Abstract
Purpose
To examine the ocular signs and symptoms in participants of the Sjögren's International Collaborative Clinical Alliance cohort, and to compare them across Sjögren's disease (SjD) status.
Methods
Our study population comprised 3380 Sjögren's International Collaborative Clinical Alliance participants who had no missing data relevant to this study. Participants’ SjD status was assessed using the updated 2016 American College of Rheumatism/European League Against Rheumatism SjD classification criteria. Participants completed baseline questionnaires of ocular symptoms and underwent ocular examinations. Differences in the ocular signs and symptoms between SjD and non-SjD groups were assessed. We used multivariable linear and linear mixed-effects models to investigate the impact of SjD on Ocular Surface Disease Index-6 and OSS.
Results
Among 1532 participants classified as SjD, their Ocular Surface Disease Index-6 did not clinically differ from those classified as non-SjD (adjusted difference, −0.97; 95% confidence interval, −1.52 to −0.41). However, SjD participants exhibited an elevated ocular staining score (adjusted difference, 3.47; 95% confidence interval, 3.36–3.57; P < 0.001) compared with non-SjD participants. In addition, SjD was associated with increased odds of ocular signs, such as reduced tear break-up time, abnormal Schirmer I test, and corneal abnormalities, and was strongly related to more intense corneal and conjunctival staining, as well as additional corneal staining points.
Conclusions
SjD is associated with a higher risk of ocular signs and pathology compared with non-SjD, whereas ocular symptoms remain similar. In addition, corneal abnormalities and corneal staining patterns could serve as a potential biomarker in identifying SjD-related dry eye.
Keywords: sjögren's disease, dry eye disease, keratoconjunctivitis sicca, ocular staining, ocular signs and symptoms
Sjögren's disease (SjD, previously known as Sjögren's syndrome) is a multisystem autoimmune disorder associated with lymphocytic infiltration of exocrine glands, particularly salivary and lacrimal glands, which produces mild to severe dry (or sicca) signs and symptoms in the mouth and eyes.1–3 Similar to other autoimmune disorders, SjD overwhelmingly affects women, typically between the ages of 30 to 60 years. More than 95% of patients complain of oral and/or ocular dryness.4 The characteristic ocular finding in SjD is keratoconjunctivitis sicca, or aqueous deficient dry eye.5 In the updated American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria for SjD, dry eye disease (DED) is assessed via the Schirmer I test (abnormal if ≤5 mm in 5 minutes in at ≥1 eye) and ocular staining score (abnormal if ≥5 in at least one eye).6–8 SjD-related DED can negatively impact quality of life by producing symptoms of ocular discomfort and affecting vision owing to poor lubrication of the ocular surface.9–11 Moreover, SjD can be associated with potentially serious vision-threatening ocular complications, including corneal melt with subsequent perforation, corneal vascularization, uveitis, scleritis, retinal vasculitis, and optic neuritis.12–17 Despite such important and potentially devastating complications, SjD remains underdiagnosed in patients with dry eye.9
The Sjögren's International Collaborative Clinical Alliance (SICCA) was established to develop and validate universally accepted classification criteria for SjD, characterize the SjD phenotype and genotype, and to develop a data and biospecimen repository to support future research.8 By virtue of participants enrolled into the cohort, SICCA intrinsically contains one of the largest dry eye cohorts and offers an opportunity to study the full extent of the ocular manifestations of SjD, particularly the lids and anterior segment.18 The purpose of the present study was to interrogate the SICCA database to identify associations between SjD classification status and anterior segment pathology. We hypothesized that SICCA participants classified as SjD would feature more potentially vision-threatening clinical manifestations compared with those not meeting ACR/EULAR SjD classification criteria.
Methods
Study Population
The SICCA cohort assemblage has been published previously.8,19 In brief, 3514 participants aged between 21 and 89 years at baseline were enrolled from nine research sites (6 international and 3 U.S. sites) between September 2004 and September 2012. Participants who had been diagnosed with SjD (using prior classification or diagnostic criteria); who had symptoms, signs, or abnormal test results suggestive of SjD; or who were suspected of having SjD by their referring ophthalmologist, rheumatologist, or oral medicine specialist were recruited into SICCA. Each participant provided informed consent, and the study protocol was approved by the institutional review boards of the University of California, San Francisco, and enrolling sites. For our analytical purpose, we excluded participants who were unable to be classified as SjD or non-SjD (n = 114) or who had more than 20% missing information (n = 20). The remaining 3380 participants comprised our study population.
Participants’ were classified as SjD or non-SjD using the 2016 ACR/EULAR classification criteria.8 Participants were queried about ocular symptoms including eye redness, itching, tearing, light sensitivity, grittiness or scratchiness, burning or stinging, blurred vision, fluctuating vision, pain, eye irritation and discomfort, and their corresponding frequencies (none of the time, some of the time, half of the time, most of the time, and all of the time). Participants were also asked if their eyes felt dry, when ocular dryness started, and the dry eye therapies they used. The six-item Ocular Surface Disease Index (OSDI-6) was calculated for each participant.20
To ensure the test accuracy and reproducibility, participants had their eyes examined by trained ophthalmologists following the specific sequence: Schirmer I test (normal ≥5 mm, abnormal <5 mm at 5 minutes), slit-lamp examination of the lids, conjunctiva, and cornea for any abnormalities, tear break-up time ( normal ≥10 seconds, abnormal <10 seconds), corneal fluorescein staining, and conjunctival lissamine green staining. The Ocular Staining Score (OSS; continuous, 0–12 points) was calculated by combining the results of corneal fluorescein staining and conjunctiva lissamine green staining.7
Statistical Analyses
For the distribution of patients’ characteristics by SjD classification status, categorical variables were expressed as counts and percentages, which were compared by chi-squared tests. Continuous variables were summarized as mean with SD or medians with interquartile ranges (IQR), and the differences between SjD groups were evaluated by t tests or Kruskal Wallis H tests, depending on whether the data were normally distributed or not. Because diagnostic measurements were eye related (rather than participant related), we used tetrachoric correlation for clinical signs having binary features (e.g., present or absent) and Pearson correlation for continuous variables to assess the correlation between paired organs (eyes) in each individual participant. To account for the correlation structure for the subunits (eyes) within a cluster (participants), univariable logistic and linear mixed-effects regression models were used for categorical and continuous outcomes, respectively, to assess the odds ratios (ORs) or differences in the clinical features between the SjD and non-SjD groups. We conducted additional analyses on ocular staining pattern to explore if there were any differences based on SjD status. The significance level was adjusted for the multiple comparisons using Bonferroni correction.
We further conducted multivariable linear regression and linear mixed-effects models, as appropriate, to assess SjD classification influence on OSDI-6 and OSS while adjusting for age, sex, region, smoking status, education, employment, and general health status. To explore whether age, sex, and health status might affect participants’ perception of ocular symptoms or ocular health, we performed subgroup analyses defined by age (≤55, >55 years, which approximated the median age of the cohort), sex (female, male), and health status (poor [self-reported poor and fair general health] or good [self-reported good and above general health]). The interaction between SjD status and subgroup factors was assessed by entering cross-product terms into the linear models. Statistical analyses were conducted using R statistical software, version 4.2.3.
Results
Demographics
Details of participants’ baseline characteristics by SjD status are shown in Table 1. Of the 3380 study participants included, 1532 (45.3%) were classified as having SjD. The overall mean age was 53.0 ± 13.2 years. The majority (91.1%) of our study population was female. A large proportion of participants were from North and South America (49.9%), were Caucasian (58.3%), had an education degree of college or above (59.2%), were currently working (61.6%), and never smoked (59.1%). One-half of the participants rated their health as good or above, whereas the other one-half reported as fair or poor. Compared with participants classified as non-SjD, SjD participants were more likely to come from Asia, be of Asian ethnicity, be used, to have never smoked, and reported an inability to produce their own tears.
Table 1.
Demographics of 3390 SICCA Participants by SjD Status
| Characteristic | All (n = 3380) | Non-SjD (n = 1848) | SjD (n = 1532) | Difference (95% CI) | P Value |
|---|---|---|---|---|---|
| Age (years) | 53.0 ± 13.2 | 53.6 ± 13.0 | 52.3 ± 13.4 | −1.3 (−2.2 to −0.5) | 0.003 |
| Sex | <0.001 | ||||
| Female | 3078 (91.1) | 1646 (89.1) | 1432 (93.5) | 4.4% (2.5% to 6.3) | |
| Male | 293 (8.7) | 196 (10.6) | 97 (6.3) | −4.3% (−6.2% to −2.4%) | |
| Region | <0.001 | ||||
| America | 1687 (49.9) | 1024 (55.4) | 663 (43.3) | −12.1% (−15.6% to −8.7%) | |
| Asia | 805 (23.8) | 278 (15.0) | 527 (34.4) | 19.4% (16.4% to 22.3%) | |
| Europe | 888 (26.3) | 546 (29.5) | 342 (22.3) | −7.2% (−10.2% to −4.2%) | |
| Race/ethnicity | <0.001 | ||||
| Caucasian | 1969 (58.3) | 1248 (67.5) | 721 (47.1) | −20.5% (−23.8% to −17.1%) | |
| Asian or Pacific Islander | 919 (27.2) | 324 (17.5) | 595 (38.8) | 21.3% (18.3% to 24.4%) | |
| Hispanic/Latino | 352 (10.4) | 202 (10.9) | 150 (9.8) | −1.1% (−3.3% to 1.0%) | |
| African | 93 (2.8) | 47 (2.5) | 46 (3.0) | 0.5% (−0.7% to 1.6%) | |
| Native American | 35 (1.0) | 19 (1.0) | 16 (1.0) | 0.0% (−0.7% to 0.7%) | |
| Education | 0.003 | ||||
| High school and lower | 1378 (40.8) | 701 (37.9) | 677 (44.2) | 6.3% (2.9% to 9.6%) | |
| College and above | 2002 (59.2) | 1147 (62.1) | 855 (55.8) | −6.3% (−9.6% to −2.9%) | |
| Employment | <0.001 | ||||
| Not Working | 547 (16.2) | 362 (19.6) | 185 (12.1) | −7.5% (−10.0% to −5.0%) | |
| Working | 2082 (61.6) | 1063 (57.5) | 1019 (66.5) | 9.0% (5.7% to 12.3%) | |
| Retired | 740 (21.9) | 416 (22.5) | 324 (21.1) | −1.4% (−4.2% to 1.5%) | |
| Others | 481 (14.2) | 248 (13.6) | 233 (14.8) | 1.2% (−1.2% to 3.6%) | |
| Smoking | <0.001 | ||||
| Never | 1995 (59.0) | 975 (52.8) | 1020 (66.6) | 13.8% (10.5% to 17.2%) | |
| Ever | 1063 (31.4) | 627 (33.9) | 436 (28.5) | −5.5% (−8.7% to −2.3%) | |
| Current | 322 (9.5) | 246 (13.3) | 76 (5.0) | −8.4% (−10.3% to −6.4%) | |
| General health | 0.004 | ||||
| Poor | 396 (11.7) | 257 (13.9) | 139 (9.1) | −4.8% (−7.0% to −2.6%) | |
| Fair | 1270 (37.6) | 687 (37.2) | 583 (38.1) | 0.9% (−2.5% to 4.2%) | |
| Good | 1149 (34.0) | 596 (32.3) | 553 (36.1) | 3.8% (0.6% to 7.1%) | |
| Very Good | 491 (14.5) | 267 (14.4) | 224 (14.6) | 0.2% (−2.3% to 2.6%) | |
| Excellent | 63 (1.9) | 33 (1.8) | 30 (2.0) | 0.2% (−0.8% to 1.2%) | |
| Ability to produce tears | <0.001 | ||||
| No | 760 (22.5) | 288 (15.6) | 472 (30.8) | 15.2% (12.3% to 18.1%) | |
| Yes | 2603 (77.0) | 1551 (83.9) | 1052 (68.7) | −15.3% (−18.2% to −12.3%) | |
| Dry eye therapy | |||||
| Artificial tears | 2140 (63.3) | 1164 (63.0) | 976 (63.7) | 0.7% (−2.6% to 4.0%) | >0.999 |
| Punctal occlusion | 317 (9.4) | 160 (8.7) | 157 (10.2) | 1.6% (−0.5% to 3.6%) | >0.999 |
| Steroids | 130 (3.8) | 75 (4.1) | 55 (3.6) | −0.5% (−1.8% to 0.9%) | >0.999 |
| Antibiotics | 192 (5.7) | 86 (4.7) | 106 (6.9) | 2.3% (0.6% to 3.9%) | 0.047 |
| Cyclosporine | 327 (9.7) | 188 (10.2) | 139 (9.1) | −1.1% (−3.2% to 1.0%) | >0.999 |
| No. of dry eye therapies | 1.000 | ||||
| 0 | 1134 (33.6) | 641 (34.7) | 493 (32.2) | −2.5% (−5.8% to 0.7%) | |
| 1 | 1600 (47.3) | 861 (46.6) | 739 (48.2) | 1.6% (−1.8% to 5.1%) | |
| ≥2 | 646 (19.1) | 346 (18.7) | 300 (19.6) | 0.9% (−1.9% to 3.6%) | |
| Length of feeling eye dry (years) | 3.91 (7.65) | 3.73 (7.46) | 4.16 (7.80) | 0.43 (−0.09 to 0.93) | 0.210 |
Values are mean ± SD, number (%), or median (IQR) unless otherwise noted.
Symptoms
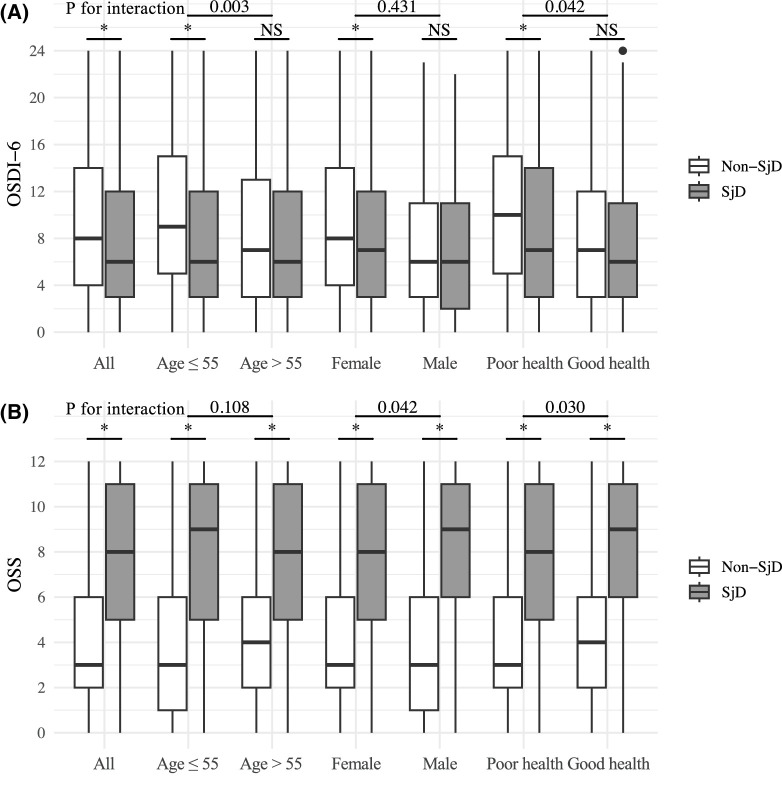
Ocular symptoms of participants are summarized in Table 2. Light sensitivity, eye irritation, and discomfort during daily activities were common complaints among our study population, whereas tearing and fluctuating vision only presented in a small proportion of participants. In those classified as having SjD, ocular symptoms such as light sensitivity, gritty or scratchy sensation, burning or stinging, eye irritation, and discomfort were less prevalent compared with those classified as non-SjD (Table 2). Most participants complained of eye dryness (85.6%) and poor vision (63.2%), which did not differ significantly between SjD and non-SjD participants. The median OSDI-6 of study participants was 8.43 (IQR, 6.21). In those classified as having SjD, the OSDI-6 was 1.27 points (95% confidence interval [CI], 0.86–1.69) lower than that of those classified as non-SjD. Participants under the age of 55 years, females, and those self-reported poor health exhibited overall higher OSDI-6 scores compared with their older, male, and healthier counterparts, respectively, particularly in the non-SjD group (Fig. 1 and Table 3). After adjusting for confounding factors, the difference in OSDI-6 between SjD and non-SjD participants remained statistically significant (−0.97; 95% CI, −1.52 to −0.41), although the clinical significance of the difference might be limited. Similar findings were observed in the younger age group, females and those self-identified as being in poor health, and there existed interactions between SjD status and age (P for interaction = 0.003), as well as health status (P for interaction = 0.042).
Table 2.
Ocular Symptoms of 3390 SICCA Participants by SjD Status
| Symptom | All (n = 3380) | Non-SjD (n = 1848) | SjD (n = 1532) | Difference (95% CI) | P Value |
|---|---|---|---|---|---|
| Redness | 0.021 | ||||
| None of the time | 1008 (29.8) | 531 (28.7) | 477 (31.1) | 2.4% (−0.8% to 5.6%) | |
| Some of the time | 1457 (43.1) | 788 (42.6) | 669 (43.7) | 1.0% (−2.4% to 4.4%) | |
| Half of the time | 298 (8.8) | 170 (9.2) | 128 (8.4) | −0.8% (−2.8% to 1.1%) | |
| Most of the time | 443 (13.1) | 237 (12.8) | 206 (13.4) | 0.6% (−1.7% to 3.0%) | |
| All of the time | 160 (4.7) | 112 (6.1) | 48 (3.1) | −2.9% (−4.4% to −1.5%) | |
| Itching | 0.020 | ||||
| None of the time | 662 (19.6) | 323 (17.5) | 339 (22.1) | 4.6% (1.9% to 7.4%) | |
| Some of the time | 1682 (49.8) | 913 (49.4) | 769 (50.2) | 0.8% (−2.7% to 4.2%) | |
| Half of the time | 443 (13.1) | 264 (14.3) | 179 (11.7) | −2.6% (−4.9% to −0.3%) | |
| Most of the time | 465 (13.8) | 268 (14.5) | 197 (12.9) | −1.6% (−4.0% to 0.7%) | |
| All of the time | 119 (3.5) | 74 (4.0) | 45 (2.9) | −1.1% (−2.4% to 0.2%) | |
| Tearing | <0.001 | ||||
| None of the time | 2389 (70.7) | 1165 (63.0) | 1224 (79.9) | 16.9% (13.8% to 19.9%) | |
| Some of the time | 806 (23.8) | 554 (30.0) | 252 (16.4) | −13.5% (−16.4% to −10.7%) | |
| Half of the time | 82 (2.4) | 61 (3.3) | 21 (1.4) | −1.9% (−3.0% to −0.9%) | |
| Most of the time | 50 (1.5) | 34 (1.8) | 16 (1.0) | −0.8% (−1.7% to 0.1%) | |
| All of the time | 41 (1.2) | 27 (1.5) | 14 (0.9) | −0.5% (−1.3% to 0.2%) | |
| Light sensitivity | <0.001 | ||||
| None of the time | 1200 (35.5) | 584 (31.6) | 616 (40.2) | 8.6% (5.3% to 11.9%) | |
| Some of the time | 979 (29.0) | 549 (29.7) | 430 (28.1) | −1.6% (−4.8% to 1.5%) | |
| Half of the time | 233 (6.9) | 135 (7.3) | 98 (6.4) | −0.9% (−2.7% to 0.9%) | |
| Most of the time | 528 (15.6) | 316 (17.1) | 212 (13.8) | −3.3% (−5.8% to −0.8%) | |
| All of the time | 430 (12.7) | 258 (14.0) | 172 (11.2) | −2.7% (−5.0% to −0.4%) | |
| Gritty | <0.001 | ||||
| None of the time | 927 (27.4) | 438 (23.7) | 489 (31.9) | 8.2% (5.1% to 11.3%) | |
| Some of the time | 1308 (38.7) | 712 (38.5) | 596 (38.9) | 0.4% (−3.0% to 3.7%) | |
| Half of the time | 371 (11.0) | 212 (11.5) | 159 (10.4) | −1.1% (−3.3% to 1.1%) | |
| Most of the time | 510 (15.1) | 323 (17.5) | 187 (12.2) | −5.3% (−7.7% to −2.8%) | |
| All of the time | 255 (7.5) | 157 (8.5) | 98 (6.4) | −2.1% (−3.9% to −0.3%) | |
| Burning | <0.001 | ||||
| None of the time | 1417 (41.9) | 697 (37.7) | 720 (47.0) | 9.3% (5.9% to 12.7%) | |
| Some of the time | 1095 (32.4) | 601 (32.5) | 494 (32.2) | −0.3% (−3.5% to 3.0%) | |
| Half of the time | 316 (9.3) | 212 (11.5) | 104 (6.8) | −4.7% (−6.7% to −2.7%) | |
| Most of the time | 387 (11.4) | 229 (12.4) | 158 (10.3) | −2.1% (−4.3% to 0.1%) | |
| All of the time | 156 (4.6) | 103 (5.6) | 53 (3.5) | −2.1% (−3.6% to −0.7%) | |
| Blurred vision | 0.058 | ||||
| None of the time | 1256 (37.2) | 663 (35.9) | 593 (38.7) | 2.8% (−0.5% to 6.2%) | |
| Some of the time | 1422 (42.1) | 766 (41.5) | 656 (42.8) | 1.4% (−2.0% to 4.8%) | |
| Half of the time | 269 (8.0) | 171 (9.3) | 98 (6.4) | −2.9% (−4.7% to −1.0%) | |
| Most of the time | 260 (7.7) | 158 (8.5) | 102 (6.7) | −1.9% (−3.7% to 0.0%) | |
| All of the time | 161 (4.8) | 82 (4.4) | 79 (5.2) | 0.7% (−0.8% to 2.2%) | |
| Fluctuating vision | 0.001 | ||||
| None of the time | 1594 (47.2) | 807 (43.7) | 787 (51.4) | 7.7% (4.3% to 11.1%) | |
| Some of the time | 1220 (36.1) | 691 (37.4) | 529 (34.5) | −2.9% (−6.2% to 0.4%) | |
| Half of the time | 212 (6.3) | 135 (7.3) | 77 (5.0) | −2.3% (−4.0% to −0.6%) | |
| Most of the time | 253 (7.5) | 153 (8.3) | 100 (6.5) | −1.8% (−3.6% to 0.1%) | |
| All of the time | 89 (2.6) | 55 (3.0) | 34 (2.2) | −0.8% (−1.9% to 0.4%) | |
| Pain | 0.003 | ||||
| None of the time | 1960 (58.0) | 1015 (54.9) | 945 (61.7) | 6.8% (3.4% to 10.1%) | |
| Some of the time | 764 (22.6) | 426 (23.1) | 338 (22.1) | −1.0% (−3.9% to 1.9%) | |
| Half of the time | 140 (4.1) | 89 (4.8) | 51 (3.3) | −1.5% (−2.9% to −0.1%) | |
| Most of the time | 306 (9.1) | 194 (10.5) | 112 (7.3) | −3.2% (−5.2% to −1.2%) | |
| All of the time | 200 (5.9) | 118 (6.4) | 82 (5.4) | −1.0% (−2.7% to 0.6%) | |
| Irritation while reading or driving | <0.001 | ||||
| None of the time | 956 (28.3) | 466 (25.2) | 490 (32.0) | 6.8% (3.6% to 9.9%) | |
| Some of the time | 1166 (34.5) | 632 (34.2) | 534 (34.9) | 0.7% (−2.6% to 3.9%) | |
| Half of the time | 318 (9.4) | 183 (9.9) | 135 (8.8) | −1.1% (−3.1% to 0.9%) | |
| Most of the time | 510 (15.1) | 295 (16.0) | 215 (14.0) | −1.9% (−4.4% to 0.5%) | |
| All of the time | 346 (10.2) | 220 (11.9) | 126 (8.2) | −3.7% (−5.8% to −1.6%) | |
| Irritation while watching TV or computer | <0.001 | ||||
| None of the time | 861 (25.5) | 418 (22.6) | 443 (28.9) | 6.3% (3.3% to 9.3%) | |
| Some of the time | 1141 (33.8) | 592 (32.0) | 549 (35.8) | 3.8% (0.5% to 7.1%) | |
| Half of the time | 352 (10.4) | 199 (10.8) | 153 (10.0) | −0.8% (−2.9% to 1.3%) | |
| Most of the time | 579 (17.1) | 350 (18.9) | 229 (14.9) | −4.0% (−6.6% to −1.4%) | |
| All of the time | 387 (11.4) | 248 (13.4) | 139 (9.1) | −4.3% (−6.5% to −2.2%) | |
| Uncomfortableness in wind | <0.001 | ||||
| None of the time | 1032 (30.5) | 500 (27.1) | 532 (34.7) | 7.7% (4.5% to 10.9%) | |
| Some of the time | 984 (29.1) | 537 (29.1) | 447 (29.2) | 0.1% (−3.0% to 3.3%) | |
| Half of the time | 206 (6.1) | 122 (6.6) | 84 (5.5) | −1.1% (−2.8% to 0.5%) | |
| Most of the time | 640 (18.9) | 376 (20.3) | 264 (17.2) | −3.1% (−5.8% to −0.4%) | |
| All of the time | 504 (14.9) | 302 (16.3) | 202 (13.2) | −3.2% (−5.6% to −0.7%) | |
| Uncomfortableness in dry or heated environment | 0.001 | ||||
| None of the time | 1070 (31.7) | 525 (28.4) | 545 (35.6) | 7.2% (3.9% to 10.4%) | |
| Some of the time | 931 (27.5) | 502 (27.2) | 429 (28.0) | 0.8% (−2.2% to 3.9%) | |
| Half of the time | 276 (8.2) | 166 (9.0) | 110 (7.2) | −1.8% (−3.7% to 0.1%) | |
| Most of the time | 628 (18.6) | 368 (19.9) | 260 (17.0) | −2.9% (−5.6% to −0.3%) | |
| All of the time | 456 (13.5) | 273 (14.8) | 183 (11.9) | −2.8% (−5.2% to −0.5%) | |
| Eye dryness | 2892 (85.6) | 1601 (86.6) | 1291 (84.3) | −2.4% (−4.8% to 0.1%) | 0.719 |
| Poor vision | 2135 (63.2) | 1160 (62.8) | 975 (63.6) | 0.9% (−2.5% to 4.2%) | 1.000 |
Values are number (%) or median (IQR) unless otherwise noted.
Figure 1.
Box plot depicting difference in ocular symptoms and signs between the SjD and non-SjD participants across all participants and subgroups defined by age, sex, and health status. Horizontal line through each box represents the median. *P < Bonferroni-corrected P value threshold. (A) OSDI-6. (B) OSS. NS, not significant.
Table 3.
Differences in OSDI-6 Between Participants Classified as SjD and Non-SjD Across Subgroups Defined by Age, Gender, and Health Status
| Subgroup | Non-SjD | SjD | Difference (95% CI) | Adjusted Difference (95% CI)* | P Value for Interaction |
|---|---|---|---|---|---|
| All | 9.01 (6.24) | 7.74 (6.09) | −1.27 (−1.69 to −0.86) | −0.97 (−1.52 to −0.41) | |
| Age (years) | 0.003 | ||||
| ≤55 | 9.83 (6.29) | 7.71 (6.20) | −2.13 (−2.70 to −1.56) | −0.97 (−1.52 to −0.41) | |
| >55 | 8.12 (6.03) | 7.81 (5.95) | −0.31 (−0.92 to −0.30) | 0.06 (−0.53 to 0.64) | |
| Sex | 0.431 | ||||
| Female | 9.26 (6.28) | 7.80 (6.10) | −1.46 (−1.90 to −1.02) | −0.50 (−0.92 to −0.08) | |
| Male | 7.17 (5.43) | 6.96 (5.87) | −0.21 (−1.61 to −1.19) | −0.32 (−1.69 to 1.05) | |
| Health status | 0.042 | ||||
| Poor | 10.20 (6.32) | 8.46 (6.46) | −1.74 (−2.36 to −1.12) | −0.76 (−1.35 to −0.16) | |
| Good | 7.82 (5.90) | 7.12 (5.67) | −0.71 (−1.26 to −0.16) | −0.38 (−0.92 to 0.17) |
Adjusted for age, sex, region, smoking status, education, employment, and general health status.
Values are mean ± SD.
Clinical Examination Findings
Clinical features of participants’ two eyes were strongly correlated (Table 3). Pinguecula was a common manifestation (23.5%; the larger percentage between the right eye and the left eye was reported, same below) among study participants, followed by meibomitis (20.5%). Entropion, ectropion, and corneal ulceration were found in relatively few participants. Compared with those classified as non-SjD, those classified as having SjD had a higher likelihood of exhibiting pathology including blepharitis (OR, 1.17; 95% CI, 1.00–1.36; P = 0.044), corneal pathology such as filaments (OR, 3.63; 95% CI, 2.76–4.78; P < 0.001), vascularization (OR, 3.02; 95% CI, 1.86–4.91; P < 0.001), scarring (OR, 1.70; 95% CI, 1.28–2.25; P < 0.001), and ulceration (OR, 5.43; 95% CI, 1.17–15.13; P = 0.031) (Table 4).
Table 4.
Anterior Segment Pathology and Clinical Signs of 3390 SICCA Participants by SjD Status
| All | Non-SjD | SjD | ||||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | OR (95% CI) | P Value | |
| Manifestation | ||||||||
| Lagophthalmos | 40 (1.2) | 36 (1.1) | 25 (1.4) | 22 (1.2) | 15 (1.0) | 14 (0.9) | 0.74 (0.46 to 1.18) | 0.204 |
| Entropion | 7 (0.2) | 8 (0.2) | 3 (0.2) | 4 (0.2) | 4 (0.3) | 4 (0.3) | 1.38 (0.50 to 3.80) | 0.538 |
| Ectropion | 9 (0.3) | 11 (0.3) | 6 (0.3) | 7 (0.4) | 3 (0.2) | 4 (0.3) | 0.65 (0.26 to 1.62) | 0.353 |
| Trichiasis | 66 (2.0) | 50 (1.5) | 32 (1.7) | 26 (1.4) | 34 (2.2) | 24 (1.6) | 1.23 (0.85 to 1.78) | 0.274 |
| Pterygium | 55 (1.6) | 62 (1.8) | 31 (1.7) | 29 (1.6) | 24 (1.6) | 33 (2.2) | 1.15 (0.79 to 1.65) | 0.467 |
| Pingueculam | 791 (23.4) | 795 (23.5) | 436 (23.6) | 441 (23.9) | 355 (23.2) | 354 (23.1) | 0.96 (0.86 to 1.08) | 0.529 |
| Meibomitis | 684 (20.2) | 694 (20.5) | 367 (19.9) | 373 (20.2) | 317 (20.7) | 321 (21.0) | 1.05 (0.93 to 1.18) | 0.423 |
| Blepharitis | 378 (11.2) | 373 (11.0) | 194 (10.5) | 189 (10.2) | 184 (12.0) | 184 (12.0) | 1.17 (1.00 to 1.36) | 0.044 |
| Corneal abnormality | ||||||||
| Filaments | 135 (4.0) | 140 (4.1) | 33 (1.8) | 38 (2.1) | 102 (6.7) | 102 (6.7) | 3.63 (2.76 to 4.78) | <0.001 |
| Corneal vascularization | 37 (1.1) | 43 (1.3) | 8 (0.4) | 15 (0.8) | 29 (1.9) | 28 (1.8) | 3.02 (1.86 to 4.91) | <0.001 |
| Corneal scarring | 100 (3.0) | 104 (3.1) | 40 (2.2) | 46 (2.5) | 60 (3.9) | 58 (3.8) | 1.70 (1.28 to 2.25) | <0.001 |
| Corneal ulceration | 3 (0.1) | 8 (0.2) | 1 (0.1) | 1 (0.1) | 2 (0.1) | 7 (0.5) | 5.43 (1.17 to 25.13) | 0.031 |
| Tear film | ||||||||
| Abnormal TBUT | 2733 (80.9) | 2726 (80.7) | 1363 (73.8) | 1372 (74.2) | 1370 (89.4) | 1354 (88.4) | 2.83 (2.47 to 3.23) | <0.001 |
| Debris | 1329 (39.3) | 1337 (39.6) | 483 (26.1) | 496 (26.8) | 846 (55.2) | 841 (54.9) | 3.40 (3.07 to 3.76) | <0.001 |
| Abnormal Schirmer I | 1126 (33.3) | 1104 (32.7) | 405 (21.9) | 384 (20.8) | 721 (47.1) | 720 (47.0) | 3.28 (2.95 to 3.65) | <0.001 |
TBUT, tear break-up time.
Values are number (%) or mean ± SD.
Dry Eye Tests
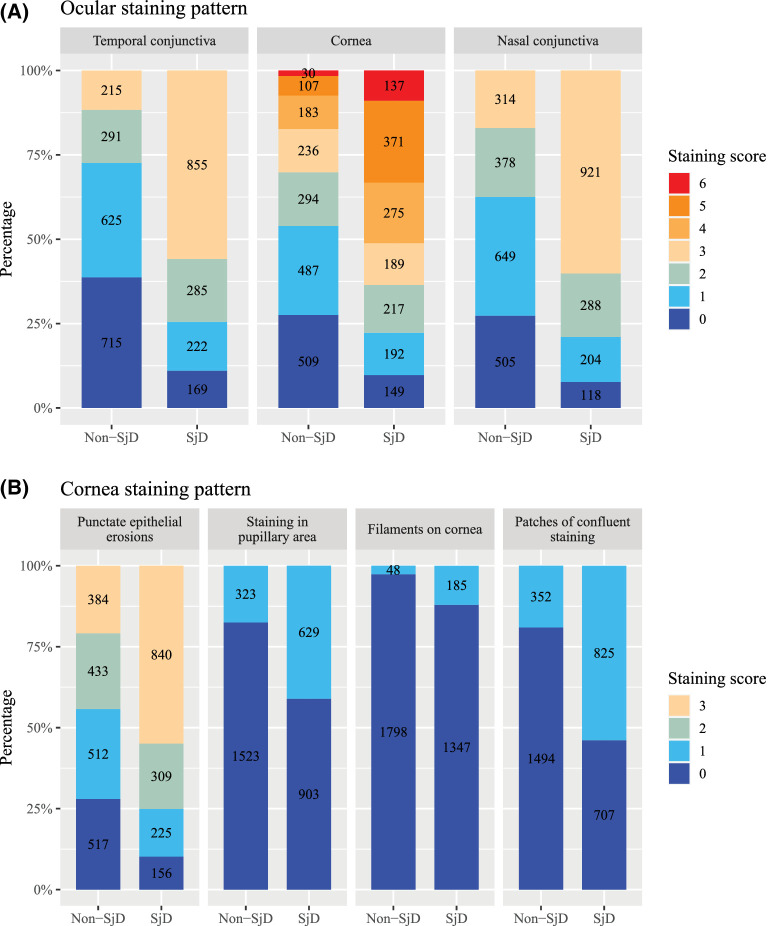
We found that 2733 participants (80.9%) had abnormal tear break-up time. The abnormal results of Schirmer I test were seen in 891 participants (26.4%). The mean OSS of the study population was greater than 5 (right: 5.04 [IQR, 3.70]; left: 5.15 [IQR, 3.69]) (Table 4). Compared with those classified as non-SjD, those classified as SjD had an increased odds of exhibiting an abnormal tear break-up time (OR, 2.83; 95% CI, 2.47–3.23; P < 0.001) and Schirmer I test (OR, 2.88; 95% CI, 2.56–3.23; P < 0.001). The OSS within the same SjD status group showed minimal variation, regardless of subgroups defined by age, sex, or health status (Table 5). Overall, SjD participants had a significantly higher OSS compared with non-SjD participants, with a mean difference of 3.70 points (95% CI, 3.49–3.91; P < 0.001). Specifically, participants classified as having SjD presented with significantly more intense ocular staining patterns, with notably increased odds of temporal conjunctival staining (OR, 4.71; 95% CI, 4.16–5.34; P < 0.001), followed by nasal conjunctival staining (OR, 4.68; 95% CI, 4.13–5.30; P < 0.001) (Fig. 2 and Supplementary Table 1). Corneal staining was also pronounced in SjD participants, but showed a relatively smaller magnitude (OR, 3.51; 95% CI, 3.10–3.98; P < 0.001) compared with conjunctival staining. However, the three additional corneal staining points, including staining in the pupillary area (OR, 3.30; 95% CI, 2.92–3.72; P < 0.001), corneal filaments (OR, 5.01; 95% CI, 3.87–6.49; P < 0.001), and patches of confluent staining (OR, 5.17; 95% CI, 4.60–5.81; P < 0.001), were also much more frequently observed in SjD participants. After adjusting for confounding factors, the higher OSS among SjD participants remained statistically and clinically significant (adjusted difference, 3.47; 95% CI, 3.36–3.57). This pattern was observed consistently across subgroups defined by age, sex, and health status, and we observed interactions between SjD status with sex (P for interaction = 0.042) and health status (P for interaction = 0.030).
Table 5.
Differences in OSS Between Participants Classified As SjD and Non-SjD Across Subgroups Defined by Age, Gender, and Health Status
| Non-SjD | SjD | ||||||
|---|---|---|---|---|---|---|---|
| Subgroup | Right | Left | Right | Left | Difference (95% CI) | Adjusted Difference (95% CI)* | P Value for Interaction |
| All | 3.36 (2.88) | 3.47 (2.94) | 7.07 (3.56) | 7.18 (3.48) | 3.70 (3.49 to 3.91) | 3.47 (3.36 to 3.57) | |
| Age (years) | 0.108 | ||||||
| ≤55 | 3.33 (2.95) | 3.39 (3.00) | 7.22 (3.58) | 7.28 (3.50) | 3.90 (3.61–4.19) | 3.51 (3.22 to 3.81) | |
| >55 | 3.41 (2.80) | 3.58 (2.87) | 6.85 (3.52) | 7.03 (3.45) | 3.45 (3.14–3.76) | 3.34 (3.03 to 3.65) | |
| Sex | 0.042 | ||||||
| Female | 3.40 (2.87) | 3.49 (2.91) | 7.03 (3.57) | 7.13 (3.49) | 3.65 (3.43–3.87) | 3.40 (3.18 to 3.63) | |
| Male | 3.14 (2.96) | 3.41 (3.18) | 7.60 (3.34) | 7.70 (3.28) | 4.37 (3.63–5.12) | 4.16 (3.42 to 4.90) | |
| Health status | 0.030 | ||||||
| Poor | 3.27 (2.76) | 3.30 (2.81) | 6.76 (3.70) | 6.84 (3.57) | 3.52 (3.23–3.82) | 3.33 (3.02 to 3.64) | |
| Good | 3.47 (3.01) | 3.67 (3.06) | 7.34 (3.40) | 7.46 (3.38) | 3.84 (3.54–4.13) | 3.59 (3.29 to 3.89) | |
Adjusted for age, sex, region, smoking status, education, employment, and general health status.
Values are mean ± SD.
Figure 2.
Ocular staining patterns based on constituent components of the OSS. (A) Ocular staining patterns by region. (B) Cornea staining patterns assessed with fluorescein.
Discussion
Using data from the SICCA cohort, our study represents the largest cohort that comprehensively compares ocular signs and symptoms between participants with SjD and non-SjD. Our findings indicated that, although the ocular symptoms were similar between SjD and non-SjD participants, those with SjD demonstrated an increased risk of anterior segment abnormalities compared with those classified as non-SjD. It was noting that some of these ocular abnormalities such as corneal filaments can be potentially vision threatening. Therefore, more aggressive interventions to avert the most deleterious sequela of DED should be considered for individuals with SjD compared with those without SjD dry eye.21,22 Moreover, corneal and conjunctival staining was more severe as assessed by the OSS in those classified as SjD compared with those classified as non-SjD.
It has been observed consistently that SjD patients may experience significant visually compromising ocular complications.14,23,24 The mechanism by which SjD is associated with corneal pathology is not entirely clear, but may be related to ocular surface inflammation as well as ocular surface dysbiosis.25 For example, the ocular surface in SjD has been demonstrated to feature less microbial diversity compared with those with non-SjD dry eye.26 Decreased microbial diversity may allow for the development of atypical ocular infections in SjD.27 It has been demonstrated that SjD-related aqueous-deficient dry eye can feature corneal filaments, and 95% of corneal filaments are secondary to keratoconjunctivitis sicca.28,29 Additionally, a previous study reported that corneal opacities, corneal ulcers, and corneal melts and perforations occur in up to 70% of patients when followed long term.30 Although our results did not feature as high a prevalence of corneal pathology, it should be noted that the present study was a cross-sectional study with participants of relatively younger age. Another study showed that there was no difference in long-term outcomes, including the development of corneal ulcers and melts, between those classified as SjD and non-SjD.31 Of importance, however, was that most patients in this previous study had their dry eye therapy escalated, which may have decreased the incidence of vision-threatening complications in both the SjD and non-SjD groups. This finding would imply that aggressive management of DED in SjD is imperative for preserving vision. Indeed, some investigators have suggested that systemic immunomodulatory therapy may be beneficial in some SjD patients with severe DED.32 Although our results did not reveal an escalated DED therapy use among participants with SjD, it could be attributed partially to the fact that our participants had varying durations of experiencing ocular dryness and might have been in the early stages of dry eye treatment, where artificial tears were the mainstay of treatment.
Despite the notable anterior segment pathology exhibited by those classified as SjD, they actually had comparable dry eye-related symptoms as those classified as non-SjD. This finding is akin to what has been found by others in the sense that ocular signs and dry eye symptoms are frequently discordant.33 The discordance in DES symptomatology could be attributed partially to the altered corneal serve density and morphology in SjD patients.34,35 Additionally, some investigators have suggested that such discordance may be related to comorbid conditions such as anxiety and depression and self-perceived health, where those with lower self-perceived health had an increased odds of complaining of DED symptoms.33,36 Mirroring these prior studies, we found that while the OSS of participants who reported poor health were generally lower compared with those who self-reported good health, their OSDI-6 scores were higher, especially in those without SjD. In addition to the rationales discussed elsewhere in this article, it is worth noting that the SICCA cohort included participants who had either diagnosed SjD or symptoms related to SjD. Participants without SjD or ocular signs must have abnormal ocular symptoms compared with the general population to meet the SICCA inclusion criteria, which led to Berkson's bias.37 As a result, SICCA participants without SjD generally showed severe symptoms while their ocular signs were absent.
Our data have revealed a significant finding of interest, suggesting that ocular staining, especially conjunctival staining and additional corneal staining patterns, was more frequently intense in SjD participants compared with participants without SjD. These results align with previous findings that such intense ocular staining could be predictive of being classified as SjD.38,39 The underlying biological mechanisms driving the progression of ocular staining in DED have not been investigated thoroughly. However, it is hypothesized that conjunctival epithelial erosions in DED begins in the nasal area and spreads to the temporal area as the disease advances, given that tears flow from the lacrimal gland to the temporal side of the globe and then toward the nasal globe.40 Consequently, temporal conjunctival staining may be more prominent in SjD because of the severity of DED in SjD. The present study adds to the existing evidence and highlights the importance of using the OSS, as well as lissagmine green staining for conjunctiva, which is not used routinely in clinical practice, in the assessment of patients with DED to help discriminate those who are suspected of having SjD. Further, novel methods are warranted to differentiate SjD keratoconjunctivitis sicca from other forms of DED. Recent evidence suggests that patients with SjD may exhibit lower corneal nerve metrics and small fiber neuropathies under in vivo confocal microscopy as compared with healthy controls, making small fiber neuropathy a potential biomarker for classifying SjD.41
It is worth noting that enrolling participants who may have already had a prior diagnosis of SjD (using a prior Sjögren's classification system) or had symptoms or signs compatible with SjD, for example, dry eye, might have created the potential selection bias in our study, because SjD and dry eye both were related to participant enrollment. However, those classified as non-SjD in the SICCA cohort are not healthy controls. Therefore, it is possible that, if compared with healthy controls (which were not recruited into SICCA), the odds of the complications we identified may actually be even higher. Moreover, we are unable to explore the causal association given the cross-sectional study design. Finally, residual confounding variables and unknown causative factors such as comorbidities, systemic medications, and lifestyle and dietary approaches may further influence our results. Nevertheless, we were able to leverage the extensive data from the SICCA cohort to examine the pattern of ocular symptoms, signs, and pathology by SjD status across a diverse geographical area and age range, which provides evidence and insights for generating hypotheses and identifying areas for future research.
Conclusions
In our study, SjD participants demonstrate more vision-threatening features of the anterior segment compared with those without SjD while DED symptoms remain similar between SjD groups. This study highlights the importance of close ophthalmological follow-up for individuals who are suspected of having SjD and the need for aggressive interventions to avert the deleterious sequela of DED. Furthermore, ocular staining could serve as a potential biomarker for classifying SjD during ophthalmologic clinics.
Supplementary Material
Acknowledgments
The authors gratefully thank the SICCA study participants, and members of the SICCA team who contributed to data collection.
The International Sjögren's Syndrome Biorepository and Data Registry was previously funded by the US PHS/NIH, the National Institute of Dental and Craniofacial Research, the National Eye Institute, and the NIH Office for Research on Women's Health (contract HHSN268201300057C) and is currently funded by NIH National Institute of Dental and Craniofacial Research grant U01-DE-028891. JAG was supported by the National Eye Institute of the National Institutes of Health under award K23EY026998. This work was supported in part by an unrestricted grant from the Research to Prevent Blindness. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Disclosure: F. Xiong, None; D. Pula, None; E.K. Akpek, None; V.Y. Bunya, None; C.H. Shiboski, None; T.L. Lietman, None; J.A. Gonzales, Dompé (C)
References
- 1. Fox RI. Sjögren's syndrome. Lancet. 2005; 366(9482): 321–331. [DOI] [PubMed] [Google Scholar]
- 2. Baer AN, Hammitt KM.. Sjögren's disease, not syndrome. Arthritis Rheumatol. 2021; 73(7): 1347–1348. [DOI] [PubMed] [Google Scholar]
- 3. Singh S, Das AV, Basu S.. Ocular involvement in Sjögren syndrome: risk factors for severe visual impairment and vision-threatening corneal complications. Am J Ophthalmol. 2021; 225: 11–17. [DOI] [PubMed] [Google Scholar]
- 4. Brito-Zerón P, Retamozo S, Ramos-Casals M.. Phenotyping Sjögren's syndrome: towards a personalised management of the disease. Clin Exp Rheumatol. 2018; 36(Suppl 112(3)): 198–209. [PubMed] [Google Scholar]
- 5. Gonzales JA, Shiboski SC, Bunya VY, et al.. Ocular clinical signs and diagnostic tests most compatible with keratoconjunctivitis sicca: a latent class approach. Cornea. 2020; 39(8): 1013–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiboski CH, Shiboski SC, Seror R, et al.. 2016 ACR-EULAR classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017; 69(1): 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whitcher JP, Shiboski CH, Shiboski SC, et al.. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren's Syndrome International Registry. Am J Ophthalmol. 2010; 149(3): 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shiboski CH, Shiboski SC, Seror R, et al.. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2017; 69(1): 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akpek EK, Bunya VY, Saldanha IJ.. Sjögren's syndrome: more than just dry eye. Cornea. 2019; 38(5): 658–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzales JA, Chou A, Rose-Nussbaumer JR, et al.. How are ocular signs and symptoms of dry eye associated with depression in women with and without Sjögren syndrome? Am J Ophthalmol. 2018; 191: 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nortey J, Shiboski C, Rose-Nussbaumer J, Bunya VY, Lietman T, Gonzales JA.. How are sicca signs and symptoms associated with depression among men classified with and without Sjögren disease? Am J Ophthalmol. 2023; 247: 96–102. [DOI] [PubMed] [Google Scholar]
- 12. Akpek EK, Mathews P, Hahn S, et al.. Ocular and systemic morbidity in a longitudinal cohort of Sjögren's syndrome. Ophthalmology. 2015; 122(1): 56–61. [DOI] [PubMed] [Google Scholar]
- 13. Murtagh P, Comer R, Fahy G.. Corneal perforation in undiagnosed Sjögren's syndrome following topical NSAID and steroid drops post routine cataract extraction. Case Reports. 2018; 2018: bcr2018225428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vivino FB, Minerva P, Huang CH, Orlin SE.. Corneal melt as the initial presentation of primary Sjögren's syndrome. J Rheumatol. 2001; 28(2): 379–382. [PubMed] [Google Scholar]
- 15. Bridges AJ, Burns RP.. Acute iritis associated with primary Sjögren's syndrome and high-titer anti-SS-A/Ro and anti-SS-B/La antibodies. Treatment with combination immunosuppressive therapy. Arthritis Rheum. 1992; 35(5): 560–563. [DOI] [PubMed] [Google Scholar]
- 16. Ahmadi-Simab K, Lamprecht P, Nölle B, Ai M, Gross WL. Successful treatment of refractory anterior scleritis in primary Sjogren's syndrome with rituximab. Ann Rheum Dis. 2005; 64(7): 1087–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi W, Lee SS, Park YG, Yoon KC.. A case of necrotizing keratoscleritis in primary Sjogren's syndrome. Korean J Ophthalmol. 2011; 25(4): 275–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonzales JA, Lietman TM.. Ocular involvement in Sjögren's syndrome: advances in therapy. Curr Treat Options in Rheum. 2018; 4(1): 99–109. [Google Scholar]
- 19. Shiboski S, Shiboski C, Criswell L, et al.. American College of Rheumatology classification criteria for Sjögren's syndrome: a data-driven, expert consensus approach in the SICCA Cohort. Arthritis Care Res (Hoboken). 2012; 64(4): 475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pult H, Wolffsohn JS.. The development and evaluation of the new Ocular Surface Disease Index-6. Ocul Surf. 2019; 17(4): 817–821. [DOI] [PubMed] [Google Scholar]
- 21. Roszkowska AM, Oliverio GW, Aragona E, et al.. Ophthalmologic manifestations of primary Sjögren's syndrome. Genes (Basel). 2021; 12(3): 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Complex case of dry eye management associated with Sjogren's syndrome: a teaching case report | The Journal of Optometric Education. Available at: https://journal.opted.org/article/complex-case-of-dry-eye-management-associated-with-sjogrens-syndrome-a-teaching-case-report/. Accessed June 5, 2023.
- 23. Deswal J, Arya SK, Raj A, Bhatti A.. A case of bilateral corneal perforation in a patient with severe dry eye. J Clin Diagn Res. 2017; 11(4): ND01–ND02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pfister RR, Murphy GE.. Corneal ulceration and perforation associated with Sjögren's syndrome. Archives of Ophthalmology. 1980; 98(1): 89–94. [DOI] [PubMed] [Google Scholar]
- 25. de Paiva CS, Trujillo-Vargas CM, Schaefer L, Yu Z, Britton RA, Pflugfelder SC.. Differentially expressed gene pathways in the conjunctiva of Sjögren syndrome keratoconjunctivitis sicca. Front Immunol. 2021; 12: 702755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim YC, Ham B, Kang KD, et al.. Bacterial distribution on the ocular surface of patients with primary Sjögren's syndrome. Sci Rep. 2022; 12: 1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shumway C, Aggarwal S, Park ST, Wade M, Kedhar S.. Complicated case of Mycobacterium abscessus conjunctivitis in Sjögren's syndrome. Am J Ophthalmol Case Rep. 2020; 19: 100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Albietz J, Sanfilippo P, Troutbeck R, Lenton LM.. Management of filamentary keratitis associated with aqueous-deficient dry eye. Optom Vis Sci. 2003; 80(6): 420–430. [DOI] [PubMed] [Google Scholar]
- 29. Kowalik BM, Rakes JA.. Filamentary keratitis–the clinical challenges. J Am Optom Assoc. 1991; 62(3): 200–204. [PubMed] [Google Scholar]
- 30. Gire A, Mathews P, Cui D, Akpek EK.. Long-term Corneal complications in patients with primary Sjögren's syndrome. Invest Ophthalmol Vis Sci. 2020; 61(7): 339. [Google Scholar]
- 31. Cui D, Mathews P, Li G, VanCourt S, Akpek E.. Outcomes of Sjögren's versus non-Sjögren's related dry eye in a longitudinal, tertiary clinic-based sample. PLoS One. 2021; 16(12): e0261241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Afsharkhamseh N, Movahedan A, Motahari H, Djalilian AR.. Cataract surgery in patients with ocular surface disease: an update in clinical diagnosis and treatment. Saudi J Ophthalmol. 2014; 28(3): 164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ong ES, Felix ER, Levitt RC, Feuer WJ, Sarantopoulos CD, Galor A.. Epidemiology of discordance between symptoms and signs of dry eye. Br J Ophthalmol. 2018; 102(5): 674–679. [DOI] [PubMed] [Google Scholar]
- 34. Li F, Zhang Q, Ying X, et al.. Corneal nerve structure in patients with primary Sjögren's syndrome in China. BMC Ophthalmol. 2021; 21: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tuominen ISJ, Konttinen YT, Vesaluoma MH, Moilanen JAO, Helintö M, Tervo TMT.. Corneal innervation and morphology in primary Sjögren's syndrome. Invest Ophthalmol Vis Sci. 2003; 44(6): 2545–2549. [DOI] [PubMed] [Google Scholar]
- 36. Vehof J, Sillevis Smitt-Kamminga N, Nibourg SA, Hammond CJ. Predictors of discordance between symptoms and signs in dry eye disease. Ophthalmology. 2017; 124(3): 280–286. [DOI] [PubMed] [Google Scholar]
- 37. Westreich D. Berkson's bias, selection bias, and missing data. Epidemiology. 2012; 23(1): 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rasmussen A, Stone DU, Kaufman CE, et al.. Reproducibility of ocular surface staining in the assessment of Sjögren syndrome–related keratoconjunctivitis sicca: implications on disease classification. ACR Open Rheumatol. 2019; 1(5): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caffery B, Simpson T, Wang S, et al.. Rose Bengal staining of the temporal conjunctiva differentiates Sjögren's syndrome from keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2010; 51(5): 2381–2387. [DOI] [PubMed] [Google Scholar]
- 40. Rolando M, Barabino S, Mingari C, Moretti S, Giuffrida S, Calabria G.. Distribution of conjunctival HLA-DR expression and the pathogenesis of damage in early dry eyes. Cornea. 2005; 24(8): 951. [DOI] [PubMed] [Google Scholar]
- 41. Nortey J, Tsang A, Lopez S, et al.. Central corneal subbasal nerve plexus abnormalities in Sjögren disease: a pilot study. Cornea. 2023. Published online February 7, 2023, doi: 10.1097/ICO.0000000000003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.