Abstract
Background:
Chronic rhinosinusitis with nasal polyposis (CRSwNP) is a type 2 (T2) inflammatory disease associated with an increased number of airway basal cells (BCs). Recent studies have identified transcriptionally distinct BCs, but the molecular pathways that support or inhibit human BC proliferation and differentiation are largely unknown.
Objective:
We sought to determine the role of T2 cytokines in regulating airway BCs.
Methods:
Single-cell and bulk RNA sequencing of sinus and lung airway epithelial cells was analyzed. Human sinus BCs were stimulated with IL-4 and IL-13 in the presence and absence of inhibitors of IL-4R signaling. Confocal analysis of human sinus tissue and murine airway was performed. Murine BC subsets were sorted for RNA sequencing and functional assays. Fate labeling was performed in a murine model of tracheal injury and regeneration.
Results:
Two subsets of BCs were found in human and murine respiratory mucosa distinguished by the expression of basal cell adhesion molecule (BCAM). BCAM expression identifies airway stem cells among P63+KRT5+NGFR+ BCs. In the sinonasal mucosa, BCAMhi BCs expressing TSLP, IL33, CCL26, and the canonical BC transcription factor TP63 are increased in patients with CRSwNP. In cultured BCs, IL-4/IL-13 increases the expression of BCAM and TP63 through an insulin receptor substrate–dependent signaling pathway that is increased in CRSwNP.
Conclusions:
These findings establish BCAM as a marker of airway stem cells among the BC pool and demonstrate that airway epithelial remodeling in T2 inflammation extends beyond goblet cell metaplasia to the support of a BC stem state poised to perpetuate inflammation. (J Allergy Clin Immunol 2023;151:1536-49.)
Keywords: Basal cell, airway stem cell, basal cell adhesion molecule, airway inflammation, type 2 inflammation, IL-4, IL-13, chronic rhinosinusitis with nasal polyposis, asthma, single-cell RNA sequencing
GRAPHICAL ABSTRACT
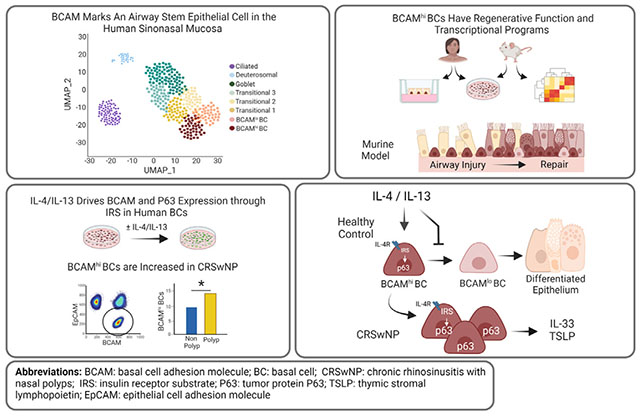
Tissue-resident stem cells exhibit remarkable plasticity, responding to local damage by regenerating diverse differentiated cell types. This plasticity ensures their ability to restore homeostasis after injury but also endows them with the capacity to remodel the tissue microenvironment and adapt to tissue stress. A canonical example of tissue remodeling is intestinal goblet cell metaplasia, which plays an essential role in host defense against helminths. Here, local immunocytes generate IL-13, which drives the differentiation of epithelial cells (EpCs) into goblet cells that secrete mucus and thereby facilitate helminth expulsion.1–3 Although appropriate tissue adaptation requires that stem cells integrate local environmental cues from diverse sources including niche mesenchymal cells, tissue immunocytes, and even their own progeny, in most circumstances the signals that mediate remodeling and the features of remodeling that are beneficial or detrimental to the host are poorly understood.
In the respiratory tract, studies using single-cell RNA sequencing (scRNA-seq) and immunofluorescence have identified variations in the abundance of EpC subsets in distinct disease states. This includes an expansion of ciliated cells in cystic fibrosis,4 an increase in IL-25–secreting tuft cells in allergic fungal rhinosinusitis and chronic rhinosinusitis with nasal polyposis (CRSwNP),5–7 and an increase in neuroendocrine cells in diffuse idiopathic pulmonary neuroendocrine cell hyperplasia,8–10 asthma,11 and neuroendocrine cell hyperplasia of infancy.12 These specialized EpCs derive from basal cell (BC) progenitors, suggesting that alterations in BC programs likely account for these variations. Indeed, scRNA-seq has detected transcriptionally distinct BC subsets in the respiratory tract,13–15 but functional annotation of these cell types is lacking.
In Western countries, CRSwNP is a type 2 (T2) inflammatory disease of the sinonasal airway that is often associated with asthma. Patients with this disorder have eosinophilia in the airways and peripheral blood and respond to treatment with mAb blockade of IL-4Rα, a component of both the T1 and T2 IL-4 receptors that bind IL-4 and IL-13.16 We previously reported that BCs from patients with CRSwNP accumulated in the sinonasal mucosa and failed to differentiate normally, but the mechanism by which this occurs is unknown.
Here, we report 2 subsets of KRT5+ BCs in the sinonasal mucosa distinguished by expression of basal cell adhesion molecule (BCAM). Using sequencing, ex vivo culture, and in vivo experimentation, we demonstrate that BCAMhi BCs are the progenitors of BCAMlo BCs, that they increase in CRSwNP, and that the T2 cytokines IL-4 and IL-13 reinforce the expression of BCAM and prevent BC differentiation through an insulin receptor substrate (IRS)–dependent signaling pathway that is overexpressed in CRSwNP. These findings demonstrate that IL-4/IL-13 can play a profound role in remodeling the airway epithelial BC compartment to drive the accumulation of a stem cell with potent proinflammatory capacity.
METHODS
Study design
This study was designed to characterize BC subsets in the human and murine airway and to understand the alterations in BC programs in T2 inflammatory diseases such as CRSwNP. This objective was addressed by (1) reanalysis of scRNA-seq13 of nonproliferating surface airway EpCs in surgical excisions of human sinonasal mucosa from patients with CRSwNP (n = 6) and those with chronic rhinosinusitis sans nasal polyposis (CRSsNP) (n = 6) to identify potential markers of BC subsets; (2) flow-cytometric analysis and ex vivo studies of primary human sinonasal BCs from subjects with chronic rhinosinusitis or healthy control subjects to demonstrate distinct BC functions; (3) development of a murine flow-cytometry panel, bulk RNA-seq data sets, BC lineage-tracing system, and a model of airway damage and regeneration to characterize BC subsets in C57BL/6 mice; and (4) assessment of human BCAMhi BCs ex vivo with and without T2 cytokines. Details of the subjects; analytic methods for scRNA-seq and bulk RNA-seq; protocols for cell isolation, staining, and culture; methods for confocal analysis; and murine Alternaria alternata (ALT) challenges are provided in this article’s Online Repository at www.jacionline.org.
Study approval
The Mass General Brigham Institutional Review Board approved the study, and all subjects provided written informed consent before participation. The use of mice for this study was in accordance with the review and approval by the Animal Care and Use Committee of Brigham and Women’s Hospital.
Data and code availability
Low-input RNA-seq data have been deposited in the Gene Expression Omnibus (GSE197274). CRSwNP single-cell data set has been deposited in ImmPort SDY1877. The public data set from healthy lung was downloaded from https://www.covid19cellatlas.org/index.healthy.html#vieira19-bronchi.14 The code for main analyses is available at https://github.com/nils-hallen/Single-Cell-Analyses.
Statistical analysis
Computational methods for RNA sequencing are detailed in this article’s Online Repository at www.jacionline.org. Other analyses were performed with the GraphPad Prism software (version 9.3.1, GraphPad, La Jolla, Calif) and R (version 4.03). All in vitro and in vivo results are either representative of or pooled across 3 to 5 independent experiments. Data indicate mean ± SEM in all bar graphs. A P value of less than .05 was considered significant.
RESULTS
BCAM marks a multipotent progenitor cell among KRT5+NGFR+ITGA6+ BCs in the human respiratory mucosa
We previously reported the impaired differentiation of airway EpCs in patients with CRSwNP.13 To better delineate EpC differentiation, we reanalyzed our scRNA-seq data set from sinus surgeries.13 Using Harmony,17 we integrated data across donors and patient subtypes (CRSsNP and CRSwNP) in principal-component space, reclustered nonproliferating surface airway secretory and ciliated EpCs (see Fig E1, A–D, and Tables E1–E3 in this article’s Online Repository at www.jacionline.org), and assessed established EpC markers (Table I) to define common EpC states across diseases (Fig E1, E). Here, we identified 2 BC subsets present in the sinonasal tissue of patients with CRSwNP and those with CRSsNP (Fig 1, A–C). One subset (cluster 0) expressed high levels of the BC markers KRT5, KRT15, and TP6318–20; the stem marker CD4421; and the cell surface receptor BCAM (false-discovery rate < 0.05; Fig 1, B and C; see also Table E4 in this article’s Online Repository at www.jacionline.org). The second BC subset (cluster 1) expressed higher levels of the oncogene MALAT122 and many ribosomal genes, suggesting ribosomal biogenesis. Differentiating transitional cells (clusters 2-4) were marked by increasing expression of the Notch pathway gene HES1,23 the luminal BC marker KRT8,24 and the club cell markers SCGB1A1 and TFF3,25,26 whereas mature secretory cells (cluster 5) were marked by MUC5AC,14 deuterosomal cells (cluster 6) were marked by CDC20B, PLK4, and FOXJ1,27 and ciliated cells (cluster 7) were marked by PIFO, CAPS, and FOXJ1.15 Cluster 5 was composed of both goblet and club secretory cells expressing MUC5AC or MUC5B and SCGB1A1, respectively (Fig E1, F).
TABLE I.
Established markers of airway epithelial cells
| Cell type | Marker | |
|---|---|---|
| BCs | NGFR | Nerve growth factor receptor |
| GSIB4* | Lectin Griffonia simplicifolia IB4 | |
| ITGA6 | Integrin subunit alpha 6 | |
| KRT5 | Keratin 5 | |
| TP63 | Tumor protein 63 | |
| CD44 | CD44 molecule | |
| PDPN | Podoplanin | |
| Luminal progenitor BCs | KRT8 | Keratin 8 |
| Secretory club cells | SCGB1A1 | Secretoglobin family 1A member 1 |
| Goblet cells | MUC5AC | Mucin 5AC |
| Ciliated cells | FOXJ1 | Forkhead box J1 |
| Acetyl-α-tubulin | Acetylated alpha tubulin |
GSIB4 lectin is a validated marker of murine, but not human, BCs.
FIG 1.
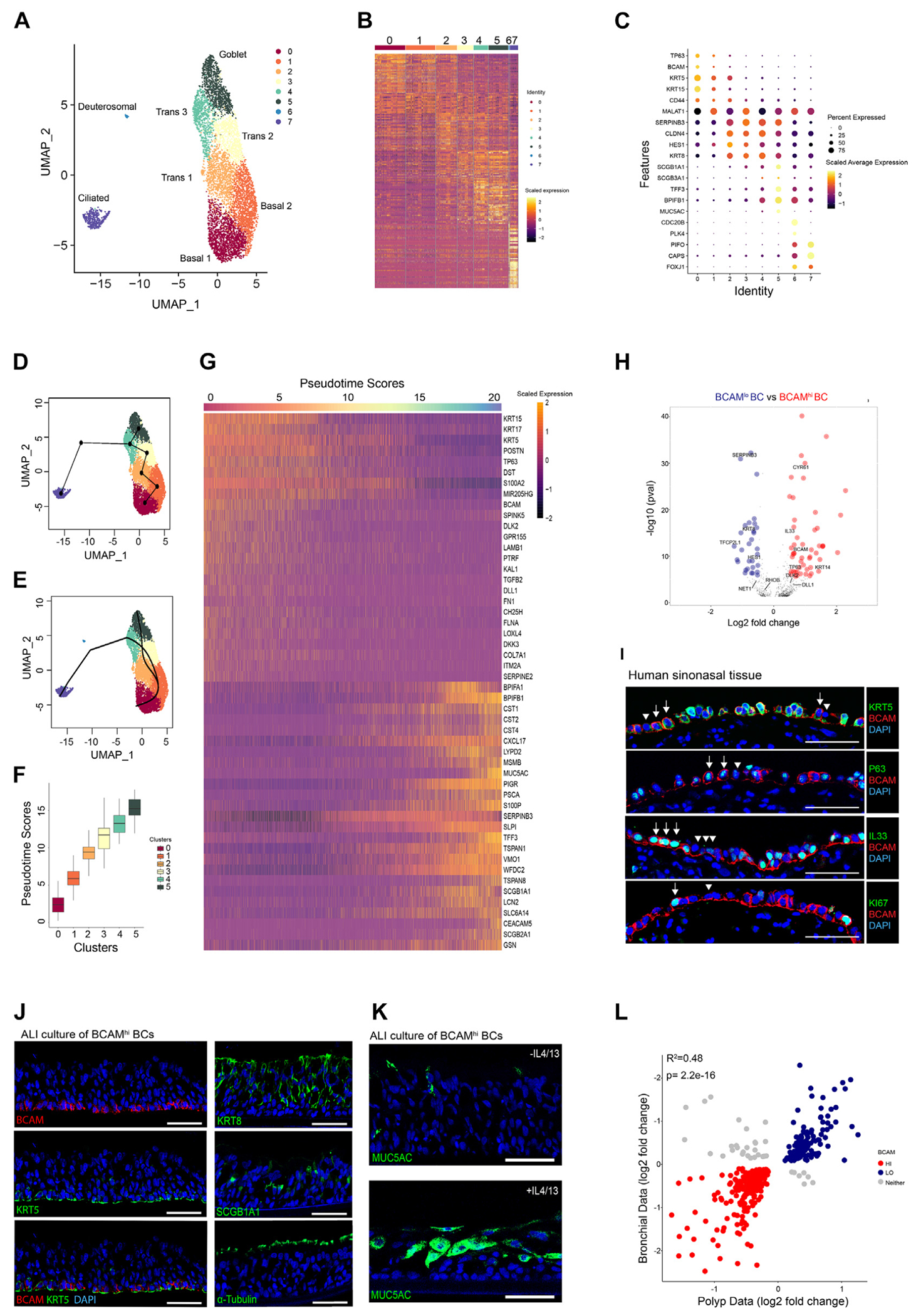
BCAM marks a multipotent progenitor among KRT5+NGFR+ITGA6+ BCs in the human respiratory mucosa. A, UMAP representation of 6970 EpCs from 12 donors. B, Heatmap of scaled gene expression for the top 30 genes identified by Wilcoxon rank-sum test and auROC analysis. C, Dot plot of epithelial markers across clusters. D, Cluster-based minimum spanning tree of lineages overlaid on UMAP. E, Cell assignment to smooth principal curves with lineage-specific pseudotimes. F, Plot of pseudotime scores from cells assigned to the secretory lineage. G, Heatmap of scaled gene expression for the topmost significant genes associated with changes in pseudotime (Bonferroni-corrected P values). H, Volcano plot of log2 fold change between basal 0 (BCAMhi) BCs and basal 1 (BCAMlo) BCs. Significant genes with increased expression in BCAMhi (red) and BCAMlo (blue) (P < 1.5 × 10−5, Bonferroni threshold). I, Representative immunostaining of human sinonasal tissue from a patient with CRSwNP. Arrow indicates BCAMhi BCs, and arrowhead indicates BCAMlo BCs. The scale bar represents 50 μm (n = 3). J, Representative immunostaining on ALI cultures derived from BCAMhi BCs. The scale bar represents 50 μm (n = 3). K, Representative immunostaining on ALI cultures derived from BCAMhi BCs treated with/without IL-4/IL-13 (n = 3). L, Coefficient of determination of log2 fold changes of shared genes from sinonasal BCs (BCAMhi vs BCAMlo) and bronchial BCs (BC1 vs BC2). auROC, Area under the receiving operator characteristic; DAPI, 4’-6-diamidino-2-phenylindole; UMAP, uniform manifold approximation and projection.
Trajectory analysis of these clusters suggested 2 distinct trajectories with a serial progression from TP63hi BC progenitors (cluster 0) to MALAT1+ BCs (cluster 1), KRT8+ HES1hi transitional cells (clusters 2-4), and then to either fully differentiated MUC5AC+ goblet cells (cluster 5) or to PIFO+ ciliated EpCs (cluster 7) (Fig 1, D and E). Accordingly, cells in the secretory trajectory from clusters 0 to 5 (Fig E1, G) demonstrated progressively increasing pseudotime scores (Fig 1, F). Using generalized linear modeling across pseudotime for EpCs in the secretory trajectory, we demonstrated 2278 genes that were either highly positively or negatively associated with the pseudotime score (P < 4.23 × 10−6, Bonferroni threshold; Fig 1, G; see also Tables E5 and E6 in this article’s Online Repository at www.jacionline.org). We detected increasing expression of canonical secretory genes across pseudotime and loss of BC markers, as expected (Fig 1, G). In addition, we saw that expression of the cluster 0 BC marker BCAM (Fig 1, C) was inversely associated with pseudotime, suggesting that its expression may distinguish states of BC differentiation.
Direct comparison of cluster 0 BCAMhi BCs and cluster 1 BCAMlo BCs demonstrated 142 genes that were differentially expressed between these clusters (P < 1.5 × 10−5, Bonferroni threshold; Fig 1, H; see also Table E7 in this article’s Online Repository at www.jacionline.org). BCAMhi BCs expressed higher levels of many genes associated with epithelial progenitor cells. These included the Yap target gene CYR61/CCN1,28 WNT target genes MMP1029 and MYC,30 the NOTCH ligand DLL131 and the NOTCH inhibitor DLK2,32 the proliferation-associated gene ZFP36L2,33 and transcription factors required for stem cell maintenance, such as TCF1234 and TP63.19,20,35 BCAMhi BCs also expressed higher levels of the T2 cytokine and transcriptional repressor IL3336,37 and showed a trend to increased TSLP that was not significant after Bonferroni adjustment. In addition, BCAMhi BCs expressed higher levels of genes encoding growth factors and extracellular matrix components such as POSTN (periostin), FN1 (fibronectin), CTGF (connective tissue growth factor), LAMB1 (laminin subunit B1), and LAMB3 (laminin subunit B3). In contrast, BCAMlo BCs expressed higher levels of genes associated with differentiation, including SERPINB3,38 HES123 KRT8,31 and the transcription factor ELF3,39,40 suggesting that increased BCAM expression identifies early airway progenitors among the BC pool.
Confocal images of the epithelium in CRSwNP confirmed variable expression of BCAM, with some KRT5+ BCs expressing BCAM solely on the basal surface (designated BCAMlo) and other KRT5+ BCs expressing BCAM circumferentially (designated BCAMhi) (Fig 1, I, top row). BCAMhi BCs expressed p63 (Fig 1, I, second row), IL-33 (Fig 1, I, third row), and Ki67 (Fig 1, l, fourth row), but none of these proteins was exclusively expressed in BCAMhi BCs. Flow cytometry also demonstrated variable BCAM expression among EpCAMloNGFRhi BCs (Fig E1, H). Remarkably, both BCAMhi and BCAMlo BCs had similar staining for integrin alpha 6,18,41 and little distinction in the expression levels of the BC markers podoplanin31 and nerve growth factor receptor (NGFR)18 (Fig E1, I). Sorted BCAMhi BCs were passaged and differentiated in air-liquid interface (ALI) cultures that supported the growth of all common EpC subsets (Fig 1, J and K). In contrast, EpCAMloNGFRhiBCAMlo BCs survived only limited passages ex vivo and could not support differentiation in ALI cultures. Taken together, these results suggested that BCAM expression distinguishes sinonasal stem cells among BCs.
Notably, examination of a published scRNA-seq data set from bronchial mucosa14 revealed that BCAM also marks a subset of bronchial BCs from healthy donors with coexpression of TP63 and IL33 (Fig E1, J). After assessment of differential gene expression between BCAMhi and BCAMlo bronchial BCs, we analyzed differentially expressed genes that were detected in both the bronchial and the polyp BC subsets. This demonstrated a strong correlation in gene programs across data sets (Fig 1, L; see also Table E8 in this article’s Online Repository at www.jacionline.org), indicating that a similar BCAMhi BC population exists in the lower airway.
BCAM marks an airway stem cell among KRT5+NGFR+ITGA6+P63+ BCs in the murine trachea
The limited progenitor capacity of BCAMlo BCs in ex vivo culture was striking, because even differentiated club cells are reported to retain some progenitor capacity in the appropriate context.42,43 Thus, we next sought to assess these populations in vivo and turned to the murine airway. In the sinonasal mucosa (see Fig E2, A, in this article’s Online Repository at www.jacionline.org) and in the trachea (Fig 2, A), confocal microscopy demonstrated 2 patterns of BCAM staining on basal EpCs, with circumferential expression of BCAM on some BCs and focal basolateral expression of BCAM on other BCs. P63 was primarily expressed in BCAMhi BCs, consistent with a BC progenitor. Because BC biology and markers are more clearly established in the trachea, we next adapted a flow-cytometric panel to assess BCAM expression in naive tracheal BCs. Within the conventional BC gate (lin−EpCAMloGSIB4hi),44,45 we again identified 2 EpC subsets distinguished by BCAM expression (Fig 2, B; see also Fig E2, B). Both populations expressed lower levels of EpCAM and higher levels of BC markers than did differentiated EpCs (Fig 2, C and D). Neither BC subset expressed markers of specialized differentiated EpCs (Fig E2, C–E). Notably, KI67+ staining was dominantly detected in BCAMhi BCs, which was confirmed by confocal microscopy (Fig 2, E). To directly assess their proliferative capacity, we first sorted tracheal BCAMhi and BCAMlo BCs and expanded them in submerged culture (Fig 2, F and G). BCAMhi BCs formed larger Ki67+ and P63+ colonies and were more efficient at closing a wound than were BCAMlo BCs (Fig 2, G and H). Thus, both classical markers of replication and functional assays for proliferation and wound healing demonstrated that BCAM distinguishes a murine airway stem cell among KRT5+ BCs.
FIG 2.
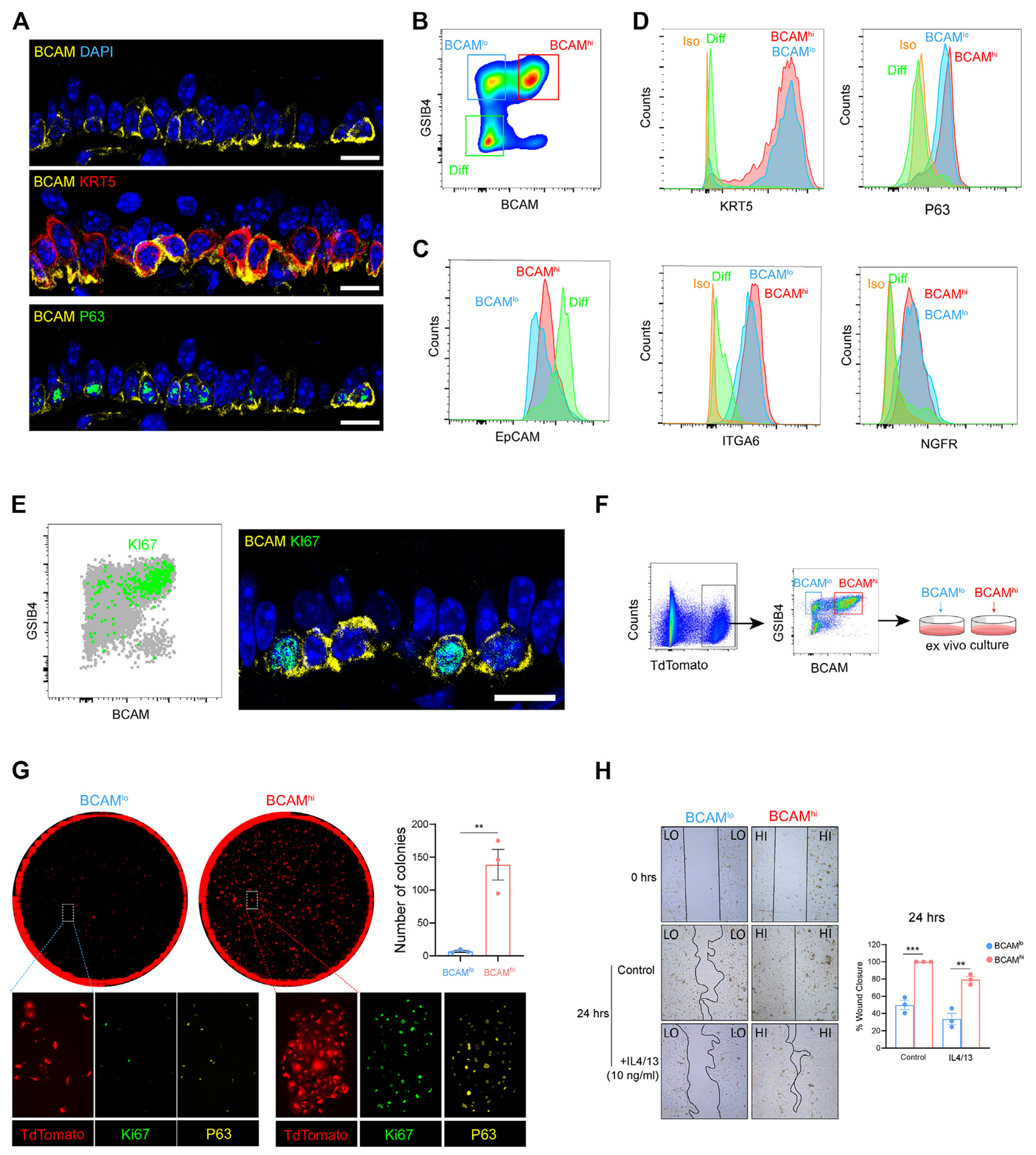
BCAM marks an airway basal stem cell among KRT5+NGFR+ITGA6+P63+ BCs in the naive murine trachea. A, Representative immunostaining in naive murine trachea. The scale bar represents 10 μm. B, Flow-cytometric panel of BCAMhi BCs, BCAMlo BCs, and differentiated EpCs (Diff). C, Expression of EpCAM in each group. D, Expression of canonical BC markers in each group. E, KI67 expression in naive tracheal airway epithelium assessed by flow-cytometric staining (left) and confocal microscopy (right). The scale bar represents 10 μm. F, Schema depicting the isolation and ex vivo culture of BCAMhi BCs and BCAMlo BCs from KRT5CrerERT2:R26tdTomato mice (for detailed information, see this article’s Methods section in the Online Repository at www.jacionline.org). G, Colony-forming assay on sorted BCAMhi BCs and BCAMlo BCs with immunostaining. Number of colonies was calculated using image J. Data are shown as mean ± SEM (n = 3; **P < .01; unpaired 2-tailed t test). H, Wound-healing assay on sorted BCAMhi BCs and BCAMlo BCs in the presence or absence of 10 ng/mL IL-4 and IL-13. Images were taken at 0 and 24 hours. The percentage of wound closure was calculated using image J. Data are shown as mean ± SEM (n = 3; P < .02; linear regression). All immunostaining is representative of n = 3. DAPI, 4’-6-Diamidino-2-phenylindole.
EpCs differentiate from BCAMhi to BCAMlo BCs in a model of airway injury and inflammation
Having established the ex vivo behavior of murine BCAMhi and BCAMlo BCs, we next assessed their behavior in a murine model of airway inflammation and injury using the repetitive inhalation of the mold aeroallergen ALT over 1 or 2 weeks (Fig 3, A). Hematoxylin and eosin staining demonstrated epithelial injury by day 7 and regeneration by day 14 (Fig 3, B). Regeneration was accompanied by a shift in the dominant BC population from BCAMhi at day 0 to BCAMint (intermediate) at day 7 and BCAMlo at day 14 (Fig 3, C). After these repetitive challenges, some BCAMlo BCs expressed the secretory markers SSEA1 and MUC5AC (Fig 3, D; see also Fig E3, A, in this article’s Online Repository at www.jacionline.org), suggesting early differentiation. In contrast, BCAMhi BCs did not express markers of differentiation and were the dominant cell type expressing Ki67 at all time points (Fig 3, E; see also Fig E3, B and C). Taken together, these results suggested that BCAM marks a renewable stem progenitor responsive to airway injury, and that BCAMhi BCs may give rise to BCAMlo BCs and then to differentiated EpCs.
FIG 3.
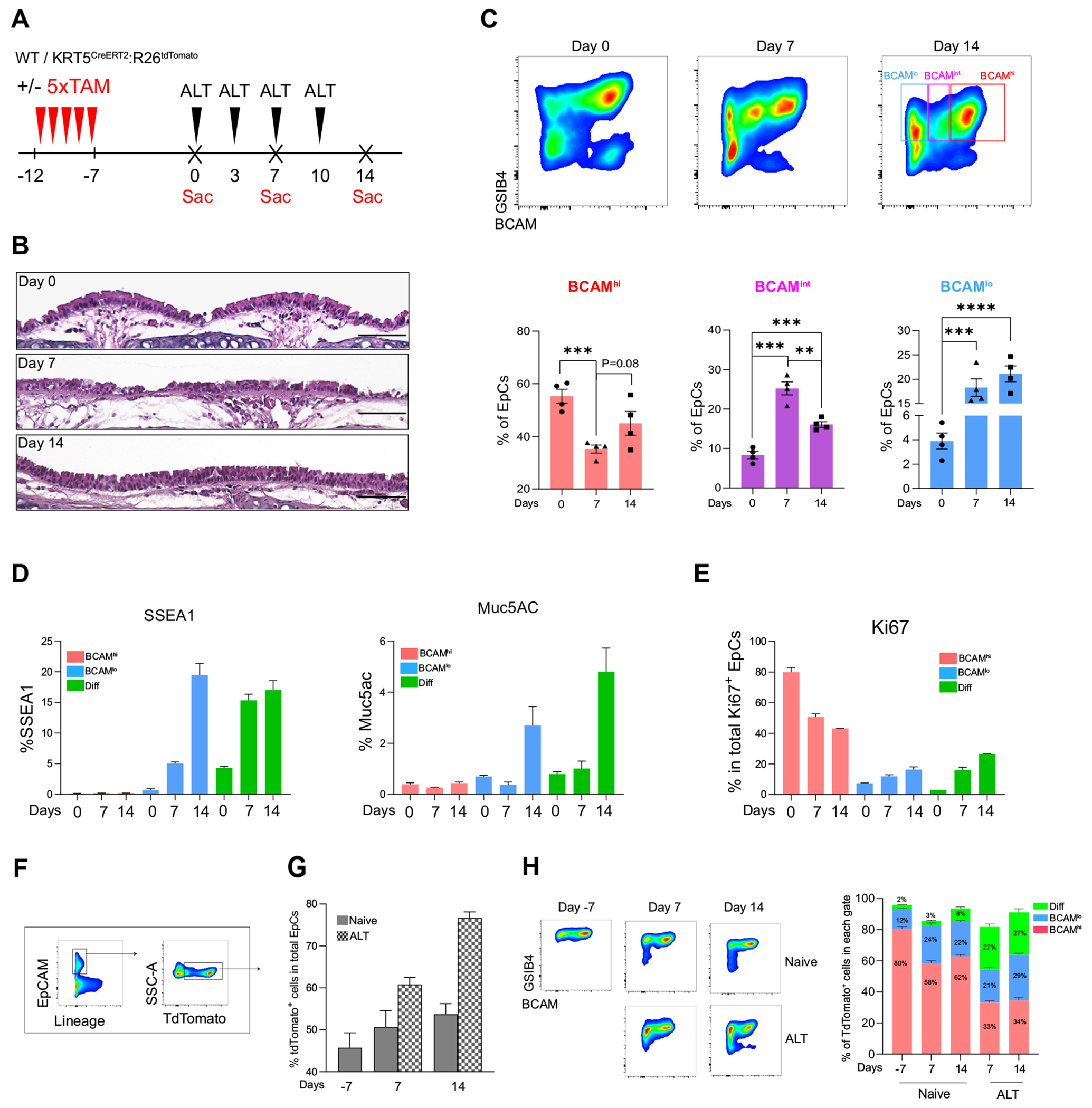
Fate labeling demonstrates a trajectory from BCAMhi BCs to BCAMlo BCs and then to differentiated EpCs. A, Experimental schema. B, H&E staining of mouse trachea. The scale bar represents 100 μm. Staining is representative of n = 3. C, Flow-cytometric panel showing the percentage of BCAMhi BCs, BCAMint BCs, and BCAMlo BCs in naive and challenged airways. Data are shown as mean ± SEM (n = 4; **P < .01, ***P < .001; unpaired 2-tailed t test). D, Percentage of SSEA1+ cells and MUC5AC+ cells in each EpC subset. Data are shown as mean ± SEM (n = 3). E, Percentage of Ki67+ cells in each EpC subset. Data are shown as mean ± SEM (n = 3). F, Gating strategy identifying tdTomato+ EpCs in KRT5CrerERT2:R26tdTomato mice. G, Percentage of tdTomato+ EpCs out of total EpCs at the indicated time points. Data are shown as mean ± SEM (n = 3). H, A representative image of GSIB4 and BCAM staining on tdTomato+ EpCs (left). Percentage of tdTomato+ EpCs that fall within each gate in naive and ALT at the indicated time points (right). Data are shown as mean ± SEM (n = 3).
To confirm that BCAMhi BCs are the progenitors of BCAMlo BCs, we performed fate mapping using tamoxifen-treated, ALT-challenged KRT5CreERT2R26tdTomato mice. Mice were treated with 5 doses of tamoxifen to label KRT5+ BCs, rested for 1 week, and then treated with intranasal ALT over 1 or 2 weeks (Fig 3, A). As expected, the percentage of EpCs labeled with tdTomato increased over the ALT challenges (Fig 3, F and G), and although most tdTomato+ cells were detected in the BCAMhi BC gate at day 7, over the ALT challenges an increasing number fell in the BCAMlo BC gate and then the differentiated EpC gate (Fig 3, H; see also Fig E3, D–F). These data are consistent with an EpC differentiation trajectory that begins with BCAMhi BCs and progresses to BCAMlo BCs and then to differentiated EpCs.
Transcriptional profile of murine BCAMhi and BCAMlo BCs
Because our low-resolution scRNA-seq provided only a limited assessment of these BC subsets that displayed such distinct ex vivo and in vivo behaviors, we next isolated them from naive murine trachea and assessed their transcriptional differences with bulk RNA-seq (see Fig E4, A, in this article’s Online Repository at www.jacionline.org). Principal-component analysis demonstrated that PC1 separated samples by stage of differentiation (Fig 4, A; see also Table E9 in this article’s Online Repository at www.jacionline.org). Direct comparison of BCAMhi and BCAMlo BCs demonstrated that BCAMhi BCs expressed higher levels of many of the same markers detected at elevated levels in human BCAMhi BCs including Krt5, Trp63, Ccn1 (CYR61), Lamb3, Jag2, Dlk2, and Bcam, and higher levels of additional BC markers Ngfr, Pdpn, Krt14, and Krt17 (FDR < 0.05; Fig 4, B and C; see also Fig E4, B, and Tables E10 and E11 in this article’s Online Repository at www.jacionline.org). Il33 was poorly detected in each group. BCAMhi and BCAMlo BCs expressed significantly different levels of genes critical for EpC differentiation and maintenance of stemness, including genes in the Wnt (Fig 4, D), Hippo-Yap (Fig 4, E), Notch (Fig 4, F), Rho (Fig 4, G), and Ras (Fig 4, H) pathways. Although only a few genes in each of these pathways were recovered in the human scRNA-seq data set, all but one of them (CCND1) were significantly increased in BCAMhi BCs, as compared with BCAMlo BCs (Fig E4, C). Transcription factors that were significantly different in the murine data included the regulator of embryonic morphogenesis Hoxd846; the driver of respiratory and epidermal BC proliferation Vdr47,48; the tumor suppressors Klf10, Klf11, and Trp5349–51; and 17 transcripts encoding zinc-finger proteins, all of which were increased in BCAMhi BCs (Fig E4, D; see also Table E12 in this article’s Online Repository at www.jacionline.org). Among the top transcription factors differentially regulated was Trp63 (Fig E4, D), the murine homologue of TP63 that was detected in human sinonasal BCAMhi BCs (Fig 1, C, and H). Assessment of a previously reported list of 175 genes with p63 binding sites52 showed that 34 were differentially expressed across BCAMhi and BCAMlo BC subsets (Fig 4, I). Moreover, 10 were among the top 250 genes upregulated in BCAMhi BCs, suggesting a potential link between BCAM and Trp63. Assessment of our previously published bulk RNA-seq data set from human sinonasal BCs from CRSwNP demonstrated strong correlation between BCAM expression and TP63 (r2 = 0.85; P = .001; Fig 4, J; see also Table E13 in this article’s Online Repository at www.jacionline.org), suggesting a potential link between BCAM and TP63 expression in human BCs.
FIG 4.
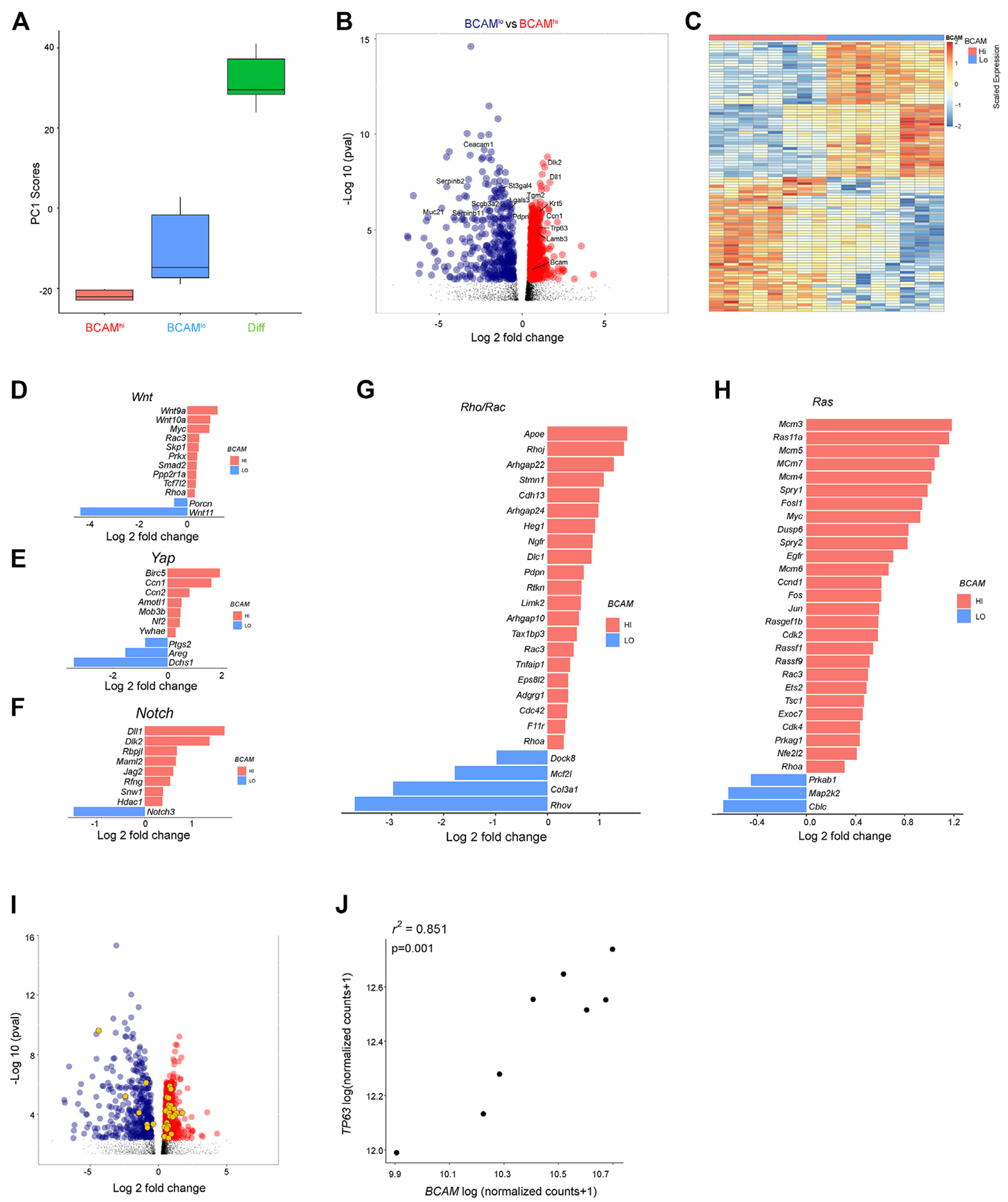
BCAMhi BCs are enriched in canonical stem cell signaling pathways. A, Box plot of principal-component 1 scores from sorted murine BCAMhi BCs, BCAMlo BCs, and differentiated EpCs (Diff). B, Volcano plot showing differentially expressed genes in BCAMhi BCs compared with BCAMlo BCs. Highlighted genes are significantly enriched in BCAMhi BCs (red) and BCAMlo BCs (dark blue), (Benjamini-Hochberg, P < .05). C, Heatmap of the top 100 most significant differentially expressed genes in BCAMhi BCs and BCAMlo BCs (Benjamini-Hochberg, P < .05; see Tables E10 and E11). D-H, Significant differentially expressed genes (Benjamini-Hochberg, P < .05) between BCAMhi and BCAMlo BCs associated with Wnt signaling (Fig 4, D), Hippo signaling (Fig 4, E), Notch signaling (Fig 4, F), Rho/Rock signaling (Fig 4, G), and Ras signaling (Fig 4, H). I, Volcano plot showing differentially expressed genes up in BCAMhi BCs (red) compared with BCAMlo BCs (blue). The significant differentially expressed genes that are P63 target genes are colored in orange. J, Correlation between BCAM and TP63 expression (normalized counts; DESeq2 median of ratios) in bulk RNA-seq from human polyp BCs.
T2-/IRS-dependent regulation of BCAM
TP63 was previously reported to be induced by the T2 cytokine IL-13 in human keratinocytes,53 and thus we hypothesized that BCAM may be similarly regulated in the sinonasal mucosa. First, we assessed BCAM and TP63 expression in bulk RNA-seq of sinonasal BCs from CRSwNP, a T2-high disease, and from nonpolyp controls (CRSsNP). Polyp BCs expressed higher levels of BCAM and TP63 and higher levels of canonical T2-inducible epithelial genes such as ALOX15, POSTN, IL33, and TSLP (Fig 5, A; see also Table E14 in this article’s Online Repository at www.jacionline.org). There was a trend to increased expression of CCL26 that was not significant. In addition, flow cytometry demonstrated that the percentage of BCAMhi BCs among lin −EpCAMloNGFR+ cells was higher in CRSwNP than in CRSsNP (Fig 5, B). Interestingly, assessment of p63-dependent genes also showed an increase in BCs from CRSwNP, as compared with CRSsNP (Fig 5, C), suggesting an increase in the p63-dependent stem program in this T2 disease.
FIG 5.
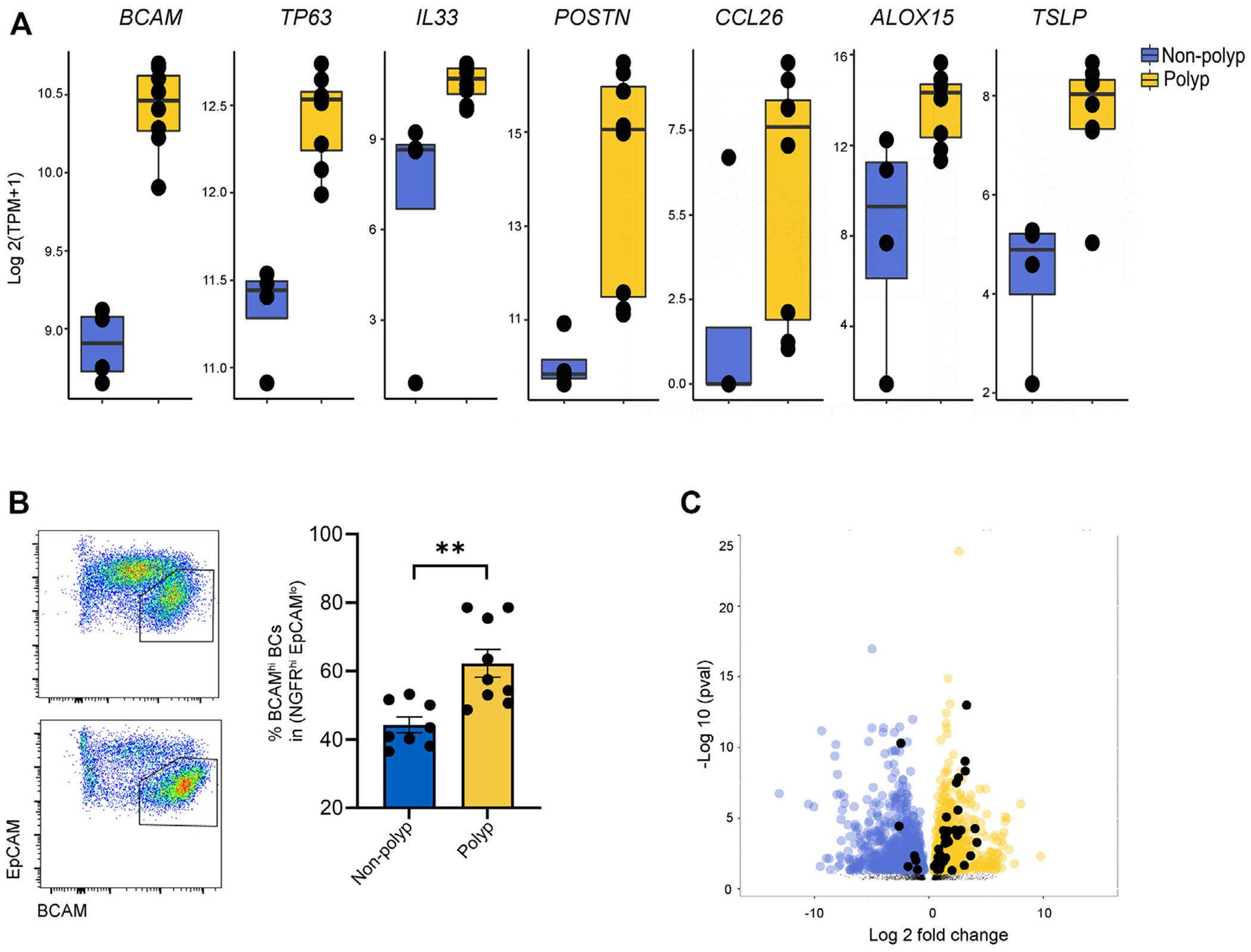
Expression of BCAM and the P63-dependent stem program is increased in CRSwNP and CRSsNP. A, Expression of the indicated genes in bulk RNA-seq from human sinonasal polyp BCs and nonpolyp BC controls. Adjusted P value (Benjamini-Hochberg) as follows: BCAM = 3.7 × 10−13, TP63 = 4.69 × 10−7, IL33 = 3.02 × 10−2, POSTN = 5.20 × 10−6, CCL26 = 3.44 × 10−1(not significant), ALOX15 = 2.95 × 10−2, and TSLP = 9.14 × 10−7. B, Flow cytometry on lin−EpCAM+ EpCs from CRSsNP and CRSwNP. Data are shown as mean ± SEM (**P < .002; unpaired 2-tailed t test). C, Volcano plot showing differentially expressed genes up in CRSwNP (yellow) compared with CRSsNP (blue). The significant differentially expressed genes that are P63 target genes are colored in black. TPM, Transcripts per million.
To understand whether BCAM and p63 were directly regulated by T2 cytokines, we cultured sinonasal BCs from healthy controls and from patients with CRSsNP or CRSwNP and treated them with either IL-4 and IL-13 or with TGF-β, which is known to induce EpC differentiation.54 In passaged unstimulated BC cultures, BCAM was expressed at high levels in all BCs with no BCAMlo BC subset detected (Fig E5, A, in this article’s Online Repository at www.jacionline.org). Addition of TGF-β downregulated the expression of BC markers NGFR and ITGA6, as expected, and also downregulated the expression of BCAM (Fig E5, B). In contrast, IL-4/IL-13 upregulated BCAM expression but reduced NGFR and had little effect on ITGA6 (Fig 6, A). IL-4/IL-13 treatment also upregulated TP63 and reduced expression of HEY1, indicating downregulation of Notch activity, which is essential for BC differentiation into secretory EpCs.23,31 Accordingly, we saw reduced SCGB1A1 that marks secretory club cells (Fig 6, B). Additional markers of terminal EpC differentiation were not altered in this short-term experiment in submerged culture, including MUC5AC, FOXJ1, IL25, and POU2F3 (Fig E5, C. Although the number of primary human BCAMlo BCs obtained from sinus tissue was too low to ask whether IL-4/IL-13 could “reverse” differentiation and drive BCAMlo BCs to BCAMhi BCs, we did note that murine IL-4/IL-13–stimulated BCAMlo BCs did not assume the robust wound-healing capacity of BCAMhi BCs (Fig 2, H), and that in vivo ALT challenge was associated with increased expression of differentiation markers in BCAMlo BCs (Fig 3, D). Taken together, these findings indicate that IL-4/IL-13 reinforces a stem program in BCAMhi BCs and prevents early steps in BC differentiation. Notably, IL-5 treatment has no such effect (Fig E5, D), possibly because of low levels of CSF2RB expression in BCs (Fig E5, E) or because of distinctions in their downstream signaling pathways.
FIG 6.
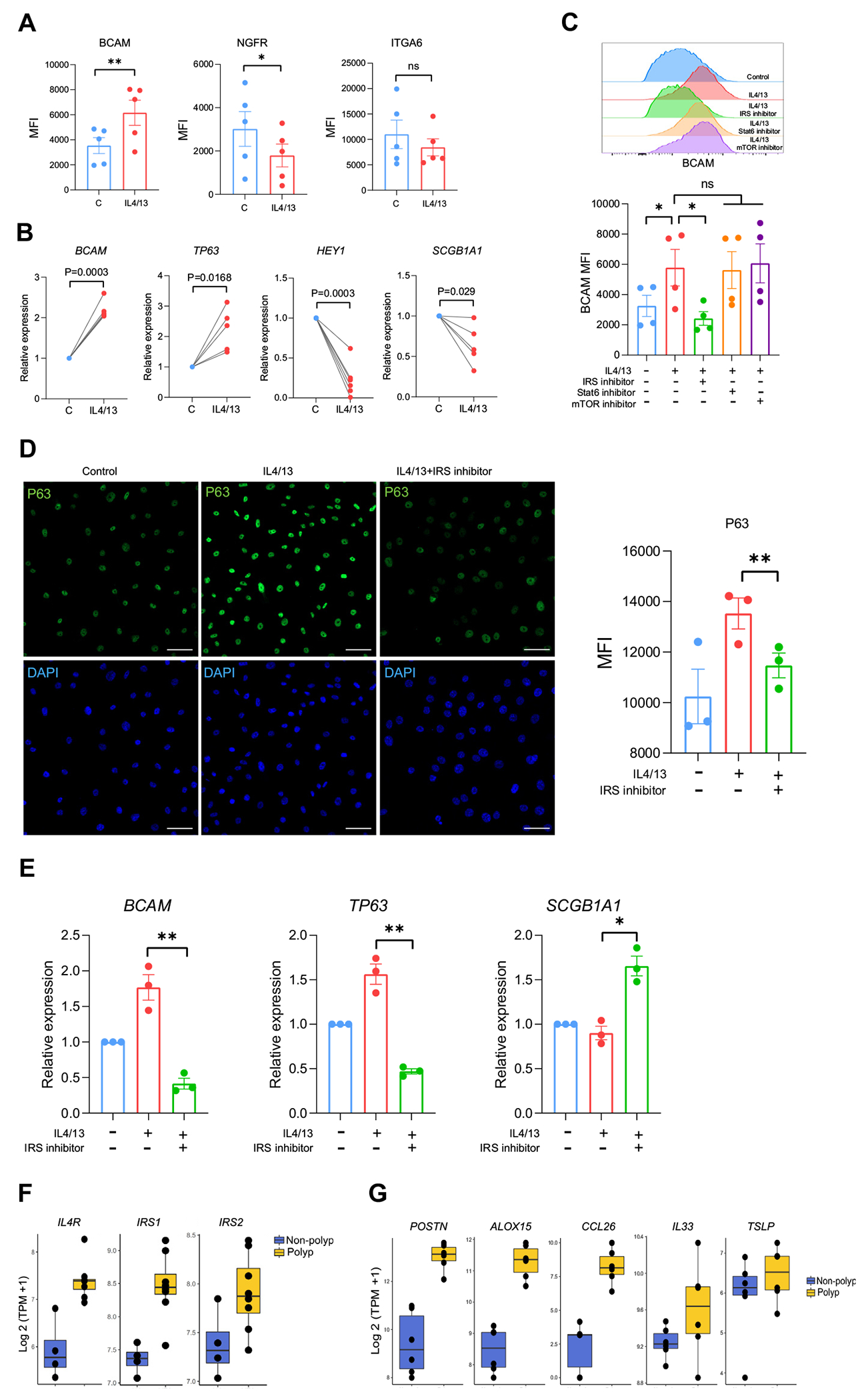
T2 cytokine–dependent and IRS-dependent regulation of BCAM. A, MFI of BCAM, NGFR, and ITGA6. Data are shown as mean ± SEM (*P < .05, **P < .01; paired 2-tailed t test). B, Quantitative PCR for the indicated genes. Data are shown as mean ± SEM (*P < .05, **P < .01; paired 2-tailed t test). C, Histogram (left) and MFI quantification (right) of BCAM expression in IL-4/IL-13-treated BCs ± inhibitors of IRS-1/2, mTOR, and STAT6. Data are shown as mean ± SEM (*P < .05; paired 2-tailed t test. D, Representative images (left) and MFI quantification (right) of P63 staining on IL-4/IL-13-treated BCs ± an IRS-1/2 inhibitor. Scale bar = 50 μm. Data are shown as mean ± SEM (*P < .05; paired 2-tailed t test). E, Quantitative PCR on IL-4/IL-13–treated BCs ± an IRS-1/2 inhibitor. Data are shown as mean ± SEM (*P < .05, **P < .01; paired 2-tailed t test). Human sinonasal BCs treated ± 10 ng/mL IL-4/IL-13 (Fig 6, A-E). F, Expression of components of IL-4R signaling pathway in bulk BC sequencing from CRSwNP or CRSsNP. IL4R and IRS1 were significantly different (Benjamini-Hochberg–adjusted P values < .05). G, Expression of the indicated genes in scRNA-seq cluster 0 (BCAMhi BCs) from CRSwNP and CRSsNP. Bonferroni threshold (1.59 × 10−5) for POSTN = 3.21 × 10−7, CCL26 = 8.97 × 10−6, and ALOX15 = 1.28 × 10−10. DAPI, 4’-6-Diamidino-2-phenylindole; MFI, mean fluorescence intensity; NS, not significant; TPM, transcripts per million.
IL-13 induces goblet cell metaplasia through a well-characterized signal transducer and activator of transcription 6 (STAT6)–dependent pathway, but STAT6-independent pathways have previously been implicated in airway epithelial wound healing.55 Thus, we next assessed whether STAT6 or other IL-4Rα signaling pathways restrain BC differentiation. Pharmacological inhibition demonstrated that IL-4/IL-13–elicited BCAM expression was independent of STAT6 and mTOR (mammalian target of rapamycin) signaling but dependent on IRS signaling (Fig 6, C). IRS inhibition also reduced P63 expression (Fig 6, D) and the expression of BCAM and TP63 transcripts (Fig 6, E) while increasing the expression of SCGB1A1. Assessment of transcripts for components of the IL-4R signaling pathway in bulk BC RNA-seq demonstrated that IL4RA and IRS1 were upregulated in polyp BCs, as compared with controls (Fig 6, F; see also Table E14), whereas IRS2 showed a trend to increase that was not significant. Taken together, these data demonstrate that IL-4Rα and IRS signaling plays an unexpected role in maintaining the BCAMhi BC stem state, which accumulates in CRSwNP. Finally, we did interrogate our scRNA-seq data set to assess whether BCAMhi BCs from CRSwNP expressed higher levels of T2-inducible epithelial genes than did BCAMhi BCs from CRSsNP. We found that although TSLP and IL33 were expressed at similar levels, POSTN, CCL26, and ALOX15 were expressed more highly in BCAMhi BCs from CRSwNP than from CRSsNP.
DISCUSSION
Identification of airway stem cells among plastic EpC types is an important prelude to defining the molecular pathways that maintain stemness, promote normal tissue regeneration, and drive pathological tissue remodeling. Previous studies have identified human and murine airway BCs as lin−EpCAMloKRT5+NGFRhi EpCs expressing ITGA6, PDPN, or GSIB413,18,56–58 and detected significant heterogeneity within the BC compartment.4,14,24,57,59–63 However, cell surface markers to distinguish BC subsets and define BC biology have been lacking. Here, we find that BCAM expression identifies molecularly and functionally distinct subsets of BCs in human and murine airways, with BCAMhi BCs expressing high levels of P63 and exhibiting increased stem functions. Remarkably, BCAMhi BCs are increased in the sinonasal mucosa of patients with the T2 inflammatory disease CRSwNP, and their P63 expression is upregulated through a T2 cytokine and IRS–dependent signaling pathway. These findings identify a robust marker of airway stem cells in mouse and human and define a T2 molecular pathway that promotes their persistence.
The top marker genes identified in BCAMhi BCs include BCAM; the keratins KRT5, KRT15, and KRT17; the canonical BC transcription factor TP6319,35 and its S100A2 target64–66; the stem cell regulator MMP1067,68; and diverse drivers of EpC proliferation including LAMB3,69 MYC,70 and the Yes-associated protein target CYR61.71 Each of these transcripts marks multipotent progenitor BCs from the lower airway.4,14,15,63,72 Moreover, analysis of differentially expressed genes between BCAMhi and BCAMlo BCs from the sinus and the lung14 demonstrates that the distinct BCAMhi and BCAMlo gene programs seen in the sinonasal mucosa are largely retained in the bronchial tree. Additional support for this BCAMhi versus BCAMlo distinction in the tracheobronchial tree is found in our murine studies. Murine tracheal BCAMhi BCs, but not BCAMlo BCs, express high levels of Ki67 in vivo, demonstrate robust colony formation and wound healing in ex vivo assays, and are rapidly labeled in lineagetracing studies. In addition, we found that murine tracheal BCAMhi BCs are enriched in Wnt, Notch, Rho, and Trp63 pathway genes expected in airway stem cells. Taken together, these findings indicate that the BCAMhi/BCAMlo distinction detected in the sinonasal mucosa is likely to be useful in characterizing bronchial BCs.
BCAM is a member of the immunoglobulin superfamily, broadly expressed in erythroid, epithelial, endothelial, and smooth muscle cells. BCAM binds the alpha chain of the extracellular matrix protein laminin 573,74 to regulate cell adhesion and migration.75 Activation of BCAM promotes extracellular signal-regulated kinase/mitogen-activated protein kinase signaling, with an increase in RhoA and a decrease in Rac1 activity, which favors cell adhesion and colony formation in fibroblasts76 and prevents biliary differentiation during liver regeneration.77 Although RhoA signaling plays a central role in airway EpC differentiation,78,79 whether BCAM is a critical regulator of BC RhoA functions will require further study. In addition, BCAM binding to laminin α5 competitively inhibits integrin binding and alters integrin-mediated functions.80 This indicates an additional potential pathway by which BCAM expression can alter the regenerative functions of laminin α581,82 that are central to epithelial homeostasis and differentiation.83,84
Notably, we found that BCAM and TP63 were both highly expressed early in pseudotime, highly correlated across BCs from subjects with CRSwNP, and similarly induced by IL-4/IL-13. Moreover, we found that human BCs from CRSwNP express higher levels of p63-dependent genes. Chromatin immunoprecipitation sequencing data demonstrate that p63 binds to a region in the BCAM promoter,85 and a recent study identified that overexpression of Np63 in 293T cells increases luciferase activity in a reporter containing the BCAM promoter region.86 Thus, the reproducibility with which BCAM expression identifies airway stem cells across species and conditions may reflect its regulation by p63. Further studies are needed to understand this relationship and to understand whether targeted inhibition of IL-4Rα reduces the expression of p63 and BCAM in vivo.
Previous studies have identified basal luminal progenitors24,31,57 and KRT4/13+ hillock BCs59,61,72 as descendants of KRT5+P63+ BCs that additionally express KRT8. In naive murine trachea and in human sinonasal scRNA-seq, KRT8 protein or transcript was consistently expressed in transitional EpCs, but it was not detected in either BCAMhi or BCAMlo BCs. Furthermore, confocal analysis demonstrated that both BCAMhi and BCAMlo BCs were detected on the basement membrane, distinct from KRT8+ luminal BCs. Taken together, these data suggest that BCAMhi and BCAMlo BCs defined here represent earlier stages of differentiation than do previously identified KRT8+ BCs.
In human sinonasal BC cultures, we found that IL-4/IL-13 upregulates the expression of BCAM and P63 while reducing Notch activity and expression of SCGB1A1, which marks differentiated club EpCs. IL-4/IL-13–elicited upregulation of BCAM and P63 was not mediated by STAT6 or mTOR signaling, but was reduced with an inhibitor of IRS-1 and IRS-2, each of which contributes to IL-4R–dependent functions in hematopoietic cells.87–89 IRS-1 has a demonstrated role in promoting murine embryonic stem cell survival in vitro90 and in maintaining the Sox9+ intestinal stem cell pool in vivo,91 indicating that this is a likely mediator of IL-4/IL-13–elicited stem function. Notably, a role for IL-4/IL-13 in reinforcing stem function was not expected, because previous studies have demonstrated that IL-4/IL-13 drives the differentiation of EpCs to goblet cells.92,93 Furthermore, T2 cytokine–driven maintenance of the BCAMhi BC stem state has potential significant pathobiological sequelae, because we find that many epithelial mediators of T2 inflammation including IL33, TSLP, ALOX15, and CCL2694 are expressed in this cell type. Taken together, these data highlight the potential for T2 cytokines to drive a type of airway remodeling that in turn supports persistent disease.
In this study, we found that the expansion of BCs in the T2 inflammatory disease CRSwNP that we previously reported13 is due to an increase in the BCAMhi BC subset. Furthermore, we found that IL4RA, IRS1, TP63, and TP63-dependent genes were significantly upregulated in CRSwNP, as compared with CRSsNP. Although there is no hallmark gene set to detect enrichment in IRS signaling, we did find that the signature of downstream Akt signaling was highly correlated with a T2-cytokine score across the spectrum of chronic rhinosinusitis (see Fig E6 in this article’s Online Repository at www.jacionline.org). Further studies will be needed to define the importance of IRS1 in CRSwNP and whether specific downstream IRS-dependent pathways may be germane therapeutic targets.
Together, our data demonstrate (1) the transcriptional and functional distinctions between multipotent progenitor BCAMhi BCs and their immediate BCAMlo BC descendants, (2) the expansion of BCAMhi BCs in a T2-high disease (CRSwNP), and (3) the IRS signaling pathway through which the T2 cytokines IL-4 and IL-13 can promote stem function. These findings highlight a role for T2 cytokines in the epithelium beyond canonical goblet cell metaplasia and support a growing literature demonstrating a role for T2 cytokines in tissue repair across diverse cell types.95–97 Moreover, resolving the cell surface phenotype of these early airway EpC precursors provides an opportunity to isolate and assess the molecular features of dysplastic BCs that are increasingly detected in a range of airway diseases from CRSwNP13 to chronic obstructive pulmonary disease98 and idiopathic pulmonary fibrosis.63,99
Supplementary Material
Key messages.
Two subsets of airway BCs have distinct transcriptional signatures and function.
High levels of BCAM expression mark the earliest BC progenitor.
IL-4 and IL-13 upregulate BCAM and P63 in an IRS-dependent fashion that prevents BC differentiation to secretory EpCs.
BCAMhi BCs are increased in CRSwNP.
Acknowledgments
This work was supported by the National Institutes of Health (grant no. U19AI095219 to J.A.B., N.A.B., T.M.L., S.R., and M.G.A. and grant nos. R01AI134989 and R01HL120952 to N.A.B.), and by the generous support of the Vinik Family Foundation and the Kaye Innovation fund.
We acknowledge Juying Lai for help with sample preparation and Adam Chicoine in the Center for Cellular Profiling for help with sorting.
Abbreviations used
- ALI
Air-liquid interface culture
- ALT
Alternaria alternata
- BC
Basal cell
- BCAM
Basal cell adhesion molecule
- CRSsNP
Chronic rhinosinusitis sans nasal polyposis
- CRSwNP
Chronic rhinosinusitis with nasal polyposis
- EpC
Epithelial cell
- EpCAM
Epithelial cell adhesion molecule
- GSIB4
Griffonia simplicifolia lectin B4
- IRS
Insulin receptor substrate
- ITGA6
Integrin alpha 6
- KRT5
Keratin 5
- mTOR
Mammalian target of rapamycin
- MUC5AC
Mucin 5AC
- NGFR
Nerve growth factor receptor
- SCGB1A1
Secretoglobin family 1A
- scRNA-seq
Single-cell RNA sequencing
- SSEA1
Stage-specific mouse embryonic antigen 1
- STAT6
Signal transducer and activator of transcription 6
- TP63
Tumor protein 63
- T2
Type 2
Footnotes
Disclosure of potential conflict of interest: J. A. Boyce has served on scientific advisory boards for Siolta Therapeutics, Third Harmonic Bio, and Sanofi/Aventis. N. A. Barrett has served on scientific advisory boards for Regeneron. K. M. Buchheit has served on scientific advisory boards for AstraZeneca, Regeneron, Sanofi, and GlaxoSmithKline. T. M. Laidlaw has served on scientific advisory boards for Regeneron, Sanofi, and GlaxoSmithKline. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Marillier RG, Michels C, Smith EM, Fick LC, Leeto M, Dewals B, et al. IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunol 2008;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oeser K, Schwartz C, Voehringer D. Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol 2015;8:672–82. [DOI] [PubMed] [Google Scholar]
- 3.Birchenough GM, Johansson ME, Gustafsson JK, Bergstrom JH, Hansson GC. New developments in goblet cell mucus secretion and function. Mucosal Immunol 2015;8:712–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carraro G, Langerman J, Sabri S, Lorenzana Z, Purkayastha A, Zhang G, et al. Transcriptional analysis of cystic fibrosis airways at single-cell resolution reveals altered epithelial cell states and composition. Nat Med 2021;27:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel NN, Triantafillou V, Maina IW, Workman AD, Tong CCL, Kuan EC, et al. Fungal extracts stimulate solitary chemosensory cell expansion in noninvasive fungal rhinosinusitis. Int Forum Allergy Rhinol 2019;9:730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohanski MA, Workman AD, Patel NN, Hung LY, Shtraks JP, Chen B, et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2018;142:460–9.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel NN, Kohanski MA, Maina IW, Triantafillou V, Workman AD, Tong CCL, et al. Solitary chemosensory cells producing interleukin-25 and group-2 innate lymphoid cells are enriched in chronic rhinosinusitis with nasal polyps [published online ahead of print May 9, 2018]. Int Forum Allergy Rhinol. 10.1002/alr.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguayo SM, Miller YE, Waldron JA Jr, Bogin RM, Sunday ME, Staton GW Jr, et al. Brief report: idiopathic diffuse hyperplasia of pulmonary neuroendocrine cells and airways disease. N Engl J Med 1992;327:1285–8. [DOI] [PubMed] [Google Scholar]
- 9.Davies SJ, Gosney JR, Hansell DM, Wells AU, du Bois RM, Burke MM, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia: an underrecognised spectrum of disease. Thorax 2007;62:248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi G, Cavazza A, Spagnolo P, Sverzellati N, Longo L, Jukna A, et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia syndrome. Eur Respir J 2016;47:1829–41. [DOI] [PubMed] [Google Scholar]
- 11.Sui P, Wiesner DL, Xu J, Zhang Y, Lee J, Van Dyken S, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 2018;360:eaan8546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young LR, Brody AS, Inge TH, Acton JD, Bokulic RE, Langston C, et al. Neuroendocrine cell distribution and frequency distinguish neuroendocrine cell hyperplasia of infancy from other pulmonary disorders. Chest 2011;139:1060–71. [DOI] [PubMed] [Google Scholar]
- 13.Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature 2018;560:649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vieira Braga FA, Kar G, Berg M, Carpaij OA, Polanski K, Simon LM, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med 2019;25:1153–63. [DOI] [PubMed] [Google Scholar]
- 15.Travaglini KJ, Nabhan AN, Penland L, Sinha R, Gillich A, Sit RV, et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 2020;587:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019;394:1638–50. [DOI] [PubMed] [Google Scholar]
- 17.Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods 2019;16:1289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 2009;106:12771–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melino G, Memmi EM, Pelicci PG, Bernassola F. Maintaining epithelial stemness with p63. Sci Signal 2015;8:re9. [DOI] [PubMed] [Google Scholar]
- 20.Memmi EM, Sanarico AG, Giacobbe A, Peschiaroli A, Frezza V, Cicalese A, et al. p63 sustains self-renewal of mammary cancer stem cells through regulation of Sonic Hedgehog signaling. Proc Natl Acad Sci U S A 2015;112:3499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thapa R, Wilson GD. The importance of CD44 as a stem cell biomarker and therapeutic target in cancer. Stem Cells Int 2016;2016:2087204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amodio N, Raimondi L, Juli G, Stamato MA, Caracciolo D, Tagliaferri P, et al. MALAT1: a druggable long non-coding RNA for targeted anti-cancer approaches. J Hematol Oncol 2018;11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomi K, Arbelaez V, Crystal RG, Walters MS. Activation of NOTCH1 or NOTCH3 signaling skews human airway basal cell differentiation toward a secretory pathway. PLoS One 2015;10:e0116507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson JK, Rulands S, Wilkinson AC, Wuidart A, Ousset M, Van Keymeulen A, et al. Clonal dynamics reveal two distinct populations of basal cells in slow-turnover airway epithelium. Cell Rep 2015;12:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, et al. The role of Scgb1a11 Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009;4:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeSimple P, van Seuningen I, Buisine MP, Copin MC, Hinz M, Hoffmann W, et al. Trefoil factor family 3 peptide promotes human airway epithelial ciliated cell differentiation. Am J Respir Cell Mol Biol 2007;36:296–303. [DOI] [PubMed] [Google Scholar]
- 27.Revinski DR, Zaragosi LE, Boutin C, Ruiz-Garcia S, Deprez M, Thome V, et al. CDC20B is required for deuterosome-mediated centriole production in multiciliated cells. Nat Commun 2018;9:4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes Dev 2012;26:2138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Song W, Yang R, Li C, Wu T, Dong XB, et al. Endothelial Wnts control mammary epithelial patterning via fibroblast signaling. Cell Rep 2021;34:108897. [DOI] [PubMed] [Google Scholar]
- 30.Yochum GS, Sherrick CM, Macpartlin M, Goodman RH. A beta-catenin/TCF-coordinated chromatin loop at MYC integrates 5’ and 3’ Wnt responsive enhancers. Proc Natl Acad Sci U S A 2010;107:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell 2011;8:639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Solana B, Nueda ML, Ruvira MD, Ruiz-Hidalgo MJ, Monsalve EM, Rivero S, et al. The EGF-like proteins DLK1 and DLK2 function as inhibitory non-canonical ligands of NOTCH1 receptor that modulate each other’s activities. Biochim Biophys Acta 2011;1813:1153–64. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Prak L, Rayon-Estrada V, Thiru P, Flygare J, Lim B, et al. ZFP36L2 is required for self-renewal of early burst-forming unit erythroid progenitors. Nature 2013;499:92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi S, Yu M, Yang S, Miron RJ, Zhang Y. Tcf12, a member of basic helix-loop-helix transcription factors, mediates bone marrow mesenchymal stem cell osteogenic differentiation in vitro and in vivo. Stem Cells 2017;35:386–97. [DOI] [PubMed] [Google Scholar]
- 35.Warner SM, Hackett TL, Shaheen F, Hallstrand TS, Kicic A, Stick SM, et al. Transcription factor p63 regulates key genes and wound repair in human airway epithelial basal cells. Am J Respir Cell Mol Biol 2013;49:978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, et al. Longterm IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest 2013;123:3967–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A 2007;104:282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009;180:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brembeck FH, Opitz OG, Libermann TA, Rustgi AK. Dual function of the epithelial specific ets transcription factor, ELF3, in modulating differentiation. Oncogene 2000;19:1941–9. [DOI] [PubMed] [Google Scholar]
- 40.Ng AY, Waring P, Ristevski S, Wang C, Wilson T, Pritchard M, et al. Inactivation of the transcription factor Elf3 in mice results in dysmorphogenesis and altered differentiation of intestinal epithelium. Gastroenterology 2002;122:1455–66. [DOI] [PubMed] [Google Scholar]
- 41.Margadant C, Frijns E, Wilhelmsen K, Sonnenberg A. Regulation of hemidesmosome disassembly by growth factor receptors. Curr Opin Cell Biol 2008;20:589–96. [DOI] [PubMed] [Google Scholar]
- 42.Alysandratos KD, Herriges MJ, Kotton DN. Epithelial stem and progenitor cells in lung repair and regeneration. Annu Rev Physiol 2021;83:529–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis JD, Wypych TP. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol 2021;14:978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu T, Nettesheim P, Mahler JF, Randell SH. Cell type-specific lectin staining of the tracheobronchial epithelium of the rat: quantitative studies with Griffonia simplicifolia I isolectin B4. J Histochem Cytochem 1991;39:7–14. [DOI] [PubMed] [Google Scholar]
- 45.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, Villoria J, Mou H, et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell 2014;30:151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mark M, Rijli FM, Chambon P. Homeobox genes in embryogenesis and pathogenesis. Pediatr Res 1997;42:421–9. [DOI] [PubMed] [Google Scholar]
- 47.Oda Y, Hu L, Nguyen T, Fong C, Zhang J, Guo P, et al. Vitamin D receptor is required for proliferation, migration, and differentiation of epidermal stem cells and progeny during cutaneous wound repair. J Invest Dermatol 2018;138:2423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brockman-Schneider RA, Pickles RJ, Gern JE. Effects of vitamin D on airway epithelial cell morphology and rhinovirus replication. PLoS One 2014;9:e86755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Memon A, Lee WK. KLF10 as a tumor suppressor gene and its TGF-beta signaling. Cancers (Basel) 2018;10:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Wu J, Chen H, Yang Y, Xiao C, Yi X, et al. Genome-wide CRISPR-Cas9 screen identified KLF11 as a druggable suppressor for sarcoma cancer stem cells. Sci Adv 2021;7:eabe3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aubrey BJ, Strasser A, Kelly GL. Tumor-suppressor functions of the TP53 pathway. Cold Spring Harb Perspect Med 2016;6:a026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riege K, Kretzmer H, Sahm A, McDade SS, Hoffmann S, Fischer M. Dissecting the DNA binding landscape and gene regulatory network of p63 and p53. Elife 2020;9:e63266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kubo T, Sato S, Hida T, Minowa T, Hirohashi Y, Tsukahara T, et al. IL-13 modulates Np63 levels causing altered expression of barrier- and inflammation-related molecules in human keratinocytes: a possible explanation for chronicity of atopic dermatitis. Immun Inflamm Dis 2021;9:734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mou H, Vinarsky V, Tata PR, Brazauskas K, Choi SH, Crooke AK, et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell 2016;19:217–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White SR, Martin LD, Abe MK, Marroquin BA, Stern R, Fu X. Insulin receptor substrate-1/2 mediates IL-4-induced migration of human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2009;297:L164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonser LR, Koh KD, Johansson K, Choksi SP, Cheng D, Liu L, et al. Flow-cytometric analysis and purification of airway epithelial-cell subsets. Am J Respir Cell Mol Biol 2021;64:308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pardo-Saganta A, Law BM, Tata PR, Villoria J, Saez B, Mou H, et al. Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell 2015;16:184–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pardo-Saganta A, Tata PR, Law BM, Saez B, Chow RD, Prabhu M, et al. Parent stem cells can serve as niches for their daughter cells. Nature 2015;523:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montoro DT, Haber AL, Biton M, Vinarsky V, Lin B, Birket SE, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018;560:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghosh M, Ahmad S, Jian A, Li B, Smith RW, Helm KM, et al. Human tracheobronchial basal cells. Normal versus remodeling/repairing phenotypes in vivo and in vitro. Am J Respir Cell Mol Biol 2013;49:1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plasschaert LW, Zilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018;560:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mou H, Yang Y, Riehs MA, Barrios J, Shivaraju M, Haber AL, et al. Airway basal stem cells generate distinct subpopulations of PNECs. Cell Rep 2021;35:109011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carraro G, Mulay A, Yao C, Mizuno T, Konda B, Petrov M, et al. Single-cell reconstruction of human basal cell diversity in normal and idiopathic pulmonary fibrosis lungs. Am J Respir Crit Care Med 2020;202:1540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hibi K, Fujitake S, Takase T, Kodera Y, Ito K, Akiyama S, et al. Identification of S100A2 as a target of the DeltaNp63 oncogenic pathway. Clin Cancer Res 2003;9:4282–5. [PubMed] [Google Scholar]
- 65.Kirschner RD, Sanger K, Muller GA, Engeland K. Transcriptional activation of the tumor suppressor and differentiation gene S100A2 by a novel p63-binding site. Nucleic Acids Res 2008;36:2969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lapi E, Iovino A, Fontemaggi G, Soliera AR, Iacovelli S, Sacchi A, et al. S100A2 gene is a direct transcriptional target of p53 homologues during keratinocyte differentiation. Oncogene 2006;25:3628–37. [DOI] [PubMed] [Google Scholar]
- 67.Justilien V, Regala RP, Tseng IC, Walsh MP, Batra J, Radisky ES, et al. Matrix metalloproteinase-10 is required for lung cancer stem cell maintenance, tumor initiation and metastatic potential. PLoS One 2012;7:e35040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mariya T, Hirohashi Y, Torigoe T, Tabuchi Y, Asano T, Saijo H, et al. Matrix metalloproteinase-10 regulates stemness of ovarian cancer stem-like cells by activation of canonical Wnt signaling and can be a target of chemotherapy-resistant ovarian cancer. Oncotarget 2016;7:26806–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, Pan YZ, Cheung M, Cao M, Yu C, Chen L, et al. LAMB3 mediates apoptotic, proliferative, invasive, and metastatic behaviors in pancreatic cancer by regulating the PI3K/Akt signaling pathway. Cell Death Dis 2019;10:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moumen M, Chiche A, Decraene C, Petit V, Gandarillas A, Deugnier MA, et al. Myc is required for beta-catenin-mediated mammary stem cell amplification and tumorigenesis. Mol Cancer 2013;12:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quan T, Xu Y, Qin Z, Robichaud P, Betcher S, Calderone K, et al. Elevated YAP and its downstream targets CCN1 and CCN2 in basal cell carcinoma: impact on keratinocyte proliferation and stromal cell activation. Am J Pathol 2014;184:937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deprez M, Zaragosi LE, Truchi M, Becavin C, Ruiz Garcia S, Arguel MJ, et al. A single-cell atlas of the human healthy airways. Am J Respir Crit Care Med 2020;202:1636–45. [DOI] [PubMed] [Google Scholar]
- 73.Parsons SF, Lee G, Spring FA, Willig TN, Peters LL, Gimm JA, et al. Lutheran blood group glycoprotein and its newly characterized mouse homologue specifically bind alpha5 chain-containing human laminin with high affinity. Blood 2001;97:312–20. [DOI] [PubMed] [Google Scholar]
- 74.Udani M, Zen Q, Cottman M, Leonard N, Jefferson S, Daymont C, et al. Basal cell adhesion molecule/lutheran protein. The receptor critical for sickle cell adhesion to laminin. J Clin Invest 1998;101:2550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kikkawa Y, Ogawa T, Sudo R, Yamada Y, Katagiri F, Hozumi K, et al. The lutheran/basal cell adhesion molecule promotes tumor cell migration by modulating integrin-mediated cell attachment to laminin-511 protein. J Biol Chem 2013;288:30990–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang HY, Chang HM, Wu TJ, Chaing CY, Tzai TS, Cheng HL, et al. The role of Lutheran/basal cell adhesion molecule in human bladder carcinogenesis. J Biomed Sci 2017;24:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miura Y, Matsui S, Miyata N, Harada K, Kikkawa Y, Ohmuraya M, et al. Differential expression of Lutheran/BCAM regulates biliary tissue remodeling in ductular reaction during liver regeneration. Elife 2018;7:e36572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eenjes E, Mertens TCJ, Buscop-van Kempen MJ, van Wijck Y, Taube C, Rottier RJ, et al. A novel method for expansion and differentiation of mouse tracheal epithelial cells in culture. Sci Rep 2018;8:7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horani A, Nath A, Wasserman MG, Huang T, Brody SL. Rho-associated protein kinase inhibition enhances airway epithelial basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol 2013;49:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kikkawa Y, Sudo R, Kon J, Mizuguchi T, Nomizu M, Hirata K, et al. Laminin alpha 5 mediates ectopic adhesion of hepatocellular carcinoma through integrins and/or Lutheran/basal cell adhesion molecule. Exp Cell Res 2008;314:2579–90. [DOI] [PubMed] [Google Scholar]
- 81.Laperle A, Hsiao C, Lampe M, Mortier J, Saha K, Palecek SP, et al. Alpha-5 laminin synthesized by human pluripotent stem cells promotes self-renewal. Stem Cell Reports 2015;5:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Godavarthy PS, Walter CB, Lengerke C, Klein G. The laminin receptors basal cell adhesion molecule/lutheran and integrin alpha7beta1 on human hematopoietic stem cells. Front Cell Dev Biol 2021;9:675240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ritie L, Spenle C, Lacroute J, Bolcato-Bellemin AL, Lefebvre O, Bole-Feysot C, et al. Abnormal Wnt and PI3Kinase signaling in the malformed intestine of lama5 deficient mice. PLoS One 2012;7:e37710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lepage M, Seltana A, Thibault MP, Tremblay E, Beaulieu JF. Knockdown of laminin alpha5 stimulates intestinal cell differentiation. Biochem Biophys Res Commun 2018;495:1510–5. [DOI] [PubMed] [Google Scholar]
- 85.Kouwenhoven EN, van Heeringen SJ, Tena JJ, Oti M, Dutilh BE, Alonso ME, et al. Genome-wide profiling of p63 DNA-binding sites identifies an element that regulates gene expression during limb development in the 7q21 SHFM1 locus. PLoS Genet 2010;6:e1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sasamoto Y, Lee CAA, Wilson BJ, Buerger F, Martin G, Mishra A, et al. Limbal BCAM expression identifies a proliferative progenitor population capable of holoclone formation and corneal differentiation. Cell Rep 2022;40:111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heller NM, Qi X, Junttila IS, Shirey KA, Vogel SN, Paul WE, et al. Type I IL-4Rs selectively activate IRS-2 to induce target gene expression in macrophages. Sci Signal 2008;1:ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang LM, Myers MG Jr, Sun XJ, Aaronson SA, White M, Pierce JH. IRS-1: essential for insulin- and IL-4-stimulated mitogenesis in hematopoietic cells. Science 1993;261:1591–4. [DOI] [PubMed] [Google Scholar]
- 89.Sun XJ, Wang LM, Zhang Y, Yenush L, Myers MG Jr, Glasheen E, et al. Role of IRS-2 in insulin and cytokine signalling. Nature 1995;377:173–7. [DOI] [PubMed] [Google Scholar]
- 90.Rubin R, Arzumanyan A, Soliera AR, Ross B, Peruzzi F, Prisco M. Insulin receptor substrate (IRS)-1 regulates murine embryonic stem (mES) cells self-renewal. J Cell Physiol 2007;213:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramocki NM, Wilkins HR, Magness ST, Simmons JG, Scull BP, Lee GH, et al. Insulin receptor substrate-1 deficiency promotes apoptosis in the putative intestinal crypt stem cell region, limits Apcmin/1 tumors, and regulates Sox9. Endocrinology 2008;149:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–61. [DOI] [PubMed] [Google Scholar]
- 93.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 1998;282:2261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hewitt RJ, Lloyd CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol 2021;21:347–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A 2013;110:9914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 2013;153:376–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walsh JT, Hendrix S,Boato F, Smirnov I, Zheng J, Lukens JR, et al. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest 2015;125:2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghosh M, Miller YE, Nakachi I, Kwon JB, Baron AE, Brantley AE, et al. Exhaustion of airway basal progenitor cells in early and established chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018;197:885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv 2020;6:eaba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Low-input RNA-seq data have been deposited in the Gene Expression Omnibus (GSE197274). CRSwNP single-cell data set has been deposited in ImmPort SDY1877. The public data set from healthy lung was downloaded from https://www.covid19cellatlas.org/index.healthy.html#vieira19-bronchi.14 The code for main analyses is available at https://github.com/nils-hallen/Single-Cell-Analyses.


