Induction of antibodies (Abs) to infectious agents requires the help of many different genes involved in selection and regulation processes. It is thus unlikely that a direct development of conventional humoral immunity has occurred in the absence of preliminary simple immune mechanisms. The germinal structure of the polyreactive Ab system is in agreement with its involvement in the intermediate steps. Polyreactive auto-Abs have been evidenced in the serum of all healthy subjects and in myeloma proteins, and a high frequency of precursor B cells displaying this auto-Ab activity has been shown elsewhere (13, 14, 20). These data were further confirmed and expanded by multiple functional and structural studies (3). Natural Abs predominate early in life and are observed even in species only distantly related to humans, such as fish and amphibians (19). They are currently encoded by variable (V) genes under their germinal configuration (4), and they bind well-conserved epitopes even from different species.
A feature of major interest is the ability of polyreactive auto-Abs to bind both self and nonself antigens such as microbial molecules. This specificity can be associated with the immune defenses against infection, especially in lower vertebrates. The antimicrobial functions of natural Abs lead us to discuss the advantages of the further development of the conventional Ab system in higher vertebrates and to suggest a scenario involving a successive evolution of these two Ab systems and their final coexistence as complementary mechanisms of protection against pathogens.
THE ANTIBODY ANCESTOR
Both V and constant (C) domains of the heavy (H) and light (L) chains of immunoglobulins (Igs) are members of a large family of proteins, which are already present in invertebrates and markedly diverse in vertebrates (23). Because of the genetic distance between vertebrates and invertebrates, most phylogenetic relationships with proteins from these animals remain elusive. However, a soluble protein containing domains of the C type was previously observed in silkworm larvae, especially during bacterial infections, as reviewed by Du Pasquier (16). Domains of the V and C types are frequently associated in molecules of the Ig superfamily, suggesting that the Ab ancestor was formed by two on-line V and C domains. As a membrane receptor, it could have resembled the CTX molecule described for Xenopus laevis (17). In a soluble form, it could have been the covalent homodimer of an unknown chain and have resembled human Bence-Jones molecules. Homodimeric L chains are capable of monoreactive (28) as well as polyreactive (29) Ab activities, and conversely, most Abs from Camelus dromedarius comprise only H chains (21). Similar to the hypothesis of J. Stewart (40), it is likely that the first molecular recognition by the Ab ancestor was directed against an endogenous molecule and occurred through a nonimmune mechanism. Indeed, most natural Abs are reactive with intracellular self-antigens, and sole specificity could have been of immediate benefit in participating in the clearance of a degraded or denatured autoantigen.
EMERGENCE OF THE PRIMORDIAL POLYREACTIVE ABS
From the putative ancestor, addition of the V loops associated with the presence of J and D segments; heterodimerization between H and L chains; and duplication of V, J, and D genes gradually extended the spectrum of specificity of the primordial Ab molecules. A small number of V genes encoding polyreactive Abs could have been sufficient for clearance of many autoantigens, provided that these genes were selected for auto-Ab reactivity. Genetic variations, in terms of auto-Ab specificity patterns, are observed in mammals and are in favor of such a selective process (7). Most illustrations of VH and VL domains represent the hypervariable regions containing the antigen-binding sites as rigid loops which can combine with a single epitope. However, interference by the CH1 domain with the affinity and/or specificity of natural Abs has demonstrated the possibility of significant plasticity of the antigen-binding area (36), which is large and mobile enough for recognition of several unrelated epitopes (5, 27). To explain the difference between monoreactive and polyreactive Abs, it has also been speculated that charge distribution within the Ab site and both length and flexibility of the CDR3 VH region could account for immunological cross-reactions with diverse and unrelated substances (26, 30). Finally, the possibility that Abs and antigens could cooperate to establish better interaction through conformational changes has also been hypothesized. In contrast to polyreactive Abs, generally encoded by germinal genes with no (1, 4) or few (24, 30) somatic mutations, conventional antigen-induced monoreactive Abs are encoded by highly mutated genes, suggesting that plasticity of the antigen-binding area of germinal genes is lost during the somatic selection process.
REACTIVITY TO PATHOGENS AS A SECONDARY EFFECT OF POLYREACTIVITY
The possible autoantigen-driven genetic selection of polyreactive Abs, described as a “know thyself” process (2), led to a wide recognition system, which could have progressively involved nonself molecules. This defense mechanism against pathogens could have occurred as a secondary effect of Ab polyreactivity. The anti-infectious agent defenses were further improved by addition of C domains and by polymerization, which increased both antigen binding and effector functions of Abs. Although some polyreactive monoclonal auto-Abs display a low affinity and have been compared with a weak nonspecific glue of minor biological significance, other studies have shown that monoclonal immunoglobulin G (IgG) (41) and polyclonal secretory IgA (37) polyreactive Abs can display intrinsic or functional affinity values within the same order of magnitude as that of monoreactive Abs. Polymerization also allows immune agglutination, while binding of the different C domains to Fc receptors gives rise to cellular activation and to transport mechanisms, extending the role of Abs. Triggering of the complement cascade also represents a major anti-infectious agent property provided by the additional C domains.
The primordial Ab system could have remained very simply organized with a limited number of random somatic mutations, and its regulation could have required nothing more than a sole balance between constant secretion and catabolism. Indeed, the multireactivity of the whole set of polyreactive Abs allows the absence of somatic induction-selection mechanisms. This primordial system could provide self-antigen clearance as well as basic protection against commensal organisms and against low inocula of pathogens. The Abs were also transported across epithelial cells towards the digestive lumen, as observed for primitive vertebrates (18). Such an economical system must have been well adapted to the low energy requirements of cold-blooded animals and to their high fecundity, preserving a species from complete extinction in case of immune failure against a pathogen.
TOWARDS MODERN DELUXE ABS
The high energy burden of mammals, their lower fecundity, and a possible adaptation of pathogens to the natural Ab spectrum have led to improvement of Ab efficiency by production of molecules designed to react solely with the corresponding antigen. Because the immune response with these à la carte (especially designed) Abs is delayed, the primordial Ab system has persisted as a basic mechanism of immunity in the higher vertebrates.
Polyreactivity of the primordial molecules does not imply that all epitopes can be recognized. Indeed, immunogenic epitopes of tubulin are not detected by human polyreactive Abs (31). Similarly, we have failed to detect significant levels of Abs to different bacterial adhesins among natural Abs. Antigens which are not detected by the species-specific set of polyreactive Abs are certainly of importance for the strategy of pathogens and may have been selected by microorganisms as factors in pathogenicity. This antigenic adaptation of virulence-associated molecules may thus have triggered the development of a novel type of immunity raising monoreactive Abs to these pathogenic molecules. This may be the reason why polyreactive Abs do not generally interfere with anti-infectious agent serodiagnostic assays. In addition, the spectrum of natural Abs is an individual characteristic with a lifelong stability. Some differences have been observed between newborn and old mice (26), and a global increase of natural Abs during parasitic infections has been reported elsewhere (42), but these changes are either slow or unrelated to a specific immune response. In contrast, the antigen-driven response is progressively adapted to the pathogen in terms of specificity and of increased specific activity and affinity. Natural Abs are thus designed for immune prevention and urgent protection, whereas antigen-induced Abs are specifically designed for recovery from damages and for immune memory against a further challenge.
Although a weak antigen-driven immune response can be observed in primitive vertebrates, the humoral immunity of these animals involves primarily polyreactive Abs (19). The branching of a monoreactive system may have preceded divergence between cold-blooded and evolved vertebrates or occurred later during their respective evolutions. In mice, a quantitatively minor set of B cells bearing the CD-5 marker (B-1 cells) has been proposed to be a major source of natural Abs, as reviewed by Murakami and Honjo (34). However, a large proportion of polyreactive Abs is synthesized by CD5− B-2 cells (12).
The respective roles of polyreactive and of monoreactive secretory IgA in the human digestive tract are easier to elucidate. These molecules are locally secreted in the mucosa or passively received from distant areas, such as from salivary glands (in adults) and from mammary glands (in newborns) (11, 33). The locally synthesized molecules serve to clear the lamina propria (25) and epithelial cells (32) of local pathogens during polymeric Ig receptor-mediated transport towards the lumen. This protective effect is delayed and dependent on an antigen-induced response initiated in Peyer’s patches containing virtually no B-1 cells. In contrast, luminal secretory IgA, which comprises a large proportion of polyreactive Abs, continuously clears the mucosal surface of the pathogens in order to prevent their entry into the body. It seems therefore that secretory IgA-associated digestive immunity represents a good model of the complementary roles of polyreactive and monoreactive Abs (Fig. 1).
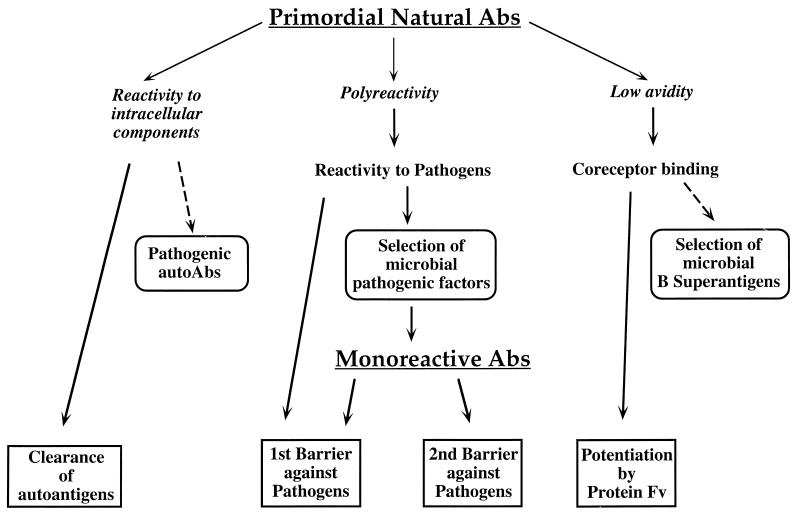
FIG. 1.
A proposed scenario of the evolution of humoral immunity from primordial natural Abs. These molecules could have been polyreactive to intracellular autoantigens, but their weak avidity impaired their efficiency. The auto-Ab specificity could have been the major selection force increasing blood and tissue clearance. In secretions, modern natural Abs impair absorption of insufficiently degraded autoantigens, preventing induction of autoimmunity. Similarly, the preimmune reactivity to microorganisms led to the first-barrier phenomenon, but its limitations could have necessitated monospecific Abs to microbial pathogenic factors. A soluble coreceptor molecule is envisioned, allowing a potentiation of Abs similar to that of the gut protein Fv towards degraded secretory Abs. The V-segment binding of microbial B-cell superantigens could be an adaptation of pathogens to induce apoptosis of corresponding B cells.
VH FAMILIES AND B-CELL SUPERANTIGENS
Binding of membrane Ig of the B-cell receptor is an important event in B-cell activation and selection. It has long been assumed that only the hypervariable regions of the Ab are involved in ligand binding. However, since the initial description of superantigens, accumulating evidence has suggested that an additional mode of interaction can occur, one whereby these molecules preferentially bind Ig structures encoded by a given V-gene segment, a V-gene family, or even a V-gene family clan (43). This interaction requires contributions from structures located in the solvent-exposed regions of FR1 and FR3 outside the conventional antigen-binding site (22, 38). Owing to this binding mechanism, the frequency of membrane and soluble Igs recognized by superantigens largely exceeds that of Abs to classical antigens.
A current opinion is that microbial superantigens are involved in pathogenicity by nonimmune stimulation of a high percentage of B cells, leading to cellular apoptosis which impairs the corresponding immune defenses. This would be the case for, namely, staphylococcal protein A (38); human immunodeficiency virus type 1 gp120 (6), which can bind VH3-positive Igs; staphylococcal enterotoxin D (VH4 specific) (15); and protein L from Peptostreptococcus magnus (Vκ specific) (35). The additional description of an endogenous Ig superantigen, called protein Fv (pFv) (10), has suggested a role for the recognized Ig structure itself. Indeed, pFv displays a VH-binding repertoire much larger than that of the reference protein A superantigen (39). It can bind not only human VH3+ Igs but also clan 3+ Igs from most mammals and Igs from inferior vertebrates, including the primitive fish sturgeon (8). Hence, the recognized structure has been highly conserved during evolution. A fascinating hypothesis is that the first Abs developed a site of recognition of a soluble coreceptor related to pFv. The primitive role of this pFv-binding site could have been to amplify the effector functions of small-sized polyreactive Abs. This is suggested by the findings for human gut secretions in which pFv binds cleavage fragments of secretory IgA to form large nonimmune complexes of 800 to 1,800 kDa (9). These complexes are called immune fortresses because they exhibit high agglutination activities for viruses and bacteria and thus play a major role in defenses against infectious agents.
CONCLUSION
According to recent data, the modern-type monoreactive Abs resulting from antigen-driven selection are included in a deluxe immune system which may have branched off from a putative primordial Ab system of polyreactive molecules. The primordial system would have gradually extended its role from solely clearance of a restricted number of autoantigens by the first Abs to the recognition of a large number of both self and nonself epitopes. A second site of recognition, located in the framework, has developed to amplify Ab activity of these germinal molecules. All these mechanisms still coexist in humans. The polyreactive Ab system seems to prevent autoimmunity and the entry of large numbers of microorganisms. It likely provides first-aid immune protection but fails to protect against the pathogens which have selected virulence-associated molecules poorly recognized by natural Abs. Modern Abs are involved in à la carte immunity to the virulence factors. After boosting, both the level of these induced Abs and their affinity for the corresponding antigens gradually increase, while an immune memory develops. The second site of recognition allows pFv to bind natural Abs in the gut lumen where their functions are restored and even largely increased. As a possible side effect, adaptation of some microorganisms to this framework site has led to the occurrence of pathogenic B-cell superantigens interfering with the VH family-associated immune response.
ACKNOWLEDGMENTS
We thank S. Iscaki for critical review of the manuscript and A. Berneman for his help in the design of Fig. 1.
REFERENCES
- 1.Adib-Conquy M, Gilbert M, Christodoulo C, Avrameas S. Reactivity and structure of a mouse anti-F(ab′)2 IgM. Comparison of its variable region sequences with those of a structurally close polyreactive natural IgM. Mol Immunol. 1994;31:555–562. doi: 10.1016/0161-5890(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 2.Avrameas S. Natural autoantibodies. From horror autotoxicus to gnothi seauton. Immunol Today. 1991;12:154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- 3.Avrameas S, Ternynck T. Natural autoantibodies: the other side of the immune system. Res Immunol. 1995;146:235–248. doi: 10.1016/0923-2494(96)80259-8. [DOI] [PubMed] [Google Scholar]
- 4.Baccala R, Quang T V, Gilbert M, Ternynck T, Avrameas S. Two murine natural autoantibodies are encoded by nonmutated germ-line genes. Proc Natl Acad Sci USA. 1989;86:4624–4628. doi: 10.1073/pnas.86.12.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentley G A, Boulot G, Riottot M M, Poljak R J. Three-dimensional structure of an idiotope-anti-idiotope complex. Nature. 1990;348:254–260. doi: 10.1038/348254a0. [DOI] [PubMed] [Google Scholar]
- 6.Berberian L, Goodglick L, Kipps T J, Braun J. Immunoglobulin VH3 gene products for HIV gp120. Science. 1993;261:1588–1591. doi: 10.1126/science.7690497. [DOI] [PubMed] [Google Scholar]
- 7.Berneman A, Ternynck T, Avrameas S. Natural mouse IgG reacts with self antigens including molecules involved in the immune response. Eur J Immunol. 1992;22:625–633. doi: 10.1002/eji.1830220303. [DOI] [PubMed] [Google Scholar]
- 8.Bouvet J P, Pirès R, Charlemagne J, Pillot J, Iscaki S. Non-immune binding of human protein Fv to immunoglobulins of various mammalian and non-mammalian species. Scand J Immunol. 1991;34:491–496. doi: 10.1111/j.1365-3083.1991.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 9.Bouvet J P, Pirès R, Iscaki S, Pillot J. Nonimmune macromolecular complexes of Ig in human gut lumen: probable enhancement of antibody functions. J Immunol. 1993;151:2562–2571. [PubMed] [Google Scholar]
- 10.Bouvet J P, Pirès R, Lunel-Fabiani F, Crescenzo-Chaigne B, Maillard P, Valla D, Opolon P, Pillot J. Protein F: a novel F(ab) binding factor, present in normal liver, and largely released in the digestive tract during hepatitis. J Immunol. 1990;145:1176–1180. [PubMed] [Google Scholar]
- 11.Brandtzaeg P. Molecular and cellular aspects of the secretory immunoglobulin system. Acta Pathol Microbiol Immunol Scand. 1995;103:1–19. doi: 10.1111/j.1699-0463.1995.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z J, Wheeler J, Notkins A L. Antigen-binding B cells and polyreactive antibodies. Eur J Immunol. 1995;25:579–586. doi: 10.1002/eji.1830250241. [DOI] [PubMed] [Google Scholar]
- 13.Dighiero G, Guilbert B, Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. II. High incidence of monoclonal Ig exhibiting antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J Immunol. 1982;128:2788–2792. [PubMed] [Google Scholar]
- 14.Dighiero G, Lymberi P, Mazié J C, Rouyre S, Butler-Browne G S, Whalen R G, Avrameas S. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983;131:2267–2272. [PubMed] [Google Scholar]
- 15.Domiati-Saad R, Attrep J F, Brezinschek H P, Cerrie A, Karp D R, Lipsky P E. Staphylococcal enterotoxin D functions as a human B cell superantigen by rescuing VH4-expressing B cells from apoptosis. J Immunol. 1996;156:3608–3620. [PubMed] [Google Scholar]
- 16.Du Pasquier L. Evolution of the immune system. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press; 1993. pp. 199–233. [Google Scholar]
- 17.Du Pasquier L, Chrétien I. CTX, a new lymphocyte receptor in Xenopus, and the early evolution of Ig domains. Res Immunol. 1996;147:218–226. doi: 10.1016/0923-2494(96)87224-5. [DOI] [PubMed] [Google Scholar]
- 18.Fellah J S, Iscaki S, Vaerman J P, Charlemagne J. Transient developmental expression of IgY and secretory component like protein in the gut of the axolotl (Ambystoma mexicanum) Dev Immunol. 1992;2:181–190. doi: 10.1155/1992/21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez R, Charlemagne J, Mahana W, Avrameas S. Specificity of natural serum antibodies present in phylogenetically distinct fish species. Immunology. 1988;63:31–36. [PMC free article] [PubMed] [Google Scholar]
- 20.Guilbert B, Dighiero G, Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982;128:2779–2787. [PubMed] [Google Scholar]
- 21.Hamers-Casterman C, Atarhouch T, Muydermans S, Robinson G, Hamers C, Bayanasonga E, Bendahman N, Hamers R. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 22.Hillson J L, Karr N S, Oppliger I R, Mannik M, Sasso E H. The structural basis of germline-encoded VH3 immunoglobulin binding of staphylococcal protein A. J Exp Med. 1993;178:331–336. doi: 10.1084/jem.178.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huncapiller T, Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44:1–63. doi: 10.1016/s0065-2776(08)60639-2. [DOI] [PubMed] [Google Scholar]
- 24.Ikematsu H, Kasaian M T, Schettino E W, Casali P. Structural analysis of the VH-D-JH segments of human polyreactive IgG mAb. Evidence for somatic selection. J Immunol. 1993;151:3604–3616. [PMC free article] [PubMed] [Google Scholar]
- 25.Kaetzel C S, Robinson J K, Chantalacharuvu K R, Vaerman J P, Lamm M E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defense function for IgA. Proc Natl Acad Sci USA. 1991;88:8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacroix-Desmazes S, Mouthon L, Coutinho A, Kazatchkine M. Analysis of the natural human IgG antibody repertoire: life-long stability of reactivities towards self antigens contrasts with age-dependent diversification of reactivities against bacterial antigens. Eur J Immunol. 1995;25:2598–2604. doi: 10.1002/eji.1830250929. [DOI] [PubMed] [Google Scholar]
- 27.Lescar L J, Pellegrini M, Souchon H, Tello D, Poljak R J, Peterson N, Greene M, Alzari P M. Crystal structure of a cross-reaction complex between Fab F9.13.7 and guinea fowl lysozyme. J Biol Chem. 1995;270:18067–18073. doi: 10.1074/jbc.270.30.18067. [DOI] [PubMed] [Google Scholar]
- 28.Mahana W, Jacquemart F, Ermonval M. A murine monoclonal multireactive immunoglobulin kappa light chain. Scand J Immunol. 1994;39:107–110. doi: 10.1111/j.1365-3083.1994.tb03347.x. [DOI] [PubMed] [Google Scholar]
- 29.Mangalo R, Iscaki S, Raynaud M. Présence d’activité anticorps dans des préparations de chaînes légères. C R Acad Sci (Paris) 1966;263D:204–207. [PubMed] [Google Scholar]
- 30.Martin T, Crouzier R, Weber J C, Kipps T J, Pasquali J L. Structure-function studies on a polyreactive (natural) autoantibody. Polyreactivity is dependent on somatically generated sequences in the third complementary-determining region of the antibody heavy chain. J Immunol. 1994;152:5988–5996. [PubMed] [Google Scholar]
- 31.Matthes T, Wolff A, Soubiran P, Gros F, Dighiero G. Antitubulin antibodies. II. Natural autoantibodies and induced antibodies recognize different epitopes on the tubulin molecule. J Immunol. 1988;141:3135–3141. [PubMed] [Google Scholar]
- 32.Mazanec M B, Kaetzel C S, Lamm M E, Fletcher D, Nedrud J G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGhee J R, Kiyono H. New perspectives to vaccine development: mucosal immunity to infections. Infect Agents Dis. 1993;2:55–73. [PubMed] [Google Scholar]
- 34.Murakami M, Honjo T. Involvement of B-1 cells in mucosal immunity and autoimmunity. Immunol Today. 1995;16:534–538. doi: 10.1016/0167-5699(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 35.Nilson B H K, Solomon A, Björck L, Åkerström B. Protein L from Peptostreptococcus magnus binds to the kappa light chain variable domain. J Biol Chem. 1992;267:2234–2239. [PubMed] [Google Scholar]
- 36.Pritsch O, Hudry-Clergeon G, Buckle M, Pétillot Y, Bouvet J P, Gagnon J, Dighiero G. Can immunoglobulin CH1 constant domain modulate antigen binding affinity of antibodies? J Clin Invest. 1996;98:2235–2243. doi: 10.1172/JCI119033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quan C, Berneman A, Pirès R, Avrameas S, Bouvet J P. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible immune barrier in humans. Infect Immun. 1997;65:3997–4004. doi: 10.1128/iai.65.10.3997-4004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman G J. Human antibody responses to bacterial antigens: studies of a model conventional antigen and a proposed model B cell superantigen. Int Rev Immunol. 1992;9:57–78. doi: 10.3109/08830189209061783. [DOI] [PubMed] [Google Scholar]
- 39.Silverman G J, Pirès R, Bouvet J P. An endogenous sialoprotein and a bacterial superantigen compete in their VH family-specific binding interactions with human Igs. J Immunol. 1996;157:496–502. [PubMed] [Google Scholar]
- 40.Stewart J. Immunoglobulin did not arise in evolution to fight infection. Immunol Today. 1992;13:396–399. doi: 10.1016/0167-5699(92)90088-O. [DOI] [PubMed] [Google Scholar]
- 41.Ternynck T, Avrameas S. Murine natural monoclonal autoantibodies: a study of their polyspecificities and affinities. Immunol Rev. 1986;94:99–112. doi: 10.1111/j.1600-065x.1986.tb01166.x. [DOI] [PubMed] [Google Scholar]
- 42.Ternynck T, Falanga P B, Unterkisher C, Grégoire J, Pereira da Silva L, Avrameas S. Induction of high levels of IgG autoantibodies in mice infected with Plasmodium chabaudii. Int Immunol. 1991;3:29–37. doi: 10.1093/intimm/3.1.29. [DOI] [PubMed] [Google Scholar]
- 43.Zouali M. B-cell superantigens: implications for selection of the human antibody repertoire. Immunol Today. 1995;16:399–405. doi: 10.1016/0167-5699(95)80009-3. [DOI] [PubMed] [Google Scholar]