Abstract
Objective
To compare the outcomes of stem cell-enrichment fat grafting (SCEFG) versus autologous fat grafting (AFG) for reconstructive purposes.
Methods
A systematic review and meta-analysis was performed as per the preferred reporting items for systematic reviews and meta-analyses. Guidelines and a search of electronic information was conducted to identify all Randomised Controlled Trials (RCTs), case-control studies and cohort studies comparing the outcomes of SCEFG versus AFG. Volume retention, fat necrosis, cancer recurrence, redness and swelling, infection, and cysts were primary outcome measures. Secondary outcome measures included patient satisfaction post-surgery, scar assessment, operation time and number of fat grafting sessions. Fixed and random effects modelling were used for the analysis.
Results
16 studies enrolling 686 subjects were selected. Significant differences between the SCEFG and AFG groups were seen in mean volume retention (standardised mean difference = 3.00, P < 0.0001) and the incidence of redness and swelling (Odds Ratio [OR] = 441, P = 0.003). No significant difference between the two groups in terms of fat necrosis (OR = 2.23, P = 0.26), cancer recurrence (OR = 1.39, P = 0.58), infection (OR = 0.30, P = 0.48) and cysts (OR = 0.88, P = 0.91). For secondary outcomes, both cohorts had similar results in patient satisfaction, scar assessment and number of fat grafting sessions. Operation time was longer for the intervention group.
Conclusions
SCEFG offers better outcomes when compared to AFG for reconstructive surgery as it improves the mean volume retention and does not worsen patient satisfaction and surgical complications except for self-limiting redness and swelling. Further clinical trials are recommended to support this argument and validate the use of SCEFG in clinical practice.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Keywords: Stem cell-enrichment fat grafts, Volume retention, Patient satisfaction
Introduction
Over the last two decades, techniques of fat transplantation have significantly improved from simple free transfers of intact adipose tissue to free composite fat-cell transplantation techniques [1]. There are various indications of fat grafting, ranging from breast and craniofacial reconstruction to improvement of joint mobility after surgery for ankylosis [2].
The application of autologous fat grafts (AFG) for soft tissue augmentation has increased in popularity, creating an inexpensive and readily available product for lipo-grafting [3]. Krastev et al. (2018) conducted a systematic review that demonstrated the effectiveness of AFGs in treating soft-tissue abnormalities with minimal complications [4]. Conversely, other studies mention that there is a lack of reliable scientific evidence on the long-term viability of the transferred fat [3]. Traditional techniques of AFG for lipo-sculpting have recorded poor rates of volume retention ranging from 25 to 80%, which often requires additional procedures to optimise cosmetic outcomes [5–8].
Stem cells have been able to augment tissue regeneration within a host matrix by differentiating along specific cell lineages [9]. This principle has been utilised in lipofilling when reconstructing defects through the enrichment of fat grafts with stromal vascular fraction [10]. Stem cell enriched fat grafts (SCEFG) have been routinely adapted to enrich harvested fat with adipose-derived stromal cells which can increase graft take and volume retention by differentiation into mature adipocytes [11, 12]. Surgeons have been able to correct deformities in the breast, face as well as other anatomical regions utilising this method [13–17].
Although randomised controlled trials (RCTs) and non-randomised studies have shown promising results [17–32], no study in the literature currently analyses the outcomes of all comparative studies. This is the first systematic review and meta-analysis focusing on all comparative studies assessing SCEFG versus routine AFG for soft tissue reconstruction.
Methods
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement standards [33], but it was not registered at the International Prospective Register of Systematic Reviews.
Eligibility Criteria
All comparative studies including randomised as well as non-randomised controlled trials and observational studies comparing SCEFG versus routine AFG for reconstructive purposes were included. SCEFG was the intervention of interest and AFGs was the comparator. All patients were included irrespective of age, gender, co-morbidity status or anatomical region reconstructed as long as they belonged to either a study or control group. Case reports and cohort studies where no comparison was conducted were excluded from the review process.
Outcome Measures
The primary outcome measures included volume retention, fat necrosis, cancer recurrence, redness and swelling, infection, and cysts. Secondary outcomes included patient satisfaction post-surgery, scar assessment, operation time and number of fat grafting sessions.
Literature Search Strategy
Two authors independently searched the electronic databases of MEDLINE, EMBASE, CINAHL and the Cochrane Central Register of Controlled Trials (CENTRAL). The last search was conducted on 1st August 2022. The search strategy was adapted according to the thesaurus headings, search operators and limits in each of the above databases. The search terms for our intervention of interest consisted of “stem cell enriched”, “stromal vascular fraction enriched”, “ADSC”, “ASCs”, “ADRCs”, “progenitor enriched”, “call assisted lipotransfer”. Search terminologies used for the control group consisted of “fat graft”, “fat harvest”, “fat transplantation”, “autologous fat harvest”, “lipofilling”, “fat transfer”, “lipograft”, All terms were combined with adjuncts of “and” as well as “or”. To extend the screening for eligible articles, the bibliographic lists were also reviewed for the relevant studies. No search restriction was placed on language as a limiting factor.
Selection of Studies
The titles and abstracts of articles retrieved from the literature were independently assessed by two authors. Articles that met the eligibility criteria were selected after their full texts were reviewed. A consultation was obtained from an independent third author for any discrepancies in study selection.
Data Extraction and Management
A Microsoft Excel data extraction spreadsheet was amalgamated that abided with Cochrane’s data collection form for intervention reviews. A pilot test was conducted with the spreadsheet extracting data from random articles and adapting it as needed. Three authors independently extracted and recorded data.
Data Synthesis
The authors aimed to perform a meta-analysis for outcomes reported by at least three studies. The odds ratio (OR) was reserved for dichotomous variables whereas the mean difference (MD) was aimed to assess continuous variables for the study and control groups.
Review Manager 5.3 and Microsoft excel was used for data analysis and the fixed and random effects models were used. Reported outcomes were given in forest plots at 95% confidence intervals (CIs).
Heterogeneity was assessed using the Cochran Q test (χ2) and we aimed to quantify inconsistency by calculating I2 which was interpreted per the following: 0% to 25% (low heterogeneity); 25% to 75% (moderate heterogeneity); and 75% to 100% (considerable heterogeneity). The authors also intended to develop funnel plots and asses their symmetry to review publication bias, however, only if a minimum of 10 studies were available.
Sensitivity and Sub-Group Analyses
To identify areas of heterogeneity and assess the robustness of results, the authors aimed to perform additional analyses for outcomes that were reported by at least four studies.
Methodological Quality and Risk Of Bias Assessment
Two authors independently assessed the methodological quality as well as the risk of bias for articles matching the inclusion criteria. Cochrane’s tool for evaluating the risk of bias for randomised trials was used. Domains assessed included selection bias, performance bias, detection bias, attrition bias, reporting bias, and other sources. It classifies studies into low, unclear, and high risk of bias. In addition, Newcastle-Ottawa Scale [34] was used for the assessment of bias of non-randomised studies in terms of three domains: selection, comparability and exposure. It uses a star scoring system with a maximum total score of nine stars for each study.
Results
Literature Search Results
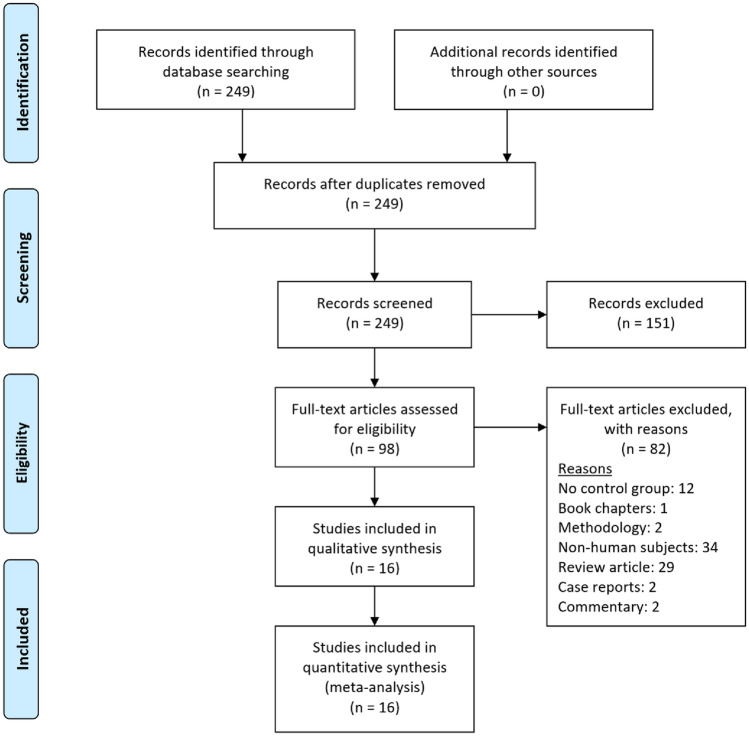
A literature search reported 248 articles in total which were reviewed by two independent authors to filter out duplicates, abstracts, review articles, studies without the intervention of interest as well as those without comparative control groups and reports involving non-human subjects. Sixteen studies were selected which met the eligibility criteria of which included 5 randomized control trials and 11 non-randomised case-control studies.
Description of Studies
Table 1 summarizes the baseline characteristic of the included studies. The studies were homogenous in the study population and interventions of interest comparing SCEFG and AFG in reconstructive surgery. The current meta-analysis included a total of 16 studies with a total sample size of 686 grafts. Eight studies focused on facial reconstruction, six studies on breast reconstruction and two studies on limb reconstruction (Fig. 1).
Table 1.
Baseline characteristics of the included studies
| Study (year) | Journal, Country | Study design | Age | Sex (male:female) | Total sample (intervention: control) | Anatomical location | Interventions compared | Method of stem cell enrichment | Volume retention assessment |
|---|---|---|---|---|---|---|---|---|---|
| Yoshimura et al. (2008) | Dermatologic surgery, Japan | Case control study | 37.5 years (mean) | 2:4 | 6 (3:3) | Face | CAL versus conventional lipoinjection (non-CAL) | The liposuction aspirate was digested with 0.075% collagenase for 30 minutes at 37 °C, which separated mature adipocytes and connective tissue from the stromal vascular fraction (SVF) containing ASCs. The SVF was then centrifuged and rinsed to isolate the cells, which took about 90 minutes. | High resolution digital camera (Model D30, Canon) |
| Sterodimas et al. (2011) | Aesthetic surgery journal, Brazil | RCT | 45.15 years (mean) | 5:15 | 20 (10:10) | Face | ADSC enriched lipografts versus autologous fat transplant | The SVF was isolated from the aspirated fat through a process involving collagenase digestion, centrifugation, and separation. The SVF and purified fat were mixed and transferred to syringes for injection | NR |
| Gentile et al. (2012) | Stem Cells translational medicine, Italy | Cohort Study | 19–60 years (range) | 0:20 | 20 (10:10) | Abdominal to breast | SVF enhanced autologous fat graft versus centrifuged fat grafting injection | Half of lipoaspirate placed in Celution system, Blood free lipid removed by wash cycle. Celase835 enzymatically digested tissue releasing SVF. Centrifugation -> extraction of SVF Added to 2nd half of lipoaspirate that is washed and filtrated. | MRI |
| Koh et al. (2012) | Annals plastic surgery, South Korea | RCT | 28 years (mean) | 5:5 | 10 (5:5) | Abdominal to face | Microfat grafts and simultaneous human ASC injection versus microfat grafts alone | Human ASCs were extracted from adipose tissue and isolated using standard protocols from the International Society for Cellular Therapy [46]. | 3-dimentional CT scan |
| Li et al. (2012) | Dermatologic surgery, China | Case control study | Intervention: 29.5 years (mean) Control: 29.1 years (mean) | 0:38 | 38 (26:12) | Abdominal or thigh to face | Transplantation with fat mixed with SVF versus fat grafting alone | The suctioned fat was digested with collagenase and then processed to obtain SVF, by separating mature adipocytes, resuspending in erythrocyte lysis buffer, washing to remove collagenase, and collecting the harvested pellet. | CT |
| Chang et al. (2013) | Dermatologic surgery, China | Case control study | 27.5 years (mean) | 8:12 | 20 (10:10) | Abdominal to face | SVF supplemented fat grafting versus fat grafting alone | The SVF was isolated from the fatty tissue using a modification of a method described by Zuk and colleagues. The fluid was filtered, washed and centrifuged to obtain the SVF, which was then mixed with the harvested adipose tissue and injected into subcutaneous tissue in a sterile operating room. | CT |
| Kølle et al. (2013) | Lancet, Denmark | RCT | 28.4 years (mean) | NR | 20 (10:10) | Abdominal to arm | ASC-enriched fat graft versus non-enriched fat graft | ASCs were isolated and expanded in a certified laboratory using GMP-grade reagents. They were then cultured for 2 weeks with a mixture of Dulbecco’s modified Eagle’s medium, antibiotics, heparin, and human platelet lysate, with regular media changes and checks for contamination. | MRI |
| Peltoniemi et al. (2013) | Journal of plastic, reconstructive & aesthetic surgery (jpras), Finland | Case control study | Intervention: 51 years (mean); 29–58 years (range) control: 39 years (mean); 33–63 years (range) | 0:18 | 18 (10:8) | Breast | WAL enriched with stromal stem cells versus WAL alone | The first part of the harvested fat using the WAL technique is processed in the Celution System. The ASCs are isolated and separated from other cells through enzymatic digestion and multiple cycles of automatic centrifugation and washing. The final ASC-rich stroma is mixed with the ordinary fat graft and immediately injected | MRI |
| Tanikawa et al. (2013) | Plastic and reconstructive surgery journal, Brazil | RCT | Intervention: 18.7 ± 7.6 years (mean ± SD) control: 12.1 ± 2.2 years (mean ± SD) | 5:9 | 14 (7:7) | Lower abdominal to face | Fat grafting with supplementation of ADSC stromal cells versus fat grafting without supplementation of ADSC | The liposuction aspirates were digested with 0.15% collagenase type IA to separate mature adipocytes and connective tissue from the SVF which contains the adipose-derived stromal cells. The SVF was resuspended in water and added to the aspirated fat, then transferred into syringes for injection after mixing and waiting for cell adherence. | CT |
| Bashir et al. (2019) | Stem Cells international, Pakistan | Quasi-experimental Study | Intervention: 30 ± 11 years (mean ± SD) control: 21 ± 5 years (mean ± SD) | 11:26 | 37 (16:21) | Abdominal or lateral thigh to face | Stem cells mixed with fat graft versus fat graft only | Fat harvest for isolation and expansion of ASCs -> expansion of ASCs -> fat harvest, preparation, and enrichment with ASCs (2–4 weeks) -> pre-enriched fat transplant | Ultrasonography |
| Gentile et al. (2015) | Plastic and reconstructive surgery — global open (prs global open), Italy | Case control study | 19–60 (range) | 0:50 | 50 (40:10) | Breast | SVF enhanced fat grafts versus centrifuged fat alone | Half of lipoaspirate placed in Celution system, Blood free lipid removed by wash cycle. Celase835 enzymatically digested tissue releasing SVF. Centrifugation using Coleman procedure -> extraction of SVF added to 2nd half of lipoaspirate that is also washed and filtrated. | MRI |
| Sasaki (2015) | Aesthetic plastic surgery, USA | Case control study | Intervention: 63.3 years (mean) control: 60.5 years (mean) | 4:126 | 130 (38:92) | Abdominal or hip rolls to face | SVF- assisted fat grafting versus combination of PRP/SVF- assisted fat grafting versus conventional fat grafting | A portion of processed adipose tissue is loaded into the Celution System for automated SVF isolation. The SVF is extracted through a process of collagenase digestion, washing, and centrifugation, resulting in a SVF-filled syringe that is mixed with processed fat within 2–4 hours. | 3D Vectra imaging |
| Tissiani et al. (2016) | Stem cells international, Brazil | Case control study | 49.68 years (mean) | 0:17 | 17 (9:8) | Breast | Fat grafts with SVF enrichment versus Fat grafts without SVF enrichment | The SG group collected 600cc of fat which was centrifuged in 50cc conic tubes. The fat was then digested with collagenase and mixed with the SVF for 15 minutes for cell adherence to occur. | MRI |
| Calabrese et al. (2018) | European review for medical and pharmacological sciences, Italy | Cohort study | Intervention: 48.8 years (mean) control: 50.3 years (mean) | 0:105 | 105 (41:64) | Breast | SVF enriched adipose tissue grafting versus AFG | Aspirated adipose tissue divided into 2 parts. 1st part added to Celution system, ADRCs released from bound matrix and concentrated. 2nd part was purified by gravity sedimentation. 1st part added to 2nd part creating ADRC enriched fat graft. | NR |
| Gentile et al. (2019) | Journal of clinical medicine, Italy | Case control study | 56.24 ± 11.44 years (mean ± SD); 25–85 years (range) | 171:0 | 171 (121:50) | Breast | EF-e-A versus engineered fat graft that was not enhanced with ASCs | Half of lipoaspirate placed in Celution system, Blood free lipid removed by wash cycle. Celase835 enzymatically digested tissue releasing SVF. Centrifugation using Coleman procedure -> extraction of SVF added to 2nd half of lipoaspirate that is also washed and filtrated. | MRI |
| Malik et al. (2019) | Indian journal of orthopaedics, India | RCT | 32.10 years (mean); 22–45 years (range) | NR | 10 (5:5) | Amputation stump | AFG enriched with SVF versus autologous fat graft alone | The lipoaspirate was processed to isolate the SVF and purified fat. The SVF was treated with 0.075% collagenase, separated through centrifugation, and analyzed for viability. The purified fat was separated from the rest of the lipoaspirate solution and both the SVF and fat were combined for injection | MRI |
NR: not reported; SVF: stromal vascular fraction; ASC: adipose-derived stem cells; CAL: cell-assisted lipotransfer; EF-e-A: engineered fat graft enhanced with adipose-derived stromal vascular fraction cells; WAL: water-assisted lipotransfer; PRP: platelet‐rich plasm ADRC: Adipose derived regenerative cells; CT: Computed tomography scan; MRI: Magnetic resonance imaging. 3D: 3 dimentional
Fig. 1.
Prisma Flow Diagram. the PRISMA diagram details the search and selection processes applied during the overview. prisma, preferred reporting items for systematic reviews and meta-analyses
Primary Outcomes
Volume Retention
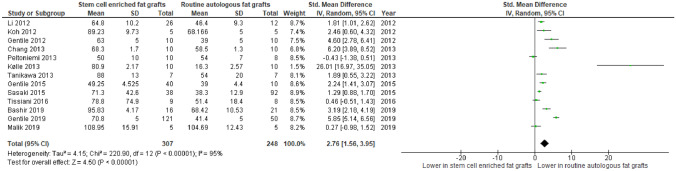
In Fig. 2, mean volume retention was reported in 13 studies enrolling 555 subjects. There was a statistically significant difference seen in the mean difference analyses showing a higher mean percentage volume retention for the SCEFG group than the AFG group (Standardised MD = 2.76, CI = 1.56 to 3.95, P < 0.000001). A considerable level of heterogeneity was found amongst the studies (I2 = 95%, P < 0.00001).
Fig. 2.
Forest plot for mean difference of cal versus aft - Volume retention. Quantitative analysis showing a statistically higher mean percentage volume retention in stem cell enhanced lipo-transfer compared with routine aft
Moreover, Yoshimura et al. revealed that the volume of fat retention showed a minimal change in the SCEFG group whereas it continued to be reduced in the AFG group. In addition, Malik et al. reported that there’s a more significant increase in the mean fat area on MRI scans pre-operatively and 6 months post-operatively in the SCEFG (17.8800 to 26.8280; P = 0.009) than the control group (24.0720 to 28.7620; P = 0.158).
Fat Necrosis
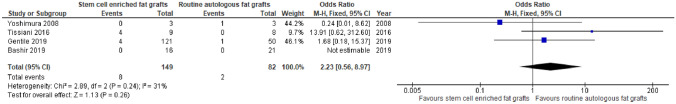
In Fig. 3, the rate of fat necrosis was reported in three studies enrolling 194 subjects. There was a statistically insignificant difference seen in the odds ratio analyses showing a lower rate of fat necrosis for the SCEFG group than the routine group (OR = 2.23, CI = 0.56 to 8.97, P = 0.26). A moderate level of heterogeneity was found amongst the studies (I2 = 31%, P = 0.24). Furthermore, Kølle et al reported a lower mean area of necrosis in the SCEFG group than the control group, with values of 4.6% and 16.1% respectively. Gentile et al. (2015), however, reported no complications in any patient in both groups.
Fig. 3.
Forest plot for odds ratio of stem cell enriched fat versus routine autologous fat transfer – fat necrosis. Quantitative analysis showing a statistically insignificant lower rate of fat necrosis in stem cell enhanced lipo-transfer compared with routine autologous fat transfer
Cancer Recurrence
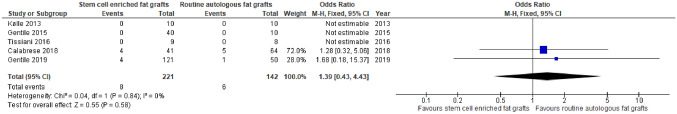
In Fig. 4, the rate of cancer recurrence was reported in five studies enrolling 363 subjects. There was a statistically insignificant difference seen in the odds ratio analyses showing a higher rate of cancer recurrence for the SCEFG group than the AFG group (OR = 1.39, CI = 0.43 to 4.43, P = 0.58). A low level of heterogeneity was found amongst the studies (I2 = 0%, P = 0.84).
Fig. 4.
Forest plot for odds ratio of stem cell enriched fat versus routine autologous fat transfer – cancer recurrence. Quantitative analysis showing a statistically insignificant higher rate of cancer recurrence in stem cell enhanced lipo-transfer compared with routine autologous fat transfer
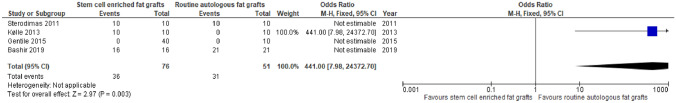
Redness and Swelling
In Fig. 5, the rate of redness and swelling was reported in three studies enrolling 90 subjects. There was a statistically significant difference seen in the odds ratio analyses showing a higher rate of redness and swelling for the SCEFG group than the AFG group (OR = 441, CI = 7.98 to 24372.70, P = 0.003). Additionally, Chang et al. reported improvement in colour at the site in both groups. Li et al., Tanikawa et al. and Bashir et al. all reported swelling in both groups in the early postoperative period that was self-limiting with no further complications. Bashir et al. also reported bruising in 11 (69%) patients in SCEFG enriched group, whereby bruising occurred after 36 sessions and resolved in 2–3 weeks. Similarly, Sasaki described transient swelling experienced by all patients lasting for 2–3 weeks until full recovery with no difference between both groups, whereby there were no recorded incidences of hematomas or tissue loss.
Fig. 5.
Forest plot for odds ratio of stem cell enriched fat versus routine autologous fat transfer – redness and swelling. Quantitative analysis showing a statistically significant higher rate of redness and swelling in stem cell enhanced lipo-transfer compared with routine autologous fat transfer
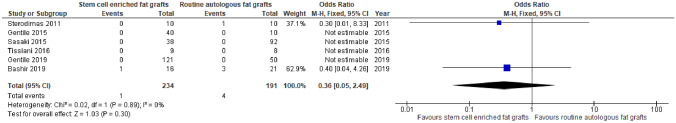
Infection
In Fig. 6, the rate of infection was reported in six studies enrolling 425 subjects. There was no significant difference seen in the odds ratio analysis between the SCEFG group and the AFG group (OR = 0.36, CI = 0.05 to 2.49, P = 0.30). Heterogeneity was revealed to be low between the studies (I2= 0%, P=0.89). Moreover, Kølle et al. and Tanikawa et al. did not report any adverse events or surgical complications respectively.
Fig. 6.
Forest plot for odds ratio of stem cell enriched fat versus routine autologous fat transfer – infection. Quantitative analysis showing a statistically insignificant lower rate of infection in stem cell enhanced lipo-transfer compared with routine autologous fat transfer
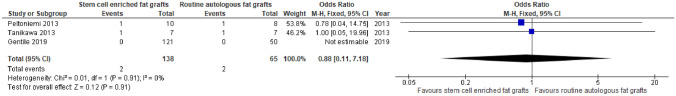
Cysts
In Fig. 7, the rate of cysts was reported in three studies enrolling 203 subjects. There was no statistically significant difference seen in the odds ratio analyses showing a similar rate of cysts for the SCEFG group and the routine group (OR = 0.88, CI = 0.11 to 7.18, P = 0.91). A low level of heterogeneity was found amongst the studies (I2 = 0%, P = 0.91). In addition, Gentile et al. (2015) highlighted oily cysts detected by ultrasound in 45.83% of study group at 12 months after the last lipofilling.
Fig. 7.
Forest plot for odds ratio of stem cell enriched fat versus routine autologous fat transfer – cysts. Quantitative analysis showing a statistically insignificant similar rate of cysts in stem cell enhanced lipo-transfer compared with routine autologous fat transfer
Patient Satisfaction Post-Surgery
Sterodimas et al. highlighted more satisfaction in the SCEFG group than the non-enriched group at 6 months; however, no difference was noted at 18 months. Visual analog scale was used by Koh et al. for the assessment of patient satisfaction, highlighting a higher score for the intervention group (4.5) than the control group (3.1). Similarly, Bashir et al. did not only report a significantly higher patient satisfaction score (mean score: 2.52 ± 0.521 [ADSC enriched group] vs. 4.25 ± 0.68 [routine AFT group]) in the intervention group but also a higher physician satisfaction score (mean score: 3.69 ± 0.79 [ADSC enriched group] vs. 2.14 ± 0.36 [routine AFT group]).
Gentile et al. (2012) reported satisfaction from all the patients in both groups in terms of the resulting softness, texture and contour. In addition, Gentile et al. (2015) reported satisfaction with the same characteristics from all the patients in the study group, although there was no report of the satisfaction of the control group. Similarly, Sasaki did not compare the satisfaction of both groups; however, 90–95% of patients were satisfied.
No significant differences between the intervention and control groups in patient satisfaction with the results of fat grafting were reported by Tissiani et al. and Gentile et al. (2019), with P values of 0.52 and 0.603 in turn.
Scar Assessment
Clinical assessment of scars by Malik et al. was performed using the observer scar assessment scale (POSAS) score preoperatively and at 1 and 6 months postoperatively, whereby both groups showed similar improvement in scar characteristics. Li et al. reported that no scars were present in patients during follow‐up.
Operative Time
Both Peltoniemi et al. and Tanikawa et al. reported that the operation time was longer in the stem-cell enriched group than the control group. Peltoniemi et al. reported a prolongation of 2–2.5 hours in the former group depending on the volume of enrichment used. Tanikawa et al. highlighted a shorter mean surgical time for the control group than the intervention group, with 80 minutes and additional 45 minutes in turn.
Number of fat grafting sessions
Five studies reported the number of fat grafting sessions that took place. Three studies (Chang et al 2013, Gentille et al 2015 and Koh et al 2012) used the same number of fat injections for both the SCEFG and AFG groups. The other two studies (Bashir et al 2019 and Sterodimas et al 2011) had an average of 1 session for the SCEFG group and 2 sessions for the AFG group.
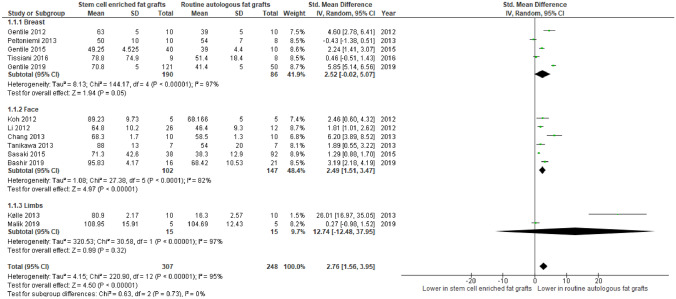
Subgroup Analysis – Volume Retention
In Fig. 8, subgroup analysis was done showcasing mean difference in volume retention across 13 different studies with a total number of 555 participants. Overall, there is a significant difference seen in the mean difference analyses favouring the intervention group whereby volume retention is higher (MD:2.76, CI: 1.56–3.95, P<0.00001) compared to the AFG group. Additionally, there is no significant difference seen between the subgroups (P=0.73).
Fig. 8.
Forest plot depicting subgroup analysis of volume retention for stem-cell enriched fat grafts vs routine autologous fat grafts- Quantitative analysis showing significant differences seen for participants undergoing breast and face grafts and no differences between the subgroups
Methodological Quality and Risk of Bias Assessment
The Cochrane Collaboration’s Tool was used to summarise the risk of bias in randomised studies (Table 2). The Newcastle-Ottawa Scale [16] was used to assess the quality of the three non-randomised studies, which offers a star system for analysis (Table 3). Although the comparability was low in most studies, selection and exposure were of higher quality. Overall, all studies were of good quality except for Sasaki et al which was of fair quality based on the AHRQ standards [16].
Table 2.
Assessment of risk of bias of the randomised trials using the cochrane collaboration’s tool
| First author | Bias | Authors’ judgement | Support for judgement |
|---|---|---|---|
| Sterodimas et al. (2011) | Random sequence generation (selection bias) | Unclear risk | No information regarding the randomisation technique is mentioned |
| Allocation concealment (selection bias) | High risk | No allocation concealment is done. | |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding of participants mentioned | |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given | |
| Incomplete outcome data (attrition bias) | Low risk | All outcome data reported | |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all data is reported in the pre-specified way. | |
| Other bias | Unclear risk | Similar baseline characteristics not mentioned | |
| Koh et al. (2012) | Random sequence generation (selection bias) | Unclear risk | No information regarding randomisation technique is mentioned |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment is mentioned. | |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information given | |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information given | |
| Incomplete outcome data (attrition bias) | High risk | Standard deviation for volume differences is missing | |
| Selective reporting (reporting bias) | Low risk | Study protocol available with no missing outcomes. | |
| Other bias | Low risk | Similar baseline characteristics | |
| Kølle et al. (2013) | Random sequence generation (selection bias) | Low risk | allocation sequence was generated using an online randomisation generator |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by a person unrelated to the trial management group and monitored by the Good Clinical Practice unit | |
| Blinding of participants and personnel (performance bias) | Low risk | “The participants, study personnel, and outcome assessors were all blinded to treatment allocation, and blinding was maintained until all data had been analysed”. | |
| Blinding of outcome assessment (detection bias) | Low risk | “The participants, study personnel, and outcome assessors were all blinded to treatment allocation, and blinding was maintained until all data had been analysed”. | |
| Incomplete outcome data (attrition bias) | Low risk | All outcome data is reported | |
| Selective reporting (reporting bias) | Low risk | Study protocol is available in the appendix | |
| Other bias | Low risk | No further biases detected | |
| Tanikawa et al. (2013) | Random sequence generation (selection bias) | Low risk | Random number generator used |
| Allocation concealment (selection bias) | Unclear risk | No information given | |
| Blinding of participants and personnel (performance bias) | Low risk | Double blinded | |
| Blinding of outcome assessment (detection bias) | Unclear Risk | No information given | |
| Incomplete outcome data (attrition bias) | Low risk | No outcome data was missing | |
| Selective reporting (reporting bias) | Low risk | Study protocol available with no missing outcomes | |
| Other bias | Low risk | No further biases detected | |
| Malik et al. (2019) | Random sequence generation (selection bias) | Low risk | Patients were selected using envelope method |
| Allocation concealment (selection bias) | Unclear risk | No information given regarding concealement | |
| Blinding of participants and personnel (performance bias) | Low risk | Patients were divided randomly using computerized methods | |
| Blinding of outcome assessment (detection bias) | Unclear risk | No mention of how outcomes were assessed | |
| Incomplete outcome data (attrition bias) | Low risk | All outcome data was reported and no patients lost to follow up | |
| Selective reporting (reporting bias) | Low risk | Study protocol available with no missing outcomes | |
| Other bias | Unclear risk | No mention of baseline characteristics between the patients |
Table 3.
Newcastle-Ottawa Scale (NOS) to assess the quality of non-randomised studies
| Study | Selection | Comparability | Exposure |
|---|---|---|---|
| Yoshimura et al. (2008) | *** | * | ** |
| Gentile et al. (2012) | *** | * | *** |
| Li et al. (2012) | *** | ** | *** |
| Chang et al. (2013) | *** | * | ** |
| Peltoniemi et al. (2013) | *** | * | ** |
| Gentile et al. (2015) | *** | * | ** |
| Sasaki (2015) | ** | * | *** |
| Tissiani et al. (2016) | *** | * | ** |
| Calabrese et al. (2018) | *** | ** | ** |
| Gentile et al. (2019) | *** | ** | ** |
| Bashir et al. (2019) | *** | * | ** |
Discussion
SCEFG produced a superior effect when compared with AFG in terms of mean volume retention shown by the results of the analyses. There was a significant (P < 0.0001) improvement in the Intervention group compared with the control groups in the combined analysis(Fig. 2) and the subgroup assessment showing no differences between them (Fig. 8). Conversely, there was a significantly (P = 0.003) increased rate of redness and swelling in the experimental group (Fig. 5). However, there were no differences observed in the analyses of fat necrosis (P = 0.26) and cancer recurrence (P = 0.58) in Fig.s 3 and 4. Similarly, the rates of infection (P = 0.48) and cysts (P = 7.18) showed no significant differences as demonstrated in Figs. 6, 7. This shows that SCEFG improves mean volume retention whilst not increasing the overall risk of complications. Regarding the between-study heterogeneity, it was low to moderate for cancer recurrence (I2 = 0%), cysts (I2 = 0%) and fat necrosis (I2 = 31%); however, it was considerably high for volume retention (I2 = 95%). The heterogeneity assessment was not applicable for the redness and swelling.
In addition to the aforementioned outcomes, the results of the current study reported several secondary outcomes that proved SCEFG to have similar effects to the AFG. In the long-term, there were no significant differences noted between both groups in terms of patient satisfaction post-operatively and scar formation. However, operative time was reported to be longer in the SCEFG group.
SCEFG have been developed to overcome the unreliability of the final volume retention of AFG as well as to reduce complications [35]. The current study findings emphasise that SCEFG is superior to AFG. This is supported by the several proposed mechanisms whereby stem cell enrichment increases adipose survival [12]. Initially stem cells differentiate into adipocytes and endothelial cells assist in adipose tissue regeneration and angiogenesis, respectively [12]. Subsequently, stem cell growth factor release helps to protect surrounding tissues from ischaemia and hypoxia [12, 36]. Finally, they survive as original adipose-derived stem cells [12]. Other studies within the literature have reported similar findings to the results of the current review with Lu et al. concluding that there is an enhancement in the survival and quality of transplanted fat tissues with adipose derived stem cells transduced with vascular endothelial growth factor (VEGF) [37]. A further study by Zhu et al. showed that the supplementation of adipose-derived regenerative cells does not only increase adipose graft retention by 2-fold at both 6 and 9 months post-operatively but also enhances the quality of the grafts with higher capillary density, demonstrating the effect of promoting neovascularisation [38].
Several studies question the safety of stem cell application in oncological cases. Stem cells are suggested by several studies to enhance breast tumour growth [39–42]. Similarly, other studies highlight an increased risk of tumour recurrence with their application [43, 44]. The quantitative assessment conducted by the current meta-analysis however shows no significant difference in the cancer recurrence rate between both stem cell enriched and standard fat transfer cohorts.
While the outcomes of this review and the numerous benefits of SCEFG offer promising results for future practise, there are several limitations that must be considered adapting them in routine clinical practice. ADSC isolation is challenging, with most included studies in this review employing SVF instead [45]. The paucity of published clinical research, a lack of standard methodology, and economic impedance all pose a hinderance to the use of ADSC- or SVF-based cell therapy in clinical activity. Furthermore, despite substantial increases in volume retention when comparing SCEFG for breast and face, none of the included studies for breast reconstruction were prospective or randomised thus reducing the quality of evidence.
Taking into consideration the results from the most reliable evidence and the physiological advantages of stem cells [12, 36], the enhancement of stem cells in AFG should be a routine practice for the management of patients undergoing reconstructive operations. However, in cases of cancer, it is important to be cautious with stem cell enrichment before the establishment of cancer remission [42].
A summary of the best available evidence was obtained by applying a systematic approach to assess the risk of bias of relevant studies [17–32]. The 16 studies were standardised in terms of their design and included patients undergoing reconstructive surgeries. The included studies were homogenous in both the intervention of interest used and the comparative routine grafting, allowing non-biased comparison. However, the current meta-analysis should be reviewed in terms of inherit limitations. 16 studies were included enrolling a total sample size of 686 subjects, only 5 of which were RCTS which could be inadequate to draw definitive conclusions as there is a higher selection bias. Further randomised clinical trials are required to fully assess the efficacy and safety of SCEFG in reconstructive cases.
Conclusions
The findings of this meta-analysis involving 16 studies comparing stem cell-enriched fat grafts and routine fat grafts suggest that stem cell enrichment improves certain outcomes in patients undergoing reconstruction surgery including the mean volume retention and patient satisfaction and surgical complications, except for redness and swelling. SCEFG remain a promising alternative to AFG bearing in mind issues with cost and lack of standardised protocols. The authors highlight the requirement of further randomised clinical studies to enhance the evidence base for the efficiency of SCEFGs.
Declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Informed Consent
For this type of study informed consent was not required
Statement of Human and Animal Rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohammad Karam and Ahmad Abul contributed equally as joint first authors.
References
- 1.Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E. Procedure, applications, and outcomes of autologous fat grafting. Annals Med Surg. 2017;1(20):49–60. doi: 10.1016/j.amsu.2017.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lexer E. Free transplantation. Ann Surg. 1914;60(2):166. doi: 10.1097/00000658-191408000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doornaert M, Colle J, De Maere E, Declercq H, Blondeel P. Autologous fat grafting: latest insights. Annals Med Surg. 2019;1(37):47–53. doi: 10.1016/j.amsu.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krastev TK, Beugels J, Hommes J, Piatkowski A, Mathijssen I, van der Hulst R. Efficacy and safety of autologous fat transfer in facial reconstructive surgery: a systematic review and meta-analysis. JAMA facial plastic surg. 2018;20(5):351–360. doi: 10.1001/jamafacial.2018.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinna R, Delay E, Garson S, Delaporte T, Toussoun G. Breast fat grafting (lipomodelling) after extended latissimus dorsi flap breast reconstruction: a preliminary report of 200 consecutive cases. J Plast Reconstr Aesthet Surg. 2010;63(11):1769–1777. doi: 10.1016/j.bjps.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Delay E, Garson S, Tousson G, Sinna R. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthetic Surg J. 2009;29(5):360–376. doi: 10.1016/j.asj.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Missana MC, Laurent I, Barreau L, Balleyguier C. Autologous fat transfer in reconstructive breast surgery: indications, technique and results. European Journal of Surgical Oncology (EJSO). 2007;33(6):685–690. doi: 10.1016/j.ejso.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura T, Hashimoto H, Nakanishi I, Furukawa M. Microvascular angiogenesis and apoptosis in the survival of free fat grafts. Laryngoscope. 2000;110(8):1333–1338. doi: 10.1097/00005537-200008000-00021. [DOI] [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg. 2006;118(3S):121S–S128. doi: 10.1097/01.prs.0000234609.74811.2e. [DOI] [PubMed] [Google Scholar]
- 11.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura K, Suga H, Eto H (2009) Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med 4(2):265–273 [DOI] [PubMed]
- 13.Tiryaki T, Findikli N, Tiryaki D. Staged stem cell-enriched tissue (SET) injections for soft tissue augmentation in hostile recipient areas: a preliminary report. Aesthetic Plast Surg. 2011;35(6):965–971. doi: 10.1007/s00266-011-9716-x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura K, Asano Y, Aoi N, Kurita M, Oshima Y, Sato K, Inoue K, Suga H, Eto H, Kato H, Harii K. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16(2):169–175. doi: 10.1111/j.1524-4741.2009.00873.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura K, Matsumoto D, Gonda K (2005) A clinical trial of soft tissue augmentation by lipoinjection with adipose-derived stromal cells. In: Proceeding of 8th Annual Meeting of Tissue Engineering Society International
- 16.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg. 2008;32(1):48–55. doi: 10.1007/s00266-007-9019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimura K, Sato K, Aoi N, Kurita M, Inoue K, Suga H, Eto H, Kato H, Hirohi T, Harii K. Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg. 2008;34(9):1178–1185. doi: 10.1111/j.1524-4725.2008.34256.x. [DOI] [PubMed] [Google Scholar]
- 18.Sterodimas A, de Faria J, Nicaretta B, Boriani F. Autologous fat transplantation versus adipose-derived stem cell–enriched lipografts: a study. Aesthetic Surg J. 2011;31(6):682–693. doi: 10.1177/1090820X11415976. [DOI] [PubMed] [Google Scholar]
- 19.Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Curcio CB, Floris M, Fiaschetti V, Floris R, Cervelli V. A comparative translational study: the combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med. 2012;1(4):341–351. doi: 10.5966/sctm.2011-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh KS, Oh TS, Kim H, Chung IW, Lee KW, Lee HB, Park EJ, Jung JS, Shin IS, Ra JC, Choi JW. Clinical application of human adipose tissue–derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann Plast Surg. 2012;69(3):331–337. doi: 10.1097/SAP.0b013e31826239f0. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Gao J, Cha P, Chang Q, Liao Y, Liu C, Li K, Lu F. Supplementing fat grafts with adipose stromal cells for cosmetic facial contouring. Dermatol Surg. 2013;39(3pt1):449–56. doi: 10.1111/dsu.12058. [DOI] [PubMed] [Google Scholar]
- 22.Chang Q, Li J, Dong Z, Liu L, Lu F. Quantitative volumetric analysis of progressive hemifacial atrophy corrected using stromal vascular fraction–supplemented autologous fat grafts. Dermatol Surg. 2013;39(10):1465–1473. doi: 10.1111/dsu.12310. [DOI] [PubMed] [Google Scholar]
- 23.Kølle SF, Fischer-Nielsen A, Mathiasen AB, Elberg JJ, Oliveri RS, Glovinski PV, Kastrup J, Kirchhoff M, Rasmussen BS, Talman ML, Thomsen C. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. The Lancet. 2013;382(9898):1113–1120. doi: 10.1016/S0140-6736(13)61410-5. [DOI] [PubMed] [Google Scholar]
- 24.Peltoniemi HH, Salmi A, Miettinen S, Mannerström B, Saariniemi K, Mikkonen R, Kuokkanen H, Herold C. Stem cell enrichment does not warrant a higher graft survival in lipofilling of the breast: a prospective comparative study. J Plast Reconstr Aesthet Surg. 2013;66(11):1494–1503. doi: 10.1016/j.bjps.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Tanikawa DY, Aguena M, Bueno DF, Passos-Bueno MR, Alonso N. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg. 2013;132(1):141–152. doi: 10.1097/PRS.0b013e3182910a82. [DOI] [PubMed] [Google Scholar]
- 26.Gentile P, Scioli MG, Orlandi A, Cervelli V (2015) Breast reconstruction with enhanced stromal vascular fraction fat grafting: what is the best method? Plast Reconstr Surg Glob Open 3(6):e406 [DOI] [PMC free article] [PubMed]
- 27.Sasaki GH. The safety and efficacy of cell-assisted fat grafting to traditional fat grafting in the anterior mid-face: an indirect assessment by 3D imaging. Aesthetic Plast Surg. 2015;39(6):833–846. doi: 10.1007/s00266-015-0533-5. [DOI] [PubMed] [Google Scholar]
- 28.Tissiani LA, Alonso N (2016) A prospective and controlled clinical trial on stromal vascular fraction enriched fat grafts in secondary breast reconstruction. Stem Cells Int 2016:2636454 [DOI] [PMC free article] [PubMed]
- 29.Calabrese C, Kothari A, Badylak S, Di Taranto G, Marcasciano M, Sordi S, Ribuffo D. Oncological safety of stromal vascular fraction enriched fat grafting in two-stage breast reconstruction after nipple sparing mastectomy: long-term results of a prospective study. Eur Rev Med Pharmacol Sci. 2018;22(15):4768–4777. doi: 10.26355/eurrev_201808_15610. [DOI] [PubMed] [Google Scholar]
- 30.Malik P, Gaba S, Ahuja C, Sharma RR, Sharma RK, Khandelwal N. Role of fat graft alone versus enriched fat graft with stromal vascular filtrate in painful amputation stump. Indian J orthop. 2019;53(3):452. doi: 10.4103/ortho.IJOrtho_385_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gentile P, Casella D, Palma E, Calabrese C. Engineered fat graft enhanced with adipose-derived stromal vascular fraction cells for regenerative medicine: Clinical histological and instrumental evaluation in breast reconstruction. J Clin Med. 2019;8(4):504. doi: 10.3390/jcm8040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bashir MM, Sohail M, Ahmad FJ, Choudhery MS (2019) Preenrichment with adipose tissue-derived stem cells improves fat graft retention in patients with contour deformities of the face. Stem Cells International 2019:5146594 [DOI] [PMC free article] [PubMed]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 34.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- 35.Matsumoto D, Sato K, Gonda K, Takaki Y, Shigeura T, Sato T, Aiba-Kojima E, Iizuka F, Inoue K, Suga H, Yoshimura K. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12(12):3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 36.Uysal AC, Mizuno H, Tobita M, Ogawa R, Hyakusoku H. The effect of adipose-derived stem cells on ischemia-reperfusion injury: immunohistochemical and ultrastructural evaluation. Plast Reconstr Surg. 2009;124(3):804–815. doi: 10.1097/PRS.0b013e3181b17bb4. [DOI] [PubMed] [Google Scholar]
- 37.Lu F, Li J, Gao J, Ogawa R, Ou C, Yang B, Fu B. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg. 2009;124(5):1437–1446. doi: 10.1097/PRS.0b013e3181babbb6. [DOI] [PubMed] [Google Scholar]
- 38.Zhu M, Zhou Z, Chen Y, Schreiber R, Ransom JT, Fraser JK, Hedrick MH, Pinkernell K, Kuo HC. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64(2):222–228. doi: 10.1097/SAP.0b013e31819ae05c. [DOI] [PubMed] [Google Scholar]
- 39.Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, Krishnappa S, Pinilla S, Droll L, Song YH. Adipose tissue derived stem cells differentiate into carcinoma-associated fibroblast-like cells under the influence of tumor derived factors. Cell Oncol. 2011;34(1):55–67. doi: 10.1007/s13402-011-0012-1. [DOI] [PubMed] [Google Scholar]
- 40.Yu JM, Jun ES, Bae YC, Jung JS. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem cells and development. 2008;17(3):463–474. doi: 10.1089/scd.2007.0181. [DOI] [PubMed] [Google Scholar]
- 41.Zhao M, Dumur CI, Holt SE, Beckman MJ, Elmore LW. Multipotent adipose stromal cells and breast cancer development: think globally, act locally. Mol Carcinog. 2010;49(11):923–927. doi: 10.1002/mc.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17(1–2):93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donnenberg VS, Zimmerlin L, Rubin JP, Donnenberg AD. Regenerative therapy after cancer: what are the risks? Tissue Eng Part B Rev. 2010;16(6):567–575. doi: 10.1089/ten.teb.2010.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eterno V, Zambelli A, Pavesi L, Villani L, Zanini V, Petrolo G, Manera S, Tuscano A, Amato A. Adipose-derived mesenchymal stem cells (ASCs) may favour breast cancer recurrence via HGF/c-Met signaling. Oncotarget. 2014;5(3):613. doi: 10.18632/oncotarget.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang J, Chen F, Liu D, Gu F, Wang Y. Adipose tissue-derived stem cells in breast reconstruction: A brief review on biology and translation. Stem Cell Res Ther. 2021;12:1–3. doi: 10.1186/s13287-020-01955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gratama JW, Kvalheim G, Orfao A. Standardization of cell analysis methods in clinical cellular therapy programs: a challenge for ISCT. Cytotherapy. 2006;8(6):528–529. doi: 10.1080/14653240601056354. [DOI] [PubMed] [Google Scholar]