Abstract
Apple (Malus domestica Borkh) is an appreciated source of polyphenols. Phenolic compounds are known as natural antioxidants and have a wide range of applications in different industries. Apple pomace has the potential of being an alternative source of polyphenols. To determine the polyphenolic profile of apple pomace, samples from the skin at two different stages of ripening were extracted with 80–20% EtOH–water/acetic acid 5% (S1) and 20–80% EtOH–water/acetic acid 5% (S2) in order to determine the solvent system. Ripe skins extracted with S1 showed a higher total polyphenol content or TPC (1.21 g of polyphenols per 100 g of fresh weight (FW)) than unripe apple skin, being the most effective system tested and a mean degree of polymerization of 2.47. Commercial apple pomace was extracted with S1, resulting in a TPC of 0.5615 ± 0.007 g of polyphenols per 100 g of FW. Meanwhile, the RP-HPLC-MS analysis led to the tentative identification of several polyphenolic compounds.
1. Introduction
Ever since the introduction of the so-called “French paradox” and the link between wine intake and lower death rates from coronary heart disease was established,1 a new wave of interest developed around the components of said beverage. Part of the benefits described by Renaud and Lorgeril were thought to be attributed to a particular group of biomolecules, casting a much favorable light over antioxidants.2
Among those molecules, there is a group of second metabolites known as phytochemicals. Within this category, over 10,000 phytochemicals, referred to as polyphenols, can be found. They are the second most abundant group in plants and are widely studied for their antioxidant capacity. Phenolic compounds are divided into simple phenols and polyphenols. Simple phenols possess only one aromatic ring, while polyphenols possess more aromatic rings. In addition to this characteristic, the aromatic rings can be substituted with one or more hydroxyl groups.3,4
Lately, the use of natural antioxidants has developed, particularly in the food, pharmaceutical, and cosmetic industries and mostly due to the ability they possess to reduce the damage caused by oxidative stress5 without the potential threats of synthetic antioxidants.3
Included in the health benefits that have been studied are action versus neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease,6 cardiovascular diseases, cancer, asthma, obesity, oral cancer cells,7,8 inflammation, high cholesterol levels, hypertension, type 2 diabetes, harmful microbiota,9 positive effect against Salmonella spp., Escherichia coli, and Listeria spp.10,11 Other uses include its addition to meats to preserve them by reducing the presence of pathogens, therefore, extending the shelf life of beef,11 and baked goods, beverages, and dairy.12
This increase in demand of bioactive compounds has resulted in the search for alternative sources with high polyphenol content, predominantly those supported in the three dimensions of sustainability (social progress, environmental protection, and economic development), achieving the recovery of byproducts without creating competition against food supplies, all this while supporting a zero waste philosophy. However, since there are other economically limiting factors such as storage (along with some pretreatments needed, like milling or drying), and, in some cases, shipping of byproducts, the use of local agricultural sources should be encouraged to obtain functional ingredients.
There are many regional agricultural products that can adhere to the requirements and principles listed above, particularly fruits. Among the fruits known to have attracted attention as potential phenolic sources are different apple cultivars. Apples are one of the main fruits cultivated in the northern region of Mexico, and it has been reported that its average annual production in the state of Chihuahua is around 584,000 tons, representing 77% of the country’s total apple production.13 The second main use for apples (after consumption as fresh fruit) is juice production. Maceration and pressing of apples in juice and cider making causes the generation of a solid residue, called apple pomace, as a consequence.
Apple pomace accounts for up to 20–35% of the weight of the fresh fruit.14 Due to its composition, its inexpensive nature, and the high volume generated, it is available for use. Apple pomace can be of great interest, with a valorization that allow us to not only being able to take advantage of the added value of the bioactive compounds that remain in the biomass after it has been processed but also contributing with providing a sustainable alternative solution to the disposal of what would otherwise be considered waste susceptible to microbial growth.15,12
In order to benefit from the underutilized functional ingredients present in apple pomace, it is of utmost importance to develop the right methodology for the extraction and recovery of phenolic compounds (due to their sensitive nature) as a means to maintain intact the main characteristics that draw attention to fruit and agro-waste derived compounds, from an economic point of view, and to allow their maximum reutilization. Being the case, the use of ultrasound-assisted extraction can overcome some of the principal obstacles faced by traditional technologies.
Ultrasound-assisted extraction (UAE) is typically selected as a green/environmental friendly recovery procedure, commonly used on vegetable materials. Using cavitation (caused by high frequency waves), UAE disrupts the cell membranes, enhancing solvent–sample contact and therefore promoting the release of potentially bioactive compounds with therapeutic properties such as polyphenols. Other great advantages over traditional techniques (such as solvent application and leaching) for phytochemical extractions are its operation conditions, low temperature (adequate for polyphenolic compounds), reduced extraction time, and the capacity to increase polyphenol extraction up to 20%, establishing UAE as a sustainable alternative.16−18 However, precautionary measures must be taken since degradation of phytochemicals can occur as consequence of cavitation-caused oxidation19,20).
With the use of agroindustrial waste to obtain bioactive molecules with a high-revalorization potential on the rise, the aim of the present study is to evaluate the suitability of apple pomace as a source of compounds of interest (polyphenols). This can be seen as a first step toward an extraction and purification process. At the same time, an alternative for the exploitation of waste generated by the apple juice and cider-making industries for their revalorization can be explored. The obtained agrowaste-derived compounds can then be directed to a wide range market of high-value products. This can be seen as early efforts to change the conception from “residue” to a revalued byproduct that can be treated as a source of polyphenols.
Furthermore, the possibility of reducing the volume of waste produced by apple juice and cider-making industries by extracting underused functional ingredients without competing with food sources is also a factor that appeals to our work.
In addition to the transformation into an extraction mix comprised of high-value compounds, the phenolic nature of apple pomace includes a high variety of oxidation products. Their presence within the apple pomace makes the characterization of the phenolic compounds more difficult, presenting a challenge on its own. Our approach of using oxidized polyphenols present in apple pomace can be original considering that it is not the usual source of phenolic compounds.
2. Materials and Methods
Materials. For preliminary studies, apples (Malus domestica Borkh Golden Delicious variety) produced in the region of Cuauhtemoc (Chihuahua, Mexico) were acquired in a local market at two different maturity stages. “Unripe” and “ripe” (commercial maturity) apples were selected. Both maturity stages were visually determined.
Apple pomace, obtained after the maceration and pressing of Golden Delicious apples, was provided by a locally based juice processing facility (Berlanga, Huertas Orgánicas de Mexico) located in the region of Cuauhtemoc (Chihuahua, Mexico).
Ethanol, methanol, hexane, and hydrochloric acid were purchased from J.T.Baker (Phillipsburg, NJ, USA) with both methanol and ethanol also being purchased from CTR Scientific (Monterrey, N.L., Mexico). Likewise, acetic acid was obtained from CTR Scientific (Monterrey, Mexico) as well. Phloroglucinol, Folin-Ciocalteu′s phenol reagent, gallic acid, chlorogenic acid, epicatechin, caffeic acid, and quercetin were used from Sigma-Aldrich (St. Louis, MO, USA). Additionally, sodium acetate from Fisher Scientific and ascorbic acid from DEQ (Nuevo Leon, Mexico) were used. All of the reagents employed were of analytical grade.
Chromatographic solvents: acetic acid from Sigma-Aldrich (St. Louis, MO, USA); water and acetonitrile were purchased from Tedia (Fairfield, MA, USA) and Sigma-Aldrich (St. Louis, MO, USA), with acetonitrile obtained from J.T.Baker (Phillipsburg, NJ, USA) as well; methanol, J.T.Baker (Phillipsburg, NJ, USA); and with the addition of formic acid purchased from Fluka Analytical (Buchs, Switzerland). All chromatographic solvents were HPLC grade.
Methods: For preliminary studies, apples were washed with mild soap, rinsed with abundant water (three times), and kept refrigerated before being peeled as a preparation step. Afterward, they were peeled, leaving as little pulp as possible attached to the skin. The skin was stored in resealable plastic bags and kept at freezing temperatures (−14 °C) until used. Before storage, the air inside the bags was removed.
Moisture determination of the unripe apple skins, ripe apple skins, and apple pomace was carried out according to the oven-drying method where the samples were placed in a hot air oven, with a set temperature of 103 ± 5 °C and allowed to dry for 24 h. After this period, the samples were allowed to cool in a desiccator for 1 h before weighting them.
Extraction was performed as a solid–liquid, 30-min single-step extraction, assisted with an ultrasonic bath (Fisher Scientific, Pittsbourgh, PA, USA) and a controlled temperature (10 °C). To determine the best solvent system for the extraction, two solvent systems were chosen: 80–20% EtOH–water/acetic acid 5% (S1) and 20–80% EtOH–water/acetic acid 5% (S2). Extraction was performed in duplicate in Erlenmeyer flasks maintaining a sample/solvent ratio of 100 g of sample/250 mL solvent.
The extracts were then concentrated under reduced pressure to a volume of 100 mL of acidified water (1% acetic acid) prior to cleaning steps consisting of: (a) a cold ethanol precipitation (with one volume on cold ethanol during 24 h) that was carried out to remove polar coextracted compounds (such as carbohydrates and proteins that are not soluble in ethanol); and (b) a subsequent liquid–liquid extraction (1:1 hexane/water) to remove nonpolar compounds (such as chlorophyll) from the sample. The cleaning step was intended to remove coextracted compounds that would present interference during analysis of the samples.
Once the solvent system was selected, apple pomace extractions were carried out under the same conditions as the preliminary extractions that took place. Following the extraction, apple pomace extracts were subjected to a liquid–liquid extraction (1:1 hexane/water) to remove coextracted compounds and then rotaevaporated under reduced pressure to prepare the samples for characterization.
Total polyphenol content (TPC) was quantified by means of both the Folin–Ciocalteu method and reversed-phase high performance liquid chromatography (RP-HPLC).
For Folin–Ciocalteu determination of TPC, the analysis was carried out using the Folin–Ciocalteu method reported by Singleton21 in which a sample aliquot of 50 μL was added to 3 mL of distilled water followed by 200 μL of Folin–Ciocalteu reagent. Afterward, the test tubes were closed, thoroughly mixed, and kept sheltered from light for 10 min. Once the 10 min were over, 600 μL of 5% (w/v) sodium carbonate solution were added, closing the test tubes and mixing completely. The test tubes were submerged in an isothermal bath with the temperature set to 37 °C during 15 min. After the reaction took place, they were removed from the isothermal bath and placed in an ice bed for 15 min. The absorbance was measured at 755 nm (Lambda 25 PerkinElmer spectrophotometer, Waltham, MA, USA) and reported as gallic acid equivalents (GAE).21
TPC, as well as the determination of polyphenols present compared with existing standards, was carried out by HPLC. Five milliliter samples were dried under reduced pressure and recovered using 5 mL of acidified water (with 1% formic acid). Samples were filtered directly onto 2 mL of Type 1 borosilicate glass vials using a 0.45-μm nylon membrane. The analysis was carried out using an Agilent 1100 Series HPLC (Santa Clara, CA, USA) equipped with a Diode Array Detector using a reversed phase (RP) column (ZORBAX SB-C18 Solvent Saver 3.0 × 250 mm; Germany) and a guard column of the same material, operated at 30 °C, flow rate of 0.3 mL/min with a 65-min gradient (water-formic acid 1% (v/v)(Solvent “A”)/acetonitrile-formic acid 1% (v/v) (Solvent “B”)) and a return to initial conditions over 10 min. The gradient was as follows: Linear gradient from 100% A to 90% A in 8 min. 90% A for 2 min. Linear gradient from 90% A to 80% A from minute 10 to minute 23. Linear gradient from 80% A to 70% A for 7 min. Linear gradient from 70% A to 60% A for 15 min. Linear gradient from 60% A to 20% A for 5 min. Linear gradient from 20% A to 100% B in 5 min. 100% B from minute 55 to 60 and a return to 100% A for minute 65.22
Total phenolic compounds were quantified at 280 nm, while the distinction between families of polyphenols was done based on the chromatographic signal of each compound, the UV spectrum, and the available standards. Phenolic acids were quantified based on their absorbance at the 320-nm signal. The compounds that showed a higher signal at 360 nm were counted as flavonols. This quantification was completed with the remaining compounds that presented a stronger signal at 280 nm. TPC, phenolic acid content, and flavonol content were reported as epicatechin equivalents (mg/L), GAEs (mg/L), and rutin equivalents (mg/L), respectively.
The identification of the remaining compounds present in the apple pomace for which a standard was not available was done by HPLC-DAD/ESI-MS (Thermo Scientific LCQ Fleet Ion Trap Mass Spectrometer System). Chromatographic separation was achieved at 30 °C, 20 μL injection volume, and 0.25 mL/min flow rate, with a gradient of water–formic acid 1% (v/v) (Solvent “A”)/acetonitrile–formic acid 1% (v/v)(Solvent “B”). The elution gradient was adapted from the previously described gradient: Linear gradient from 95% A to 90% A in 8 min. 90% A for 2 min. Linear gradient from 90% A to 80% A from minute 10 to minute 23. Linear gradient from 80% A to 70% A from minute 23 to 30. Liner gradient from 70% A to 60% A for 15 min. Linear gradient from 60% A to 20% A for 5 min. Linear gradient from 20% A to 100% B in 5 min. 100% B from minute 55 to 60 and a return to 100% A for minute 65. Initial conditions were then re-established over a 10-min postrun. Identification of the compounds was performed by MS. The mass spectrometer was operated in the negative ion mode in the range of m/z 150–2000 and under the following conditions: source voltage, 4.5 kV; capillary voltage, 23.5 V; capillary temperature, 250 °C.
In order to estimate the mean degree of polymerization (mDP) of the samples, phloroglucinolysis was carried out as established by Kennedy23 and further analyzed by HPLC.
3. Results
3.1. Solvent Selection
Preliminary studies were performed to determine which of the proposed solvent systems was to be used for further extractions. Frozen skins (either from unripe or ripe apples) were milled with S1 or S2 in order to minimize oxidation of the compounds.24 The tested solvents were evaluated based on the TPC of each extract (Table 1). Results were expressed as g per 100 g of fresh weight (FW).
Table 1. Total Polyphenol Content (TPC) of Samples From Skin of Golden Delicious Variety Apples Quantified by Folin–Ciocalteu and HPLCa.
| sample | TPC Folin-Ciocalteu | TPC HPLC | phenolic Acids HPLC | flavonoids HPLC |
|---|---|---|---|---|
| samples extracted with 80–20% EtOH-water/acetic acid 5% (S1) | ||||
| unripe | 0.44 ± 0.01 | 0.48 ± 0.07 | 0.06 ± 0.01 | 0.06 ± 0.02 |
| ripe | 0.61 ± 0.10 | 1.21 ± 0.00 | 0.10 | 0.15 ± 0.01 |
| samples extracted with 20–80% EtOH-water/acetic acid 5% (S2) | ||||
| unripe | 0.33 ± 0.07 | 0.41 ± 0.12 | 0.06 ± 0.01 | 0.07 ± 0.02 |
| ripe | 0.34 ± 0.001 | 0.57 ± 0.01 | 0.05 ± 0.001 | 0.07 ± 0.01 |
Results are expressed in g per 100 g FW.
Phenolic acids were expressed in GAEs since the equivalent is usually used for Folin–Ciocalteu and it would ease the comparative. Total phenolic content was expressed in epicatechin equivalents, as it better represents the nature of the sample (seeing that it is the main polyphenol present); and total flavonoid content was expressed in rutin equivalents (see Supporting Information).
As shown in Table 1, out of the samples tested, the two extracts with the best results were those coming from ripe apples (for the chromatogram, please see Supporting Information). However, the higher TPC was obtained by extracting ripe apple skins with S1 (1.21 g per 100 g of FW) versus the 0.57 g per 100 g of FW extracted with S2. It also presented a higher concentration of both phenolic acids (0.10 g per 100 g FW) and flavonoids (0.15 g per 100 g FW). Therefore, S1 was selected as the solvent system for further extractions.
Unriped apple skin presented 75.81 ± 0.05% humidity, while the humidity content of ripe apple skin was 76.01 ± 0.44%.
3.2. Apple Pomace Extraction
Based on the results from the preliminary studies, apple pomace was extracted with S1. 100 g samples were extracted with 250 mL of solvent in a solid–liquid extraction. The extracts were then concentrated under reduced pressure to a volume of 100 mL followed by two cleaning steps: (a) cold ethanol precipitation (to remove sugars, proteins, and other polar compounds) and (b) a subsequent liquid–liquid extraction with 1:1 hexane/water to remove coextracted compounds (to remove chlorophyll, lipids, and other nonpolar compounds).25,26
The resulting extract was enriched in polyphenols and was analyzed by HPLC in order to quantify TPC. In addition to this, the precipitate obtained from the cold ethanol precipitation was redissolved in acidified water (1% acetic acid) and analyzed by HPLC, in order to quantify polyphenols that could have precipitated as well; the hexane extract obtained after the liquid–liquid extraction was also analyzed to ensure that there was no considerable loss of polyphenolic compounds in both cleaning step (the chromatograms are available in the Supporting Information).
Table 2 shows the TPC values on the samples along with the ethanol precipitation and the hexane fraction.
Table 2. TPC of Samples From Apple Pomace (Golden Delicious Variety) Quantified by HPLCa.
| sample | TPC HPLC | flavonols HPLC |
|---|---|---|
| phenolic extract | 0.56 ± 0.01 | 0.28 ± 0.01 |
| ethanol precipitation | 0.01 ± 0.02 | |
| hexane fraction | 0.05 ± 0.01 |
Results are expressed in g per 100 g of apple pomace.
Apple pomace extract had a TPC of 0.56 g of polyphenols per 100 g of apple pomace FW and a flavonol concentration of 0.28 g per 100 g of apple pomace FW. No chromatographic signal was detected in the 520 nm wavelength, indicating the absence of anthocyanin-like compounds.
The humidity content present in the samples was 86.44 ± 0.86%.
Golden delicious pomace was obtained from the industrial production of apple juice. It has been reported that after pressing only a small amount of polyphenols is released into the juice. As a result, a large fraction of the compounds remains present in the pomace, since it is made of skin, seeds, and pulp residues.27,28 In addition to this, the difference in TPC between apple skin and apple pomace can be attributed to the fact that some of the polyphenolic compounds are located inside specific tissues of the fruit, making the phenolic profile dependent on the parts of the fruit being analyzed.29
3.3. Identification of Main Compounds Present in Apple Pomace
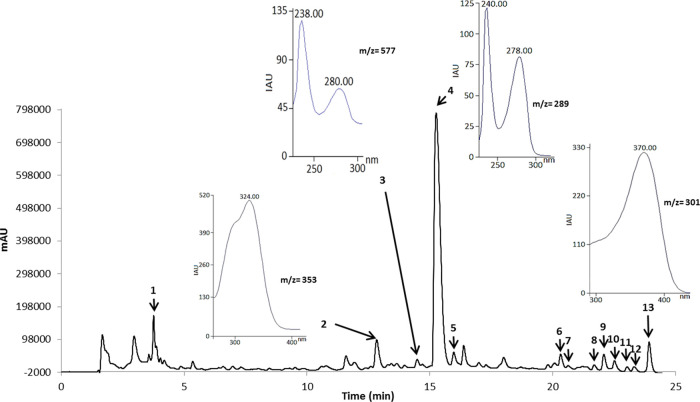
An HPLC analysis was carried out in order to identify the main polyphenols present in the apple pomace. The identification was made either by use of a standard or by a UV–vis spectrum. Figure 1 presents the HPLC chromatogram of polyphenols in samples from commercial apple. The identified peaks belong to gallic acid, chlorogenic acid, epicatechin, caffeic acid, and quercetin, respectively.
Figure 1.
Chromatogram (at 280 nm) of main polyphenols present in samples from commercial apple pomace (M. domestica) extracted with solvent system 80–20% ethanol–water/acetic acid 5% (S1). Peaks are as follows: 1 – gallic acid; 2 – chlorogenic acid; 3 – PC B2; 4 – epicatechin; 5 – caffeic acid; 6 – hyperin; 7 – isoquercetin; 8 – avicularin; 9 – quercitrin; 10 – cynaroside; 11 – phloretin; 12 – phloridzin; 13 – quercetin.
The peaks located between RT (retention time) 23 min and RT 28 min could not be identified by standard or UV–vis spectra. However, they displayed a maximum signal at 360 nm, indicating that they might belong to the flavonol family (quercetin glycosides).
Since the identification of compounds by means of HPLC is limited to the characteristic UV–vis spectrum and the availability of standards, polyphenolic profiling was carried out by HPLC-MS, as well as the confirmation of the already identified compounds.
3.4. Polyphenolic Profiling by HPLC-DAD/ESI-MS
The use of HPLC-MS was fundamental for the polyphenolic profiling since HPLC with UV–vis detection has its limitations with respect to the identification of coeluted phenolic compounds and is not a chromatographic technique which can provide molecular structure information.16Table 3 presents the tentatively identified polyphenolic compounds along with their molecular formula and molecular ion (negative ionization mode). The tentatively identified compounds can be seen in Figure 1.
Table 3. Mass Spectra Data of the Polyphenolic Compounds Tentatively Identified in Apple Pomace (Golden Delicious Variety).
| compound | type of phenolic | λmax (nm) | m/z [M – H]− | retention time RT (min) | percentage of total area (%) | |
|---|---|---|---|---|---|---|
| 1 | gallic acid | phenolic acid | 270 | 169 | 3.75 | 2.40 |
| 2 | chlorogenic acid | phenolic acid | 242, 324 | 353 | 12.81 | 9.86 |
| 3 | PC B2 | flavanol | 238, 280 | 577 | 14.47 | 0.88 |
| 4 | epicatechin | flavanol | 278 | 289 | 15.28 | 38.73 |
| 5 | caffeic acid | phenolic acid | 216, 324 | 179 | 15.94 | 2.51 |
| 6 | Hyperin | flavonol | 255, 355 | 463 | 20.30 | 13.80 |
| 7 | isoquercetin | flavonol | 260, 354 | 463 | 20.54 | 2.95 |
| 8 | avicularin | flavonol | 258, 359 | 433 | 21.64 | 1.10 |
| 9 | quercitrin | flavonol | 255, 355 | 447 | 22.06 | 4.03 |
| 10 | cynaroside | flavone | 239, 269, 340 | 447 | 22.48 | 4.56 |
| 11 | phloretin | dihydrochalcone | 286 | 273 | 22.97 | 0.42 |
| 12 | phloridzin | phenolic acid | 288 | 435 | 23.24 | 0.73 |
| 13 | quercetin | flavonol | 370 | 301 | 23.91 | 3.61 |
The previously tentatively identified compounds were confirmed by matching the m/z value to the molecular weight and the retention time established by available standards.
The presence of phloridzin is characteristic in apple pomaces but is known to be absent in pomaces of other fruit sources.30 It is of particular interest due to its use as a pigment by enzymatic oxidation and its ability as an antidiabetic agent30,31 and for being one of the most stable phytochemicals present in apple pomaces.32
3.5. Proanthocyanidins
To determine whether proanthocyanidins were present, an extract from apple peels was subjected to phloroglucinolysis. Phloroglucinolysis is based on the acid-catalyzed rupture of the interflavanic bond (IB) that links together the constitutive subunits of proanthocyanidiǹs constitutive subunits. The cleavage of the IB leads to the release of a terminal subunit in addition to the formation of an adduct between phloroglucinol and the upper subunit.23
The formation of the epicatechin–phloroglucinol adduct (see the Supporting Information for the chromatogram) allowed the estimation of an mDp of 2.47. This not only indicates that the extract contains proanthocyanidins but also specifies the type B nature of the IB, since type A bonds are not susceptible to IB cleavage, maintaining their terminal subunit and, therefore, preventing the coupling of the nucleophilic agent and the upper subunit from happening.
4. Discussion
4.1. Solvent Selection
The solvents for the experimental section were chosen from those typically employed (ethanol, methanol, ethyl acetate) for polyphenolic extractions from vegetable sources.33 The phenolic content extracted is dependent on the solvent used. As such, phenols with a low molecular weight, phenolic acids, and flavonoid aglycons can be extracted with ethyl acetate. However, in addition to the aforementioned compounds, aqueous alcoholic mixtures (which possess a higher polarity) are used to extract high molecular weight phenols and flavonoid glycosides which, in turn, is reflected in the TPC of the extract.34,35
The milling of the frozen skins was done after the addition of the solvents, in order to minimize oxidation of the compounds as ethanol has proven to decrease polyphenol oxidase (PPO) activity.24 It has been shown that polyphenol antioxidant capacity can be diminished due to enzymes present within the fruit that are released after the cell wall is damaged. It was previously reported that the higher the polyphenol content is, the higher the enzymatic oxidation catalyzed by PPO can be.28,36,37 As has been shown before by van der Sluis et al.,38 epicatechin and chlorogenic acid are the main compounds that present a decrease in total concentration due to the action of PPO (up to 28 and 25%, respectively). However, a positive effect was noted regarding total quercetin glycosides (a 17% increase).38
As depicted in Table 1, the fact that the two extracts with the higher TPC that were from ripe apples are in agreement with the nature of apple pomace, which is obtained after the pressing of mature apples for juice production.
4.2. Apple Pomace Extraction
Aside solvent type, sample–solvent ratio, and extraction time, other factors that affect the yield of polyphenols present in the extracts are temperature (as polyphenols are thermolabile and can easily undergo degradation), pH (a variable that directly affects the structure of some polyphenolic compounds), part of the plant, and particle size39 The latter is the reason behind the milling of the apple pomace, in order to homogenize the particle size as much as possible.
While various apple cultivars can present a TPC that ranges between 76 and 134 mg/100 g FW when extracted with 80% water/ethanol,40 66.2 to 211.9 mg/100 g FW,38 TPC exhibited by Golden Delicious extracts were 86.30 mg/100 g fresh fruit when extracted with 70% acetone–30% water.41 Apple pomaces have been reported to have a remaining polyphenol concentration of between 2.19 ± 0.09 and 4.59 ± 0.47 mg GAE/g dry weight when extracted with 70% methanol–30% water at (60 ± 2 °C) during 30 min,42 while Golden Delicious pomace 2.87 ± 0.75 mg GAE/g powder and 3.05 ± 0.82 mg GAE/g powder when extracted with ethanol and methanol, respectively.43 This is because the majority of polyphenols can be found in the skin, remaining in the pomace. When comparing results, it should be taken into consideration whether they are expressed in FW or dry weight, as this can be interpreted as an increase in the amount of polyphenols present due to the loss of humidity.
Moreover, the differences that can be observed within the various polyphenolic profiles depend not only on the solvent system used (solvent acidity is known to hydrolyze cellulose and pectin, helping release the polyphenols embedded in the matrix)44 but also on the cultivar of apple analyzed (apples intended for cider-making have a higher TPC than apples for human consumption),3,15 the conditions under which the fruit grew,39,45 whether or not the crop was fertilized,46 the part of the apple being used,47 how it was processed, stored, as well as the stage of maturity.
In regard to the coextraction of other molecules which do not show any interest for the purpose of this work, the sample pretreatment can be considered an important preparation step, as coextracted compounds (mainly lipidic and nonpolar compounds) can interfere with polyphenol quantification.16
4.3. Identification of Main Compounds and Polyphenolic Profiling by RP-HPLC-DAD/ESI(−)-MS of Apple Pomace
The major constituents of apple and apple pomace are reported to be chlorogenic acid, epicatechin, quercetin-3-O-glucoside, quercitrin, phloridzin, and quercetin,40 hyperin, avicularin, and rutin (were also found in M. domestica var. rayada, another apple variety cultivated in Mexico)48 all present in the pomace subject of this study. The established polyphenolic profile is in agreement with previously reported findings38 that described that chlorogenic acid and phloridzin can be found in both peel and pulp, while quercetin glycosides and epicatechin are mostly present in apple skin (even though they can be found in lower concentrations within the pulp). Caffeic acid, catechin, some quercetin glycosides and rutin are some other polyphenols that can be extracted from apple pomace.3,15
In total, 12 compounds were identified during the polyphenolic profiling of apple pomace from M. domestica Golden Delicious variety, among them gallic acid, chlorogenic acid, epicatechin, caffeic acid, and quercetin. This was achieved by means of HPLC (with both standards and UV–vis spectra) and HPLC-DAD/ESI-MS. The polyphenolic profiling deemed apple pomace as a suitable source of phenolic compounds and a possible economically interesting alternative to waste disposal of an otherwise underutilized residue with a potentially negative environmental impact.
Acknowledgments
The author’s would like to thank the Facultad de Ciencias Químicas, Universidad Autónoma de Chihuahua (UACH), and the Departamento de Medio Ambiente y Energía, Centro de Investigación en Materiales Avanzados (CIMAV) for allowing this work to be excecuted within their facilities.
Glossary
Abbreviations Used
- CHD
coronary heart disease
- NDGD’s
neurodegenerative diseases
- CVD’s
cardiovascular diseases
- UAE
ultrasound assisted extraction
- HPLC
high performance liquid chromatography
- TPC
total polyphenol content
- RP-HPLC
reversed-phase high performance liquid chromatography
- GAE
gallic acid equivalents
- RP
reversed phase
- RP-HPLC-DAD/ESI-MS
reversed-phase high performance liquid chromatography equipped with a Diode Array Detector/Electrospray ionization mass spectrometry
- mDP
mean degree of polymerization
- FW
fresh weight
- RT
retention time
- IB
interflavanic bond
- PPO
polyphenol oxidase
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c03218.
Chromatogram (at 280 nm) of sample from ripe apple skin extractions; calibration curves for the three different quantifications; chromatograms of TPC (at 280 nm) of precipitate from cold ethanol precipitation and subsequent liquid–liquid extraction; chromatogram (at 280 nm) of sample from commercial apple pomace after phloroglucinolysis (PDF)
Author Contributions
Conceptualization, E.S. and L.B.-C.; methodology, E.S., L.A.O.-F., L.B.-C., and G.G.-S.; formal analysis, B.R.-G. and M.D.R.P.-P.; resources, E.S., L.B.-C., and G.G.-S.; data curation, L.A.O.-F.; writing—original draft, L.A.O.-F.; writing—review and editing, E.S. and L.B.-C.; supervision, G.G.-S., B.R.-G., and M.D.R.P.-P.; project administration, L.B.-C.; funding acquisition, L.B.-C., and E.S. All authors have read and agreed to the published version of the manuscript.
The authors would like to thank the Mexican National Council for Science and Technology (CONACyT, Mexico) for financial support of a student via a doctoral scholarship (number 306675).
The authors declare no competing financial interest.
Special Issue
Published as part of ACS Omegavirtual special issue “Phytochemistry”.
Supplementary Material
References
- Renaud S.-D.; de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992, 339 (8808), 1523–1526. 10.1016/0140-6736(92)91277-F. [DOI] [PubMed] [Google Scholar]
- Quideau S.; Deffieux D.; Douat-Casassus C.; Pouysegu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew. Chem., Int. Ed. Engl. 2011, 50 (3), 586–621. 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- Mourtzinos I.; Goula A.. Polyphenols in agricultural byproducts and food waste. In Polyphenols in plants, 2nd ed.; Watson R.-R., Ed.; Academic Press, 2019; 23–44. [Google Scholar]
- Brglez Mojzer E.; Knez Hrnčič M.; Škerget M.; Knez Ž; Bren U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules. 2016, 21 (7), 901. 10.3390/molecules21070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde E.; Díaz Reinoso B.; González-Munoz M.-J.; Moure A.; Domínguez H.; Parajó J.-C. Recovery and concentration of antioxidants from industrial effluents and from processing streams of underutilized vegetal biomass. Food Public Health 2013, 3 (2), 69–91. 10.5923/fph.20130302.01. [DOI] [Google Scholar]
- Costa C.; Tsatsakis A.; Mamoulakis C.; Teodoro M.; Briguglio G.; Caruso E.; Tsoukalas D.; Margina D.; Dardiotis E.; Kouretas D.; Fenga C. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. 10.1016/j.fct.2017.10.023. [DOI] [PubMed] [Google Scholar]
- Rana S.; Gupta S.; Rana A.; Bhushan S. Functional properties, phenolic constituents and antioxidant potential of industrial apple pomace for utilization as active food ingredient. Food Sci. Hum Wellness. 2015, 4 (4), 180–187. 10.1016/j.fshw.2015.10.001. [DOI] [Google Scholar]
- Rana S.; Kumar S.; Rana A.; Padwad Y.; Bhushan S. Biological activity of phenolics enriched extracts from industrial apple pomace. Ind. Crops Prod. 2021, 160, 113158 10.1016/j.indcrop.2020.113158. [DOI] [Google Scholar]
- Bondonno N.-P.; Bondonno C.-P.; Ward N.-C.; Hodgson J.-M.; Croft K.-D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2017, 69, 243–256. 10.1016/j.tifs.2017.04.012. [DOI] [Google Scholar]
- Kołodziejczyk K.; Sójka M.; Abadias M.; Viñas I.; Guyot S.; Baron A. Polyphenol composition, antioxidant capacity, and antimicrobial activity of the extracts obtained from industrial sour cherry pomace. Ind. Crops Prod. 2013, 51, 279–288. 10.1016/j.indcrop.2013.09.030. [DOI] [Google Scholar]
- Márquez-Rodríguez A.-S.; Nevárez-Baca S.; Lerma-Hernández J.-C.; Hernández-Ochoa L.-R.; Nevárez-Moorillon G.-V.; Gutiérrez-Méndez N.; Muñoz-Castellanos L.-N.; Salas E. In Vitro Antibacterial Activity of Hibiscus sabdariffa L. Phenolic Extract and Its In Situ Application on Shelf-Life of Beef Meat. Foods 2020, 9 (8), 1080. 10.3390/foods9081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu F.; Luiz S.-F.; Azeredo D.-R.-P.; Cruz A.-G.; Ajlouni S.; Ranadheera C.-S. Apple pomace as a functional and healthy ingredient in food products: A Review. Processes 2020, 8 (3), 319. 10.3390/pr8030319. [DOI] [Google Scholar]
- Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (SAGARPA) . Planeación Agrícola Nacional 2017–2030. 1st ed., 2017. https://www.gob.mx/cms/uploads/attachment/file/256430/B_sico-Manzana.pdf (accessed 2022–10–08).
- Rabetafika H.-N.; Bchir B.; Blecker C.; Richel A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends in Food Sci. Technol. 2014, 40 (1), 99–114. 10.1016/j.tifs.2014.08.004. [DOI] [Google Scholar]
- Waldbauer K.; McKinnon R.; Kopp B. Apple pomace as potential source of natural active compounds. Planta Med. 2017, 83 (12/13), 994–1010. 10.1055/s-0043-111898. [DOI] [PubMed] [Google Scholar]
- Araújo M.; Pimentel F.-B.; Alves R.-C.; Oliveira M.-B.-P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends in Food Sci. Technol. 2015, 45 (2), 200–211. 10.1016/j.tifs.2015.06.010. [DOI] [Google Scholar]
- Virot M.; Tomao V.; Le Bourvellec C.; Renard C.-M.; Chemat F. Towards the industrial production of antioxidants from food processing by-products with ultrasound-assisted extraction. Ultrason Sonochemistry. 2010, 17 (6), 1066–1074. 10.1016/j.ultsonch.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Medina-Torres N.; Ayora-Talavera T.; Espinosa-Andrews H.; Sánchez-Contreras A.; Pacheco N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy. 2017, 7 (3), 47. 10.3390/agronomy7030047. [DOI] [Google Scholar]
- Vilkhu K.; Mawson R.; Simons L.; Bates D. Applications and opportunities for ultrasound assisted extraction in the food industry—A Review. Innovative Food Sci. Emerging Technol. 2008, 9 (2), 161–169. 10.1016/j.ifset.2007.04.014. [DOI] [Google Scholar]
- Kentish S.; Feng H. Applications of power ultrasound in food processing. Annu. Rev. Food Sci. Technol. 2014, 5 (1), 263–284. 10.1146/annurev-food-030212-182537. [DOI] [PubMed] [Google Scholar]
- Singleton V.-L.; Rossi J.-A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16 (3), 144–158. 10.5344/ajev.1965.16.3.144. [DOI] [Google Scholar]
- Salas E.; Fulcrand H.; Poncet-Legrand C.; Meudec E.; Köhler N.; Winterhalter P.; Cheynier V. Isolation of flavanol-anthocyanin adducts by countercurrent chromatography. J. Chromatogr Sci. 2005, 43 (9), 488–493. 10.1093/chromsci/43.9.488. [DOI] [PubMed] [Google Scholar]
- Kennedy J.-A.; Jones G.-P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49 (4), 1740–1746. 10.1021/jf001030o. [DOI] [PubMed] [Google Scholar]
- Kaur C.; Kapoor H.-C. Inhibition of enzymatic browning in apples, potatoes and mushrooms. J. Sci. Ind. Res. 2000, 59 (5), 389–394. [Google Scholar]
- Xu J.; Yue R.-Q.; Liu J.; Ho H.-M.; Yi T.; Chen H.-B.; Han Q.-B. Structural diversity requires individual optimization of ethanol concentration in polysaccharide precipitation. Int. J. Biol. Macromol. 2014, 67, 205–209. 10.1016/j.ijbiomac.2014.03.036. [DOI] [PubMed] [Google Scholar]
- Kim D.-O.; Lee C.-Y. Extraction and isolation of polyphenolics. Curr. Protoc Food Anal Chem. 2002, 6 (1), I1–2. 10.1002/0471142913.fai0102s06. [DOI] [Google Scholar]
- van der Sluis A.-A.; Dekker M.; van Boekel M.-A. Activity and concentration of polyphenolic antioxidants in apple juice. 3. Stability during storage. J. Agric. Food Chem. 2005, 53 (4), 1073–1080. 10.1021/jf040270r. [DOI] [PubMed] [Google Scholar]
- Francini A.; Sebastiani L. Phenolic compounds in apple (Malus x domestica Borkh.): compounds characterization and stability during postharvest and after processing. Antioxidants. 2013, 2 (3), 181–193. 10.3390/antiox2030181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perussello C.-A.; Zhang Z.; Marzocchella A.; Tiwari B.-K. Valorization of apple pomace by extraction of valuable compounds. Compr Rev. Food Sci. Food Saf. 2017, 16 (5), 776–796. 10.1111/1541-4337.12290. [DOI] [PubMed] [Google Scholar]
- Kammerer D.-R.; Kammerer J.; Valet R.; Carle R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res. Int. 2014, 65, 2–12. 10.1016/j.foodres.2014.06.012. [DOI] [Google Scholar]
- Masumoto S.; Akimoto Y.; Oike H.; Kobori M. Dietary phloridzin reduces blood glucose levels and reverses Sglt1 expression in the small intestine in streptozotocin-induced diabetic mice. J. Agic Food Chem. 2009, 57 (11), 4651–4656. 10.1021/jf9008197. [DOI] [PubMed] [Google Scholar]
- Lavelli V.; Corti S. Phloridzin and other phytochemicals in apple pomace: Stability evaluation upon dehydration and storage of dried product. Food Chem. 2011, 129 (4), 1578–1583. 10.1016/j.foodchem.2011.06.011. [DOI] [Google Scholar]
- Ameer K.; Shahbaz H.-M.; Kwon J.-H. Green extraction methods for polyphenols from plant matrices and their byproducts: A Review. Compr Rev. Food Sci. Food Saf. 2017, 16 (2), 295–315. 10.1111/1541-4337.12253. [DOI] [PubMed] [Google Scholar]
- Oreopoulou V.; Tzia C.. Utilization of plant by-products for the recovery of proteins, dietary fibers, antioxidants, and colorants. In Utilization of by-products and treatment of waste in the food industry. Proceedings of the Integrating Food Science and Engineering Knowledge Into Food Studies towards European Sustainable Development (ISEKI-Food); Oreopoulou V.; Russ W., Eds; Springer: Boston, MA., 2007; 3, 209–232. [Google Scholar]
- Lai W.-T.; Khong N.-M.-H.; Lim S.-S.; Hee Y.-Y.; Sim B.-I.; Lau K.-Y.; Lai O.-M. A review: Modified agricultural by-products for the development and fortification of food products and nutraceuticals. Trends Food Sci. Technol. 2017, 59, 148–160. 10.1016/j.tifs.2016.11.014. [DOI] [Google Scholar]
- Cheynier V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81 (1), 223S–229S. 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- Morante Carriel J.; Agnieszka-Obrebska A.; Bru-Martínez R.; Carranza Patiño M.; Pico-Saltos R.; Nieto Rodriguez E. Distribución, localización e inhibidores de las polifenol oxidasas en frutos y vegetales usados como alimento. Ciencia y Tecnología. 2014, 7 (1), 23–31. 10.18779/cyt.v7i1.95. [DOI] [Google Scholar]
- van der Sluis A.-A.; Dekker M.; de Jager A.; Jongen W.-M. Activity and concentration of polyphenolic antioxidants in apple: effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49 (8), 3606–3613. 10.1021/jf001493u. [DOI] [PubMed] [Google Scholar]
- Jancheva M.; Grigorakis S.; Loupassaki S.; Makris D.-P. Optimized extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J. Appl. Res. Med. Aromat Plants. 2017, 6, 31–40. 10.1016/j.jarmap.2017.01.002. [DOI] [Google Scholar]
- Lachman J.; Šulc M.; Sus J.; Pavlíková O. Polyphenol content and antiradical activity in different apple varieties. Hort Sci. (Prague). 2006, 33 (3), 95–102. 10.17221/3745-HORTSCI. [DOI] [Google Scholar]
- Vrhovsek U.; Rigo A.; Tonon D.; Mattivi F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52 (21), 6532–6538. 10.1021/jf049317z. [DOI] [PubMed] [Google Scholar]
- Rana S.; Rana A.; Gupta S.; Bhushan S. Varietal influence on phenolic constituents and nutritive characteristics of pomace obtained from apples grown in western Himalayas. J. Food Sci. Technol. 2021, 58 (1), 166–174. 10.1007/s13197-020-04526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Wei X.; Miao Z.; Hassan H.; Song Y.; Fan M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent J. 2016, 10 (1), 1–9. 10.1186/s13065-016-0195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana B.; Anwar F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108 (3), 879–884. 10.1016/j.foodchem.2007.11.053. [DOI] [PubMed] [Google Scholar]
- Guyot S.; Marnet N.; Sanoner P.; Drilleau J.-F. Variability of the polyphenolic composition of cider apple (Malus domestica) fruits and juices. J. Agric. Food Chem. 2003, 51 (21), 6240–6247. 10.1021/jf0301798. [DOI] [PubMed] [Google Scholar]
- Fratianni F.; Sada A.; Cipriano L.; Masucci A.; Nazzaro F. Biochemical characteristics, antimicrobial and mutagenic activity in organically and conventionally produced Malus domestica, Annurca. Open Food Sci. J. 2007, 1 (1), 10–16. 10.2174/1874256400701010010. [DOI] [Google Scholar]
- Petkovsek M.-M.; Slatnar A.; Stampar F.; Veberic R. The influence of organic/integrated production on the content of phenolic compounds in apple leaves and fruits in four different varieties over a 2-year period. J. Sci. Food Agric. 2010, 90 (14), 2366–2378. 10.1002/jsfa.4093. [DOI] [PubMed] [Google Scholar]
- Cerda-Tapia A.; Pérez-Chabela M.-L.; Pérez-Álvarez J.-Á.; Fernández-López J.; Viuda-Martos M. Valorization of pomace powder obtained from native Mexican apple (Malus domestica var. rayada): chemical, techno-functional and antioxidant properties. Plant Foods Hum Nutr. 2015, 70 (3), 310–316. 10.1007/s11130-015-0495-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.