Abstract
Background
Withania somnifera (L.) Dunal, known as Ashwagandha, is an adaptogen with significant importance in Ayurveda for its potential health benefits in strength ('balavardhan') and muscle growth ('mamsavardhan'). Despite numerous studies on its efficacy, limited research is reported on its clinical safety and tolerability in healthy individuals.
Objective
This research evaluated the tolerability and safety of standardized Withania somnifera root extract (WSE) capsules (AgeVel®/Witholytin®) at 1000 mg/day dose upon oral administration in healthy male participants.
Method
A non-randomized, open-label, single-treatment clinical study included eighteen healthy male participants aged 18 to 60. The participants were administered a dose of 500 mg of the WSE capsules twice daily for four weeks. Each capsule contained not less than 7.50 mg of total withanolides. The study evaluated various indicators in a cohort of healthy participants throughout the trial, including vital signs, organ function tests, urine analysis, X-ray and ECG, cardiorespiratory endurance, body fat percentage, lean body weight, adverse events profile, and tolerability of the WSE capsules.
Results
The participant's physical, hematological, and biochemical characteristics were normal, and no significant alterations or irregularities were observed in safety metrics like liver, kidney, and thyroid functions after administering AgeVel®/Witholytin®.
Conclusion
This study found that healthy male participants could consume a standardized WSE at a daily dosage of 1000 mg for four weeks without any adverse effects. Future research should focus on long-term safety assessments in male and female participants.
Keywords: Ashwagandha, Withania somnifera (L.) Dunal, Standardized extract, Clinical safety, Withanosides and withanolides
Graphical abstract
Highlights
-
•
Clinical safety and tolerability of standardized Withania somnifera (L.), Dunal root extract at 1000 mg daily in healthy male volunteers were evaluated.
-
•
Throughout the trial, a comprehensive range of assessments was conducted, including monitoring vital signs, CBC, lipid profile, TFT, LFT, KFT, urine analysis, serum B12, and C-reactive protein levels.
-
•
The study commenced and concluded with evaluating X-rays, electrocardiograms, and cardiorespiratory endurance as determined by VO2 max and anthropometric parameters.
-
•
The clinical examination and laboratory analysis indicates that W. somnifera root extract (AgeVel®/Witholytin®) was effectively tolerated by healthy participants, and no adverse effects were observed.
1. Introduction
Withania somnifera (L.) Dunal, also known as the ‘Indian Winter cherry' or ‘Indian Ginseng' or Ashwagandha, is a crucial traditional herb in Indian medicine, grown in semi-arid regions of India, the southeastern hemisphere, Congo, South Africa, Morocco, and Egypt [1]. Ashwagandha's therapeutic benefits are attributed to its bio-actives, including withanosides, withanolides, alkaloids, and numerous sitoindosides [[2], [3], [4], [5]]. The Ayurvedic texts reveal that the Ashwagandha root is used for rejuvenating, muscle-strengthening, tonic, weight-promoting, and aphrodisiac purposes. It also treats oligospermia, phthisis, poisonous disorders, pox, psychosis, scrotal swell, bleeding, chest injury, and skin disorders [6]. In Charak Samhita (1500 BC), Ashwagandha was reported for boosting weight and strength [7]. It is also mentioned in the Sushruta Samhita (2000 BC) for internal and external emaciation usage and to nourish the body [[8], [9], [10]]. Roots and extracts of Ashwagandha have been reported in pre-clinical and clinical trials to have activities including adaptogenic, immunomodulatory, anxiolytic, anti-stress, anti-inflammatory, anti-bacterial, anti-aging, cardioprotective, treatments of sleep disorders, hypothyroidism, etc. [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]].
Withanosides and withanolides-enriched Ashwagandha supplements are a significant and expanding part of dietary supplement markets for their health-promoting effects internationally. Ashwagandha has been recently deemed a top priority for human mechanistic research by the National Center for Complementary and Alternative Medicines (NCCAM) of the US National Institute of Health. It had total sales of $13.7 million in the natural retail sector in the USA and ranked third, after elderberry and cannabidiol (CBD), with a 45.2% increase in sales [24]. The global market for Ashwagandha extract, valued at $864.3 million in 2021 and is expected to grow at a CAGR of 11.4% from 2022 to 2031, despite its potential health benefits, making it crucial to understand its safety and tolerability in healthy individuals.
According to Ayurveda, W. somnifera leaves can be applied externally. Further, as per ethnopharmacological evidence, the paste of leaves is applied to painful areas, and boiling leaves are applied topically to treat boils and reduce pain [6]. Ayurveda advises the use of only roots for ingestible administration and therapeutic purposes. The Indian Ministry of ‘AYUSH', Government of India, recently released an advisory urging people to “refrain from the use of W. somnifera leaves" due to the lack of “substantial evidence and literature" in favor of the use of Ashwagandha leaves as medicine [25]. The use of additional plant parts, particularly the leaves and stem, of the W. somnifera, could have blended with root material and/or its extract for commercial purposes, which, beyond adulteration concern, may lead to adverse effects. Commercial extracts with inadequate quality control pose a risk to consumers, researchers, and regulatory organizations who expect material consistency and quality parameters. In 2023, the Indian Ministry of AYUSH also published a safety dossier on W. somnifera, analyzing 27 toxicity studies. They concluded that using W. somnifera root extract at a concentration dose of at least 2,000 mg/kg body weight is safe for human consumption without any negative impact [6].
Numerous clinical trials on Ashwagandha for treating schizophrenia, neuropathic pain, anxiety, stress, ADHD, memory and cognitive enhancement, sexual health in men and women, cardio-respiratory endurance, hyperlipidemia, and COVID-19 have been reported [[26], [27], [28], [29], [30], [31]]. However, a significant problem for the worldwide nutraceuticals and dietary supplement industry and various countries' regulatory bodies could be Ashwagandha's safety. Relatively few adequate investigations on the safety of Ashwagandha have been published. According to Raut et al.'s (2012) study, W. Somnifera roots were well tolerated when administered as an aqueous extract in capsules with gradually increasing doses of 750–1250 mg/day [32]. Recent research by Verma et al. (2021) on the safety of ashwagandha root extract in healthy volunteers at a dose of 600 mg/day shows that both male and female participants could take the supplement for eight weeks without experiencing any side effects [33]. Chandrasekhar et al. (2012) reported the ashwagandha root extract for lowering adult stress and anxiety, in which six mild side effects were reported, including drowsiness and a decreased appetite [34]. In a study by Choudhary et al. (2017), two individuals using Ashwagandha root extract for memory and cognitive enhancement reported experiencing minor giddiness, a heavy head, blurred vision, and/or hyperacidity [35]. The effectiveness and safety of ashwagandha root extract were later reported by Sharma et al. (2018) in subclinical hypothyroid patients, where one participant reported an adverse event with symptoms of mild, transient fever, asthenia, and cough [23].
Variations of commercially available WSE (including roots, leaf, or combining root and leaf extracts) are marketed globally as foods, beverages, and dietary supplements. These extracts are standardized with various analytical techniques and contain varying concentrations of withanolides (1%–5% to 35% total withanolides). More in-depth data is needed to grasp the clinical safety of the standardized WSE in the healthy population. The current study intends to investigate the safety and tolerability of WSE standardized to not less than 7.50 mg of total withanolides per capsule at a dose of 500 mg twice daily in 18 healthy male participants following repeated intake for four weeks to close this knowledge gap. Based on this study's clinical findings, standardized WSE can be successfully introduced into the global nutraceutical market while ensuring adequate safety and biological activity as a highly potent botanical ingredient.
2. Material and methods
2.1. Study design
A four-week, open-label, non-randomized clinical trial was conducted to assess the safety of WSE on healthy human volunteers. The study's primary goal was to determine the safety of WSE (AgeVel®/Witholytin®) capsules when administered to healthy human male volunteers. The study's secondary goal was to establish the product's safety and tolerability by keeping track of adverse events.
Ethics approval
The study received approval from the Lokmanya Medical Research Centre's institutional ethics committee in Pune, Maharashtra, India (clinical trial ethics approval protocol no. MHC/CT/22-23/015, approved on 28/03/2023; Ethics Committee Registration No. ECR/175/Inst/MH/2013/RR-19). The Drug and Cosmetic Act of 1940 of India, the Drug and Cosmetic Rules of 1945 of India, the Declaration of Helsinki of the World Medical Association (WMA), and the recommended harmonized tripartite guideline regarding Good Clinical Practice (ICH-GCP) of the International Conference on Harmonization (ICH) were all followed during the research. The study was carried out, documented, and reported strictly following ICH-GCP standards. The study participants' rights, safety, and general well-being were prioritized throughout the clinical trial's administration. The investigational product was prepared in a GMP (Good Manufacturing Practice) approved manufacturing facility.
2.2. Registration of clinical trial
The protocol and clinical trial were registered with the Clinical Trial Registry-India (CTRI). The registration was completed with the approval number CTRI/2023/04/051632 on April 17, 2023. The approved clinical protocol remained unchanged throughout the study.
2.3. Study participants
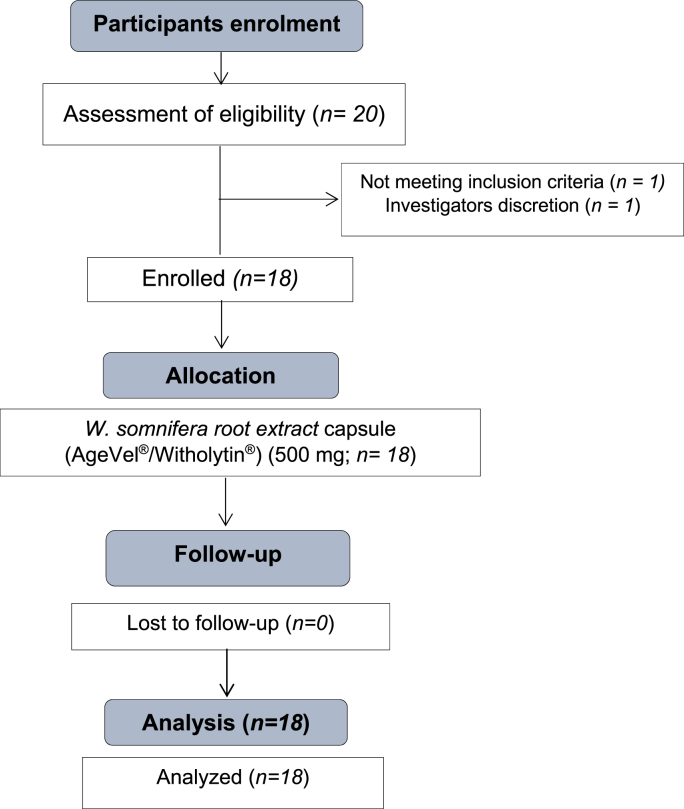
A non-randomized, open-labeled safety and tolerability study of WSE capsules included 18 healthy male volunteers. Twenty participants were initially screened to complete 18 participants for the study. Two participants were excluded from the study after being screened; one was excluded because he did not satisfy the criteria for inclusion, and the other was excluded at the investigator's discretion. There were 18 participants signed up for the study, and there were no dropouts. All 18 recruited participants completed the study. The clinical safety study's data collection period ran from April 29, 2023, to June 16, 2023. Fig. 1. displays the process of the clinical safety study as per CONSORT. The study only accepted participants who gave written informed consent, were willing to participate in ongoing follow-up visits until the end of the investigation, and met the inclusion and exclusion requirements.
Fig. 1.
CONSORT flow diagram of the clinical safety study of WSE in healthy male participants.
2.4. Screening of participants
Male participants aged 18 years and above were screened for the eligibility criteria. Participants able to provide written informed consent approved by the ethics committee and willing to complete the study intervention and follow-up were enrolled. Mentally and physically healthy males with a BMI between 18.5 and 24.9 kg/m2 (inclusive) and blood profiles within normal ranges were enrolled in the study at the investigator's discretion. Participants with normal cardiovascular function with no evidence of acute ischemic heart disease in the electrocardiogram were included in the study. Participants willing to refrain from medicines and other health supplements along with alcohol and tobacco were enrolled in the study. Participants on medically prescribed drugs were excluded from the study. Participants with reported weight loss/gain >10% of body weight in the 6-month preceding study were excluded. Participants using herbal or dietary supplements meant for improving health and well-being and subjects undergoing medical treatment that may interfere with the study outcome were excluded. Participants with a history of hypersensitivity to Ashwagandha were also excluded from the study. Participants consuming alcohol, smoking, and/or chewing tobacco during the past six months were excluded. Participants diagnosed with active disease and/or receiving pharmacological treatment prescribed for active disease and with evidence of active disease at the initial clinical examination were excluded from the study. Participants were advised to use adequate contraception and avoid fathering a child while receiving the investigational product during the study. The study did not allow the use of hormone replacement therapy and androgens or anabolic steroids.
2.5. Informed consent
Before participating, all potential volunteers received a thorough explanation of the study's objectives, design, potential risks, and any anticipated benefits. The participants could understand the language and manner used to provide this information. Participants were informed that participating in the study was voluntary and could stop without suffering consequences or losing out on prizes. Additionally, they were made aware that it was their choice to participate in the study and that each participant's written informed consent had been obtained before their inclusion. The study's objectives, the methods involved, any possible hazards and advantages, confidentiality safeguards, and the study investigators and the institutional ethics committee's contact information were all described in the consent form. All identifying information and study data were tagged and securely kept, guaranteeing participant confidentiality. All study reports, and publications kept participant confidentiality, and only authorized study personnel could access the data.
2.6. Inclusion and exclusion criteria
Healthy male participants were screened and enrolled in a non-randomized, open-labeled, single-treatment, single-dose safety study of Withania somnifera extract capsules. In the current study, we enrolled healthy male adults between 18 and 45 years of age with a BMI of 18.50-30.00 kg/m2 and a body weight of at least 50.00 Kg. During the screening and before administration of the investigational product, each participant underwent a baseline test that included a complete blood count (CBC), clinical biochemistry, liver function test (LFT), kidney function test (KFT), urine analysis, X-ray chest (PA view), and ECG. Throughout the study, the safety and well-being of the healthy participants were monitored, and any adverse events were recorded. All participants who met the following inclusion criteria were enrolled: (a) participants in normal health as determined by personal medical history, clinical examination including vital signs, and clinically acceptable results of laboratory examinations (including serological tests); (b) participants have a normal or clinically insignificant 12-lead electrocardiogram recording; (c) participants have a normal or clinically insignificant chest X-Ray (P/A view); (e) a negative alcohol breath test result; (f) participants can communicate effectively and provide written informed consent; and (g) participants are willing to follow protocol requirements as evidenced by written informed consent approved by the ethics committee; (h) participants who can provide adequate identification; (i) availability of participants for the duration of the study.
The following were the study's exclusion criteria: (a) known hypersensitivity to W. somnifera (Ashwagandha) or related drugs/botanicals, or any component of this medication; (b) participant unable to comprehend the informed consent information; (c) history or presence of significant cardiovascular, pulmonary, hepatic, renal, gastrointestinal, endocrine, immunological, dermatological, neurological, or psychiatric disease or disorder; (d) any treatment that could induce or inhibit the hepatic microsomal enzyme system within one month of beginning the study; (e) a history of alcoholism or drug abuse; (f) history of asthma, urticaria, or other allergic reactions; (g) a history of gastric and/or duodenal ulceration; (h) a history of thyroid disease, adrenal dysfunction, or an organic intracranial lesion; (i) a history of cancer; (j) consuming any prescribed medication (including herbal remedies) during the two weeks preceding the study's start date or OTC medicinal products (including herbal remedies) during the week preceding study initiation and throughout the study; (k) taking benzodiazepines, anticonvulsants, or barbiturates for one month before the start of the study and for the duration of the study; (l) smokers who consume 9 or more cigarettes per day or who are unable to abstain during the study; (m) participants had a significant illness within 90 days of screening; (n) participants took part in a drug research study within 90 days of screening and donated blood within 90 days of screening; (o) a positive screening test result for HIV, Hepatitis B, Hepatitis C, or VDRL; (p) a history or presence of easy bruising or bleeding (q) an abnormal dietary pattern for any reason (e.g., low sodium, fasting, and high protein diets); in the four weeks preceding the study.
2.7. Clinical study procedure
During a general screening organized seven days before the start of the medicine, the participant's medical history and demographic information, such as sex, age, body weight, and height, were recorded. Each participant underwent a thorough clinical examination, vital sign check, anthropometric evaluation, hematological and biochemical testing, urine analysis, ECG, chest X-ray, and cardiorespiratory endurance by step test as part of a screening process for health concerns. Medication interactions and concurrent medical problems were noted in the CRF. The participants questioned the presence of any adverse events during the screening period. The participant's allergies to the investigational product's substances were questioned. The final follow-up occurred on day 31 of the 30-day therapy period.
If a person met all inclusion criteria during the baseline visit, they were enrolled in the study. All The participants were told to take one capsule of the W. somnifera extract twice daily (morning after breakfast and evening after dinner) for 30 days. The health evaluation, which included laboratory, clinical, and diagnostic data, was carried out on days 15 and 30 of the follow-up. At the beginning and end of the trial, step tests for cardiorespiratory endurance and lean body weight evaluation were performed. Throughout the trial, adverse events, tolerability, and compliance were evaluated. The assessment of aerobic fitness was done using the VO2 max test, which measures cardiorespiratory endurance. The maximum rate at which oxygen can be taken from the air and delivered into the bloodstream for utilization by working muscles was used to measure it. Before the exam, all the participants received instructions and information on the procedure. Forty-eight hours before the exam, they were told not to do any kind of active training. The maximum aerobic capacity (VO2 max) was calculated using the Queens College Step Test [36]. A submaximal exercise like bench stepping, acceptable for participants, is a common strategy to determine maximum oxygen comprehension. Participants were instructed to warm up their lower extremities before the test. A timer and an 8-inch stepping bench were both used. The participants were required to do each one-stepping cycle at a four-step rhythm, up, up, down, and down continuously for 1 min, after which their heart rates were measured. The VO2 max was calculated by the formula VO2 max (mL/kg/min) = 111.33 − (0.42 × heart rate (bpm).
The primary efficacy assessment was done by evaluating changes in clinical laboratory examination of CBC, lipid profile, TFT, LFT, KFT, urine analysis, serum B12 levels, CRP, observations in X-ray and ECG, and vital signs from screening to the end of the study. Trained phlebotomists at the study center were responsible for collecting all blood samples. These blood samples were analyzed from all participants at a laboratory accredited by the National Accreditation Board for Testing and Calibration Laboratories (NABL), Quality Council of India. The secondary efficacy assessment was done by evaluating changes in cardiorespiratory endurance by step test, body fat percentage, and lean body weight by measuring skinfold thickness at four areas such as biceps, triceps, subscapular, and suprailiac, with a Skin Fold Caliper in millimeters at screening and end of the study.
2.8. Investigational product
2.8.1. Quality control of Withania somnifera roots
The roots of W. somnifera were collected from Madhya Pradesh, India. Botanical Survey of India, Jodhpur, India, received a voucher specimen that had been authenticated (BSI/AZRC/I.12012/Tech/19-20/PI.Id/671). For extraction and analysis, the roots were washed and then pulverized finely. The W. somnifera roots underwent rigorous examination for total withanolides, contaminants, and heavy metals for quality control. Five grams of the finely powdered W. somnifera roots were placed in a 250.00 mL round bottom flask with a reflux condenser. Then, 50 mL methanol was added to the flask, refluxed in the water bath for 15 min, cooled to room temperature, and retained the solvent [37,38]. The liquid sample was analyzed by the HPLC method. The physical parameters (identification, description, color, odour, taste, and foreign matter) were analyzed with visual, organoleptic, and HPTLC, HPLC, methods [38,39]. Further, the chemical parameters such as loss on drying, total ash content, acid insoluble ash content, alcohol soluble extractives, and heavy metals were also analyzed [39].
2.8.2. Manufacturing of Withania somnifera root extract capsule
W. somnifera roots were extracted thrice with ethanol: water (8:2 v/v) at 60 ± 5 °C for 3 h. The hydroalcoholic extract is further processed to get powdered extract. Powdered extract, after analysis, was encapsulated in a GMP-certified facility of Pharmanza Herbal Pvt. Ltd. (Anand-388430, Gujarat, India) in veggie capsules Size ‘00' (AgeVel®) containing a 500 mg WSE with thorough quality control measures and further used as an investigational product in this research [38]. The same Withania somnifera extract is marketed by Verdure Science (Noblesville, IN, 46060, USA) under the brand name Witholytin®.
2.9. Statistical analysis
The continuous variable, i.e., age, was summarized overall using summary statistics, i.e., the number of observations, mean, and standard deviation with 95% CI (among normal distribution) analyzed by student t-test. All safety parameter was checked for normality by ‘Kolmogorov-Smirnov Test'. Data of weight, BMI, cardiorespiratory endurance, body fat percentage, lean body weight and skinfold thickness, vital signs, urine analysis, and all laboratory parameters were analyzed by dependent student t-test. The statistical analysis of X-ray and ECG was performed using the chi-square test. The adverse events were expressed as the number and frequency of events in a study group. All Statistical analysis has been carried out by using SPSS version 10.0.
3. Results and discussion
3.1. Participants' demographics
The study included 18 healthy male participants. All of them completed the study, and data were examined (Fig. 1). The participants had an average age of 32.50 ± 7.69 years and an average height of 171.06 ± 8.24 cm. The participant's body weight and BMI did not alter significantly (P > 0.05), as depicted in Table 1.
Table 1.
Demographic details of healthy male participants.
| Parameter | Screening | Day 31 | P value |
|---|---|---|---|
| Weight (kg) | 65.33 ± 8.66 | 65.34 ± 8.67 | 0.903 |
| BMI (kg/m2) | 22.30 ± 2.22 | 22.31 ± 2.24 | 0.439 |
Data were analyzed by the student-dependent t-test. Significant at P-value <0.05.
3.2. Quality control of W. somnifera root extract capsule
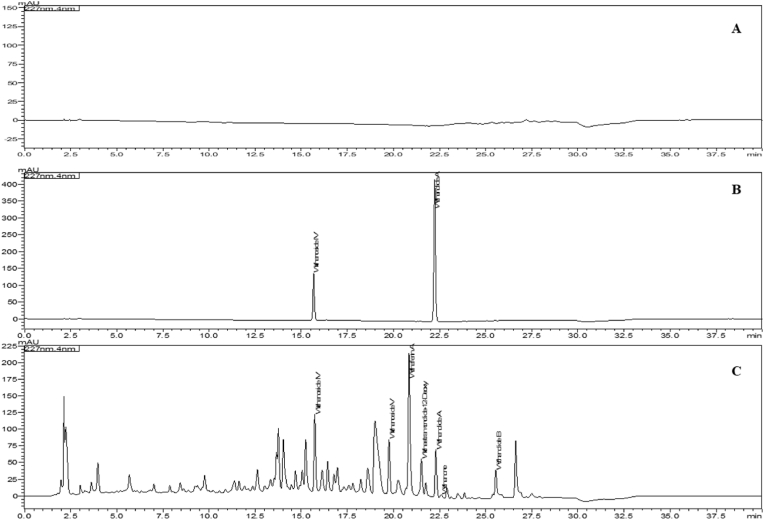
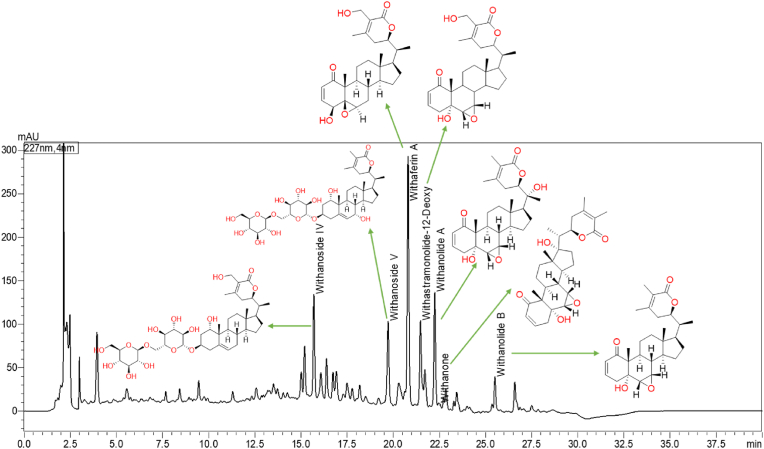
The content of withanosides and withanolides was evaluated in the W. somnifera roots and WSE capsules. The results revealed total withanolides not less than 1.5 % w/w in WSE (Table 2). Also, the WSE capsule was analyzed, and it was found that total withanolides (withanoside IV, withanoside V, withaferin A, withanolide A, withanone, withanolide B, 12 deoxy withastramonolide) not less than 7.50 mg per capsule [38]. Representative HPLC chromatogram of W. somnifera root and WSE capsules is shown in Fig. 2, Fig. 3, respectively. The physical, chemical, and microbiological specifications of the WSE capsule are depicted in Table 3.
Table 2.
Quantification of withanosides and withanolides in W. somnifera roots and WSE capsules.
| Sr. No | Analyte |
W. somnifera roots |
WSE capsules |
|---|---|---|---|
| % Content | Content mg/capsule | ||
| 1 | Withanoside IV | 0.070 | 1.950 ± 0.041 |
| 2 | Withanoside V | 0.060 | 1.565 ± 0.031 |
| 3 | Withaferin A | 0.050 | 1.995 ± 0.037 |
| 4 | 12-Deoxy-withastramonolide | 0.020 | 0.700 ± 0.011 |
| 5 | Withanolide A | 0.030 | 1.285 ± 0.026 |
| 6 | Withanone | 0.001 | 0.195 ± 0.001 |
| 7 | Withanolide B | 0.010 | 0.280 ± 0.002 |
| Total Withanolides | 0.241 | 7.970 ± 0.151 | |
Fig. 2.
Representative HPLC chromatograms of (A) Blank Methanol, (B) reference standard (withanoside IV and Withanolide A), (C) W. somnifera roots at 227 nm.
Fig. 3.
Representative HPLC chromatograms and quantified phytoconstituents WSE capsule at 227 nm.
Table 3.
Physical, chemical, and microbiological specifications of WSE capsule.
| Name of Product | Withania somnifera root extract capsule (Each capsule contains 500 mg Withania somnifera root extract) |
| Common Name | Ashwagandha root extract |
| Brand Name | AgeVel® |
| Details of Quality control | Assay Method | |
|---|---|---|
| Description | Green-green color “00″ size veggie capsule | Visual |
| Appearance | Light to dark brown color granular powder in Green-green color “00″ size veggie capsule | Visual |
| Total Withanolides | > 7.500 mg total withanolides per capsule | [38] |
| Disintegration Test | < 30 min | [40] |
| Weight variation | 580 mg ± 5% | [41] |
| Total Heavy metal | < 10 PPM | [42] |
| Total plate count | <1000 cfu/gm | [43] |
| Yeast and mold | <200 cfu/gm | [43] |
| Coliforms | Absent | [43] |
| Escherichia coli | Absent | [43] |
| Salmonella | Absent | [43] |
| S. aureus | Absent | [43] |
| Enterobacteriaceae | Absent | [43] |
3.3. Assessment of vital signs
At baseline, heart rate, blood pressure, and oral body temperature were within normal range. No clinical or statistically significant change was observed in the study participant's oral body temperature, heart rate, respiration rate, and blood pressure after 30 days of the treatment with the WSE capsule (Table 4).
Table 4.
Assessment of vital signs in healthy participants of the study.
| Vitals | Screening (n =18) | Baseline (n=18) | Day 15 (n=18) | P value | Day 31 (n=18) | P value |
|---|---|---|---|---|---|---|
| Systolic Blood Pressure (mmHg) | 122.33 ± 5.90 | 121.00 ± 4.98 | 124.56 ± 3.70 | 0.164 | 124.44 ± 4.29 | 0.263 |
| Diastolic Blood Pressure (mmHg) | 76.94 ± 3.86 | 75.61 ± 3.62 | 74.83 ± 6.24 | 0.164 | 75.39 ± 4.46 | 0.303 |
| Pulse Rate (BPM) | 72.83 ± 4.45 | 71.67 ± 2.70 | 72.67 ± 2.91 | 0.897 | 73.78 ± 2.56 | 0.349 |
| Oral temperature (°F) | 97.77 ± 0.60 | 97.77 ± 0.74 | 97.28 ± 0.80 | 0.024* | 97.59 ± 0.49 | 0.380 |
| Respiratory Rate (breaths/min) | 16.06 ± 1.92 | 16.44 ± 1.20 | 16.50 ± 1.15 | 0.331 | 16.50 ± 0.99 | 0.288 |
Data were analyzed by dependent student t-test.
3.4. Assessment of laboratory parameters
The laboratory results remained within normal ranges before and after intervention, with no clinically significant changes observed (Table 5). Serum T3 levels steadily increased on days 15 (P = 0.002) and 30 (P = 0.007) without reaching a clinically relevant threshold (70 -204 ng/mL). Compared to the baseline measurements, there was a substantial increase in blood urea nitrogen and blood urea levels at day 15 (P = 0.025). Conversely, a considerable decrease was observed in triglyceride levels (P = 0.049), the TG/LDL ratio (P = 0.046), and serum T4 (P = 0.044) at day 15. Nevertheless, no noticeable distinction was observed between day 31 and baseline measurement. Following days 15 and 31, the difference had no observable clinical significance. On day 31, a significant decrease was observed in the total bilirubin levels (P = 0.005) compared to the initial measurements. Moreover, LDL (P = 0.053), ALP (P = 0.037), and total cholesterol levels (P = 0.035) exhibit a significant increase. The investigation found no clinically significant alterations in the individuals; all biological parameters were within the reference range, as depicted in Table 5.
Table 5.
Assessment of hematological and biochemical parameters during screening and upon consumption of WSE capsule in healthy male volunteers.
| Parameters | Screening | Day 15 | P value | Day 31 | P value | Reference range |
|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 14.7 ± 0.88 | 14.69 ± 1.03 | 0.969 | 14.71 ± 0.86 | 0.977 | 13.2-16.6 |
| Hematocrit (PCV) (%) | 45.11 ± 3.19 | 44.85 ± 3.08 | 0.610 | 44.44 ± 3.58 | 0.516 | 42–52 |
| Red blood cell count (mil/cu.mm) | 5.03 ± 0.47 | 5.01 ± 0.38 | 0.771 | 4.87 ± 0.33 | 0.164 | 4.7–6.0 |
| Platelet Count (103/μL) | 247.17 ± 43.08 | 255.50 ± 43.84 | 0.250 | 255.89 ± 41.76 | 0.415 | 150 - 450 10∧3/uL |
| Total Leukocyte Count (cell/cu.mm) | 6744.44 ± 1785.31 | 6455.56 ± 1597.14 | 0.253 | 7088.89 ± 1633.43 | 0.111 | 4000–11000 |
| Neutrophils (%) | 55.00 ± 5.55 | 57.28 ± 5.46 | 0.040 | 56.28 ± 6.29 | 0.445 | 40–75 |
| Basophils (%) | 0.00 ± 0.00 | 0.00 ± 0.00 | – | 0.00 ± 0.00 | – | 0–1 |
| Lymphocytes (%) | 36.06 ± 5.23 | 32.83 ± 5.43 | 0. 004 | 33.94 ± 6.11 | 0.184 | 20–40 |
| Eosinophils (%) | 3.56 ± 0.78 | 3.83 ± 0.38 | 0.205 | 3.72 ± 0.67 | 0.563 | 1–6 |
| Monocytes (%) | 5.39 ± 1.72 | 6.06 ± 0.42 | 0.117 | 6.06 ± 0.64 | 0.192 | 2–10 |
| CRP (mg/dL) | 2.54 ± 1.03 | 2.73 ± 0.79 | 0.275 | 2.30 ± 0.72 | 0.341 | < 6 |
| Serum B12 Levels (Pg/mL) | 386 ± 97.95 | 409.94 ± 90.35 | 0.083 | 407.94 ± 42.42 | 0.359 | 200–1100 |
| Total cholesterol (mg/dL) | 155.98 ± 20.25 | 160.62 ± 13.95 | 0.057 | 164.98 ± 20.49 | 0.035* | Desirable level | < 200; Borderline High | 200-239; High | > or = 240 |
| Low-density lipoprotein (LDL) (mg/dL) | 84.25 ± 22.45 | 92.68 ± 10.66 | 0.080 | 96.04 ± 15.51 | 0.035* | Optimal <100; Near/Above Optimal 100-129; Borderline High 130-159; High 160-189; Very High > or = 190 |
| Very Low-Density Lipoprotein (VLDL) (mg/dL) | 27.60 ± 8.55 | 23.99 ± 6.90 | 0.497 | 24.47 ± 8.02 | 0.146 | 6–38 |
| Triglycerides (mg/dL) | 137.98 ± 42.74 | 119.97 ± 34.51 | 0.049* | 122.37 ± 40.09 | 0.147 | Normal: <60-165; Borderline High: 200-260; High: 260-499; Very High: ≥ 500 |
| High-density lipoprotein (HDL) (mg/dL) | 44.13 ± 8.85 | 43.94 ± 3.90 | 0.927 | 44.47 ± 4.04 | 0.885 | Normal: 35-80; Major Risk for Heart: above 80 |
| TG/LDL ratio | 1.82 ± 0.93 | 1.32 ± 0.43 | 0.046* | 1.32 ± 0.56 | 0.065 | – |
| Serum TSH (uIU/mL) | 2.21 ± 0.77 | 2.35 ± 0.60 | 0.138 | 2.64 ± 0.75 | 0.089 | First Trimester: 0.35-5.5; Second Trimester: 0.2-3.0 Third; trimester: 0.3-3.0 |
| Serum T3 (ng/mL) | 128.92 ± 18.59 | 134.94 ± 15.63 | 0.002* | 139.28 ± 11.97 | 0.007* | 70–204 |
| Serum T4 (mcg/dL) | 8.86 ± 1.87 | 8.63 ± 1.69 | 0.044* | 8.42 ± 1.70 | 0.137 | 5.1–14.1 |
| Blood Urea Nitrogen (mg/dL) | 11.96 ± 1.99 | 13.29 ± 2.49 | 0.025* | 12.55 ± 1.67 | 0.252 | 7–21 |
| Blood Urea (mg/dL) | 25.62 ± 4.27 | 28.45 ± 5.33 | 0.025* | 26.88 ± 3.58 | 0.252 | 13–45 |
| Serum Creatinine (mg/dL) | 0.86 ± 0.20 | 0.90 ± 0.10 | 0.333 | 0.85 ± 0.08 | 0.931 | 0.5–1.5 |
| Uric Acid (mg/dL) | 5.87 ± 1.44 | 5.62 ± 1.46 | 0.349 | 5.23 ± 1.56 | 0.056 | 2.6–7.7 |
| Total Bilirubin (mg/dL) | 0.84 ± 0.24 | 0.79 ± 0.18 | 0.354 | 0.65 ± 0.14 | 0.005* | 0.0–1.1 |
| Total Protein (g/dL) | 7.35 ± 0.67 | 7.56 ± 0.51 | 0.286 | 7.74 ± 0.52 | 0.104 | 6.0–8.3 |
| Albumin (g/dL) | 4.55 ± 0.53 | 4.52 ± 0.47 | 0.828 | 4.82 ± 0.31 | 0.056 | 3.2–5.5 |
| Alanine Aminotransferase (ALT)(U/L) | 33.07 ± 12.40 | 33.03 ± 9.87 | 0.981 | 32.33 ± 7.06 | 0.765 | 0–49 |
| Alkaline Phosphatase (ALP) (U/L) | 139.68 ± 35.90 | 152.57 ± 24.08 | 0.137 | 163.89 ± 19.48 | 0.037* | 64–306 |
| Aspartate Aminotransferase (AST) (U/L) | 30.74 ± 8.50 | 30.48 ± 5.55 | 0.824 | 31.15 ± 3.84 | 0.841 | 0–49 |
Data were analyzed by dependent student t-test. Statistically significant at P-value <0.05; *indicates significant changes.
3.5. Assessment of urine analysis
At screening, days 15 and 31, all physicochemical characteristics and microscopic examinations, including color, pH, clarity, specific gravity, protein, sugar, ketone bodies, RBC, WBC, pus cells, etc., of urine samples were within normal ranges. No statistical or clinically significant alterations were found (P > 0.05).
3.6. Assessment of chest X-ray and ECG
All healthy participants underwent chest X-ray and ECG exams on day 1, day 15, and day 31 of the study. None of the participants had any abnormalities or alterations that were both clinically and statistically significant (P > 0.05). Pre- and post-treatment reports from every individual were within normal limits.
3.7. Assessment of cardiorespiratory endurance by VO2 max
The total oxygen consumption capacity improvement was determined using the participants' aerobic capacity (VO2 max). During the screening, VO2 max was found to be 80.74 ± 1.87 mL/kg/min whereas after 30 days of treatment, a non-significant reduction (80.34 ± 1.07 mL/kg/min, P = 0.349) in VO2 max was observed among study participants (n = 18).
3.8. Assessment of anthropometric parameters
Skinfold thickness in millimeters at four locations, including the biceps, triceps, subscapular, and suprailiac, as well as body fat percentage and lean body weight, were carried out in each participant. The thickness of the biceps, triceps, subscapular, and suprailiac skinfolds did not alter significantly during the study treatment's first and last 30 days. Table 6 shows anthropometric measurements at the start and end of the study in healthy male individuals.
Table 6.
Assessment of anthropometric parameters in healthy male participants at screening and the end of the study.
| Anthropometric parameters | Screening (n=18) | Day 31 (n=18) | P value |
|---|---|---|---|
| Body Fat Percentage (%) | 20.01 ± 6.76 | 19.92 ± 6.74 | 0.166 |
| Lean Body Weight (kg) | 45.32 ± 7.49 | 45.37 ± 7.45 | 0.252 |
| Biceps (mm) | 8.04 ± 2.76 | 8.00 ± 2.69 | 0.421 |
| Triceps (mm) | 12.46 ± 4.22 | 12.52 ± 4.27 | 0.271 |
| Subscapular (mm) | 18.14 ± 5.78 | 18.12 ± 5.84 | 0.439 |
| Suprailiac (mm) | 18.93 ± 5.24 | 18.91 ± 5.25 | 0.686 |
Data was analyzed by the student-dependent t-test. Significant at P-value <0.05.
3.9. Assessment of adverse events and tolerability
The detection of any clinical signs and symptoms during the clinical examination or the patients' self-reporting of adverse events served as the basis for recording adverse events. During the trial, there were no adverse effects, i.e., none. All the healthy volunteers participating in the safety research demonstrated tolerability to the WSE capsule at a dose of 500 mg twice daily for 30 days.
4. Discussion
Ashwagandha has many advantageous benefits for human health, so it is regarded as a marvelous herb. The name “Ashwagandha" originates from the Sanskrit language. It combines two Sanskrit words: “Ashwa," which means “horse." “Gandha," which means “smell" or “aroma." So, “Ashwagandha" roughly translates to “smell of a horse" or “horse-like smell" in reference to the distinctive aroma of the plant's young root, which is believed to resemble the smell of a horse [44]. Traditionally, Ashwagandha has been used in Ayurveda for ages as an adaptogen and ‘Rasayana' [45]. Although the root powder of W. somnifera has reportedly been used safely for ages in the Indian Ayurvedic medical system, every substance, plant, and component must first undergo thorough scientific testing for safety, efficacy, and adverse events before being accepted and used by the general public. Additionally, it is the alarming need of the hour to check the safety profile of standardized W. somnifera extract due to global demand and the constant watch of regulatory agencies of different countries.
The results of pre-clinical safety studies suggest that Ashwagandha and its constituents demonstrate a favorable safety profile, even when supplied at high doses. Singh et al. (1982) conducted a study that reported the LD50 value of the ethanolic extract of Ashwagandha to be 1750 ± 41 mg when administered orally to Swiss albino mice. A study conducted on mice examined a single intraperitoneal administration of varying doses (1.1, 1.2, 1.3, 1.4, or 1.5 g/kg) of an ethanol extract derived from Ashwagandha. The results indicated that the 1.1 g/kg dose did not induce animal mortality. However, as the dosage was incrementally increased by 100 mg/kg, a dose-dependent lethality was observed, with all animals succumbing to death at the highest dose of 1.5 g/kg [46]. Later, Singh et al. (2001) conducted a study to assess the acute toxicity in mice. The mice were separated into multiple groups, each consisting of 10 individuals. Various groups of mice were administered increasing dosages ranging from 100 to 3,000 mg/kg of an “active" fraction derived from a 70% alcohol extract. A separate group of mice was designated as the control group. No mortality or significant alterations in behavior were recorded throughout the 72-h observation period, even at doses as high as 3,000 mg/kg. Furthermore, no adverse effects were detected for the 15-day trial course, as reported in Ref. [47]. According to a study conducted by Sharada et al. in 1993, the intraperitoneal injection of an ethanol extract of Ashwagandha at a dosage of 100 mg/kg/day for 30 days in mice led to notable reductions in the weights of the thymus, adrenal glands, and the spleen. A considerable rise in blood acid phosphatase activity was also seen [48]. According to Gupta et al. (2022), a study assessed the toxicity of withaferin-A in mice when provided orally at doses up to 2,000 mg/kg for 28 days. The study results indicated no toxicity was observed in the mice at this dosage level. The no observed adverse effect level (NOAEL) was determined to be at least 500 mg/kg when extrapolated to rats [49]. In a study conducted by Antony et al. (2018), it was observed that there were no observed harmful effects following the repeated administering of Ashwagandha root and leaf extract at a maximum dose level of 1,000 mg/kg over 90 days. The hematological and biochemical profile of the treated rats exhibited similarities to that of the control animals, and the histopathological analysis of the major organs in all animals revealed normal findings. The NOAEL was established at 1,000 mg/kg [50]. Patel et al. 2016 reported no adverse effects in rats after oral administration of a methanolic ashwagandha extract standardized to 4.5% withaferin A at dosages of 500 mg/kg/day, 1,000 mg/kg/day and 2,000 mg/kg/day for 28 days [51].
In a recent publication by Balkrishna et al. (2021), a study was conducted to investigate the sub-acute toxicity of a whole plant extract of W. somnifera. In this experiment, Sprague Dawley rats of both genders were subjected to oral administration of WS whole plant (including roots, stem, leaves, berries, and seeds) for 28 days. The doses were 100, 300, and 1000 mg/kg/day. The research also incorporated a supplementary group of animals that were administered whole plant extract for 28 days, followed by a recovery period of 14 days. The safety of W. somnifera whole plant extract was assessed, and it was determined that doses up to 1000 mg/kg/day did not result in any detectable toxicologically significant data [52]. These findings collectively support the safe use of Ashwagandha and its derivatives in pre-clinical settings. However, it's important to note that clinical research involving healthy human participants is limited.
A prospective, open-label study evaluated an ashwagandha extract's acceptability, safety, and efficacy in 18 healthy persons. The study involved administering escalating doses of an aqueous extract of Ashwagandha to participants over 30 days. The dosage regimen consisted of 750 mg/day for the initial ten days, followed by an increase to 1,000 mg/day for the subsequent ten days, and reached 1,250 mg/day for the final ten days. The participants were then evaluated for any negative effects through self-reported adverse events and various medical tests, including hemogram, liver and renal function tests, fasting sugar levels, lipid profile, and electrocardiogram. One of the participants exhibited heightened appetite, heightened libido, and hallucinatory effects accompanied by vertigo at the lowest dosage, leading to their withdrawal from the trial. Otherwise, no other adverse events [32]. Gopukumar et al. (2021) examined the sustained-release ashwagandha root extract in a single randomized, double-blind, placebo-controlled investigation. The extract was provided to a cohort of 125 participants at a dosage of 300 mg once daily for 90 days. Throughout the trial, there were no recorded instances of adverse effects [53]. In a separate study conducted by Verma et al., 40 females and 40 males were administered a relatively low dosage of 300 mg of water extract twice daily for eight weeks. This study focused on healthy adult participants [33]. The primary safety outcomes assessed in this study were hematological parameters and serum biochemistry analysis, explicitly focusing on hepatotoxicity and thyroid function. Additional outcomes included documenting reported adverse effects and measuring basic vital signs such as body temperature, respiration, heart rate, and blood pressure. Within the context of the therapy group, there were no observed occurrences of adverse events or alterations in the parameters used for evaluation. In a phase I trial conducted by Pires et al. (2019), the authors examined the pharmacokinetics and safety of withaferin-A in individuals with high-grade, advanced osteosarcoma. The study's findings indicated that the formulation was well-tolerated by patients at a maximum dosage of 4800 mg, equivalent to a daily intake of 216 mg of withaferin A. No adverse effects were seen that would restrict the dosage. Among the cohort of 11 patients, it was observed that five individuals exhibited elevated liver enzymes at grade 1, whereas two patients reported the presence of a skin rash. In addition, there were instances of fever, fatigue, edema, and diarrhea. No adverse events of grade 3 or 4 were documented [54]. These studies collectively indicate that when administered within the specified dosages and under various trial conditions, most participants find ashwagandha extract well-tolerated with minimal adverse effects. However, individual responses may vary, and further research is essential to comprehensively assess its safety, especially in diverse populations and clinical contexts.
This study's primary goal was to assess the safety of the standardized capsule containing W. somnifera root extract (commercially branded as AgeVel®/Witholytin®) in a cohort of healthy male participants. The standardized capsule containing W. somnifera root extract has effectively ensured the safety of hematological, urine analysis, diagnostic, and biochemical organ function. No clinically or statistically significant alterations were seen in the individuals' chest X-ray and ECG results. No intolerance or side effects were noticed, and there was no significant alteration in vital functions, including blood pressure, respiration rate, oral temperature, and pulse rate, when contrasting the baseline and treatment end. No notable alterations were detected in the participant's cardiorespiratory endurance, body fat percentage, lean body weight, and skinfold thickness at the biceps, triceps, suprascapular, and suprailiac areas. In the context of the safety investigation of W. somnifera extract, it is noteworthy to mention that a total of thirty human clinical trials, approved by the Clinical Trials Registry of India (CTRI), have provided evidence of the satisfactory effectiveness of root preparations in treating diverse health issues. The conditions above encompass subclinical hypothyroidism, schizophrenia, chronic stress, insomnia, anxiety, memory and cognitive enhancement, stamina and strength improvement, obsessive-compulsive disorder, rheumatoid arthritis, type-2 diabetes, male infertility, fertility promotion in females, adaptogenic effects, growth promotion in children, and chemotherapy adjuvant activity.
Furthermore, empirical investigations have substantiated the efficacy of W. somnifera in enhancing cardiovascular performance, endurance capacity, and resilience against fatigue. Ashwagandha exhibits potential benefits in the management of body weight and possesses anti-aging effects, such as the augmentation of telomerase activity [22,27,55,56]. These findings highlight the potential therapeutic value of W. somnifera root extract in addressing a diverse range of health concerns.
Further, In the present study, total body weight and BMI were not changed significantly compared to screening, like the findings reported by Raut AA et al. 2012 [32]. Although previous research has indicated enhancements in VO2 max following an eight-week ashwagandha supplementation regimen among young adults and athletes, our investigation did not corroborate these results. The observed discrepancy could be ascribed to including healthy adult participants in our investigation, suggesting that a more extended study time could be required to detect substantial consequences. The current investigation showed no substantial alterations while evaluating the hepatic, renal, and thyroid function markers. Following ingestion of the W. somnifera root extract capsule, no appreciable alterations in the participants' hematological and biochemical indicators were seen. After treatment with the extract of W. somnifera, several other investigations have documented comparable findings [28,57]. The investigation observed that using WSE did not result in any noteworthy adverse effects or substantial alterations in hematological, biochemical, or vital parameters (P > 0.05). Furthermore, the participants showed a high level of tolerability towards the intervention. This study's observed adverse event outcomes are inconsistent with the previously reported data. The preceding research studies documented a distinct pattern of adverse effects, with the most prevalent being moderate and predominantly temporary somnolence, epigastric pain or discomfort, and loose stools observed in more than 5% of the individuals participating in the study. The less frequently seen adverse effects encompassed giddiness, sleepiness, hallucinations, vertigo, coughing, colds, nausea, constipation, dry mouth, nocturnal cramps, blurred vision, hyperacidity, skin rash, weight gain, and nasal congestion (rhinitis) [58]. Notably, the safety profile of W. somnifera may exhibit variability among individuals, and standardized extract may impact it. The limitation of the current study was a small sample size, and only male participants were enrolled. Therefore, trials in male and female participants and vigilant monitoring are imperative to comprehensively comprehend potential long-term effects or interactions with concurrent drugs. This clinical study presents scientific and clinical evidence about the safe use of standardized W. somnifera root extract capsules in healthy individuals.
5. Conclusions
The clinical study on the safety of W. somnifera root extract capsule (AgeVel®/Witholytin®) revealed that it was effectively tolerated by healthy male participants when administered orally at a dosage of 500 mg twice a day. Statistical significance (P < 0.05) was observed in total cholesterol, low-density lipoprotein, triglycerides, serum T3, serum T4, blood urea nitrogen, blood urea level, and total bilirubin. Still, participants' physical, hematological, and biochemical parameters, as well as their urine analysis, chest X-ray, and ECG results, all fell within the normal reference ranges. Throughout the four-week study period, no adverse effects were associated with administering the W. somnifera root extract capsule. Considering the results obtained from this study, it is recommended that comprehensive safety assessments be conducted on standardized W. somnifera root extract in both male and female healthy participants to provide a substantial understanding of the safety profile associated with long-term usage.
Conflicts of interest and funding
The authors declare no conflict of interest pertaining to this research work and have received no external funding from any funding agencies.
Data availability statement
All the data generated in the current research work has been included in the manuscript.
Ethical approval
The Institutional Ethics Committee of Lokmanya Medical Research Center, Pune, Maharashtra 411033, India, approved the study (clinical trial ethics approval protocol no. MHC/CT/22-23/015, approved on 28/03/2023; Ethics Committee Registration No. ECR/175/Inst/MH/2013/RR-19). The protocol and clinical trial have been approved by the Clinical Trial Registry-India (CTRI) with approval number CTRI/2023/04/051632 (Approved on 17/04/2023).
Informed consent
Informed consent was obtained from all participants included in this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge Mr. Vallabh Mulay, Mr. Ankit Bhatt, and Mr. Ganesh Saste (Pharmanza Herbal Pvt Ltd, Anand-388430, Gujarat, India) for providing investigational product and analysis data. The authors also acknowledge Dr. Vaishnavi Patil (Mprex Healthcare Pvt. Ltd., Pune-411057, Maharashtra, India) for supporting the clinical safety study of Withania somnifera extract.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaim.2023.100859.
Appendix A. Supplementary data
The following is the supplementary data to this article.
References
- 1.Kulkarni S.K., Dhir A. Withania somnifera: an Indian ginseng. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:1093–1105. doi: 10.1016/j.pnpbp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Modi S.J., Tiwari A., Ghule C., Pawar S., Saste G., Jagtap S., et al. Pharmacokinetic study of withanosides and withanolides from Withania somnifera using ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) Molecules. 2022;27:1476. doi: 10.3390/molecules27051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mirjalili M.H., Moyano E., Bonfill M., Cusido R.M., Palazón J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–2393. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhary M.I., Abbas S., Jamal S.A. Withania somnifera-A source of exotic withanolides. Heterocycles. 1996;2:555–556. 3.10.22038/IJBMS.2020.44254.10378. [Google Scholar]
- 5.Bandyopadhyay M., Jha S., Tepfer D. Changes in morphological phenotypes and withanolide composition of Ri-transformed roots of Withania somnifera. Plant Cell Rep. 2007;26:599–609. doi: 10.1007/s00299-006-0260-0. [DOI] [PubMed] [Google Scholar]
- 6.Ruknuddin G., Tillu G.S., Ahmad A., Kaushik R. 2023. Ashwagandha (Withania somnifera) safety dossier. New Delhi. Indian Ministry of AYUSH (Ayurveda, Yoga, Unani, Siddha, Homeopathy) p. 81.https://www.ayush.gov.in [Google Scholar]
- 7.Sharma P.V. Varanasi Chaukhamba Bharati Acad; 2009. DravyagunaVijnana, part 2; p. 376. [Google Scholar]
- 8.Sharma P VVaranasi Chaukhamba Bharati Acad2009Sūtrasthāna. Ch. 4., Ver. 10 [7]; p. 32.
- 9.Sūtrasthāna. Ch. 3., Ver. 8-9; p. 28.
- 10.Acharya Yadav ji Tricum ji . Carakasaṁhitā of agniveśa. Sūtrasthāna. Chaukhambha, Sanskrit Sansthan; Varanasi, India: 1941. p. 32. Ch. 4., Ver. 9 [2] [Google Scholar]
- 11.Kurapati K.R.V., Atluri V.S.R., Samikkannu T., Nair M.P.N. Ashwagandha (Withania somnifera) reverses β-amyloid1-42 induced toxicity in human neuronal cells: implications in HIV-associated neurocognitive disorders (HAND) PLoS One. 2013;8 doi: 10.1371/journal.pone.0077624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das R., Rauf A., Akhter S., Islam M.N., Emran T Bin, Mitra S., et al. Role of withaferin A and its derivatives in the management of Alzheimer's disease: recent trends and future perspectives. Molecules. 2021;26:3696. doi: 10.3390/molecules26123696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad M., Saleem S., Ahmad A.S., Ansari M.A., Yousuf S., Hoda M.N., et al. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum Exp Toxicol. 2005;24:137–147. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- 14.Kumar P., Kumar A. Possible neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of Huntington's disease. J Med Food. 2009;12:591–600. doi: 10.1089/jmf.2008.0028. [DOI] [PubMed] [Google Scholar]
- 15.Haque I.M., Mishra A., Kalra B.S., Chawla S. Role of standardized plant extracts in controlling alcohol withdrawal syndrome—an experimental study. Brain Sci. 2021;11:919. doi: 10.3390/brainsci11070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanjilal S., Gupta A.K., Patnaik R.S., Dey A. Analysis of clinical trial registry of India for evidence of anti-arthritic properties of Withania somnifera (ashwagandha) Alternative Ther Health Med. 2021;27:58–66. [PubMed] [Google Scholar]
- 17.Mahdi A.A., Shukla K.K., Ahmad M.K., Rajender S., Shankhwar S.N., Singh V., et al. Withania somnifera improves semen quality in stress-related male fertility. Evidence-Based Complement Altern Med. 2011;2011 doi: 10.1093/ecam/nep138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasimi Doost Azgomi R., Nazemiyeh H., Sadeghi Bazargani H., Fazljou S.M.B., Nejatbakhsh F., Moini Jazani A., et al. Comparative evaluation of the effects of Withania somnifera with pentoxifylline on the sperm parameters in idiopathic male infertility: a triple‐blind randomised clinical trial. Andrologia. 2018;50 doi: 10.1111/and.13041. [DOI] [PubMed] [Google Scholar]
- 19.Nayak S., Nayak S., Panda B.K., Das S. A clinical study on management of stress in type-2 diabetes mellitus (madhumeha) with ashwagandha (Withania somnifera) Ayushdhara. 2015;2:413–417. [Google Scholar]
- 20.Deshpande A., Irani N., Balkrishnan R., Benny I.R. A randomized, double blind, placebo controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020;72:28–36. doi: 10.1016/j.sleep.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Bake C., Kirby J.B., O'Connor J., Lindsay K.G., Hutchins A., Harris M. The perceived impact of ashwagandha on stress, sleep quality, energy, and mental clarity for college students: qualitative analysis of a double-blind randomized control trial. J Med Food. 2022;25:1095–1101. doi: 10.1089/jmf.2022.0042. [DOI] [PubMed] [Google Scholar]
- 22.Agnihotri A.P., Sontakke S.D., Thawani V.R., Saoji A., Goswami V.S.S. Effects of Withania somnifera in patients of schizophrenia: a randomized, double blind, placebo controlled pilot trial study. Indian J Pharmacol. 2013;45:417. doi: 10.4103/0253-7613.115012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A.K., Basu I., Singh S. Efficacy and safety of ashwagandha root extract in subclinical hypothyroid patients: a double-blind, randomized placebo-controlled trial. J Alternative Compl Med. 2018;24:243–248. doi: 10.1089/acm.2017.0183. [DOI] [PubMed] [Google Scholar]
- 24.Wijeratne E.M.K., Xu Y.-M., Scherz-Shouval R., Marron M.T., Rocha D.D., Liu M.X., et al. Structure–activity relationships for withanolides as inducers of the cellular heat-shock response. J Med Chem. 2014;57:2851–2863. doi: 10.1021/jm401279n. [DOI] [PubMed] [Google Scholar]
- 25.Indian Ministry of AYUSH Government of India. Advisory for refrain from use of Aswagandha (Withania somnjfera) leaves. 2021. https://cdn.ayush.gov.in/wp-content/uploads/2021/10/advisory-on-aswagandha.pdf
- 26.Dongre S., Langade D., Bhattacharyya S. Efficacy and safety of Ashwagandha (Withania somnifera) root extract in improving sexual function in women: a pilot study. BioMed Res Int. 2015;2015 doi: 10.1155/2015/284154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wankhede S., Langade D., Joshi K., Sinha S.R., Bhattacharyya S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. J Int Soc Sports Nutr. 2015;12:43. doi: 10.1186/s12970-015-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shenoy S., Chaskar U., Sandhu J.S., Paadhi M.M. Effects of eight-week supplementation of Ashwagandha on cardiorespiratory endurance in elite Indian cyclists. J Ayurveda Integr Med. 2012;3:209. doi: 10.4103/0975-9476.104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gopal S., Ajgaonkar A., Kanchi P., Kaundinya A., Thakare V., Chauhan S., et al. Effect of an ashwagandha (Withania Somnifera) root extract on climacteric symptoms in women during perimenopause: a randomized, double‐blind, placebo‐controlled study. J Obstet Gynaecol Res. 2021;47:4414–4425. doi: 10.1111/jog.15030. [DOI] [PubMed] [Google Scholar]
- 30.Tiwari S., Gupta S.K., Pathak A.K. A double-blind, randomized, placebo-controlled trial on the effect of Ashwagandha (Withania somnifera dunal.) root extract in improving cardiorespiratory endurance and recovery in healthy athletic adults. J Ethnopharmacol. 2021;272 doi: 10.1016/j.jep.2021.113929. [DOI] [PubMed] [Google Scholar]
- 31.Lopresti A.L., Smith S.J. Ashwagandha (Withania somnifera) for the treatment and enhancement of mental and physical conditions: a systematic review of human trials. J Herb Med. 2021 doi: 10.1016/j.hermed.2021.100434. [DOI] [Google Scholar]
- 32.Raut A.A., Rege N.N., Tadvi F.M., Solanki P.V., Kene K.R., Shirolkar S.G., et al. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J Ayurveda Integr Med. 2012;3:111. doi: 10.4103/0975-9476.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma N., Gupta S.K., Tiwari S., Mishra A.K. Safety of ashwagandha root extract: a randomized, placebo-controlled, study in healthy volunteers. Compl Ther Med. 2021;57 doi: 10.1016/j.ctim.2020.102642. [DOI] [PubMed] [Google Scholar]
- 34.Chandrasekhar K., Kapoor J., Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med. 2012;34:255–262. doi: 10.4103/0253-7176.106022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choudhary D., Bhattacharyya S., Joshi K. Body weight management in adults under chronic stress through treatment with ashwagandha root extract: a double-blind, randomized, placebo-controlled trial. J Evid Based Complementary Altern Med. 2017;22:96–106. doi: 10.1177/2156587216641830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nabi T., Rafiq N., Qayoom O. Assessment of cardiovascular fitness [VO2 max] among medical students by Queens College step test. Int J Biomed Adv Res. 2015;6:418–421. doi: 10.7439/ijbar. [DOI] [Google Scholar]
- 37.Girme A., Saste G., Pawar S., Balasubramaniam A.K., Musande K., Darji B., et al. Investigating 11 withanosides and withanolides by UHPLC–PDA and mass fragmentation studies from ashwagandha (Withania somnifera) ACS Omega. 2020;5:27933–27943. doi: 10.1021/acsomega.0c03266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United States Pharmacopeial Convention . In: Dietary supplements compendium. Rockville, MD. Pharmacopeia U.S., Formulary N., editors. 2019. Ashwagandha root; powdered ashwagandha root; and powdered ashwagandha root extract. [Google Scholar]
- 39.United States Pharmacopeial chapter <561> articles of botanical origin. Page No USP 43- NF 38- 6774.
- 40.United States Pharmacopeia . USP-NF; Rockville, MD: United States Pharmacopeia: 2023. General chapter, <2040> disintegration and dissolution of dietary supplements. [Google Scholar]
- 41.United States Pharmacopeia . USP-NF; Rockville, MD: United States Pharmacopeia: 2023. General chapter, <2091> weight variation of dietary supplements. [Google Scholar]
- 42.United States Pharmacopeia . USP-NF; Rockville, MD: United States Pharmacopeia: 2023. General chapter, <233> elemental impurities—procedures. [Google Scholar]
- 43.United States Pharmacopeia . USP-NF; Rockville, MD: United States Pharmacopeia: 2023. General chapter, <2022> microbiological procedures for absence of specified microorganisms—nutritional and dietary supplements. [Google Scholar]
- 44.Mikulska P., Malinowska M., Ignacyk M., Szustowski P., Nowak J., Pesta K., et al. Ashwagandha (Withania somnifera)—current research on the health-promoting activities: a narrative review. Pharmaceutics. 2023;15:1057. doi: 10.3390/pharmaceutics15041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langade D., Kanchi S., Salve J., Debnath K., Ambegaokar D. Efficacy and safety of Ashwagandha (Withania somnifera) root extract in insomnia and anxiety: a double-blind, randomized, placebo-controlled study. Cureus. 2019;11 doi: 10.7759/cureus.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh N., Nath R., Lata A., Singh S.P., Kohli R.P., Bhargava K.P. Withania somnifera (ashwagandha), a rejuvenating herbal drug which enhances survival during stress (an adaptogen) Int J Crude Drug Res. 1982;20:29–35. doi: 10.3109/13880208209083282. [DOI] [Google Scholar]
- 47.Singh B., Saxena A.K., Chandan B.K., Gupta D.K., Bhutani K.K., Anand K.K. Adaptogenic activity of a novel, withanolide‐free aqueous fraction from the roots of Withania somnifera Dun. Phyther Res. 2001;15:311–318. doi: 10.1002/ptr.858. [DOI] [PubMed] [Google Scholar]
- 48.Sharada A.C., Solomon F.E., Devi P.U. Toxicity of Withania somnifera root extract in rats and mice. Int J Pharmacogn. 1993;31:205–212. doi: 10.3109/13880209309082943. [DOI] [Google Scholar]
- 49.Gupta S.K., Jadhav S., Gohil D., Panigrahi G.C., Kaushal R.K., Gandhi K., et al. Safety, toxicity and pharmacokinetic assessment of oral Withaferin-A in mice. Toxicol Rep. 2022;9:1204–1212. doi: 10.1016/j.toxrep.2022.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antony B., Benny M., Kuruvilla B.T., Gupta N.K., Sebastian A., Jacob S. Acute and sub chronic toxicity studies of purified Withania Somnifera extract in rats. Int J Pharm Pharmaceut Res. 2018;10:41–46. doi: 10.22159/ijpps.2018v10i12.29493. [DOI] [Google Scholar]
- 51.Patel S.B., Rao N.J., Hingorani L.L. Safety assessment of Withania somnifera extract standardized for Withaferin A: acute and sub-acute toxicity study. J Ayurveda Integr Med. 2016;7:30–37. doi: 10.1016/j.jaim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balkrishna A., Sinha S., Srivastava J., Varshney A. Withania somnifera (L.) Dunal whole-plant extract demonstrates acceptable non-clinical safety in rat 28-day subacute toxicity evaluation under GLP-compliance. Sci Rep. 2022;12 doi: 10.1038/s41598-022-14944-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gopukumar K., Thanawala S., Somepalli V., Rao T.S., Thamatam V.B., Chauhan S. Efficacy and safety of ashwagandha root extract on cognitive functions in healthy, stressed adults: a randomized, double-blind, placebo-controlled study. Evidence-Based Complement Altern Med. 2021;2021 doi: 10.1155/2021/8254344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pires N., Gota V., Gulia A., Hingorani L., Agarwal M., Puri A. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: A phase I trial. J Ayurveda Integr Med. 2020;11:68–72. doi: 10.1016/j.jaim.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choudhary D., Bhattacharyya S., Bose S. Efficacy and safety of Ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599–612. doi: 10.1080/19390211.2017.1284970. [DOI] [PubMed] [Google Scholar]
- 56.Ambiye V.R., Langade D., Dongre S., Aptikar P., Kulkarni M., Dongre A. Clinical evaluation of the spermatogenic activity of the root extract of Ashwagandha (Withania somnifera) in oligospermic males: a pilot study. Evidence-Based Complement Altern Med. 2013;2013 doi: 10.1155/2013/571420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandhu J.S., Shah B., Shenoy S., Chauhan S., Lavekar G.S., Padhi M.M. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int J Ayurveda Res. 2010;1:144. doi: 10.4103/0974-7788.72485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tandon N., Yadav S.S. Safety and clinical effectiveness of Withania Somnifera (Linn.) Dunal root in human ailments. J Ethnopharmacol. 2020;255 doi: 10.1016/j.jep.2020.112768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated in the current research work has been included in the manuscript.