Airway symptoms are often regarded as extraesophageal syndromes of gastroesophageal reflux. Although an increasingly prevalent reason for referral to gastroenterologists, extraesophageal presentations remain among the most challenging and frustrating. With clinical guidance or recommendations often leaning on low levels of evidence, or lack thereof, and expert opinions, the paradigm of extraesophageal reflux (EER) remains under scrutiny and viewed through a murky lens. Adding to the confusion is the cross-disciplinary nature, with other specialty stakeholders, such as otolaryngologists, who may have different priorities and approaches. In this commentary, we clear the lens on EER by acknowledging important updates in clinical guidance, highlighting areas ripe for research inquiry, and presenting a perspective on the future paradigm of EER (Table 1).
Table 1.
Priority Areas for Future Investigation in EER
| Knowledge gaps in diagnosis of EER | Clinical value of upper GI endoscopy |
| Optimal ambulatory reflux monitoring testing modality | |
| Whether day to day variability in EER physiology exists | |
| Significance of nonacidic or weakly acidic reflux | |
| Whether focus should remain on distal esophageal reflux or proximal reflux | |
| Diagnostic thresholds of reflux monitoring to use for EER | |
| Cost-effectiveness of upfront testing across all patients evaluated for EER | |
| Whether noninvasive screening methods can reliably stratify risk of EER | |
| Value of biochemical testing | |
| Priority research areas for EER management | |
| Low-acid or other targeted dietary approaches | |
| Psychological/behavioral interventions | |
| Multidisciplinary consensus-based management strategy | |
| Personalized phenotype-based management approach |
EER, extraesophageal reflux; GI, gastrointestinal.
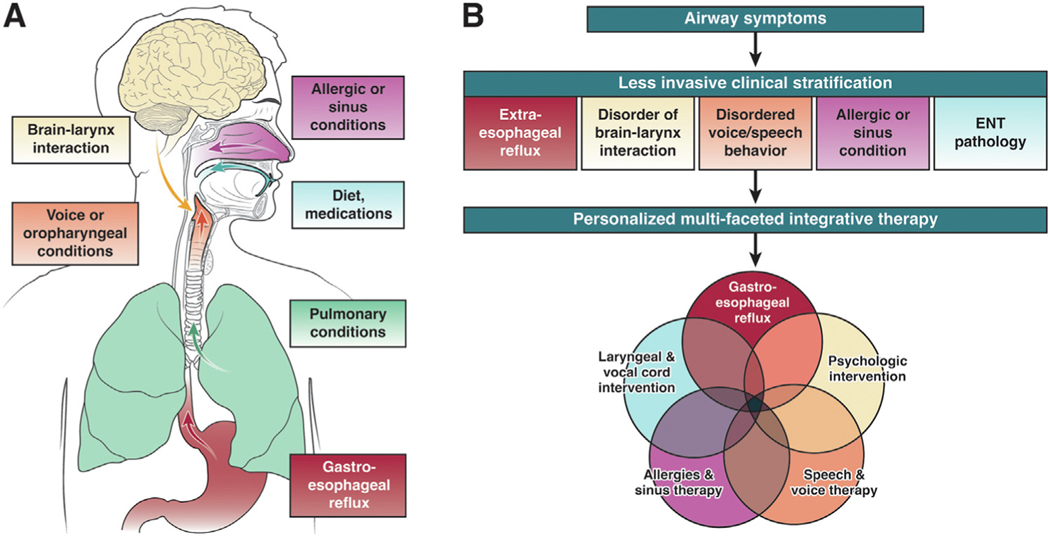
Establishing the correct diagnosis remains one of the most crucial and challenging aspects of EER, given the prevalence of these common symptoms with a wide range of underlying causes (Figure 1A). Importantly, symptoms alone are insufficient to make, or rule out, a diagnosis of EER.1 EER symptoms, particularly in the absence of esophageal symptoms, are less responsive to acid suppression than typical gastroesophageal reflux disease (GERD) because of several possible reasons, including incorrect diagnosis, other nonreflux contributors to symptoms, potential role of weakly acidic or nonacidic refluxate, and comorbid otolaryngologic conditions, such as vocal cord dysfunction or postnasal drip. Indeed, recent large prospective, randomized trials failed to show consistent benefits with empiric use of proton pump inhibitor (PPI) for suspected EER.2 In cost-utility studies, PPI trials are less cost-effective than early use of reflux testing to stratify patients for management. Therefore, empiric PPI trials are an approach of the past for EER. Instead, contemporary global guidance recommends early objective reflux testing in the evaluation of EER.3
Figure 1.
(A) Multiple potential etiologies of extraesophageal symptoms. (B) Conceptual framework of future paradigm of extraesophageal symptoms. The optimal paradigm for extraesophageal symptoms will begin with noninvasive clinical stratification to measure likelihood of each etiology of symptoms, such as extraesophageal reflux, disorder of brain-larynx interaction, and so forth. This initial clinical stratification will guide whether further testing is needed (and if, so what type) and targeted treatment strategies. Therapy will be personalized, multifaceted, and integrative.
Objective evaluation is critical to establish the presence of abnormal gastroesophageal reflux. With the lack of EER-specific consensus diagnostic criteria, the current diagnostic approach for EER generally follows the pathway for typical GERD.4 On upper endoscopy, sequelae of esophageal mucosal injury from reflux may present as erosive esophagitis, Barrett’s esophagus, and/ or peptic stricture. Since erosive disease is absent in most cases of EER, ambulatory reflux monitoring off acid suppression is commonly needed to objectively quantify reflux burden. The primary metric on ambulatory reflux monitoring is the acid exposure time, which measures the duration of acidic (pH <4) exposure in the distal esophagus. Current consensus on diagnosis of GERD classifies acid exposure time <4% as normal/physiological and acid exposure time >6% as abnormal/pathologic.5 Using these criteria, studies have shown that 40%–50% of patients with extraesophageal symptoms undergoing reflux testing at tertiary referral centers may have a positive study.6
However, the assessment of EER symptoms warrants considerations separate from those for typical GERD. The following questions remain unanswered and represent important areas for future research.
Should traditional pH thresholds for GERD also apply to EER?
What is the significance of nonacidic or weakly acidic reflux (pH >4.0) in the context of EER symptoms?
Prior studies suggest different sensitivity and susceptibility to reflux-induced injury of laryngeal mucosa compared with esophageal mucosa. Other nonacidic components of refluxate, such as pepsin, may also impact laryngeal mucosa. The definition of abnormal reflux burden may differ between traditional GERD and EER.
Should the focus remain on distal refluxate exposure or should the emphasis be on reflux events that extend proximally?
How many events (distal or proximal) should be considered abnormal for EER?
In previous studies, 2 reflux events reaching the pharynx has been proposed as abnormal.7
Among the spectrum of EER itself, should the definition of abnormal burden differ between upper airway (laryngeal) and lower airway (pulmonary) involvement?
The optimal reflux testing modality and protocol for EER remain debated. Should the preference be more days of monitoring to increase sensitivity (wireless pH monitoring) or more information over shorter duration of monitoring (catheter-based impedance-pH monitoring)?
Newer models of impedance-pH catheters allow measurement of reflux events migrating from distal esophagus to proximal esophagus or pharynx. However, their interpretation is often challenging and time-consuming, with significant interrater variability.
Although upfront testing is presently recommended for every patient with suspected EER, the costs and resources needed are seemingly substantial given the prevalence of EER symptoms. Further research is critically needed to better stratify patients according to their risk of true EER and abnormal reflux monitoring. The identification of various clinicophysiologic phenotypes of patients presenting with EER symptoms may be the beginning of developing personalized approaches.8 Risk stratification tools, such as HASBEER or other models currently under investigation, may fill the role of selecting patients with higher pretest probability for objective reflux testing. The positioning of noninvasive studies, such as biochemical testing (eg, salivary pepsin), in the overall paradigm of EER also warrants further investigation.
Management for conclusive EER should be personalized and multipronged, including 2 main treatment approaches: targeting underlying mechanisms for excessive reflux burden, and modulating other factors that may contribute to symptoms. The former may include correcting altered antireflux barrier, improving esophageal dysmotility, and reducing other risk factors, such as obesity. Patients commonly express interest in the role of dietary modifications. In addition to traditional GERD recommendations, such as avoidance of trigger food items, late night meals, and overeating, studies, primarily in the otolaryngology literature, also suggest value of low-acid diet approaches. This includes consumption of alkaline water or food/liquid with higher pH level.9 Further research of low-acid dietary approaches is needed to ascertain impact on physiologic, symptom, and quality of life outcomes.
Growing evidence supports the interplay among cognitive affective processes, behavioral mechanisms, and extraesophageal symptom burden.10 Multipronged strategies to downregulate hyperresponsive behaviors and intercept the brain-throat axis in addition to traditional GERD therapy may provide greater therapeutic success. These include use of neuromodulation, cognitive-behavioral therapy, and other psychological/behavioral interventions.11,12 Similar to disorders of gut-brain interactions, the key to success is likely interdisciplinary involvement from speech therapy, clinical psychology, and the otolaryngology community, and a patient-centered approach with emphasis on patient engagement. Overlapping nonreflux processes may also contribute to symptoms, even among patients with proven increased reflux burden. Comprehensive approaches may include assessment and intervention for vocal cord dysfunction or other concomitant laryngeal pathology, conditions that may increase laryngeal irritation, such as postnasal drip or other allergies, visceral hypersensitivity of the airway, and oropharyngeal swallowing abnormalities.
As the understanding of EER symptoms has evolved, it is abundantly clear that a multidisciplinary consensus approach is needed to define a comprehensive strategy in managing this heterogeneous population. This would involve standardization and agreement among stakeholders on terminologies and definitions, diagnostic criteria, treatment options, and outcome measurements. Development and use of clear criteria would help clarify recommendations and avoid muddled messages to practitioners, and would be paramount in minimizing heterogeneity and improving generalizability of research studies and trials.
In summary, EER symptoms are common and represent an increasingly prevalent presentation to clinicians’ practices. Rather than relying on PPI trials, the paradigm is moving toward a personalized approach for EER that requires combining clinical and physiological characteristics (Figure 1B).8 A current priority is identification of noninvasive methods to stratify likelihood of EER. When GERD is identified, antireflux management per current practice guidelines is warranted. Furthermore, other overlapping contributors to airway symptoms, such as a heightened brain-gut-larynx interaction, must be considered and addressed. Multipronged therapies may involve psychological therapy, speech/voice therapy, treatment for allergies, and/or laryngeal/vocal cord interventions. Diagnostic and treatment approaches need to be patient-centered and team-based. Increased interdisciplinary collaborations and consensus building will be the key to continue clearing the murky lens of the field, for patient care and research.
Funding
This work was supported by NIH K23 DK125266 (Yadlapati, PI).
Conflicts of interest
The authors disclose the following: Rena Yadlapati has served as a consultant for Medtronic, Ironwood Pharmaceuticals, Phathom Pharmaceuticals, StatLink MD, and Medscape; received research support from Ironwood Pharmaceuticals; and served on advisory boards for RJS Mediagnostix with stocks. Walter W. Chan has served on advisory boards for Ironwood Pharmaceuticals, Takeda Pharmaceuticals, Phathom Pharmaceuticals, and Sanofi Pharmaceuticals.
References
- 1.Salgado S, Borges LF, Cai JX, et al. Symptoms classically attributed to laryngopharyngeal reflux correlate poorly with pharyngeal reflux events on multichannel intraluminal impedance testing. Dis Esophagus 2022;36:doac041. [DOI] [PubMed] [Google Scholar]
- 2.O’Hara J, Stocken DD, Watson GC, et al. Use of proton pump inhibitors to treat persistent throat symptoms: multicentre, double blind, randomised, placebo controlled trial. BMJ 2021; 372:m4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ribolsi M, Luca Guarino MP, Balestrieri P, et al. The results from up-front esophageal testing predict proton pump inhibitor response in patients with chronic cough. Am J Gastroenterol 2021;116:2199–2206. [DOI] [PubMed] [Google Scholar]
- 4.Yadlapati R, Gyawali CP, Pandolfino JE, et al. AGA Clinical Practice Update on the Personalized Approach to the Evaluation and Management of GERD: expert review. Clin Gastroenterol Hepatol 2022;20:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67:1351–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korsunsky SRA, Camejo L, Nguyen D, et al. Resource utilization and variation among practitioners for evaluating voice hoarseness secondary to suspected reflux disease: a retrospective chart review. Medicine (Baltimore) 2022;101: e31056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lechien JR, Chan WW, Akst LM, et al. Normative ambulatory reflux monitoring metrics for laryngopharyngeal reflux: a systematic review of 720 healthy individuals. Otolaryngol Head Neck Surg 2022;166:802–819. [DOI] [PubMed] [Google Scholar]
- 8.Yadlapati R, Kaizer AM, Sikavi DR, et al. Distinct clinical physiologic phenotypes of patients with laryngeal symptoms referred for reflux evaluation. Clin Gastroenterol Hepatol 2022; 20:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zalvan CH, Hu S, Greenberg B, et al. A comparison of alkaline water and Mediterranean diet vs proton pump inhibition for treatment of laryngopharyngeal reflux. JAMA Otolaryngol Head Neck Surg 2017;143:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause AJ, Greytak M, Burger ZC, et al. Hypervigilance and anxiety are elevated among patients with laryngeal symptoms with and without laryngopharyngeal reflux. Clin Gastroenterol Hepatol. Published online October 26, 2022. 10.1016/j.cgh.2022.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiebles JL, Kwiatek MA, Pandolfino JE, et al. Do patients with globus sensation respond to hypnotically assisted relaxation therapy? A case series report. Dis Esophagus 2010; 23:545–553. [DOI] [PubMed] [Google Scholar]
- 12.Vertigan AE, Theodoros DG, Gibson PG, et al. Efficacy of speech pathology management for chronic cough: a randomised placebo controlled trial of treatment efficacy. Thorax 2006;61:1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]