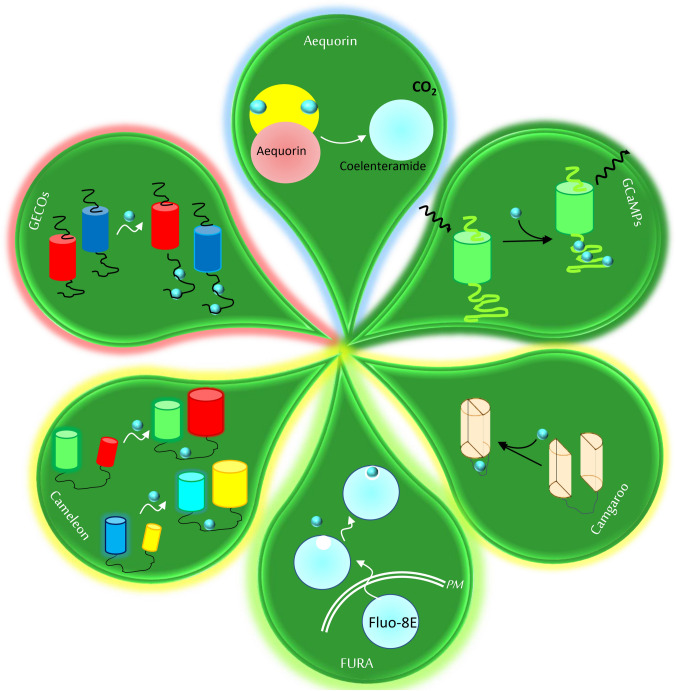
Abstract
Calcium ion (Ca2+) is a multifaceted signaling molecule that acts as an important second messenger. During the course of evolution, plants and animals have developed Ca2+ signaling in order to respond against diverse stimuli, to regulate a large number of physiological and developmental pathways. Our understanding of Ca2+ signaling and its components in physiological phenomena ranging from lower to higher organisms, and from single cell to multiple tissues has grown exponentially. The generation of Ca2+ transients or signatures for various stress factor is a well-known mechanism adopted in plant and animal systems. However, the decoding of such remarkable signatures is an uphill task and is always an interesting goal for the scientific community. In the past few decades, studies on the concentration and dynamics of intracellular Ca2+ are significantly increasing and have become a trend in modern biology. The advancement in approaches from Ca2+ binding dyes to in vivo Ca2+ imaging through the use of Ca2+ biosensors to achieve spatio-temporal resolution in micro and milliseconds range, provide us phenomenal opportunities to study live cell Ca2+ imaging or dynamics. Here, we describe the usage, improvement and advancement of Ca2+ based dyes, genetically encoded probes and sensors to achieve extraordinary Ca2+ imaging in plants and animals.
Graphical abstract
Keywords: Ca2+ imaging, Ca2+ signaling, Ca2+ signature, Genetically encoded Ca2+ sensors, Second messenger, Spatio-temporal
Introduction
Ca2+-mediated signaling, i.e., the signal-specific transient changes in the cytosolic Ca2+, [Ca2+]cyt concentration to mediate downstream signaling, has remained constant and universal for all life forms in spite of several differences (Luan and Wang 2021). The transient and spatio-temporal variations in Ca2+ concentrations generated in response to any stimulus are also known as “Ca2+ signatures” (Qudeimat and Frank 2009). The Ca2+ signatures in intracellular compartments and cytosol are very much specific for a particular stimuli or developmental event, which further regulate downstream Ca2+ signaling events. The downstream signaling components include Ca2+ sensors such as calmodulins (CaMs), calmodulin like proteins (CMLs), calcineurin-B like proteins (CBLs) and Ca2+ dependent protein kinases (CDPKs), which sense the modulations in Ca2+ concentrations. Ca2+ sensors bind to the Ca2+ and subsequently transduce the signal downstream to a set of responder proteins such as CBL-interacting protein kinases (CIPKs) and phosphatases in the designated signaling pathway (Luan et al. 2001, 2002; Cheng et al. 2002; Yang and Poovaiah 2003; Kolukisaoglu et al. 2004; Batistič et al. 2010; Hashimoto and Kudla 2011; Bender and Snedden 2013; Shankar et al. 2015). Based on the type of the stimulus, the sensor-kinase complex further binds and phosphorylate their respective targets such as transcription factors, transporter or channels and enzymes, which brings about an adaptive response. The Ca2+ transporters such as Ca2+ pumps or exchangers and channels are mainly responsible for generating as well as maintaining Ca2+ signatures and hence, are crucial components of Ca2+ signaling and homeostasis tool-kit (Berridge et al. 2003; Demidchik et al. 2018). Their synergistic transport activity is solely responsible for the maintenance of Ca2+ concentrations in the cytosol as well as internal compartments. Channels such as cyclic nucleotide gated channels (CNGC), glutamate receptor-like (GLRs) channels/Glutamate-like receptor channels, two pore channels (TPCs), annexins, mechanosensitive channels (MSLs) constitute the influx system which brings Ca2+ into the cytosol whereas Ca2+-ATPases, Ca2+/H+ exchangers (CAXs), Ca2+/cation exchangers (CCXs) act as an efflux system to remove Ca2+ out of the cytosol for restoring the resting [Ca2+]cyt2. This intricate functioning of Ca2+ influx-efflux system regulates Ca2+ levels under normal and adverse conditions (Ghosh et al. 2022).
Plants are rooted and continuously challenged with a large number of stimuli including the adverse cues in their growth habitat. In response to this, evolutionarily they have developed diverse intrinsic and sophisticated mechanisms to endure against these adverse conditions or stresses. Ca2+ signaling is involved in various physiological, biochemical, reproductive and developmental pathways (Pandey 2008; Ghosh et al. 2021, 2022). Perhaps this could be the only reason why plant based Ca2+ signaling received much attention and is well studied. Plants perceive stimuli via receptors which are considered as the first line of sensing and signaling events. Secondly, the resulting cellular events are dependent on spatial and temporal variations in apoplastic, cytoplasmic and organellar Ca2+ concentrations (Batistič and Kudla 2012; Thor 2019). In animals, Ca2+ signaling machinery is comprised of a large number of components, similar to that in plants and microbial world. Still, there remains a large distinction in the presence and governance of the Ca2+ signaling pathway. Some of the peculiar differences in animals and plants are the presence or absence of these Ca2+ signaling components such as Calcineurin (a ubiquitous Ca2+ sensor of animal world), which is absent in plants (Kudla et al. 1999; Nagata et al. 2004). However, animals also possess CaM, Ca2+ sensor that is highly conserved throughout the eukaryotes. To transduce the signal downstream in the signaling pathway, CaM binds the Ca2+ via its EF-hands in the presence of Mg2+ (Fang et al. 2002; La Verde et al. 2018; Luan and Wang 2021). The selectivity of cation Ca2+ over Mg2+ is achieved by the structural folding of the binding loop of CaM. The Ca2+/CaM complex targets a large array of functional proteins including kinases, phosphatases, channels, transporters, and cytoskeletal components (Floyd and Wray 2007; Luan and Wang 2021). Ca2+ levels in animals are also regulated by a diverse array of channels such as voltage-gated Ca2+ channels (VGCCs), CNGCs, ionotropic glutamate receptors (iGluRs), Ca2+ -permeable mechanosensitive channels like PIEZOs and Hyperosmolality-gated calcium-permeable channels (OSCAs), TPCs and transporters like exchangers and Ca2+-ATPases. In adverse conditions, the animal [Ca2+]cyt levels are elevated with the opening of Ca2+ channels localized on plasma membrane (PM)/or endomembrane followed by removal of Ca2+ by activation of PM Ca2+-ATPase (PMCAs), sarcoplasmic reticulum Ca2+-ATPases (SERCAs) and Ca2+ exchangers like NHXs. The synergistic activity of channels and transporters results in Ca2+ entry and release which maintains Ca2+ levels responsible for excitation and contraction in animal muscle cells (Floyd and Wray 2007; Luan and Wang 2021).
Growing knowledge of Ca2+ signaling has improved our understanding of various physiological and biochemical processes occuring in a cell. It also encourages researchers to develop tools and techniques to quantify and monitor Ca2+ levels or signatures in nano or micromolar (nM or µM) ranges during stressed or normal conditions. The optical quantification of Ca2+ dynamics or signatures in the isolated cell, tissue or medium with the utilization of microscope is known as “Ca2+ imaging” (Reis et al. 2020). In the early 1960s and 1970s, the dye-based indicators such as murexide, azo dyes, and chlortetracycline were used. The major drawback of using these dyes are low sensitivity, difficulty in performing live cell imaging, poor accuracy and their hazardous nature (Kanchiswamy et al. 2014). With time, the advancement in Ca2+ imaging with advanced Ca2+-based indicators came into existence. Among them, green fluorescent protein (GFP), Aequorin (AEQ) and Fluorescence resonance energy transfer (FRET) based Ca2+ imaging are very popular. These Ca2+ indicators have enabled us to understand the dynamics of Ca2+ at cellular level (Zhou et al. 1980; Kanchiswamy et al. 2014). The present study is focused on a detailed study of Ca2+ imaging aspects in plants and animals describing the ancient probes and dyes, their merits and demerits as well as evolution. We have also covered the utilization and application of these techniques in modern plant and animal sciences.
Live cell [Ca2+]cyt imaging: advancement in tools and techniques
Ca2+ imaging has benefitted from the advancements in technology based on the principle of Ca2+-based indicators. Although the Ca2+ concentration in plants is in the nM range, it can reach mM concentration in specific organelles. Ca2+ indicators can visually monitor Ca2+ concentrations in the cytoplasm because it is difficult to accurately quantify such small concentrations. Many Ca2+ indicators have been developed over time, but each one has its own advantages and disadvantages. In the beginning, intracellular Ca2+ variations were studied using the Ca2+-sensitive bioluminescent protein aequorin. However, it has certain drawbacks as well. To address these drawbacks, new indicators were created, and this series of advancements over the earlier dyes resulted in the production of a large number of new dyes. Also, the goal of acquiring high-resolution images underlies the modernity of the microscopy world. The discovery of light microscope by Anton van Leeuwenhoek (1632–1723) enabled the study of samples as uniformly as possible through the illuminating light. However, it was not sensitive enough for thicker samples where an advanced objective lens was required which could penetrate to a sufficient depth and focus in the region of interest (Evennett and Hammond 2004). Genetically encoded probes can also be covered in widefield microscope where the whole sample is illuminated by light source and can be viewed via eyepiece or displayed on monitor. The widefield microscopes are way less complexed as compared to the confocal microscope but path of light as well as out-of-focus light are a concern (Köhler and Blatt 2002; Kanchiswamy et al. 2014). In 1845, fluorescence microscope was discovered by Fredrick W. Herschel, which utilized fluorescent-based dyes. However, it could stimulate out-of-plane dye molecules as well, with out-of-focus light producing blurred images (Renz 2013). Later on, in mid-1950s, the confocal microscope was invented by Marvin Minsky to overcome the limitations of fluorescence microscopy (Merchant et al. 2005). Optical sectioning-based confocal microscope was able to reduce blurring of images by physically blocking out-of-focus light and enabling the three-dimensional reconstruction of samples. Over the years, several other confocal microscopes were invented (Merchant et al. 2005). One such example is confocal laser scanning microscopy (CLSM) which is primarily utilized for in situ 3D modelling of tissues (Stricker and Whitaker 1999; Elliott 2020). It is a fluorophore-based Ca2+ imaging method with spatial resolution that is unmatched by other Ca2+ imaging devices. CLSM visualizes Fluo-3 fluorophore emitted fluorescence and majorly transmits the powerful laser beam onto the sample (Mithöfer et al. 2009). Interestingly, CLSM has been shown to be a good tool to visualize live samples. It has been primarily used to measure spatial intracellular Ca2+ signal in eukaryotes ranging from protists, fungi, plants and animals (Mithöfer et al. 2009; Kanchiswamy et al. 2014). Confocal microscope such as Nipkow-disk-types rely on arc lamp for its illumination to acquire transient intracellular Ca2+ images (Williams 1990; Takahashi et al. 2011; Elliott 2020). The single plane illumination microscopy (SPIM) is used for 3D imaging of multicellular specimens with utmost high resolution. This was invented in the early 2000s in Ernst Stelzer’s laboratory (Gomez-Cruz et al. 2022). Alex Costa and co-workers utilized this microscopy to study the dynamics of Ca2+ in transgenic Arabidopsis expressing genetically encoded Ca2+ probe i.e., NES-YC3.6 which is based on the phenomenon of Förster Resonance Energy Transfer (FRET); (Costa et al. 2013). Deconvolution microscopy has also been used to study high resolution Ca2+ imaging. Pnevmatikakis et al., (2015) reported the position of neurons from the slow dynamics of Ca2+ indicators (Kanchiswamy et al. 2014; Pnevmatikakis et al. 2016). Allen and co-workers also used a deconvolution microscope to measure the fluorescence of Yellow Cameleon YC2.1 (Allen et al. 1999).
The advancement of Ca2+ imaging surely has paved the path of modernity of microscopy with high resolution, excellent accuracy and live cell imaging (Fig. 1). The improvement in technology will definitely enlighten the phenomenon of Ca2+ imaging in a more advanced manner.
Fig. 1.
The quantification of cellular Ca2+ dynamics measured using confocal microscope. The analysis of Ca2+ dynamics in living organisms encompasses various components. The entire plant sample is placed onto a specifically designed plate, which facilitates continuous flow within a chamber via a pump. The incident beam traverses the excitation filter and undergoes reflection upon interaction with the sample. The emitted fluorescence is collected and directed towards the detector by the return beam, which passes through the filter wheel before being visualized on the system [adapted from (Behera and Kudla 2013a)]
Measurement of Ca2+ using Ca2+ based indicators
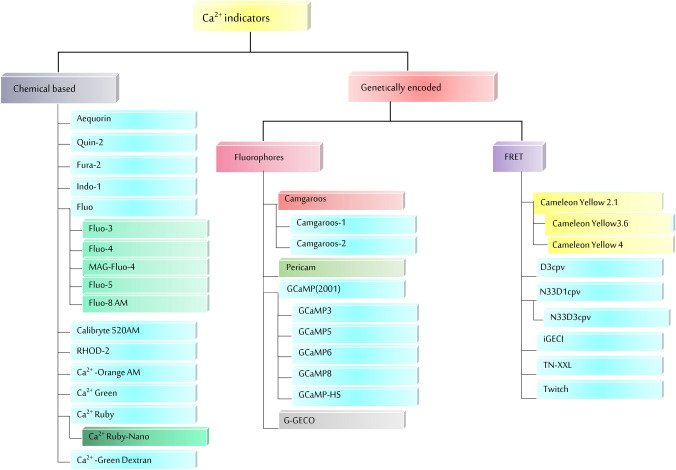
This section is dedicated to the examination of different dyes, sensors, probes, and indicators that rely on the presence of Ca2+ ions and have been employed in various instances. The indicators are categorized on the basis of their chemical nature and usage (Table 1; Fig. 2). This section provides a description of the underlying principle on which these indicators operate to analyze their respective advantages and disadvantages for their utilization in plant and animal sciences.
Table 1.
Properties of various Ca2+ dyes, probes and indicators and their special features.
| Indicator dyes | Properties | Special Features | References | |
|---|---|---|---|---|
| AEQUORIN | Kd | 2600 nM | Based on biolouminescence, which has restricted utility for rapid kinetics and allowing detection between 0.1 and 100 µM | Kaestner et al. (2014) |
| Excitation | – | |||
| Emission | 465 nm | |||
| QUIN-2 | Kd | 115 nM | Preferred for studying resting Ca2+ concentration at intracellular level due to its high affinity for Ca2+ | Grynkiewicz et al. (1985), Giacomello and Campeol (2013), Matuz-Mares et al. (2022) |
| Excitation | 339 nm | |||
| Emission | 495 nm | |||
| FURA-2 | Kd | 224 nM |
Bigger range of Ca2+-free and bound forms higher photobleaching activity resistance |
Eerbeek et al. (2004), O’Connor and Silver (2013), Tinning et al. (2018) |
| Excitation | 380–340 nm | |||
| Emission | 510 nm | |||
| INDO-1 | Kd | 822 nM |
Increased speed of measurement absence of special quartz optics |
Bassani et al. (1995), Eerbeek et al. (2004), Bannwarth et al. (2009) |
| Excitation | 351–364 nm | |||
| Emission | 405 and 485 nm | |||
| FLUO-3 | Kd | 390 nM |
Good spectroscopic qualities and better photostability |
Castell et al. (1997), Takahashi et al. (1999), Gee et al. (2000b), Contreras et al. (2010), Blass (2015) |
| Excitation | 506 nm | |||
| Emission | 526 nm | |||
| FLUO-4 | Kd | 345 nM |
Fast and high affinity Ca2+ dye used in live imaging |
Wallace et al. (2008), Blass (2015), Di Virgilio et al. (2019), Liao et al. (2021) |
| Excitation | 480 nm | |||
| Emission | 525 nm | |||
| CALIBRYTE 520 AM | Kd | 320 nM |
Do not need probenecid improve dye retention |
Liao et al. (2021) https://www.aatbio.com/products/cal-520-am |
| Excitation | 493 nm | |||
| Emission | 515 nm | |||
| CAL-590 | Kd | 561 nM |
Useful in deep imaging experiments fall in near infrared range good signal to noise ratio |
Tischbirek et al. (2015) |
| Excitation | 570 nm | |||
| Emission | 590 nm | |||
| RHOD-2 | Kd | 570 nM |
Low affinity Ca2+ indicator among all fluorescent Ca2+ probes it has the highest dissociation constant and possess the strongest signal intensity |
Hurley et al. (1992), MacGowan et al. (2001b), Liao et al. (2021) |
| Excitation | 524 nm | |||
| Emission | 589 nm | |||
| CALCIUM ORANGE AM | Kd | 7.40 nM |
signal to noise ratio is high reduce autofluorescence related issues |
Eberhard and Erne (1991), Lam et al. (2005) https://pubchem.ncbi.nlm.nih.gov/compound/16186222 |
| Excitation | 549 nm | |||
| Emission | 576 nm | |||
| CALCIUM GREEN-1 | Kd | 190 nM |
Excited by visible light reducecellular photo-damage |
Eberhard and Erne (1991), Hurley et al. (1992) https://pubchem.ncbi.nlm.nih.gov/compound/16186222 |
| Excitation | 490 nm | |||
| Emission | 531 nm | |||
| YC2.1 | Kd | 100 nM | Improved pH sensitivity | Allen et al. (1999) |
| Excitation | 488 nm | |||
| Emission | 522 nm | |||
| YC3.6 | Kd | 250 nM |
Provides a high signal-to-noise ratio has acid stability |
Miyawaki et al. (1999), Nagai et al. (2004), Saito et al. (2010), Bischof et al. (2019) |
| Excitation | 430 nm | |||
| Emission | 475/525 nm | |||
| D3cpv | Kd | 600 nM | Good Rmax/Rmin sensitivity to Ca2+ fluctuation | Rochefort and Konnerth (2008), Wallace et al. (2008), Giacomello et al. (2010) |
| Excitation | 488 nm | |||
| Emission | ||||
| N33D1cpv | Kd | 800 nM and 60 µM | Higher affinity for Ca2+ | Bischof et al. (2019), Gouriou et al. (2023b) |
| Excitation | 430 nm | |||
| Emission | 475/525 nm | |||
| N33D3cpv | Kd | 10 M |
Higher Ca2+ sensitivity greater F/F0 ratio peak provides accurate and reproducible Ca2+ measurements |
Gouriou et al. (2023b) |
| Excitation | ||||
| Emission | ||||
| GCaMP3 | Kd | 660 nm |
Possibility of cell specific expression ease of use with microscopy photostable |
Akerboom et al. (2013), Chen et al. (2013b), Cho et al. (2017), Defalco et al. (2017), Zhong and Schleifenbaum (2019), Shemetov et al. (2021) |
| Excitation | 485 nm | |||
| Emission | 513 nm | |||
| GCaMP6 | Kd | 375 nM` nM | Consists of 3 sensors i.e., GCaMP6s,6 m,and 6f which exhibits slow, medium and fast kinetics respectively | Chen et al. (2013a), Lohr et al. (2021) |
| Excitation | 496 nm | |||
| Emission | 513 nm | |||
| GCaMP-HS | Kd | 102 nM |
More stable and stronger fluorescence higher affinity for calcium higher cooperativity |
Muto et al. (2011), Muto and Kawakami (2013) |
| Excitation | 488 nm | |||
| Emission | 509 nm | |||
| G-GECO | Kd | Kd1 = 15 nM |
Longer wavelength, less photoxicity deeper penetration in tissue |
Geiger et al. (2012), Shen et al. (2018), Bi et al. (2021) |
| Kd2 = 890 nM | ||||
| Excitation | 390 nm | |||
| Emission | 640 nm and 700 nm | |||
| i-GECI | Kd | Kd1 = 15 nM |
High photostability high brightness Approximately 60% fluorescence increase upon Ca2+ binding |
Matlashov et al. (2022) |
| Kd2 = 890 nM | ||||
| Excitation | 390 nm | |||
| Emission | 670 nm and 700 nm | |||
| Twitch | Kd | A smaller size, a broad range of calcium affinities, superior photostability, a wider dynamic range, and faster kinetics compared to the TN-XXL | Thestrup et al. (2014b), Cho et al. (2017) | |
| Excitation | ||||
| Emission | ||||
| CaRuby-Nano | Kd | 295 nM |
Highly sensitive Versatile indicator |
Otsu et al. (2015), Collot et al. (2015) |
| Excitation | 575 nm | |||
| Emission | 610 nm | |||
| TN-XXL | Kd | 800 nM |
shows increased fluorescence change allows repetition of images |
Mank et al. (2008), Dedecker et al. (2013) |
| Excitation | 435 or 515 nm | |||
| Emission | 480 or 535 nm | |||
Fig. 2.
The categorization of various Ca2+ indicators utilized for studying Ca2+-dynamics is depicted. There are two main classes of Ca2+ indicators: Chemical-based and Genetically encoded Ca2+indicators (GECI). Chemical-based indicators are small molecules that can be loaded into cells, while GECIs are proteins that are expressed within the cell. Chemical-based indicators are easy to use and provide a quick visual indication of the presence or absence of a specific substance whereas GECIs are genetically modified proteins that fluoresce in the presence of Ca2+ ions
Chemical-based Ca2+ indicators
Chemical indicators have been used for intracellular Ca2+ detection over a wide range from < 50 nM to > 50 µM. High affinity chemical indicators have been optimized for [Ca2+]cyt whereas, low affinity indicators have been refined for Ca2+ of intracellular compartments (Paredes et al. 2008).
AEQUORIN, QUIN-2, FURA-2, INDO-1
Aequorin, a chemical based photoprotein obtained from the jellyfish Aequorea victoria, has attracted considerable scientific interest for its distinctive ability to demonstrate Ca2+ dynamics within living cells (Shimomura 2005). The bioluminescent protein functions as a highly sensitive Ca2+ sensor through its high-affinity binding to Ca2+ (Shimomura 2005). When aequorin and Ca2+ come into contact, the protein changes its shape, causing its coelenterazine chromophore to become oxidised. This process ultimately leads to the emission of light within the visible spectrum (Shimomura 2005; Fig. 3). The bioluminescent reaction has a linear relationship with the amount of Ca2+ in the cellular environment. This makes it easy to keep track of changes in Ca2+ levels inside cells without hurting them. Through the process of genetic modification, cells can be manipulated in order to express aequorin (Yoshimoto and Hiramoto 1991). Alternatively, targeted aequorin probes can be used to examine a wide range of Ca2+-dependent cellular processes. These processes include neurotransmission, muscle contraction, cellular signaling, and apoptosis (Wier et al. 1983; McConkey and Nutt 2004; Ryu et al. 2010).The major disadvantage of using aequorin is having low light emission which actually affects the spatio-temporal resolution of Ca2+ imaging monitored from whole seedlings or tissue. Also, mathematical simulations have proved that single-cell oscillatory Ca2+ signals cannot be detected by aequorin by using conventional microscopes (Grynkiewicz et al. 1985; Krebs et al. 2012). A number of Ca2+ imaging studies have been undertaken in plants as well as in animals with the utilization of aequorin, to elucidate the potential role of Ca2+ under stress physiology and developmental pathways. With the help of aequorin transformed tobacco cells, the involvement of Ca2+ signaling was studied during oxidative burst condition (Chandra and Low 1997). Because of the technical difficulties, mitochondrial Ca2+ concentration measurement is a little tricky. But in the year 2003, Logan et al. (2003) were able to study mitochondrial Ca2+ dynamics by generating stable Arabidopsis aequorin lines under various stresses such as cold, osmotic, mechanical and oxidative stress (Logan and Knight 2003). Aequorin has also been used in order to study [Ca2+]cyt changes in presence of flagellin (flg22), Pep1 (a plant derived DAMP; damage associated molecular pattern), and NaCl (Xie et al. 2017). Aequorin has been also utilized to visualize in vivo Ca2+ signaling in different sub-cellular compartments and comparative analysis of the same has been done (Mehlmer et al. 2012). A hybrid Ca2+ sensor which has a fusion of green fluorescent protein (GFP) and aequorin has been engineered as a chimeric protein, termed as G5A. The chimeric protein sensor G5A was based on the principle of bioluminescence resonance energy transfer (BRET). G5A was potentially used in animal systems for over ten years. Interestingly, G5A has been used in intact Arabidopsis to study the Ca2+ dynamics in whole seedling. G5A can be an influential tool to study Ca2+ dynamics with enhanced fluorescence property (Xiong et al. 2014). Kiegle et al. (2000) have studied the transient changes in Ca2+ levels under cold, osmotic and salt stress conditions in Arabidopsis (Kiegle et al. 2000). The rice harbouring aequorin was used to monitor the differences in peak durations of Ca2+ spikes under salt and oxidative stresses (Zhang et al. 2015). Ca2+ signaling in biotic stress conditions has also been studied in plants. The SlCNGC1 and SlCNGC14 silenced tomato lines expressing aequorin showed reduced Ca2+ spikes upon flg22-treatment (Zhang et al. 2018). A very interesting study by Moreno et al. (2017), showed the change in Ca2+ level in Arabidopsis root upon exposure to sound wave (200 Hz) for around 2 weeks (Moreno et al. 2017). Ca2+ imaging has also been undertaken in rice where recombinant aequorin was introduced and Ca2+ spikes were recorded upon salt stress (Zhang et al. 2015). The roots of transgenic rice showed strong luminescence during excess exogenous Ca2+ (Zhang et al. 2015). Actually, recording of [Ca2+]cyt dynamics in monocot plants is way more difficult as compared to dicots. However, Volkmann et al. (2009) were able to generate transgenic winter wheat lines which showed stable constitutive expression of aequorin in the cytosol. With the help of luminometry device, temperature-induced Ca2+ dynamics were recorded. The Ca2+ dynamics was easily detectable under cold stress implying the significance of Ca2+ signaling under stress in wheat (Volkmann et al. 2009). Aequorin-based luminescence has also been utilized to analyse the specific amino acid responsible for evoking Ca2+ signaling in rice roots. Glutamate (Glu) was found to participate in triggering Ca2+ flux in the root system (Ni et al. 2016).
Fig. 3.
The pictorial representation of aequorin bioluminescence based Ca2+ imaging. The cells that make apo-aequorin are first subjected to an incubation process with the coelenterazine compound, which gets into the cells. This incubation leads to the generation of functional aequorin within the cells. The binding of Ca2+ to aequorin induces a conformational alteration in the protein, resulting in the destabilisation of the peroxide group (-O-O-). This interaction facilitates the attachment of apoaequorin to coelenterazine, causing its decomposition into coelenteramide and CO2. The excited state of coelenteramide emits blue light with a maximum wavelength of 469 nm. When cells are subjected to an environmental stress, the second messenger, Ca2+ permeates the cytoplasm and interacts with aequorin, resulting in the emission of light by the cell. The luminescence traces provide information on both the magnitude and the duration of the Ca2+ concentration wave in the cytoplasm (Shimomura 1995; Webb et al. 2015)
Quin-2 is used in case of intact cells along with membrane permanent ester derivatives. Quin-2 works under an interesting principle. The ester group is separated by the cytosolic esterase, trapping the quin-2 and tetra anion in the cytosol. Quin-2 is excited at 339 nm that excites majorly autofluorescence, thus, causes damage to the cells. To overcome this auto-fluorescence, Quin-2 must be used in a very low concentration, for instance, one tenth of a milli-mole (mM). Upon binding Ca2+, Quin-2 does not show much shift in emission or excitation wavelength. However, fluorescence intensity is dependent on various factors such as intensity of illumination, efficiency in emission collection, concentration of dye and most importantly, on the emission-excitation shift. Therefore, it is always recommended to select a dye that shows better emission-excitation shifts (Grynkiewicz et al. 1985). Whereas, Quin-2 can be used for the Ca2+ at resting state, which is approximately 10–7 M. However, at µM concentrations, the dye loses its resolution and reaches its saturation point. Therefore, to compensate this loss of efficacy, low affinity dyes are preferably used. Quin-2 also shows affinity for Mg2+ ions, without affecting fluorescence intensity. However, variation in Mg2+ concentration could be a possible reason for effective binding of Quin-2 with Ca2+ and thus, the fluorescence intensity (Grynkiewicz et al. 1985). Quin-2 has been used to investigate unknown biological functions related to Ca2+ signaling in plants. Plasma membrane associated cation-binding protein (PCaP1) is involved in a number of developmental functions and Ca2+ signaling in plants. PCaP1 is attached to PM via N-myristoylation and a polybasic cluster. It also binds Ca2+-CaM moiety via N-terminal. Impact of myristoylation on the complex PCaP1-Ca2+-CaM and the modulation in Ca2+ sensitivity property of CaM in the complex remains unelucidated. The kinetic study of the complex in association with myristoylation and Ca2+ was studied with the help of Quin-2 and the functional role of PCaP1 and its structural aspect was demonstrated (Pedretti et al. 2023). With the utilization of Quin-2, Walton et al. (2017) reported that the divergent soybean CaMs (CaM1 and CaM4) behaved similarly on Ca2+ transient (Walton et al. 2017). Quin-2 has also been used to study the role of IQ motif in regulating function of CaM (Putkey et al. 2003; Shen et al. 2016).
The ratiometric fluorescent Ca2+ indicator was developed as an enhanced alternative to Quin-2 (Tinning et al. 2018).
FURA-2, a derivative of salicylaldehyde, represents a member of the initial cohort of Ca2+ fluorescent indicator (Liao et al. 2021). Its excitation and emission wavelengths depends on Ca2+ levels (Eerbeek et al. 2004). There is a shift in the excitation peak wavelength from 380 to 340 nm upon binding of free [Ca2+]cyt to Fura-2. However, there is no alteration in the peak emission at 510 nm. The fluorescence intensity is stimulated between the wavelengths of 340 and 380 nm with subsequent emission occurring at a wavelength of 540 nm (Tinning et al. 2018). The amount of Ca2+ required, needs to be standardized with the utilisation of a Ca2+ ionophore, such as ionomycin, to establish a stable Ca2+ detection via Fura-2 (Di Virgilio et al. 2019). The suggested optimal concentration of Fura-2 AM is 1 mM. Actually, a lower concentration of 250 nM can also be used much accurately . The FURA-2 dye is less likely to bleach under light and has a wider range of Ca2+-free and Ca2+-bound conformations (Tinning et al. 2018). Fura-2 wide-field excitation technique employs an arc lamp equipped with a monochromator as a light source. The requirement of rapid alterations of the wavelength within milliseconds is a significant constraint (Tinning et al. 2018). Additionally, the arc lamp light source exhibits a 5% amplitude instability, thereby diminishing the efficacy of precise Ca2+ concentration measurements. The use of light-emitting diodes (LEDs) presents a potential solution to address certain drawbacks associated with Arc lamps. LEDs offer advantages such as enhanced stability during rapid switching at the millisecond level and the ability to provide precise control over output. (Tinning et al. 2018). A number of studies have been reported in animals as well as in plants with the utilization of Fura-2. It has been used to study the distribution of Ca2+ levels in vegetable tissues in plants (Bonomelli et al. 2010). It has also been used as an intracellular Ca2+ indicator to study the role of chitinases in rice. Chitinase protein is involved in defense, growth and development processes in plants. Wu et al. (2017) functionally characterized one of the potential genes, OsCLP (encodes chitinase-like protein), involved in growth and developmental processes in rice. It has been observed that T-DNA insertion in OsCLP resulted in retarded root and shoot growth phenotype in rice. Additionally, Fura-2 staining and inductively coupled plasma-mass spectrometry (ICP-MS) results demonstrated the low level of Ca2+ in osclp mutants as compared to the wild type plants (Wu et al. 2017). With the utilization of Fura-2, Ca2+ oscillations have been measured in plant-bacterial/fungal symbiosis (Harper and Harmon 2005). The Ca2+ binding property and dynamics of another Ca2+ binding protein, OsCCD1, was studied via Fura-2 under osmotic and salt stress conditions in rice (Jing et al. 2016). Chitosan elicitation mediates synthesis of anthraquinone (Aq) in Rubia tinctorum L. However, stimulation of Aq can be blocked by BAPTA-AM but not by EGTA and VDCC-antagonists, verapamil and nifedipine. Interestingly, it has been observed that upon chitosan treatment, Fura-2 loaded R. tinctprum cells showed enhanced Ca2+ concentration in Ca2+ devoid media (Vasconsuelo et al. 2005). Vafadar et al., (2021) has also demonstrated the role of melatonin, which improves the rate of photosynthesis in Dracocephalum kotschyi. Also, the Ca2+ dynamic was studied with the help of Fura-2 in the presence and absence of melatonin and its respective effect on photosynthesis (Vafadar et al. 2021). With the use of Fura-2, Ca2+ signaling was studied in order to understand the somatic embryogenesis of pro-embryonic cells of Santalum album (Anil and Rao 2000). A study utilized Fura-2 and Fluo-3 to observe the Ca2+ transient in root cells under salt and osmotic stress conditions in Arabidopsis (DeWald et al. 2001). The role of Ca2+ signaling in the formation of important secondary metabolites has also been seen. Resveratrol is known to modulate cellular Ca2+ responses (Kopp et al. 2014). Interestingly, only specific Ca2+ indicators such as Fura-2 could monitor the change in [Ca2+]cyt upon resveratrol application (Kopp et al. 2014). However, advanced indicators like Fura-4 and YC3.60 could not indicate any response (Kopp et al. 2014).
Indo-1 is a Ca2+ fluorescent indicator of the first generation (Liao et al. 2021). Ultraviolet (UV) light can excite Indo-1 as it exhibits absorption within the wavelength of 351–364 nm, and its emission peaks occur at 405 nm and 485 nm (Eerbeek et al. 2004; Betzenhauser 2011). The emission ratio at 485 nm is actually influenced by the incomplete hydrolysis of Indo-1 AM (Eerbeek et al. 2004). It is well-known that a single valid calibration is very challenging task to achieve. Still, Indo-1 AM is found to be a more effective approach for analysing alterations in Ca2+ levels in heart tissues within a semi-quantitative framework. Another important fact is the Ca2+ buffering capacity of Indo-1, which is relatively low and is essential to exhibit rapid kinetic properties (Eerbeek et al. 2004). All these characteristics render Indo-1, a suitable tool for evaluating Ca2+ transients in myocytes. Most importantly, Indo-1 transients remain unaffected by the background fluorescence of NADH which makes it more prominent to use among other chemical based Ca2+ indicators (Eerbeek et al. 2004). Indo-1 has been used to study Ca2+ oscillations in many studies. Auxin-induced [Ca2+]cyt was measured in root hairs of tomato. Plant–pathogen interactions is an interesting area of research where Ca2+ signaling play essential role. In Rhizobium–legume interaction, Nodulation (NOD) factors are the essential components which are synthesized by Rhizobium and are required for interaction with plants. It has been observed that NOD factors induce intracellular Ca2+ oscillations, which have been detected by Indo-1 (Downie and Walker 1999). With the use of Indo-1, Ca2+ dynamics were studied during microspore development and embryogenesis in Brassica napus and Solanum melongena (Sendra et al. 2017). Indo-1 has been utilized to study the Ca2+ dynamics in guard cells (Gilroy et al. 1991; Israelsson et al. 2006). Studies showinghow hormones coordinate with [Ca2+]cyt and the level of [Ca2+]cyt changes have been well documented via Indo-1 in barley (Gilroy and Jones 1992).
FLUO
The Ca2+ indicator of Fluo series emits the basal level of fluorescence at resting Ca2+ concentration which gets increased more than hundreds folds with increase in the Ca2+ concentration (Paredes et al. 2008). A number of Fluo series have been discovered time to time with advancement over the previous ones.
Fluo-3 is a long-wavelength fluorescent Ca2+ indicator whose excitation and emission wavelength lies in a range of 506 nm and 526 nm, respectively. Fluo-3 can be used in flow cytometry, microplate screening assays, confocal laser scanning microscopy, or light microscopy with standard sets of fluorescence filters (Gee et al. 2000a; Liao et al. 2021). The Kd value is 0.40 µM. It is easy to use, stable, and can be loaded in cells in different forms i.e., membrane soluble, acetoxymethyl (AM) ester form, or salt form. Most of the chemical indicators impose physiological impacts in a cell. However, Fluo-3 does not impart a negative impact on normal physiological processes, which makes it suitable to use (Gee et al. 2000a). On binding Ca2+, Fluo-3 fluorescence increases up to 40 folds, thus, it does not require ratiometric recording. Researchers have utilized Fluo-3 to study the qualitative Ca2+ concentration in mitochondria under various experimental conditions. Hence, it is way more helpful in studying the kinetic changes of mitochondrial Ca2+ concentration (Saavedra-Molina et al. 1990). Also, it is used in conjugation with BAPTA-like Ca2+ chelators, which are covalently linked with fluorogenic components (Gee et al. 2000a). It has been reported that Fluo-3 is predominantly localized in the cytoplasm but in the case of fibroblast cell, it is profoundly present in cell organelles as well (Kao et al. 1989). It has also been observed that the concentration of Fluo-3 is two-fold greater in the nucleoplasm than that present in the cytoplasm under normal conditions.
Contrary to Fluo-3, Fluo-4 is a single excitation wavelength indicator, but it shares some common features with Fluo-3 such as spectral value, Kd value, stability, ease in loading the dye, or Ca2+ dependent fluorescent increase (Tinning et al. 2018). Also, it is a second generation Ca2+ fluorescent indicator and is commonly used in high throughput screening (HTS), confocal, and flow cytometry imaging techniques (Liao et al. 2021). It gets excited at 480 nm, and emitted at 525 nm, which can be used for single-cell microscopy to observe Ca2+ concentration dynamics in numerous individual cells in one setting (Di Virgilio et al. 2019). Like Fluo-3, Fluo-4 also responds to Ca2+ binding by increasing the intensity of fluorescence, but not the spectral shift (Gee et al. 2000a). However, Fluo-4 has slightly higher Ca2+ binding affinity than Fluo-3. The structural composition of Fluo-4 is somewhat similar to Fluo-3. The only difference is the substitution of two chlorines in place of two fluorines in the fluorophore. This substitution is actually a major modification which is responsible for increasing the absorbance near 488 nm, thereby, imparting a greater advantage to Fluo-4 with less photo bleaching and photo-toxicity compared to Fluo-3 (Gee et al. 2000a). Therefore, a low amount of Fluo-4 dye can generate the same intensity of fluorescence as Fluo-3 . Moreover, the lower usage of dye reduces the buffering effect of Ca2+ and lowers the production of toxic by-product such as acetoxymethyl ester which is another advantage of Fluo-4 (Gee et al. 2000a). Fluo-4 shows more intense fluorescence when it is used with an argon ion source or with a standard set of fluorescence filters (Gee et al. 2000a). It is one of the most commonly used dyes for the study of pancreatic acinar cells and imparts minimal physiological disturbances in the cell (Betzenhauser 2011).
MAG FLUO-4 is a lesser-known, low-affinity Ca2+ dye which is used in cultured cells for the quantification of endoplasmic reticulum (ER) stress. Interestingly this particular dye is sensitive for Ca2+ as well as Mg2+ ions. Actually, it has high affinity for Mg2+ than Ca2+. Its Ca2+ quantification results provide an accurate rate and time. It is also very cost-effective for ER stress examination (Lebeau et al. 2021). Fluo-5 exhibits fluorescence properties, characterised by an excitation peak at a wavelength of 494 nm and an emission peak at 516 nm. The excitation process can be achieved by utilising a laser with a wavelength of 488 nm in conjunction with a bandpass filter of 530/30 nm. Fluo-5 exhibits spectral similarities with Fluo-8, matrix metalloproteinase (MMP), Ca2+-Green, Cal-520, and Calbryte 520 (Kabbara and Allen 2001).
Fluo-8 is an innovative Ca2+ indicator that employs the fluorescence core, also present in Fluo-3 and Fluo-4, for the purpose of monitoring Ca2+ concentration and flux within cells. This allows Fluo-8 to maintain spectral characteristics that are indistinguishable from Fluo-4. However, it addresses the limitations of Fluo-3 and Fluo-4 (Rietdorf et al. 2014). The introduction of minor structural modifications has contributed to the development of Fluo-8, resulting in various improvements in its application. One notable improvement is the optimised loading conditions of Fluo-8. Similar to other fluorescent dyes, Fluo-8 lacks fluorescence in its Ca2+-free state. When it binds Ca2+, it exhibits a significant increase in fluorescence intensity, approximately 200 times greater than its initial level. This exhibits a twofold increase in brightness compared to Fluo-4 and a fourfold increase in brightness compared to Fluo-3. The temperature dependence of Fluo-8 is comparatively lower in comparison to other probes, thereby, generating more consistent and reproducible outcomes. It is frequently employed to ascertain the dynamic alterations in Ca2+ levels within the guard cells (Lock et al. 2015).
There are a number of studies where researchers have employed various versions of Fluo in plant research. [Ca2+]cyt level is of utmost importance in various developmental pathways in plants including pollen tube (PT) growth, fertilization and developmental processes in angiosperms. A very interesting study came into picture in 1997, when transient elevation of free [Ca2+]cyt was monitored during fusion of egg and sperm cells in maize during in vitro fertilization (Digonnet et al. 1997). The study was undertaken by the use of Fluo-3 dye under controlled physiological conditions to study the importance of Ca2+ transients during egg activation and early zygote development in plants (Digonnet et al. 1997). Fluo-3 was used in studying Ca2+ changes due to the infection caused by Spodoptera littoralis (Kanchiswamy et al. 2010). Ca2+ signaling plays a significant role in developing female sex organs in higher plants. In one of the reports, Fluo-3 was utilized to report Ca2+ in isolated female sex cells; egg cells and central cells of tobacco, thus, contributing to study of developmental aspects in plants (Peng et al. 2009). Glycine betaine (GB) is an important component which is responsible to enhance heat tolerance via accumulation of heat shock proteins (HSPs) in plants. But, how GB stimulates HSPs under salt stress, is not known. Li et al. (2013) observed Ca2+ transient in tobacco seedling under salt stress.
Fluo-3 has been utilized to monitor [Ca2+]cyt which contributes to anthocyanin biosynthesis in hypocotyl of radish sprouts under UV-A irradiation (Li et al. 2014). Ceramide sphingolipids are important components of cell membrane and promote programmed cell death (PCD) in plants as well as in animals. The mechanism by which ceramide induce PCD in rice is not well known. It has been speculated that Ca2+ levels go high just after ceramide application in rice. With the utilization of Fluo-4, researchers monitored Ca2+ levels on the application of ceramide in rice (Zhang et al. 2020). Fluo-4 was also used in measuring Ca2+ which worked parallelly with electric, ROS and hydraulic signaling in Arabidopsis (Fichman and Mittler 2021). Interestingly, jujube growth and development rely on Ca2+ which has been monitored by Fluo-4-AM in one of the studies (Wang et al. 2023). Apple pulp was stained by Fluo-4 to calculate the [Ca2+]cyt (Qiu et al. 2021). MAG-Fluo-4 and Fluo-5 have been used majorly in animal sciences. MAG-Fluo-4 has been used to study the involvement of Mg2+ in the development of flowers in shoot apex of Pharbitis nil (L.). The elemental distribution has been studied via Fluo-3 AM and Mag-Fluo-4 AM. The former is solely for Ca2+ detection whereas the latter can be used to detect both Ca2+ along with Mg2+ (Kobayashi et al. 2006). With the help of Fluo-8, Ca2+ change was measured in stomatal closure via ABA modulated NADK2 (NAD kinase 2) pathway in Arabidopsis guard cell (Sun et al. 2017). Protoplasts are a useful toolbox to detect plant responses under stress conditions. Fluo-8 has also been used to monitor Ca2+ level under various stress in protoplast of Arabidopsis (Gilliard et al. 2021). Submergence effect induces hypoxia condition in plants. Hypoxia signaling is evolved in submerged species in order to protect the plants from hypoxia stress. Nitric oxide (NO) plays a significant role during hypoxia stress conditions. NO signaling cross-talk with Ca2+ signaling plays an essential role in plant defense mechanism under stress conditions. With the utilization of Fluo-8, Ca2+ levels were reported under NO-induced tolerance against hypoxia stress response in maize (Li et al. 2022b).
CALIBRYTE 520 AM and RHOD-2
Calibryte 520 AM is a chemical based Ca2+ indicator, that was developed in order to get rid of the limitation of Fluo-4 AM that requires probenecid. Probenecid is an organic ion transporter inhibitor, which basically prevents leakage of dye. But, it also negatively affects the efficiency of the indicator (Liao et al. 2021). Interestingly, Calibryte 520 AM does not require probenecid, and hence, limits the harmful effects of the probenecid, or improves dye retention. It is a Ca2+ indicator with similar fluorescence and spectral properties that of Fluo-4 AM. Calibryte 520 AM shows passive diffusion across the cell membrane (Liao et al. 2021). After entry into the cell, the intracellular esterase cleaves the lipophilic blocking group of Calibryte 520 AM which subsequently, results in a negatively charged dye and hence, restricts the exit of Calibryte 520 AM from the cell. Upon binding Ca2+, its fluorescence intensity is enhanced by more than 100 folds. This dye can be used for the sample which requires a longer time for signal recording and processing and has been extensively utilized in animal research (Liao et al. 2021).
RHOD-2 is likewise one of the first-generation Ca2+ fluorescent indicators (Liao et al. 2021). It shows excitation at 524 nm and emission at 589 nm (MacGowan et al. 2001a). It has greater tissue penetration ability because of the longer excitation and emission wavelengths and has lower interference resulting from the natural occurring fluorescent compounds, e.g., NAD(P)H. The binding of the Ca2+ with the Rhod-2 increases the fluorescence by 100 folds. The major drawback of using Rhod-2 is that ratio techniques for fluorescence quantification cannot be used. It is because of the fact that this dye does not display a shift in emission or excitation spectra. For the measurement of maximum fluorescence of Rhod-2, it is saturated with Ca2+ using cyclopiazonic acid, which inhibits the ATPase and leads to the blockage of Ca2+ uptake by the sarcoplasmic reticulum (MacGowan et al. 2001a). Earlier, for the measurement of maximum fluorescence, digitonin was used. However, it causes leakage of Rhod-2 and cytosolic proteins from the cell thus, affecting maximal fluorescence value (MacGowan et al. 2001a). Rhod-2 is also extensively utilized in animal sciences, however plant based Ca2+ signaling has also been undertaken with the help of this indicator in a few studies. With the help of Rhod-2, [Ca2+]cyt was measured during mitochondrial Ca2+ release because of the disruption of actin filaments (Wang et al. 2010). Salt stress induced Ca2+ spikes were measured by Rhod-1 in the perennial herb, Glycyrrhiza uralensis (Lang et al. 2017).
Ca2+ ORANGE-AM, Ca2+ Green, Ca2+ green dextran, Ca2+ Ruby
Ca2+ Orange-AM has excitation wavelengths of 549 nm and 576 nm. The ratio of signal to noise is the highest. The fluorescence of Ca2+ Orange-AM exponentially increases with an increase in cell density up to 106 per well (Lam et al. 2005). Usually 0.04% pluronic F-127 (a non-ionic surfactant polyol) is used with Ca2+ Orange to increase its efficiency. Pluronic F-127 is a non-ionic, polyglot surfactant that helps in Ca2+ indicator AM ester dispersion. It has been observed that when 0.04% of pluronic F-127 is increased to 0.08%, there is a 60% increase in background fluorescence (Lam et al. 2005). However in 0.04% pluronic F-127, there is almost negligible or no background fluorescence (Lam et al. 2005). When the dye is stained/loaded with ionomycin (Ca2+ chelator), there is an enhancement of fluorescence intensity, whereas the fluorescence gets diminished when treated with BAPTA-AM just after 20 min of incubation. Therefore, it can be concluded that the Ca2+ Orange exhibits a change in Ca2+ concentration in presence of Ca2+ chelators and ionophores in the cell. Due to its longer excitation wavelength, it can reduce the autofluorescence-related problem, and can be visualized under confocal microscope and spectrofluorometer (Lam et al. 2005). Again, Ca2+ Orange has been utilized less in plant based research. With the help of this Ca2+-orange AM, it has been proven that intracellular Ca2+ is required for the development and pathogenesis of the fungus, Magnaporthe oryae (Rho et al. 2009). It has also been used to study Ca2+ signaling in the green alga, Ulva linza, wherein Ca2+ spiking has been recorded during settlement and adhesion of zoospores (Thompson et al. 2007).
Ca2+ Green-1 is a fluorescent chemical indicator which gets energised at visible light wavelengths. Like other indicators, it gets intensified when it binds Ca2+. The excitation and emission wavelengths are 506 nm and 531 nm, respectively. The usage of Ca2+ Green-1 in [Ca2+]cyt was compared with two regularly used dyes, i.e., Fura-2 and Fluo-3. It has been observed that Fura-2 which requires UV for its excitation, showed a quenching effect in platelet cells upon treating with menadione (UV absorbent and a quinone derivative agent). Also, Fluo-3 leaked from platelets very quickly. Additionally, Fluo-3 requires anion channel blockers (as mentioned earlier) which are known to alter platelet physiological responses (Lee et al. 1999), whereas, Ca2+ Green-1 can be used to detect the changes in [Ca2+]cyt levels without such issues (Lee et al. 1999; Kuchitsu et al. 2002). Ca2+ Green-2 is a non-ratiometric fluorescent Ca2+ indicator having lower affinity for Ca2+ (Kd = 3 µM) than more regularly used indicators such as Fura-2 and Fluo-3 etc. Because of the low Ca2+ affinity and elevated quantum yield, cells can be supplied with low concentrations of Ca2+ Green dye, hence, [Ca2+]cyt buffering can be overcome. Ca2+ Green-2, the advanced version, is even more advantageous in terms of low signal-to-noise ratio (Spencer and Berlin 1995).
Ca2+ Green Dextran (CGD) was originally created to address issues with Ca2+ -sensitive dyes in their free form, such as intracellular dye compartmentalization and dye leaking from cells. CGD stays sensitive to changes in intracellular Ca2+ after being taken up by the cell for at least 4 days, which is the longest life duration. If Ca2+ sensitivity is found to persist over longer survival durations, cells may be labelled more intensively and over a greater distance (Kreitzer et al. 2000). Ca2+ diffusion from the site of release was reduced using a high-molecular-weight Ca2+ buffer (Ca2+ green-1 dextran), which also served as a Ca2+ indicator (Lee et al. 1999). Ca2+ Green-1 has been used to study Ca2+ oscillation during touch in Arabidopsis (Legué et al. 1997). It has also been utilized to study the dynamics of Ca2+ during self-incompatibility response in Papaver rhoeas (Wang et al. 2016). Ca2+ mediated CO2 signaling pathways has been studied with the help of Ca2+ Green-1 in Chlamydomonas reinhardtii (Franklin-Tong et al. 1993). It is reported that the stress stimulus induced Ca2+ oscillation, which was monitored by Ca2+ Green-1 in guard cells of Commelina communis (McAinsh et al. 1995). Ca2+ Green-1 was also used in reporting [Ca2+]cyt change in pollen tube with the regulation of phosphoinositides and phosphatidic acid in Agapanthus umbellatus (Monteiro et al. 2005). The pollen tube orientation due to [Ca2+]cyt change was also studied by the use of Ca2+ Green-1 in A. umbellatus (Malhó and Trewavas 1996). Ca2+ green dextran has also been utilized in studying elemental propagation of Ca2+, cell-to-cell communication induced by Ca2+ fluxes, mitochondrial Ca2+ release due to disruption in actin filament, and Ca2+ wave propagation in Fucus rhizoid cells in plants (Tucker and Boss 1996; Goddard et al. 2000; Coelho et al. 2002; Wang et al. 2010).
Furthermore, the continuous need of high affinity and signal to noise ratio of red-emitting Ca2+ probes led to the creation of Ca2+ Ruby Nano, a novel functional red Ca2+ indicator with a nanomolar sensitivity (Collot et al. 2015). To get the high affinity CaRuby, a few modifications were made in the already existing CaRuby of first generation. The major change was the introduction of oxygen group on BAPTA cycle in order to make it electronically powerful. The next modification was to transfer the fluorophore moiety from the para- to the meta-position. This moiety is a positively charged rhodamine, that has an electron-depleting effect. These modifications lead to the production of a new CaRuby i.e., CaRuby-Nano (Mallet and Collot 2015). It exhibits absorption wavelength at 575 nm and emission wavelength at 610 nm with a Kd value of 295 nM (Otsu et al. 2015). Hence, it gives a bit higher affinity compared to the Fluo-4 (Kd value of 335 nM). It exhibits a 50-fold increase in fluorescence upon binding Ca2+. Therefore, it is considered as the best candidate for measuring small intracellular changes when it binds Ca2+. The quantum efficiency of CaRuby-Nano is 0.45 which is lower than another chemical Ca2+ indicator i.e., Oregon Green-488b BAPTA-1 AM (OGB-1) having quantum efficiency approx. 0.7, but greater than Fluo-4 i.e. approx. 0.14. CaRuby-Nano is suited for both in vivo and in vitro Ca2+ imaging studies. It can be used along with other probes to obtain a dual colour image of brain (Collot et al. 2015).
Genetically encoded Ca2+ indicators (GECI)
The GECI binds to Ca2+ and detect intracellular Ca2+ via emitting fluorescence and monitor in vivo cell activity. It is characterized into two basic classes i.e., single fluorophores based and fluorescence resonance energy transfer (FRET) based indicators.
Single fluorophores
Camgaroos
The Camgaroo indicator is the first discovered single fluorescent GECIs (Griesbeck et al. 2001). Camgaroos are intrinsically sensitive to the pH, thus, it is possible that the change in the fluorescence is more likely to be caused by changes in proton ([H+]) than by Ca2+ (Hasan et al. 2004). Camgaroos do not comprise of CaM binding peptide and thus, results in low level of Ca2+ binding affinity compared to the G-CaMP or Pericam (Griesbeck et al. 2001). Camgaroo-1 is a chimeric protein, yellow in color and contain CaM in its pouch with high bouncing capability in signal and hence justified the name. It has been observed that when histamine is added to the Camgaroos-1 expressing HeLa cells, there is a modest increase of fluorescence intensity i.e., 40% which results in diminished Ca2+ spiking activity, in such case Camgaroos-1 is not as bouncy as its name depicts while sensing [Ca2+]cyt. Whereas, with the usage of ionomycin, the intensity goes 7 folds high (Ai 2015; Yu et al. 2002). Camgaroo-1 has a large absorption peak at about 400 nm in the absence of Ca2+, and a dominating peak at around 490 nm under Ca2+ saturation. The deprotonation form which absorbs at 490 nm is extremely fluorescent in contrast to the protonated form, which shows absorbance at the same wavelength. An emission maximum occurs at about 515 nm after a single peak in the fluorescence excitation spectrum at 490 nm. Thus, Camgaroo-1 is a fluorescent intensometric probe (Ai 2015). However, it shows poor expression at 37 °C and is not targeted to organelles such as mitochondria which limit its usage (Griesbeck et al. 2001). Camgaroo-2 had a similar structure as Camgaroo-1since it is generated from random mutagenesis of Camgaroo-1. It also exhibits 6-to-sevenfold increase in fluorescence intensity upon Ca2+ binding as that of the Camgaroo-1 and has the same Kd value (Ai 2015; Yu et al. 2002). There is an increase in basal fluorescence and this is due to the replacement of 69th amino acid residue i.e., glutamine to methionine in Camgaroo-2 (Q69M) (Yu et al. 2002). Camgaroo-2 shows better expression at 37 °C and can be targeted to organelles such as mitochondria (Griesbeck et al. 2001).
Pericam
Pericam line of sensors were created by the Miyawaki group (Whitaker 2010). Enhanced yellow fluorescent protein (EYFP)-Val68Leu/Gln69Lys was circularly permuted to develop a novel protein which consists of Y145 and N144 as N terminal and C terminal (cpEYFP), respectively along with M13 and CaM (Ai 2015; Miyawaki et al. 1997; Whitaker 2010). It has been observed that when CaM fuses with N-terminus, the resulting construct (CaM-cpEYFP-M13) does not exhibit any Ca2+-dependent properties (Nakai et al. 2001). But in reverse conditions (M13-cpEYFP-CaM), there is about 3 fold increase in fluorescence at 520 nm under high Ca2+ levels as compared to 485 nm in Ca2+ free media (Shimozono et al. 2002). Additionally, three point mutations in Pericam resulted in the production of another Pericam, known as Flash Pericam. This point mutation resulted in the increase in fluorescence upto 8 folds at 520 nm. It is a non-ratiometric single wavelength indicator with a 0.7 µM Kd value (Whitaker 2010). Its absorbance spectrum is comparable to cpEYFP (V68L/Q69K) in the absence of Ca2+. On Ca2+ saturation, the pH titration curve shifts towards left due to the chromophore ionization which is actually caused by Ca2+ association with CaM and M13. At an optimum pH (9.0) for the ionization of chromophore, the Flash Pericam bound to Ca2+ exhibits twofold higher brightness than the free Flash Pericam. When Flash Pericam carries a substitution of phenylalanine at 203 residue within YFP, this resulted in the fluorescence on the protonated form and generation of a new construct i.e., Ratiometric Pericam, which is basically a sensor having an absorbance at 494 or 415 nm and emission at 520 nm. The functional properties of Ratiometric Pericam is analogous to Fura-2. It varies tenfold between Ca2+ free and Ca2+ saturated condition with a Kd of 1.7 µM (Whitaker 2010). Similar to Flash Pericam, it shows Ca2+-dependent changes in its absorbance spectra, and shifts the pH titration curve to the left upon binding Ca2+ (Pologruto et al. 2004). Additionally, semi-random mutagenesis in Ratiometric Pericam results in a construct named Inverse Pericam with having single wavelength and decreased intensity of fluorescence at 513–515 nm on binding with Ca2+ (Whitaker 2010). Interestingly, it can detect even low Ca2+ concentration in mitochondria and is a better option than Rhod-2 (Korzeniowski et al. 2009). The two important advantages of Inverse Pericam are the powerful brightness and the similarity of excitation/emission properties to fluorescein which is also a characteristic feature of Flash Pericam. Flash and Ratiometric Pericam are the YFP-based indicators which share common features with single wavelength Ca2+ sensors i.e., Fluo-3 and Fluo-4 (Pologruto et al. 2004). The difference between Ratiometric Pericam and Inverse Pericam is that Ratiometric Pericam shows slower decay time whereas Inverse Pericam shows constant decay time (Kaestner et al. 2014). Both Camgaroo and Pericam are used extensively in animal research.
GCaMP
GCaMP3 is a monochromatic sensor primarily used for observing live cells and performing in vivoCa2+ imaging. It relies on components such as calmodulin (CaM), the CaM binding domain RS20, circularly permuted green fluorescent protein (cpGFP), and the Ca2+/CaM-binding "M13" peptide. When the cellular Ca2+ concentration changes and Ca2+ binds to CaM, it leads to the protection of the cpGFP chromophore from the surrounding aqueous environment. Consequently, this results in an increase in fluorescence emission intensity. In mice, GCaMP3 finds utility in detecting neuronal activity in large populations within the motor cortex, barrel cortex, and hippocampus. These indicators are not suitable for quantitative ratiometric measurements because they exhibit changes in intensity without spectral shifts. Moreover, the maximum fluorescence intensity also varies with the level of expression. GEX-GECO1 (Fura-2-like) and GEM-GECO1 (Indo-1-like) were developed through random mutagenesis of GCaMP3 (Cho et al. 2017). Furthermore, each line expressing GCaMP3 was tested to observe how it responds to a non-injurious application of ice-cold water. The study aimed to understand the wound signaling in plants by using GCaMP3 as an indicator. They tested whether the mid-vein alone could transmit the wound signals to a distant leaf by removing extracellular tissue surrounding the primary vasculature of the leaf. The researchers also examined the spatial distribution of wound-stimulated GCaMP3 fluorescence (Nguyen et al. 2018). In another aspect of the study, the focus was on determining how aphid saliva affects changes in [Ca2+]cyt levels in plants. They used CaMV35S: GCaMP3 transgenic N. benthamiana and showed that saliva obtained from both Serratia-free and Serratia-infected aphids induced a powerful Ca2+ signal within the first 90 s of a 300-s observation period (Wang et al. 2020). The role of Ca2+ in biotic stress using GCaMP3 was shown through live biotic interactions between the green peach aphid (Myzus persicae) and Arabidopsis (Vincent et al. 2017). In addition, the study sought to uncover how different cell types in plant roots react to fluctuations in [Ca2+]cyt triggered by both internal and external signals. To investigate this, scientists engineered Arabidopsis plants with GCaMP3 expression in five distinct root cell types: columella, endodermis, cortex, epidermis, and trichoblasts. They observed both commonalities and differences in how [Ca2+]cyt levels changed in response to substances like adenosine tri-phosphate (ATP), glutamate, aluminium, and salt, all of which are known to induce [Ca2+]cyt increases in root cells. These specialized GCaMP3-modified plant lines provide a valuable resource for conducting more in-depth studies aimed at understanding the connections between specific environmental cues and distinct root development pathways mediated by [Ca2+]cyt signaling (Krogman et al. 2020). In another study, the altered root growth due to impaired PEIZO1 channel activity in root tips exposed to mechanical stress was studied. To investigate this, the Ca2+ response to mechanical stimulation in living plants was examined using a transgenic line that expressed GCaMP3 (Mousavi et al. 2021). Additionally, how leaves and roots react to extracellular ATP and its potential association with Ca2+ signaling and DORN1/P2K1 was examined. The alterations in [Ca2+]cyt levels in response to extracellular ATP were observed by employing wild-type roots that expressed GCaMP3 (Matthus et al. 2019).
GCaMP5s are derived from the GCCaMP3 framework through a specific library screening process that targets the interface between cpGFP/CaM and two interdomain linkers. Several GCaMP5 variants exhibit a remarkable fluorescence increase of over 150-fold upon binding Ca2+. Notably, in cultured neurons, GCaMP5G demonstrated a higher response to maximal stimulation (Akerboom et al. 2013). As RHO OF PLANTS (ROPs) are known to play key roles in controlling Ca2+ gradients, post-Golgi secretion, ROS generation, and the dynamic arrangement of actin microfilaments (MFs) during pollen tube growth, researchers were curious if these intracellular processes undergo dynamic changes during pollen germination in a BDR dependent manner. To investigate this, researchers introduced specific fluorescence probes into bdr8/bdr9 plants. These probes included GCaMP5 for monitoring Ca2+ concentrations, mRFP-RabA4b to track post-Golgi secretory vesicles, H2DCF-DA for visualizing ROS, and LifeAct-GFP to observe the dynamics of actin microfilaments (MFs;Xiang et al. 2023). G-CaMP5 was found to be an effective Ca2+ indicator for imaging Ca2+ levels in Arabidopsis pollen cells. The research utilized G-CaMP5 to reveal that Ca2+ forms a gradient focused on the tip of the pollen tube and exhibits oscillations during pollen tube growth. Additionally, a significant and temporary increase in cytosolic Ca2+ concentration was observed during pollen tube emergence. G-CaMP5 was also used to monitor changes in cytosolic Ca2+ levels in response to various treatments (Diao et al. 2018).
GCaMP6 variants are characterized by their high brightness and rapid detection of changes in [Ca2+]cyt levels, often triggered by a single action potential. These variants were developed by introducing specific point mutations into the GCaMP5G framework, resulting in the creation of the GCaMP6 series. Within this series, the selected members, GCaMP6f, GCaMP6m, and GCaMP6s, differ in their response kinetics to fluctuations in Ca2+ levels. Among them, GCaMP6s stands out as the most sensitive to Ca2+ changes.
Recently, using GCaMP6f-mCherry under the control of UBQ10 promoter, Fu et al. (2022) demonstrated the role of Ca2+ signaling in maintaining manganese uptake and homeostasis in Arabidopsis (Fu et al. 2022). Based on genetically encoded Ca2+ sensor and advanced microscopy, Vigani and Costa (2019) revealed the potential of Ca2+ signaling in nutrient homeostasis in plant (Vigani and Costa 2019). Liu et al. (2017) showed that nitrate signaling coordinates with Ca2+ signaling via live imaging. Nitrate signaling involves NRT1.1/NPF6.3 transporters; transcription factors (TFs), CIPKs, phosphatases 2C and nitrate cis-regulatory elements (NRE). NLP6 and NLP7 are the major TFs which participate in nitrate responses. Nitrate-induced Ca2+ signaling was detected using transgenic aequorin seedlings and found to be more prominent as compared to flg22-derived Ca2+ signaling. This result was further validated by using Ca2+ biosensor, GCaMP6, co-expressing with nuclear mCherry and found that unique Ca2+ spikes were enhanced in nucleus and cytosol upon supplementation with nitrate. Interestingly, the signal vanished away when EGTA (Ca2+ chelating agent) was applied (Liu et al. 2017). Ca2+ signaling plays a crucial role in plant-pathogen and plant–insect interactions. Recently, Parmagnani et al. (2022) demonstrated the evocation of Ca2+ and ROS during herbivore-associated molecular patterns (HAMPs). The group has utilized various Ca2+ dyes, modern genetic probes and indicators in plant-pathogen interactions to study Ca2+ oscillation under such conditions (Parmagnani and Maffei 2022). To study local and distal Ca2+ signaling upon herbivory attack, GCamP6s was used, which is more advanced and sensitive than GCaMP1 to GCamP5 and demonstrated the role of GLR3.3 and GLR3.6 channels in systemic Ca2+ signaling in Arabidopsis (Xue et al. 2022).
Efforts in the past to enhance GCaMP kinetics has seen limited success. However, the development of jGCaMP8 sensors marks a significant breakthrough. These sensors comprise three distinct variants: jGCaMP8s, known for its rapid rise and slow decay, combined with high sensitivity; jGCaMP8f, characterized by fast rise and fast decay; and jGCaMP8m, featuring swift rise and medium decay rates. Remarkably, all jGCaMP8 sensors exhibit nearly tenfold faster fluorescence rise times when compared to earlier GCaMP iterations. What sets the jGCaMP8 sensors apart is their improved linearity, which is a significant advantage over previous GCaMP variants. This enhanced linearity facilitates accurate spike estimation through advanced deconvolution techniques. Notably, these sensors excel in addressing key limitations seen in earlier GECIs. They demonstrate rapid kinetics in response to fluctuations in Ca2+ levels. Moreover, the jGCaMP8 sensors maintain several essential characteristics shared with other GCaMP sensors, including exclusion from the nucleus, robust fluorescence, as well as similar excitation and emission spectra. Specifically, jGCaMP8s stand out as they exhibit the most significant fluorescence change in response to a single spike compared to all other Ca2+ indicators. It also boasts a moderate half-decay time of 200 ms in the mouse brain. However, there's a trade-off to its heightened sensitivity. jGCaMP8s can saturate at a lower spike count, resulting in a reduced dynamic range. Nonetheless, this limitation is partially mitigated by its improved linearity and kinetics (Zhang et al. 2023).
The genetically encoded Ca2+ indicator, GCaMP-HS (GCaMP hyper-sensitive), represents an upgraded version of the original GCaMP, offering heightened sensitivity to changes in cellular Ca2+ ion (Ca2+) concentrations (Muto et al. 2011). This enhanced sensitivity is achieved through the incorporation of superfolder GFP mutations which is formed by amino acid substitution. The resultant construct increases folding activity but also enhances fluorescence output (Akerboom et al. 2012). Muto et al. (2011) introduced four specific superfolder mutations—S30R, Y39N, N105T in the N-terminus region of GFP, and A206V in the C-terminus region of GFP—into the previous iteration of GCaMP, known as GCaMP2 (Muto et al. 2011). This genetic modification led to a novel variant termed GCaMP-HS. GCaMP-HS exhibited an absorbance wavelength at 488 nm and emission at 509 nm, along with a Kd value of 105 nM in the presence of 300 nM Ca2+. One notable finding was that the levels of the GCaMP-HS protein consistently exceeded those of the GCaMP2 protein by a factor of 1.7. This suggests that the heightened folding efficiency induced by the superfolder mutations led to increased cellular expression levels. Consequently, this elevation in cellular expression contributed to the baseline fluorescence enhancement observed in GCaMP-HS (Muto et al. 2011).
FRET-based sensors
Genetically encoded Ca2+ biosensors are valuable tools in cell biology and neurology, but areas like signal strength and response kinetics need improvement. Forster resonance energy transfer (FRET) biosensors have revolutionized live cell imaging by allowing real-time chemical processes to be observed with high temporal resolution (Yoon et al. 2020). EGFP and Fusion-Red fluorophores were used to make a new Ca2+ biosensor called FRET-GFP-Red. This biosensor allows precise targeting of subcellular areas and repeated stimulation for longitudinal studies. The study supports a consistent 10–15% FRET response of a FRET-GFP-Red construct (Yoon et al. 2020). A new Ca2+ ion probe, H2BD3cpv, based on FRET, targets the nucleoplasm and measures Ca2+ in a precise way. The probe, adapted to mCerulean3, showed increased brightness and photostability and validated the similarity of cytoplasmic and nucleoplasmic Ca2+ concentrations in both basal and stimulated cellular states (Galla et al. 2021).
Yellow Cameleon
Yellow Cameleon indicators are majorly expressed in the cytosol and also expressed in guard cells (Allen et al. 1999). YC2.1 has been used successfully in the study of Arabidopsis guard cell Ca2+ dynamics (Allen et al. 1999). In Arabidopsis, ratiometric imaging of YC2.1 allowed Ca2+ imaging in a time-dependent manner (Grynkiewicz et al. 1985). In pollen tube growth, YC2.1 is used as an invasive method. In root hairs, the use of YC2.1 and a range of probes have limited the usefulness of YC2.1 (Lam et al. 2005). In most of the transgenic studies, increasing the expression level of YC2.1, CaMV 35S promoter driven expression is preferred. But the CaMV 35S promoter is associated with events like gene silencing and co-suppression. So, UBQ10 promoter can be used in the place of CaMV 35S as it is stable in plants, but has moderate target gene expression (Krebs et al. 2012). YC2.1 was first used for Ca2+ imaging in pollen tubes. The results showed that both Lilium longiflorum and Nicotiana tabacum had a Ca2+ gradient and tip-focused oscillations (Parton et al. 2003). Time-to-time a number of other modifications have been done to improvise the efficacy of these techniques.
Yellow Cameleon 3.6 was developed in Roger Tsien's laboratory. It is composed of CaM, donor chromophore (CFP), glycine linker, CaM-binding peptide, myosin light-chain kinase (M13), and acceptor chromophore (YFP). When Ca2+ binds to the CaM, an intramolecular interaction occurs between CaM and M13 that results in a change in the protein to a more compact form from an extended form, which increases the efficiency of FRET between CFP and YFP (Krebs et al. 2012; Behera et al. 2015). These dyes are FRET-based ratiometric Ca2+ indicators used (Fig. 4). They do not include the artifacts, which are the results of fluctuations in the Ca2+ indicator expression levels (Behera et al. 2015). It consists of a circularly permuted Venus (cpVenus). It is brighter, and on maturation, it is more efficient and has acid stability when compared with enhanced yellow fluorescent protein (EYFP) used in YC2.1 (Krebs et al. 2012). Thus, YC3.6 provides a high signal-to-noise ratio, which is why it can be used for various Ca2+ imaging experiments that were previously not possible with the other YCs (Nagai et al. 2004). Various YCs are targeted to the endoplasmic reticulum, peroxisome, and mitochondria and are thus successfully used to study the organellar Ca2+ dynamics and homeostasis (Behera et al. 2015). In guard and root cells, YC3.6 probes give efficient results for mitochondrial Ca2+ changes in response to different types of stimulus. Its Kd value for Ca2+ is 0.25 µM. The Ca2+ gradient during growth of the tip can be monitored using YC3.6 (Lam et al. 2005).
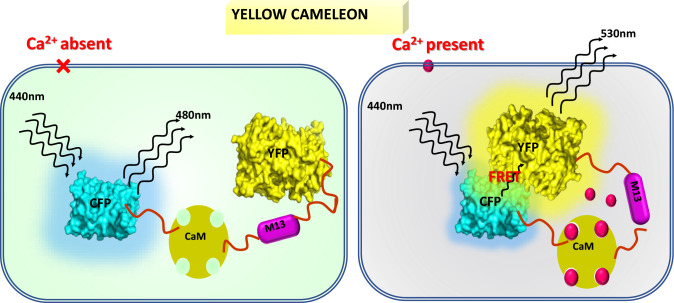
Fig. 4.
The principle model presenting YC-based Ca2+ imaging. In the absence of Ca2+ binding to the Cameleon protein, the excitation of the CFP by light with a wavelength of 488 nm leads to the emission of light with wavelengths approximately centered around 480 nm, which falls within the blue light spectrum. The process of Ca2+ binding to the M13 domain induces a conformational change in the protein, resulting in the proximity of the CFP and YFP domains. The proximity of the domains enables FRET to occur, facilitating the transfer of energy from the CFP to the YFP. Consequently, light with a wavelength of around 535 nm (yellow) is emitted. The relationship between the fluorescence intensities of YFP and CFP is directly proportional to the concentration of Ca2+ within the cell (Yellow-to-Cyan FRET Ratio = I_Yellow/I_Cyan) (adapted from Monshausen et al. 2008)
Ca2+ levels in cells are crucial signals for various biological activities. Using ratiometric Ca2+ reporter proteins like Yellow Cameleon (YC) has enabled us to understand more about the dynamic behavious of Ca2+ transient/signature in the living cells. This pioneering development in plant research has expanded to include single-cell models like pollen tubes and root hairs. However, the use of YC reporter proteins in plants has mainly focused on (Ca2+)cyt levels. YCs is composed of two forms- Green Fluorescent Protein (GFP): CFP and YFP, representing cyan and yellow, respectively (Behera et al. 2015). The M13 calcium-binding domain, derived from calmodulin, links the two forms. Under low Ca2+, the cyan fluorescent protein (CFP) emits blue light, which is induced by presence of higher Ca2+ and hence binding CaM which interact with the M13 domain and causes conformational changes. This conformational change causes the CFP and YFP domains to be near to each other, allowing FRET to occur, resulting in emission of yellow light (Fig. 4) (Behera et al. 2015). The fluorescence intensities of YFP and CFP are directly proportional to the concentration of Ca2+ in the cell or at a specific location. This ratio is advantageous as it remains unaffected by the quantity of YC3.6 protein in the cellular environment. Periodic measurements can be conducted to monitor changes in Ca2+ concentration. During development, reproduction, biotic and abiotic stress, plants generate a unique Ca2+ signatures. YC is a popular option for scholars looking to explore these characteristics. Some of the recent studies related to this sensor are briefly described here. Chlamydomonas reinhardtii, a single-cell microalgae, responds to light and the role of intracellular Ca2+ signals in these responses has been studied by using YC3.6 (Pivato et al. 2023). The ratiometric Ca2+ indicator YC3.6 was used, with a focus on the cytosol, chloroplasts, and mitochondria. In vivo single-cell confocal microscope imaging was used to examine light-induced Ca2+ signaling under various situations and genotypes, including the photoreceptor mutants, phot and acry. A genetically encoded H2O2 sensor was used to explore the potential involvement of hydrogen peroxide production in light-induced Ca2+ signaling (Pivato et al. 2023). The study found that Chlamydomonas reinhardtii cells exhibited a Ca2+ ion response reliant on light, found only inside the chloroplast. The intensity of light and photosynthetic electron transfer impacted this response. The study found a link between elevated H2O2 gradients in chloroplasts and transient increases in Ca2+ levels, confirming the role of light-induced chloroplast Ca2+ signaling (Pivato et al. 2023). Ca2+ signatures are crucial in Ca2+ signaling pathways and require high spatial and temporal resolution. Vectors and transgenic lines were developed to visualize intracellular Ca2+ dynamics in live plant cells using YC. The ubiquitin-10 (UBQ10) promoter from Arabidopsis is used to ensure consistent expression of YCs in transgenic plants. The vector repertoire includes multiple iterations directed towards specific areas. This provides an opportunity to examine Ca2+ dynamics in various cellular compartments and plant species, facilitating the development of innovative methodologies (Krebs et al. 2012). Behera et al. (2015) compared how the 35S promoter and the UBQ10 promoter works in the plants Arabidopsis thaliana and Oryza sativa. The study focused on the expression of the Ca2+ indicator YC3.6, controlled by either the UBQ10 or 35S promoters (Behera et al. 2015). The UBQ10 promoter performed better in terms of expression pattern, levels, and stability in both species. However, significant differences were observed in the spatiotemporal characteristics of the detected Ca2+ signals between the two species (Behera et al. 2015). Rice showed a decreased peak signal amplitude but a significantly prolonged signal duration compared to Arabidopsis. The study highlights the importance of comparative research for a comprehensive understanding of Ca2+ signaling in plant (Behera et al. 2015).
In eukaryotes, subcellular compartments like mitochondria, endoplasmic reticulum, lysosomes, and vacuoles can transport Ca2+ across their membranes to modulate enzyme activity or convey specific cellular signaling events. In plants, chloroplasts also display Ca2+ regulation. However, monitoring stromal Ca2+ dynamics in vivo has been limited by the use of the luminescent Ca2+ probe aequorin. A toolkit of Arabidopsis Ca2+ sensor lines expressing plastid-targeted FRET-based YC sensors has been presented. The probes reliably reportin vivo Ca2+ dynamics in the stroma of root plastids in response to extracellular ATP and leaf mesophyll and guard cell chloroplasts during light-to-low-intensity blue light illumination transitions. Applying YC sensing to single chloroplasts, the findings confirmed gradual, sustained stromal Ca2+ increases at the tissue level after light-to-low-intensity blue light illumination transitions. The toolkit identifies novel facets of chloroplast Ca2+ dynamics and refines the understanding of plastid Ca2+ regulation (Loro et al. 2013).Cells that grow from the tip are thought to have a tip-high Ca2+ gradient that controls parts of the growth machinery, such as the cytoskeleton, Ca2+-dependent regulatory proteins, and the secretory apparatus. In pollen tubes, both the Ca2+ gradient and cell elongation show oscillatory behaviour, reinforcing the link between the two (Monshausen et al. 2008). In Arabidopsis root hairs, a YC3.6 FRET-based Ca2+ sensor was used to monitor the oscillating tip-focused Ca2+ gradient (Monshausen et al. 2008). Both the elongation of root hairs and the associated tip-focused Ca2+ gradient show a similar dynamic character, oscillating with a frequency of 2 to 4 min−1. Cross-correlation analysis indicates that Ca2+ oscillations lag growth oscillations by 5.3 ± 0.3 s. However, growth never completely stops, and the concomitant tip Ca2+ level is always slightly elevated compared to the resting Ca2+ concentration along the distal shaft. Artificially increasing Ca2+ leads to an immediate cessation of elongation and thickening of the apical cell wall (Monshausen et al. 2008). Cross talk between Ca2+ and ROS signaling has been reported during root developmental processes in plants (Monshausen et al. 2007). Actually, [Ca2+]cyt is responsible for ROS generation which further stimulates downstream signaling pathway (Monshausen et al. 2007). The modulation in Ca2+, ROS and pH levels during root hair cell growth in Arabidopsis was monitored by YC3.6 Ca2+ biosensor, Oxyburst (ROS-sensitive dye), and GFP-H148D, a pH- sensitive probe, respectively (Monshausen et al. 2007). External and internal stimuli can temporarily increase Ca2+ in the cytoplasm, triggering a cascade of reactions that govern plant growth. A Ca2+oscillatory gradient at the pollen tube tip is essential for polarised growth. Disrupting this gradient can inhibit pollen tube development. Researchers have created genetically engineered Ca2+ YCs to assess the physiological function of (Ca2+)cyt in biological systems. The Cameleon uses FRET to measure changes in (Ca2+)cyt levels in developing pollen tubes (Barberini and Muschietti 2015). The transgenic plants that express the YC3.6 under the pollen-specific promoter LAT52 were employed to monitor how Ca2+ moves in the pollen tubes of tomato plants (Barberini and Muschietti 2015). The study aimed to understand the processes responsible for the generation and maintenance of the intracellular Ca2+ gradient, which is crucial for exocytosis and the transportation of fresh membrane and cell wall materials to the apex of the pollen tube (Barberini et al. 2018). Ratiometric fluorescent imaging techniques, specifically the YC3.6, have been used to investigate and interpret the Ca2+ signature generated by flg22 in individual guard cells of Arabidopsis thaliana. The determination of Ca2+ reserves and the kinds of channels involved in their creation was conducted using a pharmacological technique (Thor and Peiter 2014). The fluorescence-based Ca2+ sensors R-GECO1 and NES-YC3.6, are based on the intensity of the light. The results showed that R-GECO1 had a larger signal shift in response to different stimuli, such as the fungal elicitor chitin, which caused a strong [Ca2+]cyt oscillation in epidermal and guard cells. The elongation zone was found to be the starting point for both flg22 and chitin-induced Ca2+ signals in the root (Keinath et al. 2015). CCX2 is one such Ca2+ exchanger, localized on endoplasmic reticulum (ER), strongly expressed under salt and osmotic stress conditions (Corso et al. 2018). Cameleon Ca2+ biosensors revealed the importance of AtCCX2 gene, absence of which resulted in impaired Ca2+ dynamics in the ER and cytosol as compared to wild type (WT) and over-expression lines in Arabidopsis (Corso et al. 2018). Ca2+ imaging with fluorescent sensor, YC3.6, helped in the study of Ca2+ dynamics under dehydration and rehydration treatment in moss (Physcomitrella patens) (Storti et al. 2018).
D3cpv, N33D1cpv and N33D3cpv
D3cpv consists of a circular permutation of Venus (cpVenus). It is brighter, and on maturation, is more efficient and has acid stability compared with enhanced yellow fluorescent protein (EYFP) used in YC2.1 (Krebs et al. 2012). Since D3cpv comprises a dual fluorophore, the fluorescence ratio has the potential to be adopted to monitor Ca2+, overcoming movement aberrations (Kuhn et al. 2012). D3cpv is especially useful for researchers who are looking for minor changes in Ca2+ beyond basal levels because its Ca2+ Kd is precisely between YC2.1 and YC3.3, but it is no longer affected by excess CaM and has a fivefold increase in dynamic range. D3cpv might get localized to the plasma membrane of hippocampal neurons, where it is sensitive to both spontaneous and proven Ca2+ influx KCl-induced depolarization, exogenous glutamate addition, and electrical simulation (Palmer et al. 2006). This modified Cameleon overcomes the issue of prior Cameleons that were unable to measure Ca2+ influx when focused on the plasma membrane, enabling us to view Ca2+ influx without any artifacts. Tandem CytC signal sequence repetitions were employed to D2cpv, D3cpv, and D4cpv (Palmer et al. 2006). D3cpv, like other GECIs, has restrictions when it comes to displaying spike rates and timeframes. Because each of the currently used GECIs has positive cooperativity, the reportable Ca2+ range of concentrations for any given GECI is constrained, and Ca2+-binding saturation is quickly achieved. D3cpv also has slow response dynamics, with off-responses having a time constant of nearly two seconds (Kuhn et al. 2012). D3 Cameleon variants (D3cpv) are distinguished by a single and appropriate affinity for Ca2+ (Kd) and a good dynamic range (Rmax/Rmin) sensitivity to Ca2+ fluctuations (Greotti et al. 2019). Certain variants of the original YC protein exhibited a failure to accurately detect and report fluctuations in Ca2+ levels when localised to the plasma membrane, a cellular compartment where CaM may accumulate in mM quantities. As a result of this, Palmer et al. (2006) introduced a new lineage of Cameleons, referred to as Dcpv. In summary, the Dcpv family originates from the classical Cameleons, wherein two variants of GFP, namely CFP and cpVenus (cpv, a variant of yellow fluorescent protein (YFP) that is circularly permuted), are connected via a mutated CaM and M13 peptide (Palmer et al. 2006). This linkage serves to eliminate or significantly diminish interference from endogenous CaM. Similar to the conventional Cameleon, within the Dcpv family, the binding of Ca2+ triggers a conformational change in CaM, leading to its subsequent interaction with M13. As a consequence of the decreased distance between the CFP and the chromophore-binding protein (CPV), there is an observed augmentation in the efficiency of FRET. The rise in FRET and thus, the increase in Ca2+ may be simply quantified by measuring the increase in the ratio of emission intensities between cpv and CFP, respectively, after excitation of CFP (Palmer et al. 2006).
Mitochondria are essential organelles in the eukaryotic cell, generating unique Ca2+ patterns in response to abiotic and biotic stimuli. The discovery of the mammalian mitochondrial Ca2+ uniporter has opened up new research in plant biology. A transgenic Arabidopsis line expressing the genetically encoded Cameleon D3cpv probe, specifically targeting mitochondria, showed more fluorescence as compared to the established Cameleon YC3.6 (Loro and Costa 2013).
Giacomello et al. (2010) developed a GFP-based Ca2+ probe i.e. N33D1cpv targeted on the outer mitochondrial membrane (OMM) suitable for tracking Ca2+ hotspots (high Ca2+ levels ranging from 1 to 200–300 M) in a limited cellular area. The Ca2+ biosensor N33D1cpv comprises a structure made up of a N33, that codes for an outer mitochondrial membrane (OMM) peptide, a FRET donor (CFP-Cyan Fluorescent Protein), a Ca2+ binding domain D1, and a FRET acceptor (ccpv173-circularly permutated Venus protein); (Gouriou et al. 2023a).The Dcpv protein, often known as Cameleons, has undergone evolutionary changes resulting in the emergence of many variations, namely D1, D2, D3, and D4. These variants exhibit distinct affinities for Ca2+ ions and possess diverse cellular localization signals. Using the N33D3cpv biosensor, both genetic suppression and pharmacological inhibition of endogenous IP3R activity led to a decrease in the rate of Ca2+ leakage from the endoplasmic reticulum (ER) through the OMM when the oxygen level was low (Gouriou et al. 2023a).
Gouriou et al. (2023a, b) developed the N33D3cpv indicator by replacing the N33D1cpv D1 Ca2+ binding domain with the D3 Ca2+ binding domain of the cytosolic biosensor D3cpv, which has a greater Ca2+ affinity (Gouriou et al. 2023a). N33D3cpv having a D3 ligand domain with a dissociation constant (Kd) of 0.6 µM, is well matched to the range of low intensity Ca2+ fluctuations in the cytosol (from 0.1 to 10 µM). First, Ca2+ response amplitude with an ATP stimulus showed that the relative fluorescence intensity (F/f0) ratio peak average is higher with N33D3 than with N33D1cpv, which is 1.573 and 1.107, respectively. The overall F/F0 ratio was higher with N33D3cpv than with N33D1cpv, which is 1.426 and 1.123, and there also existed a distinction in the rate of decay of Ca2+ levels. N33D3cpv enabled precise and reliable readings of Ca2+ dynamic at OMM. Overall, these findings clearly indicate the improved sensitivity of the new N33D3cpv sensor and its capacity to recognise lower fluctuations in Ca2+ dynamic at OMM (Gouriou et al. 2023a). D3cpv, N33D1cpv, and N33D3cpv have extensively been used in recording Ca2+ in animal system.
iGECI, TN-XXL and TnC (Troponin C)-based FRET sensor (Twitch)
The near infrared Ca2+ sensor (NIR) called iGECI, is based on FRET. Mammalian cells benefit from its comparatively high effective brightness and photostability, which enables mouse cortex deep layer imaging. Although iGECI has a huge molecular weight (86 kDa) and slower signal fading, these factors may impair the effectiveness of targeting and packaging of proteins in Adeno associated virus (AAV); (Matlashov et al. 2022). iGECI exhibited a modest absorption peak at 390 nm as well as two large peak values of 640 nm and 700 nm for absorption. The fluorescence of iGECI peaked at 670 and 720 nm, respectively. iGECI showed two sensitivity constants, Kd1 = 15 nM and Kd2 = 890 nM, as a consequence of four Ca2+ binding sites (Shemetov et al. 2021).
TN-XXL is a FRET based Ca2+ biosensor and is based on Troponin C (Tian et al. 2009; Geiger et al. 2012). It is mainly used for long term Ca2+ imaging (Mank et al. 2008). TN-XXL consists of four EF-Hands, which can be divided into two classes. In one, EF-hand 3.1 and 3.2 are involved in FRET output, and in the second, 4.1 and 4.2 are involved, which are responsible for stabilising function, but not FRET output (Geiger et al. 2012). It has been detected that the change in the FRET level in TN-XXL is not only due to the various changes in the conformation but also due to the changes in fluorophore orientation. In a Ca2+-free form, it consists of fluorescent donor and acceptor proteins at opposite ends, which should not be in a close proximity with each other. However, in Ca2+-bound form, they are always in close proximity (Geiger et al. 2012). The signal amplitude of the TN-XXL is lower than that of the OGB-1 (Mank et al. 2008). In most of the studies, Ca2+ oscillations have been detected more strongly and more consistently using TN-XXL in Drosophila neurons (Whitaker 2010).
TnC (Troponin C)-based FRET sensor (Twitch) offers a linear response, a smaller size, a broad range of Ca2+ affinities, superior photostability, a wider dynamic range, and faster kinetics compared to the TN-XXL. Even with all these improvements, FRET sensors experience a low signal-to-noise ratio (SNR). In comparison to GCaMPs, the kinetics of Twitch with in on/ and off conditions is still slower (Cho et al. 2017). Twitch-5's Kd value of 9.25 µM suggests rapid kinetics, while Twitch-3's Kd value of 250 nM shows noticeably delayed kinetics. Slow kinetics produces more photons, which increases the signal and makes indicators more sensitive (Thestrup et al. 2014a). The indictors have been named because of their sensor parts are derived from Troponin C which is a Ca2+-binding protein present in the skeletal and cardiac muscle that governs muscle twitching. A single Troponin C molecule, like most Ca2+-binding proteins, binds up to four Ca2+ ions using four specialised protein domains known as EF- hands (Wilms and Häusser 2014). In addition, for measuring the accurate kinetics and function of Ca2+ binding structure, resolved spectroscopy is used due to its simple and compact structure. By decreasing the number of binding Ca2+ ions, the biocompatibility of the sensor can be improved as it reduces buffering while using it for long term expression. The structure of the Ca2+ binding domain and whole structure of Twitch-1 was determined by using nuclear magnetic resonance (NMR) and the whole prototype indicator by small angle X-ray scattering spectroscopy (Wilms and Häusser 2014). As it is a FRET indicator, it shows ratiometric detection. However it relies on change in volume and movement of Ca2+ but not the change in intensity of Ca2+. Hence, it is a reliable Ca2+ imaging indicator for motile cells. When comparing this with the previous FRET-based GECIs, which are brighter at resting condition of the cell, the Twitch has been found to be more brighter. Therefore, it allows imaging of cell structure via increasing signal-to-noise ratio and makes it suitable for doing functional imaging (Wilms and Häusser 2014). iGECI, TN-XXL and Twitch have been used in animal research extensively.
Ca2+ imaging: applications in animal sciences
Animal systems are more complex than plant and therefore, it is very important to visualize the changes occurring at cellular and sub-cellular levels. Several fields have been revolutionized from the advances in Ca2+ imaging, particularly pathology and neurosciences. Quin-2 was the first member of the first generation of fluorescent Ca2+ indicators to be utilised in biological research. Pozzan et al. (1982) used the Quin-2 indicator to determine the influence of mouse spleen lymphocyte anti-immunoglobulin on cytoplasmic Ca2+ concentration (Pozzan et al. 1982). Because Quin-2 is not an excellent Ca2+ indicator, it must be used with elevated intracellular concentrations to prevent cellular autofluorescence. Another first-generation indicator dye Fura-2 has been used by many neuroscientists since it outperforms Quin-2 in many ways (Grienberger and Konnerth 2012). Phillips et al. (2019) measured the neuronal Ca2+ levels in DFP (OP-diisopropyl fluorophosphate)-based rat model for GWI (Gulf War Illness). GWI is a chronic multi-symptom disorder that affects veterans of the First Gulf War. It includes depression and memory loss as neurological symptoms (Phillips et al. 2019). It’s utilisation is not only limited to the neurology but also includes other fields of biology. One of the common kind of muscular dystrophy is Duchenne muscular dystrophy (DMD). The severity of the illness in DMD is considered to be influenced by changes which was measured by Fura-2 (Mázala et al. 2015). Numerous other Ca2+ indicators have been developed over time, with a variety of excitation spectra and affinities for Ca2+. These include, Fluo-4 dye families, Oregon Green BAPTA families and many more. The Fluo-3AM has been used to study the intracellular Ca2+ concentration and other ROS assays to uncover whether the blood–brain barriers (BBB) in cerebrovascular endothelial cells destruction after ischemic stroke was caused by the inactivation of Wnt7/β-catenin signaling (Haiping et al. 2023). Fluo-4 is another widely used Ca2+-sensitive florescent dye. The development of Fluo-4 has had a significant impact on the study of Ca2+ signaling in biological systems, enabling researchers to gain deeper insight into intracellular Ca2+ dynamics and its role in various physiological processes. Bajnok et al. (2023) have used the Fluo-4AM to study the Ca2+ flux which plays a key role in B cell signaling pathway and might act as an important tool for drug discovery towards the development of autoimmune disease caused by B-cells. In this study, they standardised a method based on flow cytometry to study the patterns of Ca2+ flux of Peripheral Human B-cells. This approach can be used in clinical research, multi-centric studies and also facilitate the assessment of rare conditions (Bajnok et al. 2023). With time, Ca2+ indicators have been developed with the goal of enhancing the performance of Ca2+ imaging experiments. Following the Fluo family, a new synthetic Ca2+ indicator Cal-520, Cal-590, and so on have been reported. Daily et al. (2017) have shown that when iPSC-derived (induced Pluripotent Stem Cells) human cardiomyocytes for drug screening were employed, FLIPR Calcium 6 and Cal-520 were found to be appropriate dyes (Daily et al. 2017). It has been seen that Cal-590 applied ultra-short laser pulse to reduce photoxicity, showcasing the potential for deep two-photon imaging in vivo with single-cell resolution, allowing for the examination of neuronal population in all six layers of the mouse brain cortex (Birkner et al. 2017). Cal-520, a comparable alternative, offers several advantages over visible-light dyes such as Fluo-4 and Oregon Green 488 BAPTA-1 (OGB-1). It enhances intracellular retention and signal-to-noise ratio (SNR). In a study conducted by (Tada et al. 2014), Cal-520 proved to be highly effective for in vivo detection of dendritic Ca2+ transients in Purkinje cells of anesthetized mice, displaying both high SNR and rapid decay time (Tada et al. 2014).
The dysregulation of neural circuit function leads to development of neuropsychiatric disorders. Monitoring neural population and its activity under drug supplementation is still challenging for the scientific world. The regulation of behavioural addiction by neural circuits makes the monitoring of cellular granularity in brain region highly important. With the utilization of genetically encoded Ca2+ indicator (GECI), Siciliano and Tye (2019) were able to target the cells of interest and study in vivo Ca2+ dynamics to access neural activity. The researchers have developed miniaturized two-photo microscope (MINI2P) to visualize high resolution, fast and multiplane Ca2+ imaging in moving mice to study almost thousands of neurons at a time. The group were able to observe cells of medial entorhinal cortex, hippocampus and visual cortex (Zong et al. 2022). With the utilization of transgenic Pirt-GCaMP6s mice, the gauge responses of DRG (dorsal root ganglion) neurons were studied under in vivo visceral and somatic stimulation to understand the phenomenon of visceral pain sensitization and the concept of somatic hypersensitivity (Gao et al. 2021).
Ca2+ currents are very essential for proper functioning of neuron cells. To image a native neuron, Ca2+ current in brain slices, a combined approach was adopted by using low affinity Ca2+ indicators along with voltage sensitive dyes. Using this technique, Ca2+ kinetics was estimated via modulations in Ca2+ fluorescence activity which was simultaneously correlated with change in surface potential through change of voltage fluorescence (Ait Ouares et al. 2020). Ca2+ imaging in higher organisms such as Rhesus macaque is also being studied via GCaMP Ca2+ indicator, which will help in understanding the human brain function as well as neurological diseases (Bollimunta et al. 2021). With the help of genetically encoded Ca2+ indicators, Ca2+ oscillations were measured in neural cells of mammals (Jercog et al. 2016). Also, with the utilization of genetically encoded Ca2+ sensor, a protocol of brain surgeries for deep brain Ca2+ imaging via miniature fluorescence microscope (miniscope) was developed (Thapa et al. 2021).
Guillain-Barré syndrome is the a type of syndrome which causes numbness, weakness and pain in feet, hands and limbs of human body. This syndrome is majorly associated with activation of complement on the axonal PM by the attack of motor axons. In animal systems, Ca2+ influx is triggered by axonal damage which is caused by protease calpain. Cunningham et al. (2022), were able to monitor ex vivo intra-axonal Ca2+ changes in mouse affected with axonal Guillain-Barré syndrome and demonstrated the role of Ca2+ during axonal injury and explained the effects of calpain inhibition (Cunningham et al. 2022). Ca2+ act as an important messenger in neuronal system however, its function in regeneration of axon is not fully understood. A non-invasive in vivo Ca2+ imaging using GCaMP6f for studying Ca2+ oscillations was carried out during regeneration of axon in zebrafish Mauthner cells. An inward rectifying K+ channel protein i.e., Kir2.1a is solely responsible for decrease in the activity of Mauthner neural activity. The Kir2.1a overexpression resulted in the reduced prohibition of Ca2+ induced axonal regeneration (Chen et al. 2019).
Caenorhabditis elegans which is considered as an excellent model system to study Ca2+ imaging in animals was utilized to study in vivo Ca2+ dynamics in the cells of body wall muscles. It was observed that with the co-expression of presynaptic channel rhodopsin, there was a provocation of acetylcholine from excitatory motor neurons accompanied by blue light pulses, resulting in the depolarization of muscle cells and ultimately leading to transient changes in [Ca2+]cyt levels. The change in Ca2+ was monitored by genetically encoded Ca2+ sensors (Martin et al. 2019). With the utilization of GECI, the amygdala in deeper brain region of mouse was captured via Ca2+ imaging with head-mount miniaturized microscope (Lee and Han 2020).
Pancreatic islet hormones are important for the maintenance of glucose homeostasis. Interestingly, it was found that any change in blood glucose level induces change in [Ca2+]cyt in pancreatic islet cells. The excess of Ca2+ in these cells promote the secretion of three major hormones such as insulin (reside in beta-cells), glucagon (alpha-cells), and somatostatin (delta-cells). Beta cells are connected to each other to form one single entity. These are stimulated on glucose supplementation and are a major constituent of islet cells. Therefore, the excitability of others such as alpha and delta cells could show interesting variations and are of special interest. With the help of Ca2+ imaging, it has been analysed that the minor cell responses on the application of adrenaline and ghrelin which induces transient change in [Ca2+]cyt in specific populations of islets cells of the mouse (Hamilton et al. 2019).
Brugia malayi is a filarial nematode which causes lymphatic filariasis/ elephantiasis disease in human. Unlike C. elegans, Ca2+ dynamics in B. malayi is less studied (Williams et al. 2020). Williams et al. (2020) has developed Ca2+ imaging method for the first time to study the cellular Ca2+ dynamics in muscle of B. malayi. Ca2+ imaging is found to be a suitable technique to uncover the mode of action of anthelmintic drugs and their effect on the parasite. Two methods were generated to study Ca2+ dynamics: muscles of B. malayi were soaked with Fluo-3AM and the other, where Fluo-3 was directly micro injected. By using these approaches, Ca2+ dynamics as well as electrophysiological recordings were measured (Williams et al. 2020). The transgenic mouse expressing genetically encoded Ca2+ indicator, RCaMP1.07, under alpha-smooth muscle actin promoter was used to study Ca2+ fluctuations in brain cells and correlate it with hemodynamics in mice (Meza-Resillas et al. 2021).
The voltage gated calcium channel (VGCC) of the retinal ganglion mediate Ca2+ signaling which was monitored via Ca2+ imaging tool in rat (Sargoy et al. 2014). Also, the long-term memory formation is correlated with Ca2+ dynamics in animals. It is a well know mechanism that G-protein coupled receptors (GPCR) activation leads to an increase in intracellular Ca2+ concentration. Simultaneously, the activation of neuron also causes increase in intracellular Ca2+ level. It has been observed that GCaMP-expressing Drosophila larval brain cell was able to enhance Ca2+ level upon test peptide and the fluorescent signal was captured via spinning disc confocal microscope associated with CCD camera (Ishimoto and Sano 2018). With the utilization of in vivo functional and Ca2+ imaging, Rigosi et al. (2015a) has demonstrated that the right antenna of bees were better in distinguishing a target odour than left antenna (Rigosi et al. 2015b).
Single photon Ca2+ imaging via real-time processing correction (RTMC) method in animals has also been reported (Li et al. 2022a). The whole brain Ca2+ imaging with good cellular resolution was reported in freely swimming larval zebrafish by using HiLo microscopy (Kim et al. 2017). Jacob et al. (2018) described the usage of low-cost compact head-mounted endoscope (CHEndoscope) to monitor Ca2+ imaging (Jacob et al. 2018). Many such studies have brought us a clear understanding of the cellular pathways and have helped in identifying highly important mechanisms of Ca2+ dynamics and signaling in regulating diverse cellular processes.
Unveiling intricacies: important contributors in the field of Ca2+ imaging techniques
The field of Ca2+ imaging has evolved from recording Ca2+ activity in individual cells to visualising and analysing Ca2+ signals within complex tissues and organs. This has significantly improved our understanding of cellular signaling and physiological mechanisms in plants and animals. In plant biology, Ca2+ imaging is used to examine Ca2+ transitions during growth and development processes and to study plant responses to biotic and abiotic stresses. Several scientists have worked to improve Ca2+ imaging techniques in plants. This has made it possible to see and understand how Ca2+ signals work in different parts of plant physiology, growth, and responses toward diverse stimuli. In animal systems, Ca2+ imaging has enabled the observation of neuronal activity patterns, synaptic transmission, and Ca2+ signaling in various tissues and organs, including the brain, heart, and immune system. Recent advancements in imaging technology have further propelled the field of Ca2+ imaging (Kanchiswamy et al. 2014). Advancements in Ca2+ imaging have enabled high precision in observing Ca2+ dynamics in both spatial and temporal domains (Russell 2011; Nietz et al. 2022). These advancements and improvements in multiple imaging systems have improved the accuracy and sensitivity of visualising Ca2+ ion dynamics, with notable contributions from researchers over a period of time (Fig. 5).
Fig. 5.
The present roadmap aims to provide an overview of the milestones in the field of in vivo studies pertaining to Ca2+ biosensing in plants
The discovery of aequorin (1963) by Shimomura was of significant importance in understanding bioluminescence. Aequorin is a Ca2+-sensitive protein found in jellyfish that emits blue light when it binds Ca2+ ions (Prasher et al. 1985). This discovery helped scientists gain insights into the biochemical processes involved in bioluminescence and paved the way for further research on the mechanisms and applications of this phenomenon. The use of synthetic Ca2+ indicators has played a crucial and influential role in the field of Ca2+ imaging. During the early 1980s, Charles F. Stevens (1983) introduced the initial synthetic Ca2+ indicator known as Fura-2 (Neher 1992). In the 1990s, Roger Y. Tsien performed significant research on Ca2+ Green-1, a highly adaptable Ca2+ indicator that facilitated the assessment of intracellular Ca2+ levels in both plants and animals (Tsien 1998). These indicators established the fundamental methods for Ca2+ imaging. A new set of fluorescent markers have been developed to study the physiological significance of cytosolic free Ca2+. These markers have an 8-coordinate tetracarboxylate chelating site and stilbene chromophores, which improve quantum efficiency and photochemical stability. They exhibit fluorescence up to 30 times more intense than the widely used "Quin-2" dye. They also show significant wavelength changes upon binding with Ca2+, with slightly reduced affinities and longer excitation wavelengths. These features make them ideal for intracellular applications (Grynkiewicz et al. 1985). The introduction of GECIs brought about a significant transformation in the field of Ca2+ imaging (Tsien 1998). GECIs are a class of proteins that demonstrate alterations in fluorescence upon binding with Ca2+. These indicators have the ability to be expressed in particular cell types or tissues, thereby facilitating more precise and enduring investigations of Ca2+ imaging in plants and animals (Kotlikoff 2007). The initial GECI, known as “Cameleon” was developed by the research team led by Roger Y. Tsien. The development of Cameleon allowed the specific visualisation of Ca2+ through imaging techniques (Miyawaki et al. 1997). In the realm of animal research, initial investigations into Ca2+ imaging primarily centred on single-cell recognition, employing methodologies such as microinjection of Ca2+ indicators or the introduction of indicator dyes into cells (Li and Saha 2021). These techniques enabled researchers to observe the temporal dynamics of Ca2+ in individual cells with a relatively high level of precision. The initial microinjection technique for the introduction of Ca2+ indicators into individual cells was pioneered by David W. Tank in 1985 (Tank et al. 1988).
GECIs, such as Cameleon, GCaMP, and R-GECO, were devised as a means to address certain constraints associated with chemical indicators. Nakai et al. (2001) optimised GECIs, specifically GCaMP, to enhance their ability to sense changes in Ca2+ levels. Loren L. Looger and Mark H. Ellisman (2003) further developed enhanced GECIs, including various variants of GCaMP. These variants demonstrated improved sensitivity to Ca2+ and exhibited notable changes in fluorescence levels (Terai and Nagano 2008). The research conducted by Simon Gilroy has significantly contributed to the field of plant biology by developing GECIs that are specific to plants, such as GCaMP and R-GECOs variants. These indicators have enabled accurate and detailed imaging of Ca2+ in plant cells and tissue (Monshausen et al. 2008; 2011). Ca2+ ions, pH levels, and reactive oxygen species (ROS) are crucial cellular regulators in plant physiological and developmental processes. Understanding their spatial and temporal dynamics across various scales is essential to comprehending their extensive influence. Fluorescent sensors, when used with appropriate controls, provide a reliable method for quantifying dynamic changes in these entities in real-time and within living organisms. Fluorescent cellular probes can be classified into two main classes: dyes and genetically encoded sensors based on GFPs. GFP probes can be selectively directed towards specific subcellular regions, allowing for detailed mapping of these signals within the cellular environment at a high level of precision (Swanson et al. 2011).
José A. Feijó primarily centred on the utilisation of Ca2+ imaging techniques to investigate the process of polarised growth. This work provided valuable insights into the intricate dynamics of Ca2+ during the growth of pollen tubes and the subsequent fertilisation process (Feijó and Moreno 2004; Feijó and Wudick 2018). Fluorescent genetic probes and ion-sensitive vibrating probes are used to find out where and when H+ and Ca2+ are present in tobacco pollen tubes. The results showed a discernible acidity gradient, with oscillations lasting 1–4 min. The (Ca2+)cyt was visualised using confocal microscopy, revealing a V-shaped gradient spanning 40 μm from the tip. Oscillatory patterns of extracellular Ca2+ fluxes were observed in the majority of pollen tubes, ranging from 2 to 50 pmol cm−2 min−1. The study also found distinct spatial distributions and morphologies of H+ and Ca2+ inside the cellular environment, indicating a potential synergistic relationship between these two second messengers in facilitating cellular development. The findings suggest that the fluxes occurring at the apex of the pollen tube have a direct role in both the development and maintenance of the gradient (Michard et al. 2008). Another interesting study uses a genetically encoded FRET-based reporter to visualise conformational changes in Ca2+−dependent protein kinases (CDPKs and CPKs) in Arabidopsis thaliana. The researchers found that CPK21-FRET showed oscillatory changes in emission ratios, reflecting changes in (Ca2+)cyt levels. CPK23-FRET did not show similar changes, indicating different Ca2+-sensitivity and a lack of reversibility. The conformational dynamics of CPK21 in Arabidopsis guard cells indicate it interprets signal-specific Ca2+ patterns in response to abscisic acid and the flagellin peptide flg22 (Liese et al. 2023). CapHensor, a dual-reporter, was used to study signaling processes in pollen tubes, guard cells, and mesophyll cells. The study revealed previously unreported linkages between membrane voltage, Ca2+ dynamics, and pH dynamics, revealing spatio-temporal interactions (Li et al. 2021).
Zhenbiao Yang and collaborators have successfully developed enhanced variants of Ca2+ indicators, such as YC, which have been specifically optimised for utilisation in plant cells. Ca2+ is a universal second messenger that transmits unique cellular signals through a spatiotemporal signature derived from both its extracellular source and internal reserves (Endo 2006). But we don't know much about how a Ca2+ signature is made because we don't have better ways to track the changing levels of Ca2+ in different subcellular compartments at the same time. To deal with this, molecular tools called CamelliA lines have been built in Arabidopsis to make it possible to watch the movement of Ca2+ in different subcellular compartments at the same time and with high resolution. Ca2+ signatures were identified in three types of Arabidopsis cells, relating to both internal and external stimuli. Some of these signs were fast changes in the amount of Ca2+ in the cytosol and the flow of Ca2+ into the plasma membrane at the tip of fast-growing pollen tubes. Also, when root epidermal cells were put under salt stress, the spatiotemporal correlation of Ca2+ dynamics was observed in four subcellular compartments (Guo et al. 2022).
The work of Frantisek Baluska and his collaborators involved the utilisation of Ca2+ imaging techniques to investigate the occurrence of Ca2+ waves and oscillations in plant root hairs. In Arabidopsis root hairs, the study showed how actin filaments affect mitochondrial Ca2+, how much Ca2+ is in the cytoplasm, and how these two phenomena are affecting each other. The researchers used fluorescent mitochondrial dyes, MitoTracker and Rhod-2, to visualise and quantify mitochondrial Ca2+ levels (Wang et al. 2010). Another study focused on the transition zone in the root apex, which plays a crucial role in determining cellular fate and facilitating root development. This zone integrates various inputs, including hormones and sensory stimuli, into signaling and motor outputs, leading to adaptive differential growth responses. Ca2+ green-1-dextran was microinjected into the root hair and the amounts of Ca2+ in the cytoplasm were assigned pseudo-colours based on the initial intensity of green fluorescence. This understanding is essential for understanding root-apex tropisms and various aspects of adaptive root activity, as it is crucial for understanding root-apex tropisms and adaptive root activity (Baluška et al. 2010). The luminescent Ca2+ indicators, including aequorin, were developed by Marc Robert Knight and his research team. These indicators enabled the quantification of Ca2+ concentrations in plants through the use of bioluminescence methodologies. This method was a good alternative to fluorescent indicators because it made easier to spot changes in the amount of Ca2+ in different plant tissues (Gao et al. 2004; Liu et al. 2020). Julian Schroeder's study aimed to accurately measure the amount of Ca2+ in the cytosol by introducing Ca2+ Green-1 AM into guard cells in epidermal strips. The strategy involves introducing the dye and measuring the guard cells' response to abscisic acid. Their team used Arabidopsis thaliana to create Yellow Cameleon 2.1, a Ca2+ indicator based on a green fluorescent protein, to measure [Ca2+]cyt in guard cells (Allen et al. 1999). Fluorescence ratio imaging allowed time-dependent measurements of [Ca2+]cyt in guard cells, showing repetitive transients with peak values of up to 1.5 μM. Technical advancement by using combined cell biological and molecular genetic approaches will pave the path for understanding of mechanistic role of Ca2+ dynamics in plant signaling pathways (Israelsson et al. 2006).
Alex Costa, a renowned scholar in Ca2+ imaging in plant sciences, has made significant contributions to understanding the role of Ca2+ signaling in plant growth, development, and responses to environmental stimuli using advanced Ca2+ imaging methodologies. In vivo imaging of Arabidopsis cells shows that Ca2+ transients are linked to pH changes in the cytosol. This suggests that Ca2+ movement in plant cells and pH changes are linked in a fundamental way (Behera et al. 2018). Selective Plane Illumination Microscopy (SPIM) is a type of biological imaging that is often used to study transparent specimens for long periods of time in vivo. It is especially useful for studying small organs and organisms (Costa et al. 2013). A line of genetically encoded Ca2+ indicators was developed to simultaneously image Ca2+ dynamics in both the ER and cytosol. Live cell imaging revealed a wounding-induced Ca2+ wave in mature plants, enhancing high-resolution analyses of intracellular Ca2+ dynamics and paving the path for a new methodology (Resentini et al. 2021). They developed a protocol for imaging the R-GECO1 Ca2+ sensor in the roots of Arabidopsis, which can be used with other intensiometric Ca2+ sensors, as well as a method for analysing images (Himschoot et al. 2018). Loro and Costa have developed a new method to study mitochondrial Ca2+ in plant root cells using the Cameleon sensor 4mt-YC3.6. This method allows simultaneous monitoring of both nuclear and mitochondrial Ca2+ within a single cell using both nuclear and mitochondrial-targeted Cameleons by using high-resolution confocal laser scanning microscopy (Loro and Costa 2013).
Jörg Kudla, a plant biologist, has made significant contributions toward the understanding of Ca2+ signaling in plants. Kudla and team found that ratiometric fluorescence imaging works better with transgenic Arabidopsis plants that express the FRET-based Ca2+ probe Cameleon. This allows for better analysis of in vivo Ca2+ dynamics, focusing on (Ca2+)cyt concentrations (Behera and Kudla 2013a). Excessive Na+ in soils inhibits plant growth by triggering primary Ca2+ signals within the root differentiation zone. A Ca2+-sensing mechanism measures stress intensity, allowing salt detoxification responses and optimizing Na+ extrusion capacity (Steinhorst et al. 2022). Behera and Kudla developed a technique to visualize (Ca2+)cyt dynamics in roots using confocal laser scanning microscopy (CLSM) using the FRET technique, specifically using the genetically engineered Ca2+ indicator YC3.6. This method allows for precise imaging of seedlings aged 5–7 days using high-magnification objectives (Behera and Kudla 2013b). Behera and Kudla also developed a method to measure the movement of Ca2+ in the cytoplasm of Arabidopsis thaliana guard cells using the FRET method and the genetically modified Ca2+ indicator YC3.6 (Behera and Kudla 2013b; Himschoot et al. 2018).
The modulation of cellular Ca2+ concentrations is crucial for signal transduction in plant cellular processes. Carvalho-Niebel and team showed how Ca2+ oscillations in the nucleus and around the nucleus are important signs of symbiotic signaling responses in legumes. FRET yellow-based Ca2+ Cameleon probes have been effective in quantifying spatial and temporal patterns of symbiotic Ca2+ signaling in legumes. However, these sensors were limited in their capacity to measure changes in Ca2+, specifically inside individual subcellular compartments. This study examined the potential of GECOs, single fluorescent protein-based Ca2+ sensors, for simultaneous and multicolor imaging of cytoplasmic and nuclear Ca2+ signaling dynamics in root cells. Transgenic Medicago truncatula roots and Arabidopsis thaliana were used to investigate Ca2+ responses to microbial biotic and abiotic elicitors. The results showed that GECOs exhibit greater sensitivity in detecting differences in symbiosis-related Ca2+ spiking in M. truncatula compared to yellow FRET-based sensors. The dual sensor accurately analyses synchronised spatial and temporal patterns of nuclear and (Ca2+)cyt signaling inside a single root cell while the organism is alive (Kelner et al. 2018).
Wang et al. studied the pollen-tube pathway method of transformation to see how it is affected by the amount of plasmid DNA, the type of plant tissue/organ i.e., tomato used in the study, and the transformation solution. The YC3.6 vector was used as a transformation indicator. The study found a significant correlation between plasmid DNA density and transformation solution components and fruit set ratio. The optimum combination for the transformation solution was 600 ng μL−1, sucrose, and 0.05% Silwet-L-77. This technique efficiently and precisely screens transgenic plants using in vivo imaging and fluorescence microscopy, making the pollen-tube route a cost-effective approach for introducing foreign genes into tomato plants (Wang et al. 2017). [Ca2+]cyt is crucial for cellular signal transduction in plants and controls plant development. Pollen tubes establish an oscillatory Ca2+ gradient, which is essential for polarised growth. Genetically encoded Ca2+ indicators like YC quantify its role. A method for imaging (Ca2+)cyt dynamics in growing pollen tubes using laser-scanning confocal microscopy is described by Barberini and Muschietti (2015).
Advancements in the enhancement of Ca2+ imaging have been achieved not just within the discipline of plant science but also in other disciplines. Numerous scientists and researchers have made noteworthy contributions to the advancement of Ca2+ imaging resolution. Ca2+ imaging has significantly enhanced our understanding of plant and animal physiological and developmental processes. Hillman and Chédotal (2011) work enabled high-resolution images of Ca2+ movement in intact animal tissues and organs (Santi 2011). In the laboratory of Mark Schnitzer, the work focused on miniaturised microscopes and fibre-optic-based systems for Ca2+ dynamics visualisation in live animals (Jercog et al. 2016). This allowed researchers to investigate Ca2+ dynamics in various behavioural contexts (Helmchen 2002; Russell 2011; Helmchen et al. 2013). GECIs have facilitated targeted manipulation and observation of Ca2+ dynamics within neurons and brain circuits (Oh et al. 2019; Lohr et al. 2021; Zhang et al. 2023). Carlos Pantoja and Takashi Nakamura's non-invasive imaging techniques have expanded the scope of studying neural activity in live animal subjects (Albers et al. 2018; Streich et al. 2021). These advancements have contributed immensely to our understanding of Ca2+ signaling and its role in various physiological processes, including neuronal activity, synaptic transmission, and organ function.
The significant contributions made by researchers have strongly influenced the development of Ca2+ imaging in both plants and animals. The collective endeavours have established a strong basis for forthcoming breakthroughs, facilitating novel understandings of the intricate realm of cellular signaling and physiological mechanisms in both plants and animals.
Conclusion and future prospects
Ca2+ is a macronutrient which also acts as a second messenger. It participates in a number of physiological activities such as cell wall structure and integrity maintenance, nutrient uptake, stress response, blood clotting, nerve function etc., developmental (cell differentiation, seed germination, root development, meristem activity) and biochemical pathways (enzyme activity, protein regulation, cell signaling, apoptosis etc.). Plants and animals perceive stress stimuli via receptors which results in the generation of Ca2+ transient in cytosol. This change in Ca2+ is solely responsible for downstream signaling events. Interestingly, the modulation in Ca2+ concentration can be quantified by Ca2+ imaging. Time-to-time various indicators have been discovered and utilized for cellular Ca2+ quantification. With the utilization of various Ca2+ dyes, probes and indicators researchers have been able to successfully attempt in vivo live cell Ca2+ imaging in higher organisms. Ca2+ imaging unveils the mechanism of Ca2+ signaling at the cellular level and unravels the minor changes that could not have been identified accurately. It also encourages development of various tools and techniques which help researchers to perform live cell Ca2+ imaging. The association of Ca2+ imaging with the machine learning and artificial intelligence algorithms can reduce the researcher's burden of complex Ca2+ imaging dataset analysis and also improves the accuracy.
The development of miniaturized and portable super resolution Ca2+ imaging system can make the technology more accessible for field studies and for visualizing the Ca2+ dynamics with nanometre scale precision, revealing intricate sub-cellular structure and interactions. The Ca2+ indicators advancement is a crucial aspect of advancing the field of molecular biology, biochemistry and cell physiology. Some of the advancements that can be done are calibration free indicators, multiparametric indicators (indicators that can measure multiple parameters like Ca2+, pH etc.), quantum dots integration (to improve brightness, photostability and multiplexing capabilities), use of bio-orthogonal chemistry (activate or deactivate selectively in response to specific cellular events or signals), biocompatibility and many more. The modernization of the Ca2+ indicators can improvise the accuracy and quality of Ca2+ imaging, thus, helping us to picture the vital changes occurring at cellular level. Many more advancements can be done in Ca2+ imaging to deepen our knowledge regarding plant biology, stress responses and adaptations to environmental changing conditions. This is a huge support for the scientific community that is always keen to identify and make better the existing means to derive accurate representation of the responses in living systems. It has the potential to solve issues in biotechnology, environmental science and agriculture, ultimately promoting food security and plant based solutions that can adapt to a changing environment.
Authors contribution
GKP: Conceptualization, Supervision, Funding acquisition, Writing—review and editing. SG: Investigation, Methodology, Writing—original draft, Writing—review and editing. MD: Investigation, Methodology, Writing—review and editing. AK: Investigation, Methodology, Writing—review and editing. MB: Writing—review and editing.
Funding
Department of Biotechnology (DBT), Govt. of India; Science and Engineering Research Board (SERB), Govt. of India; Council for Scientific and Industrial Research (CSIR), Govt. of India; Delhi University (IoE/FRP grant), India.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Consent for publication
All authors give their consent to publish this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Monika Dahiya and Amit Kumar have contributed equally.
References
- Ai H-W. Fluorescent-protein-based probes: general principles and practices. Anal Bioanal Chem. 2015;407:9–15. doi: 10.1007/s00216-014-8236-3. [DOI] [PubMed] [Google Scholar]
- Ait Ouares K, Jaafari N, Kuczewski N, Canepari M. Imaging native calcium currents in brain slices. Adv Exp Med Biol. 2020;1131:73–91. doi: 10.1007/978-3-030-12457-1_4. [DOI] [PubMed] [Google Scholar]
- Akerboom J, Calderón NC, Tian L, et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front Mol Neurosci. 2013;6:43920. doi: 10.3389/FNMOL.2013.00002/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom J, Chen TW, Wardill TJ, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers F, Wachsmuth L, van Alst TM, Faber C. Multimodal functional neuroimaging by simultaneous BOLD fMRI and fiber-optic calcium recordings and optogenetic control. Mol Imaging Biol. 2018;20:171–182. doi: 10.1007/S11307-017-1130-6. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kwak JM, Chu SP, et al. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 1999;19:735–747. doi: 10.1046/J.1365-313X.1999.00574.X. [DOI] [PubMed] [Google Scholar]
- Anil VS, Rao KS. Calcium-mediated signaling during sandalwood somatic embryogenesis. role for exogenous calcium as second messenger. Plant Physiol. 2000;123:1301–1311. doi: 10.1104/PP.123.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajnok A, Serény-Litvai T, Temesfői V, et al. An optimized flow cytometric method to demonstrate the differentiation stage-dependent Ca2+ flux responses of peripheral human B cells. Int J Mol Sci. 2023 doi: 10.3390/IJMS24109107/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Mancuso S, Volkmann D, Barlow PW. Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci. 2010;15:402–408. doi: 10.1016/J.TPLANTS.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Bannwarth M, Corrêa IR, Sztretye M, et al. Indo-1 derivatives for local calcium sensing. ACS Chem Biol. 2009;4:179–190. doi: 10.1021/CB800258G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberini ML, Muschietti J. Imaging of calcium dynamics in pollen tube cytoplasm. Methods Mol Biol. 2015;1242:49–57. doi: 10.1007/978-1-4939-1902-4_4. [DOI] [PubMed] [Google Scholar]
- Barberini ML, Sigaut L, Huang W, et al. Calcium dynamics in tomato pollen tubes using the Yellow Cameleon 3.6 sensor. Plant Reprod. 2018;31:159–169. doi: 10.1007/S00497-017-0317-Y. [DOI] [PubMed] [Google Scholar]
- Bassani JW, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995;68:1453–1460. doi: 10.1016/S0006-3495(95)80318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistič O, Kudla J. Analysis of calcium signaling pathways in plants. Biochim Biophys Acta Gen Subj. 2012;1820:1283–1293. doi: 10.1016/j.bbagen.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Batistič O, Waadt R, Steinhorst L, et al. CBL-mediated targeting of CIPKs facilitates the decoding of calcium signals emanating from distinct cellular stores. Plant J. 2010;61:211–222. doi: 10.1111/j.1365-313X.2009.04045.x. [DOI] [PubMed] [Google Scholar]
- Behera S, Kudla J. Live cell imaging of cytoplasmic Ca2+ dynamics in Arabidopsis guard cells. Cold Spring Harb Protoc. 2013;8:665–669. doi: 10.1101/PDB.PROT072983. [DOI] [PubMed] [Google Scholar]
- Behera S, Kudla J. High-resolution imaging of cytoplasmic Ca2+ dynamics in Arabidopsis roots. Cold Spring Harb Protoc. 2013;8:670–674. doi: 10.1101/PDB.PROT073023. [DOI] [PubMed] [Google Scholar]
- Behera S, Wang N, Zhang C, et al. Analyses of Ca2+ dynamics using a ubiquitin-10 promoter-driven Yellow Cameleon 3.6 indicator reveal reliable transgene expression and differences in cytoplasmic Ca2+ responses in Arabidopsis and rice (Oryza sativa) roots. New Phytol. 2015;206:751–760. doi: 10.1111/NPH.13250. [DOI] [PubMed] [Google Scholar]
- Behera S, Xu Z, Luoni L, et al. Cellular Ca2+ signals generate defined pH signatures in plants. Plant Cell. 2018;30:2704–2719. doi: 10.1105/TPC.18.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KW, Snedden WA. Calmodulin-related proteins step out from the shadow of their namesake. Plant Physiol. 2013;163:486–495. doi: 10.1104/pp.113.221069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bi X, Beck C, Gong Y. Genetically encoded fluorescent indicators for imaging brain chemistry. Biosensors. 2021 doi: 10.3390/BIOS11040116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkner A, Tischbirek CH, Konnerth A. Improved deep two-photon calcium imaging in vivo. Cell Calcium. 2017;64:29–35. doi: 10.1016/j.ceca.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Bischof H, Burgstaller S, Waldeck-Weiermair M, et al. Live-cell imaging of physiologically relevant metal ions using genetically encoded FRET-based probes. Cells. 2019 doi: 10.3390/CELLS8050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass BE. In vitro screening systems. Basic Princ Drug Discov Dev. 2015 doi: 10.1016/B978-0-12-411508-8.00004-9. [DOI] [Google Scholar]
- Bollimunta A, Santacruz SR, Eaton RW, et al. Head-mounted microendoscopic calcium imaging in dorsal premotor cortex of behaving rhesus macaque. Cell Rep. 2021;35:109239. doi: 10.1016/J.CELREP.2021.109239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomelli C, Bravo K, Vega A, et al. Use of fura 2 fluorescent dyes as indicator for studying calcium distribution in several plant tissues. Commun Soil Sci Plant Anal. 2010;41:1061–1072. doi: 10.1080/00103621003687158. [DOI] [Google Scholar]
- Castell JV, Gómez-Lechón MJ, Ponsoda X, Bort R. In vitro investigation of the molecular mechanisms of hepatotoxicity. Arch Toxicol Suppl. 1997;19:313–321. doi: 10.1007/978-3-642-60682-3_29. [DOI] [PubMed] [Google Scholar]
- Chandra S, Low PS. Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem. 1997;272:28274–28280. doi: 10.1074/jbc.272.45.28274. [DOI] [PubMed] [Google Scholar]
- Chen L, Wang QQ, Zhou L, et al. Arabidopsis CBL-interacting protein kinase (CIPK6) is involved in plant response to salt/osmotic stress and ABA. Mol Biol Rep. 2013;40:4759–4767. doi: 10.1007/s11033-013-2572-9. [DOI] [PubMed] [Google Scholar]
- Chen M, Huang RC, Yang LQ, et al. In vivo imaging of evoked calcium responses indicates the intrinsic axonal regenerative capacity of zebrafish. FASEB J. 2019;33:7721–7733. doi: 10.1096/FJ.201802649R. [DOI] [PubMed] [Google Scholar]
- Chen Y, Song X, Ye S, et al (2013b) Structural insight into enhanced calcium indicator GCaMP3 and GCaMPJ to promote further improvement. SpringerY Chen, X Song, S Ye, L Miao, Y Zhu, RG Zhang, G JiProtein cell, 2013•Springer 4:299–309. 10.1007/s13238-013-2103-4 [DOI] [PMC free article] [PubMed]
- Cheng SH, Willmann MR, Chen HC, Sheen J. Calcium signaling through protein kinases. the Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol. 2002;129:469–485. doi: 10.1104/PP.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Swanson CJ, Chen J, et al. The GCaMP-R family of genetically encoded ratiometric calcium indicators. ACS Chem Biol. 2017;12:1066–1074. doi: 10.1021/ACSCHEMBIO.6B00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho SM, Taylor AR, Ryan KP, et al. Spatiotemporal patterning of reactive oxygen production and Ca 2 Wave propagation in Fucus Rhizoid Cells. Plant Cell. 2002;14:2369–2381. doi: 10.1105/tpc.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collot M, Wilms CD, Bentkhayet A, et al. CaRuby-Nano: a novel high affinity calcium probe for dual color imaging. Elife. 2015 doi: 10.7554/ELIFE.05808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta. 2010;1797:607–618. doi: 10.1016/J.BBABIO.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Corso M, Doccula FG, De Melo RJF, et al. Endoplasmic reticulum-localized CCX2 is required for osmotolerance by regulating ER and cytosolic Ca2+ dynamics in Arabidopsis. Proc Natl Acad Sci U S A. 2018;115:3966–3971. doi: 10.1073/pnas.1720422115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Candeo A, Fieramonti L, et al. Calcium dynamics in root cells of Arabidopsis thaliana visualized with selective plane illumination microscopy. PLoS One. 2013 doi: 10.1371/JOURNAL.PONE.0075646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ME, McGonigal R, Barrie JA, et al. Real time imaging of intra-axonal calcium flux in an explant mouse model of axonal Guillain-Barré syndrome. Exp Neurol. 2022 doi: 10.1016/J.EXPNEUROL.2022.114127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daily NJ, Santos R, Vecchi J, et al. Calcium transient assays for compound screening with human iPSC-derived cardiomyocytes: evaluating new tools. J Evol Stem Cell Res. 2017;1:1–11. doi: 10.14302/ISSN.2574-4372.JESR-16-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melo Reis RA, Freitas HR, de Mello FG. Cell calcium imaging as a reliable method to study neuron-glial circuits. Front Neurosci. 2020 doi: 10.3389/FNINS.2020.569361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedecker P, De Schryver FC, Hofkens J. Fluorescent proteins: shine on, you crazy diamond. J Am Chem Soc. 2013;135:2387–2402. doi: 10.1021/JA309768D/ASSET/IMAGES/LARGE/JA-2012-09768D_0008.JPEG. [DOI] [PubMed] [Google Scholar]
- Defalco TA, Toyota M, Phan V, et al. Using GCaMP3 to Study Ca2+ signaling in Nicotiana Species. Plant Cell Physiol. 2017;58:1173–1184. doi: 10.1093/PCP/PCX053. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala S, Isayenkov S, et al. Calcium transport across plant membranes: mechanisms and functions. New Phytol. 2018;220:49–69. doi: 10.1111/nph.15266. [DOI] [PubMed] [Google Scholar]
- DeWald DB, Torabinejad J, Jones CA, et al. Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed arabidopsis1. Plant Physiol. 2001;126:759–769. doi: 10.1104/PP.126.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Jiang LH, Roger S, et al. Structure, function and techniques of investigation of the P2X7 receptor (P2X7R) in mammalian cells. Methods Enzymol. 2019;629:115–150. doi: 10.1016/BS.MIE.2019.07.043. [DOI] [PubMed] [Google Scholar]
- Diao M, Qu X, Huang S. Calcium imaging in Arabidopsis pollen cells using G-CaMP5. J Integr Plant Biol. 2018;60:897–906. doi: 10.1111/JIPB.12642. [DOI] [PubMed] [Google Scholar]
- Digonnet C, Aldon D, Leduc N, et al. First evidence of a calcium transient in flowering plants at fertilization. Development. 1997;124:2867–2874. doi: 10.1242/DEV.124.15.2867. [DOI] [PubMed] [Google Scholar]
- Downie JA, Walker SA. Plant responses to nodulation factors. Curr Opin Plant Biol. 1999;2:483–489. doi: 10.1016/S1369-5266(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Eberhard M, Erne P. Calcium binding to fluorescent calcium indicators: calcium green, calcium orange and calcium crimson. Biochem Biophys Res Commun. 1991;180:209–215. doi: 10.1016/S0006-291X(05)81278-1. [DOI] [PubMed] [Google Scholar]
- Eerbeek O, Mik EG, Zuurbier CJ, et al. Ratiometric intracellular calcium imaging in the isolated beating rat heart using indo-1 fluorescence. J Appl Physiol. 2004;97:2042–2050. doi: 10.1152/JAPPLPHYSIOL.01125.2003/ASSET/IMAGES/LARGE/ZDG0120434660009.JPEG. [DOI] [PubMed] [Google Scholar]
- Elliott AD. Confocal microscopy: principles and modern practices. Curr Protoc Cytom. 2020;92:e68. doi: 10.1002/CPCY.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium ion as a second messenger with special reference to excitation-contraction coupling. J Pharmacol Sci. 2006;100:519–524. doi: 10.1254/jphs.CPJ06004X. [DOI] [PubMed] [Google Scholar]
- Evennett PJ, Hammond C (2004) Microscopy—overview. Encycl Anal Sci Second Ed 32–41. 10.1016/B0-12-369397-7/00376-9
- Fang L, Wu J, Lin Q, Willis WD. Calcium–calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neurosci. 2002;22:4196. doi: 10.1523/JNEUROSCI.22-10-04196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijó JA, Moreno N. Imaging plant cells by two-photon excitation. Protoplasma. 2004;223:1–32. doi: 10.1007/S00709-003-0026-2. [DOI] [PubMed] [Google Scholar]
- Feijó JA, Wudick MM. Calcium is life. J Exp Bot. 2018;69:4147–4150. doi: 10.1093/JXB/ERY279. [DOI] [Google Scholar]
- Fichman Y, Mittler R. Integration of electric, calcium, reactive oxygen species and hydraulic signals during rapid systemic signaling in plants. Plant J. 2021;107:7–20. doi: 10.1111/TPJ.15360. [DOI] [PubMed] [Google Scholar]
- Floyd R, Wray S. Calcium transporters and signalling in smooth muscles. Cell Calcium. 2007;42:467–476. doi: 10.1016/j.ceca.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong VE, Ride JP, Read ND, et al. The self-incompatibility response in Papaver rhoeas is mediated by cytosolic free calcium. Plant J. 1993;4:163–177. doi: 10.1046/J.1365-313X.1993.04010163.X. [DOI] [Google Scholar]
- Fu D, Zhang Z, Wallrad L, et al. Ca2+-dependent phosphorylation of NRAMP1 by CPK21 and CPK23 facilitates manganese uptake and homeostasis in Arabidopsis. Proc Natl Acad Sci U S A. 2022 doi: 10.1073/PNAS.2204574119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla L, Vajente N, Pendin D, et al. Generation and characterization of a new fret-based Ca2+ sensor targeted to the nucleus. Int J Mol Sci. 2021 doi: 10.3390/IJMS22189945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Knight MR, Trewavas AJ, et al. Self-reporting arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol. 2004;134:898–908. doi: 10.1104/PP.103.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Han S, Huang Q, et al. Calcium imaging in population of dorsal root ganglion neurons unravels novel mechanisms of visceral pain sensitization and referred somatic hypersensitivity. Pain. 2021;162:1068–1081. doi: 10.1097/J.PAIN.0000000000002096. [DOI] [PubMed] [Google Scholar]
- Gee KR, Brown KA, Chen WNU, et al. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/CECA.1999.0095. [DOI] [PubMed] [Google Scholar]
- Gee KR, Brown KA, Chen WNU, et al. Chemical and physiological characterization of fluo-4 Ca(2+)-indicator dyes. Cell Calcium. 2000;27:97–106. doi: 10.1054/CECA.1999.0095. [DOI] [PubMed] [Google Scholar]
- Geiger A, Russo L, Gensch T, et al. Correlating Calcium Binding, Förster Resonance Energy Transfer, and Conformational Change in the Biosensor TN-XXL. Biophys J. 2012;102:2401. doi: 10.1016/J.BPJ.2012.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Bheri M, Bisht D, Pandey GK. Calcium signaling and transport machinery: potential for development of stress tolerance in plants. Curr Plant Biol. 2022;29:100235. doi: 10.1016/J.CPB.2022.100235. [DOI] [Google Scholar]
- Ghosh S, Bheri M, Pandey GK. Delineating calcium signaling machinery in plants: tapping the potential through functional genomics. Curr Genom. 2021;22:404–439. doi: 10.2174/1389202922666211130143328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomello M, Campeol M (2013) Indicators of intracellular calcium. Encycl Biol Chem Second Ed 574–579. 10.1016/B978-0-12-378630-2.00444-8
- Giacomello M, Drago I, Bortolozzi M, et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell. 2010;38:280–290. doi: 10.1016/J.MOLCEL.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Gilliard G, Huby E, Cordelier S, et al. Protoplast: a valuable toolbox to investigate plant stress perception and response. Front Plant Sci. 2021 doi: 10.3389/FPLS.2021.749581/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Fricker MD, Read ND, Trewavas AJ. Role of calcium in signal transduction of Commelina guard cells. Plant Cell. 1991;3:333–344. doi: 10.1105/TPC.3.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Jones RL. Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secretory activity in barley aleurone protoplasts. Proc Natl Acad Sci U S A. 1992;89:3591–3595. doi: 10.1073/PNAS.89.8.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard H, Manison NFH, Tomos D, Brownlee C. Elemental propagation of calcium signals in response-specific patterns determined by environmental stimulus strength. Proc Natl Acad Sci U S A. 2000;97:1932–1937. doi: 10.1073/PNAS.020516397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cruz C, Laguna S, Bachiller-Pulido A, et al. Single plane illumination microscopy for microfluidic device imaging. Biosensors12:1110. 2022 doi: 10.3390/bios12121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouriou Y, Gonnot F, Wehbi M, et al. High-sensitivity calcium biosensor on the mitochondrial surface reveals that IP3R channels participate in the reticular Ca2+ leak towards mitochondria. PLoS ONE. 2023;18:e0285670. doi: 10.1371/JOURNAL.PONE.0285670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouriou Y, Gonnot F, Wehbi M, et al. High-sensitivity calcium biosensor on the mitochondrial surface reveals that IP3R channels participate in the reticular Ca2+ leak towards mitochondria. PLoS One. 2023 doi: 10.1371/JOURNAL.PONE.0285670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greotti E, Fortunati I, Pendin D, et al. mCerulean3-based cameleon sensor to explore mitochondrial Ca2+ dynamics in vivo. iScience. 2019;16:340–355. doi: 10.1016/J.ISCI.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienberger C, Konnerth A. Imaging calcium in neurons. Neuron. 2012;73:862–885. doi: 10.1016/J.NEURON.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Griesbeck O, Baird GS, Campbell RE et al (2001) Reducing the environmental sensitivity of yellow fluorescent protein MECHANISM AND APPLICATIONS*. 10.1074/jbc.M102815200 [DOI] [PubMed]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. doi: 10.1016/s0021-9258(19)83641-4. [DOI] [PubMed] [Google Scholar]
- Guo J, He J, Dehesh K, et al. CamelliA-based simultaneous imaging of Ca2+ dynamics in subcellular compartments. Plant Physiol. 2022;188:2253–2271. doi: 10.1093/PLPHYS/KIAC020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiping D, Haiyi X, Xiaoxiao MA, Wang Z. Mechanism of blood-brain barrier damage caused by the inhibition of Wnt7/β-catenin pathway induced by endoplasmic reticulum stress in cerebrovascular endothelial cells after stroke. J Shanghai Jiao Tong Univ Med Sci. 2023;43:829. doi: 10.3969/J.ISSN.1674-8115.2023.07.005. [DOI] [Google Scholar]
- Hamilton A, Vergari E, Miranda C, Tarasov AI. Imaging calcium dynamics in subpopulations of mouse pancreatic islet cells. J Vis Exp. 2019 doi: 10.3791/59491. [DOI] [PubMed] [Google Scholar]
- Harper JF, Harmon A. Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol. 2005;6:555–566. doi: 10.1038/nrm1679. [DOI] [PubMed] [Google Scholar]
- Hasan MT, Friedrich RW, Euler T, et al. Functional fluorescent Ca2+ indicator proteins in transgenic mice under TET control. PLOS Biol. 2004;2:e163. doi: 10.1371/JOURNAL.PBIO.0020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Kudla J. Calcium decoding mechanisms in plants. Biochimie. 2011;93:2054–2059. doi: 10.1016/j.biochi.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Helmchen F. Miniaturization of fluorescence microscopes using fibre optics. Exp Physiol. 2002;87:737–745. doi: 10.1113/EPH8702478. [DOI] [PubMed] [Google Scholar]
- Helmchen F, Denk W, Kerr JND. Miniaturization of two-photon microscopy for imaging in freely moving animals. Cold Spring Harb Protoc. 2013;2013:904–913. doi: 10.1101/PDB.TOP078147. [DOI] [PubMed] [Google Scholar]
- Himschoot E, Krebs M, Costa A, et al. Calcium ion dynamics in roots: imaging and analysis. Methods Mol Biol. 2018;1761:115–130. doi: 10.1007/978-1-4939-7747-5_9. [DOI] [PubMed] [Google Scholar]
- Hurley TW, Ryan MP, Brinck RW. Changes of cytosolic Ca2+ interfere with measurements of cytosolic Mg2+ using mag-fura-2. Am J Physiol. 1992 doi: 10.1152/AJPCELL.1992.263.2.C300. [DOI] [PubMed] [Google Scholar]
- Ishimoto H, Sano H. Ex vivo calcium imaging for visualizing brain responses to endocrine signaling in Drosophila. JoVE J Vis Exp. 2018 doi: 10.3791/57701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelsson M, Siegel RS, Young J, et al. Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr Opin Plant Biol. 2006;9:654–663. doi: 10.1016/J.PBI.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Jacob AD, Ramsaran AI, Mocle AJ, et al. A compact head-mounted endoscope for in vivo calcium imaging in freely behaving mice. Curr Protoc Neurosci. 2018 doi: 10.1002/CPNS.51. [DOI] [PubMed] [Google Scholar]
- Jercog P, Rogerson T, Schnitzer MJ. Large-scale fluorescence calcium-imaging methods for studies of long-term memory in behaving mammals. Cold Spring Harb Perspect Biol. 2016 doi: 10.1101/CSHPERSPECT.A021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing P, Zou J, Kong L, et al. OsCCD1, a novel small calcium-binding protein with one EF-hand motif, positively regulates osmotic and salt tolerance in rice. Plant Sci. 2016;247:104–114. doi: 10.1016/J.PLANTSCI.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Kabbara AA, Allen DG. The use of the indicator flou-5N to measure sarcoplasmic reticulum calcium in single muscle fibres of the cane toad. J Physiol. 2001;534:87–97. doi: 10.1111/J.1469-7793.2001.00087.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner L, Scholz A, Tian Q, et al. Genetically encoded Ca2+ indicators in cardiac myocytes. Circ Res. 2014;114:1623–1639. doi: 10.1161/CIRCRESAHA.114.303475. [DOI] [PubMed] [Google Scholar]
- Kanchiswamy CN, Malnoy M, Occhipinti A, Maffei ME. Calcium imaging perspectives in plants. Int J Mol Sci. 2014;15:3842–3859. doi: 10.3390/ijms15033842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchiswamy CN, Takahashi H, Quadro S, et al. Regulation of Arabidopsis defense responses against Spodoptera littoralis by CPK-mediated calcium signaling. BMC Plant Biol. 2010 doi: 10.1186/1471-2229-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao JPY, Harootunian AT, Tsien RY. Photochemically generated cytosolic calcium pulses and their detection by fluo-3. J Biol Chem. 1989;264:8179–8184. doi: 10.1016/S0021-9258(18)83166-0. [DOI] [PubMed] [Google Scholar]
- Keinath NF, Waadt R, Brugman R, et al. Live cell imaging with R-GECO1 sheds light on flg22- and chitin-induced transient [Ca2+]cyt patterns in Arabidopsis. Mol Plant. 2015;8:1188–1200. doi: 10.1016/J.MOLP.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelner A, Leitão N, Chabaud M, et al. Dual color sensors for simultaneous analysis of calcium signal dynamics in the nuclear and cytoplasmic compartments of plant cells. Front Plant Sci. 2018 doi: 10.3389/FPLS.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, et al. Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J. 2000;23:267–278. doi: 10.1046/J.1365-313X.2000.00786.X. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim J, Marques JC, et al. Pan-neuronal calcium imaging with cellular resolution in freely swimming zebrafish. Nat Methods. 2017;14:1107–1114. doi: 10.1038/NMETH.4429. [DOI] [PubMed] [Google Scholar]
- Kobayashi NI, Tanoi K, Nakanishi TM. Magnesium localization in shoot apices during flower induction in Pharbitis nil. Can J Bot. 2006;84:1908–1916. doi: 10.1139/B06-145/ASSET/IMAGES/LARGE/B06-145F9.JPEG. [DOI] [Google Scholar]
- Köhler B, Blatt MR. Protein phosphorylation activates the guard cell Ca2+ channel and is a prerequisite for gating by abscisic acid. Plant J. 2002;32:185–194. doi: 10.1046/J.1365-313X.2002.01414.X. [DOI] [PubMed] [Google Scholar]
- Kolukisaoglu U, Weinl S, Blazevic D, et al. Calcium sensors and their interacting protein kinases: genomics of the Arabidopsis and rice CBL-CIPK signaling networks. PLANT Physiol. 2004;134:43–58. doi: 10.1104/pp.103.033068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp RF, Leech CA, Roe MW. Resveratrol interferes with Fura-2 intracellular calcium measurements. J Fluoresc. 2014;24:279. doi: 10.1007/S10895-013-1312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski MK, Szanda G, Balla T, Spät A. Store-operated Ca2+ influx and subplasmalemmal mitochondria. Cell Calcium. 2009;46:49–55. doi: 10.1016/J.CECA.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlikoff MI. Genetically encoded Ca2+ indicators: using genetics and molecular design to understand complex physiology. J Physiol. 2007;578:55–67. doi: 10.1113/JPHYSIOL.2006.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M, Held K, Binder A, et al. FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 2012;69:181–192. doi: 10.1111/J.1365-313X.2011.04780.X. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Gee KR, Archer EA, Regehr WG. Monitoring presynaptic calcium dynamics in projection fibers by in vivo loading of a novel calcium indicator. Neuron. 2000;27:25–32. doi: 10.1016/S0896-6273(00)00006-4. [DOI] [PubMed] [Google Scholar]
- Krogman W, Sparks JA, Blancaflor EB. Cell type-specific imaging of calcium signaling in Arabidopsis thaliana seedling roots using GCAMP3. Int J Mol Sci. 2020;21:1–15. doi: 10.3390/IJMS21176385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchitsu K, Ward JM, Allen GJ, et al. Loading acetoxymethyl ester fluorescent dyes into the cytoplasm of Arabidopsis and Commelina guard cells. New Phytol. 2002;153:527–533. doi: 10.1046/J.0028-646X.2001.00346.X. [DOI] [PubMed] [Google Scholar]
- Kudla JJ, Xu Q, Harter K et al (1999) Genes for calcineurin B-like proteins in Arabidopsis are differentially regulated by stress signals [DOI] [PMC free article] [PubMed]
- Kuhn B, Ozden I, Lampi Y, et al. An amplified promoter system for targeted expression of calcium indicator proteins in the cerebellar cortex. Front Neural Circuits. 2012 doi: 10.3389/FNCIR.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Verde V, Dominici P, Astegno A. Towards understanding plant calcium signaling through calmodulin-like proteins: a biochemical and structural perspective. Int J Mol Sci. 2018 doi: 10.3390/IJMS19051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CMC, Yeung PKK, Wong JTY. Monitoring cytosolic calcium in the dinoflagellate Crypthecodinium cohnii with calcium orange-AM. Plant Cell Physiol. 2005;46:1021–1027. doi: 10.1093/PCP/PCI102. [DOI] [PubMed] [Google Scholar]
- Lang T, Deng S, Zhao N, et al. Salt-sensitive signaling networks in the mediation of K+/Na+ homeostasis gene expression in Glycyrrhiza uralensis roots. Front Plant Sci. 2017 doi: 10.3389/FPLS.2017.01403/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau PF, Platko K, Byun JH, Austin RC. Calcium as a reliable marker for the quantitative assessment of endoplasmic reticulum stress in live cells. J Biol Chem. 2021;296:100779. doi: 10.1016/j.jbc.2021.100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Han JH. Successful in vivo calcium imaging with a head-mount miniaturized microscope in the amygdala of freely behaving mouse. JoVE J Vis Exp. 2020 doi: 10.3791/61659. [DOI] [PubMed] [Google Scholar]
- Lee SK, Lee JY, Lee MY, et al. Advantages of calcium green-1 over other fluorescent dyes in measuring cytosolic calcium in platelets. Anal Biochem. 1999;273:186–191. doi: 10.1006/ABIO.1999.4212. [DOI] [PubMed] [Google Scholar]
- Legué V, Biancaflor E, Wymer C, et al. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/PP.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ES, Saha MS. Optimizing calcium detection methods in animal systems: a sandbox for synthetic biology. Biomolecules. 2021;11:1–35. doi: 10.3390/BIOM11030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Prada J, Damineli DSC, et al. An optimized genetically encoded dual reporter for simultaneous ratio imaging of Ca2+ and H+ reveals new insights into ion signaling in plants. New Phytol. 2021;230:2292–2310. doi: 10.1111/NPH.17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Guo S, Xu Y, et al. Glycine betaine-mediated potentiation of HSP gene expression involves calcium signaling pathways in tobacco exposed to NaCl stress. Physiol Plant. 2014;150:63–75. doi: 10.1111/ppl.12067. [DOI] [PubMed] [Google Scholar]
- Li M, Liu C, Cui X, et al. An open-source real-time motion correction plug-in for single-photon calcium imaging of head-mounted microscopy. Front Neural Circuits. 2022 doi: 10.3389/FNCIR.2022.891825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Nan Q, Liu Y, et al. Nitric oxide alleviates submergence-induced maize seedling root tip cell death. J Plant Growth Regul. 2022;42:1212–1221. doi: 10.1007/s00344-022-10623-3. [DOI] [Google Scholar]
- Liao J, Patel D, Zhao Q et al (2021) A novel Ca2+ indicator for long-term tracking of intracellular calcium flux. Biotechniques 70. 10.2144/BTN-2020-0161/ASSET/IMAGES/LARGE/FIGURE4.JPEG [DOI] [PubMed]
- Liese A, Eichstädt B, Lederer S, et al. Imaging of plant calcium-sensor kinase conformation monitors real time calcium-dependent decoding in planta. Plant Cell. 2023 doi: 10.1093/PLCELL/KOAD196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lenzoni G, Knight MR. Design principle for decoding calcium signals to generate specific gene expression via transcription. Plant Physiol. 2020;182:1743–1761. doi: 10.1104/PP.19.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Niu yajie, Konishi mineko et al (2017) Discovery of nitrate-CPK-NLP signalling in central nutrient-growth networks. Nat Publ Gr. 10.1038/nature22077 [DOI] [PMC free article] [PubMed]
- Lock JT, Parker I, Smith IF. A comparison of fluorescent Ca2+ indicators for imaging local Ca2+ signals in cultured cells. Cell Calcium. 2015;58:638. doi: 10.1016/J.CECA.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DC, Knight MR (2003) Scientific correspondence mitochondrial and cytosolic calcium dynamics are differentially regulated in plants 1. 10.1104/pp.103.026047 [DOI] [PMC free article] [PubMed]
- Lohr C, Beiersdorfer A, Fischer T, et al. Using genetically encoded calcium indicators to study astrocyte physiology: a field guide. Front Cell Neurosci. 2021 doi: 10.3389/FNCEL.2021.690147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loro G, Costa A. Imaging of mitochondrial and nuclear Ca2+ dynamics in Arabidopsis roots. Cold Spring Harb Protoc. 2013;2013:781–785. doi: 10.1101/PDB.PROT073049. [DOI] [PubMed] [Google Scholar]
- Loro G, Ruberti C, Zottini M, Costa A. The D3cpv Cameleon reports Ca2+ dynamics in plant mitochondria with similar kinetics of the YC3.6 Cameleon, but with a lower sensitivity. J Microsc. 2013;249:8–12. doi: 10.1111/J.1365-2818.2012.03683.X. [DOI] [PubMed] [Google Scholar]
- Luan S, Kudla JJ, Rodriguez-concepcion M, et al. Calmodulins and calcineurin B-like proteins: calcium sensors for specific signal response coupling in plants. Plant Cell Suppl. 2002 doi: 10.1105/tpc.001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan S, Ting J, Gupta R. Protein tyrosine phosphatases in higher plants. New Phytol. 2001;151:155–164. doi: 10.1046/J.1469-8137.2001.00168.X. [DOI] [PubMed] [Google Scholar]
- Luan S, Wang C. Calcium signaling mechanisms across Kingdoms. Annu Rev Cell Dev Biol. 2021;37:311–340. doi: 10.1146/annurev-cellbio-120219-035210. [DOI] [PubMed] [Google Scholar]
- MacGowan GA, Du C, Glonty V et al (2001a) Rhod-2 based measurements of intracellular calcium in the perfused mouse heart: cellular and subcellular localization and response to positive intropy 6:23–30. 10.1117/1.1316091 [DOI] [PubMed]
- MacGowan GA, Du C, Glonty V, et al. Rhod-2 based measurements of intracellular calcium in the perfused mouse heart: cellular and subcellular localization and response to positive inotropy. J Biomed Opt. 2001;6:23. doi: 10.1117/1.1316091. [DOI] [PubMed] [Google Scholar]
- Malhó R, Trewavas AJ. Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell. 1996;8:1935–1949. doi: 10.1105/TPC.8.11.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J-M, Collot M (2015) Fluorescent red emitting functionalizable calcium indicators
- Mank M, Santos AF, Direnberger S, et al. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat Methods. 2008;5:805–811. doi: 10.1038/NMETH.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AA, Alford S, Richmond JE. In vivo calcium imaging in C. elegans body wall muscles. JoVE J Vis Exp. 2019 doi: 10.3791/59175. [DOI] [PubMed] [Google Scholar]
- Matlashov ME, Vera J, Kasatkina LA, et al. Design and initial characterization of a small near-infrared fluorescent calcium indicator. Front Cell Dev Biol. 2022;10:880107. doi: 10.3389/FCELL.2022.880107/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew J. Betzenhauser(*) JHWHP and DIY (2011) Measurement of intracellular calcium concentration in pancreatic acini. Pancreapedia Exocrine Pancreas Knowl Base.10.3998/PANC.2011.34
- Matthus E, Sun J, Wang L, et al. DORN1/P2K1 and purino-calcium signalling in plants: making waves with extracellular ATP. Ann Bot. 2019;124:1227–1242. doi: 10.1093/AOB/MCZ135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuz-Mares D, González-Andrade M, Araiza-Villanueva MG, et al. Mitochondrial calcium: effects of its imbalance in disease. Antioxidants. 2022 doi: 10.3390/ANTIOX11050801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mázala DAG, Grange RW, Chin ER. The role of proteases in excitation-contraction coupling failure in muscular dystrophy. Am J Physiol Cell Physiol. 2015;308:C33–C40. doi: 10.1152/AJPCELL.00267.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Webb AA, Taylor JE, Hetherington AM. Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey DJ, Nutt L. Measurement of changes in intracellular calcium during apoptosis. Methods Mol Biol. 2004;282:117–130. doi: 10.1385/1-59259-812-9:117/COVER. [DOI] [PubMed] [Google Scholar]
- Mehlmer N, Parvin N, Hurst CH, et al. A toolset of aequorin expression vectors for in planta studies of subcellular calcium concentrations in Arabidopsis thaliana. J Exp Bot. 2012;63:1751–1761. doi: 10.1093/jxb/err406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant FA, Bartels KA, Bovik AC, Diller KR (2005) Confocal microscopy. Handb image video process second ed 1291–1309. 10.1016/B978-012119792-6/50135-2
- Meza-Resillas J, Ahmadpour N, Stobart M, Stobart J. Brain pericyte calcium and hemodynamic imaging in transgenic mice in vivo. J Vis Exp. 2021 doi: 10.3791/62725. [DOI] [PubMed] [Google Scholar]
- Michard E, Dias P, Feijó JA. Tobacco pollen tubes as cellular models for ion dynamics: improved spatial and temporal resolution of extracellular flux and free cytosolic concentration of calcium and protons using pHluorin and YC3.1 CaMeleon. Sex Plant Reprod. 2008;21:169–181. doi: 10.1007/S00497-008-0076-X. [DOI] [Google Scholar]
- Mithöfer A, Mazars C, Maffei ME. Probing spatio-temporal intracellular calcium variations in plants. Methods Mol Biol. 2009;479:79–92. doi: 10.1007/978-1-59745-289-2_5. [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci U S A. 1999;96:2135–2140. doi: 10.1073/PNAS.96.5.2135/ASSET/F87AFD77-C3B2-4A38-84D2-605A4F6687EA/ASSETS/GRAPHIC/PQ0594987005.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R et al (1997) Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin [DOI] [PubMed]
- Monshausen GB, Bibikova TN, Messerli MA, et al. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci U S A. 2007;104:20996–21001. doi: 10.1073/PNAS.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S. Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008;147:1690–1698. doi: 10.1104/PP.108.123638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Miller ND, Murphy AS, Gilroy S. Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis. Plant J. 2011;65:309–318. doi: 10.1111/J.1365-313X.2010.04423.X. [DOI] [PubMed] [Google Scholar]
- Monteiro D, Liu Q, Lisboa S, et al. Phosphoinositides and phosphatidic acid regulate pollen tube growth and reorientation through modulation of [Ca2+]c and membrane secretion. J Exp Bot. 2005;56:1665–1674. doi: 10.1093/JXB/ERI163. [DOI] [PubMed] [Google Scholar]
- Mousavi SAR, Dubin AE, Zeng WZ, et al. PIEZO ion channel is required for root mechanotransduction in Arabidopsis thaliana. Proc Natl Acad Sci U S A. 2021 doi: 10.1073/PNAS.2102188118/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Kawakami K. Prey capture in zebrafish larvae serves as a model to study cognitive functions. Front Neural Circuits. 2013 doi: 10.3389/FNCIR.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A, Ohkura M, Kotani T, et al. Genetic visualization with an improved GCaMP calcium indicator reveals spatiotemporal activation of the spinal motor neurons in zebrafish. Proc Natl Acad Sci. 2011;108:5425–5430. doi: 10.1073/PNAS.1000887108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, et al. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci U S A. 2004;101:10554–10559. doi: 10.1073/PNAS.0400417101/SUPPL_FILE/00417MOVIE2.MOV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Iizumi S, Satoh K, et al. Comparative analysis of plant and animal calcium signal transduction element using plant full-length cDNA data. Mol Biol Evol. 2004;21:1855–1870. doi: 10.1093/MOLBEV/MSH197. [DOI] [PubMed] [Google Scholar]
- Nagel-Volkmann J, Plieth C, Becker D, et al. Cold-induced cytosolic free calcium ion concentration changes in wheat. J Plant Physiol. 2009;166:1955–1960. doi: 10.1016/J.JPLPH.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;192(19):137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- Neher E (1992) Ion channels for communication between and within cells. Science (80- ) 256:498–502. 10.1126/SCIENCE.1373906/ASSET/4985D5E7-86E3-4B66-A8B9-122524714B77/ASSETS/SCIENCE.1373906.FP.PNG [DOI] [PubMed]
- Nguyen CT, Kurenda A, Stolz S, et al. Identification of cell populations necessary for leaf-toleaf electrical signaling in a wounded plant. Proc Natl Acad Sci U S A. 2018;115:10178–10183. doi: 10.1073/pnas.1807049115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Yu Z, Du G, et al. Heterologous expression and functional analysis of rice GLUTAMATE RECEPTOR-LIKE family indicates its role in glutamate triggered calcium flux in rice roots. Rice. 2016;9:9. doi: 10.1186/s12284-016-0081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nietz AK, Popa LS, Streng ML, et al. Wide-field calcium imaging of neuronal network dynamics in vivo. Biology (Basel) 2022 doi: 10.3390/BIOLOGY11111601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor N, Silver RB. Ratio imaging: practical considerations for measuring intracellular Ca2+ and pH in living cells. Methods Cell Biol. 2013;114:387–406. doi: 10.1016/B978-0-12-407761-4.00016-6. [DOI] [PubMed] [Google Scholar]
- Oh J, Lee C, Kaang BK. Imaging and analysis of genetically encoded calcium indicators linking neural circuits and behaviors. Korean J Physiol Pharmacol. 2019;23:237–249. doi: 10.4196/KJPP.2019.23.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsu Y, Couchman K, Lyons DG, et al. Calcium dynamics in astrocyte processes during neurovascular coupling. Nat Neurosci. 2015;18:210–218. doi: 10.1038/NN.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AE, Giacomello M, Kortemme T, et al. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13:521–530. doi: 10.1016/J.CHEMBIOL.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Pandey GK. Emergence of a novel calcium signaling pathway in plants: CBL-CIPK signaling network. Physiol Mol Biol Plants. 2008;14:51–68. doi: 10.1007/s12298-008-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RM, Etzler JC, Watts LT, et al. Chemical calcium indicators. Methods. 2008;46:143–151. doi: 10.1016/J.YMETH.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmagnani AS, Maffei ME. Calcium signaling in plant-insect interactions. Plants. 2022;11:2689. doi: 10.3390/PLANTS11202689/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RM, Fischer-Parton S, Trewavas AJ, Watahiki MK. Pollen tubes exhibit regular periodic membrane trafficking events in the absence of apical extension. J Cell Sci. 2003;116:2707–2719. doi: 10.1242/JCS.00468. [DOI] [PubMed] [Google Scholar]
- Pedretti M, Favretto F, Troilo F, et al. Role of myristoylation in modulating PCaP1 interaction with calmodulin. Plant Physiol Biochem. 2023 doi: 10.1016/J.PLAPHY.2023.108003. [DOI] [PubMed] [Google Scholar]
- Peng XB, Sun MX, Yang HY. Comparative detection of calcium fluctuations in single female sex cells of tobacco to distinguish calcium signals triggered by in vitro fertilization. J Integr Plant Biol. 2009;51:782–791. doi: 10.1111/J.1744-7909.2009.00857.X. [DOI] [PubMed] [Google Scholar]
- Phillips KF, Santos E, Blair RE, Deshpande LS. Targeting intracellular calcium stores alleviates neurological morbidities in a DFP-based rat model of gulf war illness. Toxicol Sci. 2019;169:567–578. doi: 10.1093/TOXSCI/KFZ070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivato M, Grenzi M, Costa A, Ballottari M. Compartment-specific Ca2+ imaging in the green alga Chlamydomonas reinhardtii reveals high light-induced chloroplast Ca2+ signatures. New Phytol. 2023;240:258–271. doi: 10.1111/NPH.19142. [DOI] [PubMed] [Google Scholar]
- Pnevmatikakis EA, Soudry D, Gao Y, et al. Simultaneous denoising, deconvolution, and demixing of calcium imaging data. Neuron. 2016;89:285. doi: 10.1016/J.NEURON.2015.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologruto TA, Yasuda R, Svoboda K. Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J Neurosci. 2004;24:9572. doi: 10.1523/JNEUROSCI.2854-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Arslan P, Tsien RY, Rink TJ. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982;94:335. doi: 10.1083/JCB.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher D, McCann RO, Cormier MJ. Cloning and expression of the cDNA coding for aequorin, a bioluminescent calcium-binding protein. Biochem Biophys Res Commun. 1985;126:1259–1268. doi: 10.1016/0006-291X(85)90321-3. [DOI] [PubMed] [Google Scholar]
- Putkey JA, Kleerekoper Q, Gaertner TR, Waxham MN. A new role for IQ motif proteins in regulating calmodulin function. J Biol Chem. 2003;278:49667–49670. doi: 10.1074/JBC.C300372200. [DOI] [PubMed] [Google Scholar]
- Qiu L, Huang D, Wang Y, Qu H. Staining the cytoplasmic Ca2+ with fluo-4/am in apple pulp. J vis Exp. 2021 doi: 10.3791/62526. [DOI] [PubMed] [Google Scholar]
- Qudeimat E, Frank W. Ca2+ signatures: the role of Ca2+-ATPases. Plant Signal Behav. 2009;4:350. doi: 10.4161/PSB.4.4.8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz M. Fluorescence microscopy-A historical and technical perspective. Cytom Part A. 2013;83:767–779. doi: 10.1002/CYTO.A.22295. [DOI] [PubMed] [Google Scholar]
- Resentini F, Grenzi M, Ancora D, et al. Simultaneous imaging of ER and cytosolic Ca2+ dynamics reveals long-distance ER Ca2+ waves in plants. Plant Physiol. 2021;187:603–617. doi: 10.1093/PLPHYS/KIAB251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho HS, Jeon J, Lee YH. Phospholipase C-mediated calcium signalling is required for fungal development and pathogenicity in Magnaporthe oryzae. Mol Plant Pathol. 2009;10:337–346. doi: 10.1111/J.1364-3703.2009.00536.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietdorf K, Chehab T, Allman SA, Bootman M (2014) Novel improved Ca2+ indicator dyes on the market-a comparative study of novel Ca2+ indicators with fluo-4
- Rigosi E, Haase A, Rath L, et al. Asymmetric neural coding revealed by in vivo calcium imaging in the honey bee brain. Proc Biol Sci. 2015 doi: 10.1098/RSPB.2014.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigosi E, Haase A, Rath L, et al. Asymmetric neural coding revealed by in vivo calcium imaging in the honey bee brain. Proc R Soc B Biol Sci. 2015 doi: 10.1098/RSPB.2014.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Sendra A, Calabuig-Serna A, Seguí-Simarro JM. Dynamics of calcium during in vitro microspore embryogenesis and in vivo microspore development in brassica napus and Solanum melongena. Front Plant Sci. 2017 doi: 10.3389/FPLS.2017.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Konnerth A. Genetically encoded Ca2+ sensors come of age. Nat Methods. 2008;59(5):761–762. doi: 10.1038/nmeth0908-761. [DOI] [PubMed] [Google Scholar]
- Rodrigo-Moreno A, Bazihizina N, Azzarello E, et al. Root phonotropism: early signalling events following sound perception in Arabidopsis roots. Plant Sci. 2017;264:9–15. doi: 10.1016/J.PLANTSCI.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Russell JT. Imaging calcium signals in vivo: a powerful tool in physiology and pharmacology. Br J Pharmacol. 2011;163:1605–1625. doi: 10.1111/J.1476-5381.2010.00988.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J-E, Choi H-S, Park H-Y, Rhee Paeng I-S. Bioluminescence immunoassay for neurotransmitter, serotonin using aequorin as a Label. Anal Sci Technol. 2010;23:60–67. doi: 10.5806/AST.2010.23.1.060. [DOI] [Google Scholar]
- Saavedra-Molina A, Uribe S, Devlin TM. Control of mitochondrial matrix calcium: studies using fluo-3 as a fluorescent calcium indicator. Biochem Biophys Res Commun. 1990;167:148–153. doi: 10.1016/0006-291X(90)91743-C. [DOI] [PubMed] [Google Scholar]
- Saito K, Hatsugai N, Horikawa K, et al. Auto-luminescent genetically-encoded ratiometric indicator for real-time Ca2+ imaging at the single cell level. PLoS One. 2010 doi: 10.1371/JOURNAL.PONE.0009935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi PA. Light sheet fluorescence microscopy: a review. J Histochem Cytochem. 2011;59:129–138. doi: 10.1369/0022155410394857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargoy A, Barnes S, Brecha NC, Pérez de Sevilla Müller L. Immunohistochemical and calcium imaging methods in wholemount rat retina. JoVE J Vis Exp. 2014 doi: 10.3791/51396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Agrawal N, Sharma M, et al. Role of protein tyrosine phosphatases in plants. Curr Genom. 2015;16:224–236. doi: 10.2174/1389202916666150424234300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemetov AA, Monakhov MV, Zhang Q, et al. A near-infrared genetically encoded calcium indicator for in vivo imaging. Nat Biotechnol. 2021;39:368–377. doi: 10.1038/S41587-020-0710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Zhang N, Zheng S, et al. Calmodulin in complex with the first IQ motif of myosin-5a functions as an intact calcium sensor. Proc Natl Acad Sci U S A. 2016;113:E5812–E5820. doi: 10.1073/PNAS.1607702113/SUPPL_FILE/PNAS.201607702SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Dana H, Abdelfattah AS, et al. A genetically encoded Ca2+ indicator based on circularly permutated sea anemone red fluorescent protein eqFP578. BMC Biol. 2018;16:1–16. doi: 10.1186/S12915-018-0480-0/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura The discovery of aequorin and green fluorescent protein. J Microsc. 2005;217:3–15. doi: 10.1111/j.0022-2720.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- Shimomura O. Luminescence of aequorin is triggered by the binding of two calcium ions. Biochem Biophys Res Commun. 1995;211:359–363. doi: 10.1006/BBRC.1995.1821. [DOI] [PubMed] [Google Scholar]
- Shimozono S, Fukano T, Nagai T, et al. Confocal imaging of subcellular Ca2+ concentrations using a dual-excitation ratiometric indicator based on green fluorescent protein. Sci STKE. 2002 doi: 10.1126/STKE.2002.125.PL4. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Tye KM. Leveraging calcium imaging to illuminate circuit dysfunction in addiction. Alcohol. 2019;74:47–63. doi: 10.1016/J.ALCOHOL.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CI, Berlin JR. A method for recording intracellular [Ca2+] transients in cardiac myocytes using calcium green-2. Pflügers Arch Eur J Physiol. 1995;430:579–583. doi: 10.1007/BF00373895. [DOI] [PubMed] [Google Scholar]
- Steinhorst L, He G, Moore LK, et al. A Ca2+-sensor switch for tolerance to elevated salt stress in Arabidopsis. Dev Cell. 2022;57:2081–2094.e7. doi: 10.1016/J.DEVCEL.2022.08.001. [DOI] [PubMed] [Google Scholar]
- Storti M, Costa A, Golin S, et al. Systemic calcium wave propagation in physcomitrella patens. Plant Cell Physiol. 2018;59:1377–1384. doi: 10.1093/PCP/PCY104. [DOI] [PubMed] [Google Scholar]
- Streich L, Boffi JC, Wang L, et al. High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy. Nat Methods. 2021;18:1253–1258. doi: 10.1038/S41592-021-01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker SA, Whitaker M. Confocal laser scanning microscopy of calcium dynamics in living cells. Microsc Res Tech. 1999;46:356–369. doi: 10.1002/(SICI)1097-0029(19990915)46:6. [DOI] [PubMed] [Google Scholar]
- Sun L, Li Y, Miao W, et al. NADK2 positively modulates abscisic acid-induced stomatal closure by affecting accumulation of H2O2, Ca2+ and nitric oxide in Arabidopsis guard cells. Plant Sci. 2017;262:81–90. doi: 10.1016/J.PLANTSCI.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Swanson SJ, Choi WG, Chanoca A, Gilroy S. In vivo imaging of Ca2+, pH, and reactive oxygen species using fluorescent probes in plants. Annu Rev Plant Biol. 2011;62:273–297. doi: 10.1146/ANNUREV-ARPLANT-042110-103832. [DOI] [PubMed] [Google Scholar]
- Tada M, Takeuchi A, Hashizume M, et al. A highly sensitive fluorescent indicator dye for calcium imaging of neural activity in vitro and in vivo. Eur J Neurosci. 2014;39:1720–1728. doi: 10.1111/EJN.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev. 1999;79:1089–1125. doi: 10.1152/PHYSREV.1999.79.4.1089. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Oba S, Yukinawa N et al (2011) High-speed multineuron calcium imaging using nipkow-type confocal microscopy. Curr Protoc Neurosci 57:2.14.1–2.14.10. 10.1002/0471142301.NS0214S57 [DOI] [PubMed]
- Tank DW, Sugimori M, Connor JA, Llinás RR (1988) Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science (80- ) 242:773–777. 10.1126/SCIENCE.2847315 [DOI] [PubMed]
- Terai T, Nagano T. Fluorescent probes for bioimaging applications. Curr Opin Chem Biol. 2008;12:515–521. doi: 10.1016/j.cbpa.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Thapa R, Liang B, Liu R, Li Y. Stereotaxic viral injection and gradient-index lens implantation for deep brain in vivo calcium imaging. JoVE J Vis Exp. 2021 doi: 10.3791/63049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thestrup T, Litzlbauer J, Bartholomäus I, et al. optimized ratiometric calcium sensors for functional in vivo imaging of neurons and t lymphocytes. Artic Nat Methods. 2014;11:175. doi: 10.1038/nmeth.2773. [DOI] [PubMed] [Google Scholar]
- Thestrup T, Litzlbauer J, Bartholomäus I, et al. Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nat Methods. 2014;11:175–182. doi: 10.1038/NMETH.2773. [DOI] [PubMed] [Google Scholar]
- Thompson SEM, Callow JA, Callow ME, et al. Membrane recycling and calcium dynamics during settlement and adhesion of zoospores of the green alga Ulva linza. Plant Cell Environ. 2007;30:733–744. doi: 10.1111/J.1365-3040.2007.01661.X. [DOI] [PubMed] [Google Scholar]
- Thor K. Calcium—nutrient and messenger. Front Plant Sci. 2019;10:440. doi: 10.3389/FPLS.2019.00440/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor K, Peiter E. Cytosolic calcium signals elicited by the pathogen-associated molecular pattern flg22 in stomatal guard cells are of an oscillatory nature. New Phytol. 2014;204:873–881. doi: 10.1111/NPH.13064. [DOI] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/NMETH.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinning PW, Franssen AJPM, Hridi SU, et al. A 340/380 nm light-emitting diode illuminator for Fura-2 AM ratiometric Ca2+ imaging of live cells with better than 5 nM precision. J Microsc. 2018;269:212–220. doi: 10.1111/JMI.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischbirek C, Birkner A, Jia H, et al. Deep two-photon brain imaging with a red-shifted fluorometric Ca2+ indicator. Proc Natl Acad Sci U S A. 2015;112:11377–11382. doi: 10.1073/PNAS.1514209112/SUPPL_FILE/PNAS.201514209SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–553. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- Tucker EB, Boss WF. Mastoparan-induced intracellular Ca2+ fluxes may regulate cell-to-cell communication in plants. Plant Physiol. 1996;111:459–467. doi: 10.1104/PP.111.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafadar F, Amooaghaie R, Ehsanzadeh P, Ghanati F. Melatonin improves the photosynthesis in Dracocephalum kotschyi under salinity stress in a Ca2+/CaM-dependent manner. Funct Plant Biol. 2021;49:89–101. doi: 10.1071/FP21233. [DOI] [PubMed] [Google Scholar]
- Vasconsuelo A, Morelli S, Picotto G, et al. Intracellular calcium mobilization: a key step for chitosan-induced anthraquinone production in Rubia tinctorum L. Plant Sci. 2005;169:712–720. doi: 10.1016/J.PLANTSCI.2005.05.022. [DOI] [Google Scholar]
- Vigani G, Costa A. Harnessing the new emerging imaging technologies to uncover the role of Ca2+ signalling in plant nutrient homeostasis. Plant Cell Environ. 2019;42:2885–2901. doi: 10.1111/PCE.13611. [DOI] [PubMed] [Google Scholar]
- Vincent TR, Avramova M, Canham J, et al. Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell. 2017;29:1460–1479. doi: 10.1105/TPC.17.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DJ, zum Alten Borgloh SM, Astori S, et al. Single-spike detection in vitro and in vivo with a genetic Ca2+ sensor. Nat Methods. 2008;5:797–804. doi: 10.1038/NMETH.1242. [DOI] [PubMed] [Google Scholar]
- Walton SD, Chakravarthy H, Shettigar V, et al. Divergent soybean calmodulins respond similarly to calcium transients: insight into differential target regulation. Front Plant Sci. 2017 doi: 10.3389/FPLS.2017.00208/PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Yamano T, Takane S, et al. Chloroplast-mediated regulation of CO2-concentrating mechanism by Ca2+-binding protein CAS in the green alga Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 2016;113:12586–12591. doi: 10.1073/PNAS.1606519113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-X, WANG L, et al. A cyclic effect of cAMP and calcium signaling contributes to jujube growth and development. J Integr Agric. 2023;22:2094–2110. doi: 10.1016/J.JIA.2023.04.039. [DOI] [Google Scholar]
- Wang Q, Yuan E, Ling X, et al. An aphid facultative symbiont suppresses plant defence by manipulating aphid gene expression in salivary glands. Plant Cell Environ. 2020;43:2311–2322. doi: 10.1111/PCE.13836. [DOI] [PubMed] [Google Scholar]
- Wang R, Li R, Xu T, Li T. Optimization of the pollen-tube pathway method of plant transformation using the Yellow Cameleon 3.6 calcium sensor in Solanum lycopersicum. Biol. 2017;72:1147–1155. doi: 10.1515/BIOLOG-2017-0127. [DOI] [Google Scholar]
- Wang Y, Zhu Y, Ling Y, et al. Disruption of actin filaments induces mitochondrial Ca2+release to the cytoplasm and [Ca2+]cchanges in Arabidopsis root hairs. BMC Plant Biol. 2010 doi: 10.1186/1471-2229-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SE, Karplus E, Miller AL. Retrospective on the development of aequorin and aequorin-based imaging to visualize changes in intracellular free [Ca(2+) ] Mol Reprod Dev. 2015;82:563–586. doi: 10.1002/MRD.22298. [DOI] [PubMed] [Google Scholar]
- Whitaker M. Genetically-encoded probes for measurement of intracellular calcium. Methods Cell Biol. 2010;99:153. doi: 10.1016/B978-0-12-374841-6.00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier WG, Kort AA, Stern MD, et al. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci U S A. 1983;80:7367. doi: 10.1073/PNAS.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA. Quantitative intracellular calcium imaging with laser-scanning confocal microscopy. Cell Calcium. 1990;11:589–597. doi: 10.1016/0143-4160(90)90013-K. [DOI] [PubMed] [Google Scholar]
- Williams PDE, Verma S, Robertson AP, Martin RJ. Adapting techniques for calcium imaging in muscles of adult Brugia malayi. Invert Neurosci. 2020;20:12. doi: 10.1007/S10158-020-00247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilms CD, Häusser M. Twitching towards the ideal calcium sensor. Nat Methods. 2014;11:139–140. doi: 10.1038/nmeth.2814. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang Y, Kim SG, et al. A secreted chitinase-like protein (OsCLP) supports root growth through calcium signaling in Oryza sativa. Physiol Plant. 2017;161:273–284. doi: 10.1111/PPL.12579. [DOI] [PubMed] [Google Scholar]
- Xiang X, Zhang S, Li E, et al. RHO OF PLANT proteins are essential for pollen germination in Arabidopsis. Plant Physiol. 2023;193:140–155. doi: 10.1093/PLPHYS/KIAD196. [DOI] [PubMed] [Google Scholar]
- Xie Q, Lee Y, Li G et al (2017) Biotic and abiotic stresses activate different Ca2+ permeable channels in arabidopsis. 10.3389/fpls.2017.00083 [DOI] [PMC free article] [PubMed]
- Xiong TC, Ronzier E, Sanchez F, 2014. Imaging long distance propagating calcium signals in intact plant leaves with the BRET-based GFP-aequorin reporter. Front Plant Sci. [DOI] [PMC free article] [PubMed]
- Xue N, Zhan C, Song J, et al. The glutamate receptor-like 3.3 and 3.6 mediate systemic resistance to insect herbivores in Arabidopsis. J Exp Bot. 2022;73:7611–7627. doi: 10.1093/JXB/ERAC399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Poovaiah BW. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003;8:505–512. doi: 10.1016/j.tplants.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Yoon S, Pan Y, Shung K, Wang Y. FRET-based Ca2+ biosensor single cell imaging interrogated by high-frequency ultrasound. Sensors (Basel) 2020;20:1–14. doi: 10.3390/S20174998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto Y, Hiramoto Y. Observation of intracellular Ca2+ with aequorin luminescence. Int Rev Cytol. 1991;129:45–73. doi: 10.1016/S0074-7696(08)60508-2. [DOI] [PubMed] [Google Scholar]
- Yu D, Baird GS, Tsien RY, Davis RL (2002) Detection of calcium transients in drosophila mushroom body neurons with camgaroo reporters [DOI] [PMC free article] [PubMed]
- Zhang QF, Li J, Bi FC, et al. Ceramide-induced cell death depends on calcium and caspase-like activity in rice. Front Plant Sci. 2020 doi: 10.3389/FPLS.2020.00145/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XR, Xu YP, Cai XZ. SLCNGC1 and SLCNGC14 suppress Xanthomonas oryzae pv. Oryzicola-induced hypersensitive response and non-host resistance in tomato. Front Plant Sci. 2018;9:285. doi: 10.3389/fpls.2018.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rózsa M, Liang Y, et al. Fast and sensitive GCaMP calcium indicators for imaging neural populations. Nature. 2023;615:884–891. doi: 10.1038/S41586-023-05828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Taylor JL, et al. Aequorin-based luminescence imaging reveals differential calcium signalling responses to salt and reactive oxygen species in rice roots. J Exp Bot. 2015;66:2535–2545. doi: 10.1093/JXB/ERV043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Schleifenbaum J. Genetically encoded calcium indicators: a new tool in renal hypertension research. Front Med. 2019;6:452253. doi: 10.3389/FMED.2019.00128/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Belavek KJ, Miller EW (1980) Origins of Ca2+ imaging with fluorescent indicators. Tsien, R Y 60:2396. 10.1021/acs.biochem.1c00350 [DOI] [PMC free article] [PubMed]
- Zong W, Obenhaus HA, Skytøen ER, et al. Large-scale two-photon calcium imaging in freely moving mice. Cell. 2022;185:1240–1256.e30. doi: 10.1016/j.cell.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Xiong TC, Ronzier E, Sanchez F, 2014. Imaging long distance propagating calcium signals in intact plant leaves with the BRET-based GFP-aequorin reporter. Front Plant Sci. [DOI] [PMC free article] [PubMed]