Xaluritamig, a novel STEAP1 × CD3 XmAb 2+1 immune therapy for metastatic castrationresistant prostate cancer, can be safely administered and shows encouraging antitumor activity, which supports further development.
Abstract
Xaluritamig (AMG 509) is a six-transmembrane epithelial antigen of the prostate 1 (STEAP1)–targeted T-cell engager designed to facilitate lysis of STEAP1-expressing cancer cells, such as those in advanced prostate cancer. This first-in-human study reports monotherapy dose exploration for patients with metastatic castration-resistant prostate cancer (mCRPC), primarily taxane pretreated. Ninety-seven patients received ≥1 intravenous dose ranging from 0.001 to 2.0 mg weekly or every 2 weeks. MTD was identified as 1.5 mg i.v. weekly via a 3-step dose. The most common treatment-related adverse events were cytokine release syndrome (CRS; 72%), fatigue (45%), and myalgia (34%). CRS occurred primarily during cycle 1 and improved with premedication and step dosing. Prostate-specific antigen (PSA) and RECIST responses across cohorts were encouraging [49% PSA50; 24% objective response rate (ORR)], with greater frequency at target doses ≥0.75 mg (59% PSA50; 41% ORR). Xaluritamig is a novel immunotherapy for prostate cancer that has shown encouraging results supporting further development.
Significance:
Xaluritamig demonstrated encouraging responses (PSA and RECIST) compared with historical established treatments for patients with late-line mCRPC. This study provides proof of concept for T-cell engagers as a potential treatment for prostate cancer, validates STEAP1 as a target, and supports further clinical investigation of xaluritamig in prostate cancer.
See related commentary by Hage Chehade et al., p. 20.
See related article by Nolan-Stevaux et al., p. 90.
This article is featured in Selected Articles from This Issue, p. 5
INTRODUCTION
Prostate cancer remains the leading cause of cancer and the second leading cause of cancer-related deaths among U.S. men in 2023, accounting for approximately 1.4 million new cancer diagnoses (7.3%) and approximately 375,000 deaths (3.8%) globally in 2020 (1, 2). The majority of patients with prostate cancer will develop resistance to androgen-suppressive therapy, resulting in castration-resistant prostate cancer (CRPC; ref. 3). Up to 20% of prostate cancer will spread to regional lymph nodes or metastasize to bone or other organs (4), and patients with metastatic CRPC (mCRPC) have a poor prognosis with a reported 5-year survival of 34% (5, 6).
Despite taxane-based chemotherapy regimens and recent approvals of novel therapies, including newer androgen deprivation therapies, prostate-specific membrane antigen (PSMA) radioligand therapy, and PARP inhibitors in subsets of patients with mCRPC (7, 8), the long-term prognosis of patients with mCRPC remains poor (6). There have been significant advances of immune therapies, such as anti–programmed death-ligand 1 (anti–PD-L1) to treat many solid tumors. However, these treatments have minimally impacted the care of patients with prostate cancer, demonstrating benefit only in a small subset with mismatch repair deficiency. Evaluation of anti–PD-L1 in combination with androgen deprivation therapy in a broader mCRPC population failed to demonstrate clinical improvement in two large, randomized phase III studies (9, 10). Furthermore, combination of anti–PD-L1 with PARP inhibitors was hypothesized to sensitize tumors to immune checkpoint inhibitor therapy; however, clinical evaluation was stopped early for futility, as no improvements in survival outcomes were observed (11).
T-cell engager (TCE) molecules represent a targeted immunotherapy approach in which TCE binding to a tumor-associated antigen on target cells and CD3 on T cells induces T-cell activation, cytokine induction, and T cell–mediated tumor cell lysis (12). This mechanism bypasses the conventional pathway of T-cell receptor activation and may enable TCE therapy to be effective against tumors resistant to other immune therapies. To date, multiple TCEs have been approved but primarily for hematologic malignancies (13–16). In prostate cancer, several PSMA-targeted TCEs have entered the clinic but have seen limited success due to minimal efficacy, toxicity, and short duration of response (DOR; refs. 7, 8, 17). For example, JNJ-63898081, a PSMA and CD3 bispecific antibody, led to transient declines in prostate-specific antigen (PSA), with two of 39 (5%) patients experiencing a confirmed PSA50 response and no radiographic responses in a phase I study of patients with mCRPC (18). PSMA-targeting TCE HPN424 reported three of 63 (5%) patients experiencing a PSA50 response and one of 34 (3%) experiencing a confirmed RECIST response with manageable safety (19). The half-life–extended TCE AMG 160 demonstrated the highest activity with a PSA50 response in 12 of 35 (34%) patients and a confirmed radiographic partial response (PR) in two of 15 (13%) patients, but it also had the highest rates of cytokine release syndrome (CRS; 91%; ref. 20). Further efforts are underway to improve PSMA TCEs, but alternative targets may be required to achieve better clinical outcomes.
The tumor-associated antigen six-transmembrane epithelial antigen of the prostate 1 (STEAP1) is expressed in most prostate cancers, including 77% to 83% of metastases, and has been associated with poor survival (21, 22). Overexpression of STEAP1 in prostate tumors, combined with low or no expression on normal tissues, makes STEAP1 an ideal potential therapeutic target (21). The only STEAP1-targeted agent explored in the clinic to date has been a STEAP1 antibody–drug conjugate (ADC) that was limited by toxicities due to the monomethyl auristatin E payload (23). Preclinical studies of chimeric antigen receptor T cells directed against STEAP1 have shown encouraging results, but clinical studies have not yet been initiated (24, 25).
Xaluritamig is a novel humanized, bispecific XmAb (registered trademark of Xencor) 2+1 TCE developed as a targeted immunotherapy for the treatment of STEAP1-expressing prostate cancer. Xaluritamig contains two identical humanized anti-STEAP1 fragment antigen–binding domains that bind STEAP1-expressing cells, an anti-CD3 single-chain variable fragment domain that binds T cells to facilitate T cell–mediated lysis, and an effectorless Fc domain that extends serum half-life. The avidity from two STEAP1-binding domains drives high potency against STEAP1-expressing tumor cells. In preclinical studies, xaluritamig induced lysis of STEAP1-expressing prostate cancer cells and showed broad anticancer effects in prostate cancer xenograft models (22, 26).
This is the first clinical report of xaluritamig and describes the monotherapy dose exploration from the first-in-human study for patients with advanced prostate cancer (NCT04221542).
RESULTS
Patients
As of March 28, 2023, 97 patients received ≥1 dose of xaluritamig. Patient characteristics are described in Table 1. Representativeness of patients is detailed in Supplementary Table S1. Median (range) age was 67 (40–86) years. A total of 77 patients (79%) had received ≥3 prior lines of therapy, and the median number of prior lines of therapy was 4 (range, 1–9). A total of 82 patients (85%) had received ≥1 prior taxane. A total of 24 (25%) patients had metastatic disease in the bone only, 15 (15%) in both the bone and lymph node, and six (6%) in the lymph node only; 51 (53%) patients had visceral metastases with other metastatic sites, including 19 (37%) with liver lesions.
Table 1.
Patient demographics and baseline disease characteristics of patients who received xaluritamig.
| Low-dose cohorts | High-dose cohorts | ||
|---|---|---|---|
| All cohorts | (1–7a) | (7b–13) | |
| (N = 97) | (n = 45) | (n = 52) | |
| Age, median (range), years | 67 (40, 86) | 67 (40, 86) | 68 (51, 85) |
| Race, n (%) | |||
| White | 59 (61) | 29 (64) | 30 (58) |
| Asian | 32 (33) | 13 (29) | 19 (37) |
| Black/African American | 5 (5) | 3 (7) | 2 (4) |
| Not available | 1 (1) | 0 | 1 (2) |
| ECOG PS, n (%) | |||
| 0 | 45 (46) | 19 (42) | 26 (50) |
| 1 | 52 (54) | 26 (58) | 26 (50) |
| Number of prior lines of therapy, n (%) | |||
| 1–2 | 20 (21) | 10 (22) | 10 (19) |
| 3 | 25 (26) | 13 (29) | 12 (23) |
| 4 | 25 (26) | 11 (24) | 14 (27) |
| ≥5 | 27 (28) | 11 (24) | 16 (31) |
| Median, range | 4 (1, 9) | 3 (1, 7) | 4 (1, 9) |
| Number of prior novel hormonal therapies, n (%) | |||
| 0 | 1 (1) | 1 (2) | 0 |
| 1 | 44 (45) | 20 (44) | 24 (46) |
| 2 | 40 (41) | 17 (38) | 23 (44) |
| 3 | 9 (9) | 6 (13) | 3 (6) |
| 4 | 3 (3) | 1 (2) | 2 (4) |
| Number of prior taxanes, n (%) | |||
| 0 | 15 (15) | 8 (18) | 7 (13) |
| 1 | 34 (35) | 18 (40) | 16 (31) |
| 2 | 42 (43) | 17 (38) | 25 (48) |
| 3 | 6 (6) | 2 (4) | 4 (8) |
| Number of prior PSMA-targeting radioligand therapies, n (%) | |||
| 0 | 93 (96) | 44 (98) | 49 (94) |
| 1 | 4 (4) | 1 (2) | 3 (6) |
| Median (range) duration of follow-up, months | 8.1 (0.5, 29.2) | 10.6 (1.2, 29.2) | 6.7 (0.5, 20.9) |
| Median (range) baseline prognostic factors | |||
| PSA, ng/mL | 113.0 (0.2, 5808.9) | 100.3 (1.7, 2740.0) | 123.8 (0.2, 5808.9) |
| Hgb, g/dL | 11.4 (8.0, 14.6) | 11.1 (8.0, 14.4) | 11.8 (8.0, 14.6) |
| Alkaline phosphatase, U/L | 119.5 (29.0, 1767.0) | 121.0 (38.0, 1767.0) | 118.0 (29.0, 1319.0) |
| LDH, U/L | 220.5 (121.0, 3022.0) | 229.0 (121.0, 824.0) | 219.0 (121.0, 3022.0) |
| Albumin, g/dL | 3.8 (2.5, 4.6) | 3.7 (3.0, 4.6) | 3.9 (2.5, 4.6) |
| Sites of metastatic disease, n (%) | |||
| Bone only | 24 (25) | 13 (29) | 11 (21) |
| Lymph node only | 6 (6) | 3 (7) | 3 (6) |
| Bone and lymph node | 15 (15) | 6 (13) | 9 (17) |
| Visceral metastases and other sites | 51 (53) | 23 (51) | 28 (54) |
| Liver | 19 (37) | 6 (26) | 13 (46) |
| No category | 1 (1) | 0 | 1 (2) |
NOTE: Number of prior lines of therapy does not include androgen deprivation therapy or first-generation androgen receptor deprivation therapy.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; Hgb, hemoglobin; LDH, lactate dehydrogenase.
Dose Escalation and MTD
Patients were initially enrolled into fixed (nonstep) dosing administered intravenously weekly in Cohorts 1 to 6 at 0.001 (n = 2), 0.003 (n = 4), 0.01 (n = 4), 0.03 (n = 4), 0.1 (n = 10), and 0.3 (n = 6) mg, respectively (Table 2; Supplementary Fig. S1). Patients in all cohorts were monitored in the hospital for 24 to 48 hours after each administration of xaluritamig until the target dose was achieved. At 0.3 mg in Cohort 6, two of six patients experienced dose-limiting toxicities (DLT) of grade 3 CRS and grade 3 encephalopathy (n = 1), and grade 3 back pain (n = 1). This 0.3-mg dose level was determined to be intolerable and exceeded the MTD for cycle 1 day 1 (C1D1).
Table 2.
Summary of cohorts for xaluritamig intravenous monotherapy dose exploration.
| C1 dosing, mg | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | N | Step dosing | D1 | D8 | D15 | D22 | Target dose, mg | Frequency | DLTs | Tolerable | PSA50 | PSA90 | RECIST OR | |
| Low dose | 1 | 2 | None | 0.001 | 0.001 | Weekly | 0/1 | Y | 0/1 | 0/1 | 0/1 | |||
| 2 | 4 | None | 0.003 | 0.003 | Weekly | 0/4 | Y | 0/4 | 0/4 | 0/4 | ||||
| 3 | 4 | None | 0.01 | 0.01 | Weekly | 0/4 | Y | 0/4 | 0/4 | 0/3 | ||||
| 4 | 4 | None | 0.03 | 0.03 | Weekly | 0/4 | Y | 1a/4 | 1/4 | 0/2 | ||||
| 5 | 10 | None | 0.1 | 0.1 | Weekly | 2/9 | Y | 3/10 | 3/10 | 0/4 | ||||
| 6 | 6 | None | 0.3 | 0.3 | Weekly | 2/6 | N | 4/6 | 1/6 | 0/4 | ||||
| 7a | 15 | 1 step | 0.1 | 0.3 | 0.3 | Weekly | 2/12 | Y | 9/14 | 3/14 | 1/12 | |||
| High dose | 7b | 12 | 2 step | 0.1 | 0.3 | 1.0 | 1.0 | Weekly | 1/8 | Y | 6/10 | 3/10 | 4/10 | |
| 7c | 7 | 2 step | 0.1 | 0.3 | 1.0 | N/A | 1.0 | Every 2 weeksb | 1/7 | Y | 3/7 | 2/7 | 2/6 | |
| 8 | 5 | 1 step | 0.3 | 1.0 | 1.0 | Weekly | 2/4 | N | 2/5 | 2/5 | 1/5 | |||
| 9 | 5 | 2 step | 0.1 | 0.3 | 0.75 | 0.75 | Weekly | 2/5 | Y | 4/4 | 2/4 | 2/4 | ||
| 10c | 5 | 1 step | 0.1 | 1.0 | 1.0 | Weekly | 3/4 | N | 2/5 | 2/5 | 2/5 | |||
| 11 | 6 | 3 step | 0.1 | 0.3 | 1.0 | 1.5 | 1.5 | Weekly | 2/5 | Y | 5/5 | 3/5 | 4/4 | |
| 12 | 6 | 3 step | 0.1 | 0.3 | 0.75 | 1.5 | 1.5 | Weekly | 0/5 | Y | 2/5 | 2/5 | 0/2 | |
| 13 | 7 | 3 step | 0.1 | 0.3 | 1.0 | 2.0 | 2.0 | Weekly | 3/4 | N | 2/3 | 0/3 | 0/1 | |
NOTE: Low dose, target dose <0.75 mg; high dose, target dose ≥0.75 mg. For DLTs, PSA50, PSA90, and RECIST OR, the denominator reflects number of patients evaluable for that endpoint.
Abbreviations: C, cycle; D, day; DLT, dose-limiting toxicity; N/A, not applicable; OR, objective response.
aPatient responded following intra-patient dose escalation to the next higher dose level.
bEvery 2 weeks administration after target dose was reached; step doses were weekly.
cTwo patients in Cohort 10 did not receive the specified step dosing schedule as DLTs were seen in initial patients receiving 10-fold step dose. An additional 0.3-mg step dose was added to the schedule for these patients (0.1 mg D1, 0.3 mg D8, 1.0 mg D15+).
Step dosing started with 0.1 mg on D1 and used either 1-step (increase on D8), 2-step (increases on D8 and D15), or 3-step (increases on D8, 15, and D22) dosing to achieve target dose by D8, D15, or D22, respectively. The evaluation of these cohorts occurred in parallel and was guided by decision-making from the dose-level review committee.
In Cohorts 7a and 10, 1-step dosing was 0.1 to 0.3 mg or 0.1 to 1.0 mg, respectively. The regimen of 0.1 to 0.3 mg (Cohort 7a) was tolerable, but the larger step dose of 0.1 to 1.0 mg (Cohort 10) was intolerable due to three of four patients experiencing DLT, consisting of grade 3 atrial fibrillation/QT interval prolongation, grade 3 fasciitis/pharyngitis, and grade 3 arthralgia (one patient each). Following adjustments of premedication and implementation of step dosing, the 0.3-mg starting dose was reevaluated (Cohort 8, 0.3 to 1.0 mg) and still determined intolerable, and the MTD for the first dose (priming dose) was confirmed to be 0.1 mg.
On the basis of the findings of the 1-step dosing, Cohorts 7b, 7c, and 9 evaluated 2-step dosing regimens with a priming dose of 0.1 mg, D8 dose of 0.3 mg, and D15 dose of 0.75 or 1.0 mg followed by weekly or every 2 weeks (Cohort 7c only) dosing; all were determined to be tolerable.
On the basis of the findings of the 2-step dosing, Cohorts 11, 12, and 13 evaluated 3-step dosing regimens with a priming dose of 0.1 mg, D8 dose of 0.3 mg, D15 dose of either 0.75 or 1.0 mg, and D22 dose of either 1.5 or 2 mg. Cohorts 11 (D15 dose of 1.0 mg) and 12 (D15 dose of 0.75 mg) had D22 target doses of 1.5, and both were determined to be tolerable. Cohort 13 tested the highest D22 dose of 2.0 mg, which was deemed not tolerable due to DLTs in three of four evaluable patients [grade 3 myalgia (n = 2); grade 3 pain in extremities and arthralgia (n = 1 each)].
In summary, the maximum tolerated priming dose with the full prophylactic regimen was 0.1 mg, and a 3-step dosing regimen consisting of D8 dose of 0.3 mg, D15 dose of 1.0 mg, and D22+ dose of 1.5 mg intravenously weekly was determined to be the MTD.
Safety
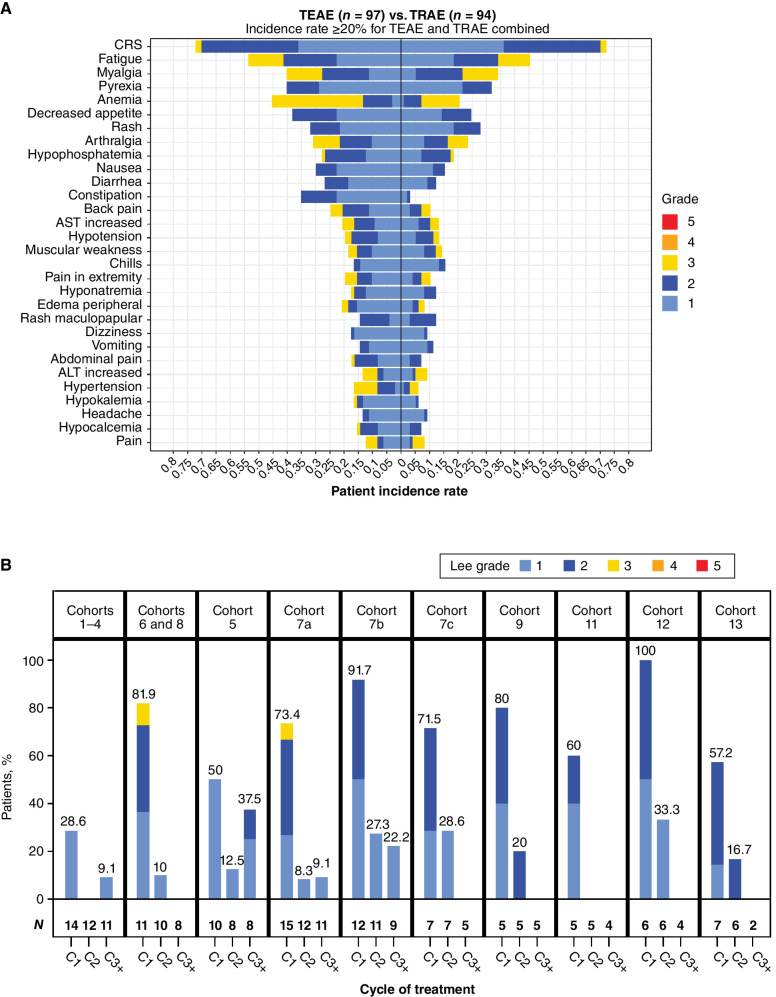
All 97 treated patients were included in the safety-evaluable population. Treatment-emergent adverse events (TEAE) were reported in 100% of patients, with 94 (97%) patients reporting at least one treatment-related adverse event (TRAE; defined as any TEAE deemed by the investigator to have a reasonable possibility of being caused by xaluritamig). The overall treatment summary and TRAEs occurring in ≥20% of patients are reported in Table 3. The most common TRAEs were primarily grade 1 and 2 and included CRS (72%), fatigue (45%), myalgia (34%), and pyrexia (32%). Grade 3 TRAEs occurred in 55% of patients, with only anemia (13%), myalgia (12%), and fatigue (11%) being reported in ≥10% of patients. There were no treatment-related fatalities on study, and the only grade 5 AE was a subdural hematoma secondary to an unrelated fall.
Table 3.
Summary of highest-grade AEs in patients receiving xaluritamig including most common TRAEs (≥20%).
| Low-dose cohorts | High-dose cohorts | ||
|---|---|---|---|
| All cohorts | (1–7a) | (7b–13) | |
| (N = 97) | (n = 45) | (n = 52) | |
| Patients with any TEAE, n (%) | 97 (100) | 45 (100) | 52 (100) |
| Related to xaluritamig | 94 (97) | 43 (96) | 51 (98) |
| Leading to discontinuation from xaluritamig | 18 (19) | 10 (22) | 8 (15) |
| Leading to xaluritamig dose interruption | 46 (47) | 17 (38) | 29 (56) |
| Leading to xaluritamig dose reduction | 7 (7) | 0 | 7 (13) |
| Serious TEAE | 55 (57) | 23 (51) | 32 (62) |
| Serious TEAE related to xaluritamig | 38 (39) | 16 (36) | 22 (42) |
| All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | |
|---|---|---|---|---|---|---|
| Most common TRAEs (≥20%), n (%) | 94 (97) | 53 (55) | 43 (96) | 24 (53) | 51 (98) | 29 (56) |
| CRS (Lee et al., 2014; ref. 32) | 70 (72) | 2 (2) | 27 (60) | 2 (4) | 43 (83) | 0 |
| Fatigue | 44 (45) | 11 (11) | 21 (47) | 6 (13) | 23 (44) | 5 (10) |
| Myalgia | 33 (34) | 12 (12) | 11 (24) | 4 (9) | 22 (42) | 8 (15) |
| Pyrexia | 31 (32) | 0 | 10 (22) | 0 | 21 (40) | 0 |
| Rash | 27 (28) | 0 | 8 (18) | 0 | 19 (37) | 0 |
| Decreased appetite | 24 (25) | 0 | 8 (18) | 0 | 16 (31) | 0 |
| Arthralgia | 23 (24) | 7 (7) | 9 (20) | 1 (2) | 14 (27) | 6 (12) |
| Anemia | 20 (21) | 13 (13) | 8 (18) | 5 (11) | 12 (23) | 8 (15) |
A total of 18 (19%) patients discontinued treatment due to a TRAE. The most common reasons for treatment discontinuation were CRS, myalgia (three patients each), and arthralgia (two patients). TRAEs led to dose interruption (missed doses) in 46 patients (47%) and dose reduction in seven patients (7%). The most common reasons for dose reduction were arthralgia, CRS, and pain in extremity (two patients each). Serious TRAEs occurred in 38 (39%) patients, with the most common events being CRS (16%), myalgia (4%), myofascitis, pain, and rash maculopapular (2% each).
CRS was the most common AE and occurred most frequently in C1. CRS was primarily grade 1 (36%) or grade 2 (33%), with two events (2%) being reported as grade 3 (Fig. 1A and B). One of the grade 3 events occurred at a higher priming dose of 0.3 mg (Cohort 6) and another before the initiation of the second predose of dexamethasone and postdose intravenous hydration initiated during Cohort 7a. Twenty-six patients in the study (27%) received tocilizumab for the treatment of CRS. There were no grade 4 or 5 CRS events.
Figure 1.
A, Frequency and highest-grade AEs occurring in ≥20% of patients treated with xaluritamig across all cohorts, TEAE vs. TRAEs (defined by the investigator as having reasonable possibility of being caused by xaluritamig). B, Incidence and grade of CRS (32) by cycle and dose schedule. Cohort 10 was excluded (0.1–1.0 mg), as dosing schedule was adjusted for the remaining patients after initial patients with a 10-fold dose increase in 1 step experienced DLTs. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Pharmacokinetics
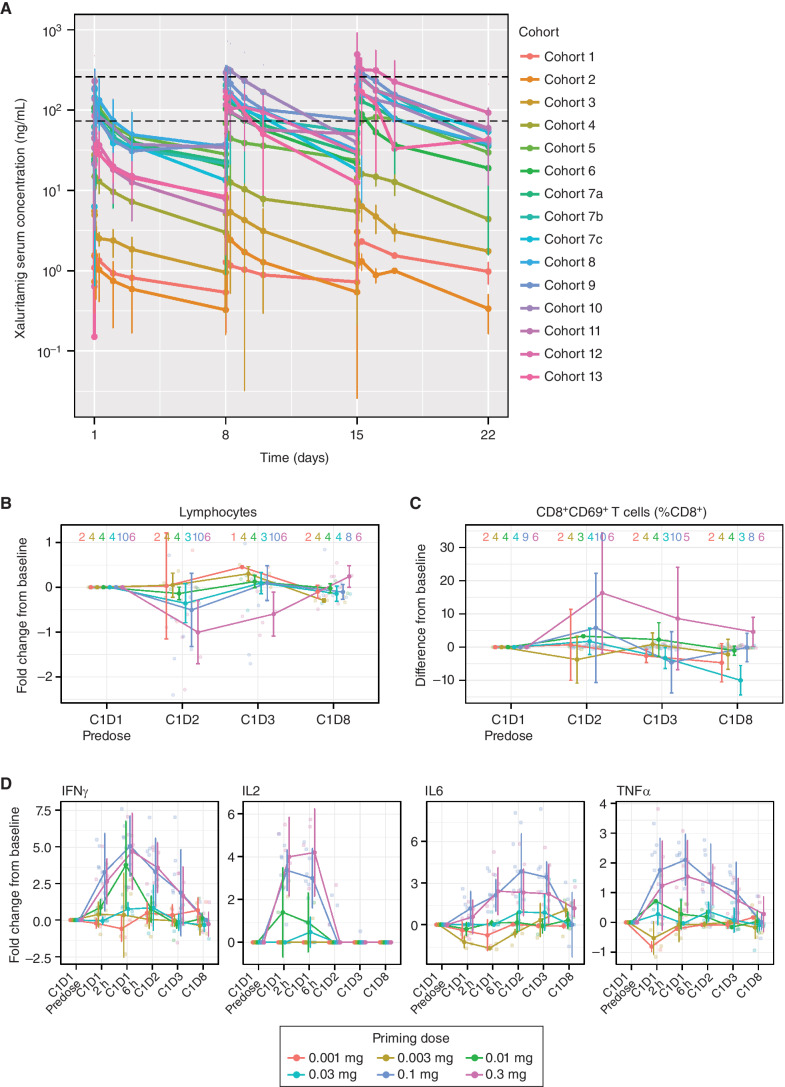
The mean (SD) xaluritamig serum concentration over time profile for C1 is shown by dose cohort in Fig. 2A. Preliminary pharmacokinetics showed a dose-proportional increase in exposure over the dose levels explored, with a mean terminal half-life of approximately 3 to 4 days. The minimum predicted efficacious exposure range of 74 to 259 ng/mL was based on preclinical studies (26). Starting at approximately the 0.75-mg target dose, Ctrough values achieved the lower end of the minimum predicted efficacious exposure. This supported an exploratory evaluation of efficacy by high dose (target dose ≥0.75 mg) and low dose (target dose <0.75 mg; Table 2).
Figure 2.
A, Mean (SD) xaluritamig serum concentration vs. time profile by individual cohorts for C1. Black dotted lines represent the lower end of the minimum predicted efficacious exposure based on EC90 for xaluritamig-mediated cell killing in vitro (74 ng/mL) and the upper end of the minimum predicted efficacious exposure based on IC50 for xaluritamig-mediated mouse tumor growth inhibition in vivo (259 ng/mL). Cohort 7c was dosed weekly for C1 and then switched to every 2 weeks starting C2 and beyond, which is not captured in this analysis. At the time of the data cut, there were 94 patients with at least one postbaseline xaluritamig concentration. T-cell pharmacodynamic biomarker response through the first infusion period is dose dependent. Peripheral lymphocyte margination (B), CD8+CD69+ activated T cells as a percentage of total CD8+ T cells (C), and induction of secreted cytokines at the indicated time points after infusion (D). Lines represent median fold change or difference of population percentage from C1D1 predose values ± median average deviation. Transparent points depict individual observations. Sample sizes within each priming dose group at each time point are shown as a strip annotation across the top of each figure. EC, effective concentration; IC, inhibitory concentration.
Pharmacodynamics
Significant changes in peripheral pharmacodynamic biomarkers of TCE activity were observed at priming doses above 0.01 mg. After the first infusion of xaluritamig, a rapid decrease of peripheral T-cell counts was observed (Fig. 2B). Lymphocyte redistribution was accompanied by transient expression of the T-cell activation marker CD69 on CD8+ T cells (Fig. 2C). T-cell margination (redistribution of T cells from blood into the periphery as a result of the mechanism of action) and T-cell activation were dose dependent with FDR-corrected P values <0.05 at 24 and 48 hours after infusion on C1D1. Measured serum cytokines, including IFNγ, IL2, IL6, and TNFα, increased from baseline following xaluritamig infusion, peaked within 6 to 24 hours, and returned to baseline before subsequent infusions (Fig. 2D). Cytokine induction was dose dependent with FDR-corrected P values <0.05 at 2, 6, 24, and 48 hours after infusion for IFNγ, IL6, and TNFα, and at 2, 6, and 24 hours after infusion for IL2.
Efficacy
During dose exploration, initial signs of clinical efficacy were observed on the basis of PSA declines starting with Cohort 5 (0.1-mg target dose; Table 2; Fig. 3A), and objective responses by RECIST criteria were first observed starting in Cohort 7a (0.3-mg target dose; Table 2; Fig. 3B).
Figure 3.
Clinical activity of xaluritamig in evaluable patients. A, Best PSA percentage change from baseline. Asterisk indicates confirmed PSA responders, and dashed lines indicate PSA50 and PSA90 declines. B, Best percentage change in size of tumor target lesions. Dashed line indicates 30% reduction in tumor SLD from baseline. C, Example of patient showing response by PSA and radiographic assessments: CT scan and PSA curve over time of a heavily pretreated 65-year-old patient with stage IV prostate adenocarcinoma. Patient was enrolled into Cohort 11 (3-step 1.5 mg target dose of xaluritamig). CT scans showed three target lesions (two liver, one lymph node) and multiple nontarget lesions in the liver as well as two lymph nodes during screening. Patient achieved 99% PSA decline from baseline on C7D1 and PR (37.3% reduction of target lesions) after 2 cycles, which was confirmed at 16 weeks and maintained after 24 weeks. AEs occurred during C1 of treatment and included recurrent CRS, tinea faciei (both grade 1), rash, and worsening of back pain (both grade 2). During further treatment cycles, rash (grade 1), myalgia, and hyperkalemia (both grade 2) were reported. Patient remains on treatment at the time of publication. Red arrows indicate sites of tumor. D, Time on treatment for patients in high-dose cohorts. PSA and RECIST responses [RECIST evaluable (gray bars) and non–RECIST evaluable (white bars)] are presented for patients in high-dose cohorts. Patients whose treatment was ongoing are noted by an arrowhead. Double parallel lines (//) represent patients who have extended beyond 48 weeks: one patient is ongoing treatment at 90 weeks, one is ongoing treatment at 84 weeks, and one ended treatment at 58 weeks. NE, not evaluable; PD, progressive disease; SD, stable disease; SLD, sum of longest diameters.
Among 97 patients treated, 67 patients had RECIST-evaluable disease per local evaluation (Table 4; Fig. 3B). Across all cohorts, 16 (24%) patients achieved a confirmed PR, 32 (48%) had stable disease (SD), 13 (19%) had progressive disease (PD), and six (9%) were not evaluable. In the low-dose (n = 30 evaluable patients) relative to high-dose cohorts (n = 37), confirmed responses were reported in one (3%) and 15 (41%) patients, respectively.
Table 4.
Summary of efficacy based on PSA and RECIST responses in patients receiving xaluritamig.
| All cohorts | Low-dose cohorts(1–7a) | High-dose cohorts (7b–13) | |
|---|---|---|---|
| PSA evaluable,an | 87 | 43 | 44 |
| PSA response, confirmed, n (%) | |||
| PSA50 | 43 (49) | 17 (40) | 26 (59) |
| PSA90 | 24 (28) | 8 (19) | 16 (36) |
| RECIST v1.1 evaluable, n | 67 | 30 | 37 |
| RECIST v1.1 response, confirmed, n (%) | |||
| PR | 16 (24) | 1 (3) | 15 (41) |
| SD | 32 (48) | 18 (60) | 14 (38) |
| PD | 13 (19) | 6 (20) | 7 (19) |
| Not evaluableb | 6 (9) | 5 (17) | 1 (3) |
aTen patients were not PSA evaluable: six patients were missing baseline PSA values, and four did not have sufficient follow-up duration.
bBest overall response of not evaluable includes five patients without postbaseline scans and one patient without sufficient follow-up duration prior to postbaseline assessment.
In the PSA-evaluable analysis set (N = 87), confirmed PSA50 responses were reported in 43 (49%) patients and confirmed PSA90 responses in 24 (28%; Table 4; Fig. 3A). In the low-dose (n = 43 evaluable patients) and high-dose (n = 44) cohorts, confirmed PSA50 responses were reported in 17 (40%) and 26 (59%) patients, respectively, and confirmed PSA90 responses occurred in eight (19%) and 16 (36%) patients.
Representative CT scans from a patient receiving xaluritamig are depicted in Fig. 3C. Both PSA and RECIST responses were rapid, typically occurring at the first evaluation (Fig. 3D; Supplementary Fig. S2). The median duration of RECIST response for patients with a confirmed response (n = 16) was 9.2 months (range, 1.9+ to 17.7+ months; +, censored); however, data remain immature due to limited time on study. As of the data cutoff, 24 of 97 (25%) patients were still on treatment, including 19 of 52 (37%) patients in the high-dose cohort. Thirteen of 52 (25%) patients in the high-dose cohorts were on treatment for more than 6 months. Swimlane plots for low- and high-dose cohorts are shown in Fig. 3D and Supplementary Fig. S2.
Immunogenicity
Treatment-emergent antidrug antibodies (ADA) were identified in 49 of 90 (54%) evaluable patients. Eight patients had a transient antibody response, in which the last time point tested for the patient was negative for ADA. Median onset of binding ADA was C2D1. Binding ADA–positive patients were evaluated for ADA impact on drug activity, exposure, and association with safety events. The ADAs observed were not associated with AEs. Of the 49 binding ADA–positive patients, 25 (51%) were found to have neutralizing antibodies in a qualified assay and 22 (45%) had an impact on xaluritamig exposure (>25% reduction compared with prior dose) after multiple cycles. The median neutralizing and/or exposure-impacting antibody onset was C3D15. Preliminary analyses on the ADA impact to xaluritamig's clinical response showed that the proportion of patients reaching landmark PSA50 at 12 weeks was comparable between the ADA-positive and ADA-negative subgroups (59.0% vs. 58.3%, respectively), 8 weeks after median ADA onset (Supplementary Table S2).
DISCUSSION
In this first-in-human dose-exploration study, xaluritamig had encouraging antitumor activity and a manageable safety profile in heavily pretreated patients with mCRPC. It provides proof of concept for leveraging STEAP1 as a target for TCEs and justifies the further exploration of this modality as a potential therapy for prostate cancer.
Utilizing the Bayesian logistic regression model (BLRM) for dose escalation, this study was able to identify the MTD through a stepwise approach. Consistent with TCE safety profile expectations and established approaches to mitigate the class effect of CRS (27, 28), this dose exploration determined the MTD for C1D1 as 0.1 mg. With 0.1 mg as the highest tolerable priming dose, several step dosing schedules were explored with the goal to achieve the highest tolerable target dose as early as possible in C1, reflecting expectations of early exposure with therapeutic dose levels leading to better responses. The target dose of 1.5 mg with a 3-step schedule was determined to be the MTD.
The safety profile in this study consisted of mostly grade 1 and 2 AEs that were clinically manageable, and no grade 5 events were found to be related to xaluritamig. Nineteen percent of patients discontinued treatment due to a TRAE, partly due to restrictions on the duration of dosing interruption. Mitigating strategies to further improve continuation on treatment are being explored, including expanding the allowed interval of dosing interruption paired with efficient measures to reduce the frequency of grade 3 AEs.
The most frequent TRAE was low-grade CRS occurring primarily in C1. CRS was expected in this study due to the biological mechanism of xaluritamig and clinical experience with other TCEs (12). Two (2%) cases of grade 3 CRS were reported; one was later reduced to grade 1 after data cutoff. The grade 3 events (Cohorts 6 and 7a) occurred before the addition of the second predose of dexamethasone and postdose intravenous hydration that was initiated in later cohorts. Almost all CRS events manifested in fever with or without hypotension, tachycardia, and rarely hypoxia. There were no grade 4 or 5 CRS events. Overall, all CRS events resolved with standard management, utilizing corticosteroids and/or tocilizumab along with intravenous hydration and acetaminophen.
Serious musculoskeletal and connective tissue disorders were reported in 11% of patients and included myalgia, myofascitis, arthralgia, arthritis, and back pain once higher dose levels were introduced. The exact mechanism for musculoskeletal pain AEs observed with xaluritamig is unclear, as STEAP1 has limited expression in muscle (29) and serum creatine kinase levels have been within normal limits for the affected patients. Further understanding of etiology and management may evolve as larger numbers of patients are treated, diagnostic imaging and biopsies are obtained, and responses to interventions are evaluated.
A favorable predictable dose–exposure relationship was observed after xaluritamig administration. The preliminary terminal half-life was approximately 3 to 4 days and supports the weekly dose schedule. Further schedules will be explored for patient convenience and optimization of toxicity management. Pharmacokinetics suggested that patients at the 0.75-mg target dose and above would have a trough concentration level at the minimum efficacious exposure based on preclinical studies. This allowed further exploratory analysis evaluating the clinical outcomes in low-dose (<0.75 mg) and high-dose (≥0.75 mg) cohorts.
The preliminary efficacy results observed with xaluritamig are numerically higher than those reported for other TCEs in prostate cancer (7, 8, 17). Efficacy as measured both by PSA and objective response by RECIST were encouraging in this heavily pretreated mCRPC population, and responses occurred with greater frequency in the higher-dose cohorts. PSA declines were seen starting with 0.1 mg xaluritamig, with 49% of patients achieving confirmed PSA50 responses and 28% of patients with PSA90 responses. At higher doses, responses were achieved in 41% of RECIST-evaluable patients. A relevant number of patients remained on treatment beyond 6 months in the higher-dose cohorts. As patients in the higher-dose cohorts had a limited time on the study [median (range) follow-up, 6.7 (0.5–20.9) months], limited conclusions on the durability of these responses can be made at this time, although preliminary DOR assessment is promising. It remains to be seen whether the PSA responses observed in patients with non–RECIST measurable disease will also translate to longer-term clinical benefit.
Targeted immunotherapy with TCEs requires binding to both CD3+ T cells and a tumor-associated antigen. Xaluritamig demonstrated dose-dependent changes in peripheral pharmacodynamic biomarkers of TCE activity, namely T-cell margination, T-cell activation, and cytokine induction. The magnitude of the biomarker changes is consistent with the observed PSA declines. Evaluation of the association between xaluritamig clinical activity and STEAP1 target expression or tumor-based biomarkers is ongoing in the expansion phase of the trial and will be critical to understanding any underlying reasons behind intrinsic or acquired resistance to xaluritamig.
The overall treatment-emergent ADA incidence was 54%, with eight patients demonstrating a transient antibody response. The ADA response was not dose dependent and did not result in AEs. With approximately one quarter of patients developing neutralizing ADAs and/or exposure-impacting ADAs, assessment of the impact on clinical responses is critical. With the onset of neutralizing ADAs being on average after C3 versus responses occurring in the first two cycles, an impact on objective response rate (ORR) is not expected, as evidenced by comparable percentages of patients reaching landmark PSA50 at 12 weeks regardless of ADA status. However, further follow-up is needed to determine whether ADAs will have an effect on the durability of the clinical response.
This trial has shown that a high proportion of patients may achieve significant clinical response, which may translate into overall clinical benefit. Successful adoption by the broader oncology community will require coordinated delivery of care, access to therapies to manage CRS, and education of practitioners on the routine management of CRS and T cell–mediated toxicities. This is particularly important during the first cycle of treatment. In this study, hospitalization was required for the first doses of xaluritamig, which may be an additional barrier for broader adoption. However, better understanding of the safety profile, including onset and management of toxicities, may allow reduction or modification of the hospitalization requirement, as has been reported with other TCE therapies (30).
This is the first clinical report of a TCE therapy targeting STEAP1 in prostate cancer. This study provides proof of concept for TCEs as a potential therapeutic modality for prostate cancer, as supported by the substantial number of radiographic and PSA responses observed. Xaluritamig also provides the first validation of STEAP1 as a target for cancer treatment, as prior clinical studies with a STEAP1 targeting ADC were limited by safety and modest efficacy. Additional characterization of xaluritamig is ongoing in dose expansion and in combination with standard-of-care therapies. Future clinical trials will need to define the optimal sequence to administer TCEs in the treatment course of advanced prostate cancer. In summary, this study demonstrates the feasibility of targeting STEAP1 with TCEs and the potential of xaluritamig as a novel treatment paradigm for patients with mCRPC.
METHODS
The study was conducted in accordance with the ethical principles derived from international guidelines including the Declaration of Helsinki, the Council for International Organizations of Medical Sciences International Ethical Guidelines, and applicable International Council for Harmonisation guidelines, laws, and regulations. The study protocol was approved by the institutional review board/independent ethics committee at each study site, and patients provided written informed consent.
Study Design
This was an open-label, multicenter phase I study in patients with advanced prostate cancer from North America, Europe, Asia, and Australia (NCT04221542). The trial commenced in March 2020 and contained multiple parts. The first part was an intravenous dose exploration for xaluritamig in patients with mCRPC enrolled between March 2020 and February 2023. Dose-escalation decisions in these cohorts were guided by the BLRM model for toxicity with overdose control (31). Data presented are as of March 28, 2023. The study schema is shown in Supplementary Fig. S1.
The primary objectives of the dose-exploration part of the study were to evaluate the safety and tolerability of xaluritamig and to determine the MTD. The secondary objectives were to evaluate the preliminary antitumor activity and characterize the pharmacokinetics. Exploratory objectives included evaluation of pharmacodynamic markers and immunogenicity.
Patient Selection
Men aged ≥18 years (or corresponding adult age depending on country) were included if they had histologically or cytologically confirmed prostate cancer (adenocarcinoma) that was refractory to a novel hormonal therapy, had disease progression following 1 to 2 taxane regimens (or were unsuitable for or had refused treatment with taxanes), and had evidence of PD as defined by the Prostate Cancer Working Group 3 (PCWG3) guidelines (32). Key inclusion criteria also required that patients had an Eastern Cooperative Oncology Group functional status 0 or 1, were either receiving continuous androgen deprivation therapy or had prior bilateral orchiectomy, and had adequate organ function. Key exclusion criteria were autoimmune disorders requiring immunosuppression and untreated central nervous system disease. Full eligibility criteria are further detailed in the Supplementary Methods.
Treatment
Xaluritamig was administered as an intravenous infusion weekly or every 2 weeks in a 28-day cycle. Patients were hospitalized for monitoring after each administration of xaluritamig until the target dose was achieved. Initial cohorts were monitored for 48 hours; however, based on initial safety data following implementation of step dosing, the hospitalization requirement was reduced to 24 hours. Dose escalation commenced at the minimum anticipated biological effect level–defined starting dose and progressed according to the dosing schema (Supplementary Fig. S1). All dose levels and schedules are provided in Table 2. Dose exploration advanced with single-patient cohorts until observation of any related grade 2 AE or DLTs, which triggered standard cohorts of two to four patients. The protocol allowed the dose-level review team to implement step dosing and prophylaxis with steroids or other medications to mitigate AEs known to be associated with TCEs (27). Step dosing consisted of a priming dose on C1D1 followed by weekly escalation until the intended target dose was achieved.
During C1, the protocol started with a requirement for prophylactic dexamethasone 8 mg (or equivalent dose of other corticosteroids) administered 1 hour prior to all doses. After observation of grade 3 CRS, the protocol was amended to add an additional dose of prophylactic dexamethasone 8 mg 6 to 16 hours before dosing and first-line intravenous hydration given immediately after dosing. In C2 and beyond, prophylactic therapies were allowed at the discretion of the treating investigator.
Treatment continued until PD, unacceptable toxicity, patient withdrawal, or investigator decision. In addition, missing more than one dose of xaluritamig for AEs required discontinuation of treatment during dose exploration. Treatment beyond progression was allowed in patients deriving clinical benefit per PCWG3 criteria (32). Intrapatient dose escalation was permitted with medical monitor approval.
Endpoints and Assessments
The primary endpoint of the study was to evaluate safety and tolerability and to determine the MTD or recommended phase II dose. The incidence and severity of AEs were assessed continuously for all patients using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0, with the exception of CRS, which was graded using the Lee 2014 criteria (33), and tumor lysis syndrome, which was graded using Cairo-Bishop criteria (34). CRS graded using Lee 2014 criteria included constitutional, neurologic, respiratory, cardiovascular, gastrointestinal, hematologic, and dermatologic signs and symptoms (33). Attribution of a final diagnosis for AEs (e.g., CRS) was per investigator determination based on overall assessments and review of clinical symptoms. TEAEs were defined as AEs starting on or after the first dose of xaluritamig up to 30 days after the last dose of xaluritamig. TRAEs were defined as TEAEs that per investigator review had a reasonable possibility of being caused by xaluritamig. DLTs were defined as AEs related to xaluritamig occurring in the first 28 days of treatment and per the criteria in the Supplementary Methods. Serious AEs were defined as any AE that met at least one of the following criteria: resulted in death, was immediately life-threatening, required in-patient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability/incapacity, or other medically important serious event. Laboratory assessments for safety were performed locally.
Secondary endpoints included pharmacokinetic assessment and evaluation of preliminary antitumor activity. Serum concentrations for xaluritamig were collected at prespecified time points. Pharmacokinetic samples were collected preinfusion, postinfusion, and 2, 6, 24, and 48 hours postinfusion during C1D1, C1D8, and C1D15 when step dosing occurred. Pharmacokinetic samples were collected preinfusion, postinfusion, and 6 hours postinfusion on C1D15 when no step dose occurred and on C1D22. Xaluritamig concentrations were determined by a validated electrochemiluminescence (ECL) assay. Concentration–time profiles were plotted using R (version 4.1.1). Pharmacokinetic parameters were calculated by noncompartmental analysis using Phoenix WinNonlin (version 8.3.4, Certara).
Efficacy analysis included assessment of objective response (OR) and DOR per RECIST version 1.1 and PSA response. Radiographic tumor burden assessments were assessed by the investigator and could include CT, MRI, and bone scans. Assessments were performed at baseline, at week 8 (defined as the baseline scan for bone scans), every 8 weeks during weeks 1 to 24, every 12 weeks thereafter, and at end of treatment or safety follow-up visits. Patients coming off treatment in the absence of disease progression continued to have scans every 3 months during follow-up. Tumor burden assessments for PSA by local laboratory analyses were performed at baseline and repeated every cycle on D1 throughout treatment and monthly until PSA progression in case of early treatment discontinuation.
A PSA50 response was defined as a ≥50% reduction in PSA level from baseline that was confirmed by a second test value ≥3 weeks later. A PSA90 response was defined as a ≥90% reduction in PSA levels from baseline confirmed ≥3 weeks later. PSA responses were assessed in all enrolled patients who had received ≥1 dose of xaluritamig, had a measurable PSA level at baseline (>0 ng/mL), and had the opportunity to be followed for ≥8 weeks starting from the first dose of xaluritamig.
Measurable disease was assessed using RECIST version 1.1, and bone scans were evaluated using PCWG3 criteria. Patients evaluable for RECIST response were those with baseline measurable disease who had received ≥1 dose of xaluritamig and who had the opportunity to be followed for ≥8 weeks starting from the first dose. OR was defined as a PR or complete response per RECIST 1.1, confirmed by a repeat assessment at least 4 weeks later.
Exploratory Endpoints
For pharmacodynamic assessments, lymphocyte subpopulations were analyzed by local laboratory assessments and by central flow cytometry. Whole peripheral blood was collected at preinfusion on C1D1, 24 and 48 hours postinfusion on C1D1, and preinfusion on C1D8 and was stained with monoclonal antibodies against the following cell-surface markers: CD3, CD4, CD8, CD25, CD357, CD45, CD197, CD69, CD127, and CD45RA. Stained cells were acquired and analyzed on a BD FACS Canto flow cytometer using FACSDIVA v9.0 software (BD Biosciences). Patient sera were collected at preinfusion; 2, 6, 24, and 48 hours during C1D1; and preinfusion during C1D8. Levels of IFNγ, IL2, IL6, and TNFα were measured centrally using a multiplex ECL assay (10-V Plex; Meso Scale Discovery).
To assess for immunogenicity, ADA formation was measured at baseline and before each dose in C1 and then every 2 weeks thereafter until end of treatment. Binding antibodies were evaluated with an ECL detection–based bridging immunoassay, and neutralizing antibodies were evaluated with a cell-based bioassay.
Statistical Considerations
The sample size in the dose-exploration phase was based on practical consideration and was consistent with conventional oncology studies with the objective to estimate the MTD. Dose-escalation decisions were guided by the BLRM for dose toxicity. After each cohort, the next dose recommended by the BLRM was the one with the highest probability of the target toxicity interval (20%–33%), subject to overdose control.
Pharmacodynamic modeling of biomarker activity was performed using linear mixed effects models (35) predicting the fold or percentage change from baseline using baseline biomarker levels as a covariate, an interactive term between categorical visit and continuous dose, and a random effect for the patient. Significance of overall increase or decrease at each time point and the dose–response relationship at each time point was determined and considered significant if the Padj was <0.05 following Benjamini–Hochberg FDR correction (36).
Data Availability
Qualified researchers may request deidentified data from Amgen clinical studies; complete details are available at http://www.amgen.com/datasharing.
Supplementary Material
Supplementary Methods: Additional methodology including full inclusion criteria, full exclusion criteria, and definition of dose-limiting toxicities.
Supplementary Table S1: Supplementary Table S1 showing representativeness of study patients, including age, race/ethnicity, geography, other considerations, and overall representativeness of this study.
Supplementary Table S2: Supplementary Table S2 showing antidrug antibody (ADA) impact to landmark prostate-specific antigen response (PSA50) at 12 weeks.
Supplementary Figure S1: Supplementary Figure S1 showing study schema for xaluritamig intravenous monotherapy dose exploration and expansion phases.
Supplementary Figure S2: Supplementary Figure S2 swimlane plot showing time on treatment for xaluritamig patients in low-dose cohorts.
Acknowledgments
Acknowledgments
We thank the patients, investigators, and study staff who contributed to this study. Medical writing support was provided by Lisa R. Denny, PhD, Maryann T. Travaglini, PharmD (ICON, Blue Bell, PA), and Lynnea R. Waters, PhD (Amgen Inc.). We also thank Judson Englert, MD, PhD, for support with interpretation of results and medical writing; Sheryl Treichel, MS, for support with statistical analyses; Bo Ye, PhD, for statistical support; Ben Decato, PhD, for pharmacodynamic modeling; and Invicro and Phil Kuo, MD, PhD, for independent central review of patient scans. This study was sponsored and funded by Amgen Inc.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
Authors’ Disclosures
W.K. Kelly reports other support from Amgen Inc. during the conduct of the study, as well as personal fees from Janssen and Bayer, and other support from Novartis, Janssen, Bayer, Exelixis, Seagen, Amgen Inc., BioClin, Roche, and Regeneron outside the submitted work. D.C. Danila reports research support from the U.S. Department of Defense, the American Society of Clinical Oncology, the Prostate Cancer Foundation, Stand Up To Cancer, Amgen Inc., Janssen Research & Development, Astellas, Medivation, Agensys, Genentech, and CreaTV, as well as consulting for Angle LLT, Janssen Research & Development, AstraZeneca, BioView LTD, Clovis, Astellas, Medivation, Pfizer, Agensys, and Merck. C.-C. Lin reports personal fees from AbbVie, Bayer, BeiGene, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly, IMPACT Therapeutics, Novartis, PharmaEngine, Roche, and Seagen outside the submitted work. J.-L. Lee reports grants from Amgen Inc. during the conduct of the study, as well as grants from Pfizer, Ipsen, Bristol Myers Squibb, MSD, Merck, Roche, Genentech, AstraZeneca, Seagen, and GI Innovation outside the submitted work. N. Matsubara reports grants from Amgen Inc. during the conduct of the study, as well as grants from Janssen, AstraZeneca, Bayer, MSD, Taiho, Astellas, Eisai, Eli Lilly, PRA Health Science, Takeda, Pfizer, Seagen, Chugai, and AbbVie, and personal fees from Sanofi outside the submitted work. P.J. Ward reports advisory board participation for Amgen Inc. (March 2023) and Fresenius Kabi (June 2023). A.J. Armstrong reports nonfinancial support from Amgen Inc. during the conduct of the study, as well as grants and personal fees from Astellas, Pfizer, Bayer, AstraZeneca, Novartis, and Bristol Myers Squibb, and personal fees from Myovant, Exelixis, Epic Sciences, Clovis, and Exact Sciences outside the submitted work. D. Pook reports other support from Amgen Inc. during the conduct of the study, as well as personal fees from Bayer and MSD, personal fees and nonfinancial support from Merck, Pfizer, Bristol Myers Squibb, and Janssen, grants, personal fees, and nonfinancial support from Astellas, and nonfinancial support from Amgen Inc. outside the submitted work. M. Kim reports personal fees from Merck Sharp & Dohme, Bristol Myers Squibb/Ono Pharmaceutical, Ipsen, Roche, Janssen, Astellas, Eisai, Bayer, Pfizer, Merck, Boryung, and Yuhan Corporation outside the submitted work. T.B. Dorff reports other support from Amgen Inc. during the conduct of the study, as well as personal fees from AstraZeneca, Bayer, and Sanofi outside the submitted work. L.G. Horvath reports other support from Amgen Inc. during the conduct of the study, as well as other support from Amgen Inc. outside the submitted work. Z. Yang reports personal fees from Amgen Inc. outside the submitted work. H.L. Penny is an Amgen Inc. employee and stockholder. J. Stieglmaier reports personal fees from Amgen Research (Munich) GmbH and other support from Amgen Inc. during the conduct of the study, as well as personal fees from Amgen Research (Munich) GmbH and other support from Amgen Inc. outside the submitted work. L.J. Appleman reports grants from Amgen Inc. during the conduct of the study. No disclosures were reported by the other authors.
Authors’ Contributions
W.K. Kelly: Investigation, writing–review and editing. D.C. Danila: Investigation, writing–review and editing. C.-C. Lin: Investigation, writing–review and editing. J.-L. Lee: Investigation, writing–review and editing. N. Matsubara: Investigation, writing–review and editing. P.J. Ward: Investigation, writing–review and editing. A.J. Armstrong: Investigation, writing–review and editing. D. Pook: Investigation, writing–review and editing. M. Kim: Investigation, writing–review and editing. T.B. Dorff: Investigation, writing–review and editing. S. Fischer: Investigation, writing–review and editing. Y.-C. Lin: Investigation, writing–review and editing. L.G. Horvath: Investigation, writing–review and editing. C. Sumey: Investigation, writing–review and editing. Z. Yang: Formal analysis, investigation, methodology, writing–review and editing. G. Jurida: Formal analysis, investigation, writing–review and editing. K.M. Smith: Formal analysis, investigation, methodology, writing–review and editing. J.N. Connarn: Formal analysis, investigation, methodology, writing–review and editing. H.L. Penny: Formal analysis, investigation, methodology, writing–review and editing. J. Stieglmaier: Conceptualization, investigation, writing–original draft, writing–review and editing. L.J. Appleman: Investigation, writing–review and editing.
References
- 1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Fact Sheets, Prostate . Lyon (France), Geneva (Switzerland): International Agency for Research on Cancer, World Health Organization; 2020 [cited 2023 Jul 3]. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf.
- 3. Wadosky KM, Koochekpour S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget 2016;7:64447–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Stat Facts: Prostate Cancer; [about 4 screens]. [cited 2023 Jul 3]. Available from: https://seer.cancer.gov/statfacts/html/prost.html.
- 5. Turco F, Gillessen S, Cathomas R, Buttigliero C, Vogl UM. Treatment landscape for patients with castration-resistant prostate cancer: patient selection and unmet clinical needs. Res Rep Urol 2022;14:339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gillessen S, Bossi A, Davis ID, de Bono J, Fizazi K, James ND, et al. Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer 2023;185:178–215. [DOI] [PubMed] [Google Scholar]
- 7. Sorrentino C, Di Carlo E. Molecular targeted therapies in metastatic prostate cancer: recent advances and future challenges. Cancers (Basel) 2023;15:2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Powers E, Karachaliou GS, Kao C, Harrison MR, Hoimes CJ, George DJ, et al. Novel therapies are changing treatment paradigms in metastatic prostate cancer. J Hematol Oncol 2020;13:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merck Provides Update on Phase 3 Trials KEYNOTE-641 and KEYNOTE-789. 2023 Feb 28 [cited 2023 Jul 20] . Available from: https://www.merck.com/news/merck-provides-update-on-phase-3-trials-keynote-641-and-keynote-789/.
- 10. Powles T, Yuen KC, Gillessen S, Kadel EE, Rathkopf D, Matsubara N, et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med 2022;28:144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antonarakis ES, Park SH, Goh JC, Shin SJ, Lee JL, Mehra N, et al. Pembrolizumab plus olaparib for patients with previously treated and biomarker-unselected metastatic castration-resistant prostate cancer: the randomized, open-label, phase III KEYLYNK-010 trial. J Clin Oncol 2023;41:3839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou S, Liu M, Ren F, Meng X, Yu J. The landscape of bispecific T cell engager in cancer treatment. Biomark Res 2021;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atallah-Yunes SA, Robertson MJ, Davé UP, Ghione P, Perna F. Novel immune-based treatments for diffuse large B-cell lymphoma: the post-CAR T cell era. Front Immunol 2022;13:901365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J-M, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 2017;376:836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nathan P, Hassel JC, Rutkowski P, Baurain J-F, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 2021;385:1196–206. [DOI] [PubMed] [Google Scholar]
- 16. Ravi G, Costa LJ. Bispecific T-cell engagers for treatment of multiple myeloma. Am J Hematol 2023;98:S13–21. [DOI] [PubMed] [Google Scholar]
- 17. Tucker MD, Zhu J, Marin D, Gupta RT, Gupta S, Berry WR, et al. Pembrolizumab in men with heavily treated metastatic castrate-resistant prostate cancer. Cancer Med 2019;8:4644–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim EA, Schweizer MT, Chi KN, Aggarwal R, Agarwal N, Gulley J, et al. Phase 1 study of safety and preliminary clinical activity of JNJ-63898081, a PSMA and CD3 bispecific antibody, for metastatic castration-resistant prostate cancer. Clin Genitourin Cancer 2023;21:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Bono JS, Fong L, Beer TM, Gao X, Geynisman DM, Burris HA III, et al. Results of an ongoing phase 1/2a dose escalation study of HPN424, a tri-specific half-life extended PSMA-targeting T-cell engager, in patients with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2021;39:5013–13. [Google Scholar]
- 20. Tran B, Horvath L, Dorff T, Rettig M, Lolkema MP, Machiels JP, et al. Results from a phase I study of AMG 160, a half-life extended (HLE), PSMA-targeted, bispecific T-cell engager (BiTE®) immune therapy for metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol 2020;31 Suppl 4:S507. Abstract nr 609O. [Google Scholar]
- 21. Xu M, Evans L, Bizzaro CL, Quaglia F, Verrillo CE, Li L, et al. STEAP1–4 (six-transmembrane epithelial antigen of the prostate 1–4) and their clinical implications for prostate cancer. Cancers (Basel) 2022;14:4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nolan-Stevaux O. AMG 509: a novel, humanized, half-life extended, bispecific STEAP1 × CD3 T cell recruiting XmAb® 2+1 antibody [abstract]. In: Proceedings of the Annual Meeting of the American Association for Cancer Research 2020; 2020 Apr 27–28 and Jun 22–24. Philadelphia (PA): AACR; Cancer Res 2020;80(16 Suppl):Abstract nr DDT02-03). [Google Scholar]
- 23. Danila DC, Szmulewitz RZ, Vaishampayan U, Higano CS, Baron AD, Gilbert HN, et al. Phase I study of DSTP3086S, an antibody-drug conjugate targeting six-transmembrane epithelial antigen of prostate 1, in metastatic castration-resistant prostate cancer. J Clin Oncol 2019;37:3518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bhatia V, Kamat NV, Pariva TE, Wu LT, Tsao A, Sasaki K, et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun 2023;14:2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee Lab, Human Biology and Clinical Research Divisions, Cancer Consortium. Boost and attack approach for metastatic prostate cancer therapy! Fred Hutch Cancer Center . Available from: https://www.fredhutch.org/en/news/spotlight/2023/06/ccg-bhatia-natcommun.html.
- 26. Nolan-Stevaux O, Li C, Liang L, Zhan J, Estrada J, Osgood T, et al. AMG 509 (xaluritamig), an anti-STEAP1 XmAb 2+1 T-cell redirecting immune therapy with avidity-dependent activity against prostate cancer. Cancer Discov 2024;14:90–103. [DOI] [PubMed]
- 27. Ball K, Dovedi SJ, Vajjah P, Phipps A. Strategies for clinical dose optimization of T cell-engaging therapies in oncology. MAbs 2023;15:2181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Project Optimus. Reforming the dose optimization and dose selection paradigm in oncology; [about 4 screens]. [cited 2023 Jul 24]. Available from: https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus.
- 29. The Human Protein Atlas. STEAP1; [about 5 screens]. [cited 2023 Jul 24]. Available from: https://www.proteinatlas.org/ENSG00000164647-STEAP1/tissue.
- 30. Dickinson MJ, Carlo-Stella C, Morschhauser F, Bachy E, Corradini P, Iacoboni G, et al. Glofitamab for relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2022;387:2220–31. [DOI] [PubMed] [Google Scholar]
- 31. Neuenschwander B, Branson M, Gsponer T. Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med 2008;27:2420–39. [DOI] [PubMed] [Google Scholar]
- 32. Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol 2016;34:1402–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol 2008;26:2767–78. [DOI] [PubMed] [Google Scholar]
- 35. Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: tests in linear mixed effects models. J Stat Softw 2017;82:1–26. [Google Scholar]
- 36. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods: Additional methodology including full inclusion criteria, full exclusion criteria, and definition of dose-limiting toxicities.
Supplementary Table S1: Supplementary Table S1 showing representativeness of study patients, including age, race/ethnicity, geography, other considerations, and overall representativeness of this study.
Supplementary Table S2: Supplementary Table S2 showing antidrug antibody (ADA) impact to landmark prostate-specific antigen response (PSA50) at 12 weeks.
Supplementary Figure S1: Supplementary Figure S1 showing study schema for xaluritamig intravenous monotherapy dose exploration and expansion phases.
Supplementary Figure S2: Supplementary Figure S2 swimlane plot showing time on treatment for xaluritamig patients in low-dose cohorts.
Data Availability Statement
Qualified researchers may request deidentified data from Amgen clinical studies; complete details are available at http://www.amgen.com/datasharing.



![Figure 3. Clinical activity of xaluritamig in evaluable patients. A, Best PSA percentage change from baseline. Asterisk indicates confirmed PSA responders, and dashed lines indicate PSA50 and PSA90 declines. B, Best percentage change in size of tumor target lesions. Dashed line indicates 30% reduction in tumor SLD from baseline. C, Example of patient showing response by PSA and radiographic assessments: CT scan and PSA curve over time of a heavily pretreated 65-year-old patient with stage IV prostate adenocarcinoma. Patient was enrolled into Cohort 11 (3-step 1.5 mg target dose of xaluritamig). CT scans showed three target lesions (two liver, one lymph node) and multiple nontarget lesions in the liver as well as two lymph nodes during screening. Patient achieved 99% PSA decline from baseline on C7D1 and PR (37.3% reduction of target lesions) after 2 cycles, which was confirmed at 16 weeks and maintained after 24 weeks. AEs occurred during C1 of treatment and included recurrent CRS, tinea faciei (both grade 1), rash, and worsening of back pain (both grade 2). During further treatment cycles, rash (grade 1), myalgia, and hyperkalemia (both grade 2) were reported. Patient remains on treatment at the time of publication. Red arrows indicate sites of tumor. D, Time on treatment for patients in high-dose cohorts. PSA and RECIST responses [RECIST evaluable (gray bars) and non–RECIST evaluable (white bars)] are presented for patients in high-dose cohorts. Patients whose treatment was ongoing are noted by an arrowhead. Double parallel lines (//) represent patients who have extended beyond 48 weeks: one patient is ongoing treatment at 90 weeks, one is ongoing treatment at 84 weeks, and one ended treatment at 58 weeks. NE, not evaluable; PD, progressive disease; SD, stable disease; SLD, sum of longest diameters.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/299b/10784743/ce9f39098fff/76fig3.jpg)