Key Points
Question
Which pathways mediate the inequity in pregnancy health associated with low educational attainment?
Findings
In this cohort study of more than 3 million individuals, an association between genetically estimated lower educational attainment and increased risk of ectopic pregnancy, hyperemesis gravidarum, gestational diabetes, preeclampsia, preterm birth, and offspring low birth weight was observed. A sizeable portion of these associations were explained by targetable risk factors.
Meaning
These findings suggest that the association of socioeconomic inequalities with adverse pregnancy outcomes may be reduced by intervening for type 2 diabetes, body mass index, smoking, high-density lipoprotein cholesterol level, and systolic blood pressure.
This cohort study evaluates the mediating pathways underlying the association between educational attainment and pregnancy complications and including ectopic pregnancy, hyperemesis gravidarum, gestational diabetes, preeclampsia, preterm birth, and offspring birth weight.
Abstract
Importance
Lower educational attainment is associated with increased risk of adverse pregnancy outcomes, but it is unclear which pathways mediate this association.
Objective
To investigate the association between educational attainment and pregnancy outcomes and the proportion of this association that is mediated through modifiable cardiometabolic risk factors.
Design, Setting, and Participants
In this 2-sample mendelian randomization (MR) cohort study, uncorrelated (R2 < 0.01) single-nucleotide variants (formerly single-nucleotide polymorphisms) associated with the exposure (P < 5 × 10−8) and mediators and genetic associations with the pregnancy outcomes from genome-wide association studies were extracted. All participants were of European ancestry and were largely from Finland, Iceland, the United Kingdom, or the US. The inverse variance–weighted method was used in the main analysis, and the weighted median, weighted mode, and MR Egger regression were used in sensitivity analyses. In mediation analyses, the direct effect of educational attainment estimated in multivariable MR was compared with the total effect estimated in the main univariable MR analysis. Data were extracted between December 1, 2022, and April 30, 2023.
Exposure
Genetically estimated educational attainment. The mediators considered were genetically estimated type 2 diabetes, body mass index, smoking, high-density lipoprotein cholesterol level, and systolic blood pressure.
Main Outcomes and Measures
Ectopic pregnancy, hyperemesis gravidarum, gestational diabetes, preeclampsia, preterm birth, and offspring birth weight.
Results
The analyses included 3 037 499 individuals with data on educational attainment, and those included in studies on pregnancy outcomes ranged from 141 014 for ectopic pregnancy to 270 002 with data on offspring birth weight. Each SD increase in genetically estimated educational attainment (ie, 3.4 years) was associated with an increased birth weight of 42 (95% CI, 28-56) g and an odds ratio ranging from 0.53 (95% CI, 0.46-0.60) for ectopic pregnancy to 0.81 (95% CI, 0.71-0.93) for preeclampsia. The combined proportion of the association that was mediated by the 5 cardiometabolic risk factors ranged from −17% (95% CI, −46% to 26%) for hyperemesis gravidarum to 78% (95% CI, 10%-208%) for preeclampsia. Sensitivity analyses accounting for pleiotropy were consistent with the main analyses.
Conclusions and Relevance
In this MR cohort study, intervening for type 2 diabetes, body mass index, smoking, high-density lipoprotein cholesterol level, and systolic blood pressure may lead to reductions in several adverse pregnancy outcomes associated with lower levels of education. Such public health interventions would serve to reduce health disparities attributable to social inequalities.
Introduction
Socioeconomic factors—educational attainment in particular—are associated with adverse pregnancy outcomes.1,2,3,4,5 However, it is challenging to modify an individual’s level of education, and opportunities to seek education are not equally distributed throughout the population. It is therefore of great importance to identify modifiable risk factors through which the association with educational attainment is mediated.6
Conventional observational studies have identified some potential mediating pathways, and cardiometabolic risk factors stand out.1 For instance, prepregnancy body mass index (BMI) and systolic blood pressure (SBP) have been observed to explain most of the association between educational level and gestational hypertension and preeclampsia.2,3 Smoking has been observed to likely mediate some of the potential association of educational level with preterm birth7 and low birth weight,4 but not with the risk of preeclampsia.2 For preterm birth, most of the likely association with educational attainment remains unexplained.1 For yet other pregnancy outcomes, such as ectopic pregnancy, hyperemesis gravidarum, and gestational diabetes, the role of any mediating pathways downstream of educational attainment remains largely unknown.5,8,9 Thus, there is a need for a systematic evaluation of targetable risk factors that may help reduce socioeconomic inequalities in pregnancy outcomes.
There are 2 other important limitations in the literature on educational attainment and pregnancy outcomes. First, many studies lack adjustment for key confounders,1 and residual confounding may have biased the results.10 Second, mediation analyses in traditional observational studies are susceptible to measurement error, such as day-to-day variations of a mediator, which in turn underestimates the mediating effect.11
Mendelian randomization (MR) studies use genetic variants as instruments to evaluate the association between an exposure and an outcome. Because genetic variants are allocated at random and are not influenced by lifestyle factors and chronic conditions, MR studies are robust to bias by both measured and unmeasured confounders. Furthermore, because genetic variants serve as proxies for the long-term effect of an exposure or mediator, MR studies are generally robust to nondifferential measurement error.12
We aimed to conduct the first MR study, to our knowledge, to evaluate the mediating pathways underlying the association between educational attainment and pregnancy complications. Specifically, we focused on 6 pregnancy complications and outcomes that are common and/or severe: ectopic pregnancy, hyperemesis gravidarum, gestational diabetes, preeclampsia, preterm birth, and offspring birth weight. For the mediating factors, we followed the statement by the American Heart Association on optimizing pregnancy health13 and investigated the mediating role of type 2 diabetes (T2D), BMI, smoking, high-density lipoprotein cholesterol (HDL-C) level, and SBP.
Methods
Study Design
In this 2-sample MR cohort study, we used publicly available, summary-level data with relevant ethical approvals, which did not require institutional review board approval or informed consent. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for MR.14 In a 2-sample MR study, summary level data from genome-wide association studies (GWAS) were used to find genetic proxies of an exposure and investigate the associations of these proxies with an outcome (detailed in the eMethods and eFigure in Supplement 1).10 Data were extracted between December 1, 2022, and April 30, 2023.
Instrumental Variable Selection for Educational Attainment
Genetic instruments for educational level were extracted from a GWAS by Okbay et al15 (Table), the largest GWAS on educational attainment at the time of analysis. In that GWAS, years of education were standardized in the participating cohorts by mapping the highest level of education to an International Standard Classification of Education 1997 category. The mean (SD) level of education was 15.4 (3.4) years. We extracted single-nucleotide variants (SNVs; formerly SNPs) that were strongly associated with educational attainment, defined as P < 5 ×10−8, and that were independent of one another, defined as a pairwise R2 < 0.01 based on the 1000 Genomes Project European ancestry superpopulation.
Table. GWAS Used as Sources for 2-Sample Mendelian Randomization Analyses.
| Trait | Source | Setting | Country | Phenotype definition | No. of participants or No. of cases/controls |
|---|---|---|---|---|---|
| Exposure | |||||
| Educational level | Okbay et al,15 2022 | 71 Cohorts, including UK Biobank and 23andMe | Many, including UK and US | Self-reported years of education | 3 037 499 |
| Mediators | |||||
| T2D | Mahajan et al,16 2018 | 32 Cohorts, including deCODE and UK Biobank | Many, including Iceland and UK | Cases: T2D status based on a combination of diagnostic testing (fasting glucose or HbA1c level), recorded diagnosis codes, or self-report; controls: not diagnosed with T2D | 74 124/824 006 |
| BMI | Pulit et al,17 2019 | 30 Cohorts, including UK Biobank | Many, including UK | Measured BMI at study participation | 806 834 |
| Lifetime smoking score | Wootton et al,18 2020 | UK Biobank | UK | Self-reported smoking behavior, lifetime smoking index constructed to reflect smoking status (ever vs never), and smoking intensity among ever smokers | 462 690 |
| HDL-C level | Graham et al,19 2021 | 146 Cohorts, including deCODE, Million Veteran Program, and UK Biobank | Many, including Iceland, UK, and US | Measured blood levels of HDL-C level | 1 244 580 |
| SBP | Neale Laboratories, release 28 | UK Biobank | UK | Automated reading of SBP | 340 159 |
| Outcomes | |||||
| Ectopic pregnancy | FinnGen, release 820 | FinnGen | Finland | Cases: ICD-8 code 631, ICD-9 code 633, or ICD-10 code O00; controls: women without mentioned ICD codes | 5052/135 962 |
| Hyperemesis gravidarum | FinnGen, release 820 | FinnGen | Finland | Cases: ICD-8 code 638, ICD-9 code 643, or ICD-10 code O21; controls: women without mentioned ICD codes | 2092/163 702 |
| Gestational diabetes | FinnGen, release 820 | FinnGen | Finland | Cases: ICD-9 code 6488A or ICD-10 code O24.4; controls: women without mentioned ICD codes | 11 279/179 600 |
| Preeclampsia | Steinthorsdottir et al,21 2020 | 6 Cohorts, including deCODE | Many, including Iceland | Cases: maternal, varied by cohort (GOPEC, ALSPAC, and MOBA, pregnancy-onset hypertension and proteinuria; deCODE, ICD-10 codes O13, O14 or O15; SSI, ICD-8 code 637.04 and ICD-10 codes 014.1, O14.2 and O15; and FINRISK, ICD-8 codes 637.03, 637.04, 637.09, 637.10, 637.99, ICD-9 codes 6424-6427A, and ICD-10 codes O14.0, O14.1, O14.9, O15.0, O15.1, O15.2, and O15.9); controls: nonpreeclampsia pregnancies, except GOPEC and deCODE, which used women without preeclampsia | 7219/155 660 |
| Preterm birth | Solé-Navais et al,22 2023 | 18 Cohorts, including deCODE and UK Biobank | Many, including Iceland and UK | Singleton live birth with spontaneous onset; cases: delivery <259 d or ICD-10 code O60; controls: delivery between 273 to 294 d | 15 419/217 871 |
| Birth weight | Juliusdottir et al,23 2021 | 23 Cohorts, including deCODE and UK Biobank | Many, including Iceland and UK | Maternal GWAS of offspring birth weight | 270 002 |
Abbreviations: ALSPAC, Avon Longitudinal Study of Parents and Children; BMI, body mass index; GOPEC, UK Genetics of Pre-eclampsia study; GWAS, genome-wide association study; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; ICD-8, International Classification of Diseases, Eighth Revision; ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; MOBA, Norwegian Mother and Child Cohort Study; SBP, systolic blood pressure; SSI, Statens Serum Institut; T2D, type 2 diabetes; UK, United Kingdom.
Outcomes
Genetic associations for 3 of the outcomes—ectopic pregnancy, hyperemesis gravidarum, and gestational diabetes—were extracted from a database of publicly available GWAS summary statistics from FinnGen (Table).20 For preeclampsia, we used data from a study by Steinthorsdottir et al21; for preterm birth, a study by Solé-Navais et al22; and for offspring birth weight, a study by Juliusdottir et al.23 All pregnancy outcomes were binary, except for birth weight, which was analyzed as a continuous outcome.
Mediators
We chose 5 cardiometabolic traits from the list of targetable risk factors to optimize pregnancy health according to the American Heart Association’s 2023 scientific statement.13 Single-nucleotide variant effects on the risk of T2D were extracted from a study by Mahajan et al16; on BMI, from a study by Pulit et al17; and on HDL-C level, from a study by Graham et al.19 For smoking behavior, we used a GWAS by Wootton et al18 on a lifetime smoking index that reflects a combination of smoking status (ever vs never) and duration, heaviness, and cessation. Finally, we used GWAS results from UK Biobank created by the Neale Laboratory (release number 2)24 that evaluated SNVs without adjustments for BMI. Type 2 diabetes was the only binary mediator, while the others were continuous (Table).
Statistical Analysis
To address the aims of this study, we sought to evaluate the following measures: (1) the association of educational attainment with each pregnancy outcome, (2) the association of educational attainment with each cardiometabolic risk factor, (3) the association of each cardiometabolic risk factor with each pregnancy outcome, and (4) the direct association of educational attainment with each pregnancy outcome after accounting for each cardiometabolic risk factor separately and combined. The first 3 measures were based on univariable MR analyses, while the fourth measure was based on multivariable MR analyses. Under the instrumental variable assumptions, the univariable MR estimate represents the total effect of the exposure, whereas the multivariable MR estimate represents the direct effect of the exposure (ie, the effect of intervening on the exposure but holding all mediators constant).
Prior to analyses, we harmonized the files to ensure that the effect estimate of a given SNV was oriented to the same allele in all files. The threshold for statistical significance was P < .05, and the tests were 2 sided. All analyses were run using R, version 4.2.0 (R Program for Statistical Computing) and the R packages MendelianRandomization, version 0.7.0, TwoSampleMR, version 0.5.6, and metaphor, version 3.4.0.
Univariable MR Analysis
For each association (eg, educational attainment and birth weight), we calculated the Wald ratio per SNV and used inverse variance–weighted (IVW) analysis to summarize the associations of all SNVs, which puts more emphasis on the estimates with the lowest variance.10 For the IVW estimate to be unbiased, however, all SNVs included in the analysis must be valid. There are 3 key assumptions that must be met for an instrument to be valid: It must be associated with the exposure; it cannot be associated with a confounder of the exposure-outcome association; and it is not associated with the outcome other than through the exposure (eFigure in Supplement 1).10 For all univariable MR analyses, we conducted 3 sensitivity analyses that provide unbiased estimates even in the presence of some invalid instruments: the weighted median, weighted mode, and MR Egger regression (eTable 1 in Supplement 1).10
Mediation Analysis
We calculated the direct association with educational attainment on each pregnancy outcome by conducting multivariable MR analyses with each of the 5 cardiometabolic mediators at a time, then with all mediators combined.10,25 The total association was provided by the aforementioned univariable MR analyses. To calculate the proportion mediated, we divided the direct by the total association and subtracted from 1. Finally, we estimated the SEs using bootstrapping.26 The mediation analyses were based on the estimates from the IVW analyses.
Results
Overall Association Among Genetically Estimated Educational Attainment, Cardiometabolic Mediators, and Pregnancy Outcomes
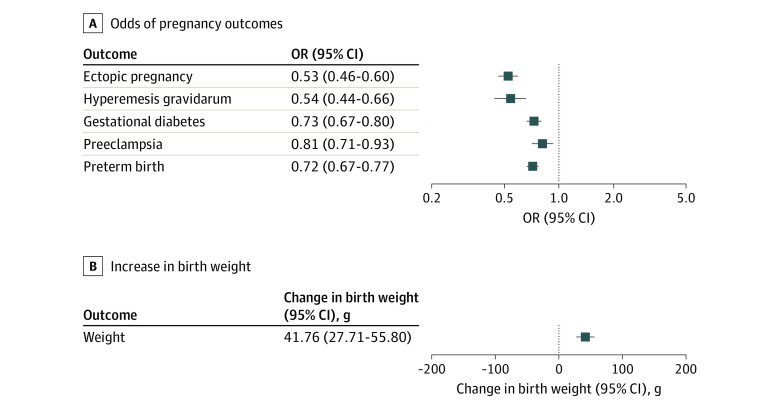
The GWAS on educational attainment evaluated 3 037 499 individuals (from 71 cohorts), and the number of individuals included in the studies on pregnancy outcomes ranged from 141 014 (ectopic pregnancy, from 1 cohort) to 270 002 (birth weight, from 23 cohorts). The genetic instruments for educational attainment explained 7.5% of its variance, with median F statistic for the individual SNVs of 49 (range, 28-576). There was a protective association of a higher level of genetically estimated educational attainment with all pregnancy outcomes (range of odds ratios [ORs], 0.53 [95% CI, 0.46-0.60] for ectopic pregnancy to 0.81 [95% CI, 0.71-0.93] for preeclampsia) and a positive association with increased offspring birth weight (42 [95% CI, 28-56] g) (Figure 1). These associations were robust in sensitivity analyses that evaluated potential bias due to genetic pleiotropy (eTable 2 in Supplement 1).
Figure 1. Associations Between Genetically Estimated Educational Attainment and Pregnancy Outcomes.
Results of inverse variance–weighted 2-sample mendelian randomization analyses are shown. Estimates are odds ratios (ORs) for pregnancy outcomes and grams of birth weight per 1-SD increase of genetically estimated years of education (3.4 years).
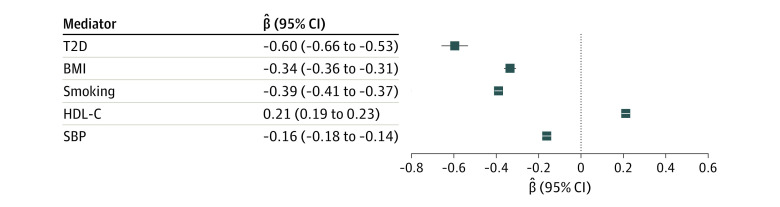
Educational attainment was also associated with each of the considered cardiometabolic mediators (β coefficient range, −0.06 [95% CI, −0.66 to −0.53] for T2D to 0.21 [95% CI, 0.19-0.23] for HDL-C level) (Figure 2). This finding was supported by the sensitivity analyses accounting for pleiotropy (eTable 3 in Supplement 1).
Figure 2. Associations Between Genetically Estimated Educational Attainment and Cardiometabolic Mediators.
Results of inverse variance–weighted 2-sample mendelian randomization analyses are shown. Estimates are the change in the mediator per 1-SD increase of genetically estimated years of education (3.4 years), and the mediators are in log(odds) units for type 2 diabetes (T2D) and in SD units for the other cardiometabolic risk factors. BMI indicates body mass index; HDL-C, high-density lipoprotein cholesterol level; and SBP, systolic blood pressure.
Association Between Genetically Estimated Cardiometabolic Mediators and Pregnancy Outcomes
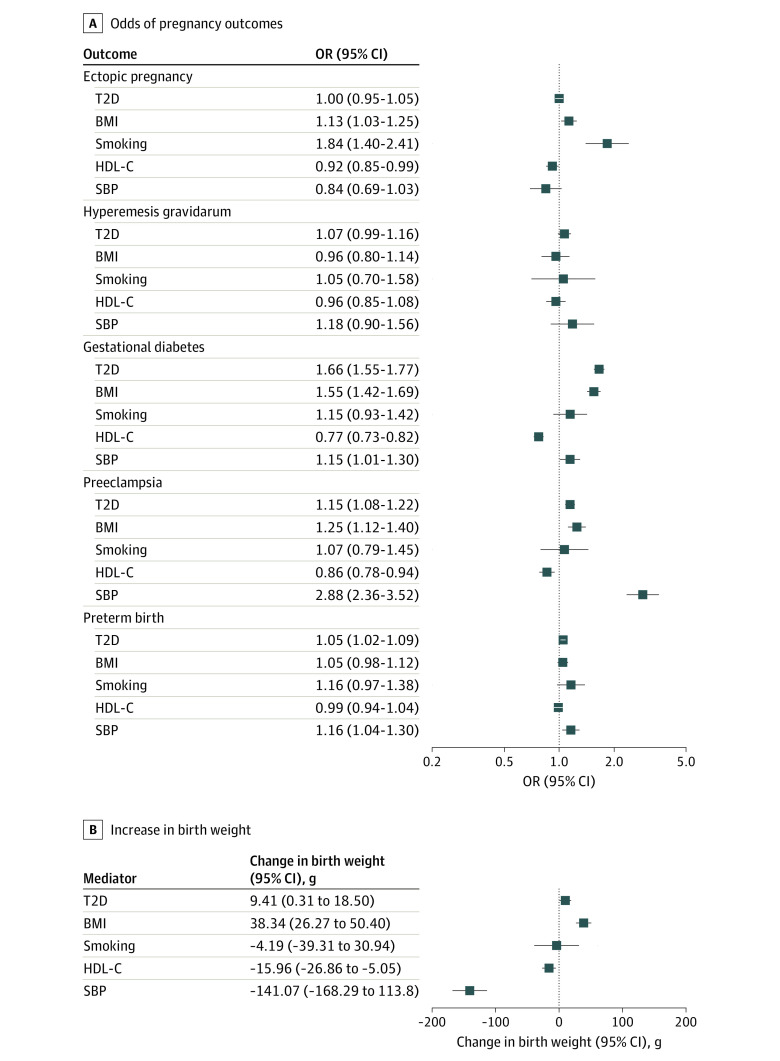
All genetically estimated cardiometabolic mediators were associated with at least 1 pregnancy outcome. The associations varied greatly between different outcomes (Figure 3). Genetically estimated higher T2D liability was associated with increased risk of gestational diabetes (OR, 1.66 [95% CI, 1.55-1.77]), preeclampsia (OR, 1.15 [95% CI, 1.06-1.22]), preterm birth (OR, 10.5 [95% CI, 1.02-1.09]), and greater birth weight (9.41 [95% CI, 0.31-18.50] g); genetically estimated higher BMI was associated with increased risk of ectopic pregnancy (OR, 1.13 [95% CI, 1.03-1.25]), gestational diabetes (OR, 1.55 [95% CI, 1.12-1.40]), preeclampsia (OR, 1.25 [95% CI, 1.12-1.40]), and greater birth weight (38.34 [95% CI, 26.27-50.40] g); genetically estimated smoking was associated with an increased risk of ectopic pregnancy (OR, 1.84 [95% CI, 1.40-2.41]); genetically estimated higher HDL-C level was associated with a reduced risk of ectopic pregnancy (OR, 0.92 [95% CI, 0.85-0.99]), gestational diabetes (OR, 0.77 [95% CI, 0.7300.82]), preeclampsia (OR, 0.86 [95% CI, 0.78-0.94]), and lower birth weight (−15.98 [95% CI, −26.86 to −5.50] g); and genetically estimated higher SBP was associated with increased risk of gestational diabetes (OR, 1.15 [95% CI, 1.01-1.30]), preeclampsia (OR, 2.88 [95% CI, 2.36-3.52]), preterm birth (OR, 1.16 [95% CI, 1.04-1.30]), and lower birth weight (OR, −141.07 [95% CI, −168.29 to 113.80] g). The sensitivity analyses accounting for pleiotropy supported these associations (eTables 4 to 8 in Supplement 1).
Figure 3. Associations Between Genetically Estimated Cardiometabolic Mediators and Pregnancy Outcomes.
Results of inverse variance–weighted 2-sample mendelian randomization analyses are shown. Estimates are odds ratios (ORs) for pregnancy outcomes and grams of birth weight per 1-unit increase of the genetically estimated log(odds) of type 2 diabetes (T2D) and 1-SD increase of the other genetically estimated cardiometabolic traits. BMI indicates body mass index; HDL-C, high-density lipoprotein cholesterol level; and SBP, systolic blood pressure.
Mediating Pathways Between Genetically Estimated Level of Education and Pregnancy Outcomes
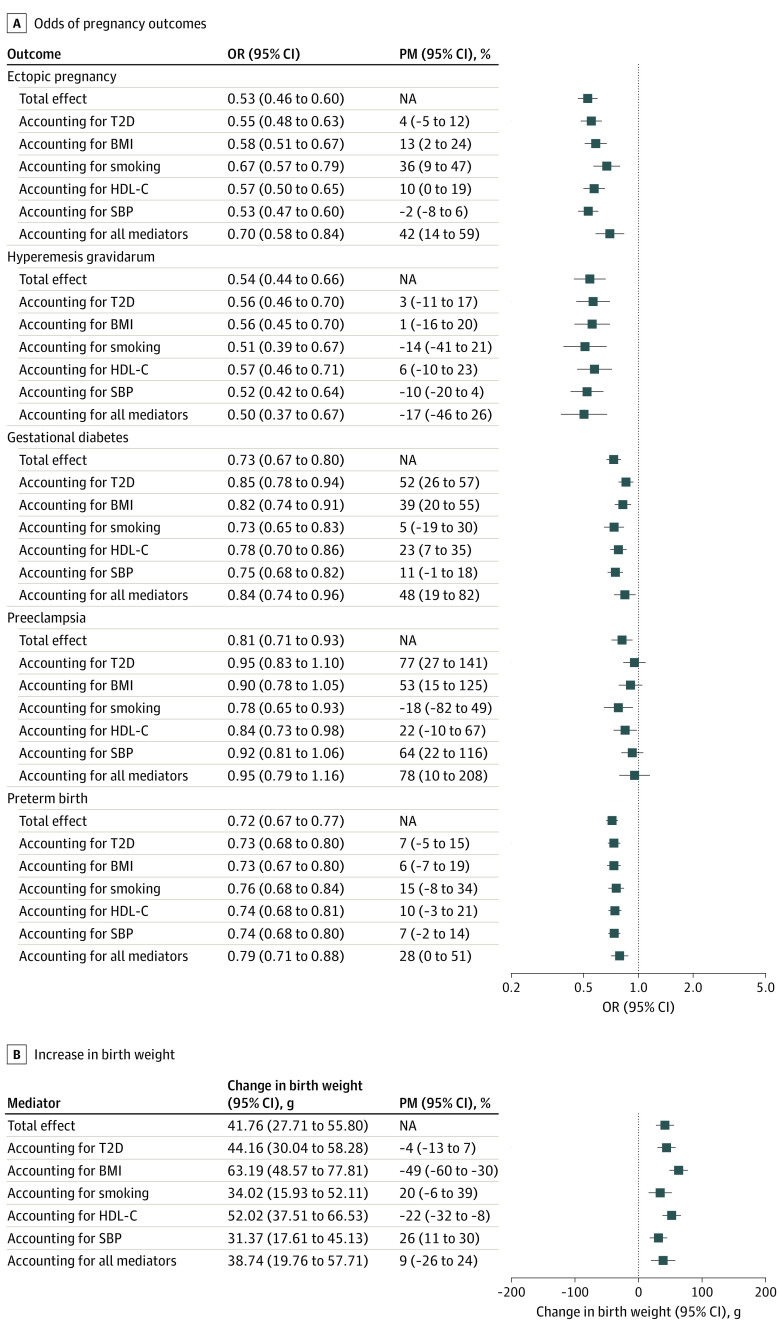
Figure 4 displays the univariable and multivariable MR estimates of educational attainment on each pregnancy outcome, representing the total and direct associations after accounting for each cardiometabolic mediator alone and all combined, as well as the attenuation in the multivariable MR estimate compared with the univariable MR estimate labeled as the proportion mediated. The attenuation in estimates on adjustment for cardiometabolic traits ranged from almost zero for the association between genetically estimated educational attainment and hyperemesis gravidarum (−17% [95% CI, −46% to 26%]) to most for the association with the risk of preeclampsia (78% [95% CI, 10%-208%]). The degree to which individual cardiometabolic factors attenuated the association between genetically estimated educational attainment and the pregnancy outcomes was largely determined by the association between genetically estimated educational attainment and the mediator and between the genetically estimated mediator and the outcome. For birth weight, the individual cardiometabolic mediators affected associations in competing directions. Thus, there was no net attenuation of the association between genetically estimated educational attainment and birth weight after accounting for all cardiometabolic mediators.
Figure 4. Associations Between Genetically Estimated Educational Attainment and Pregnancy Outcomes After Accounting for Cardiometabolic Mediators.
Results of mendelian randomization mediation analyses are shown. Estimates are odds ratios (ORs) for pregnancy outcomes and grams of birth weight per a 1-SD increase of genetically estimated years of education (3.4 years). BMI indicates body mass index; HDL-C, high-density lipoprotein cholesterol level; NA, not applicable; PM, proportion mediated; SBP, systolic blood pressure; and T2D, type 2 diabetes.
Discussion
In this 2-sample MR cohort study with mediation analyses, a high level of genetically estimated educational attainment was associated with a reduced risk of every pregnancy outcome assessed and with a higher offspring birth weight. For instance, every genetically estimated 3.4-year increase in education was associated with halved risks of ectopic pregnancy and hyperemesis gravidarum. While the cardiometabolic mediators accounted for one-half of the estimates for gestational diabetes and three-quarters of the estimates for preeclampsia, they did not account for any of the estimate for hyperemesis gravidarum.
Cardiometabolic Traits and Risk of Adverse Pregnancy Outcomes
In terms of the associations between cardiometabolic traits and pregnancy outcomes, our study generally supports the findings from previous MR studies but also evaluates several novel associations. In a previous MR study, Rogne et al27 observed that genetically estimated smoking behavior was associated with the risk of ectopic pregnancy, which was reproduced in the present study. For the other adverse pregnancy outcomes, however, there was no clear association with genetically estimated smoking behavior. While the onset of ectopic pregnancy is a few days after fertilization, the other pregnancy outcomes occur much later in pregnancy. We hypothesize that the null association of smoking with the later-onset pregnancy outcomes may in part be because the genetic associations for smoking behavior were derived from a nonpregnant population.28 For genetically estimated HDL-C level, this is the first MR study, to our knowledge, to indicate a potential protective effect on the risk of ectopic pregnancy, and our study supports previous MR studies29,30 that have found evidence of a protective association with the risk of gestational diabetes and preeclampsia. While a previous MR study observed no association between genetically estimated HDL-C level and offspring birth weight,31 we observed a negative association; the discrepancy may be explained by our study using updated genetic datasets evaluating many more individuals. We found a positive association between genetically estimated BMI and the risk of gestational diabetes, preeclampsia, and birth weight, as previously observed.29,30,32,33 We also found that high genetically estimated BMI was associated with an increased risk of ectopic pregnancy, an association that was not present in a previous MR study by Rogne et al27 due to smaller sample size. Our study supports previous MR studies reporting a positive association between genetically estimated SBP and risk of preeclampsia—expected due to shared etiology21—and lower birth weight32 and provides new data on a positive association with risk of gestational diabetes and risk of preterm birth. Last, we found that genetically estimated T2D liability was associated with an increased risk of gestational diabetes, preeclampsia, and higher offspring birth weight, as reported in previous MR studies,32,34 and a previously unreported association with increased risk of preterm birth.
Mediating Pathways Between Level of Education and Pregnancy Outcomes
To our knowledge, only 1 previous MR study33 has considered the association between educational attainment and a pregnancy outcome. That study found higher genetically estimated educational attainment to be associated with increased birth weight.33 While the investigators did not perform formal mediation analyses, they observed results comparable with those of the main analysis after conducting multivariable MR analyses, including BMI and alcohol consumption. This is in contrast to our finding of an association between genetically estimated educational attainment and birth weight after accounting for BMI. Traditional observational studies4,35 have reported that smoking mediates more than one-third of the association of educational attainment with offspring birth weight. This is more pronounced than what our findings suggest, which may be because our smoking variants were imperfect instruments for smoking during pregnancy, as discussed above. Our findings also support previous observations that BMI and smoking mediate in opposite directions, thereby masking socially differentiated healthy fetal growth.35
A recent systematic review1 summarized the literature of conventional observational studies on mediating pathways between socioeconomic status (including educational attainment) and risk of preterm birth. The individual studies included in the review had discrepant findings but generally reported mediating associations of smoking and BMI comparable with what we found (ie, fairly small mediated associations). When including T2D liability, HDL-C level, and SBP—which were not identified as potential mediators in the review—our analyses support that the 5 cardiometabolic traits combined may explain roughly one-quarter of the effect of genetically estimated educational attainment on the risk of preterm birth.
For preeclampsia and hypertensive disorders of pregnancy, previous studies2,3 have observed that the protective association of high educational attainment disappears after accounting for prepregnancy BMI or SBP, which is the same as what we find. Furthermore, smoking behavior has been observed to not mediate any of the association between educational attainment and risk of gestational hypertension,2 which is also supported by our findings. What has not previously been reported is our result suggesting that T2D liability may mediate most of the association of genetically estimated educational attainment on risk of preeclampsia.
While educational attainment has previously been observed to be linked to risk of gestational diabetes, little has been reported on potential mediating pathways.5 Not surprisingly, given their shared etiology,36 our findings support that most of the association of genetically estimated educational attainment may be mediated through T2D liability.
High genetically estimated level of education was associated with a reduced risk of hyperemesis gravidarum, similar to findings in a Norwegian register-based study.8 Interestingly, although this was one of the most pronounced associations observed in our study, the analyses suggest that it was not mediated by any of the 5 cardiometabolic traits. In other words, although educational attainment may have a strong effect on risk of hyperemesis gravidarum, this is likely due to factors other than the prenatal cardiometabolic profile.
Finally, while traditional observational studies have observed that high educational attainment is associated with a reduced risk of ectopic pregnancy,9 we are the first to show that almost half of this estimate may be explained by cardiometabolic risk factors, smoking in particular. The clinical and public health implications of our findings are described in the eAppendix in Supplement 1.
Strengths and Limitations
A major strength of our study is that we applied instrumental variable analyses using genetic instruments that allowed for assessment of the causal role of cardiometabolic mediators in the association between genetically estimated educational attainment and risk of several pregnancy outcomes. Due to the random allocation of alleles and because these alleles are static throughout an individual’s life, this design is much less likely to be affected by nondifferential measurement error of the mediator and confounding compared with traditional observational studies.10,11 This was further supported by our sensitivity analyses that did not indicate any bias due to pleiotropy. Thus, the association between genetically estimated educational attainment and adverse pregnancy outcomes may be interpreted as an approximation of the unconfounded association between observed (ie, nongenetic) educational attainment and the same outcomes.
This study also has some limitations. A potential limitation is that the genetic associations of the mediators were collected from studies that evaluated nonpregnant populations. This may particularly affect behavioral risk factors such as smoking during pregnancy. A study using data from 2 pregnancy cohorts found that a polygenic risk score for smoking explained 1% to 3% of variance of smoking during pregnancy.28 While this is lower than the 4% explained variance in smoking among nonpregnant individuals observed in the GWAS from which the polygenic risk score was based,37 it is still clear that genetic instruments of smoking behavior from a nonpregnant population reflect some of the smoking behavior during pregnancy. To our knowledge, our study is the first MR study to evaluate mediating pathways between educational attainment and pregnancy outcomes and the first to evaluate most of the associations among educational attainment, the 5 cardiometabolic traits, and the 6 pregnancy outcomes. To avoid confounding due to population stratification, we evaluated the same genetic ancestry group across all traits.10 Consequentially, we strongly encourage studies evaluating other ancestry groups.
Conclusion
By using causal genetic epidemiological models, our MR cohort study results suggest that interventions aimed at reducing BMI and SBP, reducing T2D and smoking prevalence, and increasing HDL-C level prior to and during pregnancy would lead to reductions in adverse pregnancy outcomes attributable to lower educational attainment. Except for preeclampsia, most of the association of genetically estimated educational attainment with the pregnancy outcomes considered was mediated through other pathways than these cardiometabolic risk factors, which warrants future studies on additional targetable mediators.
eMethods. Study Design and Choice of Mediators
eAppendix. Clinical and Public Health Implications
eTable 1. Overview of Analyses
eTable 2. Univariable Mendelian Randomization Analyses of Educational Attainment on Pregnancy Outcomes
eTable 3. Univariable Mendelian Randomization Analyses of Educational Attainment on Cardiometabolic Mediators
eTable 4. Univariable Mendelian Randomization Analyses of Type 2 Diabetes on Pregnancy Outcomes
eTable 5. Univariable Mendelian Randomization Analyses of Body Mass Index on Pregnancy Outcomes
eTable 6. Univariable Mendelian Randomization Analyses of Smoking on Pregnancy Outcomes
eTable 7. Univariable Mendelian Randomization Analyses of High-Density Lipoprotein Cholesterol Level on Pregnancy Outcomes
eTable 8. Univariable Mendelian Randomization Analyses of Systolic Blood Pressure on Pregnancy Outcomes
eFigure. Schematic Presentation of the Mendelian Randomization Design
eReferences
Data Sharing Statement
References
- 1.McHale P, Maudsley G, Pennington A, et al. Mediators of socioeconomic inequalities in preterm birth: a systematic review. BMC Public Health. 2022;22(1):1134. doi: 10.1186/s12889-022-13438-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jwa SC, Fujiwara T, Hata A, Arata N, Sago H, Ohya Y. BMI mediates the association between low educational level and higher blood pressure during pregnancy in Japan. BMC Public Health. 2013;13(1):389. doi: 10.1186/1471-2458-13-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva L, Coolman M, Steegers E, et al. Maternal educational level and risk of gestational hypertension: the Generation R Study. J Hum Hypertens. 2008;22(7):483-492. doi: 10.1038/jhh.2008.22 [DOI] [PubMed] [Google Scholar]
- 4.Nakamura A, Pryor L, Ballon M, et al. Maternal education and offspring birth weight for gestational age: the mediating effect of smoking during pregnancy. Eur J Public Health. 2020;30(5):1001-1006. doi: 10.1093/eurpub/ckaa076 [DOI] [PubMed] [Google Scholar]
- 5.Wang JW, Wang Q, Wang XQ, Wang M, Cao SS, Wang JN. Association between maternal education level and gestational diabetes mellitus: a meta-analysis. J Matern Fetal Neonatal Med. 2021;34(4):580-587. doi: 10.1080/14767058.2019.1611773 [DOI] [PubMed] [Google Scholar]
- 6.Cantarutti A, Franchi M, Monzio Compagnoni M, Merlino L, Corrao G. Mother’s education and the risk of several neonatal outcomes: an evidence [sic] from an Italian population-based study. BMC Pregnancy Childbirth. 2017;17(1):221. doi: 10.1186/s12884-017-1418-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulsen G, Andersen AN, Jaddoe VWV, et al. Does smoking during pregnancy mediate educational disparities in preterm delivery? findings from three large birth cohorts. Paediatr Perinat Epidemiol. 2019;33(2):164-171. doi: 10.1111/ppe.12544 [DOI] [PubMed] [Google Scholar]
- 8.Vikanes A, Grjibovski AM, Vangen S, Magnus P. Variations in prevalence of hyperemesis gravidarum by country of birth: a study of 900 074 pregnancies in Norway, 1967-2005. Scand J Public Health. 2008;36(2):135-142. doi: 10.1177/1403494807085189 [DOI] [PubMed] [Google Scholar]
- 9.Li C, Zhao WH, Zhu Q, et al. Risk factors for ectopic pregnancy: a multi-center case-control study. BMC Pregnancy Childbirth. 2015;15(1):187. doi: 10.1186/s12884-015-0613-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanderson E, Glymour MM, Holmes MV, et al. Mendelian randomization. Nat Rev Methods Primers. 2022;2(1):6. doi: 10.1038/s43586-021-00092-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blakely T, McKenzie S, Carter K. Misclassification of the mediator matters when estimating indirect effects. J Epidemiol Community Health. 2013;67(5):458-466. doi: 10.1136/jech-2012-201813 [DOI] [PubMed] [Google Scholar]
- 12.Relton CL, Davey Smith G. Two-step epigenetic mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol. 2012;41(1):161-176. doi: 10.1093/ije/dyr233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan SS, Brewer LC, Canobbio MM, et al. ; American Heart Association Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; Council on Lifestyle and Cardiometabolic Health; Council on Peripheral Vascular Disease; Stroke Council . Optimizing Prepregnancy cardiovascular health to improve outcomes in pregnant and postpartum individuals and offspring: a scientific statement from the American Heart Association. Circulation. 2023;147(7):e76-e91. doi: 10.1161/CIR.0000000000001124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the Reporting of Observational Studies in Epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375(2):n2233. doi: 10.1136/bmj.n2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okbay A, Wu Y, Wang N, et al. ; 23andMe Research Team; Social Science Genetic Association Consortium . Polygenic prediction of educational attainment within and between families from genome-wide association analyses in 3 million individuals. Nat Genet. 2022;54(4):437-449. doi: 10.1038/s41588-022-01016-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahajan A, Taliun D, Thurner M, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505-1513. doi: 10.1038/s41588-018-0241-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulit SL, Stoneman C, Morris AP, et al. ; GIANT Consortium . Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet. 2019;28(1):166-174. doi: 10.1093/hmg/ddy327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wootton RE, Richmond RC, Stuijfzand BG, et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med. 2020;50(14):2435-2443. doi: 10.1017/S0033291719002678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham SE, Clarke SL, Wu KHH, et al. ; VA Million Veteran Program; Global Lipids Genetics Consortium . The power of genetic diversity in genome-wide association studies of lipids. Nature. 2021;600(7890):675-679. doi: 10.1038/s41586-021-04064-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurki MI, Karjalainen J, Palta P, et al. ; FinnGen . FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508-518. doi: 10.1038/s41586-022-05473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinthorsdottir V, McGinnis R, Williams NO, et al. ; FINNPEC Consortium; GOPEC Consortium . Genetic predisposition to hypertension is associated with preeclampsia in European and Central Asian women. Nat Commun. 2020;11(1):5976. doi: 10.1038/s41467-020-19733-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solé-Navais P, Flatley C, Steinthorsdottir V, et al. ; Early Growth Genetics Consortium; Estonian Biobank Research Team; Danish Blood Donor Study Genomic Consortium . Genetic effects on the timing of parturition and links to fetal birth weight. Nat Genet. 2023;55(4):559-567. doi: 10.1038/s41588-023-01343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juliusdottir T, Steinthorsdottir V, Stefansdottir L, et al. Distinction between the effects of parental and fetal genomes on fetal growth. Nat Genet. 2021;53(8):1135-1142. doi: 10.1038/s41588-021-00896-x [DOI] [PubMed] [Google Scholar]
- 24.Biobank UK. Release No. 2. August 1, 2018. Accessed December 1, 2022. http://www.nealelab.is/uk-biobank/
- 25.Carter AR, Sanderson E, Hammerton G, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36(5):465-478. doi: 10.1007/s10654-021-00757-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hesterberg T. Bootstrap. Wiley Interdiscip Rev Comput Stat. 2011;3(6):497-526. doi: 10.1002/wics.182 [DOI] [Google Scholar]
- 27.Rogne T, Liew Z, Hernáez Á, Brumpton BM, Magnus MC. Modifiable risk factors for ectopic pregnancy: a Mendelian randomization study. Am J Obstet Gynecol. 2022;227(2):339-341.e4. doi: 10.1016/j.ajog.2022.03.063 [DOI] [PubMed] [Google Scholar]
- 28.Haan E, Sallis HM, Zuccolo L, et al. Prenatal smoking, alcohol and caffeine exposure and maternal-reported attention deficit hyperactivity disorder symptoms in childhood: triangulation of evidence using negative control and polygenic risk score analyses. Addiction. 2022;117(5):1458-1471. doi: 10.1111/add.15746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song X, Wang C, Wang T, Zhang S, Qin J. Obesity and risk of gestational diabetes mellitus: a two-sample mendelian randomization study. Diabetes Res Clin Pract. 2023;197(238):110561. doi: 10.1016/j.diabres.2023.110561 [DOI] [PubMed] [Google Scholar]
- 30.Hosier H, Lipkind HS, Rasheed H, DeWan AT, Rogne T. Dyslipidemia and risk of preeclampsia: a multiancestry mendelian randomization study. Hypertension. 2023;80(5):1067-1076. doi: 10.1161/HYPERTENSIONAHA.122.20426 [DOI] [PubMed] [Google Scholar]
- 31.Hwang LD, Lawlor DA, Freathy RM, Evans DM, Warrington NM. Using a two-sample mendelian randomization design to investigate a possible causal effect of maternal lipid concentrations on offspring birth weight. Int J Epidemiol. 2019;48(5):1457-1467. doi: 10.1093/ije/dyz160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ardissino M, Slob EAW, Millar O, et al. Maternal hypertension increases risk of preeclampsia and low fetal birthweight: genetic evidence from a mendelian randomization study. Hypertension. 2022;79(3):588-598. doi: 10.1161/HYPERTENSIONAHA.121.18617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Jin C, Ni LF, et al. Educational attainment and offspring birth weight: a bidirectional Mendelian randomization study. Front Genet. 2022;13(September):922382. doi: 10.3389/fgene.2022.922382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warrington NM, Beaumont RN, Horikoshi M, et al. ; EGG Consortium . Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat Genet. 2019;51(5):804-814. doi: 10.1038/s41588-019-0403-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballon M, Botton J, Forhan A, et al. Which modifiable prenatal factors mediate the relation between socio-economic position and a child’s weight and length at birth? Matern Child Nutr. 2019;15(4):e12878. doi: 10.1111/mcn.12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pervjakova N, Moen GH, Borges MC, et al. Multi-ancestry genome-wide association study of gestational diabetes mellitus highlights genetic links with type 2 diabetes. Hum Mol Genet. 2022;31(19):3377-3391. doi: 10.1093/hmg/ddac050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu M, Jiang Y, Wedow R, et al. ; 23andMe Research Team; HUNT All-In Psychiatry . Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237-244. doi: 10.1038/s41588-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Study Design and Choice of Mediators
eAppendix. Clinical and Public Health Implications
eTable 1. Overview of Analyses
eTable 2. Univariable Mendelian Randomization Analyses of Educational Attainment on Pregnancy Outcomes
eTable 3. Univariable Mendelian Randomization Analyses of Educational Attainment on Cardiometabolic Mediators
eTable 4. Univariable Mendelian Randomization Analyses of Type 2 Diabetes on Pregnancy Outcomes
eTable 5. Univariable Mendelian Randomization Analyses of Body Mass Index on Pregnancy Outcomes
eTable 6. Univariable Mendelian Randomization Analyses of Smoking on Pregnancy Outcomes
eTable 7. Univariable Mendelian Randomization Analyses of High-Density Lipoprotein Cholesterol Level on Pregnancy Outcomes
eTable 8. Univariable Mendelian Randomization Analyses of Systolic Blood Pressure on Pregnancy Outcomes
eFigure. Schematic Presentation of the Mendelian Randomization Design
eReferences
Data Sharing Statement