Abstract
This study aims to explore the anti-proliferative, pro-apoptotic, and anti-migration activities of liraglutide (LGT) in MCF-7 breast cancer (BC) cells in subjects with obesity, particularly its effects on the PI3K/Akt/mTOR/AMPK pathway. The role of AMPK/SIRT-1, an essential regulator of adipokine production, in the effect of LGT on the production of adipose-derived adipokine was also assessed. MCF-7 cells were incubated in conditioned medium (CM) generated from adipose-derived stem cells (ADSCs) of obese subjects. MCF-7 cells were then treated with LGT for 72 h. Anti-proliferative, pro-apoptotic, and anti-migration activities were investigated using alamarBlue, annexin V stain, and scratch assay, respectively. Protein levels of phosphorylated PI3K, p-Akt, p-mTOR, and p-AMPK were investigated using immunoblotting. Levels of adipokines in ADSCs were determined using RT-PCR before and after transfection of ADSCs using the specific small interference RNA sequences for AMPK and SIRT-1. LGT evoked anti-proliferative, apoptotic, and potential anti-migratory properties on MCF-7 cells incubated in CM from obese ADSCs and significantly mitigated the activity of the PI3K/Akt/mTOR survival pathway—but not AMPK—in MCF-7 cells. Furthermore, the anti-proliferative effects afforded by LGT were similar to those mediated by LY294002 (PI3K inhibitor) and rapamycin (mTOR inhibitor). Our results reveal that transfection of AMPK/SIRT-1 genes did not affect the beneficial role of LGT in the expression of adipokines in ADSCs. In conclusion, LGT elicits anti-proliferative, apoptotic, and anti-migratory effects on BC cells in obese conditions by suppressing the activity of survival pathways; however, this effect is independent of the AMPK/SIRT1 pathway in ADSCs or AMPK in BC cells.
Keywords: Breast Cancer, Obesity, Liraglutide, Akt, PI3K, mTOR
1. Introduction
In women, breast cancer (BC) is the most prevalent cancer and the leading cause of cancer death (Sung et al., 2021). Obesity is associated with increased BC cell proliferation, invasion, and migration, leading to a poor prognosis and elevated mortality rate among obese women (Haakinson et al., 2012). Data reveal that 35–40 % of all BC deaths have been attributed to overweight and obesity (Jiralerspong and Goodwin, 2016).
One mechanism that underlies obesity’s negative effect on BC cell behavior is the impact of obesity on modulating adipose tissue-secreted adipokines, including inflammatory cytokines, adiponectin, and leptin (Jarde et al., 2009, Dubois et al., 2013, Fasshauer and Bluher, 2015, Orecchioni et al., 2015, Panno et al., 2016). Adiponectin imposes pro-apoptotic, anti-proliferative, and anti-inflammatory effects on BC cells (Jarde et al., 2011, Dubois et al., 2013, Fasshauer and Bluher, 2015, Divella et al., 2016, Panno et al., 2016), while leptin exerts pro-proliferative and anti-apoptotic properties (Jarde et al., 2009, Dubois et al., 2013, Orecchioni et al., 2015). Obesity leads to an abnormal profile of adipokines, involving elevated levels of pro-inflammatory cytokines and leptin from obese adipose tissue that, together with reduced adiponectin, lead to the development and migration of BC cells (Fasshauer and Bluher, 2015).
Previous studies uncovered the antagonistic effects of adiponectin and leptin on BC cell proliferation through the modulation of numerous signaling pathways (Jarde et al., 2011, Panno et al., 2016). Among them, adiponectin inhibits the activity of phosphatidylinositol 3 kinase, Akt, and the mammalian target of rapamycin (PI3K/Akt/mTOR), a vital pathway that enhances cell proliferation, survival, metabolism, and migration (Fresno Vara et al., 2004, Sarbassov et al., 2005, Dowling et al., 2010). The activation of this pathway promotes resistance to apoptosis, cell transformation, and tumor initiation and has a significant role in BC development (Nunnery and Mayer, 2020). Evidence points to the frequent activation of PI3K/Akt/mTOR signaling in BC (Mayer and Arteaga, 2016); along these lines, approximately 70 % of breast tumorigenesis results from a gene mutation that can activate this pathway (Vasan et al., 2019). Adiponectin plays a significant part in inhibiting cancer proliferation via suppressing this pathway (Jarde et al., 2011, Dubois et al., 2013, Panno et al., 2016). Additionally, adiponectin enhances the activity of AMP-activated protein kinase (AMPK), a tumor-suppressing kinase (Li et al., 2015). Enhanced AMPK activity suppresses the activity of many downstream proteins involved in cell proliferation, such as mTOR (Xu et al., 2012, Ponnusamy et al., 2020). Through its receptors on cancer cells, phosphorylates, and activation of AMPK, adiponectin inhibits mTOR, eventually leading to suppressed carcinogenesis (Jarde et al., 2009, Jarde et al., 2011, Panno et al., 2016). In contrast to adiponectin, leptin mediates the proliferative activity of BC by activating the PI3K/Akt/mTOR survival pathway through phosphorylating Akt (Jarde et al., 2009, Jarde et al., 2011). Leptin also attenuates AMPK activation (Cirillo et al., 2008, Macciò et al., 2010). Therefore, the leptin/adiponectin pathway is a promising target for attenuating the proliferation and migration of BC cells in obese conditions.
Liraglutide (LGT), an agonist of the glucagon-like peptide-1 receptor (GLP-1R), is an anti-diabetic drug that possesses many valuable properties, including body weight reduction (Vilsboll et al., 2012), anti-inflammatory effects, (Ye et al., 2019) and anti-neoplastic activity (Miao et al., 2013, Zhao et al., 2014, Zhao et al., 2018). In the current study, we aimed to investigate the anti-proliferative, anti-migratory, and pro-apoptotic effects of LGT on BC cells in obese conditions. Furthermore, we studied whether these effects could be mediated via modulating leptin/adiponectin pathways, including the PI3K/Akt/mTOR and AMPK pathways in BC cells.
Among the adipose tissue cells that exhibit an altered secretory profile in obesity are adipose tissue-derived stem cells (ADSCs). Our recent study (Alanteet et al., 2021) and previous studies (Sabol et al., 2019, Strong et al., 2013, Strong et al., 2015, Strong et al., 2016) reported that obese subjects-derived ADSCs express higher levels of pro-inflammatory cytokines and leptin; however, the production of adiponectin was reduced. According to prior research, adipokine production from adipose tissue is regulated by many factors involving the energy sensors AMPK and sirtuin 1 (SIRT-1) (Lihn et al., 2004, Liu et al., 2007, Qiang et al., 2007, Gillum et al., 2011). In insulin resistance and metabolic diseases associated with obesity, the activation of AMPK and SIRT-1 was found to increase adiponectin levels (Lihn et al., 2004, Liu et al., 2007, Qiang et al., 2007, Granata et al., 2012) while inhibiting the adipose tissue secretion of leptin and pro-inflammatory cytokines (Lihn et al., 2004, Gillum et al., 2011, Granata et al., 2012). Furthermore, some scholars have asserted that adiponectin secretion from 3 T3-L1 cell lines is stimulated by the activation of AMPK and SIRT-1 and that their inhibition leads to decreased adiponectin secretion (Liu et al., 2007, Qiang et al., 2007, Shiota et al., 2012). In our recent work, we demonstrated, for the first time, that LGT elicits anti-proliferative activity on MCF-7 human BC cells by correcting disrupted adipokine secretions from obese ADSCs (Alanteet et al., 2021). In the current study, we aim to confirm the beneficial role of LGT in adipokine secretion from obese ADSCs and to further investigate whether the activation of AMPK/SIRT1 is implicated in these effects.
In summary, the current study aims to accomplish the following: 1) to investigate the anti-proliferative, pro-apoptotic, and anti-migratory effects of LGT on human BC cells cultured in obese ADSCs-conditioned media; 2) to investigate whether the anti-proliferative activity of LGT on BC in obese conditions is mediated via modulating leptin/adiponectin pathways, including the PI3K/Akt/mTOR and AMPK pathways in BC cells; 3) to study the role of AMPK/SIRT1 in the effects of LGT on adipokine secretion from obese ADSCs.
2. Materials and methods
2.1. Materials
LGT (sold under the brand name Victoza®, 6 mg/ml pre-filled pen, Novo Nordisk) was purchased from a local pharmacy in the city of Riyadh in the Kingdom of Saudi Arabia. MCF-7 cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). Fetal bovine serum (FBS), bovine serum albumin (BSA), Dulbecco’s Modified Eagle medium (DMEM), and collagenase type I were obtained from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies, including anti-phospho-Akt (p-Akt), anti-total Akt, anti-p-PI3K, anti-p-AMPK, anti-total AMPK, anti-p-m-TOR, and anti-total m-TOR were bought from Cell Signaling Technology, Inc. (Beverly, MA, USA). Rabbit polyclonal anti-β-actin was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The secondary antibody, anti-rabbit horseradish peroxidase-conjugated antibody LY294002 (PI3K inhibitor; Cat# 154447) and rapamycin (mTOR inhibitor; Cat# 553211), were obtained from Sigma-Aldrich.
2.2. Human subjects and isolation of ADSCs
Two groups of adipose tissues were isolated from the abdominal subcutaneous (SC) fat of healthy female subjects during plastic surgery (King Saud Medical City, Riyadh, Saudi Arabia). The first group was obtained from lean females with a body mass index (BMI) of 18.5–25 kg/m2. In contrast, the second group was obtained from obese females with a BMI of 30–35 kg/m2. The work was completed in agreement with the ethical principles for medical research outlined in the 1964 Declaration of Helsinki, and all patients provided informed consent. The protocol was approved by King Saud University Medical City, Riyadh, Saudi Arabia (Approval No. E-19–4477).
Lipoaspirates from SC adipose tissue of lean and obese subjects were incubated in 0.1 % sterile collagenase 1A solution. The collagenase and lipoaspirate mixture was shaken at 37 °C and 250 rpm for 2 h until the adipose tissue layer acquired a smooth appearance. The mixture was then centrifuged, and the top oily layer, cell debris, and floating fat cells were removed to isolate the cell pellet. ADSCs were taken by adherence after suspending the cell pellet in DMEM with penicillin, streptomycin, and 10 % FBS and seeding the cells in T25 flasks.
2.3. Generation of conditioned media (CM) from ADSCs (ADSCs-CM)
ADSCs-CM were produced by seeding ADSCs from subjects (lean or obese) at a density of 6 × 106 from passage 4–6 in DMEM with 10 % FBS until they reached 80 % confluence. After changing the media, the cells were incubated for 48 h before the supernatant was collected and used as ADSCs-CM.
2.4. Characterization of ADSCs
ADSCs differentiate into different cell types, such as adipocytes, osteocytes, and chondrocytes. Notably, this differentiation can be triggered by certain chemicals or cytokines. In the present study, ADSCs are characterized by adipogenic differentiation and flow cytometry of surface markers.
2.4.1. Adipogenic differentiation
Twelve-well plates were used to seed the cells in standard DMEM growth medium until the cells reached between 80 and 90 % confluence, then ADSCs were induced to differentiate. Adipogenic differentiation was induced by supplementing the culture medium with 1 % pen-strep, 10 % horse serum, 10 % FBS, 100 nM dexamethasone, 0.45 mM isobutylmethylxanthine, 3 μg/mL insulin, and 1 μM rosiglitazone. This medium was changed every 72 h until mature adipocytes were achieved within 10–14 days of initiating differentiation. Lipid droplets were visible beginning on day 5 and could be visualized by staining the cells with Oil Red O solution on day 10. For staining, the cells were fixed with a 10 % formalin solution for 10 min. before cells were rinsed with 3 % isopropanol and stained for one hour with Oil Red O solution.
2.4.2. Flow cytometry of surface markers
Cells were digested by trypsinization, then harvested by centrifugation at 1500 rpm for 5 min, washed in ice-cold phosphate-buffered saline (PBS), and finally resuspended at a ratio of 105 cells/antibody. Cells were then incubated for 30 min. on ice in the dark with the appropriate isotype controls or the preconjugated antibodies. Next, cells were washed and resuspended in PBS (500 μL) and then analyzed in the Becton Dickinson FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) following the method described by Elsafadi et al. (40). The following antibodies were used: APC Mouse Anti-Human CD44, FITC-PE-APC-mouse-IgG1k-isotype-control, FITC Mouse Anti-Human CD45, FITC Mouse Anti-Human CD31, PE-mouse-anti-human-CD73, PE Mouse Anti-Human CD29, and PE Mouse Anti-human HL-ADR (all available from BD Biosciences).
2.5. Evaluating the effect of LGT on the proliferation, apoptosis, and migration of MCF-7 cells cultured in lean/obese ADSCs-CM
MCF-7 BC cells were seeded in 96-well plates (7 × 103/well) and cultured in CM from lean/obese ADSCs mixed with 1 % FBS/DMEM at a ratio of 1:1 for 72 h with or without 50 µM of LGT (Alanteet et al., 2021). Next, the following tests were performed to investigate the effects of LGT on the growth, apoptosis, and migration of BC cells.
2.5.1. AlamarBlue cell proliferation assay
The percentage of MCF-7 cell proliferation was measured after 72 h via alamarBlue assay as described previously (Alanteet et al., 2021).
2.5.2. Apoptotic assay: Staining with annexinV/propidium iodide
The principle of performing an assay is as follows: Shortly after the initiation of apoptosis, the membrane phosphatidylserine is translocated from the cellular membrane to the cellular surface. Subsequently, phosphatidylserine can be detected via staining with annexin V fluorescent conjugate. Furthermore, propidium iodide can bind to DNA where the cell membrane in these cells has been completely damaged. In the current study, MCF-7 cells were treated with 50 µM LGT and various conditioned media for 72 h and then suspended in the binding buffer. Annexin V-FITC and propidium iodide (50 µg/mL) were then added to the cells, which were subsequently kept at room temperature for 5 min in the dark. Annexin V-FITC binding and phosphatidylserine staining were evaluated by flow cytometry using an FITC detector and a phycoerythrin emission detector, respectively.
2.5.3. Cell migration test: Scratch assay
In this assay, which is also called wound healing, MCF-7 cells were seeded in 12-well plates (20 × 104/well) and allowed to reach 80 % confluence in a single layer. A sterile pipette tip was used to scratch the cell bed along the diameter of the plate. Following washing by PBS, cells were cultured with LGT in the presence of obese or lean ADSCs-CM in DMEM containing 1 % FBS. Photographs were then taken under a microscope (200 × ) at 0, 24, and 48 h after scratch emergence. The relative migration distance or relative scratch closure was calculated using ImageJ software as follows:
Relative scratch closure = mean scratch width − mean remaining width/mean scratch width.
2.6. Elucidating the effect of LGT on the leptin-adiponectin pathways in MCF-7 cells
We employed immunoblotting to assess the protein levels of p-PI3K, total/phosphorylated AMPK, Akt, and mTOR in the MCF-7 cells incubated in CM from lean/obese ADSCs with or without LGT. Specifically, MCF-7 cells were seeded in 25 flasks at a density of 2 × 106 cells and treated with LGT for 18 h in the presence of lean or obese ADSCs-CM in DMEM/1% FBS. Cells were lysed with RIPA buffer containing the protease inhibitor cocktail. Samples containing 30 μg of protein were mixed with 7.5 µL of loading buffer, denatured, and separated using vertical discontinuous SDS-PA gel electrophoresis. The separated proteins were electrophoretically transferred to PVDF membranes (Immun-Blot®, Bio-Rad, CA, USA). Membranes were blocked with 3 % BSA and then incubated with the primary antibodies overnight at 4° C. The following primary antibodies were used: anti-AMPK, anti p-AMPK (1:1000 dilution), anti p-PI3K (1:1000), anti-Akt, anti p-Akt (1:1000), anti mTOR (1:1000), anti p-mTOR (1:1000), and anti-β-actin (1:2000). After washing to remove excess antibody, membranes were incubated with HRP-conjugated secondary rabbit antibody for 2 h at room temperature. Blots were settled with ECL detection reagents for 2 min, and the immunoreactive bands were pictured using the Bio-Rad Gel Doc system. Finally, the intensities of the different protein bands were quantified using ImageJ software. The relative values were adjusted to the control and expressed as fold of induction.
2.7. Elucidating the anti-proliferative activity of LGT compared to the effect of the pathway inhibitors
Cells (around 7,000) were cultured in basal media in a 96-well plate for 24 h. Next, the media were replaced with either basal media or conditioned media that included the following pathway inhibitors: LY294002 (20 µM) to block PI3K and rapamycin (20 nM) to block mTOR. These inhibitors were used with or without LGT. After 72 h, cell proliferation was evaluated h using 10 % alamarBlue as previously described (Alanteet et al., 2021).
2.8. Investigating the effect of LGT on the production of adiponectin, leptin, and IL-6 in ADSCs
The effect of LGT on the gene expression of specific adipokines, including adiponectin, leptin, and IL-6, in ADSCs was analyzed by qRT-PCR as previously described (Alanteet et al., 2021). All primer sequences (Table 1) were selected based on the previous literature (Keller et al., 2003, Jarde et al., 2009, Bullwinkle et al., 2016).
Table 1.
Primer sequences for adiponectin, leptin, and IL-6 genes.
| Primer | (5′-3′) Forward | (3′-5′) Reverse |
|---|---|---|
| Adiponectin | TGGTGAGAAGGGTGAGAA | AGATCTTGGTAAAGCGAATG |
| Leptin | GTAGGAATCGCAGCGCC | AAAGATAGGGCCAAAGCCAC |
| IL-6 | GGTACATCCTCGACGGCATCT | CACTTTCGTCGTTTCTCCGTG |
2.9. Transfection of small interfering RNA (siRNA)
To study the role of SIRT-1 and AMPK on the effect of LGT on the ADSCs-secreted adipokines, we transfected ADSCs with the specific siRNA sequences for each gene (Thermo Fischer Scientific Inc., MA), then treated with LGT, and adipokine levels were redetermined using RT-PCR. For siRNA transfection, 80 % confluent ADSCs were transfected by forward transfection with SIRT-1 siRNA (siRNA assay ID: 136457, cat. no. AM-16708) and AMPK siRNA (assay ID: 767, cat. no. AM-51331) using Lipofectamine RNAiMAX reagent and serum-free Opti-MEM® I medium, according to the manufacturer’s protocol. Cells were treated (after 24 h of transfection) with LGT for 72 h, and then cells were processed for qRT-PCR assay of leptin, adiponectin, and IL-6.
2.10. Statistical analysis
Data are presented as mean ± standard error of the mean. Two-way analysis of variance (ANOVA) followed by the Tukey-Kramer test was performed to make multiple comparisons between the different groups. A paired t-test was used to compare the data of the same group before and after treatment, while an unpaired t-test was employed to compare the data from the two different groups. GraphPad Prism software was used for statistical analysis and generating figures. Statistical differences were considered significant when P < 0.05.
3. Results
3.1. ADSCs characterization
3.1.1. Adipogenic differentiation
In a comparison of obese and lean ADSCs, no significant differences were observed in their response to adipocytic differentiation. Both types of cells were able to undergo adipogencity, and they formed lipid droplets in the mature adipocytes, as represented by Oil Red O staining (Fig. 1A).
Fig. 1.
Characterization of adipose tissue stem cells (ADSCs): Adipogenicity (Fig. 1A): cells were able to undergo adipogenicity, and they formed mature lipid-filled adipocytes that were visualized by Oil Red O staining. Expression of cell surface markers: Cells were positive for CD44, CD29, and CD73 (Fig. 1B) and negative for CD45, HLADR, and CD31 (Fig. 1C).
3.1.2. Expression of cell surface markers
According to the previous reports (Mildmay-White and Khan, 2017, Fonseca et al., 2023), the most reported positive markers for ADSCs were found to include CD73 (52.0 %), CD44 (42.1 %), and CD29 (27.6 %); in contrast, the most commonly found negative markers were CD31, CD45, and HLA-DR. Therefore, we used these markers in our study. In the present study, both lean and obese ADSCs showed the same expressed surface marker profile characteristics of human adipocyte stem cells (>90 %): Cells were positive for CD44, CD29, and CD73 (Fig. 1B) and were negative for the following marker profile CD45, HLADR, and CD31 (Fig. 1C).
3.2. Effect of LGT on proliferation, apoptosis, and migration of MCF-7 cells
3.2.1. Effect of LGT on the proliferation of MCF-7 cells
The proliferation percentage of untreated MCF-7 cells was significantly higher by approximately 33.33 % in CM from obese ADSCs compared to the proliferation percentage in CM from lean ADSCs (160.5 % ± 5.4 vs. 107 % ± 3.7, P < 0.001) (Fig. 2). The growth of LGT-treated MCF-7 cells in CM from obese ADSCs was significantly decreased by approximately 48 % compared to pre-LGT treatment (83.8 ± 0.7 vs. 160.5 ± 5.4, P < 0. 001). In addition, LGT suppressed the MCF-7 cell proliferation in CM from lean ADSCs by 37.6 % (107 % ± 3.7 vs. 66.87 % ± 1.45, P < 0.001).
Fig. 2.
Effect of liraglutide on the proliferation of MCF-7 cells in obese and lean ADSCs-CM with alamarBlue test.
3.2.2. Effect of LGT on cell apoptosis
Treatment with LGT for 72 h resulted in a significant late apoptotic effect on MCF-7 cells, as revealed by the annexin V staining test (Fig. 3). LGT increased the percentage of apoptotic MCF-7 cells in CM from obese ADSCs (6.3 ± 0.08 vs. 2.3 ± 0.09, P < 0.001) and in CM from lean ADSCs (5.1 ± 0.07 vs. 3.53 ± 0.34, P < 0.001) compared to the pre-treatment percentage.
Fig. 3.
Apoptotic effects of liraglutide on MCF-7 cells incubated in lean and obese ADSCs-CM using annexin V staining. Quantitative data are represented as mean ± SEM. ***P < 0.001 using paired t-test.
3.2.3. Effect of LGT on cell migration activity
Results of the scratch assay (Fig. 4) revealed that MCF-7 cells cultured in CM from lean or obese ADSCs showed a significant migratory property after 24 h, and the scratch was greatly healed after 48 h. The ratio of wound closure was significantly higher in MCF-7 cells incubated in CM from obese ADSCs compared to those incubated in CM from lean ADSCs after 24 h (0.57 ± 0.034 vs. 0.4 ± 0.028, P < 0.001) and 48 h (0.79 ± 0.022 vs. 0.54 ± 0.027, P < 0.001) (Fig. 4A). Treatment with LGT significantly restricted the migratory property of MCF-7 cells cultured in CM from either lean or obese ADSCs after 24 h, with the highest restriction reached at the end of 48 h. In particular, following LGT treatment, the ratio of wound closure in lean ADSCs-CM was 0.3 ± 0.013 vs. 0.4 ± 0.028 (P < 0.05) after 24 h and 0.41 ± 0.022 vs. 0.54 ± 0.027 (P < 0.01) after 48 h (Fig. 4B). In obese ADSCs-CM, the ratio of wound healing was 0.3 ± 0.026 vs. 0.57 ± 0.034 (P < 0.001) and 0.43 ± 0.031 vs. 0.79 ± 0.022 (P < 0.001) after 24 and 48 h, respectively (Fig. 4C). Thus, our results suggest that LGT can restrict cell migration activity; notably, the effect on obese conditions was significantly higher than in lean either after 24 or 48 h.
Fig. 4.
Cell migration activity determined by scratch assay (wound healing assay): A) The ratio of wound closure (mean ± SEM) in MCF-7 cells incubated in lean ADSCs-CM compared to those incubated in obese ADSCs-CM for 24 and 48 h. ***P < 0.001, using unpaired t-test. B) Cell migration activity in MCF-7 cells incubated in lean ADSCs-CM following exposure to liraglutide for 24 and 48 h. The ratio of wound closure is presented as mean ± SEM. **P < 0.01, *P < 0.05 using paired t-test. C) Cell migration activity in MCF-7 cells incubated in obese ADSCs-CM following exposure to liraglutide for 24 and 48 h. Ratio of wound closure is presented as mean ± SEM. ***P < 0.001.
3.3. Effects of LGT on adiponectin/leptin pathways
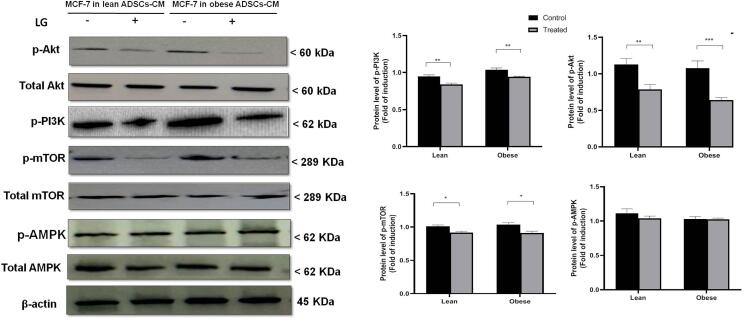
To examine the molecular mechanism of the anti-proliferative, anti-migratory, and pro-apoptotic effects of LGT on BC cells in CM from obese ADSCs, we assayed its effects on the PI3K/Akt/MTOR and AMPK. Fig. 8 As Fig. 5 illustrates, the protein levels of p-PI3K were significantly reduced in LGT-treated MCF-7 cultured in CM from either lean ADSCs (0.8 ± 0.078 fold decrease, P < 0.01) or obese ADSCs (0.75 ± 0.045 fold decrease, P < 0.01) compared to the levels in untreated cells. In addition, the p-Akt/total Akt ratio was significantly mitigated by the treatment of MCF-7 cells with LGT for 18 h in CM either from lean ADSCs (0.77 ± 0.068 fold decrease, P < 0.01) or obese ADSCs (0.62 ± 0.035 fold decrease, P < 0.001) compared to the corresponding untreated cells. Furthermore, treatment with LGT significantly attenuated the p-mTOR/total mTOR ratio in MCF-7 cells cultured in CM from either lean ADSCs (0.92 ± 0.02 fold decrease, P < 0.05) or obese ADSCs (0.91 ± 0.03 fold decrease, P < 0.05) in comparison with the corresponding untreated cells. However, no significant change emerged in the p-AMPK/total MAPK in LGT-treated MCF-7 cells in either lean or obese ADSCs–CM in comparison with untreated MCF-7 cells.
Fig. 8.
The protein levels of AMPK and SIRT-1 in lean and obese ADSCs-CM following transfection with specific small interference RNA (siRNA). The densities of immunoblots were quantified using ImageJ analysis software. Relative quantities were normalized to the control and expressed as a fold of induction. Data are presented as the mean ± SEM. ***P < 0.001.
Fig. 5.
Effects of liraglutide on the protein levels of p-PI3K, p-Akt/Akt, p-mTOR/mTOR, and p-AMPK/AMPK in MCF-7 cells cultured in lean and obese ADSCs-CM for 18 h. The densities of immunoblots were quantified using ImageJ analysis software. Relative quantities were normalized to the control and are expressed as a fold of induction. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
3.4. Anti-proliferative activity of LGT in MCF-7 BC cells using alamarBlue assay compared to pathway inhibitors
Treatment with LGT alone significantly reduced the percentage of MCF-7 proliferation to 65.4 % ± 1.2 in CM from lean ADSCs and 54.7 ± 0.52 in CM from obese ADSCs compared to 100 % proliferation in the absence of LGT (P < 0.001) (Fig. 6A). By comparison, LY294002 (PI3K inhibitor) significantly decreased the proliferation by 64.4 % ± 1.18 in lean ADSCs-CM and 55.4 % ± 0.73 in obese ADSCs-CM (P < 0.001). According to these results, the anti-proliferative activity of LGT is similar to that of the PI3K inhibitor. Notably, the combination of LY294002 with LGT displayed significant anti-proliferative activity on MCF-7 in both lean (58.8 ± 0.77) and obese (50.4 ± 1.1) ADSCs-CM compared to the 100 % proliferation in the corresponding control (P < 0.001); however, this effect was not significant compared to the effect of either LGT or LY294002 alone.
Fig. 6.
Anti-proliferative activity of liraglutide in MCF-7 BC cells using alamarBlue assay compared to (A): LY294002 (PI3K inhibitor) and (B): rapamycin (mTOR inhibitor) in lean and obese ADSCs-CM. Data are presented as mean ± SEM. ***P < 0.001, *P < 0.05.
As can be seen in Fig. 6B, treatment with LGT alone significantly mitigated the percentage of MCF-7 proliferation to 64.9 % ± 1.7 in CM from lean ADSCs and 53.4 ± 1.1 in CM from obese ADSCs compared to 100 % proliferation in the absence of LGT (P < 0.001). Rapamycin (mTOR inhibitor) significantly suppressed the proliferation in lean ADSCs-CM (58.8 ± 1.28, P < 0.001) and in obese ADSCs-CM (58.8 ± 1.28, P < 0.001). These outcomes indicate that the anti-proliferative activity of LGT is similar to that of rapamycin. The combination of rapamycin and LGT demonstrated significant anti-proliferative activity on MCF-7 in both lean (53.27 ± 0.99) and obese (49.2 ± 1.03) ADSCs-CM compared to the 100 % proliferation in the corresponding control (P < 0.001); however, this effect was not significant compared to the effect of either LGT or rapamycin alone.
3.5. Effect of LGT on adipokine levels in ADSCs and the role of AMPK/SIRT1
Another aim of our study was to confirm the effect of LGT on adipokine levels in obese ADSCs and to investigate the role of AMPK/SIRT1 in this effect. To fulfill this aim, we investigated the effect of LGT on the mRNA levels of leptin, adiponectin, and IL-6 in obese ADSCs before and after the knockdown of AMPK/SIRT1 genes.
3.6. Effect of LGT on mRNA levels of leptin, adiponectin, and IL-6 in obese ADSCs
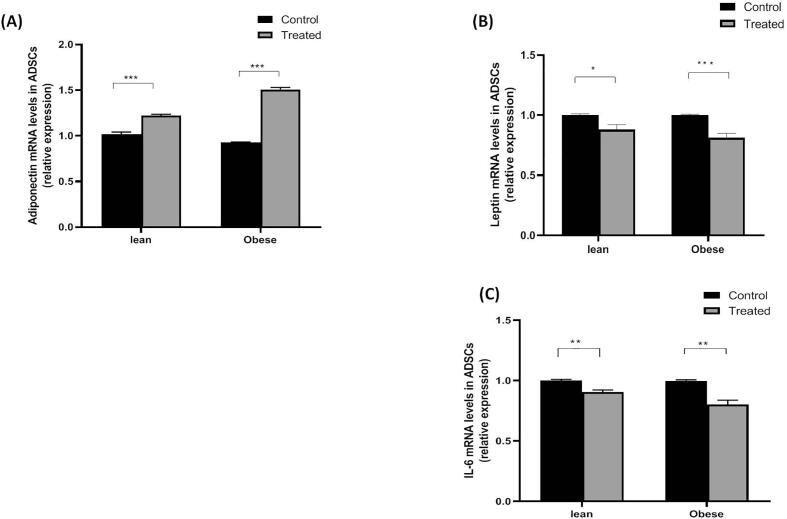
As shown in Fig. 7, obese ADSCs-CM treated with LGT demonstrated increased mRNA expression of adiponectin (1.51-fold increase, P < 0.001) and decreased mRNA levels of leptin (0.81-fold decrease, P < 0.001) and IL-6 (0.8-fold decrease, P < 0.01) in comparison with the untreated medium.
Fig. 7.
Effect of liraglutide on mRNA levels of leptin, adiponectin, and IL-6 in lean and obese ADSCs. Data are presented as mean ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05.
3.7. Effect of siRNA of AMPK/SIRT-1 on mRNA levels of adipokines in control and LGT-treated lean and obese ADSCs
The protein levels of SIRT-1 and AMPK following transfection are shown in Fig. 8. The transfected ADSCs revealed approximately 50 % inhibition of the activity of SIRT-1 and AMPK compared to non-transfected cells. The effects of transfection on adipokines were as follows:
3.8. Effect on mRNA levels of adiponectin
According to Fig. 9A, the transfection of AMPK/SIRT-1 significantly decreased the mRNA levels of adiponectin in both lean (0.59 ± 0.04-fold decrease, P < 0.001) and obese (0.52 ± 0.025, P < 0.001) ADSCs compared to the corresponding non-transfected cells. Treatment of transfected cells with LGT significantly upregulated the mRNA expression of adiponectin in comparison with untreated transfected cells in both lean (0.81 ± 0.08-fold induction vs. 0.59 ± 0.04, P < 0.05) and obese (1.05 ± 0.026 vs. 0.52 ± 0.025, P < 0.001) ADSCs. These results suggest that the effect of LGT on adiponectin expression in ADSCs is independent of AMPK/SIRT-1.
Fig. 9.
Effect of siRNA of AMPK/SIRT-1 on mRNA levels of A) adiponectin, B) leptin, and C) IL-6 in control and liraglutide-treated lean and obese ADSCs. Data are presented as mean ± SEM. ***P < 0.001, **P < 0. 01, *P < 0.05.
3.9. Effect on mRNA levels of leptin
As can be observed in Fig. 9B, transfection of AMPK/SIRT-1 led to a significant elevation in the mRNA levels of leptin in both lean (1.37 ± 0.16-fold increase, P < 0.001) and obese (1.68 ± 0.076, P < 0.001) ADSCs compared to the corresponding non-transfected cells. Treatment of transfected cells with LGT significantly attenuated the expression of leptin compared to untreated transfected cells in both lean (1.17 ± 0.05 fold vs. 1.37 ± 0.16, P < 0.05) and obese (1.26 ± 0.018 vs. 1.68 ± 0.076, P < 0.001) ADSCs. Therefore, the effect of LGT on the leptin expression in ADSCs was independent of AMPK/SIRT-1.
3.10. Effect on mRNA levels of IL-6
In Fig. 9C, it can be seen that transfection of AMPK/SIRT-1 led to a significant elevation in the mRNA levels of IL-6 in both lean (1.38 ± 0.04-fold increase, P < 0.001) and obese (1.68 ± 0.076, P < 0.001) ADSCs in comparison with the corresponding non-transfected cells. Treatment of transfected lean or obese ADSCs with LGT yielded a non-significant change in IL-6 mRNA levels compared to untreated transfected cells.
4. Discussion
Obesity has been associated with an increased prevalence of and high death rate from BC (Jiralerspong and Goodwin, 2016). In the present study, MCF-7 cell proliferation was enhanced in obese ADSCs-CM (Fig. 2) when compared to lean CM. This result is consistent with our previous research (Alanteet et al., 2021), along with findings reported in other works (Strong et al., 2015, Sabol et al., 2019). According to these studies, this enhancement can be attributed to the disturbed secretory profile of ADSCs, leading to the secretion of higher levels of pro-inflammatory cytokines and leptin and reducing the levels of adiponectin in the culture medium. According to the current study findings, LGT exerts anti-proliferative effects on MCF-7 BC cells cultured in CM from obese ADSCs, which is consistent with our previous work (Alanteet et al., 2021). This anti-proliferative effect may be mediated by correcting the gene expression of migration, leptin, and their receptors in both obese ADSCs and cancer cells (Alanteet et al., 2021).
Our results in the current research work reveal that BC cells incubated in obese ADSCs-CM demonstrated higher migration activity compared to those incubated in lean ADSCs-CM (Fig. 4A). Our findings align closely with those of previous studies (Kucerova et al., 2013, Strong et al., 2015) in which obese ADSCs enhanced BC cell migration in MCF-7 cells and the SKBR3 cell line. Variable ADSCs-secreted factors are potentially able to induce enhanced migration of BC cells (Schweizer et al., 2015). Among these factors is IL-6, which is significantly released in obese ADSCs (Alanteet et al., 2021), as well as strongly linked to increased cancer cell migration and identified as an ADSCs-secreted factor that is vital for the migratory behavior of BC (Walter et al., 2009). IL-6 can shift BC cells toward aggressiveness and metastasis (Kucerova et al., 2011, Zimmerlin et al., 2011, Strong et al., 2015). The current work adds further new data that, in addition to its anti-proliferative activity, LGT also exhibits potent anti-migration effects on human BC cells incubated in obese ADSCs-CM after 24 and 48 h of exposure (Fig. 4C). The present study and our previous work (Alanteet et al., 2021) show that LGT could ameliorate the high IL-6 levels produced in obese ADSCs, potentially linking this effect with its anti-migration efficacy in obesity. LGT also exhibited an apoptotic effect on BC cells incubated in CM from obese ADSCs, as revealed from the results of annexin V staining. According to previous data concerning the role of leptin in enhancing cancer proliferation and migration via anti-apoptotic, pro-mitogenic, and pro-inflammatory activities (Lang and Ratke, 2009, Dubois et al., 2013, Nalabolu et al., 2014), along with data revealing that adiponectin has anti-proliferative and apoptotic as well as anti-inflammatory effects on MCF-7 BC cell lines (Kang et al., 2005), the beneficial effects elicited by LGT in our study could be mediated via altering the production of adiponectin and leptin from ADSCs.
We also investigated whether the anti-proliferative, apoptotic, and anti-migration activities of LGT observed in the present work were mediated via inhibiting the PI3K/Akt/mTOR signaling pathway. The PI3K/Akt pathway is associated with BC tumorigenicity, metastasis, and migration (Guerrero-Zotano et al., 2016, Sharma et al., 2017), while mTOR, a downstream target of the PI3K/Akt pathway, enhances protein synthesis and cell proliferation (Dowling et al., 2010). Recently reported evidence points to the inhibition of PI3K/mTOR in suppressing adipocyte-mediated migration and the proliferation of MCF-7/MDA-MB-231 BCE cells (Park et al., 2020). Our findings are the first to indicate that LGT suppresses PI3K/Akt/mTOR activation in MCF-7 cultured in CM from obese ADSCs (Fig. 5). Therefore, the inhibition of PI3K/Akt/mTOR pathways by LGT may have an important role in suppressing the growth, viability, and migration of BC cells in obese women. Enhancing adiponectin production while reducing leptin and inflammatory marker secretion from obese ADSCs could be associated with this inhibitory effect of LGT. The suppressing effect of LGT on PI3K/Akt/mTOR has been reported in other types of cancers. For example, LGT promoted apoptosis and inhibited metastasis and the proliferation of pancreatic cancer via inhibiting PI3K/Akt (Zhao et al., 2014). Miao and colleagues (Miao et al., 2013) confirmed that LGT could modulate the apoptosis and proliferation of pancreatic β-cells through AMPK/mTOR signaling. Similarly, the combination of LGT and docetaxel showed synergistic effects on prostate cancer cells by reducing the activity of the PI3K/Akt pathway (Eftekhari et al., 2020). Furthermore, Ex-4 (another GLP-1R agonist) was found to reduce Akt activation in BC cells (Ligumsky et al., 2012). Interestingly, LGT showed similar effects to the PI3K inhibitors LY294002 or wortmannin in terms of inhibiting cancer migration/invasion in pancreatic cancer (Zhao et al., 2014). In the current study, and similar to the results reported by Zhao et al. (2014), LGT had an anti-proliferative effect on BC similar to LY294002 and rapamycin (Fig. 6A, 6B), suggesting the potent inhibitory effect of LGT on BC cell growth in obese women. However, the anti-proliferative effect of LGT was not potentiated when combined with rapamycin. This outcome could be because mTOR inhibition by rapamycin is associated with feedback activation of insulin receptor substrate-1, with subsequent increased activation of Akt (Zakikhani et al., 2010). Therefore, in our study, the reduced Akt activation induced by LGT might have been antagonized by rapamycin.
AMPK is a tumor-suppressing kinase that leads to the inhibition of protein synthesis, cell growth, and cellular metabolism (Ponnusamy et al., 2020). These effects may be mediated through the inhibition of mTOR (Xu et al., 2012). In our study, LGT did not affect the activity of AMPK in MCF-7 cells cultured in CM from either obese or lean ADSCs. Similar to our findings, no significant activation of AMPK was observed following LGT treatment of prostate cancer cells (Lu et al., 2018). However, Zhao et al. (Zhao et al., 2018) reported that LGT promotes apoptosis and inhibits the proliferation of MCF-7 cells by activating the miR-27a/AMPKα2 pathway. These controversial results suggest the need for further as well as more in-depth studies to explore the exact role of LGT on AMPK in BC cells in lean and obese subjects.
Our study reveals that LGT significantly corrected the disturbed mRNA levels of adiponectin, leptin, and IL-6 in obese ADSCs (Fig. 7). Thus, we aimed to investigate the role of AMPK/SIRT-1 in this valuable effect of LGT. Numerous studies have reported the crucial role of AMPK/SIRT-1 in regulating adipokine production from adipose tissue (Lihn et al., 2004, Liu et al., 2007, Qiang et al., 2007, Gillum et al., 2011, Granata et al., 2012). Our study confirms this regulatory role of AMPK/SIRT-1 in ADSCs, as the knockdown of AMPK and SIRT-1 genes led to significant increases in the mRNA expression of leptin and IL-6 along with the decreased expression of adiponectin in both lean and obese ADSCs (Fig. 9A-9C), confirming the vital role of AMPK and SIRT-1 in the secretory profile of ADSCs. Similarly, blocking the activity of AMPK or SIRT-1 suppresses the expression of adiponectin in 3 T3-L1 adipocyte cells (Liu et al., 2007, Qiang et al., 2007, Shiota et al., 2012), mitigates the wogonin action on adiponectin production (Yang et al., 2015), and results in the downregulation of adiponectin (Wang et al., 2011). Despite this regulatory role, our results suggest that the beneficial effects of LGT on adipokine secretion from ADSCs are independent of the activity of AMPK/SIRT-1 since LGT significantly upregulated the expression of adiponectin while reducing the levels of leptin and IL-6 in transfected ADSCs compared to their levels in untreated transfected cells in either lean or obese ADSCs (Fig. 9A-9C).
In conclusion, our findings demonstrate that LGT can suppress the growth and migration of BC cells cultured in CM from obese ADSCs (Fig. 10). In addition, it can induce an apoptotic influence on BC cells. The anti-proliferative, apoptotic, and anti-migratory properties of LGT on MCF-7 BC cells in obese ADSCs-CM might be mediated, at least in part, by suppressing the activity of the survival PI3K/Akt/mTOR pathway. Contrariwise, it exerted no obvious effects on the activity of AMPK. The inhibitory effect of LGT on BC cell growth was similar to that obtained by PI3K and mTOR inhibitors. The beneficial effects of LGT could also be exerted by modulating the expression of adipokines in obese ADSCs. However, this beneficial effect on adipokines is independent of the activity of the AMPK/SIRT-1 pathway in ADSCs.
Fig. 10.
Schematic presentation of cytoprotective mechanisms of liraglutide. Liraglutide exerts anti-proliferative, anti-migration, and apoptotic activities on human MCF-7 breast cancer cells cultured in obese adipose tissue stem cell-conditioned medium (ADSCs-CM). In ADSCs, liraglutide attenuates the obesity-induced alteration in adipokine secretion by ameliorating the decrease in adiponectin and attenuating the increase in leptin and inflammatory markers. In MCF-7 cells, liraglutide acts by inhibiting the PI3K/Akt/mTOR survival pathway. The net effect is the mitigation of breast cancer cell proliferation. The figure was created with BioRender.com.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSUOR3–117).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Alaa Alanteet, Email: aalanteet@ksu.edu.sa.
Hala Attia, Email: hsalem@ksu.edu.sa.
Musaed Alfayez, Email: alfayez@ksu.edu.sa.
Amer Mahmood, Email: ammahmood@ksu.edu.sa.
Khalid Alsaleh, Email: khaalsaleh@ksu.edu.sa.
Sary Alsanea, Email: salsanea@ksu.edu.sa.
References
- A.A. Alanteet, H.A. Attia, S. Shaheen, et al., 2021. Anti-Proliferative Activity of Glucagon-Like Peptide-1 Receptor Agonist on Obesity-Associated Breast Cancer: The Impact on Modulating Adipokines' Expression in Adipocytes and Cancer Cells. Dose Response. 19, 1559325821995651. https://doi.org/10.1177/1559325821995651. [DOI] [PMC free article] [PubMed]
- Bullwinkle E.M., Parker M.D., Bonan N.F., et al. Adipocytes contribute to the growth and progression of multiple myeloma: Unraveling obesity related differences in adipocyte signaling. Cancer Lett. 2016;380:114–121. doi: 10.1016/j.canlet.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Cirillo D., Rachiglio A.M., la Montagna R., et al. Leptin signaling in breast cancer: an overview. J. Cell Biochem. 2008;105:956–964. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- Divella R., De Luca R., Abbate I., et al. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer. 2016;7:2346–2359. doi: 10.7150/jca.16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling R.J., Topisirovic I., Fonseca B.D., et al. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim. Biophys. Acta. 2010;1804:433–439. doi: 10.1016/j.bbapap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Dubois V., Delort L., Billard H., et al. Breast cancer and obesity: in vitro interferences between adipokines and proangiogenic features and/or antitumor therapies? PLoS One. 2013;8:e58541. doi: 10.1371/journal.pone.0058541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari S., Montazeri H., Tarighi P. Synergistic anti-tumor effects of Liraglutide, a glucagon-like peptide-1 receptor agonist, along with Docetaxel on LNCaP prostate cancer cell line. Eur. J. Pharmacol. 2020;878 doi: 10.1016/j.ejphar.2020.173102. [DOI] [PubMed] [Google Scholar]
- Fasshauer M., Bluher M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015;36:461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Fonseca L.N., Bolivar-Mona S., Agudelo T., et al. Cell surface markers for mesenchymal stem cells related to the skeletal system: A scoping review. Heliyon. 2023;9:e13464. doi: 10.1016/j.heliyon.2023.e13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresno Vara J.A., Casado E., de Castro J., et al. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Gillum M.P., Kotas M.E., Erion D.M., et al. SirT1 regulates adipose tissue inflammation. Diabetes. 2011;60:3235–3245. doi: 10.2337/db11-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata R., Gallo D., Luque R.M., et al. Obestatin regulates adipocyte function and protects against diet-induced insulin resistance and inflammation. FASEB J. 2012;26:3393–3411. doi: 10.1096/fj.11-201343. [DOI] [PubMed] [Google Scholar]
- Guerrero-Zotano A., Mayer I.A., Arteaga C.L. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35:515–524. doi: 10.1007/s10555-016-9637-x. [DOI] [PubMed] [Google Scholar]
- Haakinson D.J., Leeds S.G., Dueck A.C., et al. The impact of obesity on breast cancer: a retrospective review. Ann. Surg. Oncol. 2012;19:3012–3018. doi: 10.1245/s10434-012-2320-8. [DOI] [PubMed] [Google Scholar]
- Jarde T., Caldefie-Chezet F., Goncalves-Mendes N., et al. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr. Relat. Cancer. 2009;16:1197–1210. doi: 10.1677/ERC-09-0043. [DOI] [PubMed] [Google Scholar]
- Jarde T., Perrier S., Vasson M.P., et al. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur. J. Cancer. 2011;47:33–43. doi: 10.1016/j.ejca.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Jiralerspong S., Goodwin P.J. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. J. Clin. Oncol. 2016;34:4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- Kang J.H., Lee Y.Y., Yu B.Y., et al. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch. Pharm. Res. 2005;28:1263–1269. doi: 10.1007/BF02978210. [DOI] [PubMed] [Google Scholar]
- Keller C., Keller P., Marshal S., et al. IL-6 gene expression in human adipose tissue in response to exercise–effect of carbohydrate ingestion. J. Physiol. 2003;550:927–931. doi: 10.1113/jphysiol.2003.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucerova L., Kovacovicova M., Polak S., et al. Interaction of human adipose tissue-derived mesenchymal stromal cells with breast cancer cells. Neoplasma. 2011;58:361–370. doi: 10.4149/neo_2011_05_361. [DOI] [PubMed] [Google Scholar]
- Kucerova L., Skolekova S., Matuskova M., et al. Altered features and increased chemosensitivity of human breast cancer cells mediated by adipose tissue-derived mesenchymal stromal cells. BMC Cancer. 2013;13:535. doi: 10.1186/1471-2407-13-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang K., Ratke J. Leptin and Adiponectin: new players in the field of tumor cell and leukocyte migration. Cell Commun. Signal. 2009;7:1–10. doi: 10.1186/1478-811X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Saud S.M., Young M.R., et al. Targeting AMPK for cancer prevention and treatment. Oncotarget. 2015;6:7365–7378. doi: 10.18632/oncotarget.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligumsky H., Wolf I., Israeli S., et al. The peptide-hormone glucagon-like peptide-1 activates cAMP and inhibits growth of breast cancer cells. Breast Cancer Res. Treat. 2012;132:449–461. doi: 10.1007/s10549-011-1585-0. [DOI] [PubMed] [Google Scholar]
- Lihn A.S., Jessen N., Pedersen S.B., et al. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem. Biophys. Res. Commun. 2004;316:853–858. doi: 10.1016/j.bbrc.2004.02.139. [DOI] [PubMed] [Google Scholar]
- Liu G., Grifman M., Macdonald J., et al. Isoginkgetin enhances adiponectin secretion from differentiated adiposarcoma cells via a novel pathway involving AMP-activated protein kinase. J. Endocrinol. 2007;194:569–578. doi: 10.1677/JOE-07-0200. [DOI] [PubMed] [Google Scholar]
- Lu R., Yang J., Wei R., et al. Synergistic anti-tumor effects of liraglutide with metformin on pancreatic cancer cells. PLoS One. 2018;13:e0198938. doi: 10.1371/journal.pone.0198938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macciò A., Madeddu C., Gramignano G., et al. Correlation of body mass index and leptin with tumor size and stage of disease in hormone-dependent postmenopausal breast cancer: preliminary results and therapeutic implications. J. Mol. Med. 2010;88:677–686. doi: 10.1007/s00109-010-0611-8. [DOI] [PubMed] [Google Scholar]
- Mayer I.A., Arteaga C.L. The PI3K/AKT pathway as a target for cancer treatment. Annu. Rev. Med. 2016;67:11–28. doi: 10.1146/annurev-med-062913-051343. [DOI] [PubMed] [Google Scholar]
- Miao X.Y., Gu Z.Y., Liu P., et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides. 2013;39:71–79. doi: 10.1016/j.peptides.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Mildmay-White A., Khan W. Cell surface markers on adipose-derived stem cells: A systematic review. Curr. Stem. Cell Res. Ther. 2017;12:484–492. doi: 10.2174/1574888X11666160429122133. [DOI] [PubMed] [Google Scholar]
- Nalabolu M.R., Palasamudram K., Jamil K. Adiponectin and leptin molecular actions and clinical significance in breast cancer. Int. J. Hematol. Oncol. Stem Cell Res. 2014;8:31–40. [PMC free article] [PubMed] [Google Scholar]
- Nunnery S.E., Mayer I.A. Targeting the PI3K/AKT/mTOR pathway in hormone-positive breast cancer. Drugs. 2020;80:1685–1697. doi: 10.1007/s40265-020-01394-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orecchioni S., Reggiani F., Talarico G., et al. Mechanisms of obesity in the development of breast cancer. Discov. Med. 2015;20:121–128. [PubMed] [Google Scholar]
- Panno M.L., Naimo G.D., Spina E., et al. Different molecular signaling sustaining adiponectin action in breast cancer. Curr. Opin. Pharmacol. 2016;31:1–7. doi: 10.1016/j.coph.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Kang S.E., Ahn K.S., et al. Inhibition of the PI3K-AKT-mTOR pathway suppresses the adipocyte-mediated proliferation and migration of breast cancer cells. J. Cancer. 2020;11:2552–2559. doi: 10.7150/jca.37975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L. Ponnusamy, S. R. Natarajan, K. Thangaraj, et al., 2020. Therapeutic aspects of AMPK in breast cancer: Progress, challenges, and future directions. Biochim. Biophys. Acta (BBA)-Reviews on Cancer. 1874, 188379. [DOI] [PubMed]
- Qiang L., Wang H., Farmer S.R. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-Lα. Mol. Cell. Biol. 2007;27:4698–4707. doi: 10.1128/MCB.02279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol R.A., Bowles A.C., Côté A., et al. Leptin produced by obesity-altered adipose stem cells promotes metastasis but not tumorigenesis of triple-negative breast cancer in orthotopic xenograft and patient-derived xenograft models. Breast Cancer Res. 2019;21:1–14. doi: 10.1186/s13058-019-1153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D.D., Guertin D.A., Ali S.M., et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schweizer R., Tsuji W., Gorantla V.S., et al. The role of adipose-derived stem cells in breast cancer progression and metastasis. Stem Cells Int. 2015;2015 doi: 10.1155/2015/120949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.R., Gupta G.K., Sharma A.K., et al. PI3K/Akt/mTOR intracellular pathway and breast cancer: Factors, mechanism and regulation. Curr. Pharm. Des. 2017;23:1633–1638. doi: 10.2174/1381612823666161116125218. [DOI] [PubMed] [Google Scholar]
- Shiota A., Shimabukuro M., Fukuda D., et al. Activation of AMPK-Sirt1 pathway by telmisartan in white adipose tissue: A possible link to anti-metabolic effects. Eur. J. Pharmacol. 2012;692:84–90. doi: 10.1016/j.ejphar.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Strong A.L., Strong T.A., Rhodes L.V., et al. Obesity associated alterations in the biology of adipose stem cells mediate enhanced tumorigenesis by estrogen dependent pathways. Breast Cancer Res. 2013;15:R102. doi: 10.1186/bcr3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong A.L., Ohlstein J.F., Biagas B.A., et al. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112. doi: 10.1186/s13058-015-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong A.L., Bowles A.C., Wise R.M., et al. Human adipose stromal/stem cells from obese donors show reduced efficacy in halting disease progression in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Stem Cells. 2016;34:614–626. doi: 10.1002/stem.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Vasan N., Toska E., Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann. Oncol. 2019;30:x3–x11. doi: 10.1093/annonc/mdz281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsboll T., Christensen M., Junker A.E., et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344 doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M., Liang S., Ghosh S., et al. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28:2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Liu M., Liu X., et al. Up-regulation of adiponectin by resveratrol: the essential roles of the Akt/FOXO1 and AMP-activated protein kinase signaling pathways and DsbA-L. J. Biol. Chem. 2011;286:60–66. doi: 10.1074/jbc.M110.188144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Ji J., Yan X.-H. Cross-talk between AMPK and mTOR in regulating energy balance. Crit. Rev. Food Sci. Nutr. 2012;52:373–381. doi: 10.1080/10408398.2010.500245. [DOI] [PubMed] [Google Scholar]
- Yang H., Bi Y., Xue L., et al. Multifaceted modulation of SIRT1 in cancer and inflammation. Crit. Rev. Oncog. 2015;20:49–64. doi: 10.1615/critrevoncog.2014012374. [DOI] [PubMed] [Google Scholar]
- Ye Y., Zhong X., Li N., et al. Protective effects of liraglutide on glomerular podocytes in obese mice by inhibiting the inflammatory factor TNF-alpha-mediated NF-kappaB and MAPK pathway. Obes. Res. Clin. Pract. 2019;13:385–390. doi: 10.1016/j.orcp.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Zakikhani M., Blouin M.-J., Piura E., et al. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res. Treat. 2010;123:271–279. doi: 10.1007/s10549-010-0763-9. [DOI] [PubMed] [Google Scholar]
- Zhao H., Wang L., Wei R., et al. Activation of glucagon-like peptide-1 receptor inhibits tumourigenicity and metastasis of human pancreatic cancer cells via PI3K/Akt pathway. Diabetes Obes. Metab. 2014;16:850–860. doi: 10.1111/dom.12291. [DOI] [PubMed] [Google Scholar]
- Zhao W., Zhang X., Zhou Z., et al. Liraglutide inhibits the proliferation and promotes the apoptosis of MCF-7 human breast cancer cells through downregulation of microRNA-27a expression. Mol. Med. Rep. 2018;17:5202–5212. doi: 10.3892/mmr.2018.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerlin L., Donnenberg A.D., Rubin J.P., et al. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng. A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]