Abstract
An 80-year-old post–coronary artery bypass graft (CABG) patient had an acute coronary syndrome with non–ST-segment elevation myocardial infarction (ACS-NSTE) with saphenous vein graft (SVG)–obtuse marginal stenosis. High-definition intravascular ultrasound revealed an underexpanded SVG stent with a hyperechoic structure. Optical coherence tomography confirmed surgical clip causing compression, resolved by post-dilation. This case underscores ACS-NSTE complexity post-CABG and the critical role of coronary imaging in optimizing interventions by addressing surgical clip–induced compression.
Key Words: acute coronary syndromes, complex coronary interventions, coronary imaging, IVUS, OCT, precision medicine
Central Illustration
The prevalence of acute coronary syndrome with non–ST-segment elevation (ACS-NSTE) has increased significantly. Intracoronary imaging devices, intravascular ultrasonography (IVUS) and optical coherence tomography (OCT), play pivotal roles in improving clinical outcomes in several scenarios, including complex percutaneous coronary interventions (PCIs) and ACS-NSTE.1 Intracoronary imaging provides comprehensive insights into vessel dimensions, plaque morphology, and stent expansion, but it also plays a crucial role in identifying the underlying cause of an ACS-NSTE.2
Case Presentation
An 80-year-old man who presented with acute chest pain received a diagnosis of ACS-NSTE as a result of elevated troponin levels. The patient had undergone coronary artery bypass graft (CABG) surgery 4 years earlier, with a saphenous vein graft (VG) connected to an obtuse marginal (OM) branch and a left internal mammary artery (LIMA) bypass to the left anterior descending artery. Coronary angiography showed a patent LIMA graft and subtotal stenosis of the VG at the anastomosis site to OM branch, with surgical clips distinctly marked at the stenotic site (Figure 1A). The absence of significant calcification prompted preliminary pre-dilatation using a compliant balloon, followed by noncompliant (NC) balloon inflation with a 3.00-mm diameter. After a barely accepted balloon inflation, a 4.00 × 24 mm drug-eluting stent was implanted. However, angiography showed an incompletely expanded stent where the surgical clips were located (Figure 1B). Consequently, a post-dilatation using a 4.00 × 6 mm NC balloon was performed, although the improvement in stent expansion remained minimal. Thus, intracoronary imaging was performed. High-definition (HD) IVUS with a 60-MHz frequency disclosed suboptimal stent expansion at both the anastomosis site and the midsection of the stent. An IVUS imaging showed a hyperechoic structure at 2 different points behind the stent struts (Figure 2A). The absence of calcification-related artifacts excluded the possibility of reverberations, instead raising suspicion about the surgical clips. To gain further insight into the nature of the hyperechoic structure, OCT was used. The subsequent OCT examination conclusively identified the hyperechoic structure as the surgical clip exerting extrinsic compression on the vessel (Figure 2B). In response, a careful post-dilatation of the stent using a 4.5-mm NC balloon was performed, achieving comprehensive stent expansion of more than 90% across all segments (Figure 3A), an outcome that was corroborated by subsequent OCT imaging (Figure 3B). The patient was discharged few days later.
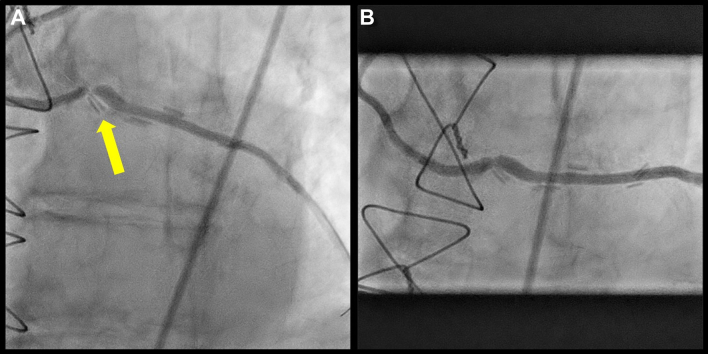
Figure 1.
Baseline Angiography
(A) Angiography showing the high-grade stenosis and the surgical clips surrounding the stenosis (yellow arrow). (B) Angiography after pre-dilatation and stent implantation with an underexpanded stent.
Figure 2.
Intravascular Ultrasound and Optical Coherence Tomography
(A) Intravascular ultrasound showing the underexpanded stent and a hyperechoic structure behind the stent struts (yellow arrow). (B) Optical coherence tomography showing the surgical clips compressing the vessel wall (yellow arrow).
Figure 3.
Final Angiography and Optical Coherence Tomography
(A) Final angiography after further stent optimization using a noncompliant balloon. (B) Final optical coherence tomography imaging showing a well-expanded stent with compression of the vessel and the surgical clips (yellow arrow).
Discussion
ACS-NSTE often includes complex scenarios, particularly when previous CABG interventions are involved. Surgical clipping of grafted vessels requires expertise and sometimes can lead to extrinsic compression of the vessels. This case shows a compressed VG resulting from surgical clips that progressed to an ACS presentation. According to European Society of Cardiology guidelines on ACS-NSTE,3 intracoronary imaging was performed to recognize the underlying cause of this ACS. The integration of intracoronary imaging devices (HD IVUS and OCT) facilitated the precise recognition of extrinsic vessel compression induced by the surgical clips, thereby enabling effective management. This case confirms how intracoronary imaging devices are essential to achieve procedural success and to identify and manage complications in both complex and ACS PCI settings.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank Prof Daniele Torella for his support in the production of this work.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Lee J.M., Choi K.H., Song Y.B., et al. Intravascular imaging-guided or angiography-guided complex PCI. N Engl J Med. 2023;388:1668–1679. doi: 10.1056/NEJMoa2216607. [DOI] [PubMed] [Google Scholar]
- 2.Kubo T., Imanishi T., Takarada S., et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 3.Collet J.-P., Thiele H., Barbato E., et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2020;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]