Abstract
Background:
Previous studies showed that Adamts9 is involved in multiple functions including ovulation, spine formation, primordial germ cell migration, and development of primary ovarian follicles in animals. However, systemic examination and high-resolution analyses of adamts9 expression are missing due to lack of a sensitive reporter assay.
Results:
In the present study, we created a new transgenic zebrafish reporter line Tg(adamts9:EGFP) and assayed its expression in various tissues and cells during development and in adults at high-resolution using confocal imaging. Reporter expression was validated with real-time quantitative PCR, whole mount in situ hybridization, and immunohistochemistry for endogenous adamts9. Strong expression of the adamts9:EGFP transgene was found in a wide range of adult and embryonic zebrafish tissues/cells including ovaries, testes, brains, eyes, pectoral fins, intestine, skin, gill, muscle, and heart; while lower expression was observed in the liver and growing ovarian follicles (stage II and III).
Conclusions:
Our results of a broad and dynamic expression pattern for this evolutionary conserved metalloprotease suggest involvement of adamts9 in the development and physiological functions of various tissues in animals.
Keywords: Metalloprotease, Folliculogenesis, Retina, Brain, Ciliary Marginal Zone
Introduction
Metalloproteases play critical roles in morphogenesis, tissue remodeling, cell migration, and cellular signaling, all of which are important in physiological and pathophysiological processes [1–3]. The ADAMTS (A disintegrin and metalloprotease domain with thrombospondin type 1 motif) proteases are a family of 19 metalloproteases in vertebrates that are highly conserved between zebrafish, mice, and humans [4,5]. Within the ADAMTS family, ADAMTS9 and ADAMTS20 form a separate phylogenetic group that was shown to be critical for primordial germ cell migration in C. elegans [1,5,6]. Genome-wide association studies (GWAS) have identified ADAMTS9 polymorphisms associated with various human diseases, such as diabetes [4,7,8], asthma [9], arthritis [10,11], artery calcification [12, 13], macular degeneration [14], and cognitive aging [15]. ADAMTS9 is expressed in most human fetal and adult tissues examined, supporting roles in various cell types during normal or disease processes [16, 17]. In mice, Adamts9 is broadly expressed during embryogenesis and knockout (KO) embryos die before gastrulation, highlighting the critical roles of ADAMTS9 in development [17–19]. Expression patterns of Adamts9 are similar in mice and Xenopus, suggesting conserved roles in tetrapods [20]. In zebrafish, adamts9 knockout results in multiple phenotypes including delayed growth, poor survival, wider central canal, kinky barbels, spinal deformities, eye abnormalities, primordial germ cell (PGC) migration delay, female infertility, and heavily male-biased sex ratios [21–23]. Despite these striking phenotypes, a systemic investigation of the cellular, spatial, and temporal expression of adamts9 at high resolution is still lacking in zebrafish.
In this study, we generated a new transgenic reporter line to visualize spatial patterns of adamts9 expression at the cellular level. The Tol2 transposon system was used to generate a transgenic zebrafish reporter line Tg(adamts9:EGFP), in which a ~4.5 kilobase (kb) proximal promoter region of adamts9 is fused to the coding sequence of EGFP. Advantages of the transgenic line include high sensitivity, the ability to rapidly observe multiple tissues without extensive staining or washing steps, and spatial resolution at the cellular level. The cloned promoter region mimicked the expression profile of endogenous adamts9 transcripts in zebrafish and reliably revealed cells in which adamts9 was previously reported in both larvae and adult, including Stage IV follicles, brain, eye, cloaca, and fin rays [5, 21–26]. We also report adamts9 expression in new domains including craniofacial muscles, neural tube, notochord, kidney, heart, gill, blood vessels, testis, and posterior lateral line ganglion in zebrafish model. Embryonic, larval, and follicular expression of adamts9 was validated by real-time quantitative PCR (qPCR), whole mount mRNA in situ hybridization (WISH), and/or immunohistochemistry. Thus, we show new domains of adamts9 expression, provide a new tool for studying adamts9 function in vivo, and also demonstrate the usefulness of studying metalloprotease expression using transgenic reporter lines in zebrafish.
Results and Discussion
In this study, we generated a highly sensitive transgenic reporter line to detect adamts9 expression in zebrafish. We found several conserved blocks in the ~30 kb upstream of adamts9 (https://useast.ensembl.org/Danio_rerio/Share/9d4c0d3e66d71fc1e7240a302b6b4681?redirect=no). A 4.5kb region upstream of adamts9 spanning the entire first conserved block, likely containing major regulatory elements, was selected, cloned, and used to drive EGFP expression.
Tg(adamts9:EGFP) expression in various tissues during zebrafish development
High levels of maternally deposited EGFP were observed in zygotes from crosses of F2 transgenic females and non-transgenic wildtype (WT, AB line) males (Fig. 1A~D). No observable EGFP signal was found at same developmental stage of embryos from crosses of F2 transgenic males and non-transgenic wildtype females (Fig. 1E~G). Therefore, the progeny of F2 transgenic males crossed to non-transgenic WT females was used for all descriptions of adamts9:EGFP zygotic expression. Zygotic expression was first detected around 10 hours post fertilization (hpf; Fig. 1H, Mov. 1).
Fig. 1. Maternal expression of adamts9 in zebrafish embryos.

A-D: Maternally-deposited EGFP signal in progeny from crosses of female Tg(adamts9:EGFP)ecu19 transgenics with wild-type males (WT, AB strain). Merged confocal images (EGFP and T-PMT channels) of embryos at the 1-cell, 2-cell, 4-cell, and 10 hours post-fertilization (hpf) stages are shown. Similar expression was confirmed in multiple F3 embryos from five independent F2 transgenic lines. E-G: No observable EGFP signal prior to 10 hpf in early dividing embryos from crosses of male Tg(adamts9:EGFP)ecu19 transgenics with WT females. H: zygote adamts9:EGFP expression. Scale bar: 200 μm.
Between 24–30 hpf, relatively weak expression of EGFP was observed in retina and muscle (Fig. 2A). Around 36 hpf, EGFP expression driven by the adamts9 promoter transiently marked the midbrain (Fig. 2B). At 48 hpf (Fig. 2C, Mov. 2), broad EGFP expression was observed in the brain (Fig. 2D), heart (Fig. 2D & E), notochord (Fig. 2F), muscle (Fig. 2C & G), pectoral fin (Fig. 2C & H), and blood vessels (Fig. 2I). EGFP expression in pectoral fins during early development suggests a role for adamts9 in teleost fin development, which is consistent with the demonstrated role of ADAMTS9 in mouse limb development [27].
Fig. 2. adamts9:EGFP expression in various tissues during zebrafish development.
A: weak adamts9:EGFP expression was observed in the developing retina and somites at 24 hpf. B: adamts9:EGFP expression at 36 hpf in middle brain. C-I: adamts9:EGFP expression at 48 hpf in whole body (C), brain and eye (D), heart (D & E), notochord and neural tube (F), trunk muscles (F), pectoral fin (H) and blood vessels (I). J-K: adamts9:EGFP expression in the posterior lateral line ganglion (pLLg) at 60 hpf. L: adamts9:EGFP expression in the retinal ciliary marginal zone (CMZ) at 72 hpf. M-O: adamts9:EGFP expression in craniofacial muscles at 72 hpf (M), 96 hpf (N) and 120 hpf (O). P: adamts9:EGFP expression in cloaca at 120 hpf. Similar expression was confirmed in multiple F3 embryos from five independent F2 transgenic lines. ah: adductor hyomandibulae; am: adductor mandibulae; do: dilatator opercula; hh: hyohyal; ih: interhyal; imp:intermandibularis posterior; ima: intermandibularis anterior; lap:levator arcus palatini; io: inferior rectus; ir: inferior oblique. Scale bar is 50 μm, except image J (100 μm) and image K (20 μm).
Around 60 hpf, EGFP expression was observed in the posterior lateral line ganglion (pLLg) (Fig. 2J & K). EGFP expression was also observed in the retinal ciliary marginal zone (CMZ, Figs. 2L & 4) and craniofacial muscles around 72 hpf (Figs. 2M~O & 4). At 5dpf, EGFP signal was observed in the cloaca (Fig. 2P), as previously reported in 13 dpf zebrafish larvae [21].
Fig. 4. Comparison of adamts9 signal profile detected by EGFP reporter, immunohistochemistry, and whole mount in situ hybridization (WISH) in retina, brain, pharyngeal arches (PAs), and ciliary marginal zone (CMZ) in zebrafish embryos.

A-C: expression in retina. D-F: expression in brain. G-I: expression in PAs. J-L: expression in CMZ. Adamts9 protein was detected using Alexa Fluor 488 conjugated secondary antibody (in green color). Nucleus was co-stained with DAPI. Purple color shows positive WISH signal. OV: otic vesicle; PA: pharyngeal arch; CMZ: ciliary marginal zone. Scale bar: 50 μm.
One worthy of note was dynamic changes of adamts9:EGFP expression in some tissues such as craniofacial muscles, retina and ovarian follicles (described later). At 48 hpf, we observed weak adamts9:EGFP expression in craniofacial muscle (Fig. 2D). Strongest adamts9:EGFP expression was observed at 72 hpf (Fig. 2M), then gradually decreases (Fig. 2N & 2O). Active changes of adamts9:EGFP expression was also observed in the retina. At 24 hpf, weak but detectable adamts9:EGFP expression was found (Fig. 2A), Stronger GFP expression observed at 36 hpf (Fig. 3). Expression of adamts9:EGFP at 60 hpf (Fig. 3) was much less, then decreasing to background levels at 72 hpf (data not shown). This onset of adamts9:EGFP retinal expression is coincident with that of the ccnb1:EGFP transgene, which labels proliferating cells during retinal development and regeneration [28], suggesting potential roles of adamts9 in retinal development.
Fig. 3. Transient adamts9:EGFP expression in the retina during zebrafish development.

Representative confocal images of EGFP expression in the retina of Tg(adamts9:EGFP) larvae at 36 hpf or 60 hpf, with or without phenylthiourea (PTU) treatment to suppress melanin pigment formation. EGFP, transmitted light (T-PMT), red fluorescent protein (RFP, for monitoring autofluorescence) or merged channels are shown. Identical confocal settings were used for both 36 and 60 hpf. Similar transient expression was confirmed in multiple F2 embryos from five independent transgenic lines. Scale bar: 50 μm.
To test the ability of our transgenic adamts9:EGFP zebrafish line to faithfully reflect endogenous patterns of adamts9 expression, transgenic reporter, adamts9 WISH and immunohistochemistry assays were conducted in parallel in the retina, CMZ, brain, and craniofacial region (Fig. 4). All three assays showed similar expression patterns in the retina (Fig. 4A~C), brain (Fig. 4D~F), and craniofacial muscle (Fig. 4G~I), suggesting that the transgenic reporter mimics endogenous adamts9 expression in these tissues. In the case of the CMZ, the adamts9:EGFP signal was clearly reproduced by WISH (Figs. 4L & 5A), while immunostaining resulted in a diffuse punctate signal (Figs. 4K & 5C). No comparable adamts9 signal was observed in control experiments where the sense probe was used (Fig. 5B) or primary antibody was omitted (Fig. 5D). Adamts9 is a secreted protein, meaning that it does not typically accumulate to high levels in the cytoplasm. Extracellular localization may explain the diffuse punctate immunostaining pattern for Adamts9 observed in the CMZ.
Fig. 5. Positive and negative control staining of whole mount in situ hybridization (WISH) and immunohistochemistry (IHC).
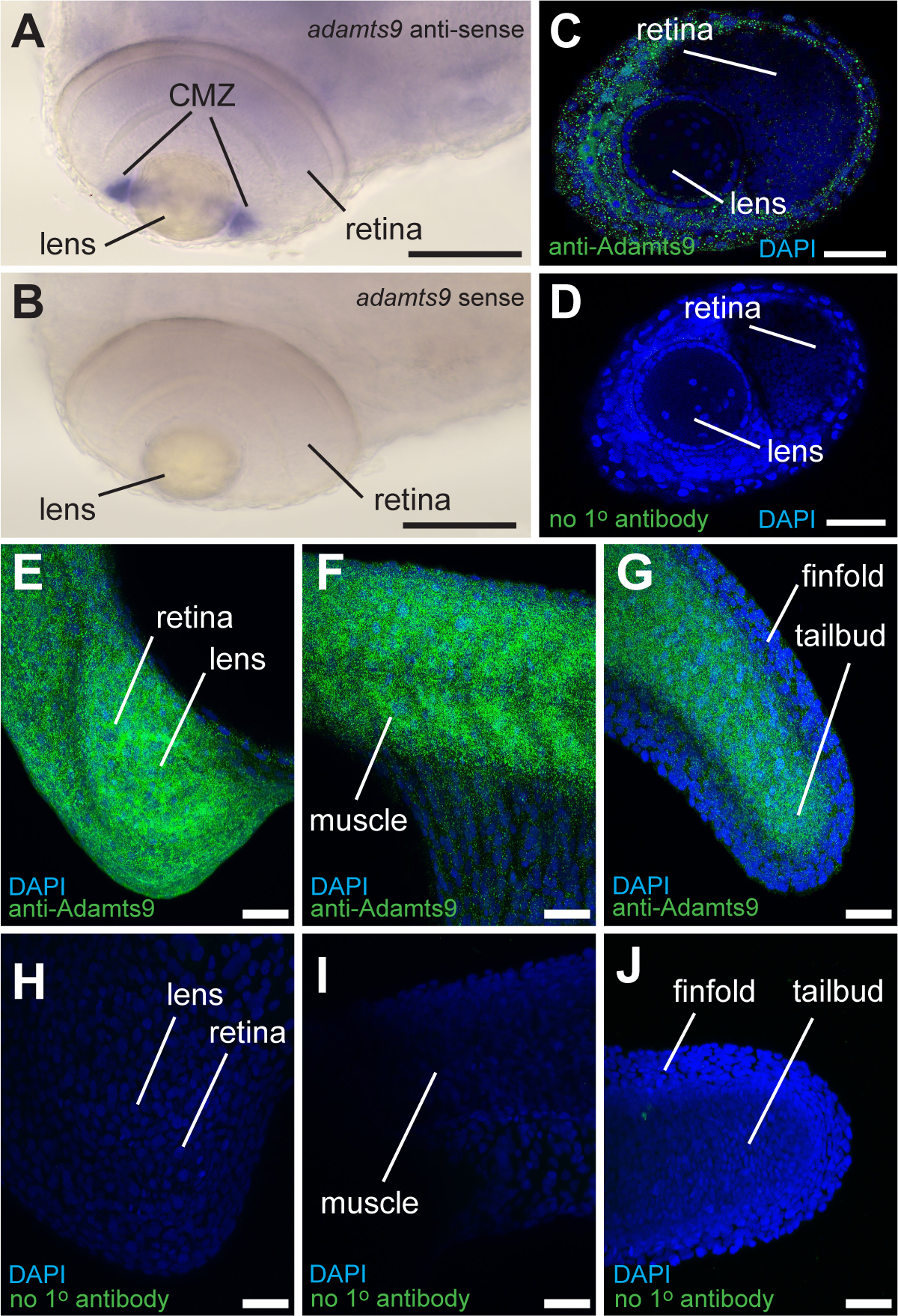
A & B: WISH signal in ciliary marginal zone (CMZ) of zebrafish embryos at 72 hours post fertilization (hpf) with adamts9-antisense (A) or adamts9-sense control (B). Scale bar: 100 μm C & D: IHC signal in eyes including the CMZ in zebrafish embryos at 72 hpf with an anti-Adamts9 primary antibody (C) or without primary antibody (D). Scale bar: 50 μm. E-J: IHC signal in zebrafish embryos at 24 hpf with anti-Adamts9 primary antibody (E-G) or without primary antibody (H-J). Scale bar: 50 μm.
The first skeletal muscles develop from the somites that form along the animal’s trunk [29]. We found that the adamts9:EGFP transgene was first expressed in zebrafish somites around 10 hpf at the 8-somite stage (Fig. 1D & H, Mov. 1) when zygotic expression of endogenous adamts9 begins [23]. Strong expression of the adamts9:EGFP transgene in muscles was observed around 48 hpf (Fig. 2C & G) and expression persisted into adult stages, as confirmed by RT-PCR (Fig. 7I) and qPCR (Fig. 7J), in accordance with previous findings in other vertebrates [16, 17]. We did not detect adamts9 expression in muscle by WISH, in agreement with a previous report [21]. Therefore, we assayed endogenous Adamts9 localization by immunohistochemistry at these stages and independently used two negative controls: primary antibody omission and adamts9 KO mutants [22]. At 24 hpf, strong immuno-positive signal was found in the brain, retina, somites, and tails (Fig. 5E~G) of WT embryos, while no comparable signal was detected in the negative controls lacking the primary antibody (Fig. 5H~J). In addition, immunostaining signal intensity was significantly different between adamts9+/+ (Fig. 6A~C), +/− (Fig. 6D~F), and −/− (Fig. 6G~I) individual embryos scored for maximum fluorescence intensity (MFI) by a blind observer at 24hpf, 72hpf and 5dpf (Fig. 6J~L). Expression of adamts9 in trunk and craniofacial muscles at the time of their first differentiation suggests potential role(s) for adamts9 in myogenesis [29].
Fig. 7. adamts9:EGFP expression in adult tissues of zebrafish.

A-H: representative merged confocal images (EGFP and T-PMT channels) of heart (A), pectoral fin (B, scale bar: 200 μm), testis (C, scale bar: 200 μm; D, scale bar: 50 μm), gill (E, scale bar: 200 μm; F, scale bar: 10 μm), kidney (G & H, scale bar: 50 μm) from a 4-month old mature male zebrafish. I: a representative gel image of RT-PCR analyses (30 cycles) of adamts9 in different tissues of adult zebrafish. J: real-time quantitative PCR (qPCR) analyses of adamts9 expression in different tissues. Results were presented as mean ± SEM (n = 6). Different letters above the error bars indicate that those groups are significantly different at p < 0.05. The expression of adamts9 in preovulatory follicles (stage IVb folic.) was omitted due to its large difference.
Fig. 6. Strong immuno-signal in wildtype zebrafish embryos.

Embryos with mixed genotype (i.e., +/+, +/−, −/−) from adamts9+/− in-cross between 24 and 72 hours post fertilization (hpf) were immune-stained together in the same reaction tubes with an anti-Adamts9 antibody and EGFP labeled second antibody. Each embryo was confocal imaged for EGFP signal and scored for maximum EGFP signal by a blinder observer, then genotyped. Scale bar: 200 μm.
Tg(adamts9:EGFP) expression in adult zebrafish
Strong EGFP expression was observed in adult heart (Fig. 7A), pectoral fin rays (Fig. 7B), testis (Fig. 7C & D), gills (Fig. 7E & F), kidney (Fig. 7G & H), and ovaries (Figs. 8&9), all of which were confirmed by RT-PCR (Fig. 7I) and qPCR (Fig. 7J) for endogenous adamts9. Kidney expression is consistent with previous reports of a requirement for ADAMTS9 in primary cilia formation and function. Furthermore, ADAMTS9 mutations are associated with Nephronophthisis-related ciliopathy (NPHP-RC) [31], a clinically and genetically heterogeneous disorder that causes dysplastic or degenerative diseases of the renal system [32].
Fig. 8. adamts9 signal in adult gonad of zebrafish.

A-C: adamts9:EGFP expression. Representative merged confocal images (EGFP and T-PMT channels) of ovaries from adult female zebrafish. No observable EGFP signal in fully-grown but immature follicles (stage IVa, A). Strong EGFP signals were found in preovulatory follicles (stage IVb, B & C). dpf: days post fertilization. D-I: immunohistochemistry staining with an anti-Adamts9 primary antibody in adult ovaries (D-F) or adult testes (H & I). G: Negative control staining without the primary antibody. Scale bar: 200 μm (A, B); 50 μm (E, G-I); 10 μm (C, D, F).
Fig. 9. adamts9 signal in zebrafish ovaries.

A-D: adamts9:EGFP expression. Representative merged confocal images (EGFP and T-PMT channels) of ovaries from juvenile female zebrafish. EGFP signal was enhanced due to low level of expression. E: Control immunohistochemistry staining without the primary antibody. F-H: immunohistochemistry staining with an anti-Adamts9 primary antibody. I-L: whole-mount in situ hybridization (WISH) in oocytes. dpf: days post fertilization. Scale bar: 200 μm (A & C); 100 μm (I & K); 50 μm (E-H); 10 μm (J & L).
In contrast with the strong adamts9:EGFP expression in adult testis (Fig. 7C & D), stage-dependent expression differences in expression were observed in ovarian follicles. Very strong EGFP signal was detected in stage IV preovulatory follicular cells (Figs. 8B & C), in correlation with the maternal deposition of EGFP observed at early embryonic stages (Fig. 1). In contrast, no or weak EGFP expression was observed in immature female follicles (stage II, III, Iva; Figs. 8A, 9A~D) or ovulated stage V oocytes [23], and relatively weak expression was observed in the ovaries of juvenile females at both 14- and 54-days post-fertilization (dpf; Fig. 9A~D).
High level of adamts9 expression has been previously reported for preovulatory follicles [22–26]. Here again, we used WISH and immunohistochemistry to test the ability of our adamts9:EGFP reporter line to faithfully reflect endogenous patterns of adamts9 expression. As expected, all three methods detected Adamts9 proteins and mRNA in ovarian follicles (Figs. 8D~F, 9F~H). No comparable signals were observed in control immunostaining experiments which omitted the anti-Adamts9 antibody (Fig. 9E), or in WISH experiments using a sense probe (Fig. 9I & J). Positive signals for Adamts9 protein and mRNA were restricted to the granulosa cells, and not found in adjacent oocyte or theca cells in mature follicles (Figs. 8D & F). Intense immunostaining was always found in the micropyle region (Fig. 8E & F). Interestingly, micropyle cells themselves seem to be devoid of Adamts9 while the surrounding follicular cells show strong expression (Fig. 8F). In testes, the EGFP reporter and immunohistochemistry assays resulted in similar profiles of Adamts9 signal (Figs. 7C, 7D, 8H, 8I). Although we detected positive signal in preovulatory stage IV follicles using WISH (Fig. 9K & L), this technique did not robustly detect adamts9 expression in stage I, II, or III follicles, or in testes (data not shown). In contrast, both the EGFP reporter and immunohistochemistry were able to pick up positive signals in early-stage ovarian follicles and testes (Fig. 9A~D, F~H, Mov. 3).
We surmise that the high sensitivity of this transgenic reporter line is advantageous for marking the presumptive cellular source of the extracellular Adamts9 protein detected by immunohistochemistry. ADAMTS9 is expressed in all human or mouse fetal tissues examined and some adult tissues by Northern blot and RT-PCR analysis [15–17]. However, cellular resolution of these previous studies was low. In this study, we demonstrated high resolution cellular images of adamts9 expression patterns using an adamts9:EGFP transgene, qPCR, WISH, and immunohistochemistry. adamts9 is broadly expressed in most tissues examined during development and in adult zebrafish, like that in mouse and Xenopus models [17,20].
The zebrafish is a gonochoristic species (two distinct sexes) of teleost that utilizes transitory hermaphroditism. Juveniles first form undifferentiated ovaries containing primordial oocytes before either undergoing apoptosis to develop as testes or maintaining the follicles to develop as ovaries [33]. Interestingly, the level of adamts9 expression was the high in the adult testis compared to other organ. This is surprising with respect to our earlier study, which indicated that adamts9 is necessary for gonadal development in female zebrafish but not in males [22]. We hypothesize that another protease whose enzymatic functions overlap with Adamts9 may be able to compensate for the loss of Adamts9 in testes development but not in the maintenance of ovarian follicles. In particular, Adamts1, Adamts5, Adamts8a/Adamts8b, or Adamts15a are possible candidates [5, 22–26]. The lowest expression of adamts9 was observed in the liver, which was similar to that reported in mouse [17]. This suggests that disrupted vitellogenin synthesis is an unlikely cause for the deficits in ovarian development and oocyte maturation observed in adamts9 KO zebrafish [22, 34–36].
Conclusions
Using an adamts9:EGFP transgene, EGFP was found in a broad array of tissue types and showed dynamic expression profiles in specific tissues such as the retina, brain, craniofacial muscles, and preovulatory follicles in zebrafish. Analyzing transgenic expression of EGFP driven by the adamts9 promoter in zebrafish provided new insights toward studying the physiological and reproductive functions of Adamts9 in different cell types during development and tissue remodeling. Further studies are required to elucidate the functions and mechanisms of Adamts9 in different cell types in vertebrates.
As we demonstrated, this new transgenic line is a highly sensitive in reporting adamts9 expression. However, it is important to note that the EGFP signal marks only those cells in which 4.5kb adamts9 promoter is active and does not label actual adamts9 mRNA nor its protein products. It is also important to acknowledge that the 4.5 Kb promoter sequence may be missing some endogenous enhancer or repressor elements for adamts9. Thus, EGFP expression driven by this 4.5kb promoter may not completely reflect endogenous adamts9 expression. Nevertheless, the majority of adamts9 expressing cell/tissue types identified by this transgenic reporter were validated by several different methods including PCR, WISH, and immunohistochemistry. The high sensitivity of this transgenic reporter is also advantageous for labeling cells with low levels of adamts9 expression. For example, RT-PCR and qPCR are more sensitive assays for detecting gene expression than in situ hybridization, but, because cells must be lysed for the PCR reaction these methods do not offer any spatial information about adamts9 expression at the cellular level. For genes with low transcript abundance or high turnover rate, transgenic reporters can be better at detecting positively expressing cells than a traditional WISH approach. Furthermore, immunohistochemistry alone may not be the best method for identifying cellular source of secreted proteins as is the case for many metalloproteases. In short, we demonstrated the usefulness of a transgenic reporter in studying the expression of a secreted metalloprotease, when other assays such as RT-PCR/qPCR, WISH, or immunohistochemistry have their limitations.
Experimental Procedures
Animal husbandry
Wild type AB line and transgenic zebrafish lines were housed in our zebrafish core facility with a 14-hour(h) light and 10h dark photoperiod, at water temperature of 28.5 °C, pH of approximately 7.2, and salinity/conductivity ranging from 500 to 1,200μS in automatically controlled zebrafish standalone rearing systems (Aquatic Habitats Z-Hab Duo systems, Florida, USA). Fish were fed to satiation three times daily with a commercial food (Otohime B2, Reed Mariculture, CA, USA) containing high contents of proteins and lipids, and supplemented with newly hatched brine shrimp Artemia (Brine Shrimp Direct, Utah, USA). The Institutional Animal Care and Use Committee (IACUC) at East Carolina University approved all experimental protocols.
Generating Tg(adamts9:EGFP) zebrafish lines.
A Tol2-based expression construct was generated using established multisite Gateway methods [37]. Briefly, we amplified 4473 base pairs upstream of the adamts9 start codon (Supplemental Fig.1), which was sub-cloned into a p5E-mcs entry vector and confirmed by Sanger sequencing. Next, multisite Gateway cloning (Invitrogen) was conducted with a 5’ entry vector containing 4.5 kb adamts9 promoter sequence, a middle entry vector containing EGFP, a 3’entry vector with stop poly A signal, and a destination vector that drives a EGFP selection marker specifically in the lens of eye (cryaa:EGFP) [38, 39]. Approximately 500 pl of a mixture containing 20 ng/ul construct, 20 ng/ul tol2 transposase mRNA, and phenol red indicator were microinjected into the one-cell stage embryos. F0 mosaic transgenic embryos were selected based on the lens EGFP marker, raised to adults, and outcrossed with AB wildtypes to produce several stable F1 lines. Subsequently, we established five independent F2 Tg(adamts9:EGFP,cryaa:EGFP)ecu19 zebrafish lines with stable expression of EGFP-driven by the adamts9 promoter.
Confocal imaging and analyses of EGFP expression in Tg(adamts9:EGFP) transgenics.
Live transgenic zebrafish of various developmental stages were collected by crossing male Tg(adamts9:EGFP)ecu19 fish with non-transgenic AB females. To examine maternal deposition of EGFP, live transgenic embryos were obtained by crossing female adamts9:EGFP transgenics with AB males. Individual embryos or larvae were placed on a depression glass slide, mounted in 1.2% low melting point agarose, and immediately imaged by confocal microscopy (Zeiss LSM 800). Five independent F2 transgenic lines were used for validating similar expression among the transgenic lines.
Fresh adult tissues were collected from adult transgenic females, placed in 15 mM HEPS buffer (pH 7.8) containing 50% L-15 medium (Fisher Scientific, #41-300-039), mounted in 1.2% low melting point agarose in a depression slide, and imaged immediately by confocal microscopy (Zeiss LSM800). An additional step was used for separation of ovarian follicles by pipetting up and down several times to separate individual follicles. Ten individual follicles of various developmental stages were placed on a depression glass slide, mounted in 1.2% low melting point agarose and immediately imaged using the confocal microscopy.
Collection of adult tissues and extraction of total RNA
Different adult tissues (preovulatory stage IVb follicles, ovary, testis, brain, eye, pectoral fin, kidney, liver, intestine, skin, gill, muscle, bone, and heart) were collected from 4-month-old AB wild type zebrafish. Samples were placed in 1.7 ml RNase-free microcentrifuge tubes (GeneMate) containing 200 μl RNAzol (Molecular Research Center, Inc., OH. Catalog: RN 190) and homogenized immediately. Total RNA was extracted from homogenized tissues according to the manufacturer’s protocol. For each sample, cDNAs were synthesized using 2 μg total RNA and a high-capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA, Catalog#4368814) following the manufacturer’s instructions.
PCR amplification of adamts9
PCR primers (forward: 5’-GCGGTACGCGTGGTAAAATC-3’; reverse: 5’- AGGCATGTGGACATAACGCA-3’) targeting 1181 bp of adamts9 3’-UTR were used for PCR amplification. PCR was performed using a Taq DNA polymerase (New England Biolabs, Ipswich, Massachusetts, USA, Catalog#0273) with initial denaturation at 95 °C for 2 minutes followed by 35 cycles of 30 seconds denaturation at 95 °C, 30 seconds annealing at 65 °C, and 60 seconds elongation at 68 °C. Zebrafish eukaryotic translation elongation factor 1 alpha 1a (eef1a1a) showed stable expression in different tissues and different developmental stages. Therefore, it was used as a housekeeping gene control. A set of PCR primers targeting 242 bp of the coding region of eef1a1a (forward: 5’-AGTGTTGCCTTCGTCCCAAT-3’; Reverse:5’-CACACGACCCACAGGTACAG-3’) was used for PCR amplification. PCR efficiency and authenticity of the amplicons were confirmed by gel electrophoresis analysis and Sanger DNA sequencing. Plasmid concentrations were quantified on a Nanodrop 2000 (Thermo Fisher Scientific, Waltham, Massachusetts, USA), serially diluted and used as DNA templates for generating the standard curves described in the following paragraph.
Real-time quantitative PCR (qPCR) amplification of adamts9
The levels of adamts9 transcripts in different tissues were determined by real-time quantitative PCR (qPCR) using SYBR green dye (Invitrogen) and a CFX Connect real-time thermal cycler (Bio-Rad Laboratories, Hercules, California, USA). The qPCR reaction was conducted with initial denaturation at 95 °C for 3 minutes, followed 45 cycles of 30 seconds denaturation at 95 °C, 30 seconds annealing at 65 °C, and 30 seconds extension at 72 °C using the specific primers (Forward: 5’- CTGTCTGCGCGGTGATTCTA -3’; Reverse: 5’- CTCTTGCAGGGGCGTGATTA -3’) and GoTaq G2 DNA polymerase (Promega, Madison, Wisconsin, USA). Each PCR mixture (15 μl) consisted of 7.795 μl DNase free water, 3 μl 5XGoTaq buffer, 1.5 μl 25 mM MgCl2, 0.3 μl 10 mM dNTP mix, 0.15 μl 10 mM forward or reverse primer, 2 μl 5X diluted cDNA, 0.03 μl 100X SYBR green dye (final concentration 0.2X), and 0.075 μl Taq. The transcript levels, expressed as absolute values (copies/μg total RNA), were determined using Ct values of samples and a standard curve generated from serial known concentrations of plasmid containing the target region of adamts9. PCR efficiency and authenticity of the PCR products were further confirmed by melting curve analyses, gel electrophoresis, and Sanger sequencing.
Whole mount mRNA in situ hybridization
An 808 bp fragment of the adamts9 coding sequence was amplified by RT-PCR from total RNA (7 dpf) using the SuperScript IV One-Step RT-PCR System (Invitrogen, Carlsbad, CA). The forward primer had a T7 RNA polymerase site on its 5’ end while the reverse included a T3 site to make sense and antisense probes, respectively (adamts9 sequence underlined - Forward: cacaTAATACGACTCACTATAGGGATACAGCGGCTCTGACCATG; Reverse: cacaCATTAACCCTCACTAAAGGGAACGACTCGGGTTTGGATGAGT). The purified PCR product was used as a template for DIG-labeled probe synthesis. Probe synthesis and mRNA ISH were performed essentially as previously described [40]. Specimens were fixed in freshly prepared 4%-PFA, permeabilized with proteinase K. Prehybridized specimens were incubated at 70 °C in 200 μl of hybridization solution containing 50 ng of either sense or antisense probe for adamts9. Samples were subsequently washed and reacted with a secondary anti-DIG antibody (Roche Diagnostics, Indianapolis, Indiana). The colorization reaction was allowed to proceed until strong signal was detected in the antisense probe. Samples were then washed with methanol to remove background staining and photographed.
Immunohistochemistry
Wild-type (AB line) or adamts9 mutant embryos, larvae, and adult organs were fixed in 10% neutral buffered formalin for four hours at room temperature. Fixed specimens were subsequently washed three times with PBS and stored in PBS at 4 °C until staining and analysis. Whole mount immunohistochemistry was conducted by permeabilizing specimens with PBS-1%Triton washes, three times for 15 minutes each; then 1 hour blocking of non-specific binding sites using a 5% normal goat serum (NGS) solution in PBS at room temperature. Specimens were then incubated overnight with a previously validated Adamts9 primary antibody [22] (Triple Point Biologics, RP5) diluted 1:500 in 1% BSA, or 1% BSA alone for negative control specimens. Following overnight incubation, specimens were washed with 1% PBS-Tween three times and then incubated with Alexa Fluor 488 conjugated secondary antibody (anti-rabbit AF488, Thermo Fisher, PA) in 1% BSA for 1 hour at room temperature. For co-staining of nuclei, specimens were incubated with 1μg/ml DAPI for thirty minutes at room temperature. For staining of F-actin in ovarian follicles and spermatogenic cysts, specimens were also incubated with Alexa Fluor 568 conjugated phalloidin (Cat. No. A12380, Thermo Fisher). Specimens were washed three times with PBS, and then returned to PBS at 4°C until imaging. For imaging, whole mount specimens were embedded in 1.2% low melting point agarose and imaged under a laser scanning confocal microscope (Zeiss LSM 800). The exact same laser, pinhole, and detector settings were used between primary antibody-stained specimens and secondary antibody only stained controls, or primary antibody-stained wild-type and knockout control larvae, respectively.
Statistical analysis
All results were presented as mean ± SEM. One-way ANOVA was used to analyze the effect of genotypes on expected outcomes of gene expression in different tissues. Statistical significance was set at p < 0.05. GraphPad Prism 8.0 (GraphPad Prism Software Inc., San Diego, CA, USA) was used to make histograms and to run statistical analyses.
Supplementary Material
Mov. 1. Confocal z-stack image of Tg(adamts9:EGFP) expression in zebrafish embryos at 10 hpf. Left movie shows EGFP signal from a representative transgenic embryo. Right movie shows no observable EGFP signal under same confocal setting from a sibling carrying no transgenic insert.
Mov. 2. Confocal z-stack image of Tg( adamts9:EGFP) expression in zebrafish embryos at 36 hpf.
Mov. 3. Confocal z-stack image of anti-Adamts9 immunostaining of immature follicles (stage III or IB).
Acknowledgements
This work was supported by NIH GM100461 and HD109785 to YZ.
Grants:
National Institute of General Medical Sciences NIH GM100461 and Eunice Kennedy Shriver National Institute of Child Health and Human Development HD109785
Reference
- 1.Blelloch R, Kimble J. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature. 1999; 399:586–590. DOI: 10.1038/21196. [DOI] [PubMed] [Google Scholar]
- 2.Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, Ramirez-Acuña JM, Perez-Romero BA, Guerrero-Rodriguez JF, Martinez-Avila N, Martinez-Fierro ML. The Roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020. 21(24):9739. doi: 10.3390/ijms21249739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41(2):271–90. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. The international journal of biochemistry & cell biology. 2004; 36:981–985. DOI: 10.1016/j.biocel.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Brunet FG, Fraser FW, Binder MJ, Smith AD, Kintakas C, Dancevic CM, Ward AC, McCulloch DR. The evolutionary conservation of the A disintegrin-like and metalloproteinase domain with thrombospondin-1 motif metzincins across vertebrate species and their expression in teleost zebrafish. BMC Ecology and Evolution. 2015; 15:22. DOI: 10.1186/s12862-015-0281-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelwick R, Desanlis I, Wheeler GN et al. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol 16, 113 (2015). 10.1186/s13059-015-0676-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artunc-Ulkumen B, Ulucay S, Pala HG, Cam S. Maternal serum ADAMTS-9 levels in gestational diabetes: a pilot study. Journal of Maternal-Fetal Medicine. 2016; 30(12):1442–1445. DOI: 10.1080/14767058.2016.1219717. [DOI] [PubMed] [Google Scholar]
- 8.Zillikens MC, et al. Large meta-analysis of genome-wide association studies identifies five loci for lean body mass. Nature Communications. 2017; 8:80. DOI: 10.1038/s41467-017-00031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulissen G, Rocks N, Gueders MM, Crahay C, Cataldo DD. Role of ADAM and ADAMTS metalloproteinases in airway diseases. Respiratory Research. 2009; 10:127. DOI: 10.1186/1465-9921-10-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang CY, Chanalaris A, Troeberg L. ADAMTS and ADAM metalloproteinases in osteoarthritis – looking beyond the ‘usual suspects’. Osteoarthritis Cartilage. 2017; 25:1000–1009. DOI: 10.1016/j.joca.2017.02.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mead TJ, Apte SS. ADAMTS proteins in human disorders. Matrix Biology. 2018; 71–72:225–239. DOI: 10.1016/j.matbio.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franceschini N, Giambartolomei C, De Vries PS, Finan C, Bis JC, Huntley RP, Lovering RC, Tajuddin SM, Winkler TW, Graff M, et al. GWAS and colocalization analyses implicate carotid intima-media thickness and carotid plaque loci in cardiovascular outcomes. Nature communications. 2018; 9:1–14. DOI: 10.1038/s41467-018-07340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suna G, Wojakowski W, Lynch M, Barallobre-Barreiro J, Mayr M. Extracellular matrix proteomics reveals interplay of aggrecan and aggrecanases in vascular remodeling of stented coronary arteries. Circulation. 2018; 137:166–183. DOI: 10.1161/CIRCULATIONAHA.116.023381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh M, Tyagi SC. Metalloproteinases as mediators of inflammation and the eyes: molecular genetic underpinnings governing ocular pathophysiology. International Journal of Ophthalmology. 2017; 10:1308–1318. DOI: 10.18240/ijo.2017.08.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clinical Science. 2017; 131(6):425–437. DOI: 10.1042/cs20160604 [DOI] [PubMed] [Google Scholar]
- 16.Clark ME, Kelner GS, Turbeville LA, Boyer A, Arden KC, Maki RA. ADAMTS9, a novel member of the ADAM-TS/Metallospondin gene family. 2000; Genomics. 67:343–350. DOI: 10.1006/geno.2000.624. [DOI] [PubMed] [Google Scholar]
- 17.Jungers KA, Le Goff, C, Somerville RPT, Apte SS. Adamts9 is widely expressed during mouse embryo development. Gene Expression Patterns. 2005; 5:609–617. DOI: 10.1016/j.modgep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Somerville RPT, Longpre J-M, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R., Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. Journal of Biological Chemistry. 2003; 278:9503–9513. DOI: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- 19.Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS, Geeting D, Nelson CM, Menick DR, Apte SS. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biology. 2010; 29:304–316. DOI: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desanlis I, Felstead HL, Edwards DR, Wheeler GN. ADAMTS9, a member of the ADAMTS family, in Xenopus development. Gene Expr Patterns. 2018;29:72–81. doi: 10.1016/j.gep.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray RS, Gonzalez R, Ackerman SD, Minowa R, Griest JF, Bayrak MN, Troutwine B, Canter S, Monk KR, Sepich DS, Solnica-Krezel L. Postembryonic screen for mutations affecting spine development in zebrafish. Dev Biol. 2021; 471:18–33. doi: 10.1016/j.ydbio.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter NJ, Roach ZA, Byrnes MM, Zhu Y. Adamts9 is necessary for ovarian development in zebrafish. General and Comparative Endocrinology. 2019; 277:130–140. DOI: 10.1016/j.ygen.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Carver JJ, He Y, Zhu Y. Delay in primordial germ cell migration in adamts9 knockout zebrafish. Scientific Reports. 2012; 11:8545. DOI: 10.1038/s41598-021-88024-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu DT, Brewer MS, Chen S, Hong W, Zhu Y. Transcriptomic signatures for ovulation in vertebrates. General and Comparative Endocrinology. 2017; 247:74–86. DOI: 10.1016/j.ygcen.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu DT, Carter NJ, Wu XJ, Hong WS, Chen SX, Zhu Y. Progestin and nuclear progestin receptor are essential for upregulation of metalloproteinase in zebrafish preovulatory follicles. Frontiers in Endocrinology. 2018; 9:517. DOI: 10.3389/fendo.2018.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu DT, Hong WS, Chen SX, Zhu Y. Upregulation of adamts9 by gonadotropin in preovulatory follicles of zebrafish. Mol Cell Endocrinol. 2020; 499:110608. DOI: 10.1016/j.mce.2019.110608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubail J, Aramaki-Hattori N, Bader HL, Nelson CM, Katebi N, Matuska B, Olsen BR, Apte SS. A new Adamts9 conditional mouse allele identifies its non-redundant role in interdigital web regression. Genesis. 2014; 52:702–12. DOI: 10.1002/dvg.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kassen SC, Thummel R, Burket CT, Campochiaro LA, Harding MJ, Hyde DR. The Tg (ccnb1: EGFP) transgenic zebrafish line labels proliferating cells during retinal development and regeneration. Molecular vision. 2008; 14:951–963. [PMC free article] [PubMed] [Google Scholar]
- 29.Sanger JW, Wang J, Holloway B, Du A, Sanger JM. Myofibrillogenesis in skeletal muscle cells in zebrafish. Cell motility and the cytoskeleton. 2009; 66(8):556–566. DOI: 10.1002/cm.20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yaffe E, Farkash-Amar S, Polten A, Yakhini Z, Tanay A, Simon I. Comparative analysis of DNA replication timing reveals conserved large-scale chromosomal architecture. PloS Genetics. 2010; 6:e1001011. DOI: 10.1371/journal.pgen.1001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi YJ, Halbritter J, Braun DA, Schueler M, Schapiro D, Rim JH, Nandadasa S, Choi WI, Widmeier E, Shril S, Körber F, Sethi SK, Lifton RP, Beck BB, Apte SS, Gee HY, Hildebrandt F. Mutations of ADAMTS9 Cause Nephronophthisis-Related Ciliopathy. American Journal of Human Genetics. 2019; 104(1):45–54. DOI: 10.1016/j.ajhg.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. Journal of the American Society of Nephrology. 2007; 18(6):1855–1871. DOI: 10.1681/asn.2006121344. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi H Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bulletin of the Faculty of Fisheries Hokkaido University. 1977; 28(2):57–65. [Google Scholar]
- 34.Levi L, Pekarski I, Gutman E, Fortina P, Hyslop T, Biran J, Levavi-Sivan B, Lubzens E. Revealing genes associated with vitellogenesis in the liver of the zebrafish (Danio rerio) by transcriptome profiling. BMC Genomics. 2009; 10:141. DOI: 10.1186/1471-2164-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hara A, Hiramatsu N, Fujita T. Vitellogenesis and choriogenesis in fishes. Fish Sci. 2016; 82, 187–202. 10.1007/s12562-015-0957-5. [DOI] [Google Scholar]
- 36.Li H, Zhang S. Functions of Vitellogenin in Eggs. Results Probl Cell Differ. 2017;63:389–401. doi: 10.1007/978-3-319-60855-6_17. [DOI] [PubMed] [Google Scholar]
- 37.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236(11):3088–99. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- 38.Kurita R, Sagara H, Aoki Y, Link BA, Arai K-i, Watanabe S. Suppression of lens growth by αA-crystallin promoter-driven expression of diphtheria toxin results in disruption of retinal cell organization in zebrafish. Developmental biology. 2003; 255:113–127. DOI: 10.1016/s0012-1606(02)00079-9. [DOI] [PubMed] [Google Scholar]
- 39.Berger J, Currie PD. 503unc, a small and muscle-specific zebrafish promoter. Genesis. 2013;51(6):443–7. doi: 10.1002/dvg.22385. [DOI] [PubMed] [Google Scholar]
- 40.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mov. 1. Confocal z-stack image of Tg(adamts9:EGFP) expression in zebrafish embryos at 10 hpf. Left movie shows EGFP signal from a representative transgenic embryo. Right movie shows no observable EGFP signal under same confocal setting from a sibling carrying no transgenic insert.
Mov. 2. Confocal z-stack image of Tg( adamts9:EGFP) expression in zebrafish embryos at 36 hpf.
Mov. 3. Confocal z-stack image of anti-Adamts9 immunostaining of immature follicles (stage III or IB).



