Abstract
BACKGROUND
Some studies have directed towards an association between diabetes mellitus (DM) and prostate cancer (PCa); however, this specific relationship remains inconclusive. In recent years, Mendelian randomization (MR) has become a widely used analytical method for inferring epidemiological causes.
AIM
To investigated the potential relationship between DM and PCa using MR.
METHODS
We downloaded relevant data on "diabetes" and "PCa" from the IEU OpenGWAS project database, performed three different methods to conduct MR, and carried out sensitivity analysis for verification.
RESULTS
The results indicated that DM was an independent risk factor for PCa. The odds ratio (OR) values obtained using the inverse variance weighted method in this study were as follows: OR = 1.018 (95% confidence interval: 1.004-1.032), P = 0.014.
CONCLUSION
We found that DM could increase the incidence rate of PCa.
Keywords: Prostate cancer, Diabetes mellitus, Mendelian randomization analysis, Risk factors, Genome-wide association study
Core Tip: Diabetes mellitus (DM) is a chronic metabolic disease caused by many factors. Prostate cancer (PCa) is a common malignant tumor in men and is the second leading cause of cancer death. The Mendelian randomization (MR) method uses genetic variation as an instrumental variable to detect and quantify causal relationships, which can avoid the impact of confounding factors on the accuracy of the research results. This makes it more reliable than observational study or even randomized controlled trial. This study aimed to clarify the relationship between DM and PCa using MR analysis. Through MR analysis of a large sample with three different methods, this study found that DM was an independent risk factor for PCa, providing new directions for the prevention and treatment of PCa.
INTRODUCTION
Diabetes mellitus (DM) is a major chronic disease worldwide, causing huge burden and harm to patients and their families[1,2]. Currently, prevention is the primary treatment for DM. Its occurrence and development are related to many factors such as diet, lifestyle, and environment[3-5]. Prostate cancer (PCa) is one of the most common cancers worldwide and the second most common cancer in men[6]. In recent years, the diagnostic and treatment modalitites for PCa have greatly improved. However, its incidence rate is steadily increasing, and the age of onset has been decreasing[7]. Currently, the recognized high-risk factors for PCa include age, family history, and ethnic background[8]. Some exogenous factors (such as obesity, diabetes, metabolic syndrome, and dietary factors) are also reportedly associated with PCa; however, this remains inconclusive[9,10]. Given the huge burden of PCa on human health, it is important to identify relevant high-risk factors for its prevention and treatment. This study aimed to investigate the effects of DM on PCa.
Mendelian randomization (MR) is a data analysis method that has been widely used in inferring epidemiological etiology in recent years. It can strengthen causal inference using genetic variation as an instrumental variable (IV). This analysis method is based on the Mendelian inheritance law, so the association between genes and diseases is free from the interference of the postpartum environment, socioeconomic status, behavioral factors, and other common confounding factors, and the resulting causal sequence is reasonable and closer to a real situation[11]. This research method is conceptually similar to a randomized controlled study in which genetic variations are randomly assigned during gamete formation before being interfered with by any confounding factors and are evenly distributed within the population. Alleles are fixed among individuals and do not change with disease occurrence or development. Therefore, the causal inference obtained from MR is not easily affected by residual confounding factors or reverse causality[12-14]. In this study, we obtained sufficient genome-wide association study (GWAS) data from relevant databases and performed a study to assess the impact of DM on PCa based on MR.
MATERIALS AND METHODS
Study design
The premise of MR analysis is that IVs must meet three preconditions: (1) Exposure correlation (correlation hypothesis); (2) no common cause with the outcome (independence assumption); and (3) outcome related only through exposure (excluding restriction assumptions). Based on these criteria, we performed MR to explore the causal relationship between DM and PCa. The entire process of the study primarily included five steps: (1) Fetching exposure factor GWAS data, (2) sifting appropriate IVs, (3) inputting the outcome GWAS data and drawing single nucleotide polymorphisms (SNPs) of the above IVs, (4) preprocessing the exposure factor and outcome GWAS data to ensure consistency in format, and (5) conducting MR and sensitivity analysis.
Data source
SNPs associated with DM were downloaded from the IEU OpenGWAS project database, using phenotype “DM” in this study. Its GWAS ID was “ukb-a-306,” the sample size was 336473 and included 10894596 SNPs. The pooled data for prostate cancer was obtained from the GWAS phenotyped “PCa” (GWAS ID: ukb-a-57; sample size: 337159; SNPs’ number: 10894596), which was also derived from the IEU OpenGWAS project database. The research data were open and transparent, and could be downloaded directly from relevant websites; therefore, no additional ethical declaration or consent was required.
Selection of IV
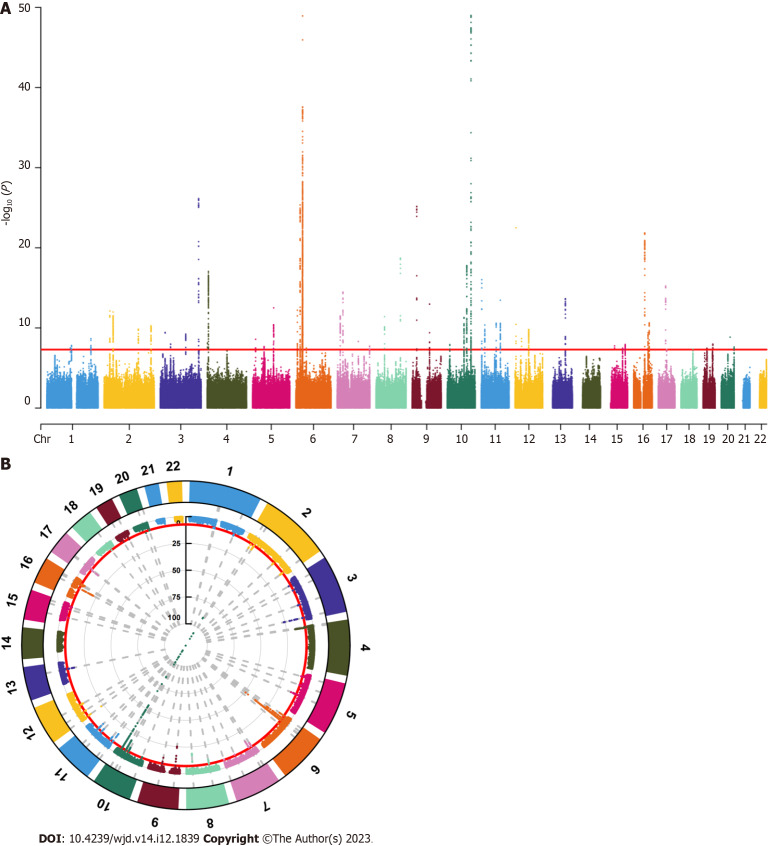
We screened SNPs under the genome-wide significance threshold (P < 5 × 10-8) related to exposure interest as potential SNPs, visualized the results of the correlation analysis, and generated Manhattan plots. In both graphs, the red lines represented the filtering conditions of P < 5 × 10-8 (Figure 1). Next, we used a clump function (r2 = 0.001, kb = 10000) to eliminate linkage disequilibrium between the selected SNPs. Further F-statistics were employed to evaluate the effect of weak IVs[15,16], when the F-statistic was less than 10, the genetic variation was considered a weak IV and might have caused bias in the research results. After removing the weak IVs, we created a comprehensive web-based genotype-phenotype association database (“phenoScanner”) to further investigate whether the remaining SNPs were related to potential risk factors for PCa, such as long-term bedridden diseases and serious diseases[17,18]. For SNPs associated with confounding factors, we conducted manual screening at the genome-wide significance level P < 5 × 10-8 to remove them. After obtaining the remaining SNPs, relevant adjustments were made to ensure that the impact of the IVs on exposure and outcomes corresponded to the same effector alleles. Finally, we removed SNPs with palindromic sequences whose orientation could not be determined and incompatible SNPs, and used the remaining SNPs as IVs for MR analysis.
Figure 1.
Selection of instrumental variables. A: Manhattan plot (line graph); B: Manhattan plot (cyclic graph). The red lines represent the filtering conditions of P < 5 × 10-8.
MR analysis
To avoid the impact of potential pleiotropy, we employed three different MR methods to assess the causal effect between DM and PCa: The inverse variance weighted (IVW), weighted median, and weighted mode methods. Among them, the result of the IVW method was considered to be the main result, as the IVW method assumed that all IVs were valid[19-21]. The results of the MR analysis were visualized as the corresponding plots.
Sensitivity analysis
To demonstrate the reliability of the results, we conducted a sensitivity analysis to evaluate pleiotropy and heterogeneity. First, Cochran’s Q test was performed to detect potential heterogeneity. When P < 0.05, Cochran’s Q statistic evaluated the heterogeneity between genetic variation and the heterogeneity that considered. The results were visualized as corresponding funnel plots. Subsequently, we performed MR-Egger intercept tests to evaluate the horizontal pleiotropy, when P < 0.05, there was pleiotropy in the result. If pleiotropy occurred, further analysis and identification of the source of pleiotropy were conducted through MR-PRESSO analysis. Finally, leave-one-out analysis was conducted to evaluate whether the causal relationship obtained in the study depended on or leaned towards a single SNP[22].
RESULTS
IVs
Through this screening process, we ultimately screened 49 SNPs as IVs for MR analysis. The F-statistic of all IVs was > 10, indicating the absence of weak IVs bias (Table 1).
Table 1.
49 Single nucleotide polymorphisms selected
|
SNP
|
Outcome
|
Exposure
|
P val. exposure
|
| rs10184004 | PCa | DM | 1.42E-10 |
| rs10196106 | PCa | DM | 1.02E-12 |
| rs10748582 | PCa | DM | 1.61E-18 |
| rs10830963 | PCa | DM | 3.45E-14 |
| rs10965247 | PCa | DM | 6.65E-26 |
| rs11671304 | PCa | DM | 1.03E-08 |
| rs11720108 | PCa | DM | 5.75E-10 |
| rs1215470 | PCa | DM | 2.17E-14 |
| rs1260326 | PCa | DM | 7.61E-13 |
| rs12910361 | PCa | DM | 3.34E-08 |
| rs1317548 | PCa | DM | 1.19E-08 |
| rs13262861 | PCa | DM | 3.90E-12 |
| rs1421085 | PCa | DM | 1.36E-22 |
| rs145762933 | PCa | DM | 3.85E-11 |
| rs1496653 | PCa | DM | 3.66E-10 |
| rs1515110 | PCa | DM | 5.35E-11 |
| rs1800961 | PCa | DM | 1.40E-09 |
| rs1801212 | PCa | DM | 8.72E-18 |
| rs2206277 | PCa | DM | 3.23E-08 |
| rs2237895 | PCa | DM | 9.39E-17 |
| rs231361 | PCa | DM | 4.68E-09 |
| rs2947793 | PCa | DM | 1.52E-10 |
| rs340882 | PCa | DM | 2.14E-09 |
| rs34715063 | PCa | DM | 1.72E-08 |
| rs34872471 | PCa | DM | 3.60E-151 |
| rs3802177 | PCa | DM | 1.90E-19 |
| rs3887925 | PCa | DM | 4.88E-09 |
| rs41463147 | PCa | DM | 1.51E-08 |
| rs464605 | PCa | DM | 2.08E-08 |
| rs4688760 | PCa | DM | 1.03E-08 |
| rs4727554 | PCa | DM | 4.59E-09 |
| rs5215 | PCa | DM | 1.49E-10 |
| rs6885132 | PCa | DM | 2.60E-09 |
| rs697239 | PCa | DM | 5.00E-12 |
| rs7018475 | PCa | DM | 3.02E-14 |
| rs7183842 | PCa | DM | 1.15E-08 |
| rs7258722 | PCa | DM | 3.37E-08 |
| rs72802365 | PCa | DM | 2.26E-11 |
| rs75199135 | PCa | DM | 4.11E-08 |
| rs757855 | PCa | DM | 1.83E-08 |
| rs7646519 | PCa | DM | 7.02E-27 |
| rs76895963 | PCa | DM | 3.07E-23 |
| rs7766070 | PCa | DM | 4.38E-26 |
| rs8118848 | PCa | DM | 2.38E-08 |
| rs849142 | PCa | DM | 3.31E-15 |
| rs9267659 | PCa | DM | 4.84E-15 |
| rs9379084 | PCa | DM | 6.03E-13 |
| rs9410573 | PCa | DM | 1.04E-13 |
| rs9667947 | PCa | DM | 2.55E-11 |
DM: Diabetes mellitus; SNP: Single nucleotide polymorphism; PCa: Prostate cancer.
MR analysis
MR is a data analysis technique used in epidemiological studies to evaluate causal inferences. It uses genetic variation as the IVs in nonexperimental data to estimate the causal relationship between the exposure factors and outcomes of interest. Using the fixed nature of genes and Mendelian laws of inheritance, the MR analysis results were not affected by common confounding factors such as the postnatal environment, socio-economic factors, and behavioral habits. The causal relationship derived from MR is more reasonable and reliable.
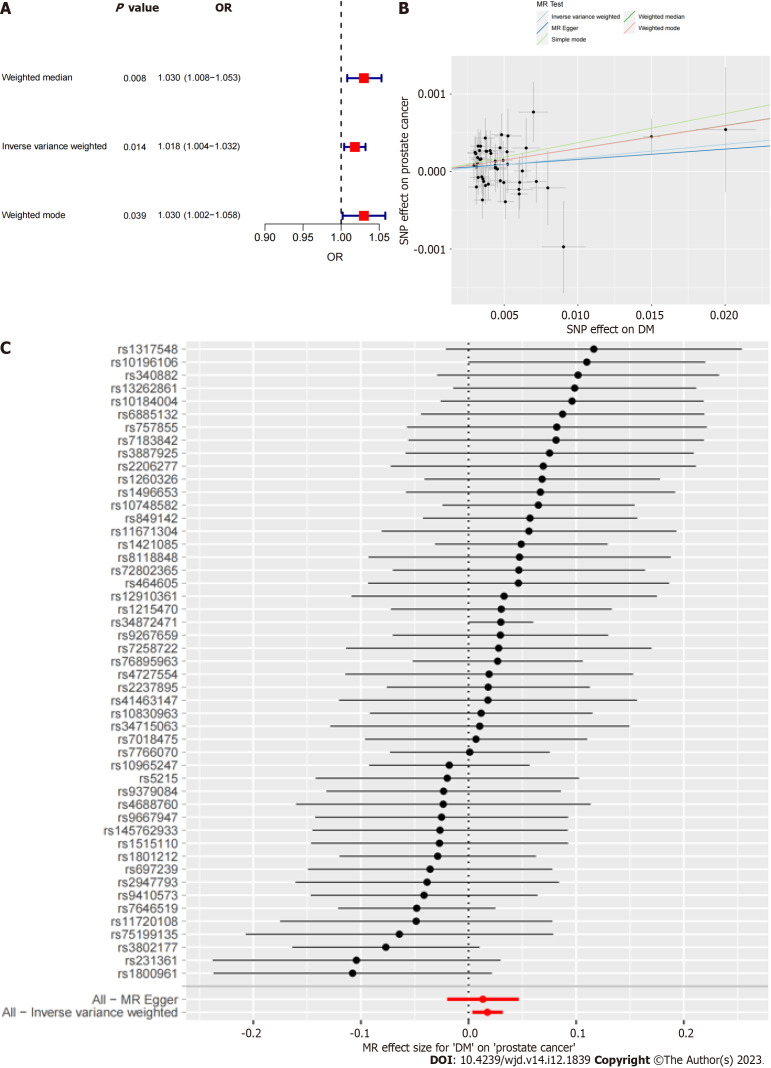
The results of all three MR methods used for analysis revealed that DM was positively correlated with the incidence of PCa. Specifically, using the IVW method as the main analysis method, the OR values obtained in this study were OR = 1.018 (95%CI: 1.004-1.032), P = 0.014. Based on these results, we plotted corresponding scatter and forest plots (Figure 2).
Figure 2.
Mendelian randomization analysis. A: Forest plot of three mendelian randomization methods; B: Scatter plot; C: Forest plot for each single nucleotide polymorphism. DM: Diabetes mellitus; MR: Mendelian randomization; SNP: Single nucleotide polymorphism.
Sensitivity analysis
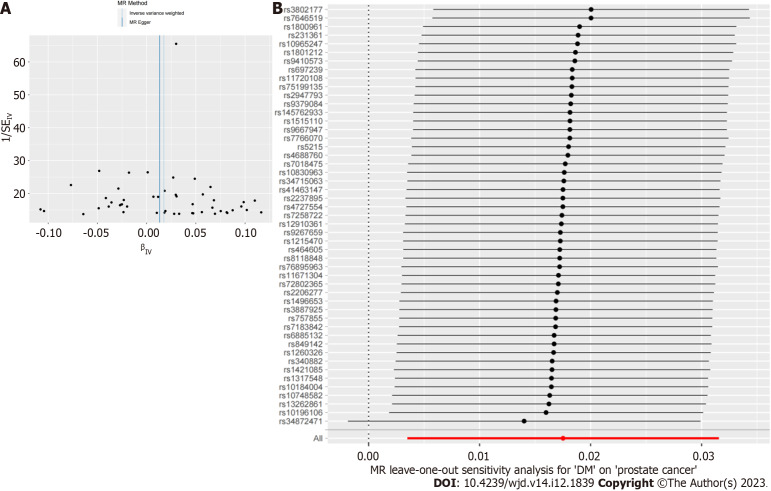
Finally, to verify the reliability of the results further, we performed a sensitivity analysis to examine the heterogeneity and pleiotropy of our conclusions. Cochran’s Q test results showed no heterogeneity in the IVs included in the study (P > 0.05), and the corresponding funnel plot was shown in Figure 3. MR-PRESSO analysis did not find significant pleiotropy in the conclusion nor did it screen for SNPs with outliers (P > 0.05). The test results of the leave-one-out method indicated that the causal relationship between DM and PCa did not depend on or lean towards any single SNP.
Figure 3.
Sensitivity analysis. A: Funnel plot; B: The result of leave-one-out method. DM: Diabetes mellitus; IV: Instrumental variable.
DISCUSSION
DM is a chronic metabolic disease caused by many factors[23,24]. Many studies indicate that the best treatment is to prevent the occurrence of diabetes by maintaining a healthy weight and increasing physical activity[25-27]. PCa is a common malignant tumor in men and is the second leading cause of cancer death[28]. Because of its inconspicuous development, most PCa patients are undiagnosed in the early stages[29]. In addition, because of the heterogeneity of tumor cells, approximately 90% of patients present with local or systemic metastasis at the time of diagnosis, losing the opportunity for radical surgery[30,31]. Therefore, early prevention of PCa and implementation of effective intervention measures are particularly important and can significantly improve patient prognosis.
The relationship between DM and PCa has long been the focus of research. Some scholars believe that DM is a protective factor for patients with PCa, whereas others believe that it is a high-risk factor for PCa. Evidence supporting both hypotheses has been reported; thus far, no conclusions have been reached. Epidemiological investigations have shown that the risk of cancer (including liver cancer, pancreatic cancer, colorectal cancer, breast cancer, and endometrial cancer) in patients with DM increases significantly, and the risk of cancer mortality also increases significantly[32]. In a 14 year cross-sectional study, Saewai et al[33] found that the long-term risk of PCa was significantly increased in patients with DM. Other studies have shown that obesity and DM are independent risk factors for PCa and may have synergistic effects, further increasing the risk of invasive PCa[34,35]. Another study also found that advanced PCa with DM was associated with a worse prognosis and a greater risk of metastasis[36]. Sánchez-Maldonado et al[37] confirmed that functional type 2 DM-related mutations may affect the risk of PCa at the genetic level. Kingshott et al[38] also found that DM could directly affect regulatory growth factors related to cancer, and that changing living habits might significantly reduce the risk of prostate and other cancers. Relevant research has shown that patients with DM have a higher risk of recurrence[39]. However, compared with other drugs, the use of metformin in patients with DM can significantly reduce the risk of new-onset PCa, which also proves that intervention in the development of DM has a positive impact on the prevention and treatment of PCa[40,41].
The MR method uses genetic variation as an IV to detect and quantify causal relationships, which can avoid the impact of confounding factors on the accuracy of the research results. This makes MR study more reliable than observational study or even randomized controlled trial. This study aimed to clarify the relationship between DM and PCa using MR. Compared to the previous observational study, this study explored the potential causal relationship between DM and PCa using three different MR methods. Through MR analysis, we found that DM was a high-risk factor for PCa, which was consistent with previous clinical experience and the results of numerous studies. The results of the sensitivity analysis validation also indicated that the obtained results were reliable. The results of the three MR methods showed that DM increased the risk of PCa. Based on the results of this study, we could conduct early clinical screening of high-risk (DM) populations, control their weight, and strengthen their exercises to further reduce the incidence rate of PCa. DM has already been regarded as a high-risk factor for PCa in some clinical guidelines and academic researches, and our results could also provide a theoretical basis.
This study had some limitations. First, the GWAS dataset obtained in our study was from the same population (European) and could be supplemented in subsequent studies to further expand the coverage of the research results. Second, DM and PCa could be divided into many subtypes, and future research should further explore the relationship between these subtypes. Third, we excluded only SNPs associated with known confounding factors, and future research was needed to further exclude other unknown confounding factors. Finally, it should be noted that the results of MR research could only partially explain the causal effect of DM on PCa. Other methods were needed to prove this result in the future.
CONCLUSION
Through MR analysis of a large sample, this study found that DM was an independent risk factor for PCa, providing new directions for the prevention and treatment of PCa.
ARTICLE HIGHLIGHTS
Research background
Some studies have shown a relationship between diabetes mellitus (DM) and prostate cancer (PCa); however, this specific relationship remains inconclusive.
Research motivation
Mendelian randomization (MR) has been a widely used analytical method in recent years for inferring epidemiological causes. We believe that MR can explain the causal relationship between DM and PCa.
Research objectives
Find the causal relationship between DM and PCa.
Research methods
We downloaded the relevant data from a public database, used three different MR methods, and conducted a sensitivity analysis for validation.
Research results
These results indicated that DM was an independent risk factor for PCa. The odds ratio (OR) values obtained using the inverse variance weighted method in this study were as follows: OR = 1.018 (95% confidence interval: 1.004-1.032), P = 0.014.
Research conclusions
Through MR analysis of a large sample, this study found that DM was an independent risk factor for PCa, providing new directions for the prevention and treatment of PCa.
Research perspectives
This study investigated the potential relationship between DM and PCa using MR.
ACKNOWLEDGEMENTS
We gratefully acknowledge the IEU OpenGWAS project for making GWAS summary-level statistics publicly available.
Footnotes
Conflict-of-interest statement: All authors declare that this study was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: September 2, 2023
First decision: November 14, 2023
Article in press: December 1, 2023
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Beg MMA, Kyrgyzstan; Katuchova J, Slovakia S-Editor: Qu XL L-Editor: A P-Editor: Zhao S
Contributor Information
Jian-Xu Yuan, Department of Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China.
Qing Jiang, Department of Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China.
Sheng-Jie Yu, Department of Surgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China. bbyddh@sina.com.
Data sharing statement
Publicly available datasets were analyzed in this study. The data are as follows: IEU OpenGWAS project:https://gwas.mrcieu.ac.uk/datasets/ukb-a-57/; https://gwas.mrcieu.ac.uk/datasets/ukb-a-306/.
References
- 1.Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17:484–495. doi: 10.1038/s41574-021-00507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34 Suppl 1:S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 5.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Kimura T, Sato S, Takahashi H, Egawa S. Global Trends of Latent Prostate Cancer in Autopsy Studies. Cancers (Basel) 2021;13 doi: 10.3390/cancers13020359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zi H, He SH, Leng XY, Xu XF, Huang Q, Weng H, Zhu C, Li LY, Gu JM, Li XH, Ming DJ, Li XD, Yuan S, Wang XH, He DL, Zeng XT. Global, regional, and national burden of kidney, bladder, and prostate cancers and their attributable risk factors, 1990-2019. Mil Med Res. 2021;8:60. doi: 10.1186/s40779-021-00354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, Bray F, Brawley O, Luckenbaugh AN, Mucci L, Morgan TM, Carlsson SV. 2022 Update on Prostate Cancer Epidemiology and Risk Factors-A Systematic Review. Eur Urol. 2023;84:191–206. doi: 10.1016/j.eururo.2023.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu D, Tang M, Wang Y, Cui H, Zhang M, Bai Y, Zeng Z, Tan Y, Wang X, Zhang B. The Causal Relationships Between Extrinsic Exposures and Risk of Prostate Cancer: A Phenome-Wide Mendelian Randomization Study. Front Oncol. 2022;12:829248. doi: 10.3389/fonc.2022.829248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rühlemann MC, Hermes BM, Bang C, Doms S, Moitinho-Silva L, Thingholm LB, Frost F, Degenhardt F, Wittig M, Kässens J, Weiss FU, Peters A, Neuhaus K, Völker U, Völzke H, Homuth G, Weiss S, Grallert H, Laudes M, Lieb W, Haller D, Lerch MM, Baines JF, Franke A. Genome-wide association study in 8,956 German individuals identifies influence of ABO histo-blood groups on gut microbiome. Nat Genet. 2021;53:147–155. doi: 10.1038/s41588-020-00747-1. [DOI] [PubMed] [Google Scholar]
- 12.Luijk R, Dekkers KF, van Iterson M, Arindrarto W, Claringbould A, Hop P, Boomsma DI, van Duijn CM, van Greevenbroek MMJ, Veldink JH, Wijmenga C, Franke L, 't Hoen PAC, Jansen R, van Meurs J, Mei H, Slagboom PE, Heijmans BT, van Zwet EW BIOS (Biobank-based Integrative Omics Study) Consortium. Genome-wide identification of directed gene networks using large-scale population genomics data. Nat Commun. 2018;9:3097. doi: 10.1038/s41467-018-05452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nolde M, Alayash Z, Reckelkamm SL, Kocher T, Ehmke B, Holtfreter B, Baurecht H, Georgakis MK, Baumeister SE. Downregulation of interleukin 6 signaling might reduce the risk of periodontitis: a drug target Mendelian randomization study. Front Immunol. 2023;14:1160148. doi: 10.3389/fimmu.2023.1160148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Guo Z, Wang X, Chen F, Wang Z. Assessing the causal relationship between sepsis and autoimmune: a mendelian randomization study. Shock. 2023 doi: 10.1097/SHK.0000000000002246. [DOI] [PubMed] [Google Scholar]
- 15.Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG EPIC- InterAct Consortium. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30:543–552. doi: 10.1007/s10654-015-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sommer A, Twig G. The Impact of Childhood and Adolescent Obesity on Cardiovascular Risk in Adulthood: a Systematic Review. Curr Diab Rep. 2018;18:91. doi: 10.1007/s11892-018-1062-9. [DOI] [PubMed] [Google Scholar]
- 17.Bamford T, Barrie A, Montgomery S, Dhillon-Smith R, Campbell A, Easter C, Coomarasamy A. Morphological and morphokinetic associations with aneuploidy: a systematic review and meta-analysis. Hum Reprod Update. 2022;28:656–686. doi: 10.1093/humupd/dmac022. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Liu C, Sun Y, Francis M, Ryu MS, Grider A, Ye K. Genetically predicted circulating levels of copper and zinc are associated with osteoarthritis but not with rheumatoid arthritis. Osteoarthritis Cartilage. 2021;29:1029–1035. doi: 10.1016/j.joca.2021.02.564. [DOI] [PubMed] [Google Scholar]
- 19.Choi KW, Chen CY, Stein MB, Klimentidis YC, Wang MJ, Koenen KC, Smoller JW Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Assessment of Bidirectional Relationships Between Physical Activity and Depression Among Adults: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry. 2019;76:399–408. doi: 10.1001/jamapsychiatry.2018.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polimanti R, Amstadter AB, Stein MB, Almli LM, Baker DG, Bierut LJ, Bradley B, Farrer LA, Johnson EO, King A, Kranzler HR, Maihofer AX, Rice JP, Roberts AL, Saccone NL, Zhao H, Liberzon I, Ressler KJ, Nievergelt CM, Koenen KC, Gelernter J Psychiatric Genomics Consortium Posttraumatic Stress Disorder Workgroup. A putative causal relationship between genetically determined female body shape and posttraumatic stress disorder. Genome Med. 2017;9:99. doi: 10.1186/s13073-017-0491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46:1985–1998. doi: 10.1093/ije/dyx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belbasis L, Mavrogiannis MC, Emfietzoglou M, Evangelou E. Environmental factors, serum biomarkers and risk of atrial fibrillation: an exposure-wide umbrella review of meta-analyses. Eur J Epidemiol. 2020;35:223–239. doi: 10.1007/s10654-020-00618-3. [DOI] [PubMed] [Google Scholar]
- 24.Fernandes Silva L, Vangipurapu J, Laakso M. The "Common Soil Hypothesis" Revisited-Risk Factors for Type 2 Diabetes and Cardiovascular Disease. Metabolites. 2021;11 doi: 10.3390/metabo11100691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan S, Larsson SC. An atlas on risk factors for type 2 diabetes: a wide-angled Mendelian randomisation study. Diabetologia. 2020;63:2359–2371. doi: 10.1007/s00125-020-05253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023;20:429–452. doi: 10.1038/s41571-023-00766-x. [DOI] [PubMed] [Google Scholar]
- 27.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 28.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 29.Cooperberg MR, Erho N, Chan JM, Feng FY, Fishbane N, Zhao SG, Simko JP, Cowan JE, Lehrer J, Alshalalfa M, Kolisnik T, Chelliserry J, Margrave J, Aranes M, Plessis MD, Buerki C, Tenggara I, Davicioni E, Carroll PR. The Diverse Genomic Landscape of Clinically Low-risk Prostate Cancer. Eur Urol. 2018;74:444–452. doi: 10.1016/j.eururo.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mo F, Lin D, Takhar M, Ramnarine VR, Dong X, Bell RH, Volik SV, Wang K, Xue H, Wang Y, Haegert A, Anderson S, Brahmbhatt S, Erho N, Wang X, Gout PW, Morris J, Karnes RJ, Den RB, Klein EA, Schaeffer EM, Ross A, Ren S, Sahinalp SC, Li Y, Xu X, Wang J, Gleave ME, Davicioni E, Sun Y, Collins CC. Stromal Gene Expression is Predictive for Metastatic Primary Prostate Cancer. Eur Urol. 2018;73:524–532. doi: 10.1016/j.eururo.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Middha M, Bicak M, Sjoberg DD, Vertosick E, Dahlin A, Häggström C, Hallmans G, Rönn AC, Stattin P, Melander O, Ulmert D, Lilja H, Klein RJ. Genome-wide Scan Identifies Role for AOX1 in Prostate Cancer Survival. Eur Urol. 2018;74:710–719. doi: 10.1016/j.eururo.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endicott M, Thirlwell C, Webster AP. Exploring genetic loci of type 2 diabetes and cancer: a review. Endocr Oncol. 2023;3:e220094. doi: 10.1530/EO-22-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saewai C, Fumaneeshoat O, Thongsuksai P, Ingviya T. Diabetes Mellitus as Cancer Risk: A 14-year, Cross-Sectional Analysis. Nutr Cancer. 2023;75:1454–1463. doi: 10.1080/01635581.2023.2205054. [DOI] [PubMed] [Google Scholar]
- 34.Zhu D, Toker M, Shyr W, Fram E, Watts KL, Agalliu I. Association of Obesity and Diabetes With Prostate Cancer Risk Groups in a Multiethnic Population. Clin Genitourin Cancer. 2022;20:299–299.e10. doi: 10.1016/j.clgc.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Kelkar S, Oyekunle T, Eisenberg A, Howard L, Aronson WJ, Kane CJ, Amling CL, Cooperberg MR, Klaassen Z, Terris MK, Freedland SJ, Csizmadi I. Diabetes and Prostate Cancer Outcomes in Obese and Nonobese Men After Radical Prostatectomy. JNCI Cancer Spectr. 2021;5 doi: 10.1093/jncics/pkab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma C, Cui D, Han B, Ding M, Zhang J, Liu S, Gao Y, Xia S. Poorly Controlled Diabetes Mellitus Increases the Risk of Deaths and Castration-Resistance in Locally Advanced Prostate Cancer Patients. Cancer Invest. 2023;41:345–353. doi: 10.1080/07357907.2023.2171050. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez-Maldonado JM, Collado R, Cabrera-Serrano AJ, Ter Horst R, Gálvez-Montosa F, Robles-Fernández I, Arenas-Rodríguez V, Cano-Gutiérrez B, Bakker O, Bravo-Fernández MI, García-Verdejo FJ, López JAL, Olivares-Ruiz J, López-Nevot MÁ, Fernández-Puerta L, Cózar-Olmo JM, Li Y, Netea MG, Jurado M, Lorente JA, Sánchez-Rovira P, Álvarez-Cubero MJ, Sainz J. Type 2 Diabetes-Related Variants Influence the Risk of Developing Prostate Cancer: A Population-Based Case-Control Study and Meta-Analysis. Cancers (Basel) 2022;14 doi: 10.3390/cancers14102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kingshott G, Biernacka K, Sewell A, Gwiti P, Barker R, Zielinska H, Gilkes A, McCarthy K, Martin RM, Lane JA, McGeagh L, Koupparis A, Rowe E, Oxley J, Holly JMP, Perks CM. Alteration of Metabolic Conditions Impacts the Regulation of IGF-II/H19 Imprinting Status in Prostate Cancer. Cancers (Basel) 2021;13 doi: 10.3390/cancers13040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ben Hadj Alouane H, Raboudi M, Maatougui J, Dridi M, Ghozzi S. Are Diabetic Patients at Increased Risk for Biochemical Recurrence After Radical Prostatectomy? Cureus. 2022;14:e24717. doi: 10.7759/cureus.24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YHA, Zhou J, Hui JMH, Liu X, Lee TTL, Hui K, Chan JSK, Wai AKC, Wong WT, Liu T, Ng K, Lee S, Dee EC, Zhang Q, Tse G. Risk of New-Onset Prostate Cancer for Metformin Versus Sulfonylurea Use in Type 2 Diabetes Mellitus: A Propensity Score-Matched Study. J Natl Compr Canc Netw. 2022;20:674–682.e15. doi: 10.6004/jnccn.2022.7010. [DOI] [PubMed] [Google Scholar]
- 41.Ozel AB, Dagsuyu E, Aydın PK, Bugan I, Bulan OK, Yanardag R, Yarat A. Brain Boron Level, DNA Content, and Myeloperoxidase Activity of Metformin-Treated Rats in Diabetes and Prostate Cancer Model. Biol Trace Elem Res. 2022;200:1164–1170. doi: 10.1007/s12011-021-02708-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. The data are as follows: IEU OpenGWAS project:https://gwas.mrcieu.ac.uk/datasets/ukb-a-57/; https://gwas.mrcieu.ac.uk/datasets/ukb-a-306/.