Abstract
Green synthesis of NPs is preferred due to its eco-friendly procedures and non-toxic end products. However, unintentional release of NPs can lead to environmental pollution affecting living organisms including plants. NPs accumulation in soil can affect the agricultural sustainability and crop production. In this context, we report the morphological and biochemical response of spinach nanoprimed with MgO–NPs at concentrations, 10, 50, 100, and 150 µg/ml. Nanopriming reduced the spinach root length by 14–26%, as a result a reduction of 20–74% in the length of spinach shoots was observed. The decreased spinach shoot length inhibited the chlorophyll accumulation by 21–55%, thus reducing the accumulation of carbohydrates and yield by 46 and 49%, respectively. The reduced utilization of the total absorbed light further enhanced ROS generation and oxidative stress by 32%, thus significantly altering their antioxidant system. Additionally, a significant variation in the accumulation of flavonoid pathway downstream metabolites myricitin, rutin, kaempferol-3 glycoside, and quercitin was also revealed on MgO-NPs nanopriming. Additionally, NPs enhanced the protein levels of spinach probably as an osmoprotectant to regulate the oxidative stress. However, increased protein precipitable tannins and enhanced oxidative stress reduced the protein digestibility and solubility. Overall, MgO-NPs mediated oxidative stress negatively affected the growth, development, and yield of spinach in fields in a concentration dependent manner.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-023-01391-9.
Keywords: Nanoparticles, Leafy Vegetable, Oxidative Stress, Phytotoxicity, Flavonoid Biosynthesis, Yield
Introduction
Agriculture is the most significant source of food for humans and economic growth for various countries around the globe. The continuously increasing world population has raised concerns of food security for several countries, especially the developing ones (Kihara et al. 2016; Praburaj et al. 2018). However, more challenging is to attain this enhancement without inducing any destructive stress/ pressure on the environment (Abebe et al. 2022). Modern agriculture relies on chemical fertilizers for plant growth and yield improvement (Kadigi et al. 2020). However, excessive chemical fertilizers hardens the soil, influences nutrient composition of the soil, reduces fertility, and bioaccumulates in the soil, thus polluting/ affecting the ecosystem (Cole et al. 2016; Pahalvi et al. 2021). The phosphate fertilizers have reportedly, led to biomagnification of heavy metals in the soil and food chain on excess usage. This has reduced the nutrient accessibility to plants, hence limiting the growth and yield of plants (Abebe et al. 2022). Hence, chemical fertilizers can meet the nutritional requirement of the growing population, but with an adverse effect on the environment and ecosystem (Babu et al. 2022). Therefore, an alternate/novel strategy to promote agricultural sustainability is required, without posing any adverse effect and threat to environment, climate, and ecosystem. Nanotechnology can potentially transform different areas including human health, diagnostics, medicine, and crop production (Savithramma et al. 2012; Ijaz et al. 2020). The nanometric range of NPs allows them to conveniently enter and stay inside the living systems (Kah et al. 2019; Nikolova et al. 2020). Therefore, NPs are considered potential replacement for chemical fertilizers to promote agricultural sustainability (Shang et al. 2019; Kumar et al. 2013, 2021).
Macronutrient based-NPs have both positive and negative effect on the plants (Tolaymat et al. 2017; Kumar et al. 2021). The 200–300 mg/L of ZnO-NPs showed reduced stem elongation and chlorophyll accumulation of Arabidopsis thaliana by more than 50% (Wang et al. 2016). The 100, 300, and 500 ppm Zn-NPs likewise induced oxidative stress in potatoes (Raigond et al. 2017). Likewise, ZnO-NPs at 1, 2, and 4 mg/L concentrations reduced the root cell viability and enhanced oxidative free radicals in exposed barley seedlings (Plaksenkova et al. 2020). The 1000 mg/L of CuO-NPs similarly, induced phytotoxicity on Brassica pekinensis in terms of affected shoot–root ratio and vitality index (Zhang et al. 2018; Wang et al. 2020). However, reports documenting the improvement in plant growth parameters on NPs exposure are also present. MnFe2O4 NPs at concentration 250 mg/L increased the growth parameters of barley on exposure (Tombuloglu et al. 2018). Similarly, Cu-NPs enhanced the biomass and flavonoid content to induce drought tolerance in maize at concentration 4.444 mg/L (Nguyen et al. 2022). Zinc oxide NPs (25, 50, 75, and 100 mg/L) and iron NPs (5, 10, 15, and 20 mg/L), likewise alleviated cadmium toxicity in wheat by improving plant growth (Rizwan et al. 2019). Similarly, reports have documented magnesium oxide NPs (MgO-NPs) mediated alteration in seedling growth, morphology, and vigor at varying concentrations such as 50–250 µg/ml, 0.5 µg/ml, 5 g, and 10, 25, 50, and 100 µg/ml (Rathore and Tarafdar 2015; Jhansi et al. 2017; Cai et al. 2018a; Cai et al. 2018b; Rani et al. 2020). MgO-NPs enhanced the germination and seedling growth of peanuts and mungbean at concentrations 0.5 µg/ml, 5 g, and 10, 25, 50, and 100 µg/ml, respectively (Jhansi et al. 2017; Anand et al. 2020; Rani et al. 2020). These NPs thus have multifarious applications in agriculture, disease diagnostics, drug delivery, medicine, biosensing, dye degradation, pollutant detection, and removal (Kumar et al. 2018). These have also been reported as potential food additives, food supplements, and seed protectants (Fernandes et al. 2020). Therefore, it is important to evaluate the positive/ negative effects of MgO-NPs on crop plants before scaling up their potential applications to high-throughput industrial levels.
The biological synthesis and characterization of MgO-NPs have earlier been validated and reported using clove extract (Kumar et al. 2018). We have earlier reported alteration in the morphological and biochemical parameters of legumes black gram, lentils, horse gram, and mungbean on in vitro MgO-NPs exposure (Sharma et al. 2021a, b, c, 2022). The plants mungbean, lentils, and black gram were negatively affected by NPs exposure, however, NPs promoted the growth parameters of horse gram. Hence, understanding the impact of any NPs on a particular plant type cannot be extrapolated to other crop types or other types of NPs. Same type of NPs can have different responses to different plant types. Furthermore, consistent NPs usage can cause accidental release into the air, soil, and water leading to cross-contamination (Nguyen et al. 2016; Mall et al. 2017). NPs can enter into soil by various natural and anthropogenic activities. Natural activities like atmospheric precipitation, absorption of gaseous compounds in soil, and sedimentation of aerosols can lead to nano-accumulation in soil (Gladkova and Terekhova 2013). Release of untreated wastewater, industrial concentrated sludge or effluents and landfills causes NPs release into soil (Rajput et al. 2019, 2020). Further, nano-products as in paints, coatings, cosmetics can easily be discharged into soil (Ahmed et al. 2021). Once inside soil, NPs undergo transformations, and are supposed to sustainably remain in soil for longer durations. Therefore, it is necessary to evaluate the influence of NPs on plant growth after germination either in vitro or in fields. In this context, we performed MgO-NPs nanopriming on spinach seeds to evaluate the plant response to NPs exposure.
Spinacia oleracea (spinach) is a nutrient-enriched annual green leafy vegetable consumed in cooked and raw forms (Morelock and Correll 2008; Roberts and Moreau 2016). The leaves consist of 91.4% water, 2.9% protein, 3.6% carbohydrates, and 0.4% fats. Compared to other green vegetables, spinach is rich in bioactive phytochemicals; polyphenolics, carotenoids, and vitamins (Hanif et al. 2006; Bunea et al. 2008). The bioactive polyphenols are responsible for the antioxidant activity of spinach to protect them against any stress-induced oxidative damage (Sun et al. 2018). Further, spinach-derived components have shown potential to alleviate and reduce instances of cancer and various metabolic syndromes in humans (Roberts and Moreau 2016).
The phenolic content of spinach, however significantly varies in response to cultivation/ agricultural conditions (Howard et al. 2002). Enhanced water levels due to increased precipitation reportedly increased the total phenolics of spinach (Buckley et al. 2020). A similar enhancement in the phenolic content of spinach in deficiency of nitrogen, potassium, and phosphorus has been reported (Xu and Mou 2016). Likewise, spinach yield is significantly reduced by various individual and multiple abiotic stresses (Ors and Suarez 2017; Nishihara et al. 2001; Xu and Leskovar 2015). The reduction in spinach yield was more than 60% at water stress levels at and beyond − 230 kPa. Similar significant reductions in the spinach yield were reported in presence of 0.3, 1.5, 3.0, 4.5, and 6.0 dS/m of water salinity (Ors and Suarez 2017; Yavuz et al. 2022). Furthermore, bioaccumulation of chemical fertilizers, herbicides, and pesticides in the soil causes environmental pollution, affecting the agricultural ecosystem and hence, crop productivity (Xu and Leskovar 2015; Kramer et al. 2006). Hence focus is on the development of an alternative biodegradable plant growth promoting agents that can support plant growth and yield during environmental variations (Shang et al. 2019).
MgO-NPs can have a significant influence on crop productivity and agricultural sustainability. Therefore, the present study evaluated the effect of MgO-NPs nanopriming on the growth and yield of spinach grown in agricultural fields. The influence of MgO-NPs exposure on the morphology, biochemical parameters, and yield of spinach was estimated. Further, the effect of nanopriming on antioxidant system of spinach was evaluated to check for any stress induced by NPs. Present study thus assessed the potential of MgO-NPs as a nanofertilizer to support growth and yield of spinach.
Materials and methods
Experimental plan
MgO-NPs were biologically synthesized through clove (Syzygium aromaticum) extract using a well characterized published protocol (Kumar et al. 2018). The MgO-NPs suspension used for nanopriming was prepared with distilled water. The concentrations used for nanopriming were 10, 50, 100, and 150 µg/ml (Sharma et al. 2021a, b, c; Kumar et al. 2018). The fresh seeds were immersed overnight in MgO-NPs suspension and also in distilled water for control. Seeds were nanoprimed once with 10, 50, 100, and 150 µg/ml MgO-NPs before sowing. The seeds were sown in the DAV University Agricultural Experimental farm for 1 month. The soil was sandy clay loam (sand 73%, silt 11%, and clay 16%) with pH 7.3. The soil properties of experimental site were 7.9 g/kg oxidizable organic carbon, 220 kg/ha available nitrogen, 27 kg/ha available potassium, and 23 kg/ha available phosphorus. The plants were regularly watered and deweeding was done on regular basis. The experiment was designed in randomized block design and maintained in three replicates to eliminate any effect of unwanted external factors during the experiment. The experiment was setup and maintained in isolated university field trial designated area. Further, the purpose of study was to compare the effect of nanopriming with respect to non-primed spinach plants i.e. control plants. Control and treated seeds were grown in the same field under exactly the same soil conditions in randomized fashion without any further addition of chemical fertilizers to the soil. The spinach seedlings treated with varying NPs concentrations were labeled as NP-10, NP-50, NP-100, and NP-150, respectively. The one month old spinach plants were morphologically characterized and used for further experiments.
Photosynthetic pigment and carbohydrate estimation of spinach
The chlorophyll, carotenoid, and carbohydrate levels of control and nanoprimed spinach were quantified. The following quantification of differentially treated spinach was determined using the method described earlier (Sharma et al. 2020; Guleria et al. 2014; Chang et al. 2002). The one month old spinach was extracted with 80% methanol and the absorbance of extract was recorded at 663 and 645 nm for chlorophyll quantification. The absorbance of the methanolic extract was measured at 480 and 510 nm for carotenoids estimation.
For carbohydrates quantification, the methanolic extract of spinach was added to 5% aqueous phenol, concentrated H2SO4 and allowed to incubate at room temperature. The incubated reaction mix was then measured at 490 nm for the quantification of carbohydrates.
Following formulae were used to quantify the photosynthetic pigments and carbohydrates (Zhang and Blumwald 2001; Chang et al. 2002; Guleria et al. 2014; Sharma et al. 2021a, b, c);
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
Estimation of polyphenols and antioxidant potential of spinach
A 1 g of oven-dried spinach shoot and root samples were extracted with 30 ml methanol and kept at 4 ºC for 48 h followed by filtration. The filtrates were vacuum dried using Genevac™ miVac, Fisher Scientific at 45ºC. The vacuum-dried methanolic extracts were reconstituted with methanol to a final solution of 20 mg/ml and stored. The isolated extracts from the shoot and root sections were used for the quantification of phenolics, total flavonoids, and total anthocyanins by following the methods described earlier (Giusti and Wrolstad 2001; Yusof et al. 2018). The antioxidant potential of the shoot and root sections of spinach was also evaluated using Ferric Reducing Antioxidant Power (FRAP) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity Assay by following the standard procedures (Yusof et al. 2018; Benzie and Strain 1999). The extract was further used for the HPLC quantification of selected flavonoids, myricitin, kaempferol 3-glycoside, quercitin, and rutin (Seo et al. 2016).
The activities of enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and lipid peroxidation levels were also quantified from the leaves of control and MgO-NPs treated spinach. The variously treated spinach leaves were extracted with phosphate buffer and EDTA for SOD extraction. The extract was incubated with nitroblue tetrazolium, and riboflavin, and the absorbance of reaction mix was measured at 560 nm. The CAT crude extract was isolated using phosphate buffer and added to reaction mix containing hydrogen peroxide. The absorbance of the reaction mix was recorded at 240 nm to estimate the enzyme activity of CAT. The spinach leaves were extracted with phosphate buffer to obtain APX crude extract. The extract was added to the reaction assay containing ascorbic acid and hydrogen peroxide. The absorbance of assay was recorded at 290 nm and activity was estimated. Lipid peroxidation was quantified in terms of levels of malondialdehyde (MDA). TCA extract of spinach leaves was mixed with thiobarbituric acid-TCA solution and measured at 532 and 600 nm using UV–Visible spectrophotometer to quantify MDA (Sharma et al. 2021a; Sharma et al. 2021b; Sharma et al. 2020; Giannopolitis and Ries 1977; Shimizu et al. 1984; Nakano and Asada 1981; Heath and Packer 1968).
Estimation of protein
The protein was isolated from spinach leaves via TCA extraction buffer and quantified (Sharma et al. 2020). The in vitro protein digestibility and solubility were also evaluated using the isolated spinach protein. The protein digestibility was estimated by digesting the isolated protein with trypsin followed by Bradford protein quantification (Sharma et al. 2020; Elkhalil et al. 2001). Likewise, the pH of isolated protein suspensions was maintained from pH 2.0 to 12.0. The protein of each protein suspension was then quantified to estimate protein solubility (Sharma et al. 2020; Bera and Mukherjee 1989).
Estimation of protein precipitable tannins
The tannins extracted from spinach leaves were incubated with 2 ml BSA, centrifuged, and resuspended in 1% (w/v) SDS (Sharma et al. 2020). SDS-triethanolamine and ferric chloride was added to the already prepared SDS suspension solution. The prepared reaction mixture was analyzed to quantify the protein precipitable tannins at 510 nm and expressed as a part percentage of total phenolics present in spinach.
Statistical analysis
The study was carried out in a randomized block design with three replications. Data in figures and tables are represented as mean ± standard deviation of three biological replicates. The data obtained in this study were subjected to statistical analysis using analysis of variance (ANOVA) and the mean values were compared using Duncan’s Multiple Range Test (DMRT). Pearson correlation analysis was also conducted to determine the relationship between the MgO-NPs treatment and the various parameters evaluated from spinach. The conclusion was drawn for a strong and significant relationship between evaluated parameters if the calculated correlation coefficient was above 0.878.
Results and discussion
Nanotechnology has been considered to potentially influence agriculture. Earlier, we reported the altered morphology and biochemical composition of legume crops horse gram, black gram, lentils, and mungbean on in vitro MgO-NPs exposure (Sharma et al. 2021a, b, c, 2022). However, as the same type of NPs induce differential responses in plants, it becomes necessary to reveal other plant responses including legumes to MgO-NPs exposure during field or in vitro lab exposures. In this context, we have performed MgO-NPs nanopriming of spinach seeds before sowing in fields. A considerable reduction in the spinach morphology, carbohydrate content, protein digestibility, and yield on NPs treatment than control was evident. Likewise, nanopriming mediated variation in the polyphenol level and free radical scavenging potential of the shoot and root sections compared to control spinach were observed.
MgO-NPs altered spinach morphology
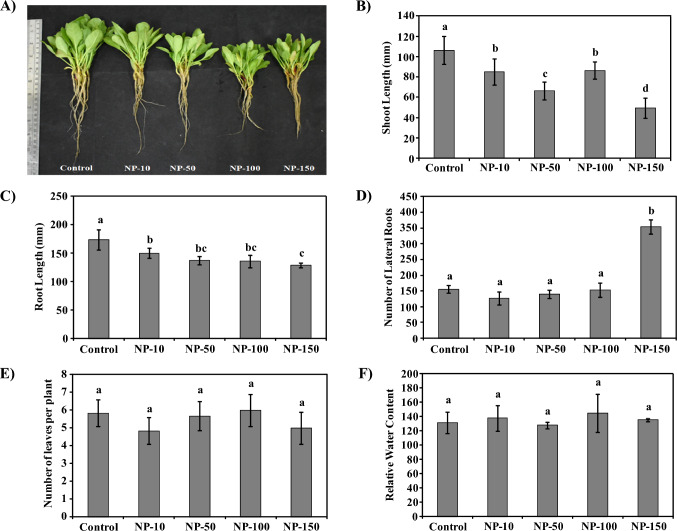
The spinach nanoprimed with 10, 50, 100, and 150 µg/ml MgO-NPs designated as NP-10, NP-50, NP-100, and NP-150, respectively were sown in the Agricultural Experimental Farm, DAV University Jalandhar, Punjab (India). One-month-old nanoprimed spinach showed significant reduction in the morphology compared to control (Fig. 1a). The stem length was reduced by 20, 38, 19, and 74% in NP-10, NP-50, NP-100, and NP-150 spinach than control, respectively (Fig. 1b). Similarly, root length was decreased by 14, 21, 22, and 26% in MgO-NPs nanoprimed spinach compared to control (Fig. 1c). The lateral root count was non-significantly altered on nanopriming except in N-150 spinach on 150 µg/ml exposure where a considerable increment in lateral root count than control was observed (Fig. 1d). Hence, nanopriming with MgO-NPs significantly affected the shoot and root length of spinach without any observed variation in leaf count per plant and relative water content (Fig. 1e, f).
Fig. 1.
Influence of MgO-NPs on spinach morphological parameters. (A) Images showing effect of MgO-NPs priming on the stem and root length of the spinach. Graphical representation of data showing MgO-NPs dependent reduction in the stem length (B), and root length (C) of spinach in comparison to control. Nanopriming mediated reduction in root length might have regulated the nutrient uptake of spinach leading to inhibition of shoot elongation. The bar diagrams representing the number of lateral roots (D), number of leaves per plant (E), and relative water content (F) in MgO-NPs treated and control spinach plants. Nanopriming showed non-significant alteration in the number of leaves per plant and relative water content of spinach. Data included is mean ± standard deviation of three independent measurements used in this study. Duncan’s multiple range test (DMRT) was applied and different superscript letters on the bars are significantly different at p ≤ 0.05
Relative water content and leaf count per plant are parameters, in addition, to shoot and root length that determines the plant growth traits during any treatment. Earlier studies have reported alterations in relative water content and leaf count per plant as an indicator of NPs mediated growth promotion and/or inhibition (Surendar et al. 2013; Çekiç et al. 2017; Sadak 2019). However, the shoot–root length and lateral root count were significantly reduced on nanopriming in this study, thus indicating that MgO-NPs influenced spinach growth.
Various other studies have documented reduction in the shoot and root length of plants on NPs exposure (Dimkpa et al. 2018; Zhang et al. 2018; Zafar et al. 2019; Singh and Kumar 2018; Dong et al. 2021; González et al. 2020; Ma et al. 2010; Kumar et al. 2013). NPs-mediated reduced morphological parameters have also been reported in wheat, Brassica nigra, Raphanus sativus, maize, and barley (Dimkpa et al. 2018; Zhang et al. 2018; Zafar et al. 2019; Singh and Kumar 2018; Dong et al. 2021; González et al. 2020). Root length reduction indicates NPs mediated toxicity in plants (Ma et al. 2010; Kumar et al. 2013). Hence, reduced shoot and root length of spinach on MgO-NPs nanopriming indicated possible phytotoxicity to spinach. In the present study, MgO-NPs reduced the root length of spinach than control, possibly affecting their nutrient uptake. This could lead to reduced shoot length in MgO-NPs exposed spinach compared to control.
Reduced photosynthetic pigments on MgO-NPs priming decreased carbohydrate content of spinach
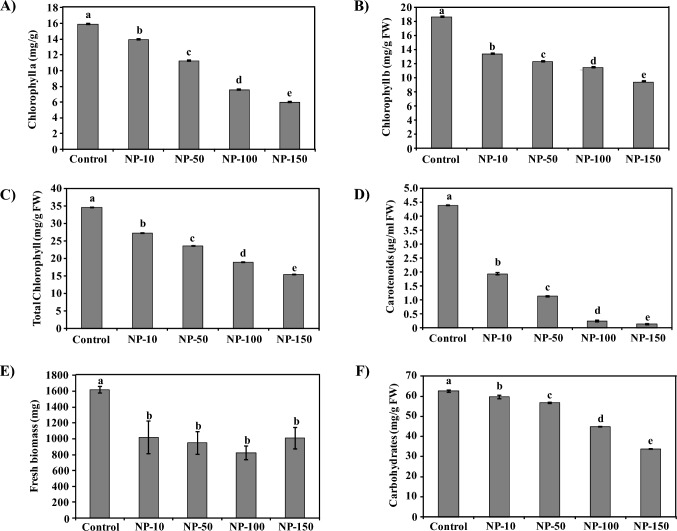
Chlorophylls and carotenoids are plant pigments essentially required for photosynthesis. Carotenoids absorb light from the spectrum and transfer the absorbed energy to chlorophylls. This transfer of energy expands the wavelength of light in a range supporting photosynthesis (Hashimoto et al. 2016). Nanopriming with MgO-NPs significantly decreased the level of chlorophylls and carotenoids compared to control (Fig. 2). The reduction of photosynthetic pigments further reduced the biomass and carbohydrate accumulation of plants (Fig. 2). The chlorophyll a content in spinach was decreased by 12, 29, 52, and 62% in NP-10, NP-50, NP-100, and NP-150 treated plants than control, respectively (Fig. 2a). Similarly, the reduction in chlorophyll b was recorded to be 28, 34, 39, and 49% compared to control in MgO-NPs exposed spinach (Fig. 2b). Likewise, 21–55% reduction in total chlorophyll content was observed on MgO-NPs nanopriming (Fig. 2c). Nanopriming was observed to similarly decrease carotenoid accumulation. A drop of 56, 74, 94, and 97% in carotenoid content of spinach on exposure to MgO-NPs compared to control was evident, respectively (Fig. 2d).
Fig. 2.
Influence of MgO-NPs exposure on the photosynthetic pigments, fresh biomass, and carbohydrates content in spinach. Graphs showing negative impact of NPs exposure on the chlorophyll a (A), chlorophyll b (B), total chlorophyll (C), carotenoids (D), fresh biomass (E), and carbohydrates (F) accumulation in spinach. MgO-NPs treatment significantly reduced the shoot length of spinach, thus leading to reduction in the amount of photosynthetic pigments. Since the amount of photosynthetic pigments are directly related to the accumulation of fresh biomass and carbohydrates. Therefore, nanopriming considerably reduced the content of biomass as well as carbohydrates. Individual bar represents the mean of three independent measurements. DMRT was applied and different superscript letters on the bars are significantly different at p ≤ 0.05
Likewise, MgO-NPs reduced the biomass and carbohydrates in spinach (Fig. 2e, f). The biomass accumulation was decreased by 37, 41, 49, and 38% in spinach exposed to MgO-NPs compared to control (Fig. 2e). The carbohydrate content was reduced by 5–46% in nanoprimed spinach (Fig. 2f). Hence, MgO-NPs significantly decreased the photosynthetic pigments that led to reduced accumulation of biomass and carbohydrates in spinach.
The level of photosynthetic pigments is directly proportional to the biomass and carbohydrate accumulation of plants (Sharma et al. 2021a, b, c; Guleria et al. 2014). In vitro MgO-NPs priming decreased the biomass and carbohydrate content because of chlorophyll reduction in legumes (Sharma et al. 2021b, c, 2022). Similarly, superparamagnetic iron oxide NPs reduced the chlorophyll and biomass accumulation of summer squash (Tombuloglu et al. 2019). CuO-NPs have reportedly, decreased the chlorophyll and carbohydrate content in Arabidopsis thaliana, Solanum lycopersicum, coriander, mungbean, and Brassica oleracea (Singh et al. 2017; Nair et al. 2014; AlQuraidi et al. 2019). NPs-mediated reduction in chlorophyll and biomass accumulation of Arabidopsis thaliana, oak leaf lettuce, and Zea mays has also been documented (Dong et al. 2021; Ma et al. 2013; Jurkow et al. 2020). MgO-NPs priming mediated reduced chlorophyll and carotenoids probably, decreased the utilization of absorbed light, thus negatively affecting the accumulation of biomass and carbohydrates in the present study. This inhibits root growth affecting nutrition absorption and retards plant growth (Farhat et al. 2016; Yang et al. 2020). Hence, spinach treated with MgO-NPs showed symptoms similar to phytotoxicity.
Altered accumulation of secondary metabolites and antioxidant potential in shoots and roots of nanoprimed spinach
Polyphenols including phenolics, flavonoids, and anthocyanins are the downstream metabolites of flavonoid biosynthetic pathway (Fig. 3). These secondary metabolites induce and maintain the antioxidant potential of the plants. Hence, we evaluated the effect of MgO-NPs nanopriming on the polyphenolics content and the resultant antioxidant potential of spinach in their shoot and root sections, respectively.
Fig. 3.
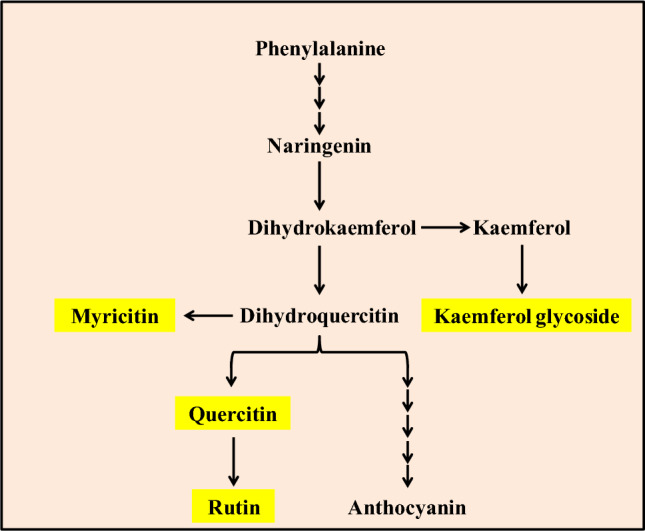
A brief outline of flavonoid biosynthesis metabolism. Phenylalanine metabolizes in several steps to synthesize naringenin. Naringenin diverts the phenylpropanoid pathway towards flavonoid biosynthesis. Among various flavonoids, the yellow highlighted metabolites, myricitn, quercitin, rutin, and kaemferol glycoside are downstream flavonoids. These highlighted flavonoid derivatives were quantified in spinach nanoprimed with MgO-NPs compared to control to assess the influence of nanopriming on flavonoid metabolism
Increased accumulation of secondary metabolites in nanoprimed spinach shoots
The MgO-NPs nanopriming was found to variously alter the accumulation of secondary metabolites like phenolics, flavonoids, and anthocyanins in shoot and root sections of spinach (Table 1). In spinach shoots, the phenolics, flavonoids, and anthocyanins content was increased in NP-10, NP-50, and NP-100 spinach followed by a reduction in NP-150 plants compared to control, with the lowest content in NP-150 spinach (Table 1). The total phenolics content was quantified as 45.5 mg/g DW in control shoots and 65.2, 111.2, 55.6, and 24.9 mg/g DW in shoots of NP-10 to NP-150 treated spinach, respectively (Table 1). Hence, except for 150 µg/ml MgO-NPs treated spinach shoots, other NPs treated spinach showed enhanced accumulation of total phenolics compared to control (Table 1).
Table 1.
Effect of MgO-NPs treatments on total phenolic, total flavonoid, total anthocyanin content and antioxidant potential in methanolic extracts of spinach shoots and roots
| Plants' parts | Treatment | TPC (mg/g DW) | TFC (mg/g DW) | TAC (mg/g DW) | FRAP (mg of FE/g DE) | DPPH IC50 (mg/ml) |
|---|---|---|---|---|---|---|
| Shoot | Control | 45.411 ± 0.69b | 121.107 ± 0.81c | 98.001 ± 4.99d | 7.902 ± 0.11a | 18.362 ± 0.44e |
| NP-10 | 65.296 ± 0.37d | 170.520 ± 0.79d | 37.044 ± 5.29b | 9.146 ± 0.12b | 13.750 ± 0.29d | |
| NP-50 | 111.210 ± 0.96e | 200.469 ± 1.04e | 71.842 ± 2.76c | 9.877 ± 0.14c | 11.107 ± 0.03b | |
| NP-100 | 55.661 ± 0.17c | 110.833 ± 0.61b | 30.699 ± 1.81ab | 7.999 ± 0.17a | 12.857 ± 0.24c | |
| NP-150 | 24.982 ± 0.13a | 65.548 ± 0.21a | 23.777 ± 0.95a | 7.751 ± 0.11a | 10.054 ± 0.09a | |
| Root | Control | 86.434 ± 1.21d | 157.290 ± 0.63d | ND | 7.766 ± 0.15c | 20.976 ± 0.72d |
| NP-10 | 100.484 ± 0.96e | 180.409 ± 0.16e | ND | 6.542 ± 0.09b | 14.693 ± 0.25c | |
| NP-50 | 68.046 ± 0.88c | 126.728 ± 0.20c | ND | 6.276 ± 0.09b | 11.417 ± 0.09a | |
| NP-100 | 17.894 ± 0.25a | 43.336 ± 0.07a | ND | 6.595 ± 0.09b | 12.834 ± 0.26b | |
| NP-150 | 28.690 ± 0.70b | 83.363 ± 0.14b | ND | 6.045 ± 0.05a | 15.558 ± 0.09c |
In shoots, FRAP and DPPH IC50 values indicated the enhanced antioxidant potential of nanoprimed spinach than control due to enhanced accumulation of phenolics, flavonoids, and anthocyanins. In roots, DPPH IC50 values indicated an enhanced antioxidant potential of spinach roots but poor reduction potential to convert ferric iron to ferrous iron
Note: Different superscript letters in the Table are significantly different at p ≤ 0.05 according to Duncan’s multiple range test (DMRT). TPC Total phenolic content, TFC Total flavonoid content, TAC Total anthocyanin content, ND not detected
An increase of 41 and 66% was observed in the flavonoid content of NP-10 and NP-50 treated spinach shoots than control. However, flavonoid content was reduced by 8 and 46% in NP-100 and NP-150 spinach shoots. Likewise, NPs priming considerably decreased the anthocyanins in spinach shoots than control. The percent reduction compared to control was found to be 62, 27, 67, and 76% in spinach shoots nanoprimed with MgO-NPs, respectively (Table 1).
FRAP assay of spinach shoots indicated the enhanced antioxidant potential of NP-10 and NP-50 spinach compared to control. The FRAP value of control and 10–150 µg/ml nanoprimed spinach shoots were estimated to be 7.9, 9.1, 9.9, 7.9, and 7.8 mg of FE/g of DE, respectively. An increase of 16 and 25% in the FRAP value of NP-10 and NP-50 spinach shoots indicated an increase in their antioxidant potential than control shoots. However, a non-significant variation in the antioxidant potential of control, NP-100, and NP-150 spinach shoots were observed (Table 1). Similarly in the DPPH IC50 assay, the MgO-NPs treated spinach shoots were observed to have significantly lower values compared to the control. The lower IC50 values denote higher DPPH radical scavenging potential. The DPPH IC50 values were estimated to be 18.4, 13.8, 11.1, 12.9, and 10.1 mg/ml in control and spinach nanoprimed with MgO-NPs, respectively (Table 1). A significant reduction of 25, 40, 30, and 45% in DPPH IC50 indicated a considerably enhanced antioxidant potential of nanoprimed spinach compared to control, respectively. Similarly, Pearson’s correlation analysis indicated a strong inverse relationship of MgO-NPs with anthocyanin accumulation and DPPH IC50 (Supplementary Table S1). Flavonoids and polyphenols are known to contribute to the antioxidant potential of plants (Shen et al. 2022). Hence, MgO-NPs mediated accumulation of flavonoids was responsible for the observed variation in the antioxidant potential of spinach. Further, MgO-NPs can potentially alter the accumulation of flavonoid biosynthesis pathway downstream metabolites.
Reduced accumulation in nanoprimed spinach roots
The total phenolic and flavonoid content was variously altered in MgO-NPs nanoprimed spinach roots than in control as was observed in the shoot sections of nanoprimed spinach (Table 2). However, anthocyanins were not detected in the roots. The roots of NP-10 spinach showed 16 and 15% enhanced accumulation of phenolics and flavonoids compared to control, respectively. On the contrary, phenolic and flavonoid accumulation was reduced in NP-100 and NP-150 spinach roots (Table 1). The phenolic accumulation was reduced by 21, 79, and 67% in the roots nanoprimed with NP-50, NP-100, and NP-150 than control, respectively. Similarly, the flavonoid content was decreased by 19, 72, and 47% in NPs exposed spinach roots compared to control (Table 1). MgO-NPs mediated reduced morphological parameters of spinach indicated stress and phytotoxicity. Therefore, increased stress with an increase in MgO-NPs concentration led to reduction of phenolic and flavonoid content on nanopriming than control spinach.
Table 2.
Quantification of flavonoids from the shoot and root of control and NPs treated spinach (µg/mg DW of the sample)
| Plants' parts | TREATMENT | Kaemferol 3-glycoside | Quercitin | Rutin | Myricitn |
|---|---|---|---|---|---|
| Shoot | Control | 0.72 ± 0.07a | 0.18 ± 0.01a | 2.0 ± 0.3a | ND |
| NP-10 | 1.05 ± 0.004c | 0.169 ± 0.001a | 17.04 ± 0.35c | ND | |
| NP-50 | 1.08 ± 0.01c | 0.171 ± 0.01a | 16.7 ± 0.4c | ND | |
| NP-100 | 1.04 ± 0.05c | 0.169 ± 0.0004a | 16.3 ± 1.6c | ND | |
| NP-150 | 0.868 ± 0.07b | 0.181 ± 0.02a | 3.7 ± 0.5b | ND | |
| Root | Control | 0.77 ± 0.001a | 0.17 ± 0.002a | 6.16 ± 0.4a | 4.5 ± 0.001a |
| NP-10 | 0.95 ± 0.001c | 0.18 ± 0.002b | 13.9 ± 0.03c | 4.5 ± 0.0003a | |
| NP-50 | 0.97 ± 0.01d | 0.18 ± 0.001b | 14.3 ± 0.19c | 4.5 ± 0.002a | |
| NP-100 | 0.89 ± 0.004b | 0.17 ± 0.001a | 11.2 ± 0.04b | 4.5 ± 0.001a | |
| NP-150 | 1.0 ± 0.01e | 0.18 ± 0.001b | 15.7 ± 0.18d | 4.5 ± 0.001a |
In shoots, myricitin was not detected and non-significant alteration in quercitin accumulation of control and NPs treated spinach was evident. Kaempferol 3-glycoside was significantly enhanced in nanoprimed spinach shoots than control. In roots, myricitin was detected but was significantly similar in control and nanoprimed spinach. Kaempferol 3-glycoside, quercitin, and rutin were significantly enhanced in MgO-NPs primed spinach roots than control
Note: Different superscript letters in the Table are significantly different at p ≤ 0.05 according to Duncan’s multiple range test (DMRT)
FRAP assay of the root sections showed a significant decrease in the antioxidant potential of nanoprimed spinach. On the contrary, the DPPH IC50 assay showed significant enhancement in the antioxidant potential (Table 1). The FRAP values for the control root were found to be 7.766 mg of FE/g DE and it was consistently reduced to 6.54, 6.27, 6.59, and 6.04 in NP-10 to NP-150 spinach roots, respectively (Table 1). The percent reduction was recorded to be 16–22% in MgO-NPs treated spinach roots compared to control, indicating a significant reduction in their antioxidant potential. However, DPPH IC50 was also reduced by 30, 46, 39, and 26% compared to control in the roots of nanoprimed spinach. The reduction of these values indicated an increase in their antioxidant potential compared to control roots. Likewise, Pearson’s correlation analysis indicated a strong negative correlation of parameters, total phenol, and total flavonoids, FRAP assay, and DPPH IC50 with the MgO-NPs treatment in spinach roots (Supplementary Table S2). Hence, increased NPs exposure concentrations lead to better DPPH scavenging potential of the extracts but poor reduction potential to convert ferric iron to ferrous iron as indicated by the decrease in FRAP values.
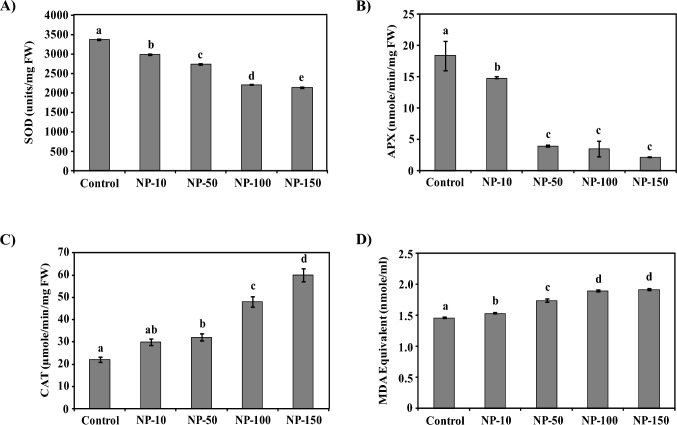
Likewise, MgO-NPs treatment variously altered the activities of spinach antioxidant enzymes SOD, CAT, and APX. The SOD and APX activity was down-regulated by 11–37 and 19–88% on MgO-NPs nanopriming than control (Fig. 4a, b). However, the CAT activity was increased by 36–172% with an increase in MgO-NPs concentration (Fig. 4c). Further, the lipid peroxidation estimated in terms of MDA accumulation indicated 6–32% enhanced oxidative stress in MgO-NPs primed spinach than control (Fig. 4d). The correlation analysis also indicated a strong and positive relation between NPs treatment and MDA accumulation. Similarly, significant relationship between NPs treatment and activity of SOD and CAT was observed in correlation analysis (see Table S3, Supporting Information).
Fig. 4.
Effect of MgO-NPs priming on the antioxidant enzyme activities of spinach. The bar graphs show MgO-NPs mediated variations in the activities of enzymes SOD (A), APX (B), and CAT (C) in spinach. SOD and APX activities were reduced in a NPs concentration dependent manner, whereas the CAT activity was increased than control. MgO-NPs mediated increment in ROS accumulation was responsible for the observed alteration in antioxidant enzyme activities. (D) The graph shows the enhanced oxidative stress in MgO-NPs primed spinach quantified in terms of MDA equivalent. The reduced utilization of absorbed light due to decreased content of photosynthetic pigments was responsible for the increased oxidative stress in spinach on MgO-NPs nanopriming. Data included is mean ± standard deviation of three independent measurements used in this study. Duncan’s multiple range test (DMRT) was applied and different superscript letters on the bars are significantly different at p ≤ 0.05
MgO-NPs significantly influenced the antioxidant system of legumes, mungbean, horse gram, black gram, and lentils (Sharma et al. 2021a, b, c, 2022). Likewise, MgO-NPs primed spinach has shown altered accumulation of polyphenols including phenols, flavonoids, and anthocyanins compared to non-treated plants in this study. Exogenous Co3O4-NPs exposure reduced the polyphenols and antioxidant enzymes of Brassica napus (Jahani et al. 2020). On the contrary, enhanced CAT activity was reported in oak leaf lettuce on Fe2O3-NPs and SnO2-NPs exposure (Jurkow et al. 2020). Earlier, inhibition in the antioxidant enzyme activities of mustard and tomato plants has been reported on Ag-NPs exposure because of oxidative damage (Çekiç et al. 2017; Vishwakarma et al. 2017). Hence, NPs tend to alter the antioxidant system of plants. In addition, enhanced MDA levels in nanoprimed spinach also reflected increased oxidative stress compared to control spinach. A significant correlation of antioxidant enzymes with MDA content furthermore, supported the findings. CuO-NPs have also enhanced lipid peroxidation in plants including Vigna radiata, Brassica oleracea var. botrytis, and Solanum lycopersicum (Nair et al. 2014; Singh et al. 2017). Likewise, iron oxide NPs upregulated lipid peroxidation in Lemna minor (Souza et al. 2019). Reduction in the use of absorbed light energy by photosynthetic pigments has been reported to enhance the ROS generation that affects plant antioxidant system (Farhat et al. 2016; Ze et al. 2009; Hauer-Jákli and Tränkner 2019). Hence, decreased photosynthetic pigments on MgO-NPs priming probably contributed to an enhanced oxidative stress leading to activation of spinach antioxidant potential. This was also reflected by a strong and negative correlation of chlorophyll content with MDA accumulation (see Table S3, Supporting Information). Thus, MgO-NPs notably induced oxidative stress in spinach in a concentration-dependent manner leading to an alteration in its antioxidant system.
Enhanced accumulation of selected flavonoids in nanoprimed spinach
As observed the total flavonoid accumulation was variously altered compared to control in the shoot and root sections of spinach on MgO-NPs exposure. The flavonoid biosynthesis is a diversion from the phenylpropanoid biosynthetic pathway. Naringenin diverts the phenylpropanoid pathway towards diverse metabolite synthesis of flavonoids (Fig. 3). The downstream metabolites of the flavonoid pathway, kaempferol 3-glycoside, quercetin, rutin, and myricitin were evaluated to assess the influence of MgO-NPs on flavonoid biosynthesis (Table 2).
Myricitin was not detected in any of the shoot samples. A non-significant alteration in the quercetin accumulation of control and NPs-treated spinach was evident (Table 2). However, the content of kaempferol 3-glycoside was significantly enhanced in nanoprimed spinach shoots than control. In control shoots, kaempferol 3-glycoside was quantified to be 0.72 µg/mg DW, and it was enhanced to 1.05, 1.08, 1.04, and 0.868 µg/mg DW in spinach shoots nanoprimed with MgO-NPs, respectively. Hence, an increase of 46, 50, 44, and 20% in the kaempferol 3-glycoside accumulation was observed in nanoprimed spinach shoots than control. Likewise, compared to control, the rutin content was increased by 751, 734, 713, and 86% in spinach shoots on MgO-NPs treatment. The rutin was recorded to be 2.0, 17.0, 16.7, 16.7, and 3.7 µg/mg DW in control and NPs primed spinach shoots, respectively (Table 2).
In roots, myricitin was detected but was significantly similar in control and nanoprimed spinach (Table 2). However, kaempferol 3-glycoside, quercetin, and rutin were significantly enhanced in MgO-NPs primed spinach roots than control. Kaempferol 3-glycoside was increased by 24, 25, 17, and 32% in the roots of spinach exposed to MgO-NPs, respectively. The kaempferol 3-glycoside amount was estimated to be 0.77, 0.95, 0.97, 0.89, and 1.0 µg/mg DW in control and 10–150 µg/ml MgO-NPs primed spinach roots, respectively. Similarly, quercetin levels were enhanced by 4.3, 4.2, and 2.8% than control in the roots of spinach treated with 10, 50, and 150 µg/ml MgO-NPs, with non-significant observed variation in spinach roots primed with 100 µg/ml MgO-NPs (Table 2). The rutin content was 6.16 µg/mg DW in control roots, but was increased to 13.9, 14.3, 11.2, 15.7 µg/mg DW in roots of MgO-NPs primed spinach (Table 2). The rutin accumulation was increased by 126, 132, 82, and 155% in NPs treated spinach roots than control roots.
The metabolite accumulation of flavonoid pathway, kaempferol 3-glycoside, and rutin were significantly increased on NPs exposure than in control spinach. This indicated MgO-NPs mediated possible regulation of the secondary metabolism in plants. Recently, graphene/nanocomposite have been reported to increase rosmarinic acid production from Melissa officinalis (Soraki et al. 2021). NPs induce phytotoxicity to plants via reactive oxygen species production, leading to the activation of enzymatic and non-enzymatic antioxidants. Therefore, activation/ alteration of secondary metabolism regulate the accumulation of antioxidant parameters (Marslin et al. 2017; Lala 2021). Similarly in this study, MgO-NPs induced phytotoxicity in spinach thus influencing the level of non-enzymatic antioxidants, in majority polyphenolics and flavonoids. The flavonoid biosynthesis pathway synthesizes polyphenolics and flavonoids. The observed variation in the content of kaempferol 3-glycoside, quercetin, rutin, and myricitin thus, shows the MgO-NPs mediated elicitation of flavonoid pathway in spinach to regulate NPs phytotoxicity. This indicated the potential of MgO-NPs to regulate the flavonoid biosynthesis pathway in spinach.
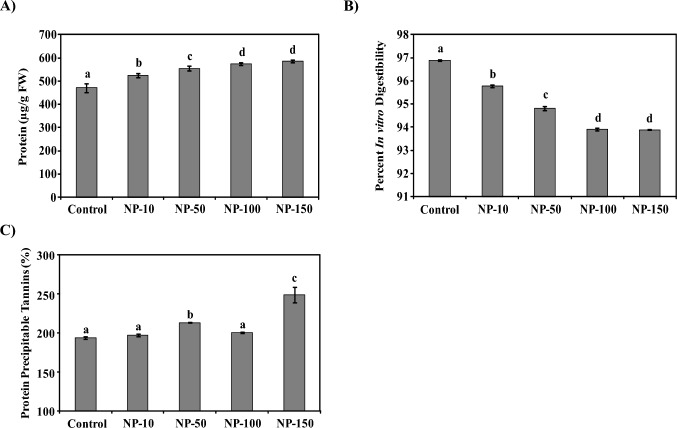
MgO-NPs enhanced protein content but reduced protein digestibility
MgO-NPs increased the total protein content of spinach (Fig. 5). In contrast to 470 µg/g FW total protein in control spinach, the protein accumulation was estimated to be 523.8, 554.2, 573.3, and 584.8 µg/g FW in NP-10 to NP-150 treated spinach (Fig. 5a). In comparison to control, the protein content was enhanced by 11–24% in nanoprimed spinach. A similar increment was observed in the spinach protein solubility on MgO-NPs priming, in NPs concentration-dependent manner (Table 3). The content and solubility of spinach protein were increased on MgO-NPs nanopriming. On the contrary, the protein digestibility was decreased in NPs nanoprimed spinach than in control (Fig. 5b). With 97% protein digestibility in control spinach, the protein digestibility was consistently reduced from 96 to 94% in NP-10 to NP-150 spinach (Fig. 5b). The digestibility was significantly decreased by 1–3% in protein isolated from spinach exposed to MgO-NPs, respectively.
Fig. 5.
MgO-NPs altered the protein parameters of spinach. (A) The bar diagram shows the increased protein content of spinach on nanopriming than control. An increase of 11–24% in the protein accumulation of nanoprimed spinach was evident. The enhanced protein acted as osmoprotectant to regulate MgO-NPs induced oxidative stress in spinach. (B) The graph depicts the protein digestibility in vitro in case of MgO-NPs treated spinach compared to control. Nanopriming was observed to considerably reduce the protein digestibility by 1–3% with an increase in MgO-NPs exposure concentration. (C) Bar diagram showing enhancement in the content of protein precipitable tannins in spinach on MgO-NPs exposure. NPs treatment was found to enhance the protein precipitable tannins that resulted into reduced protein digestibility. Data included in each bar is mean ± standard deviation of three independent measurements (*, P < 0.05). Duncan’s multiple range test (DMRT) was applied and different superscript letters on the bars are significantly different at P ≤ 0.05
Table 3.
Amount of soluble protein (mg/g) present in control and MgO-NPs primed spinach. MgO-NPs exposure evidently enhanced the content of soluble protein
| pH2 | pH3 | pH4 | pH5 | pH6 | pH7 | pH8 | pH9 | pH10 | pH11 | pH12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.171 ± 0.01a | 0.208 ± 0.03a | 0.214 ± 0.01a | 0.215 ± 0.01a | 0.224 ± 0.01a | 0.225 ± 0.01a | 0.26 ± 0.01a | 0.495 ± 0.02a | 0.53 ± 0.01a | 0.534 ± 0.01a | 0.542 ± 0.001a |
| N-10 | 0.178 ± 0.01ab | 0.21 ± 0.01a | 0.215 ± 0.03a | 0.245 ± 0.01b | 0.249 ± 0.01b | 0.257 ± 0.02b | 0.266 ± 0.01a | 0.5 ± 0.03a | 0.534 ± 0.01a | 0.538 ± 0.01a | 0.544 ± 0.001a |
| N-50 | 0.198 ± 0.01bc | 0.233 ± 0.01b | 0.25 ± 0.01b | 0.257 ± 0.01bc | 0.259 ± 0.01c | 0.29 ± 0.01c | 0.316 ± 0.01b | 0.528 ± 0.03a | 0.541 ± 0.01a | 0.543 ± 0.01ab | 0.548 ± 0.003a |
| N-100 | 0.205 ± 0.01c | 0.245 ± 0.03bc | 0.255 ± 0.01b | 0.271 ± 0.01c | 0.294 ± 0.01d | 0.333 ± 0.02d | 0.386 ± 0.01c | 0.531 ± 0.01a | 0.544 ± 0.01a | 0.55 ± 0.01b | 0.593 ± 0.01b |
| N-150 | 0.209 ± 0.03c | 0.247 ± 0.01c | 0.258 ± 0.01b | 0.309 ± 0.01d | 0.315 ± 0.01e | 0.345 ± 0.01d | 0.389 ± 0.01c | 0.533 ± 0.003a | 0.545 ± 0.003a | 0.552 ± 0.004b | 0.601 ± 0.01b |
With the change in pH from acid to basic conditions, the soluble protein was significantly increased on nanopriming than control spinach
Note: Different superscript letters in the Table are significantly different at p ≤ 0.05 according to Duncan’s multiple range test (DMRT)
The interaction of proteins with protein precipitable tannins reduces their digestibility (Sharma et al. 2020). MgO-NPs treatment enhanced the protein precipitable tannins in spinach (Fig. 5c). The NP-10 and NP-100 spinach showed non-significant variation in protein precipitable tannins to control, whereas a considerable increment in NP-50 and NP-100 plants was observed. An increase of 10 and 29% in protein precipitable tannins was evident in 50 and 150 µg/ml MgO-NPs primed spinach (Fig. 5c). Thus, the presence of tannins can be one of the probable reasons for the observed decrease in the protein digestibility of nanoprimed spinach.
Increased protein accumulation has been reported as a mechanism of plants to regulate osmotic stress, as proteins act as osmoprotectant (Azeem et al. 2023). Further, reports have documented NPs mediated enhanced protein accumulation. In vitro MgO-NPs exposure to horse gram (Sharma et al. 2021a), mungbean (Sharma et al. 2021b) and blackgram (Sharma et al. 2021c) has earlier been reported to affect their protein content and bioavailability (Sharma et al. 2021a, c). Earlier, silver and iron NPs were reported to increase the protein content of Phaseolus vulgaris, Zea mays L., and wheat (Salama 2012; Bakhtiari et al. 2015). Likewise, engineered carbon NPs reportedly upregulated the protein content in Vigna radiata (Shekhawat et al. 2021). However, increased protein precipitable tannins on MgO-NPs priming considerably reduced the protein digestibility. A strong and negative relationship between MDA accumulation and protein digestibility was reflected in Pearson correlation (see Table S3, Supporting Information). Hence, MgO-NPs increased the protein accumulation of spinach possibly to regulate oxidative stress but the enhanced protein precipitable tannins reduced protein digestibility.
MgO-NPs reduced spinach yield
The green leaves of spinach are consumed worldwide either in raw or cooked forms. Hence the fresh weight of the total leaves of control and MgO-NPs nanoprimed spinach was recorded as total plant yield (Fig. 6). The yield of 10 plants per treatment was analyzed and significant reduction in spinach yield on NPs exposure was observed. The yield was considerably reduced by 7, 29, 22, and 40% in NP-10, NP-50, NP-100, and NP-150 spinach compared to control spinach, respectively (Fig. 6). Earlier, Ag-NPs reportedly, enhanced the growth and productivity of pearl millet as they reduced reactive oxygen species level and thus, regulated oxidative stress (Khan et al. 2021). Likewise, in the present study MgO-NPs significantly reduced the shoot length, photosynthetic pigments, biomass, and carbohydrate content of spinach on nanopriming. A considerable enhancement in oxidative stress was also evident. Hence, these changes altogether could have led to the observable reductions in spinach yield.
Fig. 6.
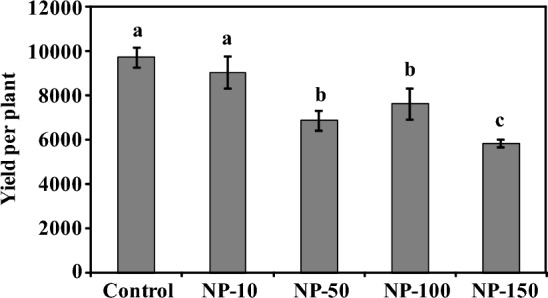
The bar graph shows the reduced yield of spinach on MgO-NPs treatment than control plants. The bars represent the yield of 10 plants per treatment. A significant reduction of 22–40% in the spinach yield on NPs exposure was observed on the exposure of NP-50, NP-100, and NP-150 MgO-NPs. Duncan’s multiple range test (DMRT) was applied and different superscript letters on the bars are significantly different at p ≤ 0.05
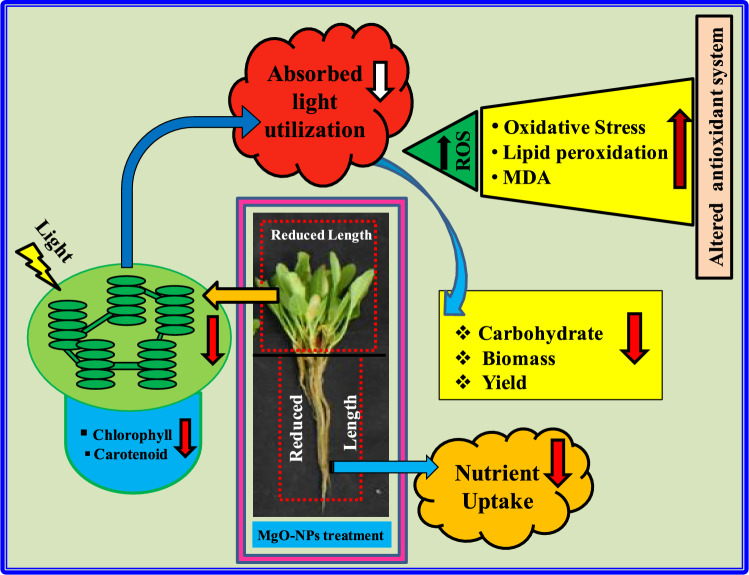
Overall, the present study depicts that reduced root length on MgO-NPs exposure probably inhibited nutrient uptake, thus decreasing the spinach shoot length (Fig. 7). Reduced leaf size on nanopriming further decreased their chlorophyll and carotenoid content. Reduction in the photosynthetic pigment downregulated the utilization of total absorbed light, thus decreasing the accumulation of biomass and carbohydrates. However, reduced utilization of absorbed light enhanced the production of reactive oxygen species, increased the oxidative stress, thus altering the antioxidant system of spinach (Fig. 7). Hence, MgO-NPs mediated reduction in the morphological parameters and increase in oxidative stress has led to significant reduction of spinach yield.
Fig. 7.
The figure depicts the mechanism of MgO-NPs mediated alteration of spinach morphology and antioxidant system, thus regulating its growth and yield. MgO-NPs mediated inhibition in root elongation limited nutrient uptake leading to reduced shoot length in spinach. The reduced leaf size in nanoprimed plants showed reduced accumulation of photosynthetic pigments. This downregulated the utilization of total absorbed light leading to reduced biomass and carbohydrates content, but increasing the production of reactive oxygen species and oxidative stress. These MgO-NPs induced changes collectively led to reduction in spinach yield
Conclusion
The present study documents a growth inhibitory effect of MgO-NPs at higher dose exposure to spinach germinated and maintained in fields. MgO-NPs priming significantly reduced the spinach root length. Roots are responsible for plant nutrient uptake and growth of aboveground plant parts. Nanopriming could have possibly reduced the spinach nutrient uptake due to root growth inhibition, thus decreasing the shoot length and photosynthetic pigment levels. These changes enhanced oxidative stress altering the antioxidant potential of spinach. In addition, MgO-NPs possibly affected the secondary metabolite accumulation of flavonoid pathway. Hence, present study documents MgO-NPs induced phytotoxicity on spinach at higher dose. However, more studies are required to develop a framework revealing the effect of NPs on agricultural sustainability.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
AG, PS, and PG are thankful to DAVU management for continuous encouragement and support to carry out research. VK would like to thank LPU management for encouragement to carry out research. SA and JSY would like to thank Universiti Malaya for continuous encouragement and support to carry out research.
Funding
Not applicable.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vineet Kumar, Email: vineetkumar22@gmail.com.
Praveen Guleria, Email: pvihbt@gmail.com.
References
- Abebe TG, Tamtam MR, Abebe AA, Abtemariam KA, Shigut TG, Dejen YA, Haile EG. Growing use and impacts of chemical fertilizers and assessing alternative organic fertilizer sources in Ethiopia. Appl Environ Soil Sci. 2022;2022:1–14. doi: 10.1155/2022/4738416. [DOI] [Google Scholar]
- Ahmed B, Rizvi A, Ali K, Lee J, Zaidi A, Khan MS, Musarrat J. Nanoparticles in the soil–plant system: a review. Environ Chem Lett. 2021;19:1545–1609. doi: 10.1007/s10311-020-01138-y. [DOI] [Google Scholar]
- AlQuraidi AO, Mosa KA, Ramamoorthy K. Phytotoxic and genotoxic effects of copper nanoparticles in coriander (Coriandrum sativum—Apiaceae) Plants. 2019;8:19. doi: 10.3390/plants8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KV, Anugraga AR, Kannan M, Singaravelu G, Govindaraju K (2020) Bio-engineered magnesium oxide nanoparticles as nano-priming agent for enhancing seed germination and seedling vigour of green gram (Vigna radiata L.). Mater Lett 271:127792.
- Azeem M, Pirjan K, Qasim M, Mahmood A, Javed T, Muhammad H, Rahimi M. Salinity stress improves antioxidant potential by modulating physio-biochemical responses in Moringa oleifera Lam. Sci Rep. 2023;13:1–17. doi: 10.1038/s41598-023-29954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu S, Singh R, Yadav D, Rathore SS, Raj R, Avasthe R, Yadav SK, Das A, Yadav V, Yadav B, Shekhawat K. Nanofertilizers for agricultural and environmental sustainability. Chemosphere. 2022;292:133451. doi: 10.1016/j.chemosphere.2021.133451. [DOI] [PubMed] [Google Scholar]
- Bakhtiari M, Moaveni P, Sani B. The effect of iron nanoparticles spraying time and concentration on wheat. Biol Forum. 2015;7:679. [Google Scholar]
- Benzie IF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27. [DOI] [PubMed]
- Bera MB, Mukherjee RK. Solubility, emulsifying, and foaming properties of rice bran protein concentrates. J Food Sci. 1989;54:142–145. doi: 10.1111/j.1365-2621.1989.tb08587.x. [DOI] [Google Scholar]
- Buckley S, Ahmed S, Griffin T, Orians C. Extreme precipitation enhances phenolic concentrations of spinach (Spinacia oleracea) J Crop Improv. 2020;34:618–636. doi: 10.1080/15427528.2020.1750521. [DOI] [Google Scholar]
- Bunea A, Andjelkovic M, Socaciu C, Bobis O, Neacsu M, Verhé R, Van Camp J. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.) Food Chem. 2008;108:649–656. doi: 10.1016/j.foodchem.2007.11.056. [DOI] [PubMed] [Google Scholar]
- Cai L, Chen J, Liu Z, Wang H, Yang H, Ding W. Magnesium oxide nanoparticles: effective agricultural antibacterial agent against Ralstonia solanacearum. Front Microbiol. 2018;9:790. doi: 10.3389/fmicb.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Liu M, Liu Z, Yang H, Sun X, Chen J, Ding W (2018b) MgO NPs can boost plant growth: Evidence from increased seedling growth, morpho-physiological activities, and Mg uptake in tobacco (Nicotiana tabacum L.). Molecules 23:3375. 10.3390/molecules23123375 [DOI] [PMC free article] [PubMed]
- Çekiç FÖ, Ekinci S, İnal MS, Özakça D. Silver nanoparticles induced genotoxicity and oxidative stress in tomato plants. Turk J Biol. 2017;41:700–707. doi: 10.3906/biy-1608-36s. [DOI] [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10.
- Cole JC, Smith MW, Penn CJ, Cheary BS, Conaghan KJ. Nitrogen, phosphorus, calcium, and magnesium applied individually or as a slow release or controlled release fertilizer increase growth and yield and affect macronutrient and micronutrient concentration and content of field-grown tomato plants. Sci Hortic. 2016;211:420–430. doi: 10.1016/j.scienta.2016.09.028. [DOI] [Google Scholar]
- Dimkpa CO, Singh U, Adisa IO, Bindraban PS, Elmer WH, Gardea-Torresdey JL, White JC (2018) Effects of manganese nanoparticle exposure on nutrient acquisition in wheat (Triticum aestivum L.). Agronomy 8:158. 10.3390/agronomy8090158
- Dong C, Jiao C, Xie C, Liu Y, Luo W, Fan S, Ma Y, He X, Lin A, Zhang Z. Effects of ceria nanoparticles and CeCl3 on growth, physiological and biochemical parameters of corn (Zea mays) plants grown in soil. NanoImpact. 2021;22:100311. doi: 10.1016/j.impact.2021.100311. [DOI] [PubMed] [Google Scholar]
- Elkhalil EAJ, El Tinay AH, Mohamed BE, Elshseikh EAE. Effect of malt pretreatment on phytic acid and in vitro protein digestibility of sorghum flour. Food Chem. 2001;72:29–32. doi: 10.1016/S0308-8146(00)00195-3. [DOI] [Google Scholar]
- Farhat N, Elkhouni A, Zorrig W, Smaoui A, Abdelly C, Rabhi M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol Plant. 2016;38:145. doi: 10.1007/s11738-016-2165-z. [DOI] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases: II. Purification and quantitative relationship with water-soluble protein in seedlings. Plant Physiol. 1977;59:315–318. doi: 10.1104/pp.59.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE (2001) Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr Protocols Food Anal Chem. 10.1002/0471142913.faf0102s00
- Gladkova MM, Terekhova VA. Engineered nanomaterials in soil: sources of entry and migration pathways. Moscow Univ Soil Sci Bull. 2013;68:129–134. doi: 10.3103/S0147687413030046. [DOI] [Google Scholar]
- González M, Jia Y, Sunahara GI, Whalen JK. Barley (Hordeum vulgare) seedling growth declines with increasing exposure to silver nanoparticles in biosolid-amended soils. Can J Soil Sci. 2020;100:189–197. doi: 10.1139/cjss-2019-0135. [DOI] [Google Scholar]
- Guleria P, Masand S, Yadav SK. Overexpression of SrUGT85C2 from Stevia reduced growth and yield of transgenic Arabidopsis by influencing plastidial MEP pathway. Gene. 2014;539:250–257. doi: 10.1016/j.gene.2014.01.071. [DOI] [PubMed] [Google Scholar]
- Hanif R, Iqbal Z, Iqbal M, Hanif S, Rasheed M. Use of vegetables as nutritional food: role in human health. J Agric Biol Sci. 2006;1:18–22. [Google Scholar]
- Hashimoto H, Uragami, C, Cogdell RJ (2016) Carotenoids and Photosynthesis. In: Stange, C. (eds) Carotenoids in Nature. Subcellular Biochemistry, Vol 79. Springer, Cham. 10.1007/978-3-319-39126-7_4 [DOI] [PubMed]
- Hauer-Jákli M, Tränkner M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: a systematic review and meta-analysis from 70 years of research. Front Plant Sci. 2019;10:766. doi: 10.3389/fpls.2019.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Howard LR, Pandjaitan N, Morelock T, Gil MI. Antioxidant capacity and phenolic content of spinach as affected by genetics and growing season. J Agric Food Chem. 2002;50:5891–5896. doi: 10.1021/jf020507o. [DOI] [PubMed] [Google Scholar]
- Ijaz M, Zafar M, Afsheen S, Iqbal TA. A review on Ag-nanostructures for enhancement in shelf time of fruits. J Inorg Organomet Polym Mater. 2020;30:1475–1482. doi: 10.1007/s10904-020-01504-x. [DOI] [Google Scholar]
- Jahani M, Khavari-Nejad RA, Mahmoodzadeh H, Saadatmand S (2020) Effects of cobalt oxide nanoparticles (Co3O4 NPs) on ion leakage, total phenol, antioxidant enzymes activities and cobalt accumulation in Brassica napus L. Not Bot Horti Agrobot Cluj Napoca 48. 10.15835/nbha48311766
- Jhansi K, Jayarambabu N, Reddy KP, Reddy NM, Suvarna RP, Rao KV, Rajendar V (2017) Biosynthesis of MgO nanoparticles using mushroom extract: effect on peanut (Arachis hypogaea L.) seed germination. 3Biotech 7:263. 10.1007/s13205-017-0894-3 [DOI] [PMC free article] [PubMed]
- Jurkow R, Sękara A, Pokluda R, Smoleń S, Kalisz A. Biochemical response of oakleaf lettuce seedlings to different concentrations of some metal(oid) oxide nanoparticles. Agronomy. 2020;10:997. doi: 10.3390/agronomy10070997. [DOI] [Google Scholar]
- Kadigi IL, Richardson JW, Mutabazi KD, Philip D, Mourice SK, Mbungu W, Bizimana JC, Sieber S (2020) The effect of nitrogen-fertilizer and optimal plant population on the profitability of maize plots in the Wami River sub-basin, Tanzania: a bio-economic simulation approach. Agricultural Syst, 185. [DOI] [PMC free article] [PubMed]
- Kah M, Tufenkji N, White JC. Nano-enabled strategies to enhance crop nutrition and protection. Nat Nanotechnol. 2019;14:532–540. doi: 10.1038/s41565-019-0439-5. [DOI] [PubMed] [Google Scholar]
- Khan I, Awan SA, Raza MA, Rizwan M, Tariq R, Ali S, Huang L. Silver nanoparticles improved the plant growth and reduced the sodium and chlorine accumulation in pearl millet: a life cycle study. Environ Sci Pollut Res. 2021;28:13712–13724. doi: 10.1007/s11356-020-11612-3. [DOI] [PubMed] [Google Scholar]
- Kihara J, Nziguheba G, Zingore S, Coulibaly A, Esilaba A, Kabambe V, Njoroge S, Palm C, Huising J. Understanding variability in crop response to fertilizer and amendments in sub-Saharan Africa. Agr Ecosyst Environ. 2016;229:1–12. doi: 10.1016/j.agee.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer SB, Reganold JP, Glover JD, Bohannan BJ, Mooney HA. Reduced nitrate leaching and enhanced denitrifier activity and efficiency in organically fertilized soils. Proc Natl Acad Sci USA. 2006;103:4522–4527. doi: 10.1073/pnas.0600359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Guleria P, Kumar V, Yadav SK. Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Sci Total Environ. 2013;461:462–468. doi: 10.1016/j.scitotenv.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Kumar V, Guleria P, Ranjan S (2021) Phytoresponse to nanoparticles exposure. In: Kumar V, Guleria P, Ranjan S, Dasgupta N, Lichtfouse E (eds) Nanotoxicology and nanoecotoxicology. Springer Nature, Switzerland AG. 10.1007/978-3-030-63241-0
- Kumar V, Jain A, Wadhawan S, Mehta SK. Synthesis of biosurfactant-coated magnesium oxide nanoparticles for methylene blue removal and selective Pb2+ sensing. IET Nanobiotechnol. 2018;12:241–253. doi: 10.1049/iet-nbt.2017.0118. [DOI] [Google Scholar]
- Lala S. Nanoparticles as elicitors and harvesters of economically important secondary metabolites in higher plants: a review. IET Nanobiotechnol. 2021;15:28–57. doi: 10.1049/nbt2.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Chhikara S, Xing B, Musante C, White JC, Dhankher OP. Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sustain Chem Eng. 2013;1:768–778. doi: 10.1007/s00128-017-2205-4. [DOI] [Google Scholar]
- Ma Y, Kuang L, He X, Bai W, Ding Y, Zhang Z, Zhao Y, Chai Z. Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere. 2010;78:273–279. doi: 10.1016/j.chemosphere.2009.10.050. [DOI] [PubMed] [Google Scholar]
- Mall RK, Gupta A, Sonkar G (2017) Effect of climate change on agricultural crops. In: Dubey SK, Pandey A, Sangwan RS (eds) Current Developments in Biotechnology and Bioengineering. Elsevier, pp 23‒46. 10.1016/B978-0-444-63661-4.00002-5
- Marslin G, Sheeba CJ, Franklin G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front Plant Sci. 2017;8:832. doi: 10.3389/fpls.2017.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelock TE, Correll JC (2008) Spinach. In: Prohens J, Nuez F (eds) Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae. Springer, New York. 10.1007/978-0-387-30443-4_6
- Nair PMG, Kim SH, Chung IM. Copper oxide nanoparticle toxicity in mung bean (Vigna radiata L.) seedlings: physiological and molecular level responses of in vitro grown plants. Acta Physiol Plant. 2014;36:2947–2958. doi: 10.1007/s11738-014-1667-9. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidise in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nguyen D, Nguyen HM, Le NT, Nguyen KH, Nguyen HT, Le HM, Nguyen AT, Dinh NTT, Hoang SA, Van Ha C. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J Plant Growth Regul. 2022;41:364–375. doi: 10.1007/s00344-021-10301-w. [DOI] [Google Scholar]
- Nguyen HC, Nguyen TT, Dao TH, Ngo QB, Pham HL, Nguyen TBN. Preparation of Ag/SiO2 nanocomposite and assessment of its antifungal effect on soybean plant (a Vietnamese species DT-26) Adv Nat Sci: Nanosci. 2016;7:045014. doi: 10.1088/2043-6262/7/4/045014. [DOI] [Google Scholar]
- Nikolova M, Slavchov R, Nikolova G (2020) Nanotechnology in medicine. In: Hock F, Gralinski M (eds) Drug discovery and evaluation: methods in clinical pharmacology. Springer, Cham, pp 533–546. 10.1007/978-3-319-68864-0_45.
- Nishihara E, Inoue M, Kondo K, Takahashi K, Nakata N. Spinach yield and nutritional quality affected by controlled soil water matric head. Agric Water Manag. 2001;51:217–229. doi: 10.1016/S0378-3774(01)00123-8. [DOI] [Google Scholar]
- Ors S, Suarez DL. Spinach biomass yield and physiological response to interactive salinity and water stress. Agric Water Manag. 2017;190:31–41. doi: 10.1016/j.agwat.2017.05.003. [DOI] [Google Scholar]
- Pahalvi HN, Rafiya L, Rashid S, Nisar B, Kamili AN (2021) Chemical fertilizers and their impact on soil health. In Microbiota and Biofertilizers, 2:1‒20. Springer, Cham.
- Plaksenkova I, Kokina I, Petrova A, Jermaļonoka M, Gerbreders V, Krasovska M. The impact of zinc oxide nanoparticles on cytotoxicity, genotoxicity, and miRNA expression in barley (Hordeum vulgare L.) seedlings. Sci World J. 2020;2020:6649746. doi: 10.1155/2020/6649746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praburaj L, Design F, Nadu T. Role of agriculture in the economic development of a country. Shanlax Int J Comm. 2018;6:1–5. [Google Scholar]
- Raigond P, Raigond B, Kaundal B, Singh B, Joshi A, Dutt S (2017) Effect of zinc nanoparticles on antioxidative system of potato plants. J Environ Biol 38:435. 10.22438/jeb/38/3/MS-209.
- Rajput V, Minkina T, Mazarji M, Shende S, Sushkova S, Mandzhieva S, Burachevskaya M, Chaplygin V, Singh A, Jatav H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Annal Agric Sci. 2020;65:137–143. doi: 10.1016/j.aoas.2020.08.001. [DOI] [Google Scholar]
- Rajput VD, Minkina T, Sushkova S, Chokheli V, Soldatov M (2019) Toxicity assessment of metal oxide nanoparticles on terrestrial plants. In: Comprehensive analytical chemistry, vol. 87, pp. 189–207, Elsevier, Amsterdam.
- Rani P, Kaur G, Rao KV, Singh J, Rawat M. Impact of green synthesized metal oxide nanoparticles on seed germination and seedling growth of Vigna radiata (Mung Bean) and Cajanus cajan (Red Gram) J Inorg Organomet Polym Mater. 2020;30:4053–4062. doi: 10.1007/s10904-020-01551-4. [DOI] [Google Scholar]
- Rathore I, Tarafdar JC. Perspectives of biosynthesized magnesium nanoparticles in foliar application of wheat plant. J Bionanosci. 2015;9:209–214. doi: 10.1166/jbns.2015.1296. [DOI] [Google Scholar]
- Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, ur Rehman MZ, Waris AA. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere. 2019;214:269–277. doi: 10.1016/j.chemosphere.2018.09.120. [DOI] [PubMed] [Google Scholar]
- Roberts JL, Moreau R (2016) Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct 2016:7. 10.1039/c6fo00051g [DOI] [PubMed]
- Sadak MS. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum) Bull Natl Res Cent. 2019;43:1–6. doi: 10.1186/s42269-019-0077-y. [DOI] [Google Scholar]
- Salama HM. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.) Int Res J Biotechnol. 2012;3:190–197. [Google Scholar]
- Savithramma N, Ankanna S, Bhumi G. Effect of nanoparticles on seed germination and seedling growth of Boswellia ovalifoliolata an endemic and endangered medicinal tree taxon. Nano Vision. 2012;2:2. [Google Scholar]
- Seo JH, Kim JE, Shim JH, Yoon G, Bang MA, Bae CS, Lee KJ, Park DH, Cho SS. HPLC analysis, optimization of extraction conditions and biological evaluation of Corylopsis coreana Uyeki Flos. Molecules. 2016;21:94. doi: 10.3390/molecules21010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Hasan MK, Ahammed GJ, Li M, Yin H, Zhou J. Applications of nanotechnology in plant growth and crop protection: a review. Molecules. 2019;24:2558. doi: 10.3390/molecules24142558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Gautam A, Kumar V, Guleria P. In vitro exposed magnesium oxide nanoparticles enhanced the growth of legume Macrotyloma uniflorum. Environ Sci Pollut Res. 2021;29:13635–13645. doi: 10.1007/s11356-021-16828-5. [DOI] [PubMed] [Google Scholar]
- Sharma P, Gautam A, Kumar V, Guleria P. In vitro exposure of magnesium oxide nanoparticles negatively regulate the growth of Vigna radiata. Int J Environ Sci Technol. 2021 doi: 10.1007/s13762-021-03738-9. [DOI] [Google Scholar]
- Sharma P, Gautam A, Kumar V, Guleria P. In vitro exposure of magnesium oxide nanoparticles adversely affects the vegetative growth and biochemical parameters of black gram. Environ Nanotechnol Monit Manag. 2021;16:100483. [Google Scholar]
- Sharma P, Gautam A, Kumar V, Guleria P (2022) MgO nanoparticles mediated seed priming inhibits the growth of lentil (Lens culinaris). Vegetos, pp 1–14. 10.1007/s42535-022-00400-8
- Sharma, P, Kumar V, Khosla R, Guleria P 2020. Exogenous naringenin improved digestible protein accumulation and altered morphology via VrPIN and auxin redistribution in Vigna radiata. 3 Biotech 10:1–14. 10.1007/s13205-020-02428-6 [DOI] [PMC free article] [PubMed]
- Shekhawat GS, Mahawar L, Rajput P, Rajput VD, Minkina T, Singh RK (2021) Role of engineered carbon nanoparticles (CNPs) in promoting growth and metabolism of Vigna radiata (L.) Wilczek: insights into the biochemical and physiological responses. Plants 10:1317. 10.3390/plants10071317 [DOI] [PMC free article] [PubMed]
- Shimizu N, Kobayashi K, Hayashi K. The reaction of superoxide radical with catalase. Mechanism of the inhibition of catalase by superoxide radical. J Biol Chem. 1984;259:4414–4418. doi: 10.3390/molecules21010094. [DOI] [PubMed] [Google Scholar]
- Singh A, Singh NB, Hussain I, Singh H. Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J Biotechnol. 2017;262:11–27. doi: 10.1016/j.jbiotec.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Singh D, Kumar A. Investigating long-term effect of nanoparticles on growth of Raphanus sativus plants: a trans-generational study. Ecotoxicol. 2018;27:23–31. doi: 10.1007/s10646-017-1867-3. [DOI] [PubMed] [Google Scholar]
- Soraki RK, Gerami M, Ramezani M. Effect of graphene/metal nanocomposites on the key genes involved in rosmarinic acid biosynthesis pathway and its accumulation in Melissa officinalis. BMC Plant Biol. 2021;21:1–14. doi: 10.1186/s12870-021-03052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza LRR, Bernardes LE, Barbetta MFS, da Veiga MAMS. Iron oxide nanoparticle phytotoxicity to the aquatic plant Lemna minor: effect on reactive oxygen species (ROS) production and chlorophyll a/chlorophyll b ratio. Environ Sci Pollut Res. 2019;26:24121–24131. doi: 10.1007/s11356-019-05713-x. [DOI] [PubMed] [Google Scholar]
- Sun F, Yan Y, Lin L. The evaluation of antioxidant properties and stability of polyphenols from Spinacia oleracea. J Biotech Res. 2018;9:8–13. [Google Scholar]
- Surendar KK, Durga Devi D, Ravi I, Jeyakumar P, Velayudham K (2013) Water stress affects plant relative water content, soluble protein, total chlorophyll content and yield of ratoon banana. Int J Hortic, 3.
- Tolaymat T, Genaidy A, Abdelraheem W, Dionysiou D, Andersen C. The effects of metallic engineered nanoparticles upon plant systems: an analytic examination of scientific evidence. Sci Total Environ. 2017;579:93–106. doi: 10.1016/j.scitotenv.2016.10.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombuloglu H, Slimani Y, Tombuloglu G, Korkmaz AD, Baykal A, Almessiere M, Ercan I. Impact of superparamagnetic iron oxide nanoparticles (SPIONs) and ionic iron on physiology of summer squash (Cucurbita pepo): a comparative study. Plant Physiol Biochem. 2019;139:56–65. doi: 10.1016/j.plaphy.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Tombuloglu H, Tombuloglu G, Slimani Y, Ercan I, Sozeri H, Baykal A. Impact of manganese ferrite (MnFe2O4) nanoparticles on growth and magnetic character of barley (Hordeum vulgare L.) Environ Pollut. 2018;243:872–881. doi: 10.1016/j.envpol.2018.08.096. [DOI] [PubMed] [Google Scholar]
- Vishwakarma K, Upadhyay N, Singh J, Liu S, Singh VP, Prasad SM, Chauhan DK, Tripathi DK, Sharma S. Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front Plant Sci. 2017;8:1501. doi: 10.3389/fpls.2017.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Ren Y, He J, Zhang L, Wang X, Cui Z. Impact of copper oxide nanoparticles on the germination, seedling growth, and physiological responses in Brassica pekinensis L. Environ Sci Pollut Res. 2020;27:31505–31515. doi: 10.1007/s11356-020-09338-3. [DOI] [PubMed] [Google Scholar]
- Wang X, Yang X, Chen S, Li Q, Wang W, Hou C, Gao X, Wang L, Wang S. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front Plant Sci. 2016;6:1243. doi: 10.3389/fpls.2015.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Leskovar DI. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci Hortic. 2015;183:39–47. doi: 10.1016/j.scienta.2014.12.004. [DOI] [Google Scholar]
- Xu C, Mou B. Responses of spinach to salinity and nutrient deficiency in growth, physiology, and nutritional value. J Am Soc Hortic Sci. 2016;141:12–21. doi: 10.21273/JASHS.141.1.12. [DOI] [Google Scholar]
- Yang Z, Xiao Y, Jiao T, Zhang Y, Chen J, Gao Y (2020) Effects of copper oxide nanoparticles on the growth of rice (Oryza Sativa L.) seedlings and the relevant physiological responses. Int J Environ Res Public Health 17:1260. [DOI] [PMC free article] [PubMed]
- Yavuz D, Kılıç E, Seymen M, Dal Y, Kayak N, Kal Ü, Yavuz N. The effect of irrigation water salinity on the morph-physiological and biochemical properties of spinach under deficit irrigation conditions. Sci Hortic. 2022;304:111272. doi: 10.1016/j.scienta.2022.111272. [DOI] [Google Scholar]
- Yusof Z, Ramasamy S, Mahmood NZ, Yaacob JS. Vermicompost supplementation improves the stability of bioactive anthocyanin and phenolic compounds in Clinacanthus nutans Lindau. Molecules. 2018;23:1345. doi: 10.3390/molecules23061345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar H, Abbasi BH, Zia M. Physiological and antioxidative response of Brassica nigra (L.) to ZnO nanoparticles grown in culture media and soil. Toxicol Environ Chem. 2019;101:281–299. doi: 10.1080/02772248.2019.1691555. [DOI] [Google Scholar]
- Ze Y, Yin S, Ji Z, Luo L, Liu C, Hong F. Influences of magnesium deficiency and cerium on antioxidant system of spinach chloroplasts. Biometals. 2009;22:941–949. doi: 10.1007/s10534-009-9246-z. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Blumwald E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol. 2001;19:765–768. doi: 10.1038/90824. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ke M, Qu Q, Peijnenburg WJGM, Lu T, Zhang Q, Ye Y, Xu P, Du B, Sun L, Qian H. Impact of copper nanoparticles and ionic copper exposure on wheat (Triticum aestivum L.) root morphology and antioxidant response. Environ Pollut. 2018;239:689–697. doi: 10.1016/j.envpol.2018.04.066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.