Abstract
Doxorubicin (DOX) is an anthracycline antibiotic widely used as a chemotherapeutic agent to treat solid tumours and hematologic malignancies. Although useful in the treatment of cancers, the benefit of DOX is limited due to its cardiotoxic effect that is observed in a large number of patients. In the literature, there is evidence that the presence of various factors may increase the risk of developing DOX-induced cardiotoxicity. A better understanding of the role of these different factors in DOX-induced cardiotoxicity may facilitate the choice of the therapeutic approach in cancer patients suffering from various cardiovascular risk factors.
In this review, we therefore discuss the latest findings in both preclinical and clinical research suggesting a link between DOX-induced cardiotoxicity and various risk factors including sex, age, ethnicity, diabetes, dyslipidaemia, obesity, hypertension, cardiovascular disease and co-medications.
Keywords: Doxorubicin, Chemotherapy, Toxicity, Diabetes, Cardiovascular risk
1. Introduction
Cancer is a life-threatening disease characterized by unregulated cell growth and the formation of malignant tumours. In 2020, an estimated 19.3 million people were diagnosed with cancer worldwide and nearly 10 million cancer deaths were recorded [1], [2]. There are multiple treatment strategies for cancer that have contributed to steadily decrease its fatality rate since the 1960s [3]. However, following treatment, many cancer survivors may experience significant side-effects of the therapy.
One such treatment is Doxorubicin (DOX), an anthracycline antibiotic frequently used as a chemotherapy for the treatment of solid tumours and hematogenous malignancies [4]. However, its use is limited due to many cancer survivors developing congestive heart failure (CHF) when receiving this treatment [5]. In an Italian cohort of 2625 cancer patients treated with anthracyclines, 9 % of the patients experienced cardiotoxicity with a mean follow up time of 5.2 years (98 % of which occurred in the first year) [6]. Similarly, in a group of patients undergoing anticancer therapy in Spain with a mean follow up of 2 years, 37.5 % of patients experienced cardiotoxicity [7].
An early indicator for DOX-induced cardiotoxicity is a decrease in left ventricular ejection fraction (LVEF) from baseline [8]. The European Society of Cardiology (ESC) defines clinical cardiotoxicity as a 10 percentage (%) point decrease in LVEF from baseline to a value below 50 % [9]. The American Society of Echocardiography and the European Association of Cardiovascular Imaging define clinical cardiotoxicity as a 10 % point decrease in LVEF from baseline to a value below 53 % and subclinical cardiotoxicity as preserved LVEF with a 15 % reduction in Global Longitudinal Strain (GLS) with/without an increase in serum biomarkers for cardiac injury such as troponin [10]. However, it is important to note that most research studies have used their own definition when referring to cardiotoxicity. Cardiotoxicity can occur at any time during or after treatment and is separated into three classes: acute, early and late onset or chronic cardiotoxicity [9]. Acute cardiotoxicity occurs during treatment and often presents as supraventricular arrhythmias, electrocardiograph (ECG) changes and left ventricular dysfunction [9]. These symptoms usually regress, however it may also progress into early or late cardiotoxicity [11]. Early cardiotoxicity occurs within the first year of treatment while late cardiotoxicity occurs several years later, but both are associated with the development of CHF [9], [11], [12].
Monitoring left ventricular systolic function with imaging modalities and cardiovascular biomarkers before and after completion of anthracycline based chemotherapy is therefore recommended to detect early myocardial damage (see Table 1). Although several imaging modalities exist, serial monitoring with two-dimensional (2D) echocardiography is routinely used in the clinical setting due to its widespread availability, avoidance of exposure to radiation, ability to obtain haemodynamic parameters and to assess cardiac structures [6], [13], [14]. However, one main challenge with 2D echocardiography is that it is not sensitive enough to detect small changes in LVEF [see review [13]]. 2D Speckle tracking echocardiography to measure myocardial global longitudinal strain (GLS) is a more sensitive method to detect a reduction in myocardial contractility prior to the onset of left ventricular systolic dysfunction [14]. In a population of breast cancer patients receiving an anthracycline based chemotherapy regimen, there was a >10 % reduction in LVEF in 23.3 % of patients at 3 months and in 25.3 % of patients at 6 months, however, LVEF remained above 50 % throughout follow up with no patients diagnosed with cardiotoxicity according to the criterion. On the other hand, a greater than 15 % reduction in GLS was observed in 61.6 % of patients at 3 months, and a relative but significant decrease in GLS in the whole population throughout follow up thus indicating that GLS is more sensitive to detect myocardial injury at an earlier timepoint following treatment [14]. Probing for cardiovascular biomarkers during treatment may assist to identify myocardial injury prior to the onset of changes in left ventricular function and could therefore assist in predicting patients at risk for a subsequent decline in left ventricular function [15]. The cardiac biomarker, high sensitivity troponin I, is released upon myocardial injury early during treatment and has demonstrated to predict a subsequent decline in LVEF [16]. Similarly, the neurohormone N-terminal pro-brain natriuretic peptide (NT pro-BNP) is released from cardiomyocytes in response to expansion and pressure overload [17]. Serum levels of NT pro-BNP increased during anthracycline treatment, an effect that was associated with a decline in left ventricular fractional shortening and ejection fraction [17]. In a different study, serum levels of NT pro-BNP increased prior to a decline in LVEF [18]. In contrast, others observed an increase in cardiac troponins I, T and NT pro-BNP during anthracycline treatment, however it was not related to changes in left ventricular dysfunction [19]. The discrepancies in findings between studies could be related to sample size, differences in the timing of assessment for left ventricular function and the biomarkers or differences in the dosage of anthracycline treatment administered where a higher cumulative dosage would have a stronger biomarker response [19]. Other biomarkers have also been considered including galectin-3, c-reactive protein and myeloperoxidase [15], [20]. Galectin-3 is related to cardiac remodelling and fibrosis and c-reactive protein is involved in systemic inflammation. Both were increased in patients receiving anthracycline treatment; however, the effect was not associated with left ventricular dysfunction [15], [19]. Similar observations were made with myeloperoxidase, an enzyme related to oxidative stress, where the serum level of myeloperoxidases increased during anthracycline treatment but was not associated with left ventricular dysfunction [15]. However, patients with baseline levels of myeloperoxidase above the median had more severe myocardial injury after anthracycline treatment [15]. Furthermore, in a meta-analysis, there was no change in serum levels of galectin-3 while myeloperoxidase increased earlier than cardiac troponin I and NT pro-BNP during anthracycline therapy and was associated with an increased risk for cardiotoxicity [21].
Table 1.
Current tools available for the early detection of anthracycline cardiotoxicity.
| Measurement | Advantage | Limitation | |
|---|---|---|---|
| Imaging modalities | |||
| 2D echocardiography | LVEF Haemodynamics Cardiac structures |
Accessible No exposure to radiation |
Sensitivity Interobserver variability |
| 3D echocardiography | LVEF Haemodynamics Cardiac structures |
Reduced variability compared to 2D echocardiography | Costly Operator dependence Limited availability |
| 2D speckle tracking echocardiography | GLS | Sensitivity Predict subsequent decline in LVEF |
Strain packages for different manufacturers vary. Abnormal strain cut off values yet to be standardized. |
| MUGA scan | LVEF | Reproducibility | Radiation Limited structural and functional information on other cardiac structures. |
| CMR imaging | LVEF Fibrosis Oedema |
Reproducible Characterization of myocardial tissue |
Costly Limited availability |
| Biomarkers | |||
| Troponin I | Myocardial injury | Sensitivity Reproducible Minimally invasive Availability |
Standardization for routine clinical use yet to be determined. |
Unfortunately, these conventional modalities to detect cardiotoxicity are only positive after damage to the myocardium has already been done. Currently, there is a need to identify patients at risk prior to the onset of any clinical manifestations [see review [22]]. A recent assessment for a biomarker utilizing technology within the omics field shows promise to offer a more targeted approach to identify patients at risk for developing anthracycline cardiotoxicity before and early during treatment [see reviews [22], [23]]. Briefly, genomic studies have identified several single nucleotide polymorphisms (SNPs) and microRNAs that are associated with a greater risk for the development of anthracycline-induced cardiotoxicity. In preclinical models, the use of a metabolomic approach has allowed to identify more than 39 metabolites that are able to predict cardiotoxicity earlier than other biochemical or histopathological analysis [see review [22]].
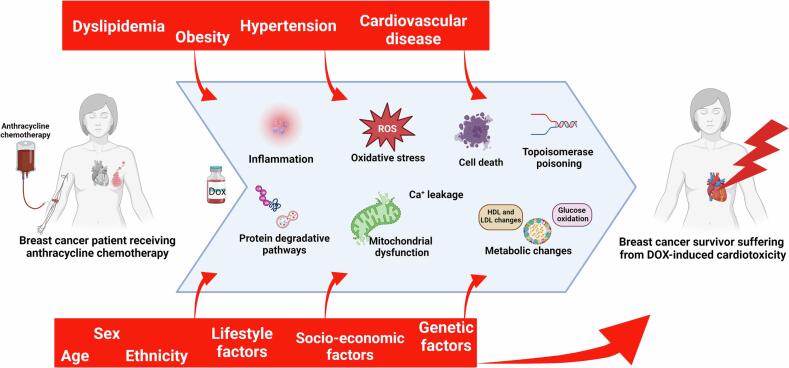
Understanding the mechanisms how DOX targets cancer cells and induces cardiotoxicity is key to ensure its efficacy while protecting patients against the side effects of DOX. As largely reviewed in the literature, DOX kills cancer cells through two main mechanisms. Firstly, by intercalation with deoxyribonucleic acid [24], leading to double stranded breaks and secondly, by topoisomerase inhibition [25], [26]. Some mechanisms involved in DOX-induced cardiotoxicity may be distinct from its anti-cancer therapeutic mechanisms while others may overlap [26]. There are two well-theorized mechanisms of DOX-induced cardiotoxicity. Firstly, upon its administration, DOX undergoes one electron reduction producing the reactive oxygen species (ROS), superoxide anion and hydrogen peroxide [27], [28]. ROS react with iron (Fe2+ and Fe3+) to produce the highly toxic hydroxyl radical promoting oxidative stress causing damage to DNA, proteins and lipids leading to cell death [28], [29]. Secondly, DOX increases intracellular iron concentration through inhibiting the iron regulatory protein-1 (IRP-1) [30]. IRP-1 regulates the expression of genes involved in iron metabolism and homeostasis, thus its inhibition leads to increased intracellular iron levels and consequently, oxidative stress [31]. Cardiac tissue has a relatively low anti-oxidative defence system which makes it more susceptible to DOX-induced ROS damage compared to other tissues [32]. In addition, DOX itself reduces the expression of anti-oxidative enzymes, glutathione and catalase, further impeding its anti-oxidative defence system and leaving the heart vulnerable to oxidative stress [33]. These two mechanisms are known as the “ROS and iron” hypothesis and are widely believed to be the major causes of DOX-induced cardiotoxicity, although many other mechanisms exist [26] [see review [34]].
Other mechanisms include targeting signalling molecules involved in apoptosis such as the pro-apoptotic gene P53, inhibition of the T-cell survival factor GATA-4 and degradation of the cell cycle regulator p300 [35], [36], [37], [38]. DOX upregulates intracellular protein degradation by increasing the activity of the ubiquitin–proteasome system (UPS) causing degradation of essential proteins [39]. The oxidative stress also promotes dysfunction of the mitochondria [40]. This results in mitochondrial swelling and apoptosis, cristae disruption, increased number of lysosomes and chromatin shrinkage [41], [42], [43], [44]. Finally, DOX has cardiotoxic mechanisms that overlap with its anticancer functions. For example, DOX causes more severe DNA damage through topoisomerase IIα than topoisomerase IIβ, the former being highly expressed in cancer cells and immature human-induced pluripotent stem cell-derived cardiomyocytes [45], [46]. Based on the current understanding of the signalling pathways involved in DOX-induced cardiotoxicity, several cardioprotective intervention strategies are currently explored to mitigate its cardiotoxic effect [see review [47]]. Briefly, modifying the administration of anthracycline chemotherapy which includes lower accumulative dosage, slow infusion, and treatment with liposomal DOX, has demonstrated to be of clinical benefit [48], [49], [50]. Concurrent or subsequent treatment with pharmacological therapies such as dexrazoxane, inhibitors of the renin-angiotensin-aldosterone system, beta adrenoceptor blockers are also investigated in clinical trials [19], [51], [52], [53], [54], [55]. Unfortunately, many clinical trials aiming to reduce or prevent the onset of cardiotoxicity are neutral despite strong preclinical studies, highlighting flaws in the translation from bench to bedside [56], [57].
There are well known risk factors associated with the development of DOX-induced cardiotoxicity, the most common one being cumulative dosage [5]. Adult and paediatric cancer patients are most at risk when cumulative dosage exceeds 400–700 mg/m2 and 300 mg/m2, respectively [58]. However, subclinical cardiac dysfunction has also been observed at a lower dosage [58]. The method of administration also plays a role, where a greater risk is associated with bolus infusion compared to continuous infusion [59]. Other than the clinical application, there are factors related to the patient profile which act as confounders, increasing susceptibility to DOX-induced cardiotoxicity.
Individuals who bear these risk factors can be considered for other therapies while those who continue to receive DOX will need to be closely monitored to recognize and treat cardiotoxicity. In this mini-review, we therefore discuss important risk factors that may favour the risk of DOX-induced cardiotoxicity, including sex, age, ethnicity, diabetes, dyslipidaemia, obesity, hypertension, cardiovascular disease, and co-medications (Fig. 1).
Fig. 1.
Major risk factors for DOX-induced cardiotoxicity. Abbreviations: ACE = Angiotensin Converting Enzyme, ARB = Angiotensin Receptor Blockers, CCB = Calcium Channel Blockers, DOX = Doxorubicin, MRT = Mediastinal Radiation Therapy, ROS = Reactive Oxygen Species, SGLT2 = Sodium Glucose Transporter 2.
2. Sex
Sex is a well-known risk factor for cardiovascular diseases in humans. It is estimated that 290 million women and 260 million men live with heart disease globally [60]. Contrastingly, an estimated 9.8 million men died of heart disease in 2019 compared to 9.2 million women [60]. Men also have a higher incidence of cancer worldwide as well as a higher total mortality due to cancer [61]. It is therefore important to consider sex-based susceptibility with cancer treatment as one sex may be more at risk of cardiotoxic effects caused by DOX (see Fig. 2).
Fig. 2.
Signalling pathways targeted by different risk factors in DOX-induced cardiotoxicity.
Sex hormones are an important mechanism behind these differences since cardiac cells express both oestrogen receptors (ER) and androgen receptors (AR), thus making these cells specifically prone to changes function to the levels of circulating sex hormones [62]. Oestrogen, specifically, has shown cardioprotective effects, in part due to prevention of apoptosis as well as alleviation of left ventricular hypertrophy [63]. Oestrogen modulates a variety of cardioprotective pathways such as the phosphatidylinositol-3-kinase (PI3K) pathway and the extracellular signal-regulated kinase (ERK) 1/2 pathway, both involved in apoptosis prevention and improved mitochondrial function [64], [65]. Other than sex hormones, differences in health are further influenced by sex-specific lifestyle habits and risk factors such as smoking, diet, stress and alcohol consumption [66]. Additionally, sex-linked genes such as Y-linked DDX3Y and X-linked XIST have also been linked to susceptibility to cardiomyopathies, indicating that genetics may play a further role in sexual dimorphism in DOX-induced cardiotoxicity [67].
Most animal studies investigating the effects of DOX have generally been assessed in male rodents to reduce the variability of results caused by fluctuating oestrogen levels in female rodents [68]. However, DOX induced greater myocardial injury in certain strains of male mice and rats compared to females. Comparing 10 collaborative cross mouse strains receiving 25 mg/kg DOX, the authors observed no changes in susceptibility between sexes in six strains, male susceptibility in three strains and female susceptibility in one strain [69]. Indeed, troponin-T levels, used as a measurement of cardiac injury, were higher in male B6C3F1 mice compared to female mice when cumulative DOX dose exceeded 21 mg/kg [70]. Similarly, LVEF was significantly reduced in male Wistar rats but not in females when DOX was given at a cumulative dose of 14 mg/kg [71]. In addition, male rats had a 50 % mortality compared to no deaths in females [71]. In another adult rat model, male rats had significantly more severe cardiomyopathy scores and myocardial lesions compared to females [72]. Finally, in a study where cumulative dose was 16 mg/kg, female Wistar Kyoto rats were better protected against DOX-induced weight loss, blood pressure increase, cardiac dysfunction, and nephrotoxicity compared to male mice [73]. The majority of evidence suggests that male rodents are more at risk of cardiotoxicity than female rodents.
In rodent studies, there is also strong evidence to support female hormones as role-players in maintaining cardiac function with DOX treatment, particularly after cardiac stress. Female ovariectomised rats treated with a subclinical dose of DOX and subject to cardiac stress in the form of swim training showed divergence from non-ovariectomised rats in maintenance of cardiac function, thus indicating that ovarian hormones may play a role in reducing DOX-induced cardiotoxicity [74]. Similarly the use of oestrogen therapy in ovariectomized rats together with DOX, improved DOX treatment-induced a decrease in ejection fraction [75]. Treatment with oestrogen was also able to prevent the release of B-myosin heavy chain in cardiac tissue, which is known to be expressed in adult hearts during heart failure. In a study examining both male and female gonadectomized mice, the hypertrophic response to DOX with adrenergic-induced cardiac stress was shown to be reduced in castrated male mice compared to non-castrated males, but no changes were seen in ovariectomized females [76]. Pre-exposure to DOX did not cause any sex-specific changes in cardiac function, but coupled with cardiac stress, DOX caused persistent cardiac atrophy in male mice only, which could not be prevented by gonadectomy, thus illustrating sexually dimorphic responses to DOX cardiotoxicity [76]. A study examining the effect of DOX treatment on cardiac contractile properties in gonadectomized rats showed that oestrogen (but not progesterone) was able to restore contractility in ovariectomized female rats, whereas testosterone was unable to restore contractility in castrated males [77].
Literature exploring sex differences in DOX induced cardiotoxicity in humans is scarce, with very few studies including adult participants. Notably, there is a difference in findings between adult and paediatric cancer patients.
Most studies report young girls being more at risk of cardiotoxicity compared to young boys [78], [79], [80]. This increased risk could theoretically relate to the low levels of oestrogen in the female body at a pre-pubescent age [81]. It is also hypothesized that females have a lower clearance of DOX than males due to their higher levels of fat mass since DOX clearance is reduced in obese patients [82], [83]. A study analysing 150 children between the ages of 11 weeks and 21 years old treated with DOX, revealed a positive association between the female sex and cardiac dysfunction [78]. The two tests used in this study were resting and exercise gated nuclear angiography, and ECG monitoring of cycle ergometry [78]. A similar study evaluating 106 children diagnosed with DOX-induced cardiotoxicity, positively correlated female sex with a higher risk of cardiotoxicity [79]. This study used patient records to measure heart failure, cardiac-related deaths, and irregular cardiac function [79]. Using mortality records, young girls may be more at risk of death due to cardiotoxicity than boys [80]. Contrastingly, in other paediatric studies, neither sex was a risk factor for DOX-induced cardiotoxicity and in others, adult females were at higher risk [78], [84], [85], [86]. These differences could be due to the cumulative dosage, grouping of paediatric and adult patients, the number of individuals of each sex in each study or a different definition used to define cardiotoxicity.
In adults, one study reported a positive correlation between male sex and risk of cardiac disease when 77 % of patients received DOX treatment [87]. These data are only partially reliable since baseline rates of heart disease are higher in males than females and can thus skew the results. Furthermore, not all patients in the cohort received DOX therapy and this may possibly interfere with the resultant correlation. Similarly, 141 adult patients with a median age of 54 and a cumulative dose of 300 mg/m2 of DOX indicated male sex as risk factor for subclinical cardiomyopathy, which was defined by decreased left ventricular fractional shortening [88]. Finally, a significantly larger proportion of men developed symptomatic HF and experienced cardiac-related deaths compared to women in a larger anthracycline study where 89 % of patients received DOX [89].
In childhood cases of cancer treated with DOX or anthracyclines in females who go on to become pregnant at a later stage, there is evidence to suggest that pregnancy exacerbates cardiovascular dysfunction induced by DOX. A population based-study in Canada has shown a higher risk for the development of heart failure, arrhythmias, valvular disease, pericardial disease, coronary artery disease, or cardiac-related death in patients diagnosed with cancer before the age of 21 when compared to matched controls without previous cancer diagnoses [90]. A study conducted in the Netherlands in survivors of childhood cancer, including 74.4 % of the study population who were treated with DOX at a cumulative mean dosage of 270 mg/m2, found that pregnancy caused left ventricular dysfunction with abnormal baseline longitudinal strain, but individuals with normal baseline values had low risk of left ventricular dysfunction during pregnancy [91]. Interestingly, none of the patients with abnormal baseline went on to develop heart failure with pregnancy, echoing the findings of another study in the Netherlands, which showed a low risk of developing peripartum anthracycline-induced congestive heart failure in survivors of childhood cancer [85]. A study conducted in the Middle-East followed a group of 37 cancer survivors, who had received DOX treatment <500 mg/m2 in their childhood (at least 2 years prior), through pregnancy [92]. Fractional shortening [93] was used as the defining measure for cardiac dysfunction, and women with normal function at baseline had no change in cardiac function during pregnancy, while those with cardiac dysfunction showed further reduced fractional shortening after pregnancy [92].
In conclusion, most animal studies elucidate a sexual dimorphism related to DOX-induced cardiotoxicity in most mice and rat strains wherein females are protected, and males are more at risk. On the other hand, clinical results allowing for a correlation between sex and disease in humans are limited and further complicated by puberty and lifestyle choices between sexes. If young females may be more are risk of developing cardiac complications than males when treated with anthracyclines, the data obtained in adults are inconclusive. Further large studies are necessary to investigate this correlation which seems to be age dependent [94].
3. Age
Aging is a well-known risk factor for both cardiovascular disease as well as cancer [60], [95]. Thus, it would follow that the incidence of DOX-induced cardiotoxicity may also increase with age. However, since the three classes of cardiotoxicity present at different times, it may be that certain age groups will be more at risk of one type of cardiotoxicity than another. For example, acute and subacute DOX-induced cardiotoxicity are more often seen in older cancer patients whereas paediatric patients display acute conditions less frequently but are more prone to late-onset cardiotoxicity [96]. This is because young individuals can incur cardiomyocyte damage with no immediate signs of cardiac dysfunction, but may start to present symptoms as the heart comes under stress with age [97]. Older individuals, on the other hand, have hearts more sensitive to damage and are more likely to suffer soon after or during treatment [98].
Investigating the effect of age on DOX-induced cardiotoxicity is difficult because most publications focus either on paediatric studies or on adult studies, few including both demographics. Furthermore, 8-year or longer follow ups are more common in paediatric analyses and near non-existent in older age groups as many elderly individuals have a limited lifespan due to other health factors. In general, in an animal study, an increase in age was associated with greater oxidative stress, inflammation, and anti-oxidant activity caused by DOX in a rat model using 72 male rats sorted into three age categories - young, adult, and elderly [99]. This sentiment is echoed in anthracycline clinical trial data which indicates an increased risk of cardiotoxicity with increasing patient age [98], [100]. Additionally, increasing patient age has been associated with decreased DOX clearance, resulting in a higher risk of cardiomyopathy [101].
In adult studies, advancing age was a risk factor for subclinical cardiomyopathy when analysing age as a continuous variable in 141 patients with a median age of 54 and a cumulative dose of 300 mg/m2 [88]. Supporting this, an increase in age was positively associated with a higher risk of cardiotoxic effects, measured by LVEF, 2 years after therapy involving 613 breast cancer patients [102]. Notably, these results only investigated age as a risk factor in women. Finally, a 3–4 year long DOX-plus-placebo study involving 620 patients of varying ages found an increased incidence of CHF in patients above 65 years old compared to those below [5]. It is also important to remember that the presence of co-morbidities (which may contribute to DOX-induced cardiotoxicity) is increasing when patients become older.
With regard to cancer treatment in children, a follow up study was performed on 115 childhood-cancer survivors where echocardiograms were obtained on average 7.8 years after completion of DOX treatment. The results showed that patients had significantly reduced left ventricular fractional shortening compared to the predicted normal value [103]. This paper, among others, has been cited as evidence that children are at a higher risk of cardiotoxic damage compared to other ages. However, it should be considered that the results were only compared to predicted healthy values and not DOX-treated individuals of other ages. Thus, these results in themselves are not categorically conclusive.
Overall, chances of heart complications are high during treatment, since acute cardiotoxicity is quite common, then decrease shortly after treatment, since subacute cardiotoxicity is rare, and then increase again over time as the likelihood of late-onset cardiotoxicity rises [11], [104]. Children are more likely to incur heart complications many years after treatment whereas the elderly, usually those above 65 years old, are more likely to present disease conditions soon after treatment.
4. Ethnicity
Ethnicity is a well-known cardiovascular risk factor with black African individuals being less at risk of coronary heart disease than other races, but more at risk of presenting with ischemic strokes and intracerebral haemorrhages compared to white individuals [105]. On the other hand, black Americans have a higher mortality rate due to heart disease than all other ethnic groups [106]. Similarly, black women in the USA have a two-fold higher rate of chronic hypertension compared to white women [106]. Differences between countries may suggest socio-economic reasons rather than ethnicity per se. When exploring the role of ethnicity in DOX-induced cardiotoxicity, it is also key to keep in mind that the physiology and the pathophysiology of cardiovascular disease may differ according to ethnicity [107], [108].
Studies separating individuals into groups based on ancestry can give insight into ethnicity as a risk factor. Comparing 100 African American patients receiving DOX with 399 DOX-treated patients of unknown age and racial distribution, a significantly increased incidence of cardiotoxicity in the African American patients for all doses of DOX (from 450 mg/m2 to 600 mg/m2) compared to the randomized study population was observed [109]. Similarly, an increased risk factor for DOX-induced cardiotoxicity was observed in African Americans compared to non-African individuals [79]. Furthermore, cancer survivors of African ancestry had an increased risk of cardiomyopathy compared to those of European ancestry [110]. Recently, a large cohort of 1084 cancer survivors treated with anthracyclines highlighted a higher incidence of HF in non-Hispanic blacks, Hispanics and Asians compared to the non-Hispanic white patients [111]. Outside of Western studies, a multi-ethnic Asian study reported that patients of Chinese ethnicity treated with anthracyclines had a significantly lower preponderance of cardiotoxicity compared to individuals from the combined group composed of Malay, Indian, Caucasian and foreign national patients [112].
In summary, all current studies suggest that individuals of African ancestry appear to be more likely to suffer from DOX-induced cardiotoxicity compared to other races while Chinese patients may be at a lower risk compared to other races. It is important to note that these correlations have not yet been linked to genetic studies and it is possible that different socio-economic, factors may explain part of these findings [113].
5. Diabetes
Diabetes is a chronic condition characterized by abnormally high blood glucose levels [114]. Diabetes is significantly related to other diseases with an increased risk of both cancer and cardiovascular diseases in diabetic patients [115]. Diabetes can result in diabetic cardiomyopathy, which is characterised by the presence of cardiac dysfunction in diabetic patients in the absence of other risk factors such as hypertension and cardiovascular disease [116]. This condition is caused by a number of factors including diastolic and systolic stress on diabetic hearts as a result of mitochondrial dysfunction, inflammation, lipotoxicity, and cardiomyocyte apoptosis (amongst others) [117]. This predisposition to cardiomyopathy in diabetic individuals may suggest that diabetic patients could be at a higher risk for developing DOX-induced cardiotoxicity, in which case this treatment strategy should be carefully considered for diabetic cancer patients.
Indeed, experimental models highlight that diabetes can aggravate DOX-induced cardiotoxicity. Interestingly, DOX promotes hyperglycaemia and insulin resistance in non-diabetic Wistar rats [118]. Surprisingly, diabetes in db/db mice does not exacerbate this insulin resistance caused by DOX [119]. However, DOX upregulated pro-inflammatory signalling and downregulated anti-inflammatory signalling in diabetic rat muscle compared to non-diabetic rat muscle [119]. Db/db mice also had an increased mortality rate and a decreased in cardiac contractile function compared to db/+ non-diabetic mice [120]. This is likely attributed to the increase in ROS generated by DOX, leading to the activation of pro-inflammatory molecules such as the signalling factor Nuclear Factor kappa-light-chain-enhancer of activated B cells (NFκB) [119], [120].
Supporting the findings of animal studies, in a recent meta-analysis study including 7488 patients, diabetes was associated with an increased risk of anthracycline-induced cardiac toxicity. Similarly, patients with diabetes have an increased risk of HF after DOX treatment compared to non-diabetic patients [121]. It is suggested that type 2 diabetes is a significant predictor of late-onset DOX-induced HF but not early-onset HF [122].
Interestingly, DOX-induced cardiotoxicity modulates many signalling pathways common to diabetes [123]. DOX inhibits peroxisome proliferator activated receptor gamma (PPAR) and causes lipotoxicity in cells. PPAR is known to relieve hyperglycaemic and high lipid conditions in diabetes as well as increasing insulin sensitivity, thus DOX inhibiting this pathway would create an excess of lipids causing lipotoxicity [124]. Much like diabetes, DOX is also known to decrease mitochondrial copy number and to reduce mitochondrial function [125]. In addition, DOX treatment reduces body weight, raises blood glucose, and serum lipid levels, effects which are also observed in patients with type II diabetes. As a result, lipotoxicity and any toxic effects caused by the abnormal metabolic state of the diabetic patient may be worsened by DOX. Additionally, it is proposed that diabetic patients would be pre-disposed to oxidative and inflammatory damage due to certain upregulated genes such as S100A8 and S100A9 which are biomarkers of inflammation that are upregulated in cardiovascular diseases [120], [126].
In summary, diabetes is likely a confounding factor for DOX-induced cardiotoxicity. The mechanism behind this predisposition is not yet fully elucidated but may involve, at least in part, an increase in lipotoxicity, hyperglycaemia, and mitochondrial damage caused by both DOX and diabetes. More research is necessary regarding the mechanisms underlying diabetes as a confounding factor for DOX-induced cardiotoxicity as this may benefit the management of diabetic patients receiving DOX.
6. Dyslipidaemia
Dyslipidaemia, defined as an abnormal level of cholesterol or fats in the blood, is a significant risk factor for cardiovascular disease [127]. Dyslipidaemia can result in the build-up of plaques in vessels, thus predisposing individuals to ischemic events [128]. Cancer itself can shift a patient’s lipid profile by decreasing high density lipoprotein (HDL) and increasing low density lipoprotein (LDL) plasma levels, leading to an increased risk of atherosclerosis [129]. DOX is also known to induce dyslipidaemia in patients post-treatment [130]. As a result, concomitant therapies of hypolipidemic drugs are often envisaged [124], [131].
A Sri Lankan study has reported dyslipidaemia to be a predictor of subclinical cardiotoxicity defined as a >10 % decrease in ejection fraction, 6 months after completion of anthracycline therapy [132]. The ANTEC study is an ongoing trial that aims to assess the prognostic value of coronary atherosclerotic lesions and the calcium score on CT scan prior to anthracycline chemotherapy for the occurrence of cardiotoxicity [133]. Hyperlipidaemia has however, been investigated in studies combining other cardiovascular risk factors, including hypertension and diabetes, suggesting that these patients had an increased risk of developing HF induced by anthracyclines compared to those with none of these risk factors [134]. In addition, having all three of these risk factors compounded the risk of HF, indicating that dyslipidaemia contributes to cardiac sensitivity when in combination with hypertension and diabetes [134]. However, dyslipidaemia was not analysed as a solitary risk factor [134].
In summary, although very little data are present in the literature, it is likely that dyslipidaemia will further enhance anthracycline-induced cardiotoxicity.
7. Obesity
Obesity is clinically defined as an unhealthy state of excess body fat. In 2016, 13 % of adults were considered obese globally [135].
Animal models use different diets to test the effect of obesity on DOX-induced cardiotoxicity. A moderately restricted diet in Sprague-Dawley rats showed protection against DOX-induced cardiotoxicity compared to a high fat, obesity-inducing diet which increased sensitivity to cardiotoxicity [136]. This study also reported that the moderate diet increased signalling in the Janus-kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signalling, a well-known cardioprotective pathway [136]. However, this evidence could point to the contribution of diet to DOX-induced cardiotoxicity rather than the contribution of obesity itself since different diets are known to contribute to heart disease independently of body mass index (BMI) [137]. Overweight C57BL/6 mice have also shown systolic dysfunction 20 days after DOX administration compared to normal mice who presented with no dysfunction at the end of the treatment [138].
In humans, a meta-analysis of 15 studies involving anthracycline-treated breast cancer patients reported that obesity or being overweight were strongly associated with cardiotoxicity [139]. However, these results were not able to exclude obesity-related risk factors such as diabetes and pre-existing heart conditions. It is likely that obesity and being overweight contribute to DOX-induced cardiotoxicity through a variety of mechanisms, including, but not exclusive to, diabetes and hypertension. Later, the same group investigated 929 patients receiving anthracyclines, and found a significant association between obesity and cardiotoxicity independent of related risk factors [140]. However, only 7 % of these patients received DOX. The remaining 93 % received other anthracyclines such as epirubicin. A recent large-scale meta-analysis including patients receiving anthracycline chemotherapy, highlighted that obesity was a risk factor for cardiotoxicity while being overweight was not associated with cardiotoxicity [141]. Others found no significant contribution of a BMI over 25 towards cardiotoxicity [102]. Finally, one study measured body fat percentage using X-ray absorptiometry in DOX-treated children and found that doxorubicinol clearance, the major metabolite of DOX, was decreased in children with a body fat percentage above 30 [142]. Two details should be noted from this study: firstly, DOX clearance itself was not significantly different between body fat percentage groups and secondly, 4 out of 6 individuals with a body fat percentage above 30 were, in fact, in the normal BMI range [142]. This data suggest that body fat percentage, and not BMI, may affect DOX pharmacokinetics. Fat cells known as adipocytes directly correlate with body fat percentage and are hypothesized to have dysregulated release of cytokines in obese individuals [143]. These adipokines have been shown to be protective against DOX cardiotoxicity in mice, perhaps illuminating a reason for the increased susceptibility in obese mice [143].
In conclusion, obesity is a risk factor for DOX-induced cardiotoxicity, both on its own and in combination with other comorbidities. Notably, BMI may be an inaccurate assessment tool, that has been questioned by researchers for years [144], [145]. A more reliable way to assess the risk of DOX-induced cardiotoxicity in obese individuals might be to take further measurements where possible such as body fat percentage and adipokines level.
8. Hypertension
High blood pressure or hypertension is a common condition which severely increases the risk of many diseases, including heart disease [60]. Both experimental and clinical studies suggest that pre-existing hypertension could potentially increase susceptibility to DOX-induced cardiotoxicity.
Spontaneous hypertensive rats treated with DOX presented with changes in cardiac electrical activity, a greater weight loss, decreased systolic blood pressure, greater cardiac lesions and mortality compared to normotensive rats [146], [147]. Similar findings were reported in 73 breast cancer patients receiving varying doses of DOX with pre-existing arterial hypertension that positively correlated with left ventricular systolic dysfunction 6 months after treatment [148]. Hypertension was found to be a significant risk factor for both early- and late-onset cardiotoxicity in 1153 American patients with more than 90 % of these patients receiving a cumulative dose above 250 mg/m2 [122]. In a retrospective study involving 58,541 Korean patients receiving DOX, 2324 of whom went on to be diagnosed with DOX-induced HF, hypertension was found to be a predictor of HF [149]. A recent systematic study screening 1330 papers, highlighted that adult patients with elevated blood pressure have an increased vulnerability to cardiotoxicity related to anthracyclines [150].
In summary all data suggest that hypertension is a significant risk factor for DOX-induced cardiotoxicity. The mechanism behind this is likely due to early damage and stress placed on the heart by hypertension prior to treatment, predisposing individuals to further damage, but thorough investigation has yet to confirm this.
9. Pre-existing cardiovascular diseases
If DOX treatment leads to cardiovascular diseases in many individuals, there is evidence in the literature supporting that pre-existing cardiovascular disease may worsen the effects of DOX-induced cardiotoxicity [151].
Pre-existing cardiac disease, defined as patients with two or more records of ischemia, atherosclerosis and/or other heart disease, was shown to increase the risk of CHF in DOX-treated cancer patients compared to individuals with no cardiac comorbidities [121]. Similarly, cardiac hospitalization (CH) before cancer diagnosis, was the largest predictor of CH within ten years after DOX treatment[87].In contrast, pre-existing coronary heart disease showed no significant contribution towards cardiotoxicity two years after DOX treatment in a cohort of 613 breast cancer patients [102]. Similarly, patients with latent atherosclerosis did not have an increased risk of chemotherapy-induced cardiotoxicity [152]. However, in this latest study, very few breast cancer patients received DOX as a treatment as most patients received trastuzumab, possibly interfering with the correlation between DOX-induced cardiotoxicity and atherosclerosis [152]. Contradicting data could be attributed to the different types and severities of pre-existing heart disease in each case.
In summary, the severity and nature of pre-existing cardiovascular disease are likely to influence the possible side effect of DOX treatment. Severe conditions such as a history of ischemia, heart attacks and strokes would need to be weighed carefully when considering DOX as a treatment whereas latent or underlying conditions may interfere less with the treatment.
10. Co-medications/co-treatments
Many cancer patients treated with DOX often receive multiple therapies which may interfere with DOX and its side effects. Trastuzumab, mediastinal radiation therapy (MRT) and diclofenac sodium are example of drugs that may affect patients treated with DOX.
Trastuzumab is an antibody for the human epidermal growth factor receptor-2 (HER-2), a receptor which precipitates a survival signal in both cancer cells and cardiomyocytes. It is often used in cancer therapies to block this signalling pathway and reduce tumorigenic activity [153]. However, this pathway is also involved in cardioprotection. When trastuzumab was first approved by the Food and Drug Administration (FDA) in 2001, it was warned against using it with any anthracyclines, DOX included. This is because one of the trials for the drug showing a 4-fold increase in CHF in patients receiving the drug concomitantly with anthracycline therapies compared to either drug on its own [154]. This was confirmed in a recent retrospective study where trastuzumab was associated with a higher risk of DOX-induced cardiotoxicity [102]. Trastuzumab may exacerbate the cardiotoxic effects of anthracycline chemotherapy mainly through interfering with the cell survival signalling pathways in relation to HER2 [see review [155]]. Binding of trastuzumab on cardiomyocytes HER2 receptors prevents the activation of downstream cell survival pathways in response to DOX-mediated ROS production [156], [157] thus potentiating DOX-mediated ROS production, oxidative stress and ultimately cardiomyocyte death. DOX in combination with trastuzumab further upregulates the expression of angiotensin II (ANG II), an inhibitor of NRG-1, a protein downstream of HER2 [158]. ANG II further activates NADPH oxidases within cardiomyocytes, increasing ROS production with subsequent oxidative stress and apoptosis [159]. In addition to inhibiting HER2 mediated cell survival signalling and promoting oxidative stress, trastuzumab alone or in combination with DOX further demonstrated to downregulate topoisomerase IIβ (topIIβ) [160].
MRT is a common anti-cancer therapy, often accompanying DOX therapy. Notably, patients receiving both treatments had an increased incidence of cardiac hospitalizations over a 10-year period compared to those receiving DOX alone [87], [161].
Digoxin is a cardiac glycoside drug used to treat heart disease symptoms such as atrial flutters and HF. Similarly to MRT, digoxin increased myocardial damage in rabbits when administered with DOX compared to either on its own [162]. Contrastingly, recent experiments conducted in mice and zebrafishes showed that digoxin may, in fact, reduce the cardiotoxicity caused by DOX [163]. This possible interaction therefore requires further investigation.
Other drugs given to the patients who are treated for other comorbidities such as hypertension and diabetes may also interfere with DOX-induced cardiotoxicity (see Table 2). Metformin improves the cancer-killing activity of DOX and there are convincing preclinical data suggesting that it may also reduce its cardiotoxicity. In rodents, Metformin alleviates LVEF reduction and cardiomyocyte damage caused by DOX [164], [165]. Cellular mechanisms affected by metformin during DOX treatment include increasing antioxidants glutathione and superoxide dismutase [165], [166], [167], [168].
Table 2.
Animal studies testing the effect of co-medications on DOX-induced cardiotoxicity.
| Drug/medication | Drug concentration | DOX concentration | Animal | Study length from first dose of DOX | Outcomes | Reference |
|---|---|---|---|---|---|---|
| Digoxin | 50ug/kg/d | 12 mg/kg once | Wistar rats | 7 days | Digoxin + DOX treated rats had a lower systolic volume and left ventricular volume in diastole compared to rats treated with DOX alone. | [204] |
| Atorvastatin | 20 mg/kg/d | 5 mg/kg/w for 4 weeks | Male C57BL/6 mice | 6 weeks | Atorvastatin with DOX increased LVEF and Survivin levels compared to mice treated with DOX alone. | [177] |
| Atorvastatin | 20 mg/kg/d or 40 mg/kg/d | 5 mg/kg/w for 4 weeks | Male C57 mice | 4 weeks | Atorvastatin increased TFEB, reduced myocardial fibrosis and enhanced lysosome function in mice receiving DOX alone. | [160] |
| Lovastatin | 10 mg/kg/d for 5 or 10 days | 1 × 10 mg/kg | Male and female Balb/c mice | 5–10 days | Lovastatin attenuated cardiomyocyte damage caused by DOX and raised CK-MB and LDH levels compared to the mice receiving DOX alone. | [205] |
| Lovastatin | 3 mg/kg 3× per week for 3 months | 3 mg/kg/w for 5 weeks | Female C57BL/6 mice | 3 months | Treatment with Lovastatin in DOX treated mice protected mice from a decrease in left ventricular posterior wall diameter with no change in ejection fraction. Lovastatin treatment also mitigated a decrease in mRNA levels of heat shock protein Hspa1b. | [206] |
| Fluvastatin | 6/mg/kg/d for 7 days | 3 × 7.5 mg/kg | Male and female Sprague Dawley rats | 1 week | Fluvastatin reduced the decrease in iNOS, eNOS, NF-κB and caspase 3 caused by DOX treatment. | [179] |
| Fluvastatin | 100 mg/kg/d for 9 days | 20 mg/kg once | Male and female C57BL/6 mice | 5 days | Co-treatment of DOX with Fluvastatin increased LV pressure and SOD activity compared to DOX treatment alone. | [207] |
| Metformin | 250 mg/kg/d for 7 days | 15 mg/kg once | Male Wistar rats | 2 days | Pre-treatment with Metformin protected against an increase in LDH, CK-MB, caspase 3 and cardiac MDA associated with DOX treatment. Metformin pre-treatment also increased SOD activity in cardiomyocytes compared to DOX alone. | [166] |
| Metformin | 500 mg/kg/d for 7 days | 20 mg/kg twice | Male Wistar albino rats | 6 days | Treatment with Metformin + DOX resulted in lower caspase 3 and TNF-α levels compared to DOX alone. | [167] |
| Metformin | 250 mg/kg for 14 days | 6 × 3 mg/kg | Female Sprague Dawley rats | 2 weeks | Metformin reduced troponin T levels and cardiomyocyte damage caused by DOX. | [165] |
| Metformin | 50 mg/kg/d or 500 mg/kg/d for 11 days | 6 × 3 mg/kg | Male Wistar albino rats | 10 days | Co-treatment of Metformin with DOX returned glutathione levels to normal or above normal and reduced cardiomyocyte injury observed with DOX alone. | [168] |
| Metformin | 250 mg/kg/d for 14 days | 4 x4mg/kg | Male Wistar albino rats | 2 weeks | Metformin protected against reduction in LVEF and cardiomyocyte apoptosis caused by DOX alone | [164] |
| Valsartan (ARB) | 15 mg/kg/d for 28 days | 5 mg/kg/w for 4 weeks | C57BL/6J mice | 4 weeks | Valsartan + DOX significantly improved LVEF, reduced cardiac inflammation and myocardial apoptosis compared to DOX alone. Valsartan + Tolvaptan was more effective in restoring cardiac function against DOX cardiotoxicity compared to Valsartan alone. | [181] |
| Valsartan (ARB) | Sacubitril/Valsartan 60 mg/kg/d for 42 days |
3 × 15 mg/kg/w | Male Sprague-Dawley rats | 6 weeks | Valsartan/Sacubitril significantly reduced cardiomyocyte apoptosis and cardiac dysfunction caused by DOX and downregulated related proteins (BAX, caspase 3, GRP78, PERK) | [184] |
| Valsartan (ARB) | 31md/kg/d for 18 days | 10 × 1.5 mg/kg/d | Male Sprague-Dawley rats | 18 days | Valsartan did not significantly change troponin T levels or ROS compared to DOX group. | [183] |
| Enalapril (ACE inhibitor) | 60 mg/ml in drinking water every other week | 15 mg/kg once or 4 mg/kg/w for 5 weeks | Male C57Bl/6J mice | 9 weeks | Enalapril protected against DOX induced cardiac dysfunction (LV volume) and cardiomyocyte atrophy in the chronic model (5 × 4 mg/kg/w) but not in the acute model (1 × 15 mg/kg). | [189] |
| Lisinopril (ACE inhibitor) | 1 mg/kg/d for 7 days | 2 mg/kg | Female New Zealand white Rabbits | 24 weeks | Lisinopril reduced the loss of cardiomyocytes caused by DOX treatment and decreased ANP in the LV. | [191] |
| Captopril + Enalapril (ACE inhibitors) | 10 mg/kg/d Captopril + 2 mg/kg/d Enalapril for 7 days | 1 × 15 mg/kg | Male rats | 30 hours | Pretreatment with ACE inhibitors reduced TBARS concentration in the heart and ameliorated the inhibition of SOD and LDH activity caused by DOX treatment. | [188] |
| Enalapril (ACE inhibitor) | 10 mg/kg/d for 70 days |
4.17 mg/kg/w for 6 weeks | Female Sprague-Dawley rats | 9 weeks | Enalapril significantly attenuated the decrease in LV contractility and abolished DOX-induced increase in free radical formation. | [190] |
| Fosinopril (ACE inhibitor) | 25 mg/kg/d for 28 days | 6 × 2.5 mg/kg | Male Sprague-Dawley rats | 2 weeks | Fosinopril attenuated increased in both heart and LV weights induced by DOX treatment and lowered LDH and AST activity. Fosinopril also prevented DOX-induced decreases in Ca2+ uptake in LV sarcoplasmic reticulum. | [192] |
| Mebudipine + Amlodipine (Calcium channel blockers) | 0.5 mg/kg/d Mebudipine + 0.35 mg/kg/d amlodipine for 14 days | 6 × 2.5 mg/kg | Male Wistar rats | 43 days | Mebudipine and amlodipine reversed the increased plasma BET-1, AST, CK-MB, and LDH caused by DOX treatment. | [199] |
| Spironolactone (Potassium-sparing diuretic) | 40 mg/kg/d for 14 days | 10 mg/kg/d for 14 days | Male Sprague-Dawley rats | 2 weeks | Spironolactone attenuated the decrease in LVEF and fractional shortening FS as well as the increase in cardiac fibrosis, apoptotic cell number, and cardiac collagen volume fraction caused by DOX treatment. | [208] |
| Empagliflozin (SGLT2 inhibitor) + Furosemide (Loop diuretic) | 10 mg/kg/d empagliflozin or 20 mg/kg/d furosemide for 35 days |
5 mg/kg/w for 5 weeks | Male C57Bl/6 mice | 6 weeks | Empagliflozin attenuated a decrease in LVEF, longitudinal shortening and circumferential strain and decreased myocardial fibrosis associated with DOX treatment. Furosemide did not have any effect. | [173] |
| Hydrochlorothiazide (Diuretic) | 10 mg/kg/d for 7 days | 3 mg/kg/w for 5 weeks | Wistar albino rats | 30 days | Co-treatment of DOX with Hydrochlorothiazide increased SOD and catalase activity and decrease TBARS levels compared to DOX alone. | [196] |
| Nifedipine (Calcium channel blocker) | 10 mg/kg/d for 14 days | 3 × 6 mg/kg | C57BL/J6 mice | 2 weeks | Nifedipine reduced DOX-induced cardiomyocyte injury and apoptosis as well as reduced phosphorylation of CaMKII and NF-κB. | [201] |
| Verapamil (Calcium channel blocker) | 25 mg/kg/d for 12 days | 12 × 5 mg/kg | Male Swiss albino mice | 4 weeks | Verapamil reduced DOX-induced cardiotoxicity through decreased levels of TNF-α and malondialdehyde in heart tissues coupled with decreased serum activity of CK-Mb and LDH. | [200] |
| Dapagliflozin (SGLT2 inhibitor) | 1 mg/kg/d for 14 days | 6 × 2.5 mg/kg | Male Sprague-Dawley albino rats | 15 days | Co-treatment of DOX with Dapagliflozin significantly reduced plasma cardiac troponin T, pro-BNP and TNF-α levels compared to DOX alone. | [174] |
| Empagliflozin (SGLT2 inhibitor) | 10 mg/kg/d for 10 days | 7 × 2.17 mg/kg/d | Female C57BL/6 mice | 1 week | Co-treatment of DOX with Empagliflozin increased EF and prevented the reduction of radial and longitudinal strain compared to DOX alone. Empagliflozin also reduced expression of pro-inflammatory cytokines, NLRP3, MyD88 and NF-κB in the heart. | [172] |
| Empagliflozin (SGLT2 inhibitor) | 10 mg/kg for 7 days | 7 × 2.57 mg/kg/ | Male Sprague-Dawley rats | 2 weeks | Co-treatment of DOX with empagliflozin lowered LVEDD and LVESD and infiltrative cell proliferation while increasing normal cell morphology and LVEF compared to DOX alone. | [24] |
ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; AST = aspartate amino transferase; CaMKII = Ca2+/calmodulin-dependent protein kinase II; CK-MB = creatine kinase myocardial band; DIC = DOX-induced cardiotoxicity; DOX = doxorubicin; eNOS = endothelial nitric oxide synthase; iNOS = inducible nitric oxide synthase; LDH = lactate dehydrogenase; LVEDD = left ventricular end-diastolic diameter; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic diameter; MyD88 = Myeloid differentiation primary response 88; NF-κB = nuclear factor kappa-B; NLRP3 = NLR family pyrin domain containing 3; pro-BNP = pro b-type natriuretic peptide; ROS = reactive oxygen species; SGLT2 = sodium/glucose transporter 2; SOD = superoxide dismutase; TBARS = thiobarbituric acid reactive substances; TFEB = transcription factor EB; TNF-α = Tumour necrosis factor alpha.
Other drugs used in treatment of diabetes are sodium-glucose cotransporter-2 (SGLT2) inhibitors. Randomized control trials highlight a cardioprotective role of these drugs in non-chemotherapeutic patients [169], [170], [171]. Current animal studies using the specific SGLT2 inhibitors empagliflozin and dapagliflozin indicate that these drugs can limit myocardial fibrosis and improve LVEF in the presence of DOX [24], [172], [173], [174]. This benefit was associated with reduced cellular expression of markers of cardiac injury and inflammation such as NLR family pyrin domain containing 3 (NLRP3), NF-κB and troponin T. The protective effects of SGLT2s are possibly related to their reduction of intracellular calcium and antioxidant properties [175], [176], [177], [178].
Statins such as atorvastatin, lovastatin and torvastatin have all been shown to reduce DOX-induced cardiotoxicity in mice at concentrations ranging from 3 mg/kg to 20 mg/kg (Table 2). While receiving the treatment prior and post-DOX has been shown to prevent cardiac damage associated with DOX, it is suggested that the prophylactic treatment is more effective [179]. The mechanism behind this protection is thought to relate to the anti-apoptotic, anti-inflammatory and antioxidant activities of the drugs [177], [179]. A retrospective study conducted in the USA suggests that co-treatment of statins with anthracyclines reduced hospitalization due to HF [180]. In contrast, a double-blind, randomized placebo study reported that atorvastatin did not affect the decline in LVEF consecutive to the DOX treatment [56]. Larger clinical trials are required to confirm the benefit of statins against DOX-induced cardiotoxicity.
Valsartan is an angiotensin receptor blocker commonly used in patients suffering from hypertension, heart failure and diabetic kidney disease [181]. In rodents, valsartan improves cardiac function in DOX-treated animals while others suggest that Valsartan alone is not enough to alleviate chemical damage caused by DOX [182], [183]. Indeed, valsartan, when given in combination with other drugs such as sacubitril and tolvaptan, significantly reduced cardiac damage caused by DOX [181], [182], [184]. Several clinical trials have confirmed the efficacy of sacubitril/valsartan in alleviating DOX induced HF by reducing LVEF when given during and after treatment with anthracyclines [93], [185], [186].
Angiotensin Converting Enzyme (ACE) inhibitors may also benefit cancer patients treated with anthracyclines. Randomized control studies have reported ACE inhibitors to reduce cardiotoxicity induced by chemotherapy, indicating the potential of these drugs to protect against DOX-induced cardiotoxicity [20], [53], [187]. Rodent studies using the ACE inhibitors enalapril, lisinopril, captopril, and fosinopril have identified possible preventative protective effects of these drugs against DOX-induced cardiotoxicity [188], [189], [190], [191], [192]. Clinical studies have led to mixed results. For example, in a randomized control study with a follow up period of 26 months, patients receiving enalapril with DOX or daunorubicin showed better outcomes in terms of cardiac biomarkers and reduction in LVEF compared to controls [193]. A multi-study cohort, with a maximum follow-up of 21 months reported no difference in LVEF or risk of new heart failure diagnosis when an ACE inhibitor was given in patients receiving anthracycline therapy [194]. More studies with longer follow up would be helpful to assess the effect of ACE inhibitors on chronic cardiotoxicity in DOX patients.
Diuretics are another first-line treatment for hypertension. In animals, eplerenone and furosemide showed no beneficial effect against DOX-induced cardiotoxicity [173], [189]. On the other hand, co-treatment of DOX with spironolactone in rodents showed promising reduction in cardiac biomarkers and an improvement in physical outcomes [195], [196]. In clinical studies, spironolactone protected against reduced LVEF caused by DOX treatment compared to controls [197]. Of important note, hypertensive patients receiving DOX and thiazide diuretics had higher prevalence of HF compared to patients receiving renin angiotensin system inhibitors and calcium channel blockers (CCBs) [198].
In rodent studies, mebudipine, amlodipine, nifedipine and verapamil were all protective against DOX-induced cardiotoxicity [199], [200], [201]. This protective mechanism most likely relates to DOX’s mechanism of increasing intracellular calcium by inhibiting sodium-calcium exchange channels and increasing calcium channel activity, ultimately leading to increased ROS, apoptosis and inflammation [see review [202]] [203]. Calcium channel blockers interact with this pathway, reducing intracellular calcium and presumably restoring some cellular functions by reducing ROS, apoptosis and inflammation.
In summary, trastuzumab and MRT appear to worsen DOX-induced cardiac damage while diabetic treatments metformin and SGLT2 inhibitors as well as lipid lowering statins reduce cardiac damage caused by DOX treatment. Hypertensive treatments including ARBS, ACE inhibitors diuretics and CCBs appear to have a protective effect against DOX-induced cardiotoxicity, it would be interesting for future studies to compare between these groups as some may be more protective than others. Finally, digoxin may worsen cardiotoxicity caused by DOX but additional studies are required to confirm these findings.
11. Conclusion
There is convincing evidence in the literature that many factors increase the risk of DOX-induced cardiotoxicity, although the mechanisms involved, and the degree of interaction is often unclear (Fig. 1).
Factors such as sex, age and ethnicity are the most complex to study. However, there is ample evidence to indicate that female sex is a risk factor in childhood. Aging positively correlates with increased risk of DOX-induced cardiotoxicity. While evidence suggests that individuals of African descent are more at risk of DOX-induced toxicity than others, whether this is due to genetic or social factors needs to be confirmed. Health complications such as diabetes, hypertension, dyslipidaemia, obesity, and cardiovascular disease are all likely candidates for risk factors of DOX-induced cardiotoxicity. Overall, knowledge on the effect of multiple factors that can increase the risk for DOX-induced cardiotoxicity are very valuable to favour a personalized approach in the treatment regime of breast cancer patients, with the aim to maximize the chances of recovery of the cancer patients while minimizing the risk of side-effects of the therapy.
12. Ethics approval and consent to participate
Not applicable.
13. Consent for publication
Not applicable.
Credit authorship contribution statement
Carl Belger: Conceptualization, Writing – original draft, Writing – review & editing. Carmelita Abrahams: Conceptualization, Supervision, Writing – review & editing. Aqeela Imamdin: Writing – review & editing. Sandrine Lecour: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The research of SL is supported by the the Cancer Association of South Africa, the South African National Research Foundation and the University of Cape Town.
References
- 1.World Health Organisation, Cancer, WHO Press, Geneva, 2021 [updated 21 September, Available from: https://www.who.int/news-room/fact-sheets/detail/cancer.
- 2.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Curry H., Parkes S., Powell J., Mann J. Caring for survivors of childhood cancers: the size of the problem. Eur. J. Cancer. 2006;42(4):501–508. doi: 10.1016/j.ejca.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Frias M.A., Lang U., Gerber-Wicht C., James R.W. Native and reconstituted HDL protect cardiomyocytes from doxorubicin-induced apoptosis. Cardiovasc. Res. 2010;85(1):118–126. doi: 10.1093/cvr/cvp289. [DOI] [PubMed] [Google Scholar]
- 5.Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer: Interdiscip. Int. J. Am. Cancer Soc. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 6.Cardinale D., Colombo A., Bacchiani G., Tedeschi I., Meroni C.A., Veglia F., et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 7.López-Sendón J., Álvarez-Ortega C., Zamora Auñon P., Buño Soto A., Lyon A.R., Farmakis D., et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur. Heart J. 2020;41(18):1720–1729. doi: 10.1093/eurheartj/ehaa006. [DOI] [PubMed] [Google Scholar]
- 8.Pardo Sanz A., Zamorano J.L. ‘Cardiotoxicity’: time to define new targets? Eur. Heart J. 2020;41(18):1730–1732. doi: 10.1093/eurheartj/ehaa013. [DOI] [PubMed] [Google Scholar]
- 9.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D., Aboyans V., Asteggiano R., Galderisi M., et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 10.Plana J.C., Galderisi M., Barac A., Ewer M.S., Ky B., Scherrer-Crosbie M., et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging. 2014;15(10):1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojtacki J., Lewicka-Nowak E., Lesniewski-Kmak K. Anthracycline-induced cardiotoxicity: clinical course, risk factors, pathogenesis, detection and prevention-review of the literature. Med. Sci. Monit. 2000;6(2):411–420. [PubMed] [Google Scholar]
- 12.Scully R.E., Lipshultz S.E. Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovasc. Toxicol. 2007;7(2):122–128. doi: 10.1007/s12012-007-0006-4. [DOI] [PubMed] [Google Scholar]
- 13.Saleh Y., Abdelkarim O., Herzallah K., Abela G.S. Anthracycline-induced cardiotoxicity: mechanisms of action, incidence, risk factors, prevention, and treatment. Heart Fail. Rev. 2021;26:1159–1173. doi: 10.1007/s10741-020-09968-2. [DOI] [PubMed] [Google Scholar]
- 14.Zito C., Manganaro R., Cusmà Piccione M., Madonna R., Monte I., Novo G., et al. Anthracyclines and regional myocardial damage in breast cancer patients. A multicentre study from the Working Group on Drug Cardiotoxicity and Cardioprotection, Italian Society of Cardiology (SIC) Eur. Heart J.-Cardiovasc. Imaging. 2021;22(4):406–415. doi: 10.1093/ehjci/jeaa339. [DOI] [PubMed] [Google Scholar]
- 15.de Barros Wanderley M.R., Jr, Ávila M.S., Fernandes-Silva M.M., das Dores Cruz F., Brandão S.M.G., Rigaud V.O.C., et al. Plasma biomarkers reflecting high oxidative stress in the prediction of myocardial injury due to anthracycline chemotherapy and the effect of carvedilol: insights from the CECCY Trial. Oncotarget. 2022;13 doi: 10.18632/oncotarget.28182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardinale D., Iacopo F., Cipolla C.M. Cardiotoxicity of anthracyclines. Front. Cardiovasc. Med. 2020;7 doi: 10.3389/fcvm.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pongprot Y., Sittiwangkul R., Charoenkwan P., Silvilairat S. Use of cardiac markers for monitoring of doxorubixin-induced cardiotoxicity in children with cancer. J. Pediatr. Hematol. Oncol. 2012;34(8):589–595. doi: 10.1097/MPH.0b013e31826faf44. [DOI] [PubMed] [Google Scholar]
- 18.De Iuliis F., Salerno G., Taglieri L., De Biase L., Lanza R., Cardelli P., et al. Serum biomarkers evaluation to predict chemotherapy-induced cardiotoxicity in breast cancer patients. Tumor Biol. 2016;37:3379–3387. doi: 10.1007/s13277-015-4183-7. [DOI] [PubMed] [Google Scholar]
- 19.Gulati G., Heck S.L., Røsjø H., Ree A.H., Hoffmann P., Hagve T.A., et al. Neurohormonal blockade and circulating cardiovascular biomarkers during anthracycline therapy in breast cancer patients: results from the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) study. J. Am. Heart Assoc. 2017;6(11) doi: 10.1161/JAHA.117.006513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulati G., Heck S.L., Ree A.H., Hoffmann P., Schulz-Menger J., Fagerland M.W., Gravdehaug B., von Knobelsdorff-Brenkenhoff F., Bratland Å., Storås T.H., Hagve T.A., Røsjø H., Steine K., Geisler J., Omland T. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur. Heart J. 2016;37(21):1671–1680. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu Y., Gao D., Xue J., Zuo Z. Galectin-3 and myeloperoxidase may monitor cancer-therapy-related cardiotoxicity? A systematic review and meta-analysis. Biomolecules. 2022;12(12):1788. doi: 10.3390/biom12121788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreyfuss A.D., Bravo P.E., Koumenis C., Ky B. Precision cardio-oncology. J. Nucl. Med. 2019;60(4):443–450. doi: 10.2967/jnumed.118.220137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madonna R. Early diagnosis and prediction of anticancer drug-induced cardiotoxicity: from cardiac imaging to “Omics” technologies. Revista Española de Cardiología (English Edition). 2017;70(7):576–582. doi: 10.1016/j.rec.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Barış V.Ö., Dinçsoy A.B., Gedikli E., Zırh S., Müftüoğlu S., Erdem A. Empagliflozin significantly prevents the doxorubicin-induced acute cardiotoxicity via non-antioxidant pathways. Cardiovasc. Toxicol. 2021;21(9):747–758. doi: 10.1007/s12012-021-09665-y. [DOI] [PubMed] [Google Scholar]
- 25.Renu K., Abilash V., Arunachalam S. Molecular mechanism of doxorubicin-induced cardiomyopathy–an update. Eur. J. Pharmacol. 2018;818:241–253. doi: 10.1016/j.ejphar.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y., Moon M., Dawood S., McManus B., Liu P. Mechanisms and management of doxorubicin cardiotoxicity. Herz. 2011;36(4):296–305. doi: 10.1007/s00059-011-3470-3. [DOI] [PubMed] [Google Scholar]
- 27.Menna P., Salvatorelli E., Minotti G. Cardiotoxicity of antitumor drugs. Chem. Res. Toxicol. 2008;21(5):978–989. doi: 10.1021/tx800002r. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Xiao M., Wang S., Guo Y., Tang Y., Gu J. Molecular mechanisms of doxorubicin-induced cardiotoxicity: novel roles of sirtuin 1-mediated signaling pathways. Cell. Mol. Life Sci. 2021:1–21. doi: 10.1007/s00018-020-03729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He H., Wang L., Qiao Y., Zhou Q., Li H., Chen S., et al. Doxorubicin induces endotheliotoxicity and mitochondrial dysfunction via ROS/eNOS/NO pathway. Front. Pharmacol. 2020;10 doi: 10.3389/fphar.2019.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minotti G., Ronchi R., Salvatorelli E., Menna P., Cairo G. Doxorubicin irreversibly inactivates iron regulatory proteins 1 and 2 in cardiomyocytes: evidence for distinct metabolic pathways and implications for iron-mediated cardiotoxicity of antitumor therapy. Cancer Res. 2001;61(23):8422–8428. [PubMed] [Google Scholar]
- 31.Minotti G., Cairo G., Monti E. Role of iron in anthracycline cardiotoxicity: new tunes for an old song? FASEB J. 1999;13(2):199–212. doi: 10.1096/fasebj.13.2.199. [DOI] [PubMed] [Google Scholar]
- 32.Odom A.L., Hatwig C.A., Stanley J.S., Benson A.M. Biochemical determinants of adriamycin® toxicity in mouse liver, heart and intestine. Biochem. Pharmacol. 1992;43(4):831–836. doi: 10.1016/0006-2952(92)90250-M. [DOI] [PubMed] [Google Scholar]
- 33.Sangomla S., Saifi M.A., Khurana A., Godugu C. Nanoceria ameliorates doxorubicin induced cardiotoxicity: possible mitigation via reduction of oxidative stress and inflammation. J. Trace Elem. Med Biol. 2018;47:53–62. doi: 10.1016/j.jtemb.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Abrahams C., Woudberg N.J., Lecour S. Anthracycline-induced cardiotoxicity: targeting high-density lipoproteins to limit the damage? Lipids Health Dis. 2022;21(1):1–16. doi: 10.1186/s12944-022-01694-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J., Mao W., Ding B., Liang C.-S. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. Am. J. Physiol.-Heart Circul. Physiol. 2008;295(5):H1956–H1965. doi: 10.1152/ajpheart.00407.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aries A., Paradis P., Lefebvre C., Schwartz R.J., Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc. Natl. Acad. Sci. 2004;101(18):6975–6980. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park A.-M., Nagase H., Liu L., Vinod Kumar S., Szwergold N., Wong C.-M., et al. Mechanism of anthracycline-mediated down-regulation of GATA4 in the heart. Cardiovasc. Res. 2011;90(1):97–104. doi: 10.1093/cvr/cvq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poizat C., Puri P.L., Bai Y., Kedes L. Phosphorylation-dependent degradation of p300 by doxorubicin-activated p38 mitogen-activated protein kinase in cardiac cells. Mol. Cell. Biol. 2005;25(7):2673–2687. doi: 10.1128/MCB.25.7.2673-2687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranek M.J., Wang X. Activation of the ubiquitin-proteasome system in doxorubicin cardiomyopathy. Curr. Hypertens. Rep. 2009;11(6):389. doi: 10.1007/s11906-009-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tokarska-Schlattner M., Zaugg M., Zuppinger C., Wallimann T., Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. J. Mol. Cell. Cardiol. 2006;41(3):389–405. doi: 10.1016/j.yjmcc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Unverferth D., Magorien R., Unverferth B., Talley R., Balcerzak S., Baba N. Human myocardial morphologic and functional changes in the first 24 hours after doxorubicin administration. Cancer Treat. Rep. 1981;65(11–12):1093–1097. [PubMed] [Google Scholar]
- 42.Ascensão A., Magalhães J., Soares J.M., Ferreira R., Neuparth M.J., Marques F., et al. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am. J. Physiol.-Heart Circul. Physiol. 2005;289(2):H722–H731. doi: 10.1152/ajpheart.01249.2004. [DOI] [PubMed] [Google Scholar]
- 43.Minotti G., Menna P., Salvatorelli E., Cairo G., Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 44.Sardão V.A., Oliveira P.J., Holy J., Oliveira C.R., Wallace K.B. Morphological alterations induced by doxorubicin on H9c2 myoblasts: nuclear, mitochondrial, and cytoskeletal targets. Cell Biol. Toxicol. 2009;25(3):227–243. doi: 10.1007/s10565-008-9070-1. [DOI] [PubMed] [Google Scholar]
- 45.Cui N., Wu F., Lu W.J., Bai R., Ke B., Liu T., et al. Doxorubicin-induced cardiotoxicity is maturation dependent due to the shift from topoisomerase IIα to IIβ in human stem cell derived cardiomyocytes. J. Cell Mol. Med. 2019;23(7):4627–4639. doi: 10.1111/jcmm.14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durbecq V., Paesmans M., Cardoso F., Desmedt C., Di Leo A., Chan S., et al. Topoisomerase-IIα expression as a predictive marker in a population of advanced breast cancer patients randomly treated either with single-agent doxorubicin or single-agent docetaxel. Mol. Cancer Ther. 2004;3(10):1207–1214. doi: 10.1158/1535-7163.1207.3.10. [DOI] [PubMed] [Google Scholar]
- 47.Omland T., Heck S.L., Gulati G. The role of cardioprotection in cancer therapy cardiotoxicity: JACC: CardioOncology state-of-the-art review. Cardio Oncol. 2022;4(1):19–37. doi: 10.1016/j.jaccao.2022.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith L.A., Cornelius V.R., Plummer C.J., Levitt G., Verrill M., Canney P., et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10(1):1–14. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Dalen E.C., Michiels E.M., Caron H.N., Kremer L.C. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst. Rev. 2006;4 doi: 10.1002/14651858.CD005006.pub4. [DOI] [PubMed] [Google Scholar]
- 50.Rafiyath S.M., Rasul M., Lee B., Wei G., Lamba G., Liu D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp. Hematol. Oncol. 2012;1:1–9. doi: 10.1186/2162-3619-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asselin B.L., Devidas M., Chen L., Franco V.I., Pullen J., Borowitz M.J., et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404. J. Clin. Oncol. 2016;34(8):854. doi: 10.1200/JCO.2015.60.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macedo A.V., Hajjar L.A., Lyon A.R., Nascimento B.R., Putzu A., Rossi L., et al. Efficacy of dexrazoxane in preventing anthracycline cardiotoxicity in breast cancer. Cardio Oncol. 2019;1(1):68–79. doi: 10.1016/j.jaccao.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardinale D., Colombo A., Sandri M.T., Lamantia G., Colombo N., Civelli M., et al. Prevention of high-dose chemotherapy–induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114(23):2474–2481. doi: 10.1161/CIRCULATIONAHA.106.635144. [DOI] [PubMed] [Google Scholar]
- 54.Kalay N., Basar E., Ozdogru I., Er O., Cetinkaya Y., Dogan A., et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J. Am. Coll. Cardiol. 2006;48(11):2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 55.Heck S.L., Mecinaj A., Ree A.H., Hoffmann P., Schulz-Menger J., Fagerland M.W., et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): extended follow-up of a 2× 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Circulation. 2021;143(25):2431–2440. doi: 10.1161/CIRCULATIONAHA.121.054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hundley W.G., D’Agostino R., Crotts T., Craver K., Hackney M.H., Jordan J.H., et al. Statins and left ventricular ejection fraction following doxorubicin treatment. NEJM Evid. 2022;1(9) doi: 10.1056/evidoa2200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avila M.S., Ayub-Ferreira S.M., de Barros Wanderley M.R., das Dores Cruz F., Goncalves Brandao S.M., Rigaud V.O.C., et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J. Am. Coll. Cardiol. 2018;71(20):2281–2290. doi: 10.1016/j.jacc.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 58.Sheppard R.J., Berger J., Sebag I.A. Cardiotoxicity of cancer therapeutics: current issues in screening, prevention, and therapy. Front. Pharmacol. 2013;4 doi: 10.3389/fphar.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El-Kareh A.W., Secomb T.W. A mathematical model for comparison of bolus injection, continuous infusion, and liposomal delivery of doxorubicin to tumor cells. Neoplasia. 2000;2(4):325–338. doi: 10.1038/sj.neo.7900096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.British Heart Foundation, Heart Statistics, 2021, Available from: https://www.bhf.org.uk/what-we-do/our-research/heart-statistics.
- 61.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 62.Dessalvi C.C., Pepe A., Penna C., Gimelli A., Madonna R., Mele D., et al. Sex differences in anthracycline-induced cardiotoxicity: the benefits of estrogens. Heart Fail. Rev. 2019;24(6):915–925. doi: 10.1007/s10741-019-09820-2. [DOI] [PubMed] [Google Scholar]
- 63.Lin D.-Y., Tsai F.-J., Tsai C.-H., Huang C.-Y. Mechanisms governing the protective effect of 17β-estradiol and estrogen receptors against cardiomyocyte injury. Biomedicine. 2011;1(1):21–28. [Google Scholar]
- 64.Filardo E.J., Quinn J.A., Bland K.I., Frackelton A.R., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14(10):1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 65.Patten R.D., Pourati I., Aronovitz M.J., Baur J., Celestin F., Chen X., et al. 17β-Estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ. Res. 2004;95(7):692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 66.Ventura-Clapier R., Moulin M., Piquereau J., Zurlo G., Garnier A. Sex differences in anthracycline cardiotoxicity. Ital. J. Gend.-Specif. Med. 2016;2(2):47–54. [Google Scholar]
- 67.Isensee J., Witt H., Pregla R., Hetzer R., Regitz-Zagrosek V., Noppinger P.R. Sexually dimorphic gene expression in the heart of mice and men. J. Mol. Med. 2008;86(1):61–74. doi: 10.1007/s00109-007-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jang Y.M., Kendaiah S., Drew B., Phillips T., Selman C., Julian D., et al. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett. 2004;577(3):483–490. doi: 10.1016/j.febslet.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 69.Zeiss C.J., Gatti D.M., Toro-Salazar O., Davis C., Lutz C.M., Spinale F., et al. Doxorubicin-induced cardiotoxicity in collaborative cross (CC) mice recapitulates individual cardiotoxicity in humans. G3: Genes Genomes Genet. 2019;9(8):2637–2646. doi: 10.1534/g3.119.400232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jenkins G.R., Lee T., Moland C.L., Vijay V., Herman E.H., Lewis S.M., et al. Sex-related differential susceptibility to doxorubicin-induced cardiotoxicity in B6C3F1 mice. Toxicol. Appl. Pharmacol. 2016;310:159–174. doi: 10.1016/j.taap.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 71.Moulin M., Piquereau J., Mateo P., Fortin D., Rucker-Martin C., Gressette M., et al. Sexual dimorphism of doxorubicin-mediated cardiotoxicity: potential role of energy metabolism remodelling. Circul. Heart Failure. 2015;8(1):98–108. doi: 10.1161/CIRCHEARTFAILURE.114.001180. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J., Knapton A., Lipshultz S.E., Cochran T.R., Hiraragi H., Herman E.H. Sex-related differences in mast cell activity and doxorubicin toxicity: a study in spontaneously hypertensive rats. Toxicol. Pathol. 2014;42(2):361–375. doi: 10.1177/0192623313482778. [DOI] [PubMed] [Google Scholar]
- 73.Zordoky B.N., Radin M.J., Heller L., Tobias A., Matise I., Apple F.S., et al. The interplay between genetic background and sexual dimorphism of doxorubicin-induced cardiotoxicity. Cardio-Oncol. 2016;2(1):1–11. doi: 10.1186/s40959-016-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calvé A., Haddad R., Barama S.-N., Meilleur M., Sebag I.A., Chalifour L.E. Cardiac response to doxorubicin and dexrazoxane in intact and ovariectomized young female rats at rest and after swim training. Am. J. Physiol.-Heart Circul. Physiol. 2012;302(10):H2048–H2057. doi: 10.1152/ajpheart.01069.2011. [DOI] [PubMed] [Google Scholar]
- 75.Phungphong S., Kijtawornrat A., Kampaengsri T., Wattanapermpool J., Bupha-Intr T. Comparison of exercise training and estrogen supplementation on mast cell-mediated doxorubicin-induced cardiotoxicity. Am. J. Physiol.-Regul. Integr. Comparative Physiol. 2020;318(5):R829–R842. doi: 10.1152/ajpregu.00224.2019. [DOI] [PubMed] [Google Scholar]
- 76.Agostinucci K., Grant M.K., Melaku W., Nair C., Zordoky B.N. Exposure to doxorubicin modulates the cardiac response to isoproterenol in male and female mice. Pharmaceuticals. 2023;16(3) doi: 10.3390/ph16030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rattanasopa C., Kirk J.A., Bupha-Intr T., Papadaki M., de Tombe P.P., Wattanapermpool J. Estrogen but not testosterone preserves myofilament function from doxorubicin-induced cardiotoxicity by reducing oxidative modifications. Am. J. Physiol.-Heart Circul. Physiol. 2019 doi: 10.1152/ajpheart.00428.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silber J.H., Jakacki R.I., Larsen R.L., Goldwein J.W., Barber G. Increased risk of cardiac dysfunction after anthracyclines in girls. Med. Pediatr. Oncol. 1993;21(7):477–479. doi: 10.1002/mpo.2950210704. [DOI] [PubMed] [Google Scholar]
- 79.Krischer J.P., Epstein S., Cuthbertson D.D., Goorin A.M., Epstein M.L., Lipshultz S.E. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J. Clin. Oncol. 1997;15(4):1544–1552. doi: 10.1200/JCO.1997.15.4.1544. [DOI] [PubMed] [Google Scholar]
- 80.Armstrong G.T., Chen Y., Yasui Y., Leisenring W., Gibson T.M., Mertens A.C., et al. Reduction in late mortality among 5-year survivors of childhood cancer. N. Engl. J. Med. 2016;374(9):833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Biro F.M., Pinney S.M., Huang B., Baker E.R., Walt Chandler D., Dorn L.D. Hormone changes in peripubertal girls. J. Clin. Endocrinol. Metab. 2014;99(10):3829–3835. doi: 10.1210/jc.2013-4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobbs N., Twelves C., Gillies H., James C., Harper P., Rubens R. Gender affects doxorubicin pharmacokinetics in patients with normal liver biochemistry. Cancer Chemother. Pharmacol. 1995;36:473–476. doi: 10.1007/BF00685796. [DOI] [PubMed] [Google Scholar]
- 83.Rodvold K.A., Rushing D.A., Tewksbury D.A. Doxorubicin clearance in the obese. J. Clin. Oncol. 1988;6(8):1321–1327. doi: 10.1200/JCO.1988.6.8.1321. [DOI] [PubMed] [Google Scholar]
- 84.Pein F., Sakiroglu O., Dahan M., Lebidois J., Merlet P., Shamsaldin A., et al. Cardiac abnormalities 15 years and more after adriamycin therapy in 229 childhood survivors of a solid tumour at the Institut Gustave Roussy. Br. J. Cancer. 2004;91(1):37–44. doi: 10.1038/sj.bjc.6601904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Dalen E.C., van der Pal H.J., Kok W.E., Caron H.N., Kremer L.C. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur. J. Cancer. 2006;42(18):3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 86.Lipshultz S.E., Lipsitz S.R., Mone S.M., Goorin A.M., Sallan S.E., Sanders S.P., et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N. Engl. J. Med. 1995;332(26):1738–1744. doi: 10.1056/nejm199506293322602. [DOI] [PubMed] [Google Scholar]
- 87.Myrehaug S., Pintilie M., Yun L., Crump M., Tsang R.W., Meyer R.M., et al. A population-based study of cardiac morbidity among Hodgkin lymphoma patients with preexisting heart disease. Blood, J. Am. Soc. Hematol. 2010;116(13):2237–2240. doi: 10.1182/blood-2010-01-263764. [DOI] [PubMed] [Google Scholar]
- 88.Hequet O., Le Q., Moullet I., Pauli E., Salles G., Espinouse D., et al. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J. Clin. Oncol. 2004;22(10):1864–1871. doi: 10.1200/JCO.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 89.Wang L., Tan T.C., Halpern E.F., Neilan T.G., Francis S.A., Picard M.H., et al. Major cardiac events and the value of echocardiographic evaluation in patients receiving anthracycline-based chemotherapy. Am. J. Cardiol. 2015;116(3):442–446. doi: 10.1016/j.amjcard.2015.04.064. [DOI] [PubMed] [Google Scholar]
- 90.Zgardau A., Ray J.G., Baxter N.N., Nagamuthu C., Park A.L., Gupta S., et al. Obstetrical and perinatal outcomes in female survivors of childhood and adolescent cancer: a population-based cohort study. JNCI: J. Natl. Cancer Inst. 2022;114(4):553–564. doi: 10.1093/jnci/djac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heemelaar J.C., Speetjens F.M., Al Jaff A.A., Evenhuis R.E., Polomski E.A., Mertens B.J., et al. Impact of age at diagnosis on cardiotoxicity in high-grade osteosarcoma and Ewing sarcoma patients. Cardio Oncol. 2023;5(1):117–127. doi: 10.1016/j.jaccao.2022.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bar J., Davidi O., Goshen Y., Hod M., Yaniv I., Hirsch R. Pregnancy outcome in women treated with doxorubicin for childhood cancer. Am. J. Obstet. Gynecol. 2003;189(3):853–857. doi: 10.1067/S0002-9378(03)00837-8. [DOI] [PubMed] [Google Scholar]
- 93.Abdin A., Schulz M., Riemer U., Hadëri B., Wachter R., Laufs U., et al. Sacubitril/valsartan in heart failure: efficacy and safety in and outside clinical trials. ESC Heart Failure. 2022;9(6):3737–3750. doi: 10.1002/ehf2.14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meiners B., Shenoy C., Zordoky B.N. Clinical and preclinical evidence of sex-related differences in anthracycline-induced cardiotoxicity. Biol. Sex Differ. 2018;9(1):1–12. doi: 10.1186/s13293-018-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Insitute NC, Age and Cancer Risk, 2017, Available from: https://www.cancer.gov/about-cancer/causes-prevention/risk/age.
- 96.Mancilla T.R., Iskra B., Aune G.J. Doxorubicin-induced cardiomyopathy in children. Compreh. Physiol. 2019;9(3):905. doi: 10.1002/cphy.c180017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi J., Zhang L., Zhang Y.-W., Surma M., Mark Payne R., Wei L. Downregulation of doxorubicin-induced myocardial apoptosis accompanies postnatal heart maturation. Am. J. Physiol.-Heart Circul. Physiol. 2012;302(8):H1603–H1613. doi: 10.1152/ajpheart.00844.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Accordino M.K., Neugut A.I., Hershman D.L. Cardiac effects of anticancer therapy in the elderly. J. Clin. Oncol. 2014;32(24):2654. doi: 10.1200/JCO.2013.55.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmadian M., Roshan V.D. Modulatory effect of aerobic exercise training on doxorubicin-induced cardiotoxicity in rats with different ages. Cardiovasc. Toxicol. 2018;18(1):33–42. doi: 10.1007/s12012-017-9411-5. [DOI] [PubMed] [Google Scholar]
- 100.Von Hoff D.D., Layard M.W., Basa P., Davis H.L., Jr, Von Hoff A.L., Rozencweig M., et al. Risk factors for doxorubicin-lnduced congestive heart failure. Ann. Intern. Med. 1979;91(5):710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 101.Robert J., Hoerni B. Age dependence of the early-phase pharmacokinetics of doxorubicin. Cancer Res. 1983;43(9):4467–4469. [PubMed] [Google Scholar]
- 102.Cho H., Lee S., Sim S.H., Park I.H., Lee K.S., Kwak M.H., et al. Cumulative incidence of chemotherapy-induced cardiotoxicity during a 2-year follow-up period in breast cancer patients. Breast Cancer Res. Treat. 2020;182:333–343. doi: 10.1007/s10549-020-05703-5. [DOI] [PubMed] [Google Scholar]
- 103.Lipshultz S.E., Lipsitz S.R., Sallan S.E., Dalton V.M., Mone S.M., Gelber R.D., et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2005;23(12):2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 104.Buzdar A.U., Marcus C., Blumenschein G.R., Smith T.L. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer. 1985;55(12):2761–2765. doi: 10.1002/1097-0142(19850615)55:12%3C2761::AID-CNCR2820551206%3E3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 105.George J., Mathur R., Shah A.D., Pujades-Rodriguez M., Denaxas S., Smeeth L., et al. Ethnicity and the first diagnosis of a wide range of cardiovascular diseases: associations in a linked electronic health record cohort of 1 million patients. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0178945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Virani S.S., Alonso A., Aparicio H.J., Benjamin E.J., Bittencourt M.S., Callaway C.W., et al. Heart disease and stroke statistics—2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 107.Chaturvedi N. Ethnic differences in cardiovascular disease. Heart. 2003;89(6):681–686. doi: 10.1136/heart.89.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chandra A., Skali H., Claggett B., Solomon S.D., Rossi J.S., Russell S.D., et al. Race-and gender-based differences in cardiac structure and function and risk of heart failure. J. Am. Coll. Cardiol. 2022;79(4):355–368. doi: 10.1016/j.jacc.2021.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hasan S., Dinh K., Lombardo F., Kark J. Doxorubicin cardiotoxicity in African Americans. J. Natl Med. Assoc. 2004;96(2):196. [PMC free article] [PubMed] [Google Scholar]
- 110.Sapkota Y., Qin N., Ehrhardt M.J., Wang Z., Chen Y., Wilson C.L., et al. Genetic variants associated with therapy-related cardiomyopathy among childhood cancer survivors of African ancestry. Cancer Res. 2021;81(9):2556–2565. doi: 10.1158/0008-5472.CAN-20-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.L. Zhang, J. Song, R. Clark, A. Mangoni, Y. Goldberg, L. Slipczuk, et al., Racial and ethnic differences in anthracycline cardiotoxicity, Circulation 144 (Suppl_1) (2021) A13090.
- 112.Tan V.Z.Z., Chan N.M., Ang W.L., Mya S.N., Chan M.Y., Chen C.K. Cardiotoxicity after anthracycline chemotherapy for childhood cancer in a multiethnic Asian population. Front. Pediatr. 2021;9 doi: 10.3389/fped.2021.639603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fazal M., Malisa J., Rhee J.-W., Witteles R.M., Rodriguez F. Racial and ethnic disparities in cardio-oncology: a call to action. Cardio Oncol. 2021;3(2):201–204. doi: 10.1016/j.jaccao.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.WHO. Diabetes [fact sheet], 2021, Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes.
- 115.Nanayakkara N., Curtis A.J., Heritier S., Gadowski A.M., Pavkov M.E., Kenealy T., et al. Impact of age at type 2 diabetes mellitus diagnosis on mortality and vascular complications: systematic review and meta-analyses. Diabetologia. 2020:1–13. doi: 10.1007/s00125-020-05319-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Boudina S., Abel E.D. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010;11:31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bugger H., Abel E.D. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57(4):660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Lima Junior E.A., Yamashita A.S., Pimentel G.D., De Sousa L.G., Santos R.V.T., Gonçalves C.L., et al. Doxorubicin caused severe hyperglycaemia and insulin resistance, mediated by inhibition in AMPk signalling in skeletal muscle. J. Cachexia. Sarcopenia Muscle. 2016;7(5):615–625. doi: 10.1002/jcsm.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Supriya R., Tam B.T., Pei X.M., Lai C.W., Chan L.W., Yung B.Y., et al. Doxorubicin induces inflammatory modulation and metabolic dysregulation in diabetic skeletal muscle. Front. Physiol. 2016;7 doi: 10.3389/fphys.2016.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pei X.M., Tam B.T., Sin T.K., Wang F.F., Yung B.Y., Chan L.W., et al. S100A8 and S100A9 are associated with doxorubicin-induced cardiotoxicity in the heart of diabetic mice. Front. Physiol. 2016;7 doi: 10.3389/fphys.2016.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hershman D.L., Eisenberger A., Wang J., Jacobson J., Grann V., McBride R., et al. Doxorubicin, cardiac risk factors and cardiac toxicity in elderly patients with diffuse b-cell non-Hodgkin's lymphoma [Abstract] J. Clin. Oncol. 2007;25(18_suppl) doi: 10.1200/jco.2007.25.18_suppl.9050. [DOI] [PubMed] [Google Scholar]
- 122.Qin A., Thompson C.L., Silverman P. Predictors of late-onset heart failure in breast cancer patients treated with doxorubicin. J. Cancer Surviv. 2015;9(2):252–259. doi: 10.1007/s11764-014-0408-9. [DOI] [PubMed] [Google Scholar]
- 123.Arunachalam S., Pichiah P.T., Achiraman S. Doxorubicin treatment inhibits PPARγ and may induce lipotoxicity by mimicking a type 2 diabetes-like condition in rodent models. FEBS Lett. 2013;587(2):105–110. doi: 10.1016/j.febslet.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 124.Olorundare O.E., Adeneye A.A., Akinsola A.O., Sanni D.A., Koketsu M., Mukhtar H. Clerodendrum volubile ethanol leaf extract: a potential antidote to doxorubicin-induced cardiotoxicity in rats. J. Toxicol. 2020;2020 doi: 10.1155/2020/8859716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hao E., Mukhopadhyay P., Cao Z., Erdélyi K., Holovac E., Liaudet L., et al. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol. Med. 2015;21(1):38–45. doi: 10.2119/molmed.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cai Z., Xie Q., Hu T., Yao Q., Zhao J., Wu Q., et al. S100A8/A9 in myocardial infarction: a promising biomarker and therapeutic target. Front. Cell Dev. Biol. 2020:1310. doi: 10.3389/fcell.2020.603902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodríguez Á.D. Guidelines for the management of dyslipidemia. SEMERGEN. 2014;40:19–25. doi: 10.1016/s1138-3593(14)74393-x. [DOI] [PubMed] [Google Scholar]
- 128.Mi T., Sun S., Zhang G., Carora Y., Du Y., Guo S., et al. Relationship between dyslipidemia and carotid plaques in a high-stroke-risk population in Shandong Province, China. Brain Behavior. 2016;6(6) doi: 10.1002/brb3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shah F.D., Shukla S.N., Shah P.M., Patel H.R., Patel P.S. Significance of alterations in plasma lipid profile levels in breast cancer. Integr. Cancer Ther. 2008;7(1):33–41. doi: 10.1177/1534735407313883. [DOI] [PubMed] [Google Scholar]
- 130.Klinnikova M., Lushnikova E., Koldysheva E., Tolstikova T., Sorokina I., Yuzhik E., et al. Cardiotoxic and dyslipidemic effects of doxorubicin and betulinic acid amide. Bull. Exp. Biol. Med. 2016;162(2):277–282. doi: 10.1007/s10517-016-3594-9. [DOI] [PubMed] [Google Scholar]
- 131.Haybar H., Goudarzi M., Mehrzadi S., Aminzadeh A., Khodayar M.J., Kalantar M., et al. Effect of gemfibrozil on cardiotoxicity induced by doxorubicin in male experimental rats. Biomed. Pharmacother. 2019;109:530–535. doi: 10.1016/j.biopha.2018.10.101. [DOI] [PubMed] [Google Scholar]
- 132.Sandamali J.A.N., Hewawasam R.P., Fernando M.A.C.S.S., Jayatilaka K.A.P.W., Madurawe R.D., Sathananthan P.P., et al. Anthracycline-induced cardiotoxicity in breast cancer patients from Southern Sri Lanka: an echocardiographic analysis. Biomed Res. Int. 2020;2020 doi: 10.1155/2020/1847159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borowiec A., Ozdowska P., Rosinska M., Jagiello-Gruszfeld A., Jasek S., Waniewska J., et al. Prognostic value of coronary atherosclerosis and CAC score for the risk of chemotherapy-related cardiac dysfunction (CTRCD): The protocol of ANTEC study. PLoS One. 2023;18(8) doi: 10.1371/journal.pone.0288146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Salz T., Zabor E.C., de Nully Brown P., Dalton S.O., Raghunathan N.J., Matasar M.J., et al. Preexisting cardiovascular risk and subsequent heart failure among non-Hodgkin lymphoma survivors. J. Clin. Oncol. 2017;35(34):3837. doi: 10.1200/JCO.2017.72.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.World Health Organization, Obesity and Overweight, WHO Press, Geneva, 2021, Available from: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight.
- 136.Mitra M.S., Donthamsetty S., White B., Mehendale H.M. High fat diet-fed obese rats are highly sensitive to doxorubicin-induced cardiotoxicity. Toxicol. Appl. Pharmacol. 2008;231(3):413–422. doi: 10.1016/j.taap.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 137.Méjean C., Droomers M., Van Der Schouw Y.T., Sluijs I., Czernichow S., Grobbee D.E., et al. The contribution of diet and lifestyle to socioeconomic inequalities in cardiovascular morbidity and mortality. Int. J. Cardiol. 2013;168(6):5190–5195. doi: 10.1016/j.ijcard.2013.07.188. [DOI] [PubMed] [Google Scholar]
- 138.Guenancia C., Hachet O., Aboutabl M., Li N., Rigal E., Cottin Y., et al. Overweight in mice, induced by perinatal programming, exacerbates doxorubicin and trastuzumab cardiotoxicity. Cancer Chemother. Pharmacol. 2016;77:777–785. doi: 10.1007/s00280-016-2995-9. [DOI] [PubMed] [Google Scholar]
- 139.Guenancia C., Lefebvre A., Cardinale D., Yu A.F., Ladoire S., Ghiringhelli F., et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J. Clin. Oncol. 2016;34(26):3157. doi: 10.1200/JCO.2016.67.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kaboré E.G., Guenancia C., Vaz-Luis I., Di Meglio A., Pistilli B., Coutant C., et al. Association of body mass index and cardiotoxicity related to anthracyclines and trastuzumab in early breast cancer: French CANTO cohort study. PLoS Med. 2019;16(12) doi: 10.1371/journal.pmed.1002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Qiu S., Zhou T., Qiu B., Zhang Y., Zhou Y., Yu H., et al. Risk factors for anthracycline-induced cardiotoxicity. Front. Cardiovasc. Med. 2021:1174. doi: 10.3389/fcvm.2021.736854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thompson P.A., Rosner G.L., Matthay K.K., Moore T.B., Bomgaars L.R., Ellis K.J., et al. Impact of body composition on pharmacokinetics of doxorubicin in children: a Glaser Pediatric Research Network study. Cancer Chemother. Pharmacol. 2009;64(2):243. doi: 10.1007/s00280-008-0854-z. [DOI] [PubMed] [Google Scholar]
- 143.Maruyama S., Shibata R., Ohashi K., Ohashi T., Daida H., Walsh K., et al. Adiponectin ameliorates doxorubicin-induced cardiotoxicity through Akt protein-dependent mechanism. J. Biol. Chem. 2011;286(37):32790–32800. doi: 10.1074/jbc.M111.245985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ahima R.S., Lazar M.A. The health risk of obesity—better metrics imperative. Science. 2013;341(6148):856–858. doi: 10.1126/science.1241244. [DOI] [PubMed] [Google Scholar]
- 145.Carnethon M.R., De Chavez P.J.D., Biggs M.L., Lewis C.E., Pankow J.S., Bertoni A.G., et al. Association of weight status with mortality in adults with incident diabetes. J. Am. Med. Assoc. 2012;308(6):581–590. doi: 10.1001/jama.2012.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hazari M.S., Haykal-Coates N., Winsett D.W., Costa D.L., Farraj A.K. Continuous electrocardiogram reveals differences in the short-term cardiotoxic response of Wistar-Kyoto and spontaneously hypertensive rats to doxorubicin. Toxicol. Sci. 2009;110(1):224–234. doi: 10.1093/toxsci/kfp092. [DOI] [PubMed] [Google Scholar]
- 147.Sharkey L.C., Radin M.J., Heller L., Rogers L.K., Tobias A., Matise I., et al. Differential cardiotoxicity in response to chronic doxorubicin treatment in male spontaneous hypertension-heart failure (SHHF), spontaneously hypertensive (SHR), and Wistar Kyoto (WKY) rats. Toxicol. Appl. Pharmacol. 2013;273(1):47–57. doi: 10.1016/j.taap.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 148.Vaitiekus D., Muckiene G., Vaitiekiene A., Maciuliene D., Vaiciuliene D., Ambrazeviciute G., et al. Impact of arterial hypertension on doxorubicin-based chemotherapy-induced subclinical cardiac damage in breast cancer patients. Cardiovasc. Toxicol. 2020;20(3):321–327. doi: 10.1007/s12012-019-09556-3. [DOI] [PubMed] [Google Scholar]
- 149.Kim Y.A., Cho H., Lee N., Jung S.Y., Sim S.H., Park I.H., et al. Doxorubicin-induced heart failure in cancer patients: a cohort study based on the Korean National Health Insurance Database. Cancer Med. 2018;7(12):6084–6092. doi: 10.1002/cam4.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Philip L.J., Findlay S.G., Gill J.H. Baseline blood pressure and development of cardiotoxicity in patients treated with anthracyclines: a systematic review. Int. J. Cardiol. Cardiovasc. Risk Prevent. 2022 doi: 10.1016/j.ijcrp.2022.200153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paulenka Y., Cotto M., Rickette C., Hunter K. Latent atherosclerosis as a risk factor in chemotherapy-induced cardiomyopathy [Abstract] J. Clin. Oncol. 2021;39 doi: 10.1200/JCO.2021.39.15_suppl.e12525. [DOI] [Google Scholar]
- 152.Paulenka Y., Cotto M., Rickette C., Hunter K. Wolters Kluwer Health; 2021. Latent Atherosclerosis as a Risk Factor in Chemotherapy-Induced Cardiomyopathy. [Google Scholar]
- 153.Hudis C.A. Trastuzumab—mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 154.Slamon D.J., Leyland-Jones B., Shak S., Fuchs H., Paton V., Bajamonde A., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 155.Anjos M., Fontes-Oliveira M., Costa V.M., Santos M., Ferreira R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci. 2021;280 doi: 10.1016/j.lfs.2021.119760. [DOI] [PubMed] [Google Scholar]
- 156.Gianni L., Salvatorelli E., Minotti G. Anthracycline cardiotoxicity in breast cancer patients: synergism with trastuzumab and taxanes. Cardiovasc. Toxicol. 2007;7:67–71. doi: 10.1007/s12012-007-0013-5. [DOI] [PubMed] [Google Scholar]
- 157.Jerusalem G., Lancellotti P., Kim S.-B. HER2+ breast cancer treatment and cardiotoxicity: monitoring and management. Breast Cancer Res. Treat. 2019;177:237–250. doi: 10.1007/s10549-019-05303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zeglinski M., Ludke A., Jassal D.S., Singal P.K. Trastuzumab-induced cardiac dysfunction: a ‘dual-hit’. Exp. Clin. Cardiol. 2011;16(3):70. [PMC free article] [PubMed] [Google Scholar]
- 159.Zablocki D., Sadoshima J. Angiotensin II and oxidative stress in the failing heart. Antioxid. Redox Signal. 2013;19(10):1095–1109. doi: 10.1089/ars.2012.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jiang J., Mohan N., Endo Y., Shen Y., Wu W.J. Type IIB DNA topoisomerase is downregulated by trastuzumab and doxorubicin to synergize cardiotoxicity. Oncotarget. 2018;9(5):6095. doi: 10.18632/oncotarget.23543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Myrehaug S., Pintilie M., Tsang R., Mackenzie R., Crump M., Chen Z., et al. Cardiac morbidity following modern treatment for Hodgkin lymphoma: supra-additive cardiotoxicity of doxorubicin and radiation therapy. Leuk. Lymphoma. 2008;49(8):1486–1493. doi: 10.1080/10428190802140873. [DOI] [PubMed] [Google Scholar]
- 162.Reeves W.C., Griffith J.W., Wood M.A., Whitesell L. Exacerbation of doxorubicin cardiotoxicity by digoxin administration in an experimental rabbit model. Int. J. Cancer. 1990;45(4):731–736. doi: 10.1002/ijc.2910450427. [DOI] [PubMed] [Google Scholar]
- 163.Wang Y., Ma Q., Zhang S., Liu H., Zhao B., Du B., et al. Digoxin enhances the anticancer effect on non-small cell lung cancer while reducing the cardiotoxicity of adriamycin. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Argun M., Üzüm K., Sönmez M.F., Özyurt A., Karabulut D., Soyersarica Z., et al. Cardioprotective effect of metformin against doxorubicin cardiotoxicity in rats. Anat. J. Cardiol. 2016;16(4):234. doi: 10.5152/akd.2015.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zilinyi R., Czompa A., Czegledi A., Gajtko A., Pituk D., Lekli I., et al. The cardioprotective effect of metformin in doxorubicin-induced cardiotoxicity: the role of autophagy. Molecules. 2018;23(5) doi: 10.3390/molecules23051184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kelleni M.T., Amin E.F., Abdelrahman A.M. Effect of metformin and sitagliptin on doxorubicin-induced cardiotoxicity in rats: impact of oxidative stress, inflammation, and apoptosis. J. Toxicol. 2015;2015 doi: 10.1155/2015/424813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sheta A., Elsakkar M., Hamza M., Solaiman A. Effect of metformin and sitagliptin on doxorubicin-induced cardiotoxicity in adult male albino rats. Hum. Exp. Toxicol. 2016;35(11):1227–1239. doi: 10.1177/0960327115627685. [DOI] [PubMed] [Google Scholar]
- 168.Ashour A.E., Sayed-Ahmed M.M., Abd-Allah A.R., Korashy H.M., Maayah Z.H., Alkhalidi H., et al. Metformin rescues the myocardium from doxorubicin-induced energy starvation and mitochondrial damage in rats. Oxid. Med. Cell. Longevity. 2012;2012 doi: 10.1155/2012/434195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 170.Zinman B., Inzucchi S.E., Wanner C., Hehnke U., George J.T., Johansen O.E., et al. Empagliflozin in women with type 2 diabetes and cardiovascular disease–an analysis of EMPA-REG OUTCOME®. Diabetologia. 2018;61:1522–1527. doi: 10.1007/s00125-018-4630-2. [DOI] [PubMed] [Google Scholar]
- 171.Fitchett D., Inzucchi S.E., Cannon C.P., McGuire D.K., Scirica B.M., Johansen O.E., et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation. 2019;139(11):1384–1395. doi: 10.1161/CIRCULATIONAHA.118.037778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Quagliariello V., De Laurentiis M., Rea D., Barbieri A., Monti M.G., Carbone A., et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 2021;20(1):1–20. doi: 10.1186/s12933-021-01346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sabatino J., De Rosa S., Tammè L., Iaconetti C., Sorrentino S., Polimeni A., et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc. Diabetol. 2020;19(1):1–11. doi: 10.1186/s12933-020-01040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.E. Belen, I. Canpolat, G. Yiğittürk, Ö. Cetinarslan, Cardio-protective effect of dapagliflozin against doxorubicin induced cardiomyopathy in rats, 2022. [DOI] [PubMed]
- 175.Pabel S., Wagner S., Bollenberg H., Bengel P., Kovacs A., Schach C., et al. Empagliflozin directly improves diastolic function in human heart failure. Eur. J. Heart Fail. 2018;20(12):1690–1700. doi: 10.1002/ejhf.1328. [DOI] [PubMed] [Google Scholar]
- 176.Li C., Zhang J., Xue M., Li X., Han F., Liu X., et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019;18:1–13. doi: 10.1186/s12933-019-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oh J., Lee B.S., Lim G., Lim H., Lee C.J., Park S., et al. Atorvastatin protects cardiomyocyte from doxorubicin toxicity by modulating survivin expression through FOXO1 inhibition. J. Mol. Cell. Cardiol. 2020;138:244–255. doi: 10.1016/j.yjmcc.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 178.Ng K.-M., Lau Y.-M., Dhandhania V., Cai Z.-J., Lee Y.-K., Lai W.-H., et al. Empagliflozin ammeliorates high glucose induced-cardiac dysfuntion in human iPSC-derived cardiomyocytes. Sci. Rep. 2018;8(1):14872. doi: 10.1038/s41598-018-33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kuşçu G.C., Gürel Ç., Buhur A., Karabay Yavaşoğlu N.Ü., Köse T., Yavaşoğlu A., et al. Fluvastatin alleviates doxorubicin-induced cardiac and renal toxicity in rats via regulation of oxidative stress, inflammation, and apoptosis associated genes expressions. Drug Chem. Toxicol. 2023;46(2):400–411. doi: 10.1080/01480545.2022.2043351. [DOI] [PubMed] [Google Scholar]
- 180.Seicean S., Seicean A., Plana J.C., Budd G.T., Marwick T.H. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J. Am. Coll. Cardiol. 2012;60(23):2384–2390. doi: 10.1016/j.jacc.2012.07.067. [DOI] [PubMed] [Google Scholar]
- 181.Mustafa N.H., Jalil J., Zainalabidin S., Saleh M.S., Asmadi A.Y., Kamisah Y. Molecular mechanisms of sacubitril/valsartan in cardiac remodeling. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.892460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yan F., Zhu H., He Y., Wu Q., Duan X. Combination of tolvaptan and valsartan improves cardiac and renal functions in doxorubicin-induced heart failure in mice. Eur. J. Histochemi.: EJH. 2022;66(4) doi: 10.4081/ejh.2022.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Miyoshi T., Nakamura K., Amioka N., Hatipoglu O.F., Yonezawa T., Saito Y., et al. LCZ696 ameliorates doxorubicin-induced cardiomyocyte toxicity in rats. Sci. Rep. 2022;12(1):4930. doi: 10.1038/s41598-022-09094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim B.S., Park I.-H., Lee A.-H., Kim H.-J., Lim Y.-H., Shin J.-H. Sacubitril/valsartan reduces endoplasmic reticulum stress in a rat model of doxorubicin-induced cardiotoxicity. Arch. Toxicol. 2022;96(4):1065–1074. doi: 10.1007/s00204-022-03241-1. [DOI] [PubMed] [Google Scholar]
- 185.Kim H., Oh J., Lee S., Ha J., Yoon M., Chun K.-h., et al. Clinical evidence of initiating a very low dose of sacubitril/valsartan: a prospective observational analysis. Sci. Rep. 2021;11(1):16335. doi: 10.1038/s41598-021-95787-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Di Lenarda A., Di Gesaro G., Sarullo F.M., Miani D., Driussi M., Correale M., et al. Sacubitril/valsartan in heart failure with reduced ejection fraction: real-world experience from Italy (the REAL. IT study) J. Clin. Med. 2023;12(2) doi: 10.3390/jcm12020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bosch X., Rovira M., Sitges M., Domènech A., Ortiz-Pérez J.T., de Caralt T.M., Morales-Ruiz M., Perea R.J., Monzó M., Esteve J. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies) J. Am. Coll. Cardiol. 2013;16(23):2355–2362. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 188.Abd El-Aziz M., Othman A., Amer M., El-Missiry M. Potential protective role of angiotensin-converting enzyme inhibitors captopril and enalapril against adriamycin-induced acute cardiac and hepatic toxicity in rats. J. Appl. Toxicol.: Int. J. 2001;21(6):469–473. doi: 10.1002/jat.782. [DOI] [PubMed] [Google Scholar]
- 189.Hullin R., Métrich M., Sarre A., Basquin D., Maillard M., Regamey J., et al. Diverging effects of enalapril or eplerenone in primary prevention against doxorubicin-induced cardiotoxicity. Cardiovasc. Res. 2018;114(2):272–281. doi: 10.1093/cvr/cvx162. [DOI] [PubMed] [Google Scholar]
- 190.Hiona A., Lee A.S., Nagendran J., Xie X., Connolly A.J., Robbins R.C., et al. Pretreatment with angiotensin-converting enzyme inhibitor improves doxorubicin-induced cardiomyopathy via preservation of mitochondrial function. J. Thoracic Cardiovasc. Surg. 2011;142(2):396–403.e393. doi: 10.1016/j.jtcvs.2010.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Boucek R.J., Steele A., Miracle A., Atkinson J. Effects of angiotensin-converting enzyme inhibitor on delayed-onset doxorubicin-induced cardiotoxicity. Cardiovasc. Toxicol. 2003;3:319–329. doi: 10.1385/CT:3:4:319. [DOI] [PubMed] [Google Scholar]
- 192.Zhang Y.-c., Tang Y., Zhang M., Chen J., Zhou Q., Sun Y.-g., et al. Fosinopril attenuates the doxorubicin-induced cardiomyopathy by restoring the function of sarcoplasmic reticulum. Cell Biochem. Biophys. 2012;64:205–211. doi: 10.1007/s12013-012-9386-6. [DOI] [PubMed] [Google Scholar]
- 193.Gupta V., Kumar Singh S., Agrawal V., Bali S.T. Role of ACE inhibitors in anthracycline-induced cardiotoxicity: a randomized, double-blind, placebo-controlled trial. Pediatr. Blood Cancer. 2018;65(11) doi: 10.1002/pbc.27308. [DOI] [PubMed] [Google Scholar]
- 194.Gujral D.M., Lloyd G., Bhattacharyya S. Effect of prophylactic betablocker or ACE inhibitor on cardiac dysfunction & heart failure during anthracycline chemotherapy±trastuzumab. Breast. 2018;37:64–71. doi: 10.1016/j.breast.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 195.Liu W., Gong W., He M., Liu Y., Yang Y., Wang M., et al. Spironolactone protects against diabetic cardiomyopathy in streptozotocin-induced diabetic rats. J Diabetes Res. 2018;2018 doi: 10.1155/2018/9232065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chakraborty M., Kamath J.V., Bhattacharjee A. Potential interaction of green tea extract with hydrochlorothiazide against doxorubicin-induced myocardial damage. J. Ayurveda Integr. Med. 2015;6(3):187. doi: 10.4103/0975-9476.146555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Akpek M., Ozdogru I., Sahin O., Inanc M., Dogan A., Yazici C., et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur. J. Heart Fail. 2015;17(1):81–89. doi: 10.1002/ejhf.196. [DOI] [PubMed] [Google Scholar]
- 198.Hwang H.-J., Han S.-A. Differential cardiovascular outcomes of each antihypertensive drug class in patients with hypertension and breast cancer undergoing doxorubicin-containing chemotherapy. J. Breast Cancer. 2022:26. doi: 10.4048/jbc.2023.26.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Aali E., Ghaznavi H., Soltanpour M.S., Mahmoudian M., Shafiei M. Cardioprotective effects of mebudipine in a rat model of doxorubicin-induced heart failure. Iran. J. Med. Sci. 2021;46(2):136. doi: 10.30476/ijms.2019.82057.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Abo Mansour H., El-Batsh M., Badawy N., Mehanna E., Mesbah N., Abo-Elmatty D. Effect of co-treatment with doxorubicin and verapamil loaded into chitosan nanoparticles on diethylnitrosamine-induced hepatocellular carcinoma in mice. Hum. Exp. Toxicol. 2020;39(11):1528–1544. doi: 10.1177/0960327120930266. [DOI] [PubMed] [Google Scholar]
- 201.Ikeda S., Matsushima S., Okabe K., Ikeda M., Ishikita A., Tadokoro T., et al. Blockade of L-type Ca2+ channel attenuates doxorubicin-induced cardiomyopathy via suppression of CaMKII-NF-κB pathway. Sci. Rep. 2019;9(1):9850. doi: 10.1038/s41598-019-46367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rawat P.S., Jaiswal A., Khurana A., Bhatti J.S., Navik U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111708. [DOI] [PubMed] [Google Scholar]
- 203.Keung E., Toll L., Ellis M., Jensen R. L-type cardiac calcium channels in doxorubicin cardiomyopathy in rats morphological, biochemical, and functional correlations. J. Clin. Invest. 1991;87(6):2108–2113. doi: 10.1172/JCI115241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.da Silva Ferreira R., Fernandes P.B.U., da Cruz J.P.O., Silva F.L.A., Lempek M.R., Canta G.N., et al. Comparative therapeutic potential of cardioactive glycosides in doxorubicin model of heart failure. Cardiovasc. Toxicol. 2022:1–10. doi: 10.1007/s12012-021-09702-w. [DOI] [PubMed] [Google Scholar]
- 205.Sikandar A., Ajmal K., Afzal A., Rafique S., Zafar A., Tehseen T. Evaluating the protective effects of lovastatin against doxorubicin induced cardiotoxicity in Balb-C mice. J. Ayub Med. Coll. Abbottabad-Pak. 2023;35(2) doi: 10.55519/JAMC-02-10528. [DOI] [PubMed] [Google Scholar]
- 206.Henninger C., Huelsenbeck S., Wenzel P., Brand M., Huelsenbeck J., Schad A., et al. Chronic heart damage following doxorubicin treatment is alleviated by lovastatin. Pharmacol. Res. 2015;91:47–56. doi: 10.1016/j.phrs.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 207.Riad A., Bien S., Westermann D., Becher P.M., Loya K., Landmesser U., et al. Pretreatment with statin attenuates the cardiotoxicity of Doxorubicin in mice. Cancer Res. 2009;69(2):695–699. doi: 10.1158/0008-5472.CAN-08-3076. [DOI] [PubMed] [Google Scholar]
- 208.Liu G., Liu Y., Wang R., Hou T., Chen C., Zheng S., et al. Spironolactone attenuates doxorubicin-induced cardiotoxicity in rats. Cardiovasc. Ther. 2016;34(4):216–224. doi: 10.1111/1755-5922.12189. [DOI] [PubMed] [Google Scholar]