Abstract
Over the last two decades, increased availability of human pancreatic tissues has allowed for major expansions in our understanding of islet biology in health and disease. Indeed, studies of fixed and frozen pancreatic tissues, as well as efforts using viable isolated islets obtained from organ donors, have provided significant insights toward our understanding of diabetes. However, the procedures associated with islet isolation result in distressed cells that have been removed from any surrounding influence. The pancreas tissue slice technology was developed as an in situ approach to overcome certain limitations associated with studies on isolated islets or fixed tissue. In this Perspective, we discuss the value of this novel platform and review how pancreas tissue slices, within a short time, have been integrated in numerous studies of rodent and human islet research. We show that pancreas tissue slices allow for investigations in a less perturbed organ tissue environment, ranging from cellular processes, over peri-islet modulations, to tissue interactions. Finally, we discuss the considerations and limitations of this technology in its future applications. We believe the pancreas tissue slices will help bridge the gap between studies on isolated islets and cells to the systemic conditions by providing new insight into physiological and pathophysiological processes at the organ level.
Article Highlights
Human pancreas tissue slices represent a novel platform to study human islet biology in close to physiological conditions.
Complementary to established technologies, such as isolated islets, single cells, and histological sections, pancreas tissue slices help bridge our understanding of islet physiology and pathophysiology from single cell to intact organ.
Diverse sources of viable human pancreas tissue, each with distinct characteristics to be considered, are available to use in tissue slices for the study of diabetes pathogenesis.
Introduction
For decades, information regarding the human endocrine pancreas and its functional unit, the islet of Langerhans, has been somewhat limited; a facet largely related to structural anatomy and islets being obtained from autopsy or organ donor cases under somewhat uncontrolled conditions (1–3). As a result, a majority of data on the function and physiology of the human endocrine pancreas have been derived nearly exclusively from systemic metabolic studies from living subjects. Specifically, analysis of blood plasma samples following the application of various standardized metabolic tests (e.g., glucose tolerance tests or mixed meal tests) have, and remain, a standard method to provide a valuable picture of pancreatic function within the systemic environment (4). This representation of endocrine pancreas function includes the integrative response of all endocrine cells within the organ, but in addition, their modulation and regulation via the nervous and vascular system (5). Within this setting, the complexity of the obtained readout is further increased by the potential alteration through other organs and tissues (e.g., through changes in insulin resistance, insulin clearance, or glucose uptake) (6). Thus, investigations of systemic metabolism are multifaceted, and the challenge is to pinpoint the contribution and cellular mechanisms of the endocrine pancreas to the systemic status. Nevertheless, systemic studies are indispensable for successful implementation of interventions and therapeutic approaches into clinical use as they offer a unique and important option to illustrate effects on the complete organism.
However, understanding human islet and pancreas pathogenesis in detail ultimately requires studies of viable human tissue to enable analysis and direct assessment of islet cell function, regulation, and their cellular interactions. Importantly, animal models, although useful for experimental manipulations and basic mechanistic studies, display fundamental differences and have provided fairly limited translational value (7–16). This situation has sparked the formation of several initiatives over the last two decades, efforts that aimed to expand access for researchers to human pancreatic specimens (17–21). As a result, the number of studies investigating pancreas histopathology and function of human islet biology has grown immensely.
Specifically, frozen and fixed sections of intact human pancreatic tissues have enabled the detailed study of pancreas morphology, cell identity, and cell status (3). This said, investigations of endocrine pancreas function and physiology have predominantly been performed on isolated islets from brain-dead organ donors (22,23). For this purpose, islets are isolated from the organ donor pancreas by chemical and mechanical methods. The resulting preparation allows for investigation of endocrine pancreas cell physiology while maintaining the three-dimensional (3D) cellular structure of the islets as a functional unit (24). Depending on the technologies used, isolated islets can be investigated in large numbers simultaneously to provide knowledge on the integrated function and status of the endocrine pancreas, or individually to address potential heterogeneities between islets (25). In addition, certain methods, including electrophysiology and live-cell imaging, also allow for assessment of single-cell physiology within the context of an islet (26). Thus, isolated human islets facilitate the study of biological processes in pancreatic endocrine cells within a preserved juxtacrine and paracrine signaling environment. In this way, isolated islets have and will continue to occupy a central role in acquiring knowledge on the human endocrine pancreas in health and disease.
However, there are a number of limitations one should consider in terms of study design and data interpretation, with the notion that isolated islets are highly susceptible to the preparation procedure itself. Naturally, extraction of islets from their surrounding tissue prevents one from assessing any islet-extrinsic aspects of endocrine pancreas physiology and pathophysiology. More importantly, the isolation process has been shown to alter islet morphology, gene expression, and cell biology (27,28). The necessary recovery period following their isolation and extended culture times might lead to an additional adaptation of the islet to its in vitro environment. Finally, an isolation procedure based on chemical and mechanical properties of the tissue is susceptible to a selection bias for islets within a specific range of physical and biochemical properties. This, along with the enzymatic and mechanical stress imparted during the isolation process, could lead to loss or destruction of islets that have been structurally compromised in pathophysiological processes (e.g., a type 1 diabetes autoimmune attack).
In addition to investigating intact isolated islets, the study of pancreatic endocrine cell biology can be resolved to a cellular level by dispersing islets into single cells in vitro. This process further eases cell handling and the application of additional technologies, including high-resolution imaging or electrophysiology. In recent years, their use in the constantly growing field of single-cell “omics” technologies has become an increasingly important focus of attention (29,30). These approaches allow for the assessment of cell status at an extraordinarily detailed level and, therefore, have vastly increased our information on human islet cell biology and heterogeneity (25).
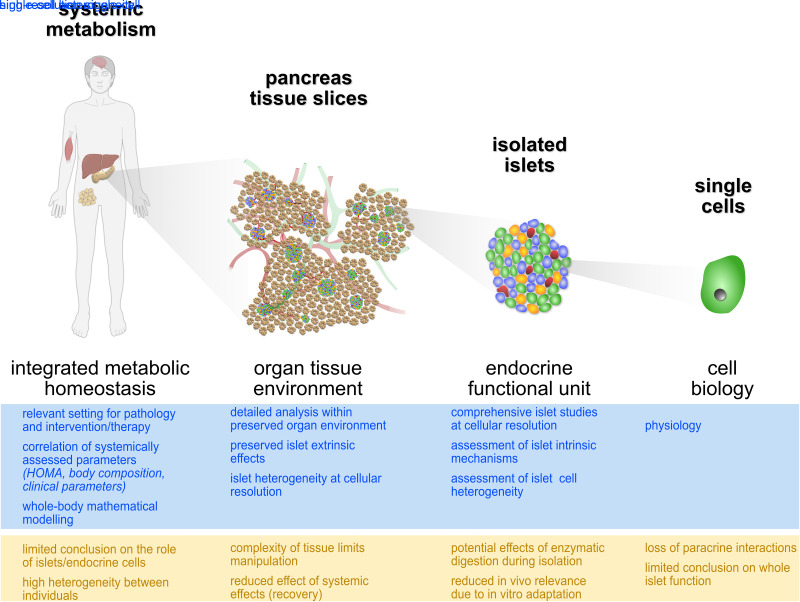
To provide a platform for the study of islet cell physiology and pathophysiology that circumvents many, but not all, of the known methodological limitations of existing technologies and bridges the methodological gap between isolated islets and systemic metabolism, we developed the human pancreas tissue slice platform (Fig. 1) (31,32). Herein, we discuss the potential use of human pancreas tissue slices for the study of islet physiology and function from single-cell to organ tissue level, the added value and limitations of studies on tissue slices, and the considerations that must be made depending on the source of human pancreatic tissue.
Figure 1.
Pancreas tissue slices bridge cellular and systemic approaches for the study of human islet physiology. Available platforms to investigate physiology of the endocrine pancreas in health and disease from the systemic in vivo setting to single-cell analysis, with selected advantages (blue) and limitations (yellow).
Tissue Slices Enable Studies of the Endocrine Pancreas at Different Levels
The preparation of tissue slices was initially established and successfully applied to several other organs, including intestine, colon, liver, brain, and heart (33–35). For the study of the pancreas, the tissue slice platform was originally developed to investigate the endocrine pancreas of the mouse, which as noted earlier, has proven its value by contributing to an increased understanding of islet physiology and pathophysiology in diabetes (36). Recently, we were able to transfer this technology to the study of the human endocrine pancreas by using pancreas tissue obtained from patients undergoing pancreatectomy or from organ donors (31,32,37). By immersing pieces of viable human pancreas tissue in low-temperature gelling agarose for stabilization, small tissue blocks (∼2–5-mm edge length) can be readily cut into slices of variable thickness (80–200 µm) using a vibratome (38). Although the slicing procedure will inevitably result in some partially damaged islets, especially at the cutting surface, the injured cells are washed off shortly after preparation. Subsequently, islets compromised by the slicing procedure can be easily identified and excluded in imaging or electrophysiological studies. In addition, digestive enzymes leaked from damaged exocrine cells due to slicing or secreted during culture will need to be inactivated by application of enzyme inhibitors to allow islet studies after slice culture (37). The main benefit of tissue slices for the study of pancreas physiology is related to the apparent preserved morphological arrangement of the different cellular compartments within viable organ tissue (38). In addition, the fast and minimally destructive preparation procedure imposes no enzymatic and only limited mechanical stress. This also promotes the almost immediate use of tissue slices for functional assessment, circumventing the need for a resting culture period and reducing a potential adaption of cells to the culture conditions (39). Thus, tissue slices facilitate the investigation of a close reflection of in vivo pancreas cell physiology and pathogenesis from individual cells to organ tissue.
Investigating Endocrine Pancreas Physiology in Tissue Slices—From Cells to Tissue
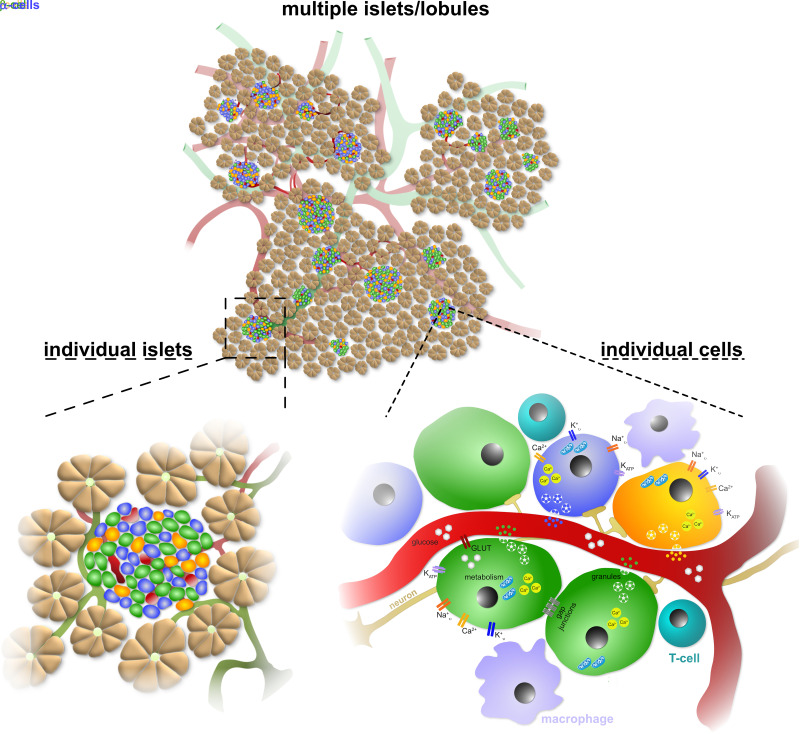
Similar to isolated islets, pancreas tissue slices enable the study of islet physiology at different levels, from individual cells within islets to groups of islet cells or several islets simultaneously (Fig. 2). A number of studies on mouse pancreas tissue slices have used electrophysiological techniques to provide single-cell resolution of islet physiology. Among others, the tissue slice approach was the first preparation to demonstrate KATP channel regulation by physiological intracellular ATP concentrations (40). In addition, tissue slices have allowed for the study of various aspects of β-cell biology using whole-cell patch-clamp measurements, including functional maturation (41) and modulation of coordinated islet cell function by gap junctions (42). Electrophysiological investigations of α-cell function have also been performed in living tissue slices of streptozotocin-induced diabetes, where the tissue slice technique is particularly advantageous as the damaged islets of this mouse model cannot be easily isolated or result in an extremely low yield of isolated islets (43,44). An important display of the in situ character of pancreas tissue slices was achieved by combining different imaging methods for studies of β-cell polarity. This was possible due to the preserved 3D endocrine-vascular niche in tissue slices (45,46). Studies using 3D confocal, two-photon, and block-face serial electron microscopy in mouse and human samples were able to identify three distinct β-cell domains, and the basal domain facing the vasculature was suggested to be responsible for insulin secretion into the circulation (46,47).
Figure 2.
Pancreas tissue slices enable the investigation of human islet cell physiology at a cellular to multi-islet level.
Zooming out from individual cells to the simultaneous assessment of multiple endocrine cells, live-cell imaging methods have provided important data from studies on islets in tissue slices (Fig. 2). Glucose-stimulated metabolic flux was investigated in a newly designed microfluidic device, where a clear increase in NAD(P)H autofluorescence was observed in endocrine cells with only minimal changes in the surrounding exocrine tissue in living pancreas tissue slices from rats (48). As in other preparations, the most commonly used parameter to investigate islet cell physiology by live-cell imaging in slices has been intracellular calcium ([Ca2+]i) dynamics (38,49–51). Hereby, [Ca2+]i imaging of numerous β-cells in acute tissue slices revealed that [Ca2+]i waves originate in a group of cells and that fast [Ca2+]i oscillations superimposed on a sustained plateau is the predominant type of response in β-cells during glucose stimulation (52). In addition, these studies enabled the analysis of the detailed network characteristics of β-cells within intact islets, which could be activated by glucose, modulated by N-methyl-d-aspartate receptor inhibition, and regulated by extracellular pH (53–57). Furthermore, a critical role of intracellular Ca2+ stores in glucose-stimulated [Ca2+]i oscillations in β-cell collectives was highlighted in a recent study using mouse pancreas tissue slices (51). Interestingly, calcium imaging on a large number of α-cells in tissue slices revealed a high degree of functional heterogeneity in this cell population in the pancreas (58) and that glutamate receptor signaling in α-cells is impaired in type 1 diabetes but could be rescued with positive allosteric modulators together with a restoration of glucagon secretion (59). To assess several signal transduction parameters at the same time, calcium imaging has been combined with membrane potential recording to explore the relationship between glucose-evoked membrane potential oscillations and [Ca2+]i oscillations in β-cells (49), or with membrane capacitance measurements to investigate the correlation of exocytosis and [Ca2+]i in β-cells in the setting of diabetes (60).
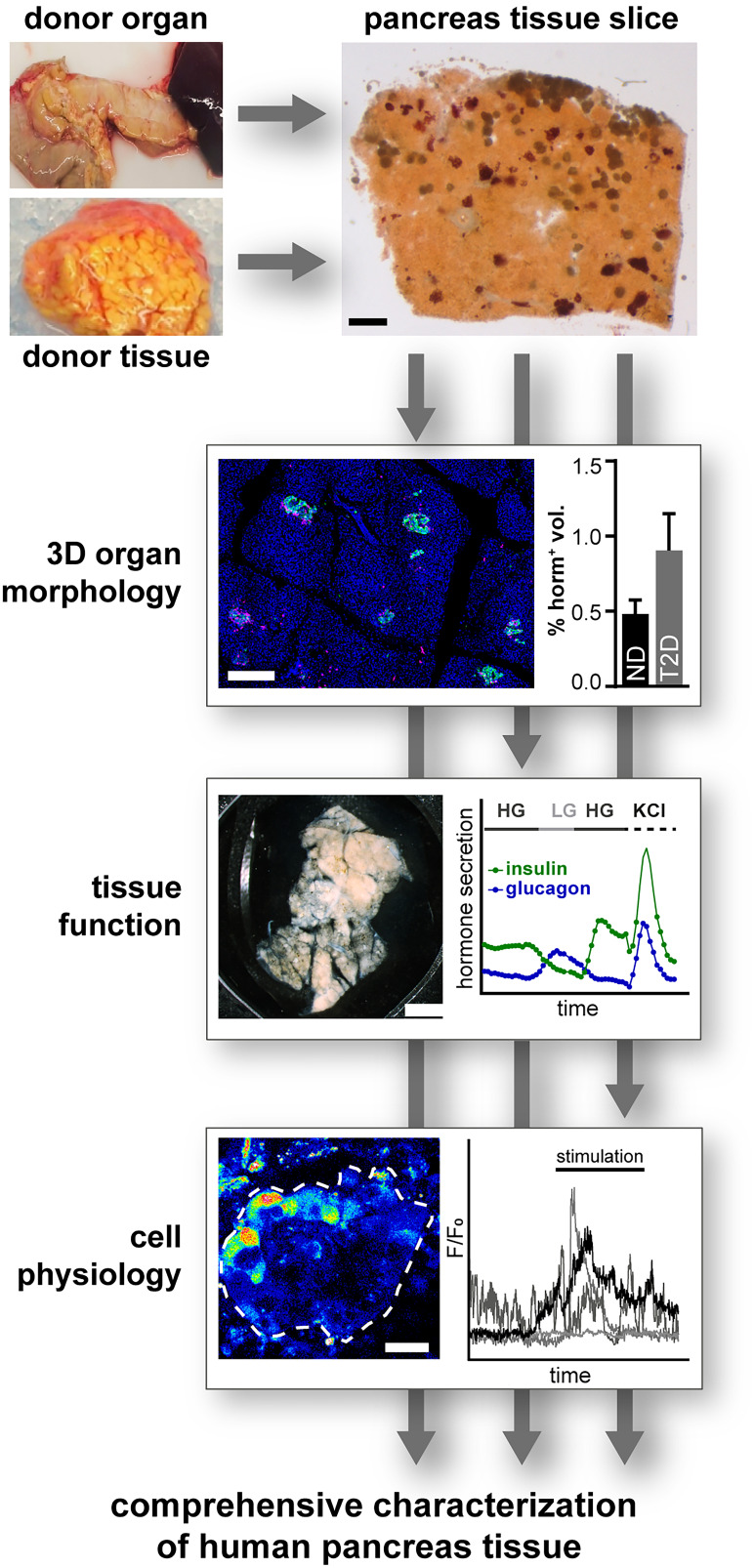
Finally, pancreas tissue slices also enable the study of several islets simultaneously (Fig. 2). By measuring hormone release from one to several slices in response to various stimuli, the integrated secretory function of a distinct number of islets can be assessed (31,32). Moreover, high-speed live-cell imaging approaches enable assessment of cell physiology in different islets throughout a slice, allowing the investigation of islet heterogeneity within a given tissue region. Importantly, the preserved 3D organ environment of pancreas tissue slices allows in-depth histomorphometric analyses of the same samples used for functional assessments. Indeed, we recently combined morphometric and functional assays on the same and adjacent human pancreas tissue slices to dissect the contribution of β-cell mass and function in the pathogenesis of type 1 diabetes (32) and type 2 diabetes (31). These analyses facilitate the assessment and correlation of islet function with a multitude of parameters within the same tissue, including morphology, function, and cell physiology (Fig. 3).
Figure 3.
Pancreas tissue slices facilitate simultaneous assessment of diverse aspects of islet cell physiology. Pancreas tissue slices from organ and tissue donors allow assessment of morphology, cell function, and cell physiology within the same tissue, facilitating a comprehensive characterization of islet physiology and pathophysiology. HG, high glucose; LG, low glucose; ND, no diabetes; T2D, type 2 diabetes.
Pancreas Tissue Slices for the Assessment of Nonendocrine Modulators of Islet Physiology and Pathogenesis
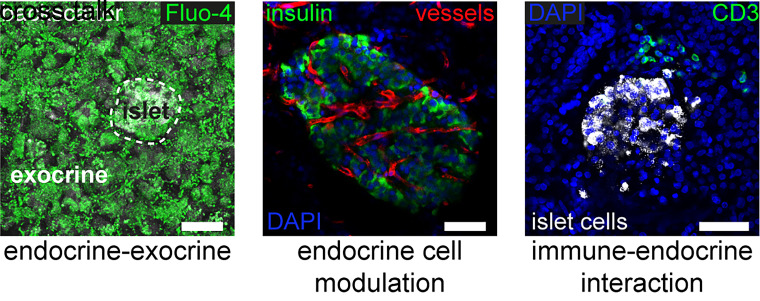
In addition to the study of islet cell physiology within an intact tissue environment, pancreas tissue slices allow the investigation of nonendocrine pancreatic cells and tissues that modulate islet cell biology and that might play a role in diabetes pathogenesis. These different cell types, which are located within and/or outside of the islet, are preserved in tissue slices but are often lost or compromised during the isolation and resting culture period of islets (Fig. 4) (24). Among these, intraislet vascular structures, immune cells, and neurons have been shown to be conserved in pancreas tissue slices, allowing for the acquisition of novel insight into their role in islet biology (36). Functional imaging of intraislet vessels in living tissue slices revealed that glucose stimulation in β-cells leads to islet capillary dilation via adenosine-induced inhibition of pericytes that cover the islet microvasculature in the mouse pancreas (61) and that pericytes are major targets of sympathetic nerves in controlling blood vessels in the human endocrine pancreas (62). In another study of pancreas tissue slices, islet macrophages were found to monitor local concentrations of ATP released from β-cells as a measure to sense β-cell activity and thereby presumably promote a stable islet microenvironment (63). Interestingly, the pericyte coverage of human islet capillaries (61) and the expression of purinergic receptors and matrix metallopeptidase 9 in macrophages sorted from type 2 diabetes donor islets (63) were both found to be downregulated, indicating a potential role of these cells in type 2 diabetes pathogenesis. Furthermore, vagal sensory axons that innervate pancreatic islets were recently noted to express the serotonin 5-hydroxytryptamine receptor 3, thereby allowing neuronal sensing of β-cell activity by serotonin, which is co-released with insulin upon β-cell stimulation (64).
Figure 4.
Investigation of nonendocrine modulators of islet cell physiology in pancreas tissue slices. In addition to islet cell physiology and pathophysiology, pancreas tissue slices enable the investigation of extraislet influences, including endocrine-exocrine cross talk, endocrine cell modulation by the vascular system, or the interaction with immune cells. Scale bars = 50 µm.
Outside of the islet, pancreas tissue slices allow for the study of the surrounding exocrine pancreas compartment and its ductal system (38,65,66). This aspect of pancreas physiology and pathophysiology has recently attained increasing interest, as interaction of the endocrine and exocrine pancreas has been implicated in the development of diabetes (67). On the one hand, exocrine acini display heterogeneous properties regarding their distance to islets; additionally, pancreas weight, which is almost entirely determined by the exocrine tissue, is already decreased in pretype 1 diabetes (68). Concomitantly, exocrine pancreas dysfunction and insufficiency are often diagnosed in patients with diabetes (69–72). On the other hand, islet function is believed to be compromised in some disorders of the exocrine pancreas (73,74). Pancreas tissue slices now allow for the investigation of both components of the pancreas simultaneously for the first time, facilitating investigations on the cross talk of the endocrine and exocrine part. Calcium imaging in intact acinar cells has revealed a well synchronized calcium activity within acini, but not between acini in mouse tissue slices (75). Simultaneous calcium imaging on both β-cells and acinar cells discovered a dual mode of action of acetylcholine, where a physiological concentration increases glucose-stimulated calcium oscillations frequency in both cell types and synchronizes acinar cells, whereas a supraphysiological concentration inhibits the synchronization between β-cells and abolishes oscillatory activity in acinar cells (76). In addition, the exocrine tissue and ductal cells have been suggested to serve as a source for regenerated islet cells via neogenesis (77). To address this hypothesis for the first time in situ, Qadir et al. (37) developed a novel long-term culture system for human and mouse pancreas tissue slices, allowing for the longitudinal study of potential neogenic processes. With this approach, they were able to show duct–to–β-cell neogenesis in mouse tissue, as well as bone morphogenetic protein-7–induced β-cell regeneration in human tissue slices (37).
The immediate surroundings of islets within the pancreas are also of particular interest in the pathogenesis of type 1 diabetes. Immune cells implicated in autoimmune processes are often located in the periphery or outside of islets and, therefore, are mostly lost during isolation (24). This hampers the study of islet immune cell interactions in disease pathogenesis. Here, pancreas tissue slices allow the study of immune cells within the pancreatic tissue and their effect on islet biology independent of their location (78). Furthermore, in advanced stages of the autoimmune attack, the tissue-preserving nature of pancreas tissue slices additionally holds an advantage to allow studies of islets that are structurally compromised and, as such, would be destroyed or unused during islet isolation. Using tissue slices prepared from the Network for Pancreatic Organ donors with Diabetes (nPOD) donor organs with and without diabetes, we were able to perform the first live-cell studies of the human pancreas in type 1 diabetes pathogenesis (32). β-Cell function, including insulin secretion and single-cell calcium live imaging of the residual β-cell mass, could be evaluated in tissue from subjects with type 1 diabetes and compared with control subjects without diabetes. This study demonstrated that tissue slices represent a novel approach for investigating structurally compromised islets in this disease as well as the potential interactions of immune cells with these islets.
Thus, pancreas tissue slices allow for investigation of human islet cell physiology and pathophysiology at different levels within the organ tissue. The platform distinguishes itself from other preparations, such as isolated islets and dispersed cells, by presenting less perturbed cells in a more intact tissue environment and, together with an almost immediate use of the viable tissue, thereby provides a closer physiological reflection of the in vivo setting. Conversely, the complexity of the tissue slice preparation impedes sample manipulation and results in a reduced experimental throughput compared with isolated islets or single cells (50,78). These biological and technical features make human pancreas tissue slices a valuable complementary platform, linking cellular and systemic studies.
Sources of Viable Tissue for the Preparation of Human Pancreas Tissue Slices
Another benefit of the tissue slice preparation is the possibility to study human islet physiology even in small pancreas tissue samples, from which isolation of islets is possible, but rather tedious and of limited success (79,80). This further broadens the use of different sources of human pancreas tissue for the study of islet biology. In recent years, several efforts have been undertaken to provide researchers with an increased number of specimens of viable human pancreas for the study of organ physiology and disease pathogenesis. Thereby, pancreas tissue for research was obtained by three different approaches: 1) the procurement of organs from brain-dead or donation after cardiac death organ donors, 2) the retrieval of complete organs or pieces of tissue from patients undergoing pancreatectomy, and 3) for one study, the acquisition of tissue from pancreas biopsies of volunteers. Each of these sources provides distinct advantages for the investigation of human islet physiology and pathophysiology. However, different aspects of each tissue source also have to be considered when interpreting the obtained results (Table 1).
Table 1.
Strengths and limitations of human pancreas tissue sources
| Organ donors | Surgical resections | Biopsies | |
|---|---|---|---|
| Advantages | • Detailed investigation of entire organ • Availability of other organs/tissues |
• Rapid organ procurement with short ischemia times • Full clinical assessment, metabolic stratification |
• Living donors • Comorbidity-free tissue |
| Limitations | • Deleterious effects of brain and/or cardiac death • Intensive care unit treatment • Prolonged cold ischemia time |
• Regional restrictions based on resected tissue • Influence of tumor/pancreas disease on islet physiology |
• Regional restriction (tail biopsy) • Postsurgical complications |
Pancreas From Brain-Dead or Donation After Cardiac Death Organ Donors
Pancreata procured from cadavers enable detailed investigations of the morphometry and histology of entire organs. However, postmortem changes within the organ, uncontrolled procurement conditions, and prolonged cold ischemia times might promote autolysis, and proteins or RNA are prone to destruction (17), thus limiting functional analyses of the organs. In order to overcome these limitations, several new sources of pancreas tissue became available for research purposes, such as the PanFin Network in Europe (17) or nPOD, the Human Islet Research Network (HIRN)–supported Human Pancreas Analysis Program (HPAP), and Human Atlas of Neonatal Development and Early Life-Pancreas/Immune (HANDEL-P/I) in the U.S. (81), as well as the Alberta IsletCore in Canada (20,21). In these programs of controlled and well-organized organ procurement, pancreata retrieved from organ donors represent the most comprehensive source to study diabetes in humans, providing a considerable amount of living tissue to allow for both the isolation of islets and the preparation of pancreas tissue slices. In addition, these organ procurement programs often present access to additional tissues, such as spleen, lymph nodes, blood, and more. However, potential deleterious effects of brain death seen in the liver and kidneys (82) are also debated for the pancreas. For instance, macrophage infiltration and the elevated release of inflammatory cytokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor-α, and MCP-1 were recently shown to be a consequence of brain death in rats (83), but could only be confirmed for tumor necrosis factor-α and IL-6 serum levels in humans, whereas immune cell infiltration and expression of other cytokines showed no significant changes (84,85). Nevertheless, quantification of inflammatory responses and immune cell infiltration might differ in the context of type 1 diabetes and needs additional investigations. In addition, β-cells dissected from deceased donor pancreases were shown to exhibit significant upregulation of genes related to endoplasmic reticulum stress and the unfolded protein response (86).
However, effects of brain death on the human pancreas itself are hard to assess, as length and intensity of medical care prior to death are usually highly variable. Several brain death management protocols require administration of dextrose-containing fluids, insulin, or steroids that can lead to insulin resistance and contribute to terminal hyperglycemia, potentially affecting pancreatic endocrine cell function (84). Additionally, it is still debated whether prolonged hospital stay in the intensive care unit and time of brain death before retrieval increases β-cell replication in young organ donors (84,87).
Finally, organ preservation is an important parameter that might affect tissue integrity or cellular metabolism. Studies showed that warm ischemia times during organ procurement leads to a decreased islet yield at isolation and, more importantly, affects islet function compared with pancreata procured in cooled conditions (88,89). Although endocrine and exocrine tissue morphology have both been shown to be preserved in tissue procured from organ donors, these organs are typically flushed with preservative solutions and have prolonged cold ischemia times. Most of the time, this factor is unavoidable as tissues must be shipped to research laboratories. Increased cold ischemia time is well known to affect islet isolation outcomes (88,90). However, these effects can be counteracted by optimization of transport media depending on research purposes.
Surgical Resections of Pancreas Tissue From Live Donors
An alternative tissue source that can overcome some of the limitations from brain-dead organ donors originates from surgical resections. These become clinically necessary based on a localized benign or malignant neoplasm, some nonpancreatic tumors, or an exocrine disease such as chronic pancreatitis (91). As tissue usually becomes accessible shortly after resection, adverse effects imparted by hospital care and brain/cardiac death management protocols do not apply. Furthermore, warm ischemia times are usually reduced to the time period of organ clamping during surgery as tissue is cooled immediately after resection to be transferred for pathological examination. Subsequently and dependent on the proximity of the laboratory to the clinic, cold ischemia times are considerably shorter compared with donated organs. Additionally, clinically assessed parameters or even comprehensive metabolic stratification of patients, as shown by Solimena et al. (18,92), provide an unprecedented repertoire of in vivo parameters for correlation with experimentally assessed factors after resection (e.g., the in situ assessment of islet morphology and function) (93,94).
Despite these advantages, tissues obtained from surgical resections also have potential drawbacks that must be taken into account. First, with the exception of total pancreatectomies, the amount of nontumorous tissue available for research is often low, and the observations made are limited to the resected region of the pancreas. Second, although its preserved tissue integrity and microenvironment could be advantageous and valuable for exocrine-endocrine interaction studies, whether the underlying diseases of the patients may affect physiological responses within the endocrine pancreas is still being debated. For example, in cases of cystic fibrosis and chronic pancreatitis, patients showed decreased insulin secretion as well as glucose intolerance, suggesting impaired islet function due to the compromised structure of the pancreatic tissue (95). In pancreatic cancer, bidirectional effects of tumor and islets have been suggested as a possible cause of diabetes (96), but the causal relationships are still being discussed (97). Enhanced cancer growth in pancreatic ductal adenocarcinomas was shown to be associated with type 2 diabetes-related hyperglycemia in 47% of the patients (98). In addition, pancreatic ductal adenocarcinomas were shown to promote islet cell death as well as islet distortion in close proximity to the tumor (99), whereas coculture studies of cancer cells and isolated islets suggested that tumor cells cause reduced insulin secretion (100–102). However, cancer-related diabetes was suggested to occur within 2–3 years of a cancer diagnosis (103), implying that earlier manifestation of diabetes developed independent of the cancer. Additionally, the tissue, which was procured from the healthy surgical margins of the resection based on pathological examination, showed no cancer-related alterations of islet cell gene expression profiles (18). In distinct cases, localization and size of the tumor can result in papillary compression, leading to functional liver impairment and insulin resistance, which eventually leads to diabetes onset that is reversible after tumor resection and provides an interesting model for the acute compensation of liver insulin resistance with no apparent direct insult of the tumor on the islets (91,104–106). The disease-related treatment regimens of the tissue donors are also important for the study of pancreas tissue from surgical resections. Although the effects of localized tumors are potentially avoided by studying distant nontumorous tissue, resections from patients receiving chemotherapy or anticancer medication prior to surgery may not be suitable for analyses of diabetes pathogenesis due to the systemic actions of drugs. Indeed, it has been shown that various chemotherapeutic agents, including doxorubicin or l-asparaginase, as well as treatment with glucocorticoids, can directly or indirectly induce hyperglycemia (107,108).
Pancreas Tissue From Live Donors Obtained by Laparoscopic Biopsy
Laparoscopically procured pancreata from tail biopsies performed as part of the Diabetes Virus Detection (DiViD) program represent an exception to the above-described methods for pancreas procurement. This approach was used to obtain tissues from six individuals with recent-onset type 1 diabetes and provided valuable insights into various aspects of the pancreatic pathogenesis in type 1 diabetes, including islet function (39), islet amyloid polypeptide deposition, (109), islet density and size distribution (110), and characteristics of insulitic islet infiltration (111). However, an unexpectedly high complication rate (in three of the six participants), including postoperative bleeding, pancreatic drainage, pain, and fever, led to the cessation of the study and, for now, speaks against the use of this tissue source on a larger scale (112).
Considerations and Limitations for Future Applications
We and others have recently shown that human transplant-grade pancreata, as well as tissue from localized surgical resections, can readily be used in combination with the tissue slice platform to study various aspects of islet biology and its pathophysiology in the natural history of type 1 and type 2 diabetes (31,32,65,113). Further investigations on slices, islets, and/or single cells using the respective tissue sources will provide essential insights into currently unanswered questions of human pancreas biology and diabetes research. Notably, the distinct sources will enable the study and shed light onto different aspects of physiology and pathology. The use of organ donor tissue with its broad donor age spectrum will facilitate, in particular, the investigation of developmental processes, intraorgan heterogeneity, and, thanks to programs like nPOD, type 1 diabetes pathogenesis. Tissue from surgical resections related to intestinal disease might be more applicable for the study of type 2 diabetes pathogenesis and the detailed correlation of pancreas function with a larger set of clinical parameters. Importantly, although most likely neither the pancreas of organ donors nor tissue from medically induced surgical resections fully reflects the in vivo setting of the human pancreas, these invaluable resources have and without a doubt will continue to move translational research a huge step closer to finding new therapeutic options. As with single cells and isolated islets, combining this platform with faster and more versatile imaging methods will allow investigators to record various cellular processes simultaneously in the near future. Tissue slices will additionally enable these recordings within multiple islets with preserved spatial information that can deepen our understanding of interislet, endocrine-exocrine, or even endocrine-immune cell cross talk in a preserved native environment. Moreover, live-cell analysis within the tissue slices will not be limited to studies of endocrine diseases but will also allow studying the impact of exocrine diseases or tumors on the different compartments within the pancreas. Finally, integrating novel techniques, such as imaging mass cytometry or spatial transcriptomics after the functional assessment of tissue slices in combination with, for example, novel clinical β-cell mass imaging techniques could be a valuable addition in the future to further synergize in vivo and in vitro knowledge in pancreas pathophysiology.
As every novel and versatile platform that opens up new possibilities, pancreas tissue slices are also associated with limitations that need to be considered when interpreting the obtained data. Compared with isolated islets, which are usually produced from the entire organ, tissue slices are prepared from small tissue blocks from a particular region (or regions) of the pancreas. Therefore, although tissue slices are advantageous in studying regional heterogeneity within and between sliced region(s), isolated islets might be better to represent a good average of all isolatable islets of the entire pancreas and, therefore, potentially display a closer correlation to the systemic metabolic data. In addition, when using tissue slices acutely to study a close to in vivo setting, it has to be considered that the quality of the donor organ or tissue, including ischemia time and disease-related treatment regimens, might have a stronger impact on the generated data than those obtained from isolated islets that are acquired after a resting and adaptation culture period in vitro. Importantly, given the different cellular and technical complexity of the preparations, isolated islets can be produced in larger numbers, are more easily cultured and shipped for longer time periods, and allow for more experimental manipulation and throughput. Therefore, isolated islets can be made accessible to a larger number of investigators and approaches around the world, enabling more comprehensive investigations of islet intrinsic biology of the same organ. Finally, although there is no selection bias for islets with any specific physical properties in the tissue slice preparation, the maximal size of islets will be limited by the chosen slice thickness (usually 120–150 µm). Thus, despite the fact that pancreas tissue slices excel in studying diabetes pathogenesis in a sustained, more physiological local environment and beyond the endocrine compartment, isolated islets will continue to be the preparation of choice for numerous approaches to study human endocrine pancreas physiology and pathophysiology.
Conclusions
Human pancreas tissue slices represent a novel platform to study human islet biology in health and disease. Importantly, the unique characteristics of the technique make pancreas tissue slices a welcome complementary approach to existing preparations such as isolated islets and fixed/frozen tissue sections. Slices show limitations in throughput, applicable experimental manipulations, and single-cell “omics” but possess benefits in reduced inflicted cell stress, a conserved tissue environment, and the unique opportunity to study extraislet influences on islet physiology and pathophysiology. Fortunately, different sources of human pancreas tissue have been made available to an increasing number of researchers during recent years, which make it possible to combine pancreas tissue slices with other cell platforms. Therefore, the characteristics of the human tissue source must be carefully evaluated and its implications considered when interpreting any resulting data. By further integrating innovative techniques after live-cell analyses, thereby facilitating the correlation of cellular function with morphology and omics technologies, the pancreas tissue slices platform can present new perspectives and further synergize in vivo and in vitro pancreas research. In summary, studies using pancreas tissue slices, particularly in combination with isolated islets and fixed or frozen tissue sections from the same organ/tissue, will provide unprecedented insight into the human endocrine pancreas and its pathogenesis in diabetes.
Article Information
Acknowledgments. The authors wholeheartedly thank the tissue and organ donors and their families for their invaluable contribution to science. Furthermore, we thank Amanda Posgai and Sara Williams, University of Florida, for editorial assistance in the preparation of the manuscript.
Funding. The authors’ work was supported in part by the DFG Collaborative Research Centre/Transregio 127, the DFG–International Research Training Group 2251, the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK123292 and R01DK131059, JRDF grant 2-SRA-2019-696-S-B, the Paul Langerhans Institute Dresden (PLID) of the Helmholtz Zentrum München at the University Clinic Carl Gustav Carus of Technische Universität Dresden, and the German Ministry for Education and Research to the German Centre for Diabetes Research. This research was also performed with the support of the Network for Pancreatic Organ donors with Diabetes (nPOD, RRID:SCR_014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD, 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (grant 2018#PG-T1D053, G-2108-04793).
The content and views expressed are the responsibility of the authors and do not necessarily reflect the official view of nPOD. Organ procurement organizations partnering with nPOD to provide research resources are listed at https://www.jdrfnpod.org/for-partners/npod-partners/.
Duality of Interest. No potential conflicts of interest relevant to the article were reported.
Author Contributions. C.M.C., C.C., M.A.A., D.M.D., and S.S. contributed to the preparation and writing of the manuscript. S.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of data and the accuracy of data analysis.
Funding Statement
The authors’ work was supported in part by the DFG Collaborative Research Centre/Transregio 127, the DFG–International Research Training Group 2251, the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK123292 and R01DK131059, JRDF grant 2-SRA-2019-696-S-B, the Paul Langerhans Institute Dresden (PLID) of the Helmholtz Zentrum München at the University Clinic Carl Gustav Carus of Technische Universität Dresden, and the German Ministry for Education and Research to the German Centre for Diabetes Research. This research was also performed with the support of the Network for Pancreatic Organ donors with Diabetes (nPOD, RRID:SCR_014641), a collaborative type 1 diabetes research project sponsored by JDRF (nPOD, 5-SRA-2018-557-Q-R) and The Leona M. & Harry B. Helmsley Charitable Trust (grant 2018#PG-T1D053, G-2108-04793).
References
- 1. Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 1985;4:110–125 [DOI] [PubMed] [Google Scholar]
- 2. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab 2008;10(Suppl. 4):32–42 [DOI] [PubMed] [Google Scholar]
- 3. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 4. Stumvoll M, Mitrakou A, Pimenta W, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23:295–301 [DOI] [PubMed] [Google Scholar]
- 5. Osundiji MA, Lam DD, Shaw J, et al. Brain glucose sensors play a significant role in the regulation of pancreatic glucose-stimulated insulin secretion. Diabetes 2012;61:321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic β-cell function: review of methods and clinical applications. Curr Diabetes Rev 2014;10:2–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arrojo e Drigo R, Ali Y, Diez J, Srinivasan DK, Berggren PO, Boehm BO. New insights into the architecture of the islet of Langerhans: a focused cross-species assessment. Diabetologia 2015;58:2218–2228 [DOI] [PubMed] [Google Scholar]
- 8. Leiter EH, von Herrath M. Animal models have little to teach us about type 1 diabetes: 2. In opposition to this proposal. Diabetologia 2004;47:1657–1660 [DOI] [PubMed] [Google Scholar]
- 9. Roep BO, Atkinson M. Animal models have little to teach us about type 1 diabetes: 1. In support of this proposal. Diabetologia 2004;47:1650–1656 [DOI] [PubMed] [Google Scholar]
- 10. Dybala MP, Butterfield JK, Hendren-Santiago BK, Hara M. Pancreatic islets and gestalt principles. Diabetes 2020;69:1864–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noguchi GM, Huising MO. Integrating the inputs that shape pancreatic islet hormone release. Nat Metab 2019;1:1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rorsman P, Ashcroft FM. Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol Rev 2018;98:117–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levetan CS, Pierce SM. Distinctions between the islets of mice and men: implications for new therapies for type 1 and 2 diabetes. Endocr Pract 2013;19:301–312 [DOI] [PubMed] [Google Scholar]
- 14. Bonner-Weir S, Sullivan BA, Weir GC. Human islet morphology revisited: human and rodent islets are not so different after all. J Histochem Cytochem 2015;63:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skelin Klemen M, Dolenšek J, Slak Rupnik M, Stožer A. The triggering pathway to insulin secretion: functional similarities and differences between the human and the mouse β cells and their translational relevance. Islets 2017;9:109–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 2013;75:155–179 [DOI] [PubMed] [Google Scholar]
- 17. Tauriainen S, Salmela K, Rantala I, Knip M, Hyöty H. Collecting high-quality pancreatic tissue for experimental study from organ donors with signs of β-cell autoimmunity. Diabetes Metab Res Rev 2010;26:585–592 [DOI] [PubMed] [Google Scholar]
- 18. Solimena M, Schulte AM, Marselli L, et al. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia 2018;61:641–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell-Thompson M, Wasserfall C, Kaddis J, et al. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev 2012;28:608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lyon J, Manning Fox JE, Spigelman AF, et al. Research-focused isolation of human islets from donors with and without diabetes at the Alberta Diabetes Institute IsletCore. Endocrinology 2016;157:560–569 [DOI] [PubMed] [Google Scholar]
- 21. Cooper TT, Hess DA. The IsletCore Program: improving the supply of human islets to satisfy the demand for research. See article in Endocrinology 2016;157:560–569. Endocrinology 2016;157:997–1002 [DOI] [PubMed] [Google Scholar]
- 22. Marchetti P, Schulte AM, Marselli L, et al. Fostering improved human islet research: a European perspective. Diabetologia 2019;62:1514–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hart NJ, Powers AC. Use of human islets to understand islet biology and diabetes: progress, challenges and suggestions. Diabetologia 2019;62:212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes 1988;37:413–420 [DOI] [PubMed] [Google Scholar]
- 25. Arrojo E Drigo R, Roy B, MacDonald PE. Molecular and functional profiling of human islets: from heterogeneity to human phenotypes. Diabetologia 2020;63:2095–2101 [DOI] [PubMed] [Google Scholar]
- 26. Fu J, Githaka JM, Dai X, et al. A glucose-dependent spatial patterning of exocytosis in human β-cells is disrupted in type 2 diabetes. JCI Insight 2019;5:e127896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenberg L, Wang R, Paraskevas S, Maysinger D. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery 1999;126:393–398 [PubMed] [Google Scholar]
- 28. Bottino R, Balamurugan AN, Tse H, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 2004;53:2559–2568 [DOI] [PubMed] [Google Scholar]
- 29. Wang YJ, Schug J, Won KJ, et al. Single-cell transcriptomics of the human endocrine pancreas. Diabetes 2016;65:3028–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Segerstolpe Å, Palasantza A, Eliasson P, et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 2016;24:593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohrs CM, Panzer JK, Drotar DM, et al. Dysfunction of persisting β cells is a key feature of early type 2 diabetes pathogenesis. Cell Rep 2020;31:107469. [DOI] [PubMed] [Google Scholar]
- 32. Panzer JK, Hiller H, Cohrs CM, et al. Pancreas tissue slices from organ donors enable in situ analysis of type 1 diabetes pathogenesis. JCI Insight 2020;5:e134525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Graaf IA, Olinga P, de Jager MH, et al. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat Protoc 2010;5:1540–1551 [DOI] [PubMed] [Google Scholar]
- 34. Ting JT, Daigle TL, Chen Q, Feng G. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol 2014;1183:221–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kang C, Qiao Y, Li G, et al. Human organotypic cultured cardiac slices: new platform for high throughput preclinical human trials. Sci Rep 2016;6:28798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Speier S, Rupnik M. A novel approach to in situ characterization of pancreatic beta-cells. Pflugers Arch 2003;446:553–558 [DOI] [PubMed] [Google Scholar]
- 37. Qadir MMF, Álvarez-Cubela S, Weitz J, et al. Long-term culture of human pancreatic slices as a model to study real-time islet regeneration. Nat Commun 2020;11:3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marciniak A, Cohrs CM, Tsata V, et al. Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nat Protoc 2014;9:2809–2822 [DOI] [PubMed] [Google Scholar]
- 39. Krogvold L, Skog O, Sundström G, et al. Function of isolated pancreatic islets from patients at onset of type 1 diabetes: insulin secretion can be restored after some days in a nondiabetogenic environment in vitro: results from the DiViD Study. Diabetes 2015;64:2506–2512 [DOI] [PubMed] [Google Scholar]
- 40. Speier S, Yang SB, Sroka K, Rose T, Rupnik M. KATP-channels in beta-cells in tissue slices are directly modulated by millimolar ATP. Mol Cell Endocrinol 2005;230:51–58 [DOI] [PubMed] [Google Scholar]
- 41. Rozzo A, Meneghel-Rozzo T, Delakorda SL, Yang SB, Rupnik M. Exocytosis of insulin: in vivo maturation of mouse endocrine pancreas. Ann N Y Acad Sci 2009;1152:53–62 [DOI] [PubMed] [Google Scholar]
- 42. Speier S, Gjinovci A, Charollais A, Meda P, Rupnik M. Cx36-mediated coupling reduces beta-cell heterogeneity, confines the stimulating glucose concentration range, and affects insulin release kinetics. Diabetes 2007;56:1078–1086 [DOI] [PubMed] [Google Scholar]
- 43. Huang YC, Rupnik M, Gaisano HY. Unperturbed islet α-cell function examined in mouse pancreas tissue slices. J Physiol 2011;589:395–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang YC, Rupnik MS, Karimian N, et al. In situ electrophysiological examination of pancreatic α cells in the streptozotocin-induced diabetes model, revealing the cellular basis of glucagon hypersecretion. Diabetes 2013;62:519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Low JT, Zavortink M, Mitchell JM, et al. Insulin secretion from beta cells in intact mouse islets is targeted towards the vasculature. Diabetologia 2014;57:1655–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gan WJ, Zavortink M, Ludick C, et al. Cell polarity defines three distinct domains in pancreatic β-cells. J Cell Sci 2017;130:143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cottle L, Gan WJ, Gilroy I, et al. Structural and functional polarisation of human pancreatic beta cells in islets from organ donors with and without type 2 diabetes. Diabetologia 2021;64:618–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rafiei N, Moghadam MG, Au A, et al. Design of a versatile microfluidic device for imaging precision-cut-tissue slices. Biofabrication 2022;14:041001. [DOI] [PubMed] [Google Scholar]
- 49. Dolenšek J, Stožer A, Skelin Klemen M, Miller EW, Slak Rupnik M. The relationship between membrane potential and calcium dynamics in glucose-stimulated beta cell syncytium in acute mouse pancreas tissue slices. PLoS One 2013;8:e82374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stožer A, Dolenšek J, Križančić Bombek L, Pohorec V, Slak Rupnik M, Klemen MS. Confocal laser scanning microscopy of calcium dynamics in acute mouse pancreatic tissue slices. J Vis Exp 2021:e62293. [DOI] [PubMed] [Google Scholar]
- 51. Postić S, Sarikas S, Pfabe J, et al. High-resolution analysis of the cytosolic Ca2+ events in β cell collectives in situ. Am J Physiol Endocrinol Metab 2023;324:E42–E55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stožer A, Dolenšek J, Rupnik MS. Glucose-stimulated calcium dynamics in islets of Langerhans in acute mouse pancreas tissue slices. PLoS One 2013;8:e54638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stožer A, Gosak M, Dolenšek J, et al. Functional connectivity in islets of Langerhans from mouse pancreas tissue slices. PLoS Comput Biol 2013;9:e1002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Postić S, Gosak M, Tsai WH, et al. pH-dependence of glucose-dependent activity of beta cell networks in acute mouse pancreatic tissue slice. Front Endocrinol (Lausanne) 2022;13:916688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zmazek J, Klemen MS, Markovič R, et al. Assessing different temporal scales of calcium dynamics in networks of beta cell populations. Front Physiol 2021;12:612233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stožer A, Skelin Klemen M, Gosak M, et al. Glucose-dependent activation, activity, and deactivation of beta cell networks in acute mouse pancreas tissue slices. Am J Physiol Endocrinol Metab 2021;321:E305–E323 [DOI] [PubMed] [Google Scholar]
- 57. Šterk M, Križančić Bombek L, Skelin Klemen M, et al. NMDA receptor inhibition increases, synchronizes, and stabilizes the collective pancreatic beta cell activity: insights through multilayer network analysis. PLoS Comput Biol 2021;17:e1009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Panzer JK, Caicedo A. Targeting the pancreatic α-cell to prevent hypoglycemia in type 1 diabetes. Diabetes 2021;70:2721–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Panzer JK, Tamayo A, Caicedo A. Restoring glutamate receptor signaling in pancreatic alpha cells rescues glucagon responses in type 1 diabetes. Cell Rep 2022;41:111792. [DOI] [PubMed] [Google Scholar]
- 60. Rose T, Efendic S, Rupnik M. Ca2+-secretion coupling is impaired in diabetic Goto Kakizaki rats. J Gen Physiol 2007;129:493–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Almaça J, Weitz J, Rodriguez-Diaz R, Pereira E, Caicedo A. The pericyte of the pancreatic islet regulates capillary diameter and local blood flow. Cell Metab 2018;27:630–644.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tamayo A, Gonçalves LM, Rodriguez-Diaz R, et al. Pericyte control of blood flow in intraocular islet grafts impacts glucose homeostasis in mice. Diabetes 2022;71:1679–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weitz JR, Jacques-Silva C, Qadir MMF, et al. Secretory functions of macrophages in the human pancreatic islet are regulated by endogenous purinergic signaling. Diabetes 2020;69:1206–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Makhmutova M, Weitz J, Tamayo A, et al. Pancreatic islets communicate with the brain via vagal sensory neurons. Gastroenterology 2021;160:875–888.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liang T, Dolai S, Xie L, et al. Ex vivo human pancreatic slice preparations offer a valuable model for studying pancreatic exocrine biology. J Biol Chem 2017;292:5957–5969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gál E, Dolenšek J, Stožer A, Pohorec V, Ébert A, Venglovecz V. A novel in situ approach to studying pancreatic ducts in mice. Front Physiol 2019;10:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mastracci TL, Apte M, Amundadottir LT, et al. Integrated physiology of the exocrine and endocrine compartments in pancreatic diseases: workshop proceedings. Diabetes 2023;72:433–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Atkinson MA, Campbell-Thompson M, Kusmartseva I, Kaestner KH. Organisation of the human pancreas in health and in diabetes. Diabetologia 2020;63:1966–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Larger E, Philippe MF, Barbot-Trystram L, et al. Pancreatic exocrine function in patients with diabetes. Diabet Med 2012;29:1047–1054 [DOI] [PubMed] [Google Scholar]
- 70. Alexandre-Heymann L, Mallone R, Boitard C, Scharfmann R, Larger E. Structure and function of the exocrine pancreas in patients with type 1 diabetes. Rev Endocr Metab Disord 2019;20:129–149 [DOI] [PubMed] [Google Scholar]
- 71. Altay M. Which factors determine exocrine pancreatic dysfunction in diabetes mellitus? World J Gastroenterol 2019;25:2699–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Radlinger B, Ramoser G, Kaser S. Exocrine pancreatic insufficiency in type 1 and type 2 diabetes. Curr Diab Rep 2020;20:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rickels MR, Norris AW, Hull RL. A tale of two pancreases: exocrine pathology and endocrine dysfunction. Diabetologia 2020;63:2030–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chen N, Unnikrishnan I R, Anjana RM, Mohan V, Pitchumoni CS. The complex exocrine-endocrine relationship and secondary diabetes in exocrine pancreatic disorders. J Clin Gastroenterol 2011;45:850–861 [DOI] [PubMed] [Google Scholar]
- 75. Marolt U, Paradiž Leitgeb E, Pohorec V, et al. Calcium imaging in intact mouse acinar cells in acute pancreas tissue slices. PLoS One 2022;17:e0268644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sluga N, Postić S, Sarikas S, Huang YC, Stožer A, Slak Rupnik M. Dual mode of action of acetylcholine on cytosolic calcium oscillations in pancreatic beta and acinar cells in situ. Cells 2021;10:1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xu X, D’Hoker J, Stangé G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008;132:197–207 [DOI] [PubMed] [Google Scholar]
- 78. Huber MK, Drotar DM, Hiller H, et al. Observing islet function and islet-immune cell interactions in live pancreatic tissue slices. J Vis Exp 2021:e62207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brissova M, Haliyur R, Saunders D, et al. α Cell function and gene expression are compromised in type 1 diabetes. Cell Rep 2018;22:2667–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Martín F, Soria B. Glucose-induced [Ca2+]i oscillations in single human pancreatic islets. Cell Calcium 1996;20:409–414 [DOI] [PubMed] [Google Scholar]
- 81. Campbell-Thompson M. Organ donor specimens: what can they tell us about type 1 diabetes? Pediatr Diabetes 2015;16:320–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Van Erp AC, Rebolledo RA, Hoeksma D, et al. Organ-specific responses during brain death: increased aerobic metabolism in the liver and anaerobic metabolism with decreased perfusion in the kidneys. Sci Rep 2018;8:4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Toyama H, Takada M, Suzuki Y, Kuroda Y. Activation of macrophage-associated molecules after brain death in islets. Cell Transplant 2003;12:27–32 [DOI] [PubMed] [Google Scholar]
- 84. Kusmartseva I, Beery M, Philips T, et al. Hospital time prior to death and pancreas histopathology: implications for future studies. Diabetologia 2018;61:954–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rech TH, Crispim D, Rheinheimer J, et al. Brain death-induced inflammatory activity in human pancreatic tissue: a case-control study. Transplantation 2014;97:212–219 [DOI] [PubMed] [Google Scholar]
- 86. Ebrahimi A, Jung MH, Dreyfuss JM, et al. Evidence of stress in β cells obtained with laser capture microdissection from pancreases of brain dead donors. Islets 2017;9:19–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. In’t Veld P, De Munck N, Van Belle K, et al. Beta-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes 2010;59:1702–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Henquin JC. Influence of organ donor attributes and preparation characteristics on the dynamics of insulin secretion in isolated human islets. Physiol Rep 2018;6:e13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lakey JR, Kneteman NM, Rajotte RV, Wu DC, Bigam D, Shapiro AM. Effect of core pancreas temperature during cadaveric procurement on human islet isolation and functional viability. Transplantation 2002;73:1106–1110 [DOI] [PubMed] [Google Scholar]
- 90. Hilling DE, Bouwman E, Terpstra OT, Marang-van de Mheen PJ. Effects of donor-, pancreas-, and isolation-related variables on human islet isolation outcome: a systematic review. Cell Transplant 2014;23:921–928 [DOI] [PubMed] [Google Scholar]
- 91. Ehehalt F, Sturm D, Rösler M, et al. Blood glucose homeostasis in the course of partial pancreatectomy--evidence for surgically reversible diabetes induced by cholestasis. PLoS One 2015;10:e0134140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wigger L, Barovic M, Brunner AD, et al. Multi-omics profiling of living human pancreatic islet donors reveals heterogeneous beta cell trajectories towards type 2 diabetes. Nat Metab 2021;3:1017–1031 [DOI] [PubMed] [Google Scholar]
- 93. Barovic M, Distler M, Schöniger E, et al. Metabolically phenotyped pancreatectomized patients as living donors for the study of islets in health and diabetes. Mol Metab 2019;27S(Suppl.):S1–S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Khamis A, Canouil M, Siddiq A, et al. Laser capture microdissection of human pancreatic islets reveals novel eQTLs associated with type 2 diabetes. Mol Metab 2019;24:98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Norris AW, Ode KL, Merjaneh L, et al. Survival in a bad neighborhood: pancreatic islets in cystic fibrosis. J Endocrinol 2019;241:R35–R50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li J, Cao G, Ma Q, Liu H, Li W, Han L. The bidirectional interation between pancreatic cancer and diabetes. World J Surg Oncol 2012;10:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dugnani E, Gandolfi A, Balzano G, et al. Diabetes associated with pancreatic ductal adenocarcinoma is just diabetes: results of a prospective observational study in surgical patients. Pancreatology 2016;16:844–852 [DOI] [PubMed] [Google Scholar]
- 98. Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008;134:981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Parajuli P, Nguyen TL, Prunier C, Razzaque MS, Xu K, Atfi A. Pancreatic cancer triggers diabetes through TGF-β-mediated selective depletion of islet β-cells. Life Sci Alliance 2020;3:e201900573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. McAuliffe JC, Christein JD. Type 2 diabetes mellitus and pancreatic cancer. Surg Clin North Am 2013;93:619–627 [DOI] [PubMed] [Google Scholar]
- 101. Tan J, You Y, Guo F, Xu J, Dai H, Bie P. Association of elevated risk of pancreatic cancer in diabetic patients: a systematic review and meta-analysis. Oncol Lett 2017;13:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wang F, Larsson J, Adrian TE, Gasslander T, Permert J. In vitro influences between pancreatic adenocarcinoma cells and pancreatic islets. J Surg Res 1998;79:13–19 [DOI] [PubMed] [Google Scholar]
- 103. Magruder JT, Elahi D, Andersen DK. Diabetes and pancreatic cancer: chicken or egg? Pancreas 2011;40:339–351 [DOI] [PubMed] [Google Scholar]
- 104. Dugnani E, Balzano G, Pasquale V, et al. Insulin resistance is associated with the aggressiveness of pancreatic ductal carcinoma. Acta Diabetol 2016;53:945–956 [DOI] [PubMed] [Google Scholar]
- 105. Kang MJ, Jung HS, Jang JY, et al. Metabolic effect of pancreatoduodenectomy: resolution of diabetes mellitus after surgery. Pancreatology 2016;16:272–277 [DOI] [PubMed] [Google Scholar]
- 106. Sohn SY, Lee EK, Han SS, et al. Favorable glycemic response after pancreatoduodenectomy in both patients with pancreatic cancer and patients with non-pancreatic cancer. Medicine (Baltimore) 2018;97:e0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Heart EA, Karandrea S, Liang X, et al. Mechanisms of doxorubicin toxicity in pancreatic β-cells. Toxicol Sci 2016;152:395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hwangbo Y, Lee EK. Acute hyperglycemia associated with anti-cancer medication. Endocrinol Metab (Seoul) 2017;32:23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Westermark GT, Krogvold L, Dahl-Jørgensen K, Ludvigsson J. Islet amyloid in recent-onset type 1 diabetes-the DiViD study. Ups J Med Sci 2017;122:201–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Seiron P, Wiberg A, Kuric E, et al. Characterisation of the endocrine pancreas in type 1 diabetes: islet size is maintained but islet number is markedly reduced. J Pathol Clin Res 2019;5:248–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Krogvold L, Wiberg A, Edwin B, et al. Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia 2016;59:492–501 [DOI] [PubMed] [Google Scholar]
- 112. Krogvold L, Edwin B, Buanes T, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia 2014;57:841–843 [DOI] [PubMed] [Google Scholar]
- 113. Karimian N, Qin T, Liang T, et al. Somatostatin receptor type 2 antagonism improves glucagon counterregulation in biobreeding diabetic rats. Diabetes 2013;62:2968–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]