Abstract
Vascular and valvular calcifications, commonly seen in renal patients, increase operative mortality and can preclude conventional valvular management. We show a novel approach to treat aortic stenosis and degenerative mitral regurgitation under hypothermic circulatory arrest in a hemodialysis patient with aortic, mitral disease and porcelain aorta with surgical and transcatheter contraindications.
Key Words: deep hypothermic circulatory arrest, mitral annulus calcification, mitral repair, porcelain aorta, TAVR
Central Illustration
Introduction
Aortic stenosis and mitral regurgitation are the 2 most common valvular pathologies in the United States,1 which occur frequently in patients with end-stage renal disease (ESRD).2 Extensive circumferential aortic calcification, known as porcelain aorta, is present in up to 34% of patients with ESRD.3 Porcelain aorta carries increased operative mortality because manipulation of calcified aortic segments can lead to catastrophic aortic dissection, stroke, and uncontrollable bleeding, and porcelain aorta can be resistant to conventional surgical management.3 One option can be replacement of the ascending aorta, but this is dependent on an aortic arch that is amenable to hemi-arch implantation. As a result, surgical correction of aortic stenosis is considered very high risk in patients with porcelain aorta and transcatheter aortic valve replacement (TAVR) is indicated,3 although individualized strategies have also been suggested.4 Patients with ESRD also have higher rates of mitral valve disease including mitral annular calcification (MAC).5 Surgical repair of the mitral valve with MAC is a formidable technical challenge that is independently associated with poorer outcomes and higher operative mortality,6 including devastating atrioventricular groove disruption.
Learning Objectives
-
•
Vascular and valvular calcifications can preclude conventional management of the cardiac patient.
-
•
We demonstrate a hybrid surgical-TAVR approach to manage a ‘technically inoperable’ patient with concurrent porcelain aorta and coronary cusp calcifications.
In this report, we describe an ESRD patient with severe aortic stenosis and porcelain aorta who was not a TAVR candidate due to leaflet calcium immediately adjacent to the left main coronary artery. This patient also had severe mitral regurgitation and MAC. Here we describe a novel approach of direct TAVR and mitral repair under deep hypothermic circulatory arrest.
Case Presentation
A 64-year-old female patient was referred to us with severe aortic stenosis, an ejection fraction of 50%, and a past medical history of hemodialysis for ESRD. Preoperative imaging revealed porcelain aorta from aortic root to arch (Figure 1A, Video 1). A calcified 2-cm nodule on the left coronary cusp of the aortic valve was prominent (Figure 1B). This prohibited safe peripherally implanted TAVR owing to concerns for acute left main coronary artery obstruction. Preoperative transthoracic echocardiography computed tomography imaging suggested moderate mitral regurgitation with severe trigone to trigone MAC (Figure 2).
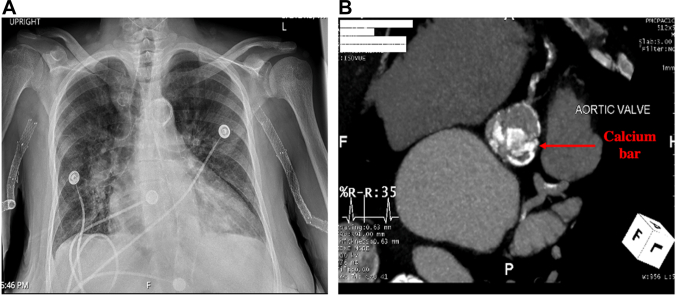
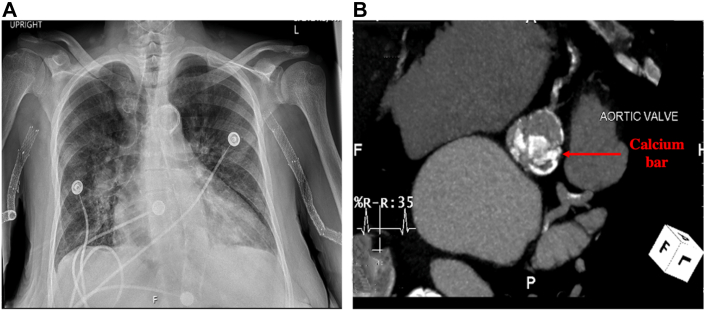
Figure 1.
Extent of Vascular and Valvular Calcifications
(A) Chest x-ray showing porcelain aorta associated with extensive calcifications from ascending to descending aorta. (B) Heavily calcified aortic valve and coronary artery calcifications. Notice labelled (red arrow) leaflet calcifications overlying the ostium projection of the left main coronary artery.
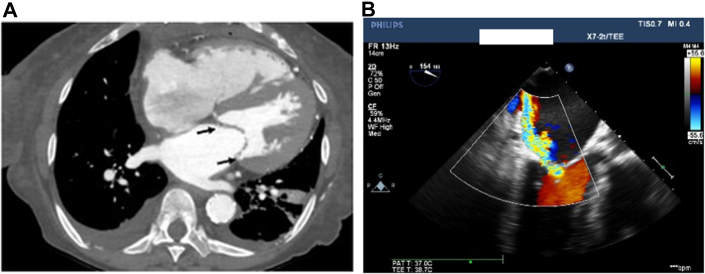
Figure 2.
Calcified Mitral Annulus and Mitral Regurgitation
(A) Axial computed tomography scan showing mitral annulus calcification (arrows). (B) Transesophageal echo focal lateral A2 flail and severe posteriorly directed mitral regurgitation.
In the operating room, the patient was anesthetized, and transesophageal echocardiography revealed severe mitral insufficiency due to P1 focal flail, as well as moderate to severe aortic insufficiency (Videos 2 and 3). The right axillary artery was exposed and cannulated and used for arterial access of cardiopulmonary bypass (CPB). The patient was placed in the standard supine position and the heart was exposed through a full median sternotomy. Venous access was obtained via bicaval canula. CPB was instituted and the patient was cooled to a core body temperature of 18 °C. Once the desired temperature was reached, and confirmed by suppressed electroencephalogram activity, CPB was stopped, achieving circulatory arrest. Retrograde cerebral perfusion was administered via the superior vena cava targeting a central venous pressure of 20 mm Hg.
First, the left atrium was accessed via a left atriotomy through the interatrial groove of Sondergaard. The mitral annulus was invaded by severe MAC. The flail segment of the mitral valve was stabilized using an Alfieri stitch between A2 and P2 (Video 4).The left atrium was left open to minimize the duration of deep hypothermic circulatory arrest, and we then turned our attention to the aorta.
We opened the aorta in a small noncalcified aortic segment (0.5 × 1.5 cm) that was identified by palpation and preoperative planning on computed tomography. A linear arteriotomy was then made on the anterolateral aspect of the aorta ∼6 cm distal to the aortic valve (Figure 3A).7 This arteriotomy would not be sufficient for surgical aortic valve replacement. The large nodule of calcium immediately anterior to the left main coronary artery was identified and excised, whereas the remainder of the native valve was left in place to support the TAVR valve. An Edwards Sapien3 23-mm valve (Edwards Lifesciences) was then positioned transaortically through the arteriotomy and deployed under direct visualization (Figure 3B). Detailed description of patient anatomical features is provided in the Supplemental Appendix. Patency of both coronary ostia was confirmed by direct probing. A slightly oversized Dacron patch was sewn along the incision to close the aorta in a tension-free manner.
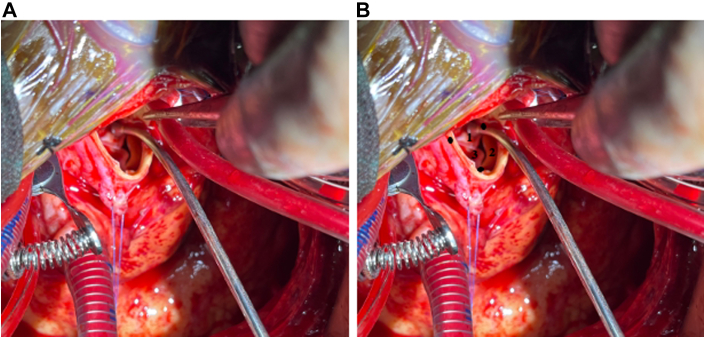
Figure 3.
Operative View of Aortic Valve
(A) Calcified aortic valve with severe aortic stenosis. The aortic root is small and calcified. A large bar of calcium threatening the left main was removed, whereas the rest of the valve was left in place to support the valve undergoing transcatheter aortic valve replacement. (B) Layout of the aortic valve commissures and leaflet cusps used for direct transcatheter aortic valve replacement: left coronary cusp, right coronary cusp, and noncoronary cusp.
CPB was restored, the brain was deaired, the left atriotomy was closed, and the patient was rewarmed and weaned off CPB using minimal inotropes. Intraoperative transesophageal echocardiography demonstrated a normal functioning aortic prosthesis, trace mitral regurgitation, and normal left and right ventricular function. Patient recovery was good, and she was discharged to home without any complications. Furthermore, the patient was seen in clinic 18 months after the operation and had a mean mitral valve gradient of 3 mm Hg without insufficiency, normal biventricular function, and an aortic mean gradient of 8 mm Hg without aortic valve regurgitation.
Discussion
The pathophysiology of ESRD promotes the development of cardiovascular calcifications, which can manifest as aortic stenosis and porcelain aorta, as well as MAC.3
For management of aortic stenosis, the choice of TAVR vs surgical aortic valve replacement depends on surgical risk and patient-specific anatomic features. Because porcelain aorta can significantly increase risks associated with surgical aortic valve replacement, aortic stenosis in such patients is typically corrected using TAVR.3,7 However, this case was complicated by the presence of a large calcification bar on the left coronary cusp leaflet immediately adjacent the left main coronary ostia. Although coronary artery obstruction is a relatively rare complication of TAVR, leaflet calcifications can increase the risk of this potentially fatal complication and marks an absolute contraindication for TAVR.8 This combination of porcelain aorta and cusp calcification made management of the aortic stenosis “technically inoperable.” We were able to circumvent this by combining a hybrid surgical-TAVR approach, in which the arteriotomy enabled direct visualization and excision of the calcified bar, whereas the rest of the native valve was left intact to support the TAVR. To the best of our knowledge, this was the first time this approach has been deployed.
Surgical mitral valve repair in MAC can be challenging and can involve either “resection” (en bloc decalcification and annular reconstruction) or “respect” (oversized annuloplasty with minimal decalcification) approaches.6 In our case, an Alfieri stitch was used to correct the degenerative mitral regurgitation, whereby the flail segments of the anterior and posterior segment are sutured, creating a double orifice mitral valve. The Alfieri stitch allowed us to minimize handling of the calcified annulus.9 Herein, rapid repair afforded by the Alfieri stitch was desirable given that our patient was under time-sensitive deep hypothermic circulatory arrest and suffered from double valve disease.
We have discussed a complicated case of porcelain aorta, aortic leaflet calcifications, and MAC in an ESRD patient. We demonstrate a novel hybrid surgical-TAVR approach combined with Alfieri stitch that allows effective correction of complex valvular pathology in this patient population.
Funding Support and Author Disclosures
Dr Szeto has been an investigator, speaker, and member of an advisory board for Edwards Lifesciences and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For detailed description of patient anatomical features as well as supplemental videos, please see the online version of this paper.
Appendix
Axial CT Scan of Patient; Note MAC as Well as Porcelain Aorta
Intraoperative TEE Showing Severe Mitral Regurgitation
Second Intraoperative TEE Showing Regurgitant Jets
Intraoperative TEE Following Alfieri Stitch and TAVR
References
- 1.Czarny M.J., Resar J.R. Diagnosis and management of valvular aortic stenosis. Clin Med Insights Cardiol. 2014;8(suppl 1):15–24. doi: 10.4137/CMC.S15716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marwick T.H., Amann K., Bangalore S., et al. Chronic kidney disease and valvular heart disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;96(4):836–849. doi: 10.1016/j.kint.2019.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Abramowitz Y., Jilaihawi H., Chakravarty T., Mack M.J., Makkar R.R. Porcelain aorta: a comprehensive review. Circulation. 2015;131(9):827–836. doi: 10.1161/circulationaha.114.011867. [DOI] [PubMed] [Google Scholar]
- 4.Carrel T., Vogt P.R. Porcelain aorta does not mean inoperability but needs special strategies. Interact Cardiovasc Thorac Surg. 2022;35(4) doi: 10.1093/icvts/ivac222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexis S.L., Malik A.H., El-Eshmawi A., et al. Surgical and transcatheter mitral valve replacement in mitral annular calcification: a systematic review. J Am Heart Assoc. 2021;10(7) doi: 10.1161/JAHA.120.018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feindel C.M., Tufail Z., David T.E., Ivanov J., Armstrong S. Mitral valve surgery in patients with extensive calcification of the mitral annulus. J Thorac Cardiovasc Surg. 2003;126(3):777–782. doi: 10.1016/s0022-5223(03)00081-3. [DOI] [PubMed] [Google Scholar]
- 7.Bax J.J., Delgado V., Bapat V., et al. Open issues in transcatheter aortic valve implantation. Part 2: procedural issues and outcomes after transcatheter aortic valve implantation. Eur Heart J. 2014;35(38):2639–2654. doi: 10.1093/eurheartj/ehu257. [DOI] [PubMed] [Google Scholar]
- 8.Holmes D.R., MacK M.J., Kaul S., et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59(13):1200–1254. doi: 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Sherlock K.E., Muthuswamy G., Basu R., Mitchell I.M. The Alfieri stitch: the advantages for mitral valve repair in difficult circumstances. J Card Surg. 2011;26(5):475–477. doi: 10.1111/j.1540-8191.2011.01295.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Axial CT Scan of Patient; Note MAC as Well as Porcelain Aorta
Intraoperative TEE Showing Severe Mitral Regurgitation
Second Intraoperative TEE Showing Regurgitant Jets
Intraoperative TEE Following Alfieri Stitch and TAVR