibacterial strategies. A multifunctional dressing (CHBC) was designed by the membrane-liquid interface multiple spraying method using the antibacterial agent hydroxypropyltrimethyl ammonium chloride chitosan, collagen I, and bacterial cellulose. The CHBC dressing had outstanding antibacterial properties and achieved wound healing for 8 days in a wound infection model (Fig. 4B) [81].
3.1.3. Adhesion and hemostasis
Hemostasis is the first stage of wound healing, and uncontrolled hemorrhage can lead to exsanguination. Therefore, achieving hemostasis is a basic and essential function in developing skin substitutes. Skin substitutes with adhesive properties can facilitate hemostasis by absorbing wound extract to congregate coagulation factors [82] or by sealing the wound though adhesiveness [83]. In addition to promoting hemostasis, wound dressings with adhesive properties can also help prevent infection by sealing the wound. Wang et al. developed a hydrogen-bond-based hydrogel with tough, wet, and underwater adhesion. The hydrogel was formed by polymerizing acrylic acid and 1-vinylimidazole to create hydrogen bonding and electrostatic interaction with the tissues. This hydrogel demonstrated excellent hemostatic ability in both a mice-tail docking model and a mice-liver hemorrhage model (Fig. 4C) [84]. Another approach to hemostasis is the application of hemostatic chitosan and kaolin in the preparation of chitosan/polyethylene oxide/kaolin nanofiber membranes using electrospinning technology. The hemostatic time of the nanofiber membranes was significantly lower (43 ± 1.4 s) than that of commercial QuikClot® Combat Gauze (55.7 ± 1.2 s) [85].
3.2. Advanced functions
3.2.1. Mechanical properties
The skin is a mechanically strong biological material, the collagen network and heterogeneous arrangement of hydrated matrix in the skin exhibit nonlinear stress-strain curve when subjected to external forces, redistributing pressure and absorbing strain to protect human organs from physical trauma and infection. Skin substitutes lie directly on the skin interface and will bear external stresses during application, so appropriate strength and softness (stiffness) are important factors for wound dressing. Strength refers to the maximum stress that a material can withstand when an external force is applied, and skin substitute needs to have a certain tensile strength to prevent rupture due to stretching during use. It also needs to have enough extensibility to be placed on joint areas such as knees, hips, and hands. Appropriate stiffness can ensure that the material is not easily deformed, in order to better adapt to the curvature and shape of the skin surface. In addition, stiffness can also affect the biomechanical properties of the material, such as sensation and comfort when in contact with the skin.
The skin substitutes will bear external stresses during application, so appropriate strength and softness are important factors for wound dressing. Silk fibroin and konjac glucomannan sponges in wet or dry state were reported to have similar compressive modulus with native skin tissue, the addition of konjac glucomannan can form interlocking entangled chains and intermolecular hydrogen bond to get tunable mechanical properties for wound dressing application [86].
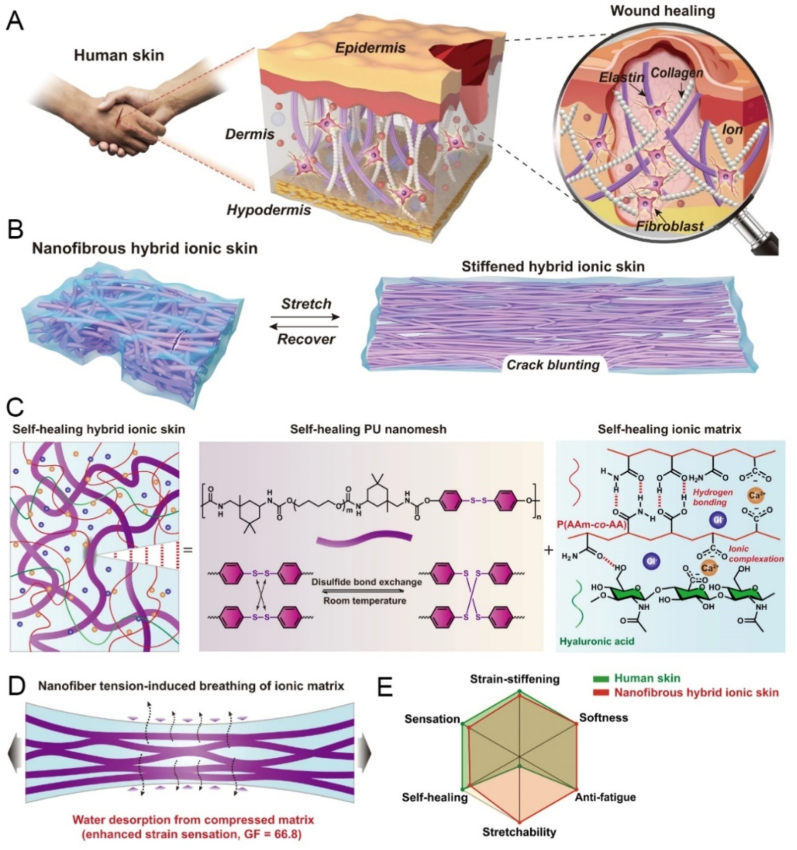
The fatigue resistance refers to the stability of the material under multiple cyclic loading, which may be related to the service life of the material. For example, high fatigue resistance is required in joint areas such as the knee. To prevent failure after repeated stretching and to extend the service life without deformation or damage, the skin substitutes should have the ability to resist crack propagation during fatigue loads. Most fatigue resisting structure are produced by incorporating dynamic covalent or physical crosslinking in the network which rearrange through deformation [87]. Wang et al. reported a fatigue-free and self-healable hybrid ionic skin by polyurethane (PU) nano mesh and poly (acrylamide-co-acrylic acid) copolymer ionic matrix. The high-energy elastic PU nano mesh scaffold was embedded into the soft copolymer ionic matrix. Such a hybrid design leads to superhigh fracture energy (16.3 kJ m−2) and fatigue threshold (2950 J m−2) while maintaining skin-like self-healability, softness (modulus ∼1.8 MPa), stretchability (680 %), and strain-stiffening response (37 times stiffness enhancement) (Fig. 5) [88].
Fig. 5.
Fatigue-free and self-healable hybrid ionic skin. A. Composite structure and wound-repairing mechanism. B. Schematic structural changes of self-healable nanofibrous hybrid ionic skin upon reversible stretching. C. Schematic illustrations of the hybrid structure and respective self-healing mechanisms of PU nanomesh scaffold and ionic matrix. D. Nanofiber tension reversibly breathe moisture in air. E. A rough comparison of the sensing and mechanical performance between human skin and nanofibrous hybrid ionic skin [88]. Copyright 2022, Nature.
3.2.2. Anti-scarring
Early-stage human fetuses and oral mucosal wounds undergo much less scarring during skin healing than cutaneous wounds, possibly due to a weaker inflammatory response in these cases. This observation suggests that the inflammation in adult cutaneous wounds may have evolved as a compromise to combat microbial infection at the expense of scarring [89].
During the process of wound healing, fibrosis can occur as a result of the body's inflammatory and connective tissue repair response, which facilitates physiological repair. However, fibroproliferative disorders may cause hypertrophic scars and keloid formation, resulting in functional problems [90]. Deep dermal fibroblasts are known to be pro-fibrotic, so adjusting the degree of fibrosis is essential for anti-scar treatment [91]. Zhang et al. developed an anti-scar wound dressing consisting of carboxymethyl chitosan, poly-γ-glutamic acid, and anti-fibrotic polypeptide (AF38Pep). The genipin crosslinked hydrogel network exhibited wound healing and scar prevention properties. The RNA-Seq data revealed that AF38Pep in the network suppressed genes related to the focal adhesion pathway in heterograft keloid fibroblasts and thus regulate the deposition and distribution of favorable collagen to prevent scar [92]. Many 3D printing [93], electrospinning [94], and hydrogel have been used to prepare artificial skin to prevent scar formation. Ni et al. developed Aloe vera-chitosan hydrogel, the cationicity of chitosan demonstrated potential to mitigate hypertrophic scar in wound healing by suppressing the expression of a-smooth muscle actin (a-SMA) and promoting secretion of type I matrix metalloproteinases (MMP-1) [95].
3.2.3. Regulation during wound healing process
The wound healing process involves a complex cascade of cellular and biochemical events, and chronic or non-healing wounds may result in the dysregulation of these biological activities [96]. Skin substitutes that combine exogenous growth factors and cytokines can serve as a motivating factor in coordinating the behavior of cells and promoting tissue repair. The TGF family acts as a chemotactic agent for inflammatory cells and fibroblasts, promoting fibroblast proliferation and differentiation into myofibroblasts [97,98]. FGF has the ability to facilitate mitogenic and proangiogenic activities, promoting granulation tissue formation and re-epithelialization [99]. VEGF promotes endothelial cell proliferation, while angiopoietins act as blood vessel stabilizers or participate in their remodeling [100]. Products containing growth factors such as PDGF, EGF, and FGF are already approved and available for human use. These growth factors play a crucial role in coordinating the cellular and biochemical events during wound healing, making them promising candidates for skin substitutes in tissue repair.
3.2.4. Oxygen-releasing structures
Oxygen is critical to the survival, function, and fate of mammalian cells. Oxygen tension controls cellular behavior through metabolic programming, which in turn controls tissue regeneration. Oxygen also acts as an essential substance and a signaling molecule involved in metabolism. Oxygen deficiency can lead to tissue necrosis and programmed cell death [101]. In some chronic wounds, the local blood supply is limited, which inhibits the migration of inflammatory cells and the proliferation of endothelial cells in wounds [102]. However, simple oxygen diffusion has a very limited range of 100–200 μm [103], so the introduction of oxygen releasing structures can overcome this limitation by providing precise and on-demand oxygen supply. A variety of oxygen-releasing biomaterials have been developed and applied in for regenerative medicine. Some organic systems include haemoglobin, photoautotrophical Synechococcus elongatus, or Cyanobacteria [104,105], inorganic systems include CaO2, MgO2, Na2CO3, and H2O2 et al. [106,107] are applied.
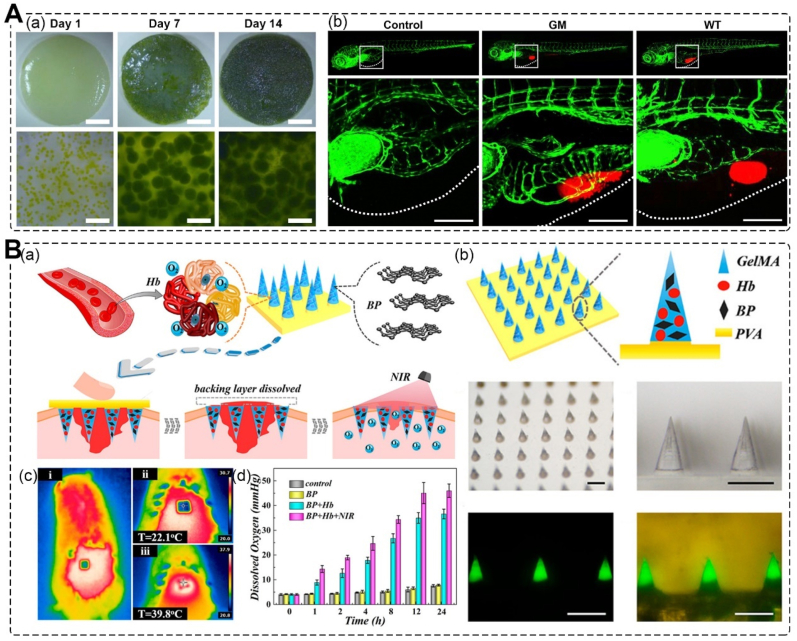
Chavez et al. incorporated Chlamydomonas reinhardtii microalgae into an integra dermal regeneration template for oxygenic photosynthesis (Fig. 6A). This led to significantly increased VEGF expression of genetically engineered algae 2 weeks after implantation into mice. The dermal regeneration template led to an increased recruitment of vascular endothelial cells and alpha smooth actin-positive vessels [108]. Zhang et al. developed black phosphorus (BP)-loaded separable responsive microneedles (MNs) with oxygen-carrying and controllable oxygen-delivering abilities for wound healing (Fig. 6B). The MNs are loaded with BP quantum dots (BP QDs) and hemoglobin. Due to the photothermal effect of BP QDs and the reversible oxygen binding properties of hemoglobin, the local temperature of the skin will increase after near-infrared irradiation, resulting in responsive oxygen release. The MNs exhibited practical wound healing ability in treating the full-thickness cutaneous wounds of a type I diabetes rat model [109].
Fig. 6.
Oxygen-releasing Structures. A. Genetically modified microalgae to engineer photosynthetic scaffolds; (a) gene modified microalgae seeded into photosynthetic dermal scaffolds; (b) bioactivity of recombinant human VEGF expressed by C. reinhardtii in vivo (Microalgae, red, chlorophyll auto fluorescence) [108]. Copyright 2016, Elsevier. B. Black phosphorus-loaded separable microneedles as responsive oxygen delivery carriers for wound healing; (a) schematic illustrations of wound healing using NIR-responsive separable MNs which encapsulate BP QDs and oxygen-carrying Hb; (b) scheme and characterization of the design of responsive separable MNs; (c) thermal images of MNs applied to the rat dorsal skin before and after 2 min NIR irradiation; (d) oxygen releasing conditions of different groups [109]. Copyright 2020, American Chemical Society.
3.3. Intelligent skins
In addition to their basic function as wound dressings, current skin substitutes are designed to replace or enhance the function of natural skin [110]. Advanced skins substitutes have been developed that can detect, perceive, and respond to changes in temperature or stains, while wearable devices that can interact with users and the environment for health monitoring are also being widely studied.
3.3.1. Detection and perception
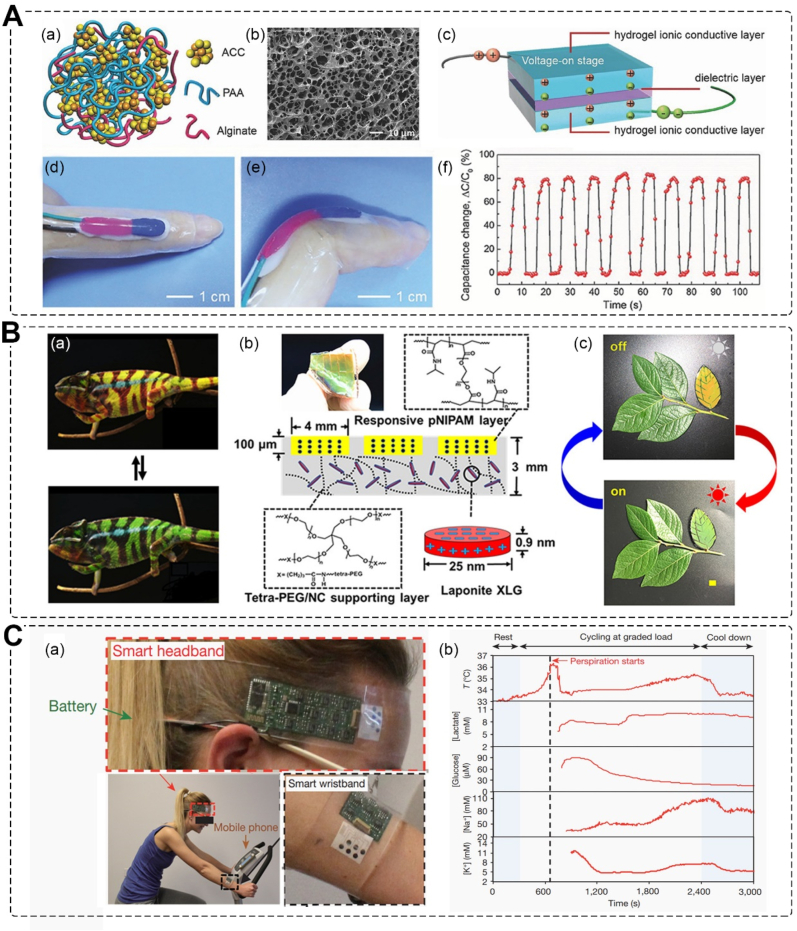
The skin contains a variety of sensory receptors distributed throughout each layer, forming a vast nerve network. These receptors are responsible for specific stimuli, and through the use of electronics, the intelligent skins can process the data and provide artificial sensation and other functions [111,112]. Currently, pressure detection in electronic skin is achieved through two main approaches: resistive and capacitive sensing. Zhou et al. have developed a highly sensitive, mechanically compliant, and autonomously self-healable ionic skin. This hydrogel-based ionic skin exhibits high pressure sensitivity up to 1 kPa, enabling it to detect gentle finger touches, human motion, and even small water droplets (mimicking rain) (Fig. 7A) [9]. Some animals, such as pit vipers, possess sensitive membranes that enable them to locate prey. Raffaele et al. have prepared a pectin film that mimics the sensing mechanism of pit membranes. This film holds a sensitivity of at least 10 mK and can be integrated as a layer in skin substitutes [113].
Fig. 7.
Intelligent skin substitutes. A. Pressure Sensing electronic skin; (a) Schematic structure and (b) SEM image of the hydrogel; (c) the interfaces between the ionic conductive layers and the dielectric layer accumulate electric charge when a voltage is applied; photos of the self-healing hydrogel sensor attached to a (d) straight or (e) bent finger; (f) real-time capacitance signal recorded when the finger was bent cyclically [9]. Copyright 2017, Wiley. B. Chameleon-inspired strain-accommodating skin; (a) color change of chameleon; (b) photograph and schematic of the smart skin includes dimensions and chemical structure of polymers; (c) camouflaged “leaf” and positioned alongside real leaves before (left) and after (right) sunlight exposure for 10 min [10]. Copyright 2019, American Chemical Society. C. Health monitoring skin-like wearable devices; (a) photographs of the subject wearing a ‘smart headband’ and a ‘smart wristband’; (b) data collection for each sensor from sweat sample [11]. Copyright 2016, Nature.
3.3.2. Stimuli-responsive
The stimuli-responsive skin can respond to complex changes in the external environment, such as strain response, microenvironment response, photothermal response, and more. A chameleon-inspired strain-accommodating skin was developed (Fig. 7B), which can change its color in response to thermal heating, mechanical stretching, and illumination, including ambient sunlight. This strain-accommodating skin embeds responsive materials within a scaffolding polymer and provides a general framework to guide the future design of artificial intelligent skins [10].
Diabetic ulcers have a distinctive physiological microenvironment, including an acidic pH, high levels of glucose, proteases, and matrix metalloproteinases. Zhu et al. developed a pH/glucose dual-responsive injectable hydrogels through the covalent crosslinking of imine bonds and phenylboronate esters. During in situ crosslinking, insulin and fibroblasts were incorporated into the hydrogels. The decrease in pH and increase in glucose levels lead to the release of insulin. The fibroblast-incorporated hydrogels exhibited accelerated wound healing in diabetic wounds [114]. Wang et al. developed an NIR-responsive nanocomposite membrane by incorporating Cu2S nanoparticles into biopolymer fibers via a modified electrospinning method. The membranes exhibited excellent and controllable photothermal performance under near-infrared irradiation, effectively inhibiting tumor growth in mice (>90 %). Moreover, the angiogenic effects of Cu2+ released from the scaffolds improved skin tissue regeneration in tumor-induced wounds [115].
3.3.3. Wearable products
Skin-like wearable devices are already being used for personal health monitoring, including the detection of glucose, uric acid, lactose, heart rate, blood pressure, ion levels, stress levels, strain, tactile input, temperature, and humidity [116,117]. These sensors are also being embedded into soft robots to enable intelligent interaction with users and the environment. The concentration of ions, small molecules, and proteins in perspiration can provide valuable health information. To enable in situ perspiration analysis, Javey and co-workers developed a flexible and wearable device that can measure and analyze perspiration metabolites, electrolytes, and skin temperature (Fig. 7C). An integrated sensing array was created by electrically decoupling the operating points of each sensor's interface. After strenuous exercise, dehydration was detected through sweat analysis [11].
4. Manufacturing methods
The physicochemical properties of skin substitutes and artificial skin are highly dependent on the materials and usage [118]. Traditional skin substitutes mainly include gauze, bandages dressings etc. They can absorb exudate and secretion from wound sites and protect wounds. However, they will dry out and cause secondary damage if prolonged used. Natural dermal substitutes, represented by “acellular substitutes”, are prepared by removing cells from the epidermis and dermis, leaving intact ECM components such as collagen fibers and basement membranes to reduce rejection reactions. Integra® is commoditized acellular substitute consists of collagen crosslinked with glycosaminoglycan and function as a temporary epidermis [119]. Dermagraft contains human neonatal fibroblasts plated on biodegradable polylactic acid scaffolds to be more simulate natural skin [120]. These substitutes are more expensive. Modern approaches are applied in various clinical forms, such as hydrogels, films, sponges, and 3D skin scaffolds. These products can be prepared using methods like (in situ) 3D bioprinting, electrospinning, microfluidics, and spraying [121] (Table 1).
Table 1.
Manufacturing methods for preparing skin substitutes.
| Materials | Methods used | Active ingredient | Properties | Ref. |
|---|---|---|---|---|
| Cholesterol-modified hyaluronic acid micelles; Phenylboronic acid grafted alginate hydrogel |
In situ injectable hydrogel | Antibiotic amikacin and anti-inflammatory drug naproxen | pH responsive; ROS responsive; antibacterial properties; accelerating bacterial infections wound healing |
[123] |
| Chitosan-graft-aniline tetramer and dibenzaldehyde-terminated poly (ethylene glycol) | In situ injectable hydrogel | Exosomes | Regulation of macrophage polarization; enhance angiogenesis; diabetic wound healing | [124] |
| Dopamine-modified gelatin | In situ injectable hydrogel | Dopamine-modified gelatin@Ag nanoparticles | Injectable and self-healing performance; antioxidant activity; near-infrared (NIR) laser irradiation synergistic antibacterial behavior; accelerate wound healing |
[125] |
| Gelatin/alginate/hyaluronic acid | 3D bioprinting | Photoactive cationic conjugated poly (phenylene vinylene); laminin-derived peptide A5G81 | Photodynamic therapy; cell adhesion, migration, and proliferation; anti-infection ability; wound repair properties |
[131] |
| Plasma-derived fibrin matrix and fibrin scaffold | 3D bioprinting | Human fibrinogen, primary human fibroblasts, and keratinocytes | Bilayered dermo-epidermal equivalents; structure similar to normal human skin | [133] |
| Platelet-rich plasma; alginate-gelatin | In situ extrusion bioprinting | Dermal fibroblasts and epidermal stem cells | Regulate the tube formation of vascular endothelial cells and macrophage polarization | [134] |
| Alginate and enzymatically cross-linkable proteins | Handheld skin printer | Dermal and epidermal cells | The capacity of depositing onto inclined and compliant wound surfaces that are subject to respiratory motion. | [135] |
| PCL and quaternized chitosan-graft-polyaniline | Electrospinning | Quaternized chitosan-graft-polyaniline | Antibacterial, anti-oxidant, and electroactive properties; full-thickness skin repair | [144] |
| PCL | Hand-held electrospinning | Aggregation-induced emission luminogens | Antibacterial activity; accelerated wound healing | [145] |
| Methacryloyl chondroitin sulfate | Microfluidics | VEGF | Wound healing applications | [148] |
| Alkylated chitosan/alginate microcapsules | Microfluidics | Polycationic surface of alkylated chitosan | Persistent antimicrobial activities hemostatic properties; time-sequential functions for tissue repair |
[149] |
| Alginate | Droplet microfluidics | Bubble-propelled nanomotors | Improve the healing effects of wounds in the type I diabetes rat models | [150] |
| Alginate | In situ spray-by-spray deposition | Tea tree oil microemulsion | Antimicrobial properties; Infected wounds |
[153] |
4.1. Hydrogel
The extracellular microenvironment of the skin is a highly hydrated network due to the presence of glycosaminoglycan chains, which make the structure of hydrogels highly similar to that of ECM. Polymer hydrogels provide an ideal moist environment for wound healing while protecting the wound. They have the advantage of providing a cooling effect and not adhering to the wound tissue. Polymer hydrogels can be used as permanent or temporary dressings to support the regeneration and healing of injured epidermis or dermis. Developing advanced hydrogel dressings is an active research field aimed at improving skin regeneration and wound healing according to clinical needs, such as in situ injectable hydrogels and stimulus-responsive hydrogels [122].
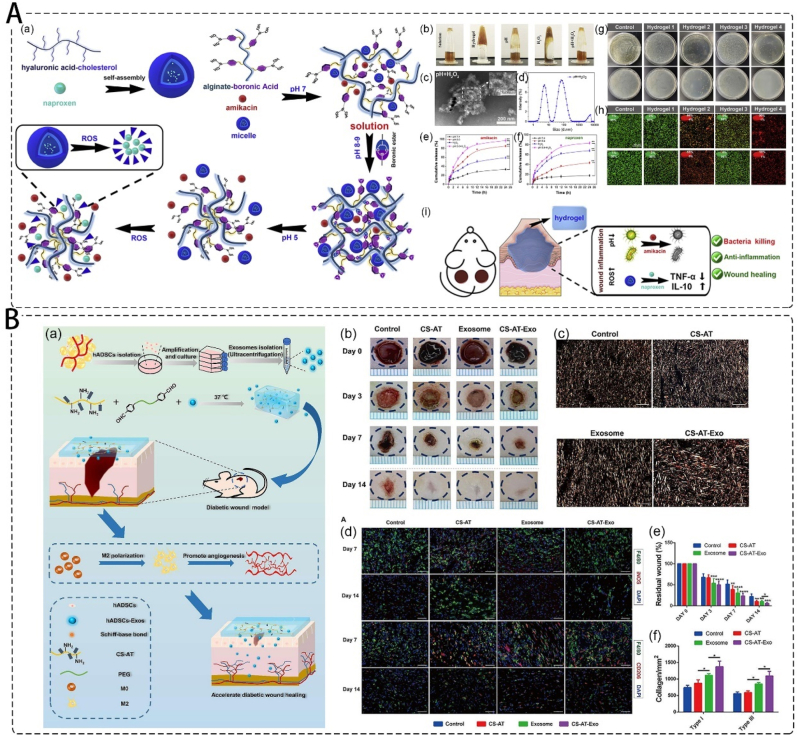
The in situ injectable hydrogel can adapt to the exact shape of the defect and fit perfectly with the wound edge. Hu et al. developed a dual-responsive injectable hydrogel wherein the antibiotic amikacin and anti-inflammatory drug naproxen were preloaded into the cholesterol-modified hyaluronic acid (HA-CHOL) micelles to prepare the phenylboronic acid grafted alginate hydrogel. The hydrogel showed dual responsiveness to low pH and high reactive oxygen species (ROS), and over 80 % amikacin and naproxen were released from the hydrogel at 24 h, effectively exhibiting antibacterial properties and accelerating bacterial infections wound healing (Fig. 8A) [123]. Wang et al. developed an exosome-loaded injectable hydrogel based on chitosan-graft-aniline tetramer (CS-AT) and dibenzaldehyde-terminated poly (ethylene glycol). The CS-AT hydrogel and exosomes work synergistically to induce local regulation of M2 macrophage polarization and enhance angiogenesis, resulting in improved diabetic wound healing in vivo (Fig. 8B) [124]. Zhang et al. developed dopamine-modified gelatin@Ag nanoparticles (Gel-DA@Ag NPs) in guar gum-based hydrogels for bacteria-derived wound infection and other tissue repair related to reactive oxygen species overexpression [125]. Conventional hydrogels are usually soft, weak, and have limited stretchability and adhesion [126]. Moreover, hydrogels are not stable under extreme conditions, and cannot maintain mechanical properties and electrical conductivity [127]. The toughness and self-repairing ability of hydrogels can be improved by using reversible covalent bonds in a double-network structure [128].
Fig. 8.
Injectable hydrogel as wound dressing. A. pH and ROS responsive injectable hydrogels encapsulating drug-loaded micelles; (a) schematic illustration for the formation of hydrogels; (b) the photograph of the hydrogel to pH and H2O2 variations; (c) the TEM images and (d) size distribution of HA-CHOL micelles; (e) the amikacin and (f) naproxen cumulative release of the hydrogel at different time points; the antibacterial ability for hydrogels on (g) CFU results and (h) live/dead staining of S. aureus (top) and P. aeruginosa (bottom) after treated with different hydrogels for 12 h; (i) schematic illustration for the mechanisms that anti-bacteria and wound healing [123]. Copyright 2020, Elsevier. B. Exosomes loaded self-healing injectable CS-AT-Exo hydrogel; (a) schematic description of the fabrication of CS-AT-Exo hydrogel and the application in the diabetic wound healing process; (b) representative images of diabetic wound healing in rats; (c) picrosirius red staining of collagen (yellow: type I collagen, green: type III collagen); (d) images of iNOS (red) and F4/80 (green) immunostaining showed acc umulation of M1 macrophages, CD206 (red) and F4/80 (green) immunostaining showed accumulation of M2 macrophages at the wound bed on day 7 and day 14; (e) rate of the residual wound after day 3, day 7, and day 14 during the healing process; (f) quantification analysis of type I and type III collagen of wound area at day 14 [124]. Copyright 2022, Elsevier.
4.2. 3D bioprinting
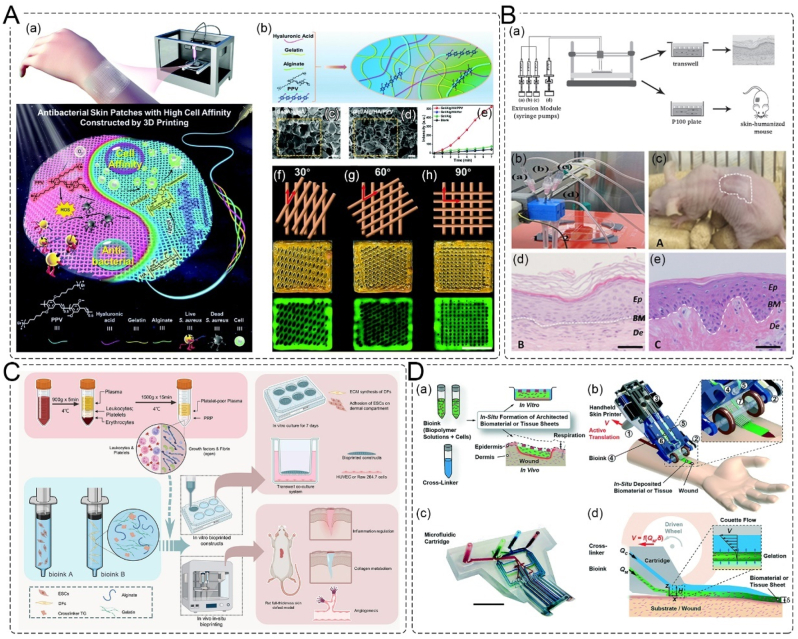
3D bioprinting is a technique used for tissue engineering and wound-healing, which involves layer-by-layer deposition of biomaterials on a 3D controllable platform. This technique provides personalized shapes and tailored structures. To fabricate skin substitutes, many works have been done in the development of a suitable bioink, and using appropriate mechanisms and patterns based on inkjet, extrusion, laser-assisted, and stereolithography [129]. The selection of appropriate materials and patterns depends on several factors, including printability, biocompatibility, degradation kinetics and by-products, and structural and mechanical properties [130]. For instance, Zhao et al. used a photoactive cationic conjugated poly (phenylene vinylene) (PPV) derivative as a photosensitizer and gelatin/alginate/hyaluronic acid as the ink to develop 3D printed skin substitutes patches. They further covalently modified the patches with laminin-derived peptide A5G81 to enhance cell adhesion, migration, and proliferation. The skin patch showed photodynamic therapy (PDT)-based anti-infection ability and in vitro and in vivo wound repair properties (Fig. 9A). [131].
Fig. 9.
3D printing skin substitutes patch. A. Photodynamic therapy 3D printing skin substitutes patch; (a) schematic of the 3D printed antibacterial skin patches with high cell affinity for wound repair; (b) preparation scheme of the Gel/Alg/HA/PPV ink; FSEM images of the Ca2+-crosslinked (c) Gel/Alg/HA and (d) Gel/Alg/HA/PPV ink, scale bar: 200 μm; (e) ROS-production ability of the skin patches; pattern diagram, digital and fluorescence images of 3D printed structures with (f) 30°, (g) 60° and (h) 90° pore geometry [131]. Copyright 2022, Royal Society of Chemistry. B. Bilayered skin containing primary human fibroblasts, and keratinocytes; (a) scheme of the bioprinting process; (b) picture of the head showing the tubes connection, (i) hFBs, (ii) plasma, (iii) CaCl2, and (iv)hKCs; (c) visual appearance of the grafted human skin on the immunodeficient mice; H/E staining of the (d)regenerated human skin and (e) normal human skin [133]. Copyright 2016, IOP Science. C. Schematic illustration of PRP-integrated AG composite hydrogel bioinks. DFs and ESCs-laded 3D bioprinted constructs were fabricated according to a single-layer or double-layered structure [134]. Copyright 2022, Elsevier. D. Handheld skin printer; (a) schematic diagram illustrating working principle of handheld bioprinter; (b) rendered image of handheld bioprinter: ① handle, ② stepper motor, pulley and drive mechanism, ③ on-board syringe pump modules, ④ bioink, ⑤ cross-linker solution, ⑥ syringe holder, ⑦ 3D printed microfluidic cartridge; (c) photograph of 3D printed microfluidic cartridge, scale bar 10 mm; (d) schematic side-view image showing sheet formation between moving microfluidic cartridge and deposition surface or wound [135]. Copyright 2018, Royal Society of Chemistry.
It is relatively easy to incorporate a single cell type in skin substitutes, but due to limitations in current seeding methods, it is quite difficult to incorporate more than two cell types into one skin substitutes. 3D bioprinting shows potential for adding multiple cell lines to a skin substitute in a precise manner, resulting in a better resemblance to human skin [132]. Cubo et al. developed a human bilayered skin using bioinks containing human fibrinogen, primary human fibroblasts, and keratinocytes to imitate the dermis and epidermis structure (Fig. 9B). The regenerated human skin was then grafted onto the backs of immunodeficient mice, and histological analysis showed that it presented a structure similar to that of normal human skin [133].
4.3. In-situ 3D printing and 4D printing
In situ 3D printing involves the direct printing of bio-ink to create or repair tissue or organs at defective sites in clinical [136]. Recent technological advancements have led to the development of handheld skin printers, which can be used to restore skin tissue in situ at the wound site. Zhao et al. utilized the extrusion strategy to fabricate 3D bioprinted constructs embedded with dermal fibroblasts and epidermal stem cells. The platelet-rich plasma (PRP) was integrated in the bioink and acted as a source of patient-specific autologous growth factors. The bioprinting was utilized for in-situ extrusion bioprinting on full-thickness rat cutaneous defects (Fig. 9C) [134]. Hakimi et al. also developed a portable handheld extruded skin printer (<0.8 kg) capable of printing patches containing cells (Fig. 9D) [135]. These developments have made it possible to print on skin in situ, which is applicable for real-time treatment of burns, scalds, wars, and other emergencies [137]. However, the safety and sterility of the handheld 3D bioprinting technology need to be rigorously tested before it can be used clinically.
4D printing is defined as multi-material printing with the ability to change over time, adding a temporal dimension to the existing 3D spatial dimension [138]. When exposed to predetermined stimuli such as light, heat, or magnetism, or based on identification of bio information such as body temperature, stress or sweat, the shape, property, or function of the material can change [139,140]. This technology can be used for the manufacture of wearable medical implants that are printed directly on the human body, allowing for clinical diagnosis and assistance in wound repair or tissue regeneration. 4D printing is an emerging area of research in biomedicine, with promising potential for tissue engineering techniques and biomedical devices [141].
4.4. Electrospinning
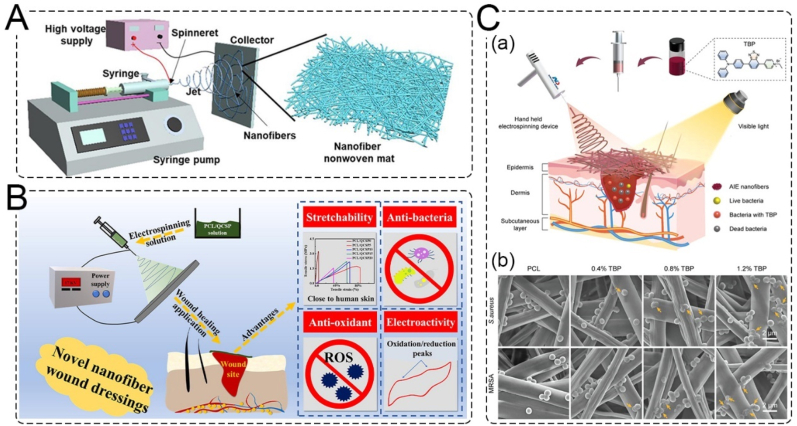
Electrospinning has recently attracted significant attention in the tissue engineering and healthcare fields. The electrospinning technique can be used to produce scaffolds/patches with controllable 3D fibrous structures and porosity. Materials such as fibrinogen, collagen, gelatin, chitosan, PU, poly (lactic acid), and PCL, either alone or in combination, have been used in electrospinning. Furthermore, drugs and other biologically active molecules can be added to the nanofibers to obtain a controlled release system with multifunctional or antibacterial activity [142]. The electrospinning device mainly consists of four parts: a high-voltage generator, fluid driver, spinneret, and collection device (Fig. 10A) [143]. The morphology and size of nanofibers can be regulated by adjusting the process parameters and raw materials, and nanofibers with suitable pore size, porosity, and ECM-like structure can be obtained. He et al. developed an electroactive nanofibrous membrane using electrospinning PCL and quaternized chitosan-graft-polyaniline (QCSP) polymer solutions. The nanofibrous wound dressings exhibited antibacterial, anti-oxidant, and electroactive properties. In a mouse full-thickness wound defect model, the wound dressing exhibited higher collagen deposition, granulation tissue thickness, and more angiogenesis compared to a commercial dressing (Tegaderm™ Film) (Fig. 10B) [144].
Fig. 10.
Electrospinning and its application. A. Schematic of a conventional electrospinning device [146]. Copyright 2022, MDPI. B. Electrospinning PCL/QCSP nanofiber membrane with anti-bacteria, anti-oxidant, stretchability and electroactivity [144]. Copyright 2020, Elsevier. C. in situ deposited AIE nanofibrous electrospinning. (a) Schematic illustration of electrospinning AIEgen-incorporated antibacterial dressing. (b) Morphological changes of S. aureus and MRSA imaged by SEM [145]. Copyright 2022, Wiley.
Hand-held electrospinning devices have been designed to miniaturize electrospinning equipment for greater portability, convenience, and comfort of use. In situ electrospinning offers the potential to provide customized wound dressings that better cover the wound, reduce edema or pain, and promote faster healing [120]. Dong et al. have used a handheld electrospinning device to electrospin an aggregation-induced emission luminogens (AIEgens)-incorporated PCL solution into a nanofibrous dressing (Fig. 10C). This AIE nanofibrous dressing exhibited excellent antibacterial activity and significantly accelerated wound healing. Hand-held in-situ deposited electrospinning could offer personalized therapies for emergency wounds [145].
4.5. Microfluidics
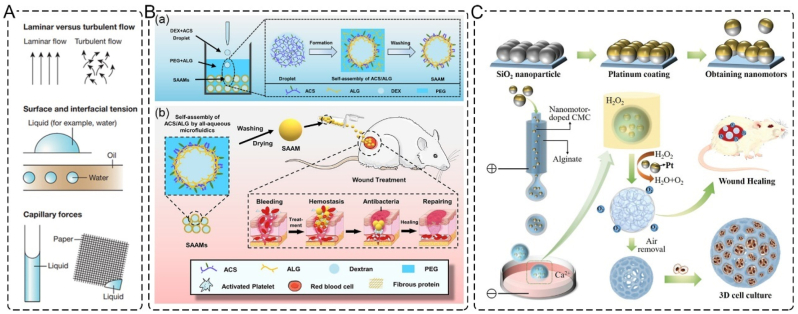
Microfluidics is a technique that employs channels ranging from tens to hundreds of microns to process small amounts of fluid, as shown in Fig. 11A. This method enables better monodispersity and higher reaction efficiency, along with continuous production and reduced by-product formation. Therefore, microfluidics can achieve the production of materials that are difficult to complete using traditional processing methods [147]. Microfluidics is considered one of the most promising and versatile technologies for manufacturing high-performance functional dressings. It allows for the preparation of spherical (porous) particles, microspheres, microcapsules, microfibers, and nanofibers in common formats.
Fig. 11.
Applications of microfluidics. A. Schematic diagram of the basic principle of microfluidics [151]. Copyright 2014, Nature. B. Self-Assembled Alkylated Chitosan Microcapsules (SAAMs); (a) schematic illustration of the preparation of the SAAMs and the self-assembly of ACS and ALG; (b) design of SAAMs from all-aqueous microfluidics and their biomedical functions in the entire wound healing process [149]. Copyright 2022, American Chemical Society. C. Schematic illustrations of the generation process of PSNs and the porous microparticles and their applications [150]. Copyright 2022, Wiley.
Zhang et al. have developed drug-loaded methacryloyl chondroitin sulfate (CSMA) microspheres to promote wound healing. The CSMA droplets were generated from microfluidic electrospray and polymerized using ultraviolet light. The drug-loaded CSMA microspheres exhibited excellent biocompatibility and wound healing properties [148]. Similarly, Gao et al. fabricated self-assembled, multifunctional alkylated chitosan/alginate microcapsules (SAAMs) using microfluidics. The polycationic surface and internal porous dextran-rich cores in SAAMs provide excellent hemostatic properties. The size-tailored soft microcapsules can realize time-sequential functions for tissue repair (Fig. 11B) [149]. Liu et al. developed novel droplet microfluidics for continuous and direct generation of porous particles by introducing bubble-propelled nanomotors into the system (Fig. 11C). The obtained platinum-coated silica nanoparticles (PSNs) porous microparticles can serve as microcarriers for 3D cell culture. Due to the existence of oxygen in these microparticles, they can be used to improve the healing effects of wounds in type I diabetes rat models [150].
4.6. Spray
Sprays are widely used in clinical practice due to their versatility and applicability in the treatment of both acute and chronic wounds, particularly those that are large or have irregular surfaces [152]. They can be categorized into two types based on their composition: acellular skin sprays and cellular skin sprays. Acellular skin sprays generally consist of hydrogels that act as a protective dressing to prevent wound infection and drying. For example, Catanzano et al. developed an in-situ forming alginate hydrogel for delivering tea tree oil microemulsion. This hydrogel were prepared via a spray-by-spray deposition method and exhibits antimicrobial properties against Escherichia coli and has potential for treating infected wounds [153]. On the other hand, cell spray autografting is an innovative technique that employs sprays as a delivery system for fibroblasts and keratinocytes. Cells are obtained from a biopsy of undamaged skin from the patient and can be sprayed directly onto the wound or cultured in vitro to obtain sufficient cell numbers before being sprayed. However, cell spray technology is not yet widely applicable or available for general use, as it is still under clinical evaluation [154].
5. Clinical/commercial progress and design requirements of skin substitutes
5.1. Clinical research of skin substitutes
In the last few years, various artificial skin or skin substitutes have shifted from cell or animal experiments to clinical trials to explore clinical applications and improve treatment efficacy. To show the recent clinical research advancements in this field, a search was conducted on the ClinicalTrials.gov website from January 2015 to January 2023, involving clinical studies on wound dressings and skin substitutes. The search terms used were “skin substitutes”, “artificial skin”, and “wound dressing”. A preliminary screening yielded 400 results, including cell-containing, acellular, natural, and synthetic polymer-based substitutes (Table 2). Many decellularized skin substitutes and cellular skin substitutes have been adopted in clinical practice, primarily aimed at diabetic foot ulcers and burn wounds. CellMist System conducted by RenovaCare, is a stem cell-based skin substitutes treatment for burn patients (NCT04890574). Altrazeal (NCT05424354) aims to evaluate the effectiveness and safety of a composite material of hydroxyapatite and artificial dermis-based skin substitute for the treatment of chronic wounds. These new skin substitutes products are expected to improve treatment efficacy and comfort, and expand the applications of skin substitutes in the medical field.
Table 2.
Clinical Trial of artificial skin or skin substitutes.
| Product | NCT Number | Title | Conditions | Phase | State |
|---|---|---|---|---|---|
| Fitostimoline® hydrogel | NCT05661474 | Fitostimoline® Hydrogel Versus Saline Gauze Dressing in Diabetic Foot Ulcers | Diabetic Foot | IV | Completed |
| Altrazeal Transforming Powder Dressing | NCT05424354 | Acute Partial Thickness Burn Study Comparing Transforming Powder Dressing to Standard of Care Dressing | Wounds and Injuries | IV | Active, not recruiting |
| Novadress, Mepilex Ag, and Xeroform Occlusive dressings | NCT05499104 | A Trial Comparing a Cellulose Dressing to Two Standard of Care Dressings in Treating Split Thickness Donor Sites in Burn and Wound Patients. | Donor Sites; Wound Drainage |
III | Completed |
| hyaluronic acid (Gengiegel 0.2 % oral gel) | NCT04390100 | Palatal Wound Healing Evaluation After Application of Platelet Rich Fibrin Versus 0.2 % Hyaluronic Acid Dressings | Free Gingival Graft | IV | Completed |
| Platelet-lysate loaded sustained release thermo-gelling formulation | NCT05671250 | Bioactive Smart Dressings for Diabetic Foot Ulcers: Randomized Controlled Trial | Diabetic Foot Ulcer | II | Completed |
| Sulfadiazine Silver | NCT03592498 | Study to Evaluate the Use of Tilapia Skin (Oreochromis Niloticus), in the Treatment of Burn Wounds | Burns | II | Completed |
| Nile Tilapia Fish Skin | NCT04202289 | Use of Nile Tilapia Fish Skin as a Xenograft for Burn Treatment: Phase III Study | Burns | III | Completed |
| Xeno-Skin™ | NCT03695939 | Evaluation of Safety, Tolerability and Efficacy of Xeno-Skin® for Temporary Closure of Severe Burn Wounds | Deep Full-thickness Burn | I/II | Completed |
| CellMist™ | NCT04890574 | CellMist™ Autologous Cells to Treat Deep Second-Degree Burns | Second degree burns | I | Active, not recruiting |
| DenovoSkin | NCT03227146 | Study With an Autologous Dermo-epidermal Skin Substitute for the Treatment of Burns in Adults and Adolescents | Burns | II/III | Active, not recruiting |
5.2. Current research of commercial products
In the 1970s, Burke and Yannas developed the first biodegradable scaffold using collagen derived from cattle and pigs, and polysaccharides derived from shark cartilage. The scaffold was inoculated with fibroblasts and named Apligraf®. Apligraf® was subsequently approved for sale by the US FDA for the treatment of diabetic foot ulcers and venous leg ulcers [155].
There are many artificial skin substitutes available on the market (Table 3). Acellular substitutes are typically derived from cadaveric or animal dermis, and the use of acellular cadaveric dermal matrix can preserve the ECM while reducing the risk of rejection and inflammation [34]. Examples of commercial allografts from cadaveric dermis include Allover™ and Graf®. Allover™ (LifeCell Corp., Branchburg, N.J.), extracted from acellular human dermis, is commonly used for burns and soft tissue defect repairs, but it may lead to scar formation due to skin contraction. Matriderm® (Dr. Suwelack Skin & Health Care AG), an animal-derived acellular skin substitute made from bovine type I collagen and elastin, is used for full-thickness burns and chronic wounds.
Table 3.
Commercially available skin substitutes used in the treatment of wounds.
| Commercial Product | corporation | Composition | Cell type | Skin structure | Indications | Defect | Ref. |
|---|---|---|---|---|---|---|---|
| Integra® | Ethicon, Inc., Somerville, N.J. | Collagen from bovine tendon crosslinked with glycosaminoglycan obtained from shark cartilage | / | Dermal | Extensive burns and limited autotransplant donor area | Allergic to cow collagen, chondroitin, or silicone material. | [166] |
| Matriderm® | Dr. Suwelack Skin & Health Care AG | Type I collagen from bovine and elastin | / | Dermal | Full-thickness burns and chronic wounds | Scar formation due to skin contraction | [166] |
| Dermagraft | Smith and Nephew, Largo, FL | Human neonatal fibroblasts plated on biodegradable polylactic acid scaffolds | human dermal fibroblast | Dermal | Restore the dermal bed in a diabetic foot ulcer | Complications, such as infection | [120] |
| Biobrane® | Bertec Pharmaceuticals, Morgantown, WV, USA | Chemically adhering collagen to nylon fabric embedded in a silicone film | / | Dermo-epidermal | Partial and full thickness burns in children | Not for contaminate wounds | [31] |
| BioSeed® | BioTissue Technologies GmbH, Freiburg, Germany | Autologous keratinocytes cultured in fibrin sealant | Keratinocyte | Epidermal | Chronic leg ulcer | / | [167] |
| Laserskin® | Fidia Advanced Biopolymers Ltd, Abano Terme, Italy | Benzyl-esterified hyaluronic acid derivative with Keratinocyte on the surface | Keratinocyte | Epidermal | Burns or chronic full-thickness ulcers | Late graft rejection | [168] |
| AWBAT-D™ | Aubrey Inc., Carlsbad, CA, USA | A thin silicone membrane attached to nylon fabric and porcine collagen | / | Epidermal | Transplantation donor site | Non-adhesive and not elastic | [169] |
| Allover ™ | LifeCell Corp., Branchburg, N.J. | Acellular human dermis | / | Dermal | Burns and other wounds; repair of soft tissue defects | Infection, seroma, dehiscence, hypersensitive, Allergic or other immune response | [170] |
| NovoSorb | BTM; PolyNovo Ltd, Port Melbourne, Victoria, Australia | Biodegradable polyurethane foam | / | Dermal | Reconstruction of deep burn and soft tissue defect | Potential failure to integrate especially in cases of borderline vascularity or infection | [119] |
| TransCyte® | Advanced Tissue Sciences, La Jolla, Calif | The scaffold made of type I collagen from porcine and human neonatal fibroblasts cultured on the scaffold coated with bioabsorbable polyglactin. | extracellular matrix of allogeneic human dermal fibroblasts | Dermo-epidermal | partial thickness burns | Infectious issues however, prohibitive in children. | [171] |
| Hyalomatrix® | Anika Therapeutics S.r.l., Abano Terme, Padova, Italy | Bilayer of an esterified hyaluronan scaffold beneath a silicone membrane | / | Dermal | partial thickness and full thickness burns | The silicone layer had to be removed as it peeled off | [172] |
| Epigard® | / | Polytetrafluorethylene, polyurethane | / | Epidermal | Preparation of wound bed before skin transplantation | / | [173] |
| MySkin™ | / | Silicone | (keratinocytes) | Epidermal | Diabetic foot ulcers | / | [174] |
| Apligraf® | Organogenesis, Inc., Canton, MA | Bovine type I collagen | Fibroblasts, keratinocytes | Dermo-epidermal | Partial- and full-thickness burns, chronic ulcers | Alloantigens rejection | [175] |
| MyDerm™ Cellular | Universiti Kebangsaan Malaysia | Fibrin | Fibroblasts, keratinocytes | Dermo-epidermal | Full-thickness wounds | Soft and fragile | [176] |
There are many other synthetic polymer-based skin substitutes available on the market, such as PLGA, polycaprolactone, polylactic acid, and hyaluronan. Laserskin® (Fidia Advanced Biopolymers Ltd, Abano Terme, Italy) is made from a benzyl-esterified hyaluronic acid derivative and is cultured with autologous keratinocytes for the treatment of burns or chronic full-thickness ulcers. Integra® (Ethicon, Inc., Somerville, N.J.) is perhaps the most established and promising artificial dermal template and is used in full-thickness burns as well as reconstruction of scar contractures. Integra® consists of a matrix of purified collagen from bovine tendon crosslinked with glycosaminoglycan obtained from shark cartilage, and may be supplied with a removable silicone layer that functions as a temporary epidermis [119]. However, there are risks associated with the use of bovine collagen-derived skin substitutes. Recently, many products have been developed and used in therapeutic applications to mimic natural skin structures and their microenvironmental properties.
5.3. The design requirements of skin substitutes
Skin substitutes play an indispensable role in saving patients' lives and improving their health with the continuous growth of medical demand. When designing skin substitutes, many factors should be taken into consideration, such as suitable mechanical properties, suitable skin anisotropy, suitable porosity and permeability, low rejection reaction and meet the comply with regulations.
5.3.1. Structural characteristics
Skin is anisotropic, viscoelastic, and relatively incompressible tissue with low modulus, self-stiffness, high toughness and tear resistance [156]. The Young's modulus of the human skin ranges from 4.5 to 8 kPa [157]. Therefore, skin substitutes should have rheology comparable to the native skin and can withstand shear forces and mechanical tensions that are applied during the substitute placement. The arrangement of collagen in ECM not only supports the mechanical strength and elasticity of the skin, but also plays a key role in skin anisotropy [158]. Therefore, it is important to consider mimicking the arrangement of collagen tissue in order to reduce scarring when designing skin substitutes [159].
The shape and structure of a skin substitute scaffold, including pore size and interconnectivity, can have a significant impact on cell function and the tissue healing process. Micron-sized pores can enhance cellular attachment and proliferation, while pores of around 100 μm or more can improve cell and capillary ingrowth [160]. Biomaterials with gradient or graded pores can further accelerate tissue reconstruction [161]. An ideal skin substitute needs to transmit water in a similar way to the normal skin, in order to avoid fluid loss or accumulation [162]. An appropriate water absorption ratio will enhance the biological activity of skin equivalents and contributes to the maintenance of 3D structure [163]. Scaffolds with high water absorption ratio are appropriate in excess exudates wounds such as the full-thickness wounds while scaffolds with a lower water absorption ratio are more suitable for partial-thickness wounds [164].
5.3.2. Performance and regulatory requirements
Implants are recognized by the host as “foreign bodies,” triggering a complex signaling cascade known as the foreign body response (FBR) during wound healing [38]. The FBR involves four stages: protein adsorption, acute inflammation, chronic inflammation, and collagen encapsulation. Skin grafts typically trigger an acute inflammatory reaction in the host within a few hours of transplantation, causing inflammatory cells such as neutrophils and monocytes to migrate to the implantation site, release proinflammatory cytokines, recruit and activate macrophages, and trigger a cascade immune response [39]. The fibroblasts that migrate to the wound continually deposit collagen fibers around the implants, resulting in a highly fibrotic structure in the outer layer of the implant, which is the phrase of chronic inflammation [40]. While FBR is the natural protective mechanism of the body, both chronic inflammation and acute inflammation will bring strong discomfort to patients, greatly affecting the function of implanted materials. Therefore, immune regulation design is necessary to reduce the inflammatory reaction of skin substitutes in order to alleviate immune rejection and improve the biocompatibility of skin substitutes.
The International Organization of Standardization (ISO) has defined several tests to ensure the biocompatibility of skin substitutes (ISO 10 993; biological evaluation of medical devices), based on the type and length of body contact. Tests such as cytotoxicity, sensitization, irritation, systemic (acute) toxicity, subacute, and subchronic toxicity, genotoxicity, hemocompatibility, chronic toxicity, carcinogenicity, reproductive (developmental) toxicity, biodegradation—identification and toxicokinetic studies, physicochemical, morphological, and topographical characterization, and immunotoxicology should be evaluated [3]. The US Food and Drug Administration (FDA) approves a range of natural and synthetic biomaterials, such as collagen, silk, Pluronic F-127, and poly (ε-caprolactone) (PCL) used in biocompatible scaffold either individually or collectively. Clinical trials for the development of cell-containing skin substitutes should comply with Good Manufacturing Practice (GMP) standards, and quality items such as biological tests of cell components, microbiological examination, and environmental control are strictly controlled [165].
6. Conclusions
Skin substitutes have revolutionized wound management by shortening time to wound closure and decreasing wound-associated morbidities, ultimately resulting in overall cost reduction compared with standard-of-care therapy. Although skin substitutes may have high initial costs, their benefits are significant. These advancements in skin substitutes serve as a model for regenerative medicine, cosmetic, and pharmaceutical applications.
In addition to the development of new biomaterials and the use of more human skin phenotypes or cytokines, bioprinting in situ has also emerged as a promising approach for skin substitute fabrication. Despite the lack of a perfect skin substitute currently available, rapid advancements in our understanding of skin development and wound repair, as well as progress in stem cell and tissue bioengineering, provide hope that the development of such a product is a feasible goal in the future. Future research on skin substitutes will likely focus on simulating the functional and aesthetic characteristics of natural skin, including the introduction of sensory perception, skin pigments, and appendages, which will enable skin substitutes to be customized in terms of performance and appearance for more personalized treatment.
However, there are still many challenges that need to be addressed. Natural biomaterials have biocompatibility and similarity to the native skin while suffer from low mechanical properties and fast degradation. Synthetic biomaterials have controlled good mechanical and physical properties but with less ideal cell interaction and overlong degradation rate. Reasonable combine the natural and synthetic biomaterials can design the potential candidate for a successful and economical skin substitute. Currently, most artificial skin substitutes are still in the early stages of development. In order to bring these substitutes to clinical application as soon as possible, the materials, cells, and equipment used in research must meet corresponding regulatory, clinical, and aseptic requirements. Furthermore, the cost of producing these substitutes remains a significant obstacle that needs to be overcome. To achieve this, it is essential for clinicians to collaborate with scientists to develop better skin substitutes and make appropriate improvements to existing products.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the financial support through the National Natural Science Foundation of China (No. 22104068), Natural Science Foundation of Shandong Province (No. ZR2021QB051), Natural Science Foundation of Shandong Province (No. ZR2022QH201), National Natural Science Foundation of China (No. 32300788).
Contributor Information
Xinlin Liu, Email: lxl2021910024@qdu.edu.cn.
Dongming Xing, Email: xdm_tsinghua@163.com.
Data availability
Data will be made available on request.
References
- 1.Karimkhani C., Dellavalle R.P., Coffeng L.E., Flohr C., Hay R.J., Langan S.M., Nsoesie E.O., Ferrari A.J., Erskine H.E., Silverberg J.I. Global skin disease morbidity and mortality: an update from the global burden of disease study 2013. JAMA dermatology. 2017;153:406–412. doi: 10.1001/jamadermatol.2016.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang M., Zhao X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020;162:1414–1428. doi: 10.1016/j.ijbiomac.2020.07.311. [DOI] [PubMed] [Google Scholar]
- 3.Hosseini M., Shafiee A. Engineering bioactive scaffolds for skin regeneration. Small. 2021;17 doi: 10.1002/smll.202101384. [DOI] [PubMed] [Google Scholar]
- 4.Bhardwaj N., Chouhan D., Mandal B.B. Tissue engineered skin and wound healing: current strategies and future directions. Curr. Pharmaceut. Des. 2017;23:3455–3482. doi: 10.2174/1381612823666170526094606. [DOI] [PubMed] [Google Scholar]
- 5.Chortos A., Liu J., Bao Z. Pursuing prosthetic electronic skin. Nat. Mater. 2016;15:937–950. doi: 10.1038/nmat4671. [DOI] [PubMed] [Google Scholar]
- 6.Cheng H., Li C., Jiang Y., Wang B., Wang F., Mao Z., Xu H., Wang L., Sui X. Facile preparation of polysaccharide-based sponges and their potential application in wound dressing. J. Mater. Chem. B. 2018;6:634–640. doi: 10.1039/c7tb03000b. [DOI] [PubMed] [Google Scholar]
- 7.An S., Jeon E.J., Jeon J., Cho S.-W. A serotonin-modified hyaluronic acid hydrogel for multifunctional hemostatic adhesives inspired by a platelet coagulation mediator. Mater. Horiz. 2019;6:1169–1178. doi: 10.1039/c9mh00157c. [DOI] [Google Scholar]
- 8.Kim H.S., Sun X., Lee J.-H., Kim H.-W., Fu X., Leong K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2019;146:209–239. doi: 10.1016/j.addr.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Lei Z., Wang Q., Sun S., Zhu W., Wu P. A bioinspired mineral hydrogel as a self‐healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv. Mater. 2017;29 doi: 10.1002/adma.201700321. [DOI] [PubMed] [Google Scholar]
- 10.Dong Y., Bazrafshan A., Pokutta A., Sulejmani F., Sun W., Combs J.D., Clarke K.C., Salaita K. Chameleon-inspired strain-accommodating smart skin. ACS Nano. 2019;13:9918–9926. doi: 10.1021/acsnano.9b04231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao W., Emaminejad S., Nyein H.Y.Y., Challa S., Chen K., Peck A., Fahad H.M., Ota H., Shiraki H., Kiriya D. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529:509–514. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheikholeslam M., Wright M.E., Jeschke M.G., Amini‐Nik S. Biomaterials for skin substitutes. Adv. Healthcare Mater. 2018;7 doi: 10.1002/adhm.201700897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mine S., Fortunel N.O., Pageon H., Asselineau D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: a new view of skin morphogenesis and aging. PLoS One. 2008;3 doi: 10.1371/journal.pone.0004066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbas O., Mahalingam M. Epidermal stem cells: practical perspectives and potential uses. Br. J. Dermatol. 2009;161:228–236. doi: 10.1111/j.1365-2133.2009.09250.x. [DOI] [PubMed] [Google Scholar]
- 15.Rehder J., Souto L.R.M., Issa C.M.B.M., Puzzi M.B. Model of human epidermis reconstructed in vitro with keratinocytes and melanocytes on dead de-epimerized human dermis. Sao Paulo Med. J. 2004;122:22–25. doi: 10.1590/s1516-31802004000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markeson D., Pleat J.M., Sharpe J.R., Harris A.L., Seifalian A.M., Watt S.M. Scarring, stem cells, scaffolds and skin repair. J. Tissue Engin. Regener. Med. 2015;9:649–668. doi: 10.1002/term.1841. [DOI] [PubMed] [Google Scholar]
- 17.Sonnemann K.J., Bement W.M. Wound repair: toward understanding and integration of single-cell and multicellular wound responses. Annu. Rev. Cell Dev. Biol. 2011;27:237–263. doi: 10.1146/annurev-cellbio-092910-154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavakoli S., Klar A.S. Bioengineered skin substitutes: advances and future trends. Appl. Sci. 2021;11:1493. doi: 10.3390/app11041493. [DOI] [Google Scholar]
- 19.Tavakoli S., Klar A.S. Advanced hydrogels as wound dressings. Biomolecules. 2020;10:1169. doi: 10.3390/biom10081169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhett J.M., Ghatnekar G.S., Palatinus J.A., O'Quinn M., Yost M.J., Gourdie R.G. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol. 2008;26:173–180. doi: 10.1016/j.tibtech.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Du P., Suhaeri M., Ha S.S., Oh S.J., Kim S.-H., Park K. Human lung fibroblast-derived matrix facilitates vascular morphogenesis in 3D environment and enhances skin wound healing. Acta Biomater. 2017;54:333–344. doi: 10.1016/j.actbio.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Sun G. Pro‐regenerative hydrogel restores Scarless skin during cutaneous wound healing. Adv. Healthcare Mater. 2017;6 doi: 10.1002/adhm.201700659. [DOI] [PubMed] [Google Scholar]
- 23.Festa E., Fretz J., Berry R., Schmidt B., Rodeheffer M., Horowitz M., Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolarsick P.A., Kolarsick M.A., Goodwin C. Anatomy and physiology of the skin. J. Dermatol. Nurses' Assoc. 2011;3:203–213. doi: 10.1097/jdn.0b013e3182274a98. [DOI] [Google Scholar]
- 25.T. Xiang, Q. Guo, L. Jia, T. Yin, W. Huang, X. Zhang, S. Zhou, Multifunctional Hydrogels for the Healing of Diabetic Wounds, Advanced Healthcare Materials. n/a 2301885. https://doi.org/ 10.1002/adhm.202301885.. [DOI] [PubMed]
- 26.Cho H., Blatchley M.R., Duh E.J., Gerecht S. Acellular and cellular approaches to improve diabetic wound healing. Adv. Drug Deliv. Rev. 2019;146:267–288. doi: 10.1016/j.addr.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Yu Z., Li Q., Wang J., Yu Y., Wang Y., Zhou Q., Li P. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res. Lett. 2020;15:115. doi: 10.1186/s11671-020-03344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laiva A.L., O'Brien F.J., Keogh M.B. Innovations in gene and growth factor delivery systems for diabetic wound healing. J. Tissue Engin. Regen. Med. 2018;12:e296–e312. doi: 10.1002/term.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widjaja W., Tan J., Maitz P.K. Efficacy of dermal substitute on deep dermal to full thickness burn injury: a systematic review. ANZ J. Surg. 2017;87:446–452. doi: 10.1111/ans.13920. [DOI] [PubMed] [Google Scholar]
- 30.Amini-Nik S., Yousuf Y., Jeschke M.G. Scar management in burn injuries using drug delivery and molecular signaling: current treatments and future directions. Adv. Drug Deliv. Rev. 2018;123:135–154. doi: 10.1016/j.addr.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vig K., Chaudhari A., Tripathi S., Dixit S., Sahu R., Pillai S., Dennis V.A., Singh S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017;18:789. doi: 10.3390/ijms18040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holl J., Kowalewski C., Zimek Z., Fiedor P., Kaminski A., Oldak T., Moniuszko M., Eljaszewicz A. Chronic diabetic wounds and their treatment with skin substitutes. Cells. 2021;10:655. doi: 10.3390/cells10030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pliszczyński J., Nita M., Kowalewski C., Woźniak K., Eljaszewicz A., Moniuszko M., Kamiński A., Śladowski D., Zimek Z., Majewski S. Transplantation Proceedings. Elsevier; 2020. Transplantation of a new biological product in rare diseases, such as epidermolysis bullosa: response and clinical outcome; pp. 2239–2243. [DOI] [PubMed] [Google Scholar]
- 34.Chocarro‐Wrona C., López‐Ruiz E., Perán M., Gálvez‐Martín P., Marchal J. Therapeutic strategies for skin regeneration based on biomedical substitutes. J. Eur. Acad. Dermatol. Venereol. 2019;33:484–496. doi: 10.1111/jdv.15391. [DOI] [PubMed] [Google Scholar]
- 35.Sun W.Q., Leung P. Calorimetric study of extracellular tissue matrix degradation and instability after gamma irradiation. Acta Biomater. 2008;4:817–826. doi: 10.1016/j.actbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Lima Junior E.M., de Moraes Filho M.O., Costa B.A., Fechine F.V., Vale M.L., Diogenes A.K.d.L., Neves K.R.T., Uchoa A.M.d.N., Soares M.F.A.d.N., de Moraes M.E.A. Nile tilapia fish skin–based wound dressing improves pain and treatment-related costs of superficial partial-thickness burns: a phase III randomized controlled trial. Plast. Reconstr. Surg. 2021;147:1189–1198. doi: 10.1097/prs.0000000000007895. [DOI] [PubMed] [Google Scholar]
- 37.Brown P., Will R.G., Bradley R., Asher D.M., Detwiler L. Bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease: background, evolution, and current concerns. Emerg. Infect. Dis. 2001;7:6. doi: 10.3201/eid0701.010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang D., Chen Q., Shi C., Chen M., Ma K., Wan J., Liu R. Dealing with the foreign‐body response to implanted biomaterials: strategies and applications of new materials. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202007226. [DOI] [Google Scholar]
- 39.Anderson J.M., Rodriguez A., Chang D.T. Seminars in Immunology. Elsevier; 2008. Foreign body reaction to biomaterials; pp. 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labow R.S., Meek E., Santerre J.P. Neutrophil‐mediated biodegradation of medical implant materials. J. Cell. Physiol. 2001;186:95–103. doi: 10.1002/1097-4652(200101)186:1<95::AID-JCP1008>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Monteiro I.P., Shukla A., Marques A.P., Reis R.L., Hammond P.T. Spray‐assisted layer‐by‐layer assembly on hyaluronic acid scaffolds for skin tissue engineering. J. Biomed. Mater. Res. 2015;103:330–340. doi: 10.1002/jbm.a.35178. [DOI] [PubMed] [Google Scholar]
- 42.Souto L.R.M., Rehder J., Vassallo J., Cintra M.L., Kraemer M.H.S., Puzzi M.B. Model for human skin reconstructed in vitro composed of associated dermis and epidermis. Sao Paulo Med. J. 2006;124:71–76. doi: 10.1590/s1516-31802006000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sierra-Sánchez Á., Kim K.H., Blasco-Morente G., Arias-Santiago S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regenerative Medicine. 2021;6:1–23. doi: 10.1038/s41536-021-00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi C., Zhu Y., Su Y., Cheng T. Stem cells and their applications in skin-cell therapy. Trends Biotechnol. 2006;24:48–52. doi: 10.1016/j.tibtech.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Sellheyer K., Krahl D. Skin mesenchymal stem cells: prospects for clinical dermatology. J. Am. Acad. Dermatol. 2010;63:859–865. doi: 10.1016/j.jaad.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 46.Koppula P.R., Chelluri L.K., Polisetti N., Vemuganti G.K. Histocompatibility testing of cultivated human bone marrow stromal cells–A promising step towards pre-clinical screening for allogeneic stem cell therapy. Cell. Immunol. 2009;259:61–65. doi: 10.1016/j.cellimm.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Phinney D.G. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J. Cell. Biochem. 2012;113:2806–2812. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 48.Liu L., Yu Y., Hou Y., Chai J., Duan H., Chu W., Zhang H., Hu Q., Du J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell (Cambridge, MA, U. S.) 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Dash B.C., Xu Z., Lin L., Koo A., Ndon S., Berthiaume F., Dardik A., Hsia H. Stem cells and engineered scaffolds for regenerative wound healing. Bioengineering. 2018;5:1–19. doi: 10.3390/bioengineering5010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilousova G., Chen J., Roop D.R. Differentiation of mouse induced pluripotent stem cells into a multipotent keratinocyte lineage. J. Invest. Dermatol. 2011;131:857–864. doi: 10.1038/jid.2010.364. [DOI] [PubMed] [Google Scholar]
- 52.Muller Q., Beaudet M.-J., De Serres-Bérard T., Bellenfant S., Flacher V., Berthod F. Development of an innervated tissue-engineered skin with human sensory neurons and Schwann cells differentiated from iPS cells. Acta Biomater. 2018;82:93–101. doi: 10.1016/j.actbio.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues M., Kosaric N., Bonham C.A., Gurtner G.C. Wound healing: a cellular perspective. Physiol. Rev. 2019;99:665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronfard V., Rives J.-M., Neveux Y., Carsin H., Barrandon Y. Long-term regeneration of human epidermis on third degree burns transplanted with autologous cultured epithelium grown on a fibrin matrix1, 2. Transplantation. 2000;70:1588–1598. doi: 10.1097/00007890-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 55.Zhao X., Lang Q., Yildirimer L., Lin Z.Y., Cui W., Annabi N., Ng K.W., Dokmeci M.R., Ghaemmaghami A.M., Khademhosseini A. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv. Healthcare Mater. 2016;5:108–118. doi: 10.1002/adhm.201500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhardwaj N., Sow W.T., Devi D., Ng K.W., Mandal B.B., Cho N.-J. Silk fibroin–keratin based 3D scaffolds as a dermal substitute for skin tissue engineering. Integr. Biol. 2015;7:53–63. doi: 10.1039/c4ib90045f. [DOI] [PubMed] [Google Scholar]
- 57.Shi L., Xiong L., Hu Y., Li W., Chen Z., Liu K., Zhang X. Three‐dimensional printing alginate/gelatin scaffolds as dermal substitutes for skin tissue engineering. Polym. Eng. Sci. 2018;58:1782–1790. doi: 10.1002/pen.24779. [DOI] [Google Scholar]
- 58.Chong C., Wang Y., Fathi A., Parungao R., Maitz P.K., Li Z. Skin wound repair: results of a pre-clinical study to evaluate electrospun collagen–elastin–PCL scaffolds as dermal substitutes. Burns. 2019;45:1639–1648. doi: 10.1016/j.burns.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 59.Lin H.-Y., Chen S.-H., Chang S.-H., Huang S.-T. Tri-layered chitosan scaffold as a potential skin substitute. J. Biomater. Sci. Polym. Ed. 2015;26:855–867. doi: 10.1080/09205063.2015.1061350. [DOI] [PubMed] [Google Scholar]
- 60.Still J., Glat P., Silverstein P., Griswold J., Mozingo D. The use of a collagen sponge/living cell composite material to treat donor sites in burn patients. Burns. 2003;29:837–841. doi: 10.1016/s0305-4179(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 61.Peramo A., Marcelo C.L., Feinberg S.E. Tissue engineering of lips and muco-cutaneous junctions: in vitro development of tissue engineered constructs of oral mucosa and skin for lip reconstruction. Tissue Eng. C Methods. 2012;18:273–282. doi: 10.1089/ten.TEC.2011.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aleemardani M., Trikić M.Z., Green N.H., Claeyssens F. The importance of mimicking dermal-epidermal junction for skin tissue engineering: a review. Bioengineering. 2021;8:148. doi: 10.3390/bioengineering8110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei J.C., Edwards G.A., Martin D.J., Huang H., Crichton M.L., Kendall M.A. Allometric scaling of skin thickness, elasticity, viscoelasticity to mass for micro-medical device translation: from mice, rats, rabbits, pigs to humans. Sci. Rep. 2017;7:1–16. doi: 10.1038/s41598-017-15830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong X., Wu T., He S. Physical forces make rete ridges in oral mucosa. Med. Hypotheses. 2013;81:883–886. doi: 10.1016/j.mehy.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Estrach S., Legg J., Watt F.M. Syntenic mediates Delta1-induced cohesiveness of epidermal stem cells in culture. J. Cell Sci. 2007;120:2944–2952. doi: 10.1242/jcs.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramos-Rodriguez D.H., MacNeil S., Claeyssens F., Ortega Asencio I. Fabrication of topographically controlled electrospun scaffolds to mimic the stem cell microenvironment in the dermal-epidermal junction. ACS Biomater. Sci. Eng. 2021;7:2803–2813. doi: 10.1021/acsbiomaterials.0c01775. [DOI] [PubMed] [Google Scholar]
- 67.Malara M.M., Blackstone B.N., Baumann M.E., Bailey J.K., Supp D.M., Powell H.M. Cultured epithelial autograft combined with micropatterned dermal template forms rete ridges in vivo. Tissue Eng. 2020;26:1138–1146. doi: 10.1089/ten.TEA.2020.0090. [DOI] [PubMed] [Google Scholar]
- 68.Winter G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–294. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 69.Chen F., Zhou D., Wang J., Li T., Zhou X., Gan T., Handschuh‐Wang S., Zhou X. Rational fabrication of anti‐freezing, non‐drying tough organohydrogels by one‐pot solvent displacement. Angew. Chem. 2018;130:6678–6681. doi: 10.1002/anie.201803366. [DOI] [PubMed] [Google Scholar]
- 70.Peng W., Han L., Gao Y., Gong Z., Lu T., Xu X., Xu M., Yamauchi Y., Pan L. Flexible organohydrogel ionic skin with Ultra-Low temperature freezing resistance and Ultra-Durable moisture retention. J. Colloid Interface Sci. 2022;608:396–404. doi: 10.1016/j.jcis.2021.09.125. [DOI] [PubMed] [Google Scholar]
- 71.Zhou N., Zheng S., Xie W., Cao G., Wang L., Pang J. Konjac glucomannan: a review of structure, physicochemical properties, and wound dressing applications. J. Appl. Polym. Sci. 2022;139 doi: 10.1002/app.51780. [DOI] [Google Scholar]
- 72.Gruppuso M., Iorio F., Turco G., Marsich E., Porrelli D. Hyaluronic acid/lactose-modified chitosan electrospun wound dressings–Crosslinking and stability criticalities. Carbohydr. Polym. 2022;288 doi: 10.1016/j.carbpol.2022.119375. [DOI] [PubMed] [Google Scholar]
- 73.Feng Y., Wang Q., He M., Zhang X., Liu X., Zhao C. Antibiofouling zwitterionic gradational membranes with moisture retention capability and sustained antimicrobial property for chronic wound infection and skin regeneration. Biomacromolecules. 2019;20:3057–3069. doi: 10.1021/acs.biomac.9b00629. [DOI] [PubMed] [Google Scholar]
- 74.Sharifi E., Sadati S.A., Yousefiasl S., Sartorius R., Zafari M., Rezakhani L., Alizadeh M., Nazarzadeh Zare E., Omidghaemi S., Ghanavatinejad F. Cell loaded hydrogel incorporating Ag‐doped bioactive glass‐ceramic nanoparticles as skin substitute: antibacterial properties, immune response and scarless cutaneous wound regeneration. Bioengin. Translat. Med. 2022;7 doi: 10.1002/btm2.10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sasidharan S., Pottail L. Biodegradable polymers and gold nanoparticle–decorated skin substitutes: synthesis, characterization, and in vitro biological activities. Appl. Biochem. Biotechnol. 2021;193:3232–3252. doi: 10.1007/s12010-021-03600-1. [DOI] [PubMed] [Google Scholar]
- 76.Augustine R., Kalarikkal N., Thomas S. Advancement of wound care from grafts to bioengineered smart skin substitutes. Progress in Biomater. 2014;3:103–113. doi: 10.1007/s40204-014-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phan T.T.V., Huynh T.-C., Oh J. Photothermal responsive porous membrane for treatment of infected wound. Polymers. 2019;11:1679. doi: 10.3390/polym11101679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He J., Shi M., Liang Y., Guo B. Conductive adhesive self-healing nanocomposite hydrogel wound dressing for photothermal therapy of infected full-thickness skin wounds. Chem. Eng. J. 2020;394 doi: 10.1016/j.cej.2020.124888. [DOI] [Google Scholar]
- 79.Li Y., Fu R., Duan Z., Zhu C., Fan D. Mussel-inspired adhesive bilayer hydrogels for bacteria-infected wound healing via NIR-enhanced nanozyme therapy. Colloids Surf. B Biointerfaces. 2022;210 doi: 10.1016/j.colsurfb.2021.112230. [DOI] [PubMed] [Google Scholar]
- 80.Chen M., Zhang J., Qi J., Dong R., Liu H., Wu D., Shao H., Jiang X. Boronic acid-decorated multivariate photosensitive metal–organic frameworks for combating multi-drug-resistant bacteria. ACS Nano. 2022;16:7732–7744. doi: 10.1021/acsnano.1c11613. [DOI] [PubMed] [Google Scholar]
- 81.Zhou C., Yang Z., Xun X., Ma L., Chen Z., Hu X., Wu X., Wan Y., Ao H. De novo strategy with engineering a multifunctional bacterial cellulose-based dressing for rapid healing of infected wounds. Bioact. Mater. 2022;13:212–222. doi: 10.1016/j.bioactmat.2021.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wen J., Weinhart M., Lai B., Kizhakkedathu J., Brooks D.E. Reversible hemostatic properties of sulfabetaine/quaternary ammonium modified hyperbranched polyglycerol. Biomaterials. 2016;86:42–55. doi: 10.1016/j.biomaterials.2016.01.067. [DOI] [PubMed] [Google Scholar]
- 83.Cidreira A.C.M., de Castro K.C., Hatami T., Linan L.Z., Mei L.H.I. Cellulose nanocrystals-based materials as hemostatic agents for wound dressings: a review. Biomed. Microdevices. 2021;23:1–23. doi: 10.1007/s10544-021-00581-0. [DOI] [PubMed] [Google Scholar]
- 84.Wang X., Guo Y., Li J., You M., Yu Y., Yang J., Qin G., Chen Q. Tough wet adhesion of hydrogen-bond-based hydrogel with on-demand debonding and efficient hemostasis. ACS Appl. Mater. Interfaces. 2022;14:36166–36177. doi: 10.1021/acsami.2c10202. [DOI] [PubMed] [Google Scholar]
- 85.Liu T., Zhang Z., Liu J., Dong P., Tian F., Li F., Meng X. Electrospun kaolin-loaded chitosan/PEO nanofibers for rapid hemostasis and accelerated wound healing. Int. J. Biol. Macromol. 2022;217:998–1011. doi: 10.1016/j.ijbiomac.2022.07.186. [DOI] [PubMed] [Google Scholar]
- 86.Feng Y., Li X., Zhang Q., Yan S., Guo Y., Li M., You R. Mechanically robust and flexible silk protein/polysaccharide composite sponges for wound dressing. Carbohydr. Polym. 2019;216:17–24. doi: 10.1016/j.carbpol.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 87.Webber M.J., Tibbitt M.W. Dynamic and reconfigurable materials from reversible network interactions. Nat. Rev. Mater. 2022;7:541–556. doi: 10.1038/s41578-021-00412-x. [DOI] [Google Scholar]
- 88.Wang J., Wu B., Wei P., Sun S., Wu P. Fatigue-free artificial ionic skin toughened by self-healable elastic nanomesh. Nat. Commun. 2022;13:1–12. doi: 10.1038/s41467-022-32140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun B.K., Siprashvili Z., Khavari P.A. Advances in skin grafting and treatment of cutaneous wounds. Science. 2014;346:941–945. doi: 10.1126/science.1253836. [DOI] [PubMed] [Google Scholar]
- 90.Wynn T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol.: A Journal of the Patholog. Soc. Great Britain and Ireland. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Varkey M., Ding J., Tredget E.E. Advances in skin substitutes—potential of tissue engineered skin for facilitating anti-fibrotic healing. J. Funct. Biomater. 2015;6:547–563. doi: 10.3390/jfb6030547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang L., Tai Y., Liu X., Liu Y., Dong Y., Liu Y., Yang C., Kong D., Qi C., Wang S. Natural polymeric and peptide-loaded composite wound dressings for scar prevention. Appl. Mater. Today. 2021;25 doi: 10.1016/j.apmt.2021.101186. [DOI] [Google Scholar]
- 93.Liang J., Zeng H., Qiao L., Jiang H., Ye Q., Wang Z., Liu B., Fan Z. 3D printed piezoelectric wound dressing with dual piezoelectric response models for scar-prevention wound healing. ACS Appl. Mater. Interfaces. 2022;14:30507–30522. doi: 10.1021/acsami.2c04168. [DOI] [PubMed] [Google Scholar]
- 94.Mulholland E.J. Electrospun biomaterials in the treatment and prevention of scars in skin wound healing. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang N., Gao T., Wang Y., Liu J., Zhang J., Yao R., Wu F. Modulating cationicity of chitosan hydrogel to prevent hypertrophic scar formation during wound healing. Int. J. Biol. Macromol. 2020;154:835–843. doi: 10.1016/j.ijbiomac.2020.03.161. [DOI] [PubMed] [Google Scholar]
- 96.Trengove N.J., Bielefeldt‐Ohmann H., Stacey M.C. Mitogenic activity and cytokine levels in non‐healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8:13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 97.Zubair M., Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: a detailed review. Rev. Endocr. Metab. Disord. 2019;20:207–217. doi: 10.1007/s11154-019-09492-1. [DOI] [PubMed] [Google Scholar]
- 98.Zhang R., Liu W., Zeng J., Meng J., Jiang H., Wang J., Xing D. Niemann-Pick C1-Like 1 inhibitors for reducing cholesterol absorption. Eur. J. Med. Chem. 2022;230 doi: 10.1016/j.ejmech.2022.114111. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y., Luo J., Zhang Q., Deng T. Growth factors, as biological macromolecules in bioactivity enhancing of electrospun wound dressings for diabetic wound healing: a review. Int. J. Biol. Macromol. 2021;193:205–218. doi: 10.1016/j.ijbiomac.2021.09.210. [DOI] [PubMed] [Google Scholar]
- 100.Bitto A., Minutoli L., Galeano M.R., Altavilla D., Polito F., Fiumara T., Calò M., Lo Cascio P., Zentilin L., Giacca M. Angiopoietin-1 gene transfer improves impaired wound healing in genetically diabetic mice without increasing VEGF expression. Clin. Sci. 2008;114:707–718. doi: 10.1042/Cs20070250. [DOI] [PubMed] [Google Scholar]
- 101.Landman K.A., Cai A.Q. Cell proliferation and oxygen diffusion in a vascularising scaffold. Bull. Math. Biol. 2007;69:2405–2428. doi: 10.1007/s11538-007-9225-x. [DOI] [PubMed] [Google Scholar]
- 102.Yu J., Lu S., McLaren A.M., Perry J.A., Cross K.M. Topical oxygen therapy results in complete wound healing in diabetic foot ulcers. Wound Repair Regen. 2016;24:1066–1072. doi: 10.1111/wrr.12490. [DOI] [PubMed] [Google Scholar]
- 103.Wang Z., Chen T., Li X., Guo B., Liu P., Zhu Z., Xu R.X. Oxygen-releasing biomaterials for regenerative medicine. J. Mater. Chem. B. 2023 doi: 10.1039/d3tb00670k. [DOI] [PubMed] [Google Scholar]
- 104.Zhu Y., Wang Z., Bai L., Deng J., Zhou Q. Biomaterial-based encapsulated probiotics for biomedical applications: current status and future perspectives. Mater. Des. 2021;210 doi: 10.1016/j.matdes.2021.110018. [DOI] [Google Scholar]
- 105.Wang J., Su Q., Lv Q., Cai B., Xiaohalati X., Wang G., Wang Z., Wang L. Oxygen-generating cyanobacteria powered by upconversion-nanoparticles-converted near-infrared light for ischemic stroke treatment. Nano Lett. 2021;21:4654–4665. doi: 10.1021/acs.nanolett.1c00719. [DOI] [PubMed] [Google Scholar]
- 106.Farris A.L., Rindone A.N., Grayson W.L. Oxygen delivering biomaterials for tissue engineering. J. Mater. Chem. B. 2016;4:3422–3432. doi: 10.1039/C5TB02635K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Willemen N.G., Hassan S., Gurian M., Li J., Allijn I.E., Shin S.R., Leijten J. Oxygen-releasing biomaterials: current challenges and future applications. Trends Biotechnol. 2021;39:1144–1159. doi: 10.1016/j.tibtech.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chávez M.N., Schenck T.L., Hopfner U., Centeno-Cerdas C., Somlai-Schweiger I., Schwarz C., Machens H.-G., Heikenwalder M., Bono M.R., Allende M.L., Nickelsen J., Egaña J.T. Towards autotrophic tissue engineering: photosynthetic gene therapy for regeneration. Biomaterials. 2016;75:25–36. doi: 10.1016/j.biomaterials.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 109.Zhang X., Chen G., Liu Y., Sun L., Sun L., Zhao Y. Black phosphorus-loaded separable microneedles as responsive oxygen delivery carriers for wound healing. ACS Nano. 2020;14:5901–5908. doi: 10.1021/acsnano.0c01059. [DOI] [PubMed] [Google Scholar]
- 110.Low Z.W.K., Li Z., Owh C., Chee P.L., Ye E., Kai D., Yang D.P., Loh X.J. Using artificial skin devices as skin replacements: insights into superficial treatment. Small. 2019;15 doi: 10.1002/smll.201805453. [DOI] [PubMed] [Google Scholar]
- 111.Wang M., Luo Y., Wang T., Wan C., Pan L., Pan S., He K., Neo A., Chen X. Artificial skin perception. Adv. Mater. 2021;33 doi: 10.1002/adma.202003014. [DOI] [PubMed] [Google Scholar]
- 112.Yan L., Zhao C., Wang Y., Qin Q., Liu Z., Hu Y., Xu Z., Wang K., Jiang X., Han L., Lu X. Adhesive and conductive hydrogel-based therapy simultaneously targeting neuroinflammation and neurofunctional damage after brain injury. Nano Today. 2023;51 doi: 10.1016/j.nantod.2023.101934. [DOI] [Google Scholar]
- 113.Di Giacomo R., Bonanomi L., Costanza V., Maresca B., Daraio C. Biomimetic temperature-sensing layer for artificial skins. Sci. Robot. 2017;2 doi: 10.1126/scirobotics.aai9251. eaai9251. [DOI] [PubMed] [Google Scholar]
- 114.Zhao L., Niu L., Liang H., Tan H., Liu C., Zhu F. pH and glucose dual-responsive injectable hydrogels with insulin and fibroblasts as bioactive dressings for diabetic wound healing. ACS Appl. Mater. Interfaces. 2017;9:37563–37574. doi: 10.1021/acsami.7b09395. [DOI] [PubMed] [Google Scholar]
- 115.Wang X., Lv F., Li T., Han Y., Yi Z., Liu M., Chang J., Wu C. Electrospun micropatterned nanocomposites incorporated with Cu2S nanoflowers for skin tumor therapy and wound healing. ACS Nano. 2017;11:11337–11349. doi: 10.1021/acsnano.7b05858. [DOI] [PubMed] [Google Scholar]
- 116.Yu Y., Nyein H.Y.Y., Gao W., Javey A. Flexible electrochemical bioelectronics: the rise of in situ bioanalysis. Adv. Mater. 2020;32 doi: 10.1002/adma.201902083. [DOI] [PubMed] [Google Scholar]
- 117.Trung T.Q., Lee N.E. Flexible and stretchable physical sensor integrated platforms for wearable human‐activity monitoringand personal healthcare. Adv. Mater. 2016;28:4338–4372. doi: 10.1002/adma.201504244. [DOI] [PubMed] [Google Scholar]
- 118.Qihui Z., Lu G., Guimarães C.F., T K.P., Liangliang Y., Patrick V.R. Development of a novel orthogonal double gradient for high-throughput screening of mesenchymal stem cells-materials interaction. Adv. Mater. Interfac. 2018 doi: 10.1002/admi.201800504. [DOI] [Google Scholar]
- 119.Cheshire P.A., Herson M.R., Cleland H., Akbarzadeh S. Artificial dermal templates: a comparative study of NovoSorb™ biodegradable temporising matrix (BTM) and Integra® dermal regeneration template (DRT) Burns. 2016;42:1088–1096. doi: 10.1016/j.burns.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 120.Chouhan D., Dey N., Bhardwaj N., Mandal B.B. Emerging and innovative approaches for wound healing and skin regeneration: current status and advances. Biomaterials. 2019;216 doi: 10.1016/j.biomaterials.2019.119267. [DOI] [PubMed] [Google Scholar]
- 121.Savoji H., Godau B., Hassani M.S., Akbari M. Skin tissue substitutes and biomaterial risk assessment and testing. Front. Bioeng. Biotechnol. 2018;6:86. doi: 10.3389/fbioe.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang D., Mei L., Hao Y., Yi B., Hu J., Wang D., Zhao Y., Wang Z., Huang H., Xu Y., Deng X., Li C., Li X., Zhou Q., Lu Y. A hydrogel-based first-aid tissue adhesive with effective hemostasis and anti-bacteria for trauma emergency management. Biomater. Res. 2023;27:56. doi: 10.1186/s40824-023-00392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hu C., Zhang F., Long L., Kong Q., Luo R., Wang Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Contr. Release. 2020;324:204–217. doi: 10.1016/j.jconrel.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 124.Wang K., Dong R., Tang J., Li H., Dang J., Zhang Z., Yu Z., Guo B., Yi C. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem. Eng. J. 2022;430 doi: 10.1016/j.cej.2021.132664. [DOI] [Google Scholar]
- 125.Zhang H., Sun X., Wang J., Zhang Y., Dong M., Bu T., Li L., Liu Y., Wang L. Multifunctional injectable hydrogel dressings for effectively accelerating wound healing: enhancing biomineralization strategy. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202100093. [DOI] [Google Scholar]
- 126.Zhang Y.S., Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356 doi: 10.1126/science.aaf3627. eaaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuk H., Lu B., Zhao X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019;48:1642–1667. doi: 10.1039/c8cs00595h. [DOI] [PubMed] [Google Scholar]
- 128.Ying B., Liu X. Skin-like hydrogel devices for wearable sensing, soft robotics and beyond. iScience. 2021;24 doi: 10.1016/j.isci.2021.103174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang Y., Zhang X.F., Gao G., Yonezawa T., Cui X. 3D bioprinting and the current applications in tissue engineering. Biotechnol. J. 2017;12 doi: 10.1002/biot.201600734. [DOI] [PubMed] [Google Scholar]
- 130.Pedde R.D., Mirani B., Navaei A., Styan T., Wong S., Mehrali M., Thakur A., Mohtaram N.K., Bayati A., Dolatshahi‐Pirouz A. Emerging biofabrication strategies for engineering complex tissue constructs. Adv. Mater. 2017;29 doi: 10.1002/adma.201606061. [DOI] [PubMed] [Google Scholar]
- 131.Zhao H., Xu J., Yuan H., Zhang E., Dai N., Gao Z., Huang Y., Lv F., Liu L., Gu Q. 3D printing of artificial skin patches with bioactive and optically active polymer materials for anti-infection and augmenting wound repair. Mater. Horiz. 2022;9:342–349. doi: 10.1039/d1mh00508a. [DOI] [PubMed] [Google Scholar]
- 132.Ng W.L., Wang S., Yeong W.Y., Naing M.W. Skin bioprinting: impending reality or fantasy? Trends Biotechnol. 2016;34:689–699. doi: 10.1016/j.tibtech.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 133.Cubo N., Garcia M., Del Canizo J.F., Velasco D., Jorcano J.L. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication. 2016;9 doi: 10.1088/1758-5090/9/1/015006. [DOI] [PubMed] [Google Scholar]
- 134.Zhao M., Wang J., Zhang J., Huang J., Luo L., Yang Y., Shen K., Jiao T., Jia Y., Lian W. Functionalizing multi-component bioink with platelet-rich plasma for customized in-situ bilayer bioprinting for wound healing. Materials Today Bio. 2022;16 doi: 10.1016/j.mtbio.2022.100334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hakimi N., Cheng R., Leng L., Sotoudehfar M., Ba P.Q., Bakhtyar N., Amini-Nik S., Jeschke M.G., Günther A. Handheld skin printer: in situ formation of planar biomaterials and tissues. Lab Chip. 2018;18:1440–1451. doi: 10.1039/c7lc01236e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guillemot F., Souquet A., Catros S., Guillotin B., Lopez J., Faucon M., Pippenger B., Bareille R., Rémy M., Bellance S. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2010;6:2494–2500. doi: 10.1016/j.actbio.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 137.Singh S., Choudhury D., Yu F., Mironov V., Naing M.W. In situ bioprinting–Bioprinting from beachside to bedside? Acta Biomater. 2020;101:14–25. doi: 10.1016/j.actbio.2019.08.045. [DOI] [PubMed] [Google Scholar]
- 138.Tibbits S. 4D printing: multi‐material shape change. Architect. Des. 2014;84:116–121. doi: 10.1002/ad.1710. [DOI] [Google Scholar]
- 139.Mallakpour S., Tabesh F., Hussain C.M. 3D and 4D printing: from innovation to evolution. Adv. Colloid Interface Sci. 2021;294 doi: 10.1016/j.cis.2021.102482. [DOI] [PubMed] [Google Scholar]
- 140.Chen X., Han S., Wu W., Wu Z., Yuan Y., Wu J., Liu C. Harnessing 4D printing bioscaffolds for advanced orthopedics. Small. 2022;18 doi: 10.1002/smll.202106824. [DOI] [PubMed] [Google Scholar]
- 141.Wang Y., Cui H., Esworthy T., Mei D., Wang Y., Zhang L.G. Emerging 4D printing strategies for next‐generation tissue regeneration and medical devices. Adv. Mater. 2022;34 doi: 10.1002/adma.202109198. [DOI] [PubMed] [Google Scholar]
- 142.Bhardwaj N., Kundu S.C. Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv. 2010;28:325–347. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 143.Aidana Y., Wang Y., Li J., Chang S., Wang K., Yu D.-G. Fast dissolution electrospun medicated nanofibers for effective delivery of poorly water-soluble drug. Curr. Drug Deliv. 2022;19:422–435. doi: 10.2174/1567201818666210215110359. [DOI] [PubMed] [Google Scholar]
- 144.He J., Liang Y., Shi M., Guo B. Anti-oxidant electroactive and antibacterial nanofibrous wound dressings based on poly(ε-caprolactone)/quaternized chitosan-graft-polyaniline for full-thickness skin wound healing. Chem. Eng. J. 2020;385 doi: 10.1016/j.cej.2019.123464. [DOI] [Google Scholar]
- 145.Dong R., Li Y., Chen M., Xiao P., Wu Y., Zhou K., Zhao Z., Tang B.Z. In situ electrospinning of aggregation‐induced emission nanofibrous dressing for wound healing. Small Methods. 2022 doi: 10.1002/smtd.202101247. [DOI] [PubMed] [Google Scholar]
- 146.Li T., Sun M., Wu S. State-of-the-art review of electrospun gelatin-based nanofiber dressings for wound healing applications. Nanomaterials. 2022;12:784. doi: 10.3390/nano12050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao W., Zhang Y., Liu L., Gao Y., Sun W., Sun Y., Ma Q. Microfluidic-based functional materials: new prospects for wound healing and beyond. J. Mater. Chem. B. 2022;10:8357–8374. doi: 10.1039/d2tb01464e. [DOI] [PubMed] [Google Scholar]
- 148.Zhang H., Xu R., Yin Z., Yu J., Liang N., Geng Q. Drug-loaded chondroitin sulfate microspheres generated from microfluidic electrospray for wound healing. Macromol. Res. 2022;30:36–42. doi: 10.1007/s13233-022-0001-4. [DOI] [Google Scholar]
- 149.Gao Y., Sun W., Zhang Y., Liu L., Zhao W., Wang W., Song Y., Sun Y., Ma Q. All-aqueous microfluidics fabrication of multifunctional bioactive microcapsules promotes wound healing. ACS Appl. Mater. Interfaces. 2022;14:48426–48437. doi: 10.1021/acsami.2c13420. [DOI] [PubMed] [Google Scholar]
- 150.Liu Y., Cheng Y., Zhao C., Wang H., Zhao Y. Nanomotor‐derived porous biomedical particles from droplet microfluidics. Adv. Sci. 2022;9 doi: 10.1002/advs.202104272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sackmann E.K., Fulton A.L., Beebe D.J. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–189. doi: 10.1038/nature13118. [DOI] [PubMed] [Google Scholar]
- 152.Pleguezuelos-Beltrán P., Gálvez-Martín P., Nieto-García D., Marchal J.A., López-Ruiz E. Advances in spray products for skin regeneration. Bioact. Mater. 2022;16:187–203. doi: 10.1016/j.bioactmat.2022.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Catanzano O., Straccia M., Miro A., Ungaro F., Romano I., Mazzarella G., Santagata G., Quaglia F., Laurienzo P., Malinconico M. Spray-by-spray in situ cross-linking alginate hydrogels delivering a tea tree oil microemulsion. Eur. J. Pharmaceut. Sci. 2015;66:20–28. doi: 10.1016/j.ejps.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 154.Esteban-Vives R., Choi M.S., Young M.T., Over P., Ziembicki J., Corcos A., Gerlach J.C. Second-degree burns with six etiologies treated with autologous noncultured cell-spray grafting. Burns. 2016;42:e99–e106. doi: 10.1016/j.burns.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 155.Rivers J. Advances in Skin Regeneration. SAGE Publications Sage CA; Los Angeles, CA: 2013. pp. 75–76. [Google Scholar]
- 156.Kalra A., Lowe A., Al-Jumaily A. Mechanical behaviour of skin: a review. J. Mater. Sci. Eng. 2016;5 doi: 10.4172/2169-0022.1000254. [DOI] [Google Scholar]
- 157.Lohmann N., Schirmer L., Atallah P., Wandel E., Ferrer R.A., Werner C., Simon J.C., Franz S., Freudenberg U. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aai9044. [DOI] [PubMed] [Google Scholar]
- 158.Sakai S., Yamanari M., Lim Y., Nakagawa N., Yasuno Y. In vivo evaluation of human skin anisotropy by polarization-sensitive optical coherence tomography. Biomed. Opt Express. 2011;2:2623–2631. doi: 10.1364/BOE.2.002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xing J., Liu N., Xu N., Chen W., Xing D. Engineering complex anisotropic scaffolds beyond simply uniaxial alignment for tissue engineering. Adv. Funct. Mater. 2022;32 doi: 10.1002/adfm.202110676. [DOI] [Google Scholar]
- 160.Dang H.P., Vaquette C., Shabab T., Pérez R.A., Yang Y., Dargaville T.R., Shafiee A., Tran P.A. Porous 3D printed scaffolds for guided bone regeneration in a rat calvarial defect model. Appl. Mater. Today. 2020;20 doi: 10.1016/j.apmt.2020.100706. [DOI] [Google Scholar]
- 161.Ma P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008;60:184–198. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Woodroof E.A. The search for an ideal temporary skin substitute: awbat. Eplasty. 2009;9:e10. [PMC free article] [PubMed] [Google Scholar]
- 163.Hilmi A.B.M., Halim A.S. Vital roles of stem cells and biomaterials in skin tissue engineering. World J. Stem Cell. 2015;7:428. doi: 10.4252/wjsc.v7.i2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gupta B., Agarwal R., Alam M. Textile-based smart wound dressings. Indian J. Fibre Text. Res. 2010;35:174–187. doi: 10.1016/j.nantod.2021.101290. [DOI] [Google Scholar]
- 165.Gálvez P., Clares B., Bermejo M., Hmadcha A., Soria B. Standard requirement of a microbiological quality control program for the manufacture of human mesenchymal stem cells for clinical use. Stem Cell. Dev. 2014;23:1074–1083. doi: 10.1089/scd.2013.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schneider J., Biedermann T., Widmer D., Montano I., Meuli M., Reichmann E., Schiestl C. Matriderm® versus Integra®: a comparative experimental study. Burns. 2009;35:51–57. doi: 10.1016/j.burns.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 167.Vanscheidt W., Ukat A., Horak V., Brüning H., Hunyadi J., Pavlicek R., Emter M., Hartmann A., Bende J., Zwingers T. Treatment of recalcitrant venous leg ulcers with autologous keratinocytes in fibrin sealant: a multinational randomized controlled clinical trial. Wound Repair Regen. 2007;15:308–315. doi: 10.1111/j.1524-475X.2007.00231.x. [DOI] [PubMed] [Google Scholar]
- 168.Lam P.K., Chan E.S., To E.W., Lau C.H., Yen S.C., King W.W. Development and evaluation of a new composite Laserskin graft. J. Trauma Acute Care Surg. 1999;47:918. doi: 10.1097/00005373-199911000-00017. [DOI] [PubMed] [Google Scholar]
- 169.Solanki N., Mackie I., Greenwood J. A randomised prospective study of split skin graft donor site dressings: AWBAT-D™ vs. Duoderm®, Burns. 2012;38:889–898. doi: 10.1016/j.burns.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 170.Park S., Kim Y., Jang S. The application of an acellular dermal allograft (AlloDerm) for patients with insufficient conjunctiva during evisceration and implantation surgery. Eye. 2018;32:136–141. doi: 10.1038/eye.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Amani H., Dougherty W.R., Blome-Eberwein S. Use of Transcyte® and dermabrasion to treat burns reduces length of stay in burns of all size and etiology. Burns. 2006;32:828–832. doi: 10.1016/j.burns.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 172.Motolese A., Vignati F., Brambilla R., Cerati M., Passi A. Interaction between a regenerative matrix and wound bed in nonhealing ulcers: results with 16 cases. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/849321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lucas D., Di Rocco D., Müller C.T., Jurjus A.R., Raffoul W., di Summa P.G., Watfa W. Application of dermal skin substitutes for hand and finger palmar soft tissue loss. Plastic and Reconstruc. Surgery Global Open. 2019;7 doi: 10.1097/GOX.0000000000002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yoon D., Cho Y.S., Joo S.Y., Seo C.H., Cho Y.S. A clinical trial with a novel collagen dermal substitute for wound healing in burn patients. Biomater. Sci. 2020;8:823–829. doi: 10.1039/c9bm01209e. [DOI] [PubMed] [Google Scholar]
- 175.Curran M.P., Plosker G.L. Bilayered bioengineered skin substitute (Apligraf®) A review of its use in the treatment of venous leg ulcers and diabetic foot ulcers. BioDrugs. 2002;16:439–455. doi: 10.2165/00063030-200216060-00005. [DOI] [PubMed] [Google Scholar]
- 176.Haflah N.H.M., Ng M.H., Yunus M.H.M., Naicker A.S., Htwe O., Razak K.A.A., Idrus R. Massive traumatic skin defect successfully treated with autologous, bilayered, tissue-engineered MyDerm skin substitute: a case report. JBJS case connector. 2018;8:e38. doi: 10.2106/jbjs.cc.17.00250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.